Abstract
The nr allele at the mouse Fv1 restriction locus governs resistance to B-tropic and some N-tropic murine leukemia viruses (MLVs). Sequence analysis and site-specific mutagenesis of N-tropic MLVs identified a single amino acid difference responsible for this restriction that is distinct from the site that governs N or B tropism. Viruses with other substitutions at this site were evaluated for altered replication patterns.
The virus resistance gene Fv1 governs the relative sensitivity of mouse cells to different subgroups of murine leukemia viruses (MLVs) (17). Initial observations by Hartley and her coworkers led to the identification of two Fv1 alleles. Prototypical Fv1n mouse, NIH, cells are more sensitive to N-tropic virus, whereas BALB/c cells (Fv1b) are more sensitive to B-tropic virus (7). NB-tropic viruses replicate to equal titers in Fv1n and Fv1b cells. Subsequent studies identified at least three additional alleles at this locus. Fv1nr mice are highly sensitive to some, but not all, N-tropic viruses (17, 21), Fv1d strain DBA/2 shows altered sensitivity to both N- and B-tropic viruses (17, 21), and many strains of wild mouse lack Fv1 restriction altogether and are equally sensitive to all MLVs (6, 11).
Although the Fv1 gene has now been cloned and shown to be related to the presumptive gag region of the endogenous HERV-L elements (2), the mechanism by which this gene is responsible for resistance is still undetermined. Part of the difficulty in studying Fv1 restriction is the fact that resistance is not absolute; virus titers are reduced 100- to 1,000-fold in resistant cells. Furthermore, replication in restricted cells generally shows two-hit kinetics, suggesting that restriction can be overcome or abrogated by the presence of multiple infecting particles (3, 18).
In an effort to further characterize this resistance, we have focused on the viral target of Fv1 restriction. Early studies had demonstrated that the responsible sequences are found in CAgag (1, 5, 9) and that there are only two amino acids that distinguished N- and B-tropic viruses (4, 15). In our previous studies, we used site-specific mutagenesis to demonstrate that alteration of only one of these amino acids, residue 110, is sufficient for conversion of an N-tropic virus to a B-tropic virus (12). In the present study, we extended this approach to determine the basis for Fv1nr restriction.
Fv1nr is responsible for relative resistance to B-tropic viruses and some, but not all, N-tropic viruses. This allele is found in 129 mice, as well as F and NZB mice (11; J. W. Hartley, unpublished data), and cells from these strains, as well as some wild mouse species (11), show resistance to most AKV type N-tropic MLVs, as well as B-tropic MLVs. In contrast, the isolate designated AKR-L1 grows to high titer in cells of these mice, as well as in cells of Fv1n mice. AKR-L1 was originally isolated from the spleen, thymus, and lymph nodes of a mouse with spontaneous AKR leukemia (17), but the basis for the unique replication pattern of this AKR-derived ecotropic MLV is unknown. To address this question, we first biologically cloned the virus by passaging it through two rounds of limiting dilution. The resulting virus, designated AKR-L1J, was used to infect prototype Fv1n, Fv1b, Fv1nr, and Fv1o cells (Table 1). Subconfluent cell cultures were infected with 10-fold dilutions of virus-containing medium in the presence of Polybrene (4 μg/ml; Aldrich, Milwaukee, Wis.). Replication was scored by the XC test (19) 4 to 5 days after infection. As shown in Table 1, AKR-L1J resembled AKR-L1 in its ability to replicate in 129 cells.
TABLE 1.
Replication of wild-type and mutant viruses in cells of various laboratory strainsa
| Virus | Log10 virus titer
|
Tropism | |||
|---|---|---|---|---|---|
| SC-1 (o)b | NFS (n) | 129 (nr) | BALB/c (b) | ||
| AKV | 6.2 | 5.2 | 1.8 | 3.7 | N |
| AKR-L1 | 5.4 | 4.7 | 4.4 | 3.4 | NR |
| WN1802B | 5.2 | 2.1 | 3.0 | 4.8 | B |
| Moloney MLV | 4.0 | 4.3 | 4.9 | 4.3 | NB |
| M1 | 5.5 | 3.9 | 4.1 | 1.8 | NR |
| M2 | 6.1 | 3.7 | 4.1 | 3.0 | NR |
| M3 | 5.4 | 3.4 | 1.7 | 1.0 | N |
The four reference viruses (Moloney MLV, AKV, AKR-L1, and WN1802B) were obtained originally from Janet Hartley (National Institute of Allergy and Infectious Diseases, Bethesda, Md.). NIH 3T3 (Fv1n), SC-1 (Fv1o), NFS (Fv1n), 129 (Fv1nr), BALB/c (Fv1b), M. dunni, and rat XC cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum and antibiotics. Embryo fibroblast cells were prepared from NFS/N-Bxv1 mice bred in our own colony and from BALB/cJ and 129/J mice obtained from The Jackson Laboratory (Bar Harbor, Maine). The underlined titers indicate restriction.
