ABSTRACT
Lithiated organic cathode materials show great promise for practical applications in lithium-ion batteries owing to their Li-reservoir characteristics. However, the reported lithiated organic cathode materials still suffer from strict synthesis conditions and low capacity. Here we report a thermal intermolecular rearrangement method without organic solvents to prepare dilithium hydroquinone (Li2Q), which delivers a high capacity of 323 mAh g−1 with an average discharge voltage of 2.8 V. The reversible conversion between orthorhombic Li2Q and monoclinic benzoquinone during charge/discharge processes is revealed by in situ X-ray diffraction. Theoretical calculations show that the unique Li–O channels in Li2Q are beneficial for Li+ ion diffusion. In situ ultraviolet-visible spectra demonstrate that the dissolution issue of Li2Q electrodes during charge/discharge processes can be handled by separator modification, resulting in enhanced cycling stability. This work sheds light on the synthesis and battery application of high-capacity lithiated organic cathode materials.
Keywords: lithium-ion batteries, cathode materials, lithiated organic materials, dilithium hydroquinone, separator modification
The lithiated organic cathode material, dilithium hydroquinone, has been synthesized by a facile thermal intermolecular rearrangement method, and shows a good promise as high-capacity cathodes for lithium-ion batteries.
INTRODUCTION
Lithium-ion batteries (LIBs) have dominated the market of portable electronics and shown great promise for large-scale energy storage applications since their commercialization in the early 1990s [1,2]. The current electrochemistry of cathode materials in commercial LIBs is based on Li-ion interaction/de-interaction in transition-metal oxides or phosphates [3–5]. The limitations of inorganic cathode materials mainly include scarce natural resources, recyclability issues, and high CO2 emissions and energy consumption during the material production process [6–8]. Thus, the development of new cathode materials is urgently needed. In recent years, organic electrode materials have attracted much attention because of their high abundance, environmental friendliness and renewability [9–11]. Unfortunately, the reported organic cathode materials are mostly free of Li initially and used at oxidation states, which have to match with Li-containing anodes such as Li metal [12–15]. The immaturity of Li-containing anodes inevitably hinders the practical application of most organic cathode materials. In this regard, developing lithiated organic cathode materials at reduced states as Li reservoirs to match with commercial Li-free anode materials, such as graphite, is of great significance for future commercial applications.
In fact, the investigation of lithiated organic cathode materials is still in its infancy [16]. The most widely studied lithiated organic cathode materials are dilithium (2,5-dilithium-oxy)-terephthalate (Li4-p-DHT) and its derivatives [17–22]. Li4-p-DHT can be prepared by the reaction between CH3OLi and 2,5-dihydroxyterephthalic acid in absolute methanol, showing a discharge capacity of 223 mAh g−1 at 0.1 C after morphology optimization [17]. The capacity is lower for the derivatives of Li4-p-DHT owing to the introduction of inactive atoms/groups [19,20,22]. Recently, Vlad's group prepared a series of lithiated organic cathode materials based on conjugated sulfonamides by using CH3OLi and LiH as lithiation reagents [23,24]. Among them, dilithium 1,4-phenylenebis((methylsulfonyl)amide) exhibits the highest reversible capacity, ∼166 mAh g−1. Similarly, the same group subsequently developed families of conjugated triflimides and cyanamides as high-voltage lithiated cathode materials by using CH3OLi and LiH as lithiation reagents [25]. In addition, other types of lithiated organic cathode materials based on different active sites such as C=N–Li and N–O–Li were also reported [26,27]. For example, lithium tetracyanoquinodimethane featuring active groups of C=N–Li was demonstrated to be electrochemically reversible, however, with a low capacity of 127 mAh g−1 [26]. To date, the most common lithiation reagents used to synthesize lithiated organic cathode materials have been LiH and CH3OLi, which are very chemically reactive and hard to apply to large-scale practical applications. Moreover, the reversible capacities of lithiated organic cathode materials are generally limited. In short, the strict synthesis conditions derived from the use of LiH or CH3OLi and the limited reversible capacity are the two common challenges facing lithiated organic cathode materials.
Herein, we develop a thermal intermolecular rearrangement method under mild conditions to realize the scalable synthesis of the lithiated organic cathode material dilithium hydroquinone (Li2Q), which shows a high reversible capacity of 323 mAh g−1 with an average discharge voltage of 2.8 V at 0.1 C, corresponding to an energy density of ∼900 Wh kg−1 at the active material level. The pristine Li2Q with an orthorhombic structure converts to 1,4-benzoquinone (BQ) with a monoclinic structure after charge, and the conversion is reversible during the subsequent discharge process, as revealed by in situ X-ray diffraction (XRD) patterns. Density functional theory (DFT) calculations found that there are unique Li–O channels for facile Li+ ion diffusion in Li2Q. The cycling stability of Li2Q can be improved by separator optimization owing to the mitigated dissolution issue, as observed via in situ ultraviolet-visible (UV-vis) spectroscopy. This work illustrates the facile synthesis and battery applications of lithiated organic cathode materials.
