Abstract
Provided herein are novel CD73 inhibitors, pharmaceutical compositions, use of such compounds in treating cancer, and processes for preparing such compounds.
Important Compound Classes
Title
CD73 Compounds
Patent Publication Number
WO 2024/006929 A1
Publication Date
January 4, 2024
Priority Application
US 63/357,948
Priority Date
July 1, 2022
Inventors
Bartlett, M. J.; Chin, G. F.; Cosman Ellis, J. L.; Mackman, R. L.; Mish, M. R.
Assignee Company
Gilead Sciences Inc., USA
Disease Area
Cancer
Biological Target
CD73
Summary
The glycosyl-phosphatidylinositol (GPI)-anchored CD73 antigen (also known as Cluster of Differentiation 73, ecto-5′-nucleotidase, ecto-5′-NT, 5′-NT, and NT5E) is considered the rate-limiting enzyme in the generation of extracellular adenosine. CD73 is a 70-kDa GPI-anchored protein normally expressed on endothelial cells and subsets of hematopoietic cells. CD73, together with CD39, regulates adenosine triphosphate (ATP) metabolism. CD39 converts ATP into AMP, with only trace amounts of ADP being released, while CD73 catalyzes the conversion of AMP to adenosine (Ado).
Extracellular Ado accumulates in cancerous tissues and constitutes an important mechanism of tumor immune escape. CD73 can be found constitutively expressed at high levels on various types of cancer cells. CD73-generated adenosine is assumed to suppress adaptive antitumor immune responses, thereby promoting tumor growth and metastasis. The studies in animal models have shown that blockade of CD73 activity suppresses tumor growth and prolongs survival by promoting antitumor adaptive immunity.
The present application describes a series of novel CD73 inhibitors for the treatment of cancer. Further, the application discloses compounds, their preparation, use, and pharmaceutical composition, and treatment.
Definitions
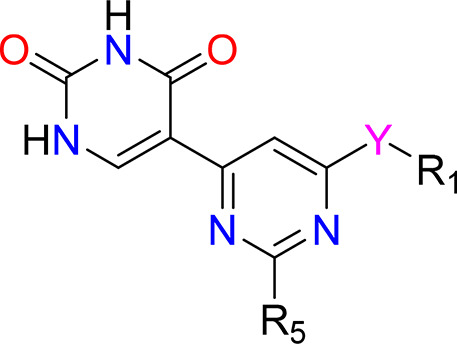
Y = C1–6 alkyl, C3–7 cycloalkyl, O-C1–6 alkyl-O, 4–8 membered heterocyclyl or O-4–8 membered heterocyclyl;
R1 = H, C1–6 alkyl, O-C1–6 alkyl, O-C1–6 alkyl-O, C3–7 cycloalkyl, O-(4–12 membered heteroaryl), C6–10 aryl, 4–12 membered heteroaryl, C1–6 alkyl-C6–10 aryl, C1–6 alkyl-4–12 membered heteroaryl, C(O)N(R4)(R4), C(O)N(H)C6–12 aryl; and
R5 = H, C1–6 alkyl, CN, C3–7 cycloalkyl, O-C1–6 alkyl, C1–6 alkyl-O-C1–6 alkyl.
