Abstract
Stable isotopes such as 2H, 13C, and 15N have important applications in chemistry and drug discovery. Late-stage incorporation of uncommon isotopes via isotopic exchange allows for direct conversion of complex molecules into their valuable isotopologues without requiring a de novo synthesis. While synthetic methods exist for the conversion of hydrogen and carbon atoms into their less abundant isotopes, a corresponding method for accessing 15N-primary amines from their naturally occurring 14N-analogues has not yet been disclosed. We report an approach to access 15N-labeled primary amines via late-stage isotopic exchange using a simple benzophenone imine as the 15N source. By activating α-1° and α-2° amines to Katritzky pyridinium salts and α-3° amines to redox-active imines, we can engage primary alkyl amines in a deaminative amination. The redox-active imines proceed via a radical-polar-crossover mechanism, whereas the Katritzky salts are engaged in copper catalysis via an electron donor-acceptor complex. The method is general for a variety of amines, including multiple drug compounds, and results in complete and selective isotopic labeling.
Keywords: Isotope, labeling, photoredox catalysis, radical-polar crossover, copper catalysis
Graphical Abstract
INTRODUCTION
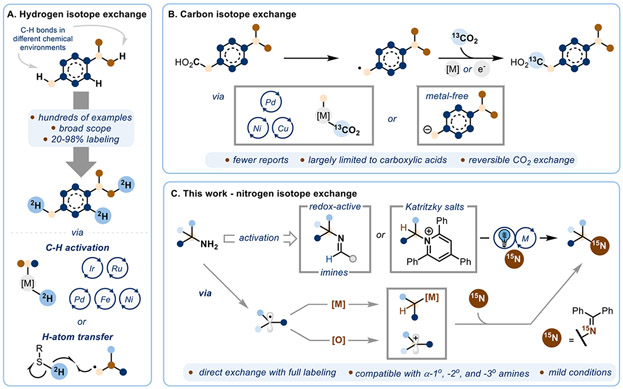
The incorporation of isotopes into organic molecules is of paramount interest across many areas of science. Stable isotopes such as 2H, 13C, and 15N have had important applications in elucidating chemical mechanisms1-5 and in drug discovery as tracers for metabolic studies.6-13 In addition, 13C and 15N-labeled amino acids have been extensively used as labeling tracers in quantitative proteomics (SILAC).14,15 More specialized uses, such as in hyperpolarized NMR,16 are also emerging, increasing the demand for reliable syntheses of these labeled materials. As molecules for these advanced applications become more complex, new synthetic routes to prepare isotopically enriched materials must advance to minimize the length and cost of synthesis.17 Selective replacement of an atom for its isotope through late-stage functionalization is attractive because it obviates the need for a costly and time consuming de novo synthesis. However, such a realization requires very mild reaction conditions and ideally complete isotopic exchange.
Significant efforts towards late-stage atom exchange18 of hydrogen- and carbon-containing molecules have been previously described. Installing 2H in organic frameworks has been achieved by exploiting the inherent acidity of many C-H bonds, or by using isotopically enriched metal-hydrides or hydrogen-atom-transfer reagents (Scheme 1A).19 Although powerful, the C-H functionalization step is often in equilibrium between the labeled and unlabeled substrate, which can lead to incomplete labeling. Strategies aiming to incorporate carbon isotopes, specifically via carbon atom exchange, have been developed largely using decarboxylative carboxylation with 13CO2 (Scheme 1B).20-25 This strategy again relies on the reversibility of the decarboxylation process, which makes it challenging to obtain high levels of the desired isotopic enrichment without biasing the system, often by using large excess of labeled starting material.26
Scheme 1.
Overview of Late-Stage Stable Isotope Incorporation.
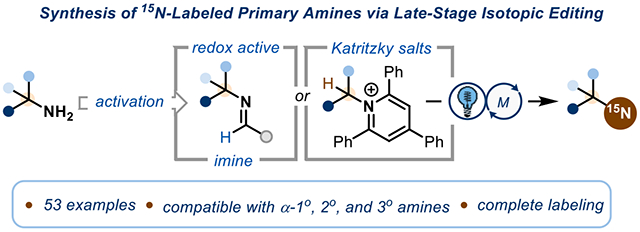
In contrast, due to the strength of C-N bonds, as well as the inherent nucleophilicity and poor leaving group ability of amines, direct exchange of a nitrogen atom for its isotope is a non-trivial transformation. The unique properties of 15N, such as wide chemical shift range and optimal relaxation lifetime, makes it an ideal nucleus for emerging biological imaging techniques.16 The current technology for obtaining an 15N-labeled primary amine requires a de novo synthesis of the desired molecule,27-30 precluding fast and modular diversification of existing medicinally relevant or naturally abundant nitrogen containing molecules for their 15N counterparts.31-33 In addition, some of these examples also result in incomplete labeling. Herein, we present strategies for direct conversion of a broad range of primary amines to their isotopic counterparts (Scheme 1C). Judicious choice of reaction conditions renders the reaction feasible for α-primary, -secondary, and -tertiary amines, including many pharmaceutical compounds. The isotope is introduced using 15N-labeled benzophenone imine, easily synthesized from 15N-ammonium chloride, the most economic and abundant form of 15N. The isotopically labeled products are isolated with complete labeling, as the unreacted 14N-starting material is functionalized and separated. The reaction proceeds under mild conditions, making it amenable to many complex drug targets.