RESULTS AND DISCUSSION
Three different approaches have been tried to prepare Li2Q from hydroquinone (H2Q), as shown in Fig. 1a. The first method utilizes LiH as the lithiation reagent and anhydrous 1,2-dimethoxyethane (DME) as the solvent under conditions free of H2O and O2. After adequate stirring, the DME solvent was evaporated in a vacuum to obtain the product. The infrared (IR) spectra and 1H nuclear magnetic resonance (NMR) spectra of the pristine H2Q and obtained product are shown in Figs S1 and S2 (Supplementary Data), respectively. The results show that there are obvious peaks assigned to the stretching vibration of residual O–H, indicating that the neutralization reaction between H2Q and LiH cannot proceed completely. Moreover, the industrial production of LiH is challenging and dangerous because it generally comes from the reaction between Li metal and H2 at high temperature and/or high pressure [28]. We then used LiOH·H2O as the lithiation reagent under Ar atmosphere with H2O as the solvent. The IR and 1H NMR spectra of the obtained product in Figs S3 and S4 indicate that there is also residual O–H and the neutralization reaction between H2Q and LiOH is incomplete. This could be attributed to the weak dissociation of the second H+ of H2Q (pKa2 = 11.4) [29]. To sum up, it is hard to synthesize pure Li2Q using the two aforementioned methods (Fig. 1a). Thus, new approaches for synthesizing pure Li2Q are urgently needed.
Figure 1.

Synthesis of the lithiated organic cathode material Li2Q. (a) Schematic diagrams of three different methods used to synthesize Li2Q and the corresponding characteristics. (b) Synthesis mechanism of obtaining pure Li2Q by using insufficient LiOH·H2O to react with H2Q, and then annealing to generate Li2Q.
The incomplete reactions between H2Q and LiOH·H2O lead to the emergence of monolithium hydroquinone (LiHQ). On the basis of the intermolecular interaction of LiHQ and the sublimation property of H2Q, we develop a new method, thermal intermolecular rearrangement, to synthesize pure Li2Q. As shown in Fig. 1a, we firstly used excess H2Q to react with insufficient LiOH·H2O in water under Ar atmosphere, to generate the mixture containing LiHQ. Then, the mixture was annealed at 180°C to make LiHQ decompose to Li2Q and H2Q. All H2Q escapes from this system in a gas state (Fig. 1b). The sublimated material is indeed pure H2Q, as confirmed by IR and 1H NMR spectra (Figs S5 and S6). The thermal intermolecular rearrangement reaction is entropy-increasing and thus easy to carry out, and is similar to the thermal decomposition of NaHCO3 to Na2CO3 and H2CO3 (i.e. CO2, H2O). The synthesis of Li2Q by this method is green because no organic solvent was used during the reaction and isolation processes. This is superior to the previously reported methods of synthesizing lithiated organic cathode materials using LiH or CH3OLi with regard to toxicity, cost and safety. More importantly, this method is generalizable and could be extended to synthesize other similar lithiated organic cathode materials. The basic prerequisite of this method, used for other lithiated organic materials, is that the raw material (phenol) can sublimate at a proper temperature before the decomposition of the target product.
The product obtained by the thermal intermolecular rearrangement method was characterized by various approaches (Fig. 2). The IR spectra of H2Q, LiOH·H2O and the product in Fig. 2a indicate that there is no peak assigned to O–H of H2Q or LiOH·H2O, implying the complete substitution of H in O–H of H2Q by Li. The results can also be confirmed by the Raman spectra of H2Q, LiOH·H2O and the product (Fig. 2b). The liquid-state 1H NMR spectrum of the Li2Q product is shown in Fig. S7. Except for the solvent peak, no other peaks can be observed, implying that the solubility of Li2Q in deuterium dimethyl sulfoxide (DMSO-d6) with high polarity is limited. Thus, we used solid-state NMR to further characterize the raw material, intermediate mixture and products. The solid-state 13C NMR spectra in Fig. 2c show that there are two types of C in H2Q, that is, 149 ppm (C of C–OH) and 116 ppm (other C of the benzene ring). From the intermediate mixture to Li2Q, the peak belonging to C of C–OH disappears completely and meanwhile the peak at 154 ppm, which can be assigned to C of C–OLi, emerges, suggesting the successful synthesis of Li2Q. Furthermore, we obtained the solid-state 7Li NMR spectra of LiOH·H2O, intermediate mixture and Li2Q. The results in Fig. 2d show that Li2Q has the lowest chemical shift of −0.947 ppm when compared with LiOH·H2O and the intermediate mixture.
