Abstract

Dysregulation of the Hippo pathway has been observed in various cancers. The transcription factor TEAD, together with its coactivators YAP/TAZ, plays a crucial role in regulating the transcriptional output of the Hippo pathway. Recently, extensive research has focused on small molecule inhibitors targeting TEAD, but studies on TEAD degraders are comparatively rare. In this study, we designed and synthesized a series of TEAD PROTACs by connecting a pan-TEAD inhibitor with the CRBN ligand thalidomide. A representative compound, 27, exhibited potent antiproliferative activity against NF2-deficient NCI-H226 cells. It dose-dependently induced TEAD degradation dependent on CRBN and proteasome system and decreased key YAP target genes CYR61 and CTGF expressions in NCI-H226 cells. Further degradation selectivity studies revealed that 27 exhibited more potent activity against TEAD2 compared to those of the other three family members in Flag-TEADs transfected 293T cells. Therefore, 27 may serve as a valuable tool for advancing biological studies related to TEAD2.
Keywords: Hippo pathway, TEAD, PROTAC, Selectivity
The transcriptional enhanced associate domains (TEADs) are evolutionarily conserved transcription factors that play critical roles in a variety of physiological processes and human diseases.1 The TEAD family in mammals comprises four highly homologous members, TEAD1–4, each differentially expressed in tissues.1 Typically, TEAD possesses low intrinsic transcriptional activity and requires coactivators to initiate gene transcription.2 The most important and well-studied coactivators are Yes-associated protein (YAP) and its paralog, transcriptional coactivator with PDZ-binding motif (TAZ).3 The formation of the YAP/TAZ–TEAD transcriptional complex is tightly regulated by the Hippo pathway.4 Under normal conditions, the upstream mammalian STE20-like protein kinase 1/2 (MST1/2) phosphorylate and activate the large tumor suppressor 1/2 (LATS1/2),4 and this process can be facilitated by the tumor suppressor Merlin which is encoded by NF2 (Neurofibromatosis Type 2).5 The activated LATS1/2 phosphorylate YAP/TAZ, leading to their retention in the cytoplasm and subsequent degradation via the ubiquitin–proteasome system, thereby limiting tissue growth and cell proliferation.4 Dysregulation of the Hippo signaling pathway has been implicated in various carcinomas, including lung,6 gastric,7 prostate,8 and colorectal cancers,9 and is associated with poor outcomes. In cancer cells, the dephosphorylated YAP/TAZ translocate into the nucleus, bind to TEAD, and drive the transcriptions of target oncogenes critical for cancer development.1 Apart from interacting with the coactivators, the transcriptional activity of TEAD is also modulated by the post-translational modifications such as phosphorylation10 and palmitoylation.11 Specifically, the S-palmitoylation is important for TEAD’s stability and function, while its depalmitoylation disrupts the association with YAP and the consequent transcriptional activity.11,12
Targeting the Hippo pathway has been engaged as a promising strategy for cancer therapy. As the upstream kinases LATS1/2 and MST1/2 act as tumor suppressors,9 the conventional kinase inhibitors are unlikely to work. Directly targeting the intrinsically disordered proteins YAP/TAZ is also challenging. However, since TEAD requires pairing with YAP/TAZ to promote cell growth, disrupting the YAP–TEAD protein–protein interaction (PPI) could be an efficient therapeutic strategy in cancer.13 Several peptide-based inhibitors have been designed to bind to the TEAD surface pockets, thereby directly disrupting the YAP–TEAD interaction.14 Additionally, small molecule PPI inhibitors, such as compound 1 (Figure 1), have also achieved impressive progression.15 A representative PPI inhibitor, IAG-933, is currently being evaluated in a Phase I clinical trial (NCT04857372).
Figure 1.
Representative small molecule TEAD ligands.