Fv1 types are in parentheses.
To determine the nucleotide sequence of the CA gene of this virus, Hirt DNA (8) extracted from AKR-L1J-infected Mus dunni cells (13) was used as a PCR substrate. The primers used to amplify the CA gene were as follows: CA-1 (forward; nucleotides [nt] 1022 to 1041), 5′-TTACCCTGCTCTTACCCCCT-3′; CA-2 (reverse; nt 2124 to 2143), 5′-TGATCCTTGTCAAGTTGGGG-3′. PCR products were cloned into the pGEM-T vector and directly sequenced. As shown in Fig. 1, the amino acid sequence of CA differs at only one residue, 114, from that of AKV MLV (GenBank accession no. AF222714). At this site, His is replaced by Asn. At the N or B site at amino acid 110, AKR-L1 resembles N-tropic AKV MLV.
FIG. 1.
Nucleotide sequence of CAgag of AKR-L1J (line 3) compared with those of N-tropic AKV MLV, B-tropic WN1802B (15), and the NB-tropic Rauscher, Moloney, and Friend MLVs (10, 16, 20).
To confirm that this change at residue 114 is responsible for the phenotypic difference, mutations were generated by PCR using the Stratagene (La Jolla, Calif.) QuickChange Site-Directed Mutagenesis kit. PCR product segments of the CA gene were subcloned into pGEM-T. Amino acid changes were introduced into the CA gene using oligonucleotides designed to incorporate specific base changes. The mutated fragment was then removed as a 723-bp BsiWI-PshAI fragment corresponding to nt 1126 to 1849 of the viral genome. This fragment was ligated to pAKV34, from which the corresponding 723-bp fragment had been deleted. Plasmid pAKV34 contains an 8.2-kb PstI fragment of pAKR623 subcloned into pBR322 (14) and was a gift of J. Lenz (Albert Einstein College of Medicine, New York, N.Y.). All mutations were confirmed by DNA sequencing.
To determine the effect of the alteration of 114His to 114Asn on virus replication, the mutated proviral DNA was introduced into SC-1 cells (6) by transfection. DNA was first digested with PstI to release the viral insert, treated with T4 ligase overnight, and then transfected using the Qiagen (Valencia, Calif.) SuperFect Transfection Kit. A day after transfection, cells were trypsinized and divided into T-75 flasks. At 3- to 4-day intervals, each culture was tested for virus and then passed into larger flasks to prepare a virus pool.
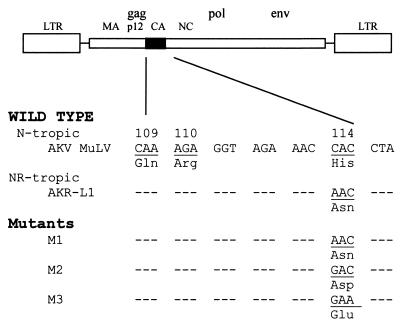
Culture supernatants collected from transfected cells were tested for viral tropism as shown in Table 1. The initial mutant, M1, in which AKV MLV residue 114His was converted to 114Asn, has a replication pattern comparable to that of AKR-L1J (Fig. 2; Table 1), confirming the role of this substitution in the n and nr phenotypes. Our previous mutagenesis studies had focused on residues 109 and 110 and demonstrated that when residue 110 contains acidic amino acids, the virus is B tropic, but when basic amino acids are substituted at this site, N-tropic viruses result (12). Therefore, we introduced several additional changes at residue 114 to determine the range of sequence variation within the two phenotypic classes (Fig. 2). We converted 114His, a basic amino acid, to the acidic amino acids Glu and Asp. Both substitutions produced infectious virus. However, the virus containing 114Asp resembles AKR-L1 in its high titer on 129 cells whereas the virus with 114Glu was restricted by these cells, like AKV MLV. These results indicate that these substitutions are tolerated and produce infectious virus with replication patterns that are comparable to the known patterns of sensitivity to Fv1 restriction.
FIG. 2.
Site-directed mutagenesis in the Fv1-sensitive region of the AKV MLV CA gene. Nucleotide and amino acid sequences are given for the prototype virus, for AKR-L1J, and for three mutants. LTR, long terminal repeat.