Figure 2.

Characterization of Li2Q. (a) IR and (b) Raman spectra of H2Q, LiOH·H2O and Li2Q. (c) Solid-state 13C NMR spectra of H2Q, the intermediate mixture and Li2Q. (d) Solid-state 7Li NMR spectra of LiOH·H2O, the intermediate mixture and Li2Q. The evolution of IR spectra of Li2Q at different exposure times (e) in ambient air and (f) in dry air (dew point: about −40°C).
For practical applications, the air stability, thermal stability and electronic conductivity of cathode materials are very important, especially for lithiated organic materials at reduced states. Figure 2e shows the evolution of the IR spectra of Li2Q in ambient air. The results indicate that Li2Q tends to absorb H2O in air, which leads to hydrolysis and oxidation, as proved by the appearance of peaks attributed to H2O, LiOH and C=O after exposure for 1 h and 4 h. This is the reason why the synthetic process was conducted in Ar atmosphere instead of air. In contrast, the stability of Li2Q in dry air (dew point: about −40°C) is much better, as confirmed by the IR spectra in Fig. 2f, where no change was observed after being placed for 12 h. The high stability of Li2Q in dry air means it can be handled and processed in practical applications. We also evaluated the thermal stability of Li2Q, which affects the safety of batteries. The weight loss of Li2Q in Ar atmosphere starts at ∼568°C, implying its high thermal stability (Fig. S8). Moreover, the electronic conductivity of Li2Q tested via digital multimeter is 9.37 × 10−10 S cm−1, which is close to that of inorganic LiFePO4 cathode material [30]. In addition, the scanning electron microscopy (SEM) images of Li2Q show that it exists in the form of micrometer-level particles (Fig. S9).
We then studied the crystal structure of the obtained Li2Q through XRD testing and theoretical calculations. At first, we optimized the crystal structure of Li2Q through theoretical calculations. Then, Rietveld refinement was conducted to simulate the experimental XRD pattern, as shown in Fig. 3a. The results reveal that the crystal system of Li2Q is orthorhombic. Detailed crystal parameters can be seen in Table S1 (Supplementary Data). The three main peaks at 2θ = 11.7°, 22.2° and 26.7° are assigned to the crystal plane of (1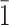 0), (11
0), (11 ) and (121) of Li2Q, respectively. Figure 3b shows the crystal structure of Li2Q with different views, where Li atoms are fixed by the neighboring three organic molecules and the length of the Li–O bond is ∼2.0 Å. To the best of our knowledge, this is the first time the crystal structure of Li2Q has been obtained.
) and (121) of Li2Q, respectively. Figure 3b shows the crystal structure of Li2Q with different views, where Li atoms are fixed by the neighboring three organic molecules and the length of the Li–O bond is ∼2.0 Å. To the best of our knowledge, this is the first time the crystal structure of Li2Q has been obtained.
Figure 3.

Crystal structure of Li2Q and evolution during charge and discharge processes. (a) XRD pattern and corresponding Rietveld refinement, and (b) crystal structure with different views of Li2Q. (c) In situ XRD patterns of Li2Q in the first cycle, where the main diffraction peaks and corresponding crystal planes of Li2Q and charge product BQ are marked.
The crystal structure evolution of Li2Q during charge and discharge processes was further investigated by in situ XRD. As shown in Fig. 3c, the three main diffraction peaks attributed to the crystal plane of (1 0), (11
0), (11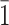 ) and (121) of Li2Q can be detected easily in the pristine electrode. These peaks gradually decrease and disappear completely after charging to 3.5 V (vs. Li+/Li). Meanwhile, the diffraction peaks at 2θ = 14.4°, 17.5°, 28.4°, 29.2°, 30.0°, 31.3° and 32.9° gradually emerge and become stronger and stronger with the charge process. As shown in Figs S10 and S11, these peaks are well consistent with the crystal plane of (100), (011), (120), (10
) and (121) of Li2Q can be detected easily in the pristine electrode. These peaks gradually decrease and disappear completely after charging to 3.5 V (vs. Li+/Li). Meanwhile, the diffraction peaks at 2θ = 14.4°, 17.5°, 28.4°, 29.2°, 30.0°, 31.3° and 32.9° gradually emerge and become stronger and stronger with the charge process. As shown in Figs S10 and S11, these peaks are well consistent with the crystal plane of (100), (011), (120), (10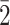 ), (012), (210) and (102) of BQ, featuring a monoclinic crystal system, respectively. During the subsequent discharge process, these peaks of BQ gradually disappear. Meanwhile, the two main peaks at 2θ = 22.2° and 26.7° assigned to the crystal plane of (11
), (012), (210) and (102) of BQ, featuring a monoclinic crystal system, respectively. During the subsequent discharge process, these peaks of BQ gradually disappear. Meanwhile, the two main peaks at 2θ = 22.2° and 26.7° assigned to the crystal plane of (11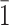 ) and (121) of Li2Q emerge but are weak after being fully discharged, implying that the crystallinity of Li2Q after the first cycle decreases when compared with pristine Li2Q. In subsequent cycles, the crystallinity of the charge product (BQ) does not change significantly and only becomes slightly weak when compared with the first cycle (Fig. S12), which could be attributed to the dissolution of BQ in electrolyte. In contrast, the crystallinity of the discharge product (Li2Q) in subsequent cycles remains weak and almost unchanged when compared with the first cycle. These results demonstrate the reversible conversion between orthorhombic Li2Q with low crystallinity and monoclinic BQ with relatively high crystallinity during repeated charge and discharge processes after the first cycle. The lower crystallinity of Li2Q than BQ during cycles may be ascribed to the fact that the latter is arranged in the same orientation (Fig. S11).