Given that the palmitoylation of TEAD is also critical for its transcriptional activity, small molecules targeting the central lipophilic palmitate-binding pocket have gained significant interest in recent years. Two distinct types of compounds, based on their binding modes, have been reported. These include reversibly binding compounds and irreversible inhibitors. Compounds that reversibly bind to this pocket elicit varying effects on the Hippo pathway. For instance, compound 2 (Q2, Figure 1) acts as an activator of TEAD, enhancing YAP–TEAD transcriptional activity.16 In contrast, other reported reversible compounds 3–9 (Figure 1) function as inhibitors.14 Additionally, compounds capable of forming covalent bonds with the conserved cysteine residue in the palmitate-binding pocket have been extensively studied. Representative covalent inhibitors with electrophilic warheads such as vinylsulfonamide (10),17 acrylamide (11 and 12),18,19 chloromethyl ketone (13),20 kojic acide (14),21 and cyanamide (15)22 are shown in Figure 1. Furthermore, the inhibitors vary in their effectiveness in disrupting the YAP–TEAD interaction. For example, compound 5 only inhibits the palmitoylation of TEAD,23 while others can simultaneously inhibit the YAP–TEAD interaction.14 Notably, two inhibitors binding to the central pocket, VT3989 (NCT04665206) and IK-930 (NCT05228015), are currently undergoing clinical developments. VT3989 has demonstrated favorable tolerance and preliminary clinical efficiency in its Phase I clinical trial.24
In addition to the targeted inhibition strategy, over the past decades, targeted protein degradation mediated by proteolysis targeting chimeras (PROTACs) or molecular glues has emerged as a promising approach for modulating the function of disease-related proteins. A PROTAC is a heterobifunctional molecule composed of a ligand for the protein of interest (POI), a chemical linker, and a ligand for the E3 ubiquitin ligase. PROTACs can recruit the E3 complex to the targeted protein, leading to its ubiquitination and subsequent degradation.25 This mode of action results in the destruction of all possible functions of the targeted protein.26 Furthermore, the development of isoform-selective inhibitors could be a significant challenge owing to the high structural similarity among traditional inhibitor binding sites across various isoforms. Nevertheless, a range of PROTACs derived from pan-inhibitors have successfully achieved isoform-selective degradation.27−29 This may be attributed to the fact that PROTAC requires the formation of a POI–PROTAC–E3 ternary complex to induce protein degradation. Each isoform can potentially form distinct ternary complexes, which differ in their capacities of inducing protein degradation.25,26,28
Currently, most of the reported TEAD inhibitors are pan-TEAD inhibitors. Only a few inhibitors, such as IK-93030 and VT103,31 have demonstrated a degree of selectivity for TEAD1 among the four family members. Considering the distinct modes of action between inhibitors and degraders along with the potential for achieving isoform-selective degradation, we thought it would be valuable to explore TEAD degraders based upon the PROTAC concept. During the course of this project, some related patents disclosed their TEAD PROTACs.32,33 Herein, we report our efforts in the design, synthesis, and bioevaluation of a series of TEAD PROTACs.
The reported pan-TEAD inhibitor VT107 was initially docked into the TEAD3 protein (PDB: 7CNL).31 The results indicated that the amino group on the pyridine ring is oriented toward the solvent region, which presents a suitable site for attaching the E3 ligase ligand (Figure 2A). Considering the wide use of the CRBN ligand in developing clinical oral PROTACs, thalidomide was selected as the E3 ligand. The putative TEAD PROTACs 16–28 were then designed by connecting VT107 from the amino group to both positions 1′ and 2′ of thalidomide using various linkers (Figure 2B).
Figure 2.

(A) Docking model of VT107 with TEAD3 (PDB: 7CNL). (B) Design of putative TEAD PROTACs 16–28.
The general synthesis route is briefly outlined in Scheme 1. We began with commercially available 5-bromo-2-naphthoic acid (29). Compound 31 was synthesized by coupling 29 with (4-(trifluoromethyl)phenyl)boronic acid (30) through a Suzuki coupling reaction. The resulting acid 31 was then reacted with amine 32 to yield compound 33. This compound underwent an Ullmann coupling reaction with indicated amine 34, producing compounds 35. The Boc protecting group of 35 was removed by using trifluoroacetic acid (TFA), and the resulting amines were subsequently reacted with F-substituted thalidomides 36 under conditions used for nucleophilic aromatic substitution reactions, yielding the final compounds 16–28.