RESULTS AND DISCUSSION
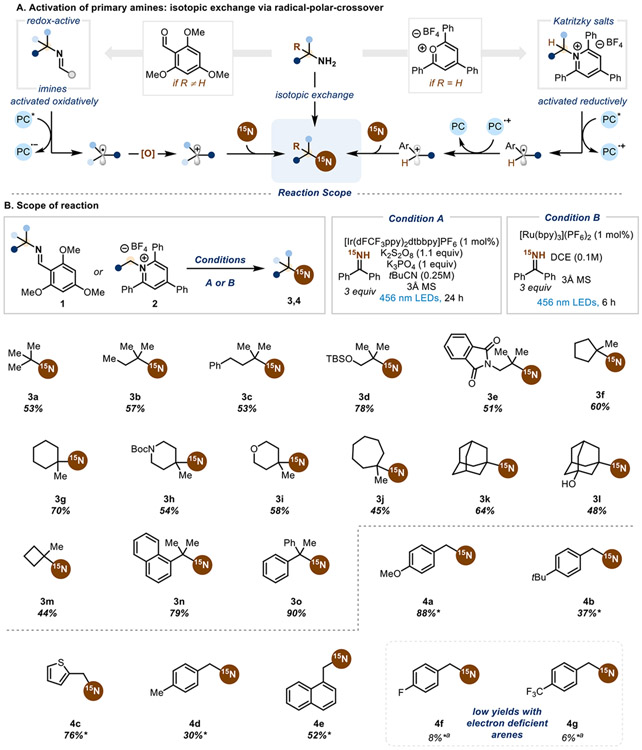
We envisioned that we could utilize existing amine activation strategies (Scheme 2A) to engage primary amines in isotopic editing. State-of-the-art amine activation for α-1° and αa-2° amines relies on the formation of Katritzky pyridinium salts, which can undergo single-electron reduction via transition metal (TM) catalysis34 or photoredox catalysis35 to generate a primary or secondary carbon-centered radical. For sterically hindered α-3° amines, Katritzky salt formation is not possible due to steric clash of the 2,6-phenyl substitution and the α-3° amine. Instead, the use of redox-active imines that engage in a single-electron oxidation enables the formation of a tertiary alkyl radical.36,37 We posited that these radical species could be further oxidized and trapped with a 15N-labeled nucleophile in a radical-polar-crossover (RPC) mechanism. RPC has gained traction over the years as a reliable method for coupling nucleophiles to sterically hindered electrophiles via a carbocation intermediate.38-45
Scheme 2. Reaction Design and Scope.
All yields are isolated yields with >99% 15N labeling. Compounds without an asterisk indicates that the reaction was conducted with coupling partner 1 and Condition A. An asterisk indicates that the reaction was conducted with coupling partner 2 and Condition B. (a) NMR yield with mesitylene as an internal standard.
It became immediately apparent that using the redox-active imines for RPC posed several significant challenges: a sacrificial oxidant is needed for photocatalyst turnover and radical oxidation; the alkyl radical could be reduced via hydrogen-atom-transfer; the carbocation trapping could be outcompeted by water (hydration product) or by elimination to form an alkene. Extensive optimization (see Supporting Information for full optimization efforts) led us to suitable conditions to minimize these deleterious pathways and successfully form the isotopically labeled amines, showing a clear trend with respect to yield and carbocation stability. We found that irradiating the redox-active imines with 456 nm light in combination with an oxidizing iridium photocatalyst, potassium persulfate as a sacrificial oxidant, potassium phosphate tribasic, and 15N-benzophenone imine in pivalonitrile (Condition A) provides good yields of the desired 15N-labeled α-3° amines (Scheme 2B). The reaction tolerates acyclic α-3° amines (3a-e) with myriad protected functionalities, such as alcohols (3d) and amines (3e). Cyclic α-3° amines with various ring sizes are also competent coupling partners in the reaction conditions (3f-m), including an example with an unprotected alcohol (3m). Benzylic α-3° amines (3n, 3o) give higher yields of desired product, likely due to the stability of both the radical and carbocation intermediates, which mitigates undesired reactivity. However, electron-deficient benzylic α-3° amines are not suitable coupling partners, instead forming the homodimer of the radical species as the major product (see SI).
In an effort to engage α-1° and α-2° amines in isotopic exchange, we turned to Katritzky salts for a redox-neutral RPC. The oxidized photocatalyst after radical generation can be reduced by the resulting radical, generating the desired carbocation intermediate. We found that a reducing ruthenium photocatalyst and 15N-benzophenone imine as the nucleophile (Condition B) affords labeled primary benzylic amines in high yields (4a-c). Electron-rich benzylic amines are good coupling partners, while electron neutral ones lead to a drop in yield (4d, 4e) and electron-deficient ones result in trace yields (4f, 4g), likely due to the challenging generation of the carbocation intermediate. In addition, using nonbenzylic Katritzky salts in the RPC system results in trace yields of desired product. It was apparent that the dependence on carbocation stability limited this system to electron-rich α-primary amines.