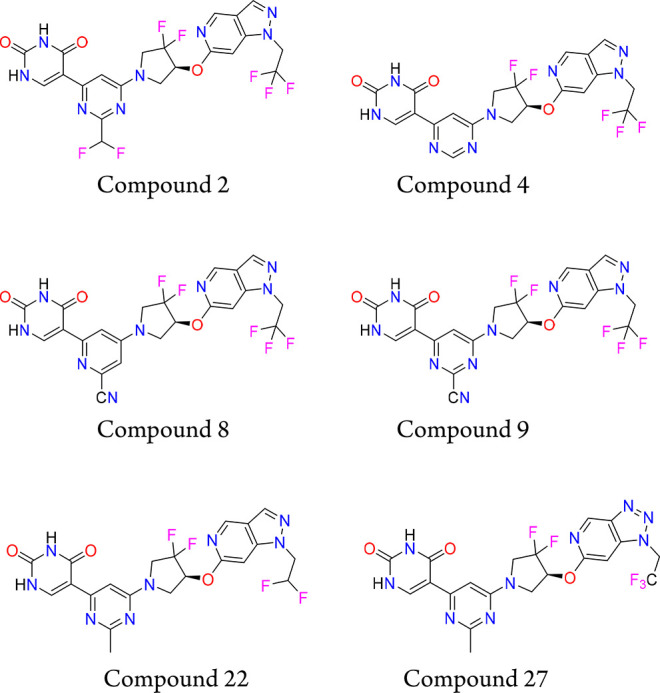
Key Structures
Biological Assay
The CD73 biochemical assay was performed. The compounds described in this application were tested for their ability to inhibit CD73. The CD73 IC50 values (nM) are shown in the following table.
Biological Data
The table below shows representative
compounds that were tested for CD73 inhibition and the biological
data obtained from testing representative examples.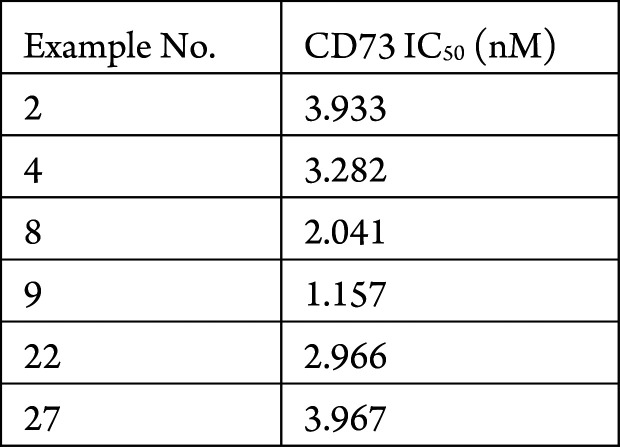
Claims
Total claims: 15
Compound claims: 13
Pharmaceutical composition claims: 1
Method of treatment claims: 1
Recent Review Articles
The author declares no competing financial interest.
References
- Ge G.; Wang Q.; Zhang Z.; Zhang X.; Guo S.; Zhang T.; Meng F. Small molecular CD73 inhibitors: Recent progress and future perspectives. Eur. J. Med. Chem. 2024, 264, 116028. 10.1016/j.ejmech.2023.116028. [DOI] [PubMed] [Google Scholar]
- Vilbois S.; Xu Y.; Ho P. Metabolic interplay: tumor macrophages and regulatory T cells. Trends Cancer 2024, 10, 242–255. 10.1016/j.trecan.2023.11.007. [DOI] [PubMed] [Google Scholar]
- Bisht K.; Fukao T.; Chiron M.; Richardson P.; Atanackovic D.; Chini E.; Chng W. J.; Van De Velde H.; Malavasi F. Immunomodulatory properties of CD38 antibodies and their effect on anticancer efficacy in multiple myeloma. Cancer Med. 2023, 12, 20332–20352. 10.1002/cam4.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsleichter N. E.; Azambuja J. H.; Rubenich D. S.; Braganhol E. CD73 in glioblastoma: Where are we now and what are the future directions?. Immunol. Lett. 2023, 256–257, 20–27. 10.1016/j.imlet.2023.03.005. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Dai X.; Xiang Y.; Xie L.; Sun M.; Shi J. Advances in CD73 inhibitors for immunotherapy: Antibodies, synthetic small molecule compounds, and natural compounds. Eur. J. Med. Chem. 2023, 258, 115546. 10.1016/j.ejmech.2023.115546. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Jin S.; Zhuang Q.; Liu N.; Chen R.; Adam S. A.; Jin J.; Sun J. Chimeric antigen receptor natural killer cells: a promising antitumor immunotherapy. MedComm 2023, 4, e422. 10.1002/mco2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]