Scheme 1. Synthesis of Compounds 16–28.
Reagents and conditions: (a) Pd(dppf)CH2Cl2, Na2CO3, 1,4-dioxane: H2O = 10:1 (v/v), 90 °C, 16 h; (b) HOBt, EDCI, DIPEA, THF, r.t., 16 h; (c) l-proline, CuI, K3PO4, DMSO, 100 °C, 16 h; (d) two steps: (1) TFA, DCM, r.t., 0.5 h; (2) DIPEA, DMSO, 90 °C, 16 h.
We first evaluated the antiproliferative activities of our synthesized compounds in NF2-deficient NCI-H226 cells, with the results outlined in Table 1. Compounds 16–22, which bear alkyl linkers of varying lengths, displayed comparable antiproliferative activities, with potency around 1 μM. However, these compounds were significantly less potent than the pan-TEAD inhibitor VT107. To improve the solubility of the putative PROTACs, we synthesized and tested the three compounds 23–25 featuring ether linkers. These compounds exhibited improved inhibitory activities, with IC50 values of 0.34, 0.29, and 0.26 μM, respectively. Additionally, we explored the design of PROTACs by using the different tethering position of thalidomide. Three new compounds 26–28 with the same linkers as 23–25 were obtained. These new compounds displayed similar antiproliferative activities to their parental compounds, with the most potent one being 27 (IC50 = 0.21 μM). Compound 27 was further assessed in NCI-H2452 cells with wild-type NF2, and it was much less potent in NCI-H2452 cells (IC50 > 10 μM, Figure S1).
Table 1. Anti-proliferative Activities of Compounds 16–28 and VT107 in NCI-H226 Cells.

NCI-H226 cells were treated with compounds at indicated concentrations for 5 days. IC50 values are shown as mean ± SEM from four independent replicates.
Compounds 24, 25, 27, and 28, which showed promising cellular activities, were selected to assess the degradation activity in NCI-H226 cells. Given that these putative PROTACs were generated from a pan-TEAD inhibitor, we initially assessed the degradation of TEAD using the pan-TEAD antibody in Western blot assays. The cells were treated with the selected compounds at three concentrations of 0.1, 1, and 10 μM for 24 h. All the tested compounds displayed some degree of degradation activity at 1 and 10 μM, in which compounds 25 and 27 induced TEAD degradation by 45.1% and 42.4% at 0.1 μM, respectively (Figure 3A). Compounds 25 and 27 with better degradation potency were further tested in a broad range of concentrations, and the DC50s (half-maximal degradation concentrations) were obtained as 39.7 and 32.7 nM, respectively (Figure 3B).
Figure 3.
Degradation activities of PROTACs 24, 25, 27, and 28. (A) NCI-H226 cells were treated with 0.1, 1, and 10 μM of different compounds for 24 h. (B) NCI-H226 cells were treated with indicated concentrations of 25 or 27 for 24 h. Quantification for pan-TEAD is shown on the right. Quantified data represent mean ± SEM from three independent replicates.