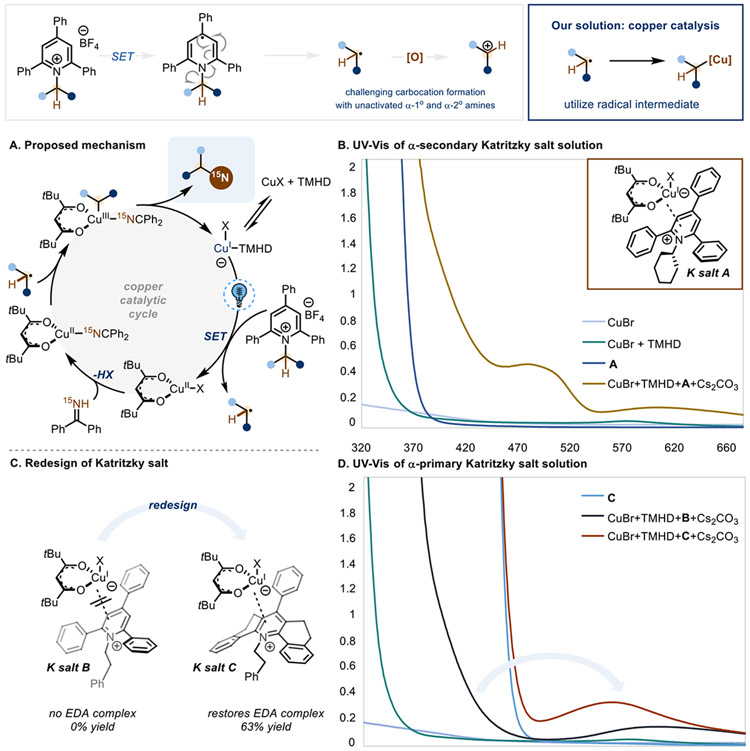
To address this limitation, we sought a method suitable for isotopic exchange of unactivated α-1° and α-2° amines by functionalizing the intermediate radical through a divergent pathway that does not involve carbocation formation (Scheme 3). Radicals generated from Katritzky salts are known to interact with a wide variety of electrophilic coupling partners because of the polarity match with the nucleophilic carbon-centered radical.46 To instead encourage coupling with nucleophilic amine sources, we turned to copper catalysis as a viable approach for a C–N cross coupling given recent precedent using benzophenone imine as a coupling partner.47,48 In this approach, we hypothesized that a photoredox catalyst could reduce the Katritzky salt to the corresponding radical, which can be subsequently trapped by a copper catalyst.
Scheme 3.
Activating Amines for Isotopic Exchange via Copper Catalysis.
We were pleased to find that this approach proved effective for performing isotopic exchange on α-secondary amines. However, control studies revealed that omitting photocatalyst has little effect on the yield of the reaction, but light is necessary for productive reactivity (see SI for optimization and control studies). Therefore, we propose that the single-electron transfer event occurs with the copper catalyst and the Katritzky salt through a transiently formed electron donor-acceptor (EDA) complex,49,50 enabling formation of the desired radical and the oxidized Cu(II) catalyst (Scheme 3A). Alternatively, the Cu(I)(TMHD)imido species, which would be formed if benzophenone imine exchanged with Br, can still form an EDA complex with the Katritzky salt (see Figure S1 in SI). Ligand exchange and radical trapping (or vice versa) results in the formation of a Cu(III) species that is poised to undergo reductive elimination to yield the desired product. UV-Vis studies confirm that upon mixing the copper catalyst with the Katritzky salt in the presence of base, a new complex is formed that can absorb light. This complex can then undergo photoinduced single-electron transfer, allowing isotopic exchange for unactivated α-secondary amines (Scheme 3B).
Interestingly, replacing Katritzky salt A for Katritzky salt B containing an α-1° amine disrupts the EDA complex with a concomitant loss of reactivity (Scheme 3C and 3D). Because the redox potentials of A and B differ by around 50 mV (see SI for cyclic voltammetry data), we wondered if this loss in yield was due to the slight shift in electronics. However, utilizing an α-1° Katritzky salt with electron-deficient aryl substituents, thus shifting the redox potential past that of A, also results in low yield (see SI). Therefore, we mainly attribute this loss of reactivity to steric differences between the two pyridinium salts. In the case of A, the α-2° amine is twisted out of plane, creating a rigid complex for the copper catalyst to interact with and twisting the pyridinium ring out of planarity. Conversely, B has more degrees of freedom and less steric clash, resulting in a more planarized pyridinium ring and possibly affecting the manner in which it interacts with the Cu(I)(TMHD) catalyst.51 Redesigning the Katritzky salt52 and installing ethylene bridges between the triphenyl core to rigidify the structure (Katritzky salt C) results in the formation of a new EDA complex while restoring reactivity and yield (Scheme 3D).