These studies confirm that CA contains the target of the Fv1nr gene product and, together with our previous study (12), also show that altered Fv1-mediated replication patterns can result from changes in at least two sites within CAgag. Sequence comparisons of NB-tropic viruses (Fig. 1) further indicate that, while these viruses contain multiple substitutions in CAgag, there are no consistent differences at residue 110 or 114, indicating that still further sites are likely to influence the interaction of NB-tropic viruses with the Fv1 gene product during replication.
We plan to continue to produce and test mutants with changes in CAgag for two reasons. First, mutant viruses with more extreme patterns of restricted replication would be potentially useful reagents in studies to determine the mechanism of Fv1 restriction. Second, previous studies with laboratory and wild mouse species suggest that, in addition to the well-defined Fv1-mediated replication patterns, there exist additional, more subtle but clearly reproducible, variations in virus sensitivity (11, 12). It is hoped that additional mutagenesis will produce viruses that intensify these subtle differences in replication patterns and will aid in the complete description of Fv1 allelic variation. In fact, the possibility that mutagenesis in CAgag will produce novel phenotypes was demonstrated in our previous mutagenesis studies (12), in which we generated one virus with a substitution at residue 110 that had a replication pattern unlike that described for any naturally occurring virus.
Acknowledgments
Y.T.J. was supported in part by the Korea Research Foundation in the program year 1997.
REFERENCES
- 1.Benade L E, Ihle J N, Decleve A. Serological characterization of B-tropic viruses of C57BL mice: possible origin by recombination of endogenous N-tropic and xenotropic viruses. Proc Natl Acad Sci USA. 1978;75:4553–4557. doi: 10.1073/pnas.75.9.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best S, Le Tissier P, Towers G, Stoye J P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- 3.Boone L R, Innes C L, Heitman C K. Abrogation of Fv-1 restriction by genome-deficient virions produced by a retrovirus packaging cell line. J Virol. 1990;64:3376–3381. doi: 10.1128/jvi.64.7.3376-3381.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DesGroseillers L, Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983;48:685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gautsch J W, Elder J H, Schindler J, Jensen F C, Lerner R A. Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc Natl Acad Sci USA. 1978;75:4170–4174. doi: 10.1073/pnas.75.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley J W, Rowe W P. Clonal cell lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975;65:128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- 7.Hartley J W, Rowe W P, Huebner R J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970;5:221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins N, Schindler J, Hynes R. Six NB-tropic murine leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977;21:309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khimani A H, Lim M, Graf T G, Smith T F, Ruprecht R M. Phylogenetic relationship of the complete Rauscher murine leukemia virus genome with other murine leukemia virus genomes. Virology. 1997;238:64–67. doi: 10.1006/viro.1997.8814. [DOI] [PubMed] [Google Scholar]
- 11.Kozak C A. Analysis of wild-derived mice for the Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J Virol. 1985;55:281–285. doi: 10.1128/jvi.55.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak C A, Chakraborti A. Single amino acid changes in the murine leukemia virus capsid protein gene define the target of Fv1 resistance. Virology. 1996;225:300–305. doi: 10.1006/viro.1996.0604. [DOI] [PubMed] [Google Scholar]
- 13.Lander M R, Chattopadhyay S K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ecotropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984;52:695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenz J, Crowther R, Straceski A, Haseltine W. Nucleotide sequence of Akv env gene. J Virol. 1982;42:519–529. doi: 10.1128/jvi.42.2.519-529.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou C-Y, Boone L R, Koh C-K, Tennant R W, Yang W K. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J Virol. 1983;48:779–784. doi: 10.1128/jvi.48.3.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perryman S, Nishio J, Chesebro B. Complete nucleotide sequence of Friend murine leukemia virus, strain FB29. Nucleic Acids Res. 1991;19:6950. doi: 10.1093/nar/19.24.6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pincus T, Hartley J W, Rowe W P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971;133:1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pincus T, Hartley J W, Rowe W P. A major genetic locus affecting resistance to infection with murine leukemia virus. IV. Dose-response relationships in Fv-1 sensitive and resistant cell cultures. Virology. 1975;65:333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- 19.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 20.Shinnick T M, Lerner R A, Sutcliffe J G. Nucleotide sequence of Moloney leukaemia virus. Nature. 1981;293:543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- 21.Steeves R, Lilly F. Interactions between host and viral genomes in mouse leukemia. Annu Rev Genet. 1977;11:277–296. doi: 10.1146/annurev.ge.11.120177.001425. [DOI] [PubMed] [Google Scholar]