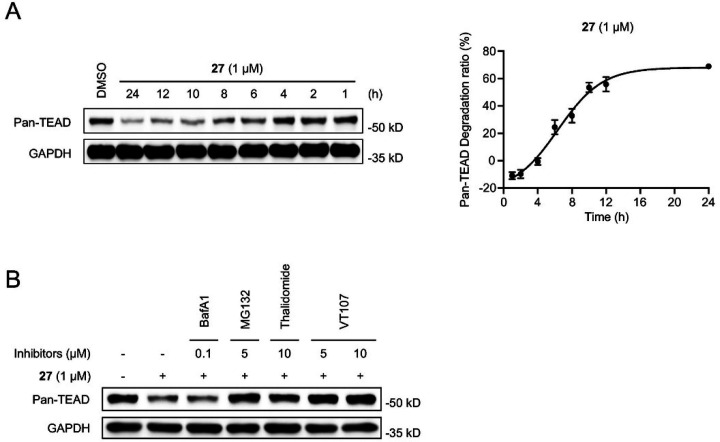
Compound 27 was further employed to investigate the degradation kinetics in the NCI-H226 cells. The cells were exposed to 1 μM 27 for durations ranging from 1 to 24 h. As illustrated in Figure 4A, significant TEAD degradation was observed at 10 h, and maximal degradation was achieved at 24 h. We then explored the mechanisms of action of the newly synthesized TEAD PROTAC. A series of rescue experiments were performed (Figure 4B). The TEAD degradation was significantly attenuated when the NCI-H226 cells were pretreated with excess TEAD inhibitor VT107 or CRBN ligand thalidomide. These data indicate that the degradation of TEAD by compound 27 requires its binding to the TEAD and CRBN proteins. Additionally, pretreatment with the proteasome inhibitor MG132 blocked TEAD degradation, implying proteasome-mediated degradation. Conversely, pretreatment with the lysosomal inhibitor Bafilomycin A1 (BafA1) did not significantly reverse the reduction of TEAD protein levels, indicating that the degradation is not dependent on the lysosomal pathway.
Figure 4.
Degradation kinetics and mechanisms of action of PROTAC 27. (A) Western blot analysis of pan-TEAD degradation kinetics with compound 27 in NCI-H226 cells. NCI-H226 cells were treated with 1 μM 27 for indicated time points. Quantification for pan-TEAD is plotted on the right. Quantified data represents mean ± SEM from three independent replicates. (B) NCI-H226 cells were pretreated with BafA1, MG132, Thalidomide, and VT107 for 1 h, followed by an 8 h cotreatment with 27. The indicated protein levels were determined by Western blot analysis.
NF2 deficiency induces YAP activation and nuclear localization, subsequently impacting Hippo pathway target genes expression through interaction with TEAD. To further explore the effect of compound 27 on the Hippo pathway, the NCI-H226 cells were treated with 27 at two concentrations of 0.5 and 1 μM for 8 h. Compound 27 concentration-dependently decreased YAP target genes CYR61, IGFBP3, AMOTL2, CTGF, and NPPB expression (Figure 5A), while it induced the upregulation of the FBXO32 gene (Figure 5B).
Figure 5.
Effects on transcription of CYR61, IGFBP3, AMOTL2, CTGF, NPPB (A) and FBXO32 (B) in NCI-H226 cells measured by qPCR. Cells were treated with 27 at 0.5 or 1 μM for 8 h. Data represent mean ± SEM from three independent replicates.
The degradation selectivity of compound 27 was finally evaluated. Recently, Wenzhi Ji et al. reported the first-in-class programmed cell death protein 2 (PDCD2) degraders, employing the same POI ligand and E3 ligand as those used in compound 27, but with different linker moieties.34 To assess the PDCD2 degradation activity of our compound, NCI-H226 cells were treated with compound 27 at four concentrations of 0.01, 0.1, 1, and 10 μM for 24 h. The results revealed that compound 27 did not induce significant PDCD2 degradation at these concentrations (Figure S2). Considering that PROTACs derived from nonselective ligands can potentially achieve isoform-selective degradation, we then explored the degradation selectivity of compound 27 among the four TEAD family members. Due to the lack of highly specific TEAD1–4 antibodies, an alternative approach was performed to assess the degradation selectivity. The 293T cells were transfected with Flag-tagged TEAD1–4, respectively. These cells were pretreated with cycloheximide for 2 h and subsequently treated with compound 27 at various concentrations for another 24 h. As shown in Figure 6, 27 exhibited significant degradation activity against Flag-TEAD2 with a DC50 value of 54.1 nM in 293T cells, and it showed relatively low Flag-TEAD1 degradation activity with maximal degradation around 40.0%. In contrast, compound 27 induced no obvious Flag-TEAD3 or Flag-TEAD4 degradation in the corresponding Flag-TEAD transfected 293T cells. These results suggest that compound 27 acts as a TEAD2 selective degrader. However, the degradation selectivity of compound 27 toward endogenous TEAD family proteins requires further investigation across more cancer cell lines. Additionally, more explorations are needed to determine whether the linker structure influences isoform selectivity and to elucidate the underlying molecular mechanisms responsible for the observed degradation selectivity.