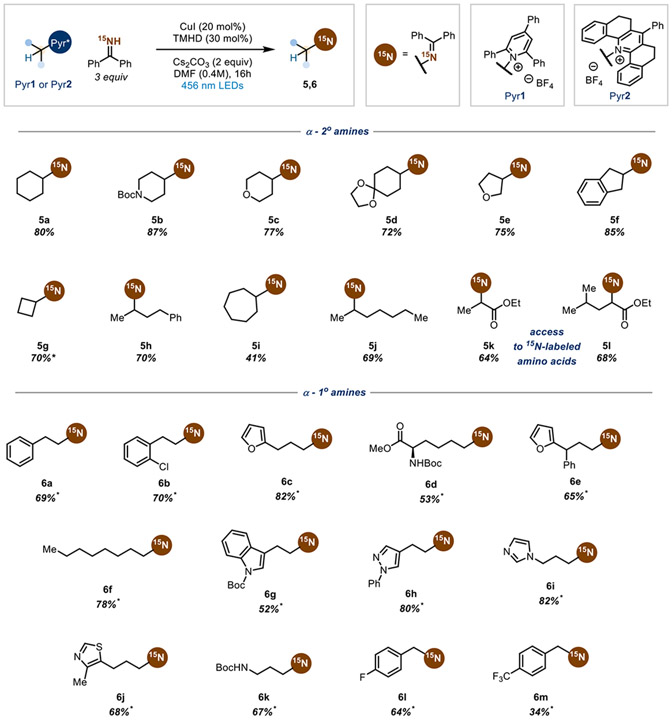
Having established a viable strategy for isotopic exchange of unactivated primary amines, we proceeded to examine the scope of amines for the transformation (Scheme 4). Cyclic α-2° amines containing various functionalities provide good yields, including six- (5a-5d), five- (5e, 5f), four- (5g), and seven-membered rings (5h). Linear α-2° amines are also competent coupling partners (5i, 5j). Excitingly, amino acid motifs (5k, 5l) are successful coupling partners in this transformation, affording valuable 15N-labeled amino acid derivatives53,54 and complimenting recent carbon isotope exchange methodologies on amino acids.55
Scheme 4. Scope of Amines for Isotopic Exchange with Cu Catalysis.
All yields are isolated yields with >99% 15N labeling. Compounds without an asterisk indicates that the reaction was conducted with Pyr1. An asterisk indicates the reactions conducted with Pyr2.
Utilizing Pyr2 allows for isotopic exchange of a wide variety of α-1° amines. Various heterocycles are tolerated, including furans (6c, 6e), indoles (6g), pyrazoles (6h), imidazoles (6i), and thiazoles (6j). Protected lysine 6d undergoes desired coupling efficiently, showcasing the ability to label either nitrogen of this amino acid. Lastly, electron-deficient arenes that were low yielding under the radical-polar crossover conditions can be efficiently converted in the Cu catalytic system (6l, 6m).56
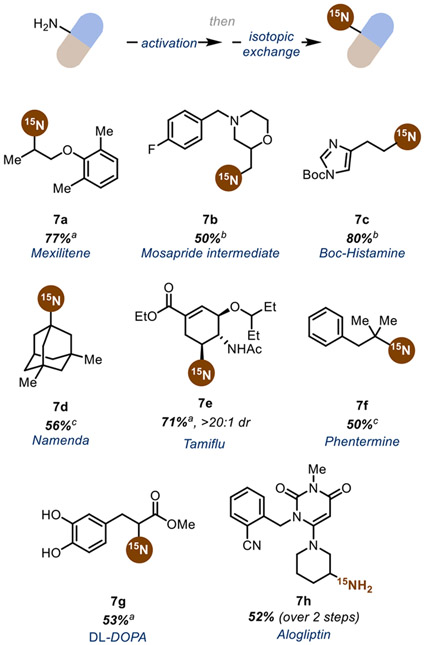
Lastly, we sought to demonstrate the versatility and applicability of our reaction conditions by labeling a variety of drug-like molecules (Scheme 5). Amine functionality is one of the most prevalent functional groups in pharmaceutical targets,57 with primary amines occurring in a variety of market drugs or being used as late-stage intermediates (see 7b) towards substituted amine and amide containing drugs.58 Installing an isotopic label as the last step in a complex molecule synthesis is ideal, as the yield of the isotopically enriched substrate is maximized and there is minimal isotopically labeled waste.
Scheme 5. Scope of Reaction Conditions with Drug-Like Molecules.
All yields are isolated yields with >99% 15N labeling. (a) 1 equiv Pyr1, 20 mol% CuI, 30 mol% TMHD, 2 equiv Cs2CO3, 3 equiv 15N-benzophenone imine, DMF (0.4M), 456 nm LED irradiation for 16 hours. (b) Reaction conditions are the same as in (a) but with 1 equiv Pyr2 instead of Pyr1. (c) 1 equiv redox-active imine, 1 mol% [Ir(dFCF3ppy)2dtbbpy]PF6,2 equiv potassium persulfate, 1 equiv potassium phosphate tribasic, 3 equiv 15N-benzophenone imine, ~25 mg 3Å MS in pivalonitrile (0.25M) with 456 nm irradiation for 24 hours.
