Abstract
This Patent Highlight delves into the ground-breaking impact of Proteolysis Targeting Chimeras (PROTACs) on targeted protein degradation, offering novel strategies to eliminate pathogenic proteins. By exploring the cutting-edge development of compounds targeting IRAK-4 and CDK2, this work illuminates PROTACs’ role in treating immune disorders and cancer. The analysis not only highlights the specificity and potential of PROTACs in transforming disease treatment but also addresses the challenges and future directions of this technology, emphasizing its broad applicability and the promise of more effective therapeutic strategies.
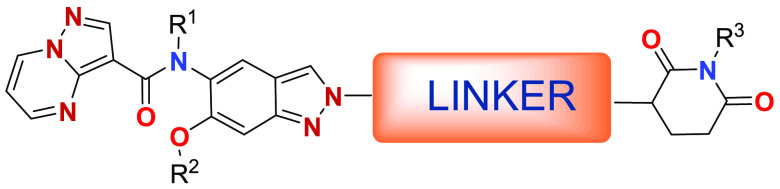
Important Compound Classes
Titles
Compounds and Methods for the Targeted Degradation of IRAK-4; CDK2 Degraders and Uses Thereof; Novel PROTAC Chimeric Compound, and Pharmaceutical Composition Comprising Same for Preventing, Ameliorating, or Treating Diseases Through Target Protein Degradation; and Methods and Materials for Treating Cancer
Patent Publication Numbers
WO 2024/020522 A1 (URL: https://patents.google.com/patent/WO2024020522A1/en?oq=WO+2024%2f020522+A1);
WO 2024/039901 A2 (https://patents.google.com/patent/WO2024039901A2/en?oq=WO+2024%2f039901+A2);
US 2023/0405134 A1 (URL: https://patents.google.com/patent/US20230405134A1/en?oq=US+2023%2f0405134+A1);
WO 2023/249994 A1 (URL: https://patents.google.com/patent/WO2023249994A1/en?oq=WO+2023%2f249994+A1)
Publication Dates
January 25, 2024; February 22, 2024; December 21, 2023; and December 28, 2023
Priority Applications
US 63/369,115; US 63/522,640; KR 18/148,653; and US 63/354,370
Priority Dates
July 22, 2022; June 22, 2023; December 30, 2022; and June 22, 2022
Inventors
Araujo, E. M. V.; Cantley, J. L.; Dong, H. (WO 2024/020522 A1); Collier, P.; Zheng, X.; Zhu, X.; Ford, M.; Weiss, M. M.; Aversa, R.; Pennington, L. D.; Comer, E.; Mahasenan, K. V.; Zhang, Y. (WO 2024/039901 A2); Lim, H. S.; Lee, Y. J. (US 2023/0405134 A1); and Mansfiedl, A. S.; Borad, M. J.; Yang, L.; Natarajan, A.; Mallareddy, J. R. (WO 2023/249994 A1)
Assignee Companies
Arvinas Operations, Inc. [US/US]; 5 Science Park, 395 Winchester Ave, New Haven, Connecticut 06511, United States (WO 2024/020522 A1); Kymera Therapeutics, Inc. [US/US]; 200 Arsenal Yards Blvd., Suite 230, Watertown, Massachusetts 02472, United States (WO 2024/039901 A2). Postech Research and Business Development Foundation, Pohang-si, (KR) (US 2023/0405134 A1) and Mayo Foundation for Medical Education and Research [US/US]; 200 First Street SW, Rochester, Minnesota 55905, United States. University of Nebraska Medical Center [US/US]; Intellectual Property Office, 986099 Nebraska Medical Center, Omaha, Nebraska 68198–6099, United States (WO 2023/249994 A1)
Disease Area
Cancer
Biological Targets
IRAK-4, CDK2, SRC-1 and MET polypeptide
Summary
The field of modern medicine is in constant pursuit of more refined and selective therapeutic strategies to tackle complex diseases. The emergence of targeted protein degradation (TPD) technologies, notably Proteolysis Targeting Chimeras (PROTACs), introduces an innovative paradigm in drug development. Unlike conventional drugs that inhibit protein function, PROTACs aim to eradicate undesirable proteins from cells. This novel approach leverages the body’s natural degradation machinery, offering a precise method to combat diseases at their molecular core. This Patent Highlight delves into the essence of PROTAC technology, emphasizing its significance and pioneering advancements, including innovative patents that have opened new avenues for treating cancer and immune-related diseases.
The Targeted Degradation of IRAK-4 and CDK2
The recent advancements in PROTAC technology are highlighted by two notable patents: WO 2024/020522 A1 and WO 2024/039901 A2. These patents underscore the innovative approaches to targeting and degrading specific proteins—Interleukin-1 Receptor-Associated Kinase 4 (IRAK-4) and cyclin-dependent kinase 2 (CDK2)—which play pivotal roles in immune disorders and cancer, respectively.
Patent application WO 2024/020522 A1 details the development of compounds for the targeted degradation of IRAK-4, a crucial enzyme in the innate immunity and inflammation pathways. Overactivity of IRAK-4 is implicated in various immune disorders and certain types of cancer. The ability to specifically target and degrade IRAK-4 using PROTACs illustrates the technology’s versatility and potential in addressing diseases where IRAK-4 is a key player. This advancement opens up new possibilities for modulating immune responses and treating conditions that have eluded traditional therapeutic approaches.