Figure 6.
Degradation selectivity of PROTAC 27. 293T cells transfected with Flag-TEAD1, Flag-TEAD2, Flag-TEAD3, or Flag-TEAD4 were pretreated with 200 μg/mL cycloheximide (CHX) for 2 h, followed by a 24 h treatment of 27 at the indicated concentrations. Different Flag-tagged TEAD isoforms were detected by using an anti-Flag antibody. Quantified data represents mean ± SEM from three independent replicates.
In summary, we designed and synthesized a series of heterobifunctional molecules by connecting pan-TEAD inhibitor VT107 and CRBN ligand thalidomide by using various linkers. The antiproliferative activities of the obtained compounds were first assessed in the NF2-deficient NCI-H226 cancer cells, and resulted in several compounds with submicromolar activities. The representative compound 27 induced TEAD degradation in a dose-dependent manner, relying on CRBN and the proteasome in NCI-H226 cells. It also down-regulated YAP target genes CYR61, IGFBP3, AMOTL2, CTGF, and NPPB expressions. Preliminary isoform degradation selectivity studies in Flag-TEAD transfected 293T cells revealed that compound 27 is an effective and selective degrader of TEAD2. Further structural modifications, particularly focused on the linker and the E3 ligase ligand, may provide us with more potent and isoform-selective TEAD degraders, which can be used for biological research and drug development.
Acknowledgments
The authors would like to thank the financial support from the National Natural Science Foundation of China (22107110 to T. Xu, T2225002 to M. Zheng) and the Youth Innovation Promotion Association CAS (2023296 to S. Zhang).
Glossary
ABBREVIATIONS
- TEAD
transcriptional enhanced associate domain
- YAP
yes-associated protein
- TAZ
transcriptional coactivator with PDZ-binding motif
- MST1/2
mammalian STE20-like protein kinase 1/2
- LATS1/2
large tumor suppressor 1/2
- NF2
neurofibromatosis type 2
- PPI
protein–protein interaction
- PROTACs
proteolysis targeting chimeras
- POI
protein of interest
- HOBt
1-hydroxybenzotriazole
- EDCI
1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide
- DIPEA
N,N-diisopropylethylamine
- THF
tetrahydrofuran
- TFA
trifluoroacetic acid
- DCM
dichloromethane
- DMSO
dimethyl sulfoxide
- BafA1
Bafilomycin A1
- DC50s
half-maximal degradation concentrations
- CTGF
connective tissue growth factor
- IGFBP3
insulin-like growth factor-binding protein 3
- AMOTL2
angiomotin-like 2
- CYR61
cysteine-rich angiogenic inducer 61
- NPPB
natriuretic peptide B
- FBXO32
F-box only protein 32
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.4c00029.