Utilizing α-1° amines such as Mosapride intermediate 7b and Boc-Histamine 7c give the desired product in moderate to good yields. Drug derivatives containing α-2° amines such as Mexilitene 7a, Tamiflu 7e, DL-DOPA 7g, and Alogliptin also give desired isotopic exchange product in synthetically useful yields, while demonstrating the feasibility of deprotection to the primary amine (7h). In addition, α-3° amines such as Namenda 7d and Phentermine 7f also perform well in the isotopic exchange.
CONCLUSIONS
In conclusion, we have developed a general method for the synthesis of 15N-labeled primary amines. This expands the range of isotopic labeling to include nitrogen-isotope exchange of primary amines, complementary to hydrogen- and carbon-isotope exchange methodologies. By condensing α-1° and -2° amines to the Katritzky pyridinium salt and α-3° amines to redox-active imines, we have developed the first isotopic-exchange conditions suitable for all three categories of primary alkyl amines. The reaction tolerates a variety of functionality and is amenable to the isotopic exchange of late-stage drug derivatives. We hope that unlocking this new approach to the synthesis of 15N-labeled materials will broaden their already prevalent uses in mechanistic studies, amino acid labeling, hyperpolarization probes, and labels in clinical pharmacology.
Supplementary Material
ACKNOWLEDGMENT
Research reported pertaining to cryoprobe NMR data in this publication was supported by the Office of the Director of the NIH under Award Number S10OD026749.
Funding Sources
We thank NIGMS (GM125206) for support. JRD would like to acknowledge NSF-GRFP DGE-2036197 for funding.
Footnotes
The authors declare a conflict of interest: A provisional patent has been filed on this work.
Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org
Experimental procedures, optimization tables, compound characterization data
REFERENCES
- (1).Caner J; Vilarrasa J 15N Double-Labeled Guanosine from Inosine through Ring-Opening–Ring-Closing and One-Pot Pd-Catalyzed C–O and C–N Cross-Coupling Reactions. J. Org. Chem 2010, 75 (14), 4880–4883. 10.1021/jo100808w. [DOI] [PubMed] [Google Scholar]
- (2).Ma Y; Stivala CE; Wright AM; Hayton T; Liang J; Keresztes I; Lobkovsky E; Collum DB; Zakarian A Enediolate–Dilithium Amide Mixed Aggregates in the Enantioselective Alkylation of Arylacetic Acids: Structural Studies and a Stereochemical Model. J. Am. Chem. Soc 2013, 135 (45), 16853–16864. 10.1021/ja403076u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Wu Z-C; Houk KN; Boger DL; Svatunek D Mechanistic Insights into the Reaction of Amidines with 1,2,3-Triazines and 1,2,3,5-Tetrazines. J. Am. Chem. Soc 2022, 144 (24), 10921–10928. 10.1021/jacs.2c03726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Yu X; Chen K; Yang F; Zha S; Zhu J Oxadiazolone-Enabled Synthesis of Primary Azaaromatic Amines. Org. Lett 2016, 18 (20), 5412–5415. 10.1021/acs.orglett.6b02814. [DOI] [PubMed] [Google Scholar]
- (5).Dale HJA; Leach AG; Lloyd-Jones GC Heavy-Atom Kinetic Isotope Effects: Primary Interest or Zero Point? J. Am. Chem. Soc 2021, 143 (50), 21079–21099. 10.1021/jacs.1c07351. [DOI] [PubMed] [Google Scholar]
- (6).Schellekens RCA; Stellaard F; Woerdenbag HJ; Frijlink HW; Kosterink JGW Applications of Stable Isotopes in Clinical Pharmacology. Br. J. Clin. Pharmacol 2011, 72 (6), 879–897. 10.1111/j.1365-2125.2011.04071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Kim I-Y; Suh S-H; Lee I-K; Wolfe RR Applications of Stable, Nonradioactive Isotope Tracers in in Vivo Human Metabolic Research. Exp. Mol. Med 2016, 48 (1), e203–e203. 10.1038/emm.2015.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Parr A; Badman G; Bowen CL; Coffin M; Gupta M; Jones L; Kurtinecz M; Naderer O; Travis E; Zhu J; Patel P Application of a Stable Isotope Approach to Evaluate Impact of Changes in Manufacturing Parameters for an Immediate-Release Tablet. J. Clin. Pharmacol 2016, 56 (7), 801–805. 10.1002/jcph.664. [DOI] [PubMed] [Google Scholar]