Patent application WO 2024/039901 A2 introduces a strategy for the development of CDK2 degraders. CDK2 is essential for cell cycle regulation, and its dysregulation is a common feature in many cancers. While inhibitors of CDK2 have been previously explored, the advent of PROTACs targeting CDK2 represents a groundbreaking approach, potentially offering more effective and lasting therapeutic outcomes. This patent highlights the promise of PROTAC technology in cancer treatment, especially in targeting critical proteins involved in cell cycle regulation and tumor growth.
SRC-1 and MET polypeptides
The patents application US 20230405134 A1 and WO 202324999 A1 illustrate another practical application of PROTAC technology in targeting disease-relevant proteins. The first patent (US 20230405134 A1) focuses on the degradation of Steroid Receptor Coactivator-1 (SRC-1), a protein involved in cancer progression and immune response modulation. By designing PROTACs that target SRC-1 for degradation, this approach offers a novel pathway to treat cancers and immune-related conditions where SRC-1 is overexpressed. Similarly, WO 202324999 A1 explores the use of PROTACs to degrade oncogenic MET polypeptides, crucial in tumorigenesis, metastasis, and resistance to therapies. The targeted degradation of MET polypeptides represents a strategic advance in combating MET-driven cancers, showcasing the potential of PROTAC technology to address the root causes of disease progression.
A Leap Toward Therapeutic Realities with PROTACs in Clinical Trials
After nearly two decades of research, PROTAC technology has hit a significant stride, with over 20 drugs entering clinical trials by 2022, marking a crucial phase in validating their therapeutic potential, particularly in oncology. Notably, ARV-471 targets the estrogen receptor, advancing to phase III trials, underscoring PROTACs’ wide-reaching implications. The year 2022 witnessed key advancements, including the first reversible covalent degrader for KRASG12C, significant progress in HDCA1 degrader activity, and the discovery of novel ligands like EN106 for FEM1B. Innovations also included the first PROTAC targeting SOS1, specialized degraders for BRD4 isoforms, and the pioneering degrader for CDK9 and Cyclin T1. These breakthroughs not only demonstrate PROTACs’ evolving landscape but also their practical impact in cancer treatment, highlighting promising design strategies and anticancer efficacy.
Challenges and Prospects
Despite the encouraging progress, the journey of PROTAC technology is not without challenges. Achieving high specificity to avoid off-target effects and circumventing potential resistance mechanisms developed by cancer cells are among the hurdles to be overcome. The design and optimization of E3 ligase recruiters remain critical, as the efficacy of PROTACs relies on the precise and efficient tagging of target proteins for degradation.
The future of PROTAC technology is promising, with ongoing research poised to deepen our understanding of protein–protein interactions and the ubiquitin-proteasome system. Such knowledge is expected to lead to the development of more sophisticated and efficient PROTAC molecules. Additionally, exploring alternative degradation pathways and combining PROTACs with other therapeutic strategies hold great promise for the advancement of disease treatment.
As such, PROTAC technology signifies a monumental advance in the field of targeted protein degradation, offering new therapeutic options for complex diseases. With the incorporation of recent patents focusing on the degradation of IRAK-4, CDK2, SRC-1 and MET polypeptides, the scope of PROTAC research and its application in medicine continue to widen. These developments not only underscore the technology’s adaptability but also its potential to address a diverse array of diseases. Through continued innovation and exploration, PROTACs are set to redefine the landscape of therapeutic strategies, heralding a new era of precision medicine.
Key Structures
Biological Assay
IRAK-4 ELISA protocol
Biological Data
The table provides PBMC degradation data for exemplary compounds represented by DC90, where A = < 10.0 nM; B = ≥ 10.0 nM and <25.0 nM; DC50, A = < 1.0 nM; B = ≥ 1.0 nM and <5.0 nM; and DCmax, A = ≥ 90; B = ≥ 80 and <90.
Recent Review Articles
The author declares no competing financial interest.
References
- Chen Y.; Xue H.; Jin J. Applications of Protein Ubiquitylation and Deubiquitylation in Drug Discovery. J. Biol. Chem. 2024, 107264. 10.1016/j.jbc.2024.107264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Byun I.; Kim D. Y.; Joh H.; Kim H. J.; Lee M. J. Targeted Protein Degradation Directly Engaging Lysosomes or Proteasomes. Chem. Soc. Rev. 2024, 53, 3253–3272. 10.1039/D3CS00344B. [DOI] [PubMed] [Google Scholar]
- Rutherford K. A.; McManus K. J. PROTACs: Current and Future Potential as a Precision Medicine Strategy to Combat Cancer. Mol. Cancer Ther. 2024, 23, 454–463. 10.1158/1535-7163.MCT-23-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R.; Xie S.; Ran J.; Li T. Restraining the Power of Proteolysis Targeting Chimeras in the Cage: A Necessary and Important Refinement for Therapeutic Safety. J. Cell Physiol. 2024, 10.1002/jcp.31255. [DOI] [PubMed] [Google Scholar]
- Hajjo R.; Sabbah D. A.; Bardaweel S. K.; Zhong H. A. Targeting the EGFR/RAS/RAF Signaling Pathway in Anticancer Research: A Recent Update on Inhibitor Design and Clinical Trials (2020–2023). Expert Opin. Ther. Pat. 2024, 34, 51. 10.1080/13543776.2024.2327307. [DOI] [PubMed] [Google Scholar]
- Wang X.; Qin Z. L.; Li N.; Jia M. Q.; Liu Q. G.; Bai Y. R.; Song J.; Yuan S.; Zhang S. Y. Annual Review of PROTAC Degraders as Anticancer Agents in 2022. Eur. J. Med. Chem. 2024, 267, 116166. 10.1016/j.ejmech.2024.116166. [DOI] [PubMed] [Google Scholar]