Details of biological assays and chemical synthesis (PDF)
Author Contributions
# H. Li and Z. Ge contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Lin K. C.; Park H. W.; Guan K. L. Regulation of the Hippo Pathway Transcription Factor TEAD. Trends Biochem. Sci. 2017, 42 (11), 862–872. 10.1016/j.tibs.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J. H.; Davidson I.; Matthes H.; Garnier J. M.; Chambon P. Cloning, Expression, and Transcriptional Properties of the Human Enhancer Factor Tef-1. Cell 1991, 65 (4), 551–568. 10.1016/0092-8674(91)90088-G. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Ye X.; Yu J. D.; Li L.; Li W. Q.; Li S. M.; Yu J. J.; Lin J. D.; Wang C. Y.; Chinnaiyan A. M.; Lai Z. C.; Guan K. L. TEAD mediates YAP-dependent gene induction and growth control. Gene Dev 2008, 22 (14), 1962–1971. 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z. P.; Moroishi T.; Guan K. L. Mechanisms of Hippo pathway regulation. Gene Dev 2016, 30 (1), 1–17. 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli A. M.; Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016, 35 (5), 537–548. 10.1038/onc.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau A. N.; Curtis S. J.; Fillmore C. M.; Rowbotham S. P.; Mohseni M.; Wagner D. E.; Beede A. M.; Montoro D. T.; Sinkevicius K. W.; Walton Z. E.; Barrios J.; Weiss D. J.; Camargo F. D.; Wong K. K.; Kim C. F. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014, 33 (5), 468–481. 10.1002/embj.201386082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. H.; Li B. Z.; Chen Y.; Wang J. TEADs serve as potential prognostic biomarkers and targets for human gastric cancer. Bmc Gastroenterol 2022, 22 (1), 308. 10.1186/s12876-022-02386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem O.; Hansen C. G. The Hippo Pathway in Prostate Cancer. Cells 2019, 8 (4), 370. 10.3390/cells8040370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K. F.; Zhang X. M.; Thomas D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 2013, 13 (4), 246–257. 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- Gupta M. P.; Kogut P.; Gupta M. Protein kinase-A dependent phosphorylation of transcription enhancer factor-1 represses its DNA-binding activity but enhances its gene activation ability. Nucleic Acids Res. 2000, 28 (16), 3168–3177. 10.1093/nar/28.16.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P.; Han X.; Zheng B. H.; Deran M.; Yu J. Z.; Jarugumilli G. K.; Deng H.; Pan D. J.; Luo X. L.; Wu X. Autopalmitoylation of TEAD proteins regulates transcriptional output of the Hippo pathway. Nat. Chem. Biol. 2016, 12 (4), 282–289. 10.1038/nchembio.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland C. L.; Gierke S.; Schnier P. D.; Murray J.; Sandoval W. N.; Sagolla M.; Dey A.; Hannoush R. N.; Fairbrother W. J.; Cunningham C. N. Palmitoylation of TEAD Transcription Factors Is Required for Their Stability and Function in Hippo Pathway Signaling. Structure 2016, 24 (1), 179–186. 10.1016/j.str.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Pobbati A. V.; Kumar R.; Rubin B. P.; Hong W. J. Therapeutic targeting of TEAD transcription factors in cancer. Trends Biochem. Sci. 2023, 48 (5), 450–462. 10.1016/j.tibs.2022.12.005. [DOI] [PubMed] [Google Scholar]
- Lou J. F.; Lu Y. H.; Cheng J.; Zhou F. L.; Yan Z. Q.; Zhang D. Z.; Meng X. J.; Zhao Y. J. A chemical perspective on the modulation of TEAD transcriptional activities: Recent progress, challenges, and opportunities. Eur. J. Med. Chem. 2022, 243, 114684 10.1016/j.ejmech.2022.114684. [DOI] [PubMed] [Google Scholar]