- (9).Atkinson AJ The Stable Isotope Method for Determining Absolute Bioavailability. Transl. Clin. Pharmacol 2017, 25 (2), 53–58. 10.12793/tcp.2017.25.2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Parr A; Gupta M; Montague TH; Hoke F Re-Introduction of a Novel Approach to the Use of Stable Isotopes in Pharmacokinetic Studies. AAPS J. 2012, 14 (3), 639–645. 10.1208/s12248-012-9371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Polepally AR; Remmel RP; Brundage RC; Leppik IE; Rarick JO; Ramsay RE; Birnbaum AK Steady-State Pharmacokinetics and Bioavailability of Immediate-Release and Extended-Release Formulations of Lamotrigine in Elderly Epilepsy Patients: Use of Stable Isotope Methodology. J. Clin. Pharmacol 2015, 55 (10), 1101–1108. 10.1002/jcph.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Jiang H; Zeng J; Li W; Bifano M; Gu H; Titsch C; Easter J; Burrell R; Kandoussi H; Aubry A-F; Arnold ME Practical and Efficient Strategy for Evaluating Oral Absolute Bioavailability with an Intravenous Microdose of a Stable Isotopically-Labeled Drug Using a Selected Reaction Monitoring Mass Spectrometry Assay. Anal. Chem 2012, 84 (22), 10031–10037. 10.1021/ac3024558. [DOI] [PubMed] [Google Scholar]
- (13).Elmore CS Chapter 25 The Use of Isotopically Labeled Compounds in Drug Discovery. In Annual Reports in Medicinal Chemistry; Macor JE, Ed.; Academic Press, 2009; Vol. 44, pp 515–534. 10.1016/S0065-7743(09)04425-X. [DOI] [Google Scholar]
- (14).Ong S-E; Mann M A Practical Recipe for Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC). Nat. Protoc 2006, 1 (6), 2650–2660. 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- (15).Ong S-E; Blagoev B; Kratchmarova I; Kristensen DB; Steen H; Pandey A; Mann M Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteomics 2002, 1 (5), 376–386. 10.1074/mcp.M200025-MCP200. [DOI] [PubMed] [Google Scholar]
- (16).Zhang L; Liang D; Wang Y; Li D; Zhang J; Wu L; Feng M; Yi F; Xu L; Lei L; Du Q; Tang X State-of-the-Art Accounts of Hyperpolarized 15N-Labeled Molecular Imaging Probes for Magnetic Resonance Spectroscopy and Imaging. Chem. Sci 2018, 9 (1), 44–51. 10.1039/C7SC03842A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Maxwell BD New Radical Methods for the Potential Synthesis of Carbon-13 and Carbon-14 Labeled Complex Products. J. Label. Compd. Radiopharm 2018, 61 (14), 1024–1035. 10.1002/jlcr.3680. [DOI] [PubMed] [Google Scholar]
- (18).Jurczyk J; Woo J; Kim SF; Dherange BD; Sarpong R; Levin MD Single-Atom Logic for Heterocycle Editing. Nat. Synth 2022, 1 (5), 352–364. 10.1038/s44160-022-00052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kang Q-K; Shi H Catalytic Hydrogen Isotope Exchange Reactions in Late-Stage Functionalization. Synlett 2022, 33 (04), 329–338. 10.1055/a-1354-0367. [DOI] [Google Scholar]
- (20).Kong D; Munch M; Qiqige Q; Cooze CJC; Rotstein BH; Lundgren RJ Fast Carbon Isotope Exchange of Carboxylic Acids Enabled by Organic Photoredox Catalysis. J. Am. Chem. Soc 2021, 143 (5), 2200–2206. 10.1021/jacs.0c12819. [DOI] [PubMed] [Google Scholar]
- (21).Destro G; Loreau O; Marcon E; Taran F; Cantat T; Audisio D Dynamic Carbon Isotope Exchange of Pharmaceuticals with Labeled CO2. J. Am. Chem. Soc 2019, 141 (2), 780–784. 10.1021/jacs.8b12140. [DOI] [PubMed] [Google Scholar]
- (22).Destro G; Horkka K; Loreau O; Buisson D-A; Kingston L; Del Vecchio A; Schou M; Elmore CS; Taran F; Cantat T; Audisio D Transition-Metal-Free Carbon Isotope Exchange of Phenyl Acetic Acids. Angew. Chem. Int. Ed 2020, 59 (32), 13490–13495. 10.1002/anie.202002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Kong D; Moon PJ; Lui EKJ; Bsharat O; Lundgren RJ Direct Reversible Decarboxylation from Stable Organic Acids in Dimethylformamide Solution. Science 2020, 369 (6503), 557–561. 10.1126/science.abb4129. [DOI] [PubMed] [Google Scholar]
- (24).For an example of carbon-isotope exchange with benzonitriles, see: Reilly SW; Lam Y; Ren S; Strotman NA Late-Stage Carbon Isotope Exchange of Aryl Nitriles through Ni-Catalyzed C─CN Bond Activation. J. Am. Chem. Soc 2021, 143 (12), 4817–4823. 10.1021/jacs.1c01454. [DOI] [PubMed] [Google Scholar]
- (25).For an example of carbon-isotope exchange with nitriles, see: Feng M; De Oliveira J; Sallustrau A; Destro G; Thuéry P; Roy S; Cantat T; Elmore CS; Blankenstein J; Taran F; Audisio D Direct Carbon Isotope Exchange of Pharmaceuticals via Reversible Decyanation. J. Am. Chem. Soc 2021, 143 (15), 5659–5665. 10.1021/jacs.1c01923. [DOI] [PubMed] [Google Scholar]
- (26).For an example with complete carbon-isotope labeling, see: Ton SJ; Neumann KT; Nørby P; Skrydstrup T Nickel-Mediated Alkoxycarbonylation for Complete Carbon Isotope Replacement. J. Am. Chem. Soc 2021, 143 (42), 17816–17824. 10.1021/jacs.1c09170. [DOI] [PubMed] [Google Scholar]
- (27).Chandrashekhar VG; Baumann W; Beller M; Jagadeesh RV Nickel-Catalyzed Hydrogenative Coupling of Nitriles and Amines for General Amine Synthesis. Science 2022, 376 (6600), 1433–1441. 10.1126/science.abn7565. [DOI] [PubMed] [Google Scholar]
- (28).Zhou Z; Kweon J; Jung H; Kim D; Seo S; Chang S Photoinduced Transition-Metal-Free Chan–Evans–Lam-Type Coupling: Dual Photoexcitation Mode with Halide Anion Effect. J. Am. Chem. Soc 2022, 144, (20), 9161–9171. 10.1021/jacs.2c03343. [DOI] [PubMed] [Google Scholar]
- (29).Finkelstein P; Reisenbauer JC; Botlik BB; Green O; Florin A; Morandi B Nitrogen Atom Insertion into Indenes to Access Isoquinolines. Chem. Sci 2023, 14, 2954–2959. 10.1039/D2SC06952K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).He J; Zhang X; He Q; Guo H; Fan R Synthesis of 15N-Labeled Heterocycles via the Cleavage of C─N Bonds of Anilines and Glycine-15N. Chem. Commun 2021, 57 (44), 5442–5445. 10.1039/D1CC01734A. [DOI] [PubMed] [Google Scholar]
- (31).Chisholm MH; Delbridge EE; Kidwell AR; Quinlan KB Nitrogen Atom Exchange between Molybdenum, Tungsten and Carbon. A Convenient Method for N-15 Labeling. Chem. Commun 2003, 126–127. 10.1039/B210286B. [DOI] [PubMed] [Google Scholar]
- (32).Fier PS; Maloney KM NHC-Catalyzed Deamination of Primary Sulfonamides: A Platform for Late-Stage Functionalization. J. Am. Chem. Soc 2019, 141 (4), 1441–1445. 10.1021/jacs.8b11800. [DOI] [PubMed] [Google Scholar]
- (33).Chukanov NV; Kidd BE; Kovtunova LM; Bukhtiyarov VI; Shchepin RV; Chekmenev EY; Goodson BM; Kovtunov KV; Koptyug IV A Versatile Synthetic Route to the Preparation of 15N Heterocycles. J. Label. Compd. Radiopharm 2019, 62 (13), 892–902. 10.1002/jlcr.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Basch CH; Liao J; Xu J; Piane JJ; Watson MP Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C─N Bond Activation. J. Am. Chem. Soc 2017, 139 (15), 5313–5316. 10.1021/jacs.7b02389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Klauck FJR; James MJ; Glorius F Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem. Int. Ed 2017, 56 (40), 12336–12339. 10.1002/anie.201706896. [DOI] [PubMed] [Google Scholar]
- (36).Ashley MA; Rovis T Photoredox-Catalyzed Deaminative Alkylation via C─N Bond Activation of Primary Amines. J. Am. Chem. Soc 2020, 142 (43), 18310–18316. 10.1021/jacs.0c08595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Dorsheimer JR; Ashley MA; Rovis T Dual Nickel/Photoredox-Catalyzed Deaminative Cross-Coupling of Sterically Hindered Primary Amines. J. Am. Chem. Soc 2021, 143 (46), 19294–19299. 10.1021/jacs.1c10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).(a) Leibler IN-M; Tekle-Smith MA; Doyle AG A General Strategy for C(Sp3)–H Functionalization with Nucleophiles Using Methyl Radical as a Hydrogen Atom Abstractor. Nat. Commun 2021, 12 (1), 6950. 10.1038/s41467-021-27165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Das M; Zamani L; Bratcher C; Musacchio PZ Azolation of Benzylic C-H Bonds via Photoredox-Catalyzed Carbocation Generation. J. Am. Chem. Soc 2023, 145 (7), 3861–3868. 10.1021/jacs.2c12850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Go SY; Chung H; Shin SJ; An S; Youn JH; Im TY; Kim JY; Chung TD; Lee HG A Unified Synthetic Strategy to Introduce Heteroatoms via Electrochemical Functionalization of Alkyl Organoboron Reagents. J. Am. Chem. Soc 2022, 144 (20), 9149–9160. 10.1021/jacs.2c03213. [DOI] [PubMed] [Google Scholar]
- (40).Li P; Zbieg JR; Terrett JA A Platform for Decarboxylative Couplings via Photoredox Catalysis: Direct Access to Carbocations from Carboxylic Acids for Carbon─Oxygen Bond Formation. ACS Catal. 2021, 11 (17), 10997–11004. 10.1021/acscatal.1c03251. [DOI] [Google Scholar]