- Sellner H.; Chapeau E.; Furet P.; Voegtle M.; Salem B.; Le Douget M.; Bordas V.; Groell J. M.; Le Goff A. L.; Rouzet C.; Wietlisbach T.; Zimmermann T.; McKenna J.; Brocklehurst C. E.; Chene P.; Wartmann M.; Scheufler C.; Kallen J.; Williams G.; Harlfinger S.; Traebert M.; Dumotier B. M.; Schmelzle T.; Soldermann N. Optimization of a Class of Dihydrobenzofurane Analogs toward Orally Efficacious YAP-TEAD Protein-Protein Interaction Inhibitors. ChemMedChem. 2023, 18, e202300051 10.1002/cmdc.202300051. [DOI] [PubMed] [Google Scholar]
- Pobbati A. V.; Mejuch T.; Chakraborty S.; Karatas H.; Bharath S. R.; Gueret S. M.; Goy P. A.; Hahne G.; Pahl A.; Sievers S.; Guccione E.; Song H. W.; Waldmann H.; Hong W. J. Identification of Quinolinols as Activators of TEAD-Dependent Transcription. ACS Chem. Biol. 2019, 14 (12), 2909–2921. 10.1021/acschembio.9b00786. [DOI] [PubMed] [Google Scholar]
- Lu W. C.; Wang J.; Li Y.; Tao H. R.; Xiong H.; Lian F. L.; Gao J.; Ma H. N.; Lu T.; Zhang D.; Ye X. Q.; Ding H.; Yue L. Y.; Zhang Y. Y.; Tang H. Y.; Zhang N. X.; Yang Y. X.; Jiang H. L.; Chen K. X.; Zhou B.; Luo C. Discovery and biological evaluation of vinylsulfonamide derivatives as highly potent, covalent TEAD autopalmitoylation inhibitors. Eur. J. Med. Chem. 2019, 184, 111767 10.1016/j.ejmech.2019.111767. [DOI] [PubMed] [Google Scholar]
- Kaneda A.; Seike T.; Danjo T.; Nakajima T.; Otsubo N.; Yamaguchi D.; Tsuji Y.; Hamaguchi K.; Yasunaga M.; Nishiya Y.; Suzuki M.; Saito J. I.; Yatsunami R.; Nakamura S.; Sekido Y.; Mori K. The novel potent TEAD inhibitor, K-975, inhibits YAP1/TAZ-TEAD protein-protein interactions and exerts an anti-tumor effect on malignant pleural mesothelioma. Am. J. Cancer Res. 2020, 10 (12), 4399–4415. [PMC free article] [PubMed] [Google Scholar]
- Fan M. Y.; Lu W. C.; Che J. W.; Kwiatkowski N. P.; Gao Y.; Seo H. S.; Ficarro S. B.; Gokhale P. C.; Liu Y.; Geffken E. A.; Lakhani J.; Song K. J.; Kuljanin M.; Ji W. Z.; Jiang J.; He Z. X.; Tse J.; Boghossian A. S.; Rees M. G.; Ronan M. M.; Roth J. A.; Mancias J. D.; Marto J. A.; Dhe-Paganon S.; Zhang T. H.; Gray N. S. Covalent disruptor of YAP-TEAD association suppresses defective Hippo signaling. eLife 2022, 11, e78810 10.7554/eLife.78810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bum-Erdene K.; Zhou D. H.; Gonzalez-Gutierrez G.; Ghozayel M. K.; Si Y. B.; Xu D.; Shannon H. E.; Bailey B. J.; Corson T. W.; Pollok K. E.; Wells C. D.; Meroueh S. O. Small-Molecule Covalent Modification of Conserved Cysteine Leads to Allosteric Inhibition of the TEAD center dot Yap Protein-Protein Interaction. Cell Chem. Biol. 2019, 26 (3), 378–389. 10.1016/j.chembiol.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Karatas H.; Akbarzadeh M.; Adihou H.; Hahne G.; Pobbati A. V.; Ng E. Y.; Gueret S. M.; Sievers S.; Pahl A.; Metz M.; Zinken S.; Dotsch L.; Nowak C.; Thavam S.; Friese A.; Kang C. B.; Hong W. J.; Waldmann H. Discovery of Covalent Inhibitors Targeting the Transcriptional Enhanced Associate Domain Central Pocket. J. Med. Chem. 2020, 63 (20), 11972–11989. 10.1021/acs.jmedchem.0c01275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bum-Erdene K.; Yeh I. J.; Gonzalez-Gutierrez G.; Ghozayel M. K.; Pollok K.; Meroueh S. O. Small-Molecule Cyanamide Pan-TEAD.YAP1 Covalent Antagonists. J. Med. Chem. 2023, 66 (1), 266–284. 10.1021/acs.jmedchem.2c01189. [DOI] [PubMed] [Google Scholar]