- (41).Xiang J; Shang M; Kawamata Y; Lundberg H; Reisberg SH; Chen M; Mykhailiuk P; Beutner G; Collins MR; Davies A; Del Bel M; Gallego GM; Spangler JE; Starr J; Yang S; Blackmond DG; Baran PS Hindered Dialkyl Ether Synthesis with Electrogenerated Carbocations. Nature 2019, 573 (7774), 398–402. 10.1038/s41586-019-1539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Sharma S; Singh J; Sharma A Visible Light Assisted Radical-Polar/Polar-Radical Crossover Reactions in Organic Synthesis. Adv. Synth. Catal 2021, 363 (13), 3146–3169. 10.1002/adsc.202100205. [DOI] [Google Scholar]
- (43).Sheng T; Zhang H-J; Shang M; He C; Vantourout Julien. C.; Baran Phil. S. Electrochemical Decarboxylative N-Alkylation of Heterocycles. Org. Lett 2020, 22 (19), 7594–7598. 10.1021/acs.or-glett.0c02799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Shibutani S; Kodo T; Takeda M; Nagao K; Tokunaga N; Sasaki Y; Ohmiya H Organophotoredox-Catalyzed Decarboxylative C(Sp3)─O Bond Formation. J. Am. Chem. Soc 2020, 142 (3), 1211–1216. 10.1021/jacs.9b12335. [DOI] [PubMed] [Google Scholar]
- (45).Kobayashi R; Shibutani S; Nagao K; Ikeda Z; Wang J; Ibáñez I; Reynolds M; Sasaki Y; Ohmiya H Decarboxylative N-Alkylation of Azoles through Visible-Light-Mediated Organophotoredox Catalysis. Org. Lett 2021, 23 (14), 5415–5419. 10.1021/acs.or-glett.1c01745. [DOI] [PubMed] [Google Scholar]
- (46).Correia JTM; Fernandes VA; Matsuo BT; Delgado JAC; de Souza WC; Paixão MW Photoinduced Deaminative Strategies: Katritzky Salts as Alkyl Radical Precursors. Chem. Commun 2020, 56 (4), 503–514. 10.1039/C9CC08348K. [DOI] [PubMed] [Google Scholar]
- (47).Mao R; Balon J; Hu X Cross-Coupling of Alkyl Redox-Active Esters with Benzophenone Imines: Tandem Photoredox and Copper Catalysis. Angew. Chem. Int. Ed 2018, 57 (30), 9501–9504. 10.1002/anie.201804873. [DOI] [PubMed] [Google Scholar]
- (48).Barzanò G; Mao R; Garreau M; Waser J; Hu X Tandem Photoredox and Copper-Catalyzed Decarboxylative C(Sp3)–N Coupling of Anilines and Imines Using an Organic Photocatalyst. Org. Lett 2020, 22 (14), 5412–5416. 10.1021/acs.orglett.0c01769. [DOI] [PubMed] [Google Scholar]
- (49).Lima CGS; de M Lima T; Duarte M; Jurberg ID; Paixão MW Organic Synthesis Enabled by Light-Irradiation of EDA Complexes: Theoretical Background and Synthetic Applications. ACS Catal. 2016, 6 (3), 1389–1407. 10.1021/acscatal.5b02386. [DOI] [Google Scholar]
- (50).Crisenza GEM; Mazzarella D; Melchiorre P Synthetic Methods Driven by the Photoactivity of Electron Donor–Acceptor Complexes. J. Am. Chem. Soc 2020, 142 (12), 5461–5476. 10.1021/jacs.0c01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Wu J; Grant PS; Li X; Noble A; Aggarwal VK Catalyst-Free Deaminative Functionalizations of Primary Amines by Photoinduced Single-Electron Transfer. Angew. Chem. Int. Ed 2019, 58 (17), 5697–5701. 10.1002/anie.201814452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ma Y; Pang Y; Chabbra S; Reijerse EJ; Schnegg A; Niski J; Leutzsch M; Cornella J Radical C–N Borylation of Aromatic Amines Enabled by a Pyrylium Reagent. Chem. – Eur. J 2020, 26 (17), 3738–3743. 10.1002/chem.202000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Hoerrner ME; Baker KM; Basch CH; Bampo EM; Watson MP Deaminative Arylation of Amino Acid-Derived Pyridinium Salts. Org. Lett 2019, 21 (18), 7356–7360. 10.1021/acs.orglett.9b02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Sun S-Z; Cai Y-M; Zhang D-L; Wang J-B; Yao H-Q; Rui X-Y; Martin R; Shang M Enantioselective Deaminative Alkylation of Amino Acid Derivatives with Unactivated Olefins. J. Am. Chem. Soc 2022, 144 (3), 1130–1137. 10.1021/jacs.1c12350. [DOI] [PubMed] [Google Scholar]
- (55).Bsharat O; Doyle MGJ; Munch M; Mair BA; Cooze CJC; Derdau V; Bauer A; Kong D; Rotstein BH; Lundgren RJ Aldehyde-Catalysed Carboxylate Exchange in α-Amino Acids with Isotopically Labelled CO2. Nat. Chem 2022, 14 (12), 1367–1374. 10.1038/s41557-022-01074-0. [DOI] [PubMed] [Google Scholar]
- (56).In some cases, 15NH4Cl can be used directly as the nucleophile, yielding the direct 15N-amine. See supporting information for more details on scope and limitations.
- (57).Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem 2014, 57 (24), 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- (58).McGrath NA; Brichacek M; Njardarson JT A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ 2010, 87 (12), 1348–1349. 10.1021/ed1003806. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.