- Holden J. K.; Crawford J. J.; Noland C. L.; Schmidt S.; Zbieg J. R.; Lacap J. A.; Zang R.; Miller G. M.; Zhang Y.; Beroza P.; Reja R.; Lee W.; Tom J. Y. K.; Fong R.; Steffek M.; Clausen S.; Hagenbeek T. J.; Hu T. S.; Zhou Z.; Shen H. C.; Cunningham C. N. Small Molecule Dysregulation of TEAD Lipidation Induces a Dominant-Negative Inhibition of Hippo Pathway Signaling. Cell Rep 2020, 31 (12), 107809 10.1016/j.celrep.2020.107809. [DOI] [PubMed] [Google Scholar]
- Yap T. A.; Kwiatkowski D. J.; Desai J.; Dagogo-Jack I.; Millward M.; Kindler H. L.; Tolcher A. W.; Frentzas S.; Thurston A. W.; Post L.; Dorr F. A. First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. Cancer Res. 2023, 83 (8), CT006. 10.1158/1538-7445.AM2023-CT006. [DOI] [Google Scholar]
- Nalawansha D. A.; Crews C. M. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem. Biol. 2020, 27 (8), 998–1014. 10.1016/j.chembiol.2020.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neklesa T. K.; Winkler J. D.; Crews C. M. Targeted protein degradation by PROTACs. Pharmacol Therapeut 2017, 174, 138–144. 10.1016/j.pharmthera.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Jiang B. S.; Wang E. S.; Donovan K. A.; Liang Y. K.; Fischer E. S.; Zhang T. H.; Gray N. S. Development of Dual and Selective Degraders of Cyclin-Dependent Kinases 4 and 6. Angew. Chem. Int. Edit 2019, 58 (19), 6321–6326. 10.1002/anie.201901336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak R. P.; DeAngelo S. L.; Buckley D.; He Z. X.; Donovan K. A.; An J.; Safaee N.; Jedrychowski M. P.; Ponthier C. M.; Ishoey M.; Zhang T. H.; Mancias J. D.; Gray N. S.; Bradner J. E.; Fischer E. S. Plasticity in binding confers selectivity in ligand-induced protein degradation. Nat. Chem. Biol. 2018, 14 (7), 706–714. 10.1038/s41589-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J. T.; Hu B.; Xu F. M.; Wang M.; Qin C.; McEachern D.; Stuckey J.; Wang S. M. Precise Conformational Control Yielding Highly Potent and Exceptionally Selective BRD4 Degraders with Strong Antitumor Activity. J. Med. Chem. 2023, 66 (12), 8222–8237. 10.1021/acs.jmedchem.3c00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N.; Punkosdy G.; Cavanaugh J.; Bantle C.; Constan A.; Li B.; Conley J.; Sanchez-Martin M.; Xu L.; McGovern K.; Castro A.; Zhang M.; Ecsedy J. IK-930, a paralog-selective TEAD inhibitor for treating YAP/TAZ-TEAD dependent cancers. Cancer Res. 2023, 83 (7_Suppl), 1646. 10.1158/1538-7445.AM2023-1646.36892426 [DOI] [Google Scholar]
- Tang T. T.; Konradi A. W.; Feng Y.; Peng X.; Ma M.; Li J.; Yu F.-X.; Guan K.-L.; Post L. Small Molecule Inhibitors of TEAD Auto-palmitoylation Selectively Inhibit Proliferation and Tumor Growth of NF2-deficient Mesothelioma. Mol. Cancer Ther 2021, 20 (6), 986–998. 10.1158/1535-7163.MCT-20-0717. [DOI] [PubMed] [Google Scholar]
- Chapeau E.; Chene P.; Furet P.; Kieffer L.; Machauer R.; Picard A.; Schmelzle T.; Sellner H.; Soldermann N.; Voegtle M.; Wartmann M.. Bifunctional degraders comprising a tead binder. WO/2023031801, March 09, 2023.
- Gray N. S.; Zhang T. H.; Ji W. Z.; Lu W. C.. Transcriptional enhanced associate domain (tead) degraders and uses thereof. WO/2023154811, August 17, 2023.
- Ji W. Z.; Byun W. S.; Lu W. C.; Zhu X. J.; Donovan K. A.; Dwyer B. G.; Che J. W.; Yuan L. J.; Abulaiti X.; Corsello S. M.; Fischer E. S.; Zhang T. H.; Gray N. S. Proteomics-Based Discovery of First-in-Class Chemical Probes for Programmed Cell Death Protein 2 (PDCD2). Angew. Chem. Int. Ed 2023, 62 (43), e202308292 10.1002/anie.202308292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.