) and (121) of Li2Q emerge but are weak after being fully discharged, implying that the crystallinity of Li2Q after the first cycle decreases when compared with pristine Li2Q. In subsequent cycles, the crystallinity of the charge product (BQ) does not change significantly and only becomes slightly weak when compared with the first cycle (Fig. S12), which could be attributed to the dissolution of BQ in electrolyte. In contrast, the crystallinity of the discharge product (Li2Q) in subsequent cycles remains weak and almost unchanged when compared with the first cycle. These results demonstrate the reversible conversion between orthorhombic Li2Q with low crystallinity and monoclinic BQ with relatively high crystallinity during repeated charge and discharge processes after the first cycle. The lower crystallinity of Li2Q than BQ during cycles may be ascribed to the fact that the latter is arranged in the same orientation (Fig. S11).
After revealing the crystal structure evolution of Li2Q during cycles, we used DFT calculations to study the accessible Li+ ion diffusion pathways in the crystal structure of pristine Li2Q and the charge product BQ. As shown in Fig. 4a, two diffusion pathways, i.e. along ab and along c directions, are included in Li2Q based on its bulk structure. The diffusion of Li+ ion along the ab direction goes through the interlayer of benzene rings. Due to the weak interaction between benzene rings and Li+ ions, the diffusion energy barrier along the ab direction is as high as 1.31 eV (Fig. 4b). The diffusion of Li+ ion along the c direction is along the channel composed of O atoms, and this unique Li–O channel enables facile Li+ diffusion through the Li2Q crystal structure (Fig. 4a). Thus, the diffusion along the c direction shows a lower energy barrier of 0.47 eV, suggesting that the Li+ ion diffusion along the c direction is more favorable than that along the ab direction (Fig. 4b).
Figure 4.
Li+ ion diffusion behaviors in Li2Q and BQ. (a) The calculated different Li+ ion diffusion pathways and (b) corresponding relative energy of each pathway of Li2Q. (c) The calculated different Li+ ion diffusion pathways and (d) corresponding relative energy of each pathway of BQ.
For the charge product BQ, there are three possible Li+ ion diffusion pathways, that is, along a, b and c directions (Fig. 4c). The diffusion of Li+ ion along the a direction goes through the organic layers composed of benzene rings. The diffusions of Li+ ion along the b and c directions are both along the interlayer between organic layers. As shown in Fig. 4d, the diffusion energy barriers along a, b and c directions are 0.77 eV, 0.90 eV and 0.67 eV, respectively. Among them, the Li+ diffusion along the c direction in BQ is the most favorable one, and similar to the result in Li2Q. When compared with other organic electrode materials in previous works [31,32], the Li+ ion diffusion energy barriers in Li2Q and BQ along the c direction are relatively low, implying the effective diffusion of Li+ ions through both Li2Q and BQ. This might explain the high rate performance of batteries, which will be discussed later.
One of the major issues facing most organic electrode materials is their high solubility, and/or the high solubility of redox intermediates in electrolyte, leading to a shuttle effect and poor cycling stability [33,34]. Thus, prior to the electrochemical performance test, we used in situ UV-vis spectra to investigate the dissolution behavior of Li2Q electrodes in electrolyte during charge and discharge processes. The in situ UV-vis spectra tests were conducted using a homemade cell with ∼3 mL electrolyte (Fig. S13). As shown in Fig. 5a and Fig. S14a, pristine Li2Q shows limited dissolution in 1 mol kg−1 lithium bis(trifluoromethanesulfonyl) imide (LiTFSI) dissolved in ethylene carbonate and dimethyl carbonate (EC:DMC = 1:1 vol%) electrolyte. However, the solubility gradually increases with the charging process, especially at the end of charging (3.5 V vs. Li+/Li). In the following discharge process, the UV-vis spectra do not change obviously, implying that the dissolved charge products cannot be fully utilized during the discharge process.
Figure 5.
Electrochemical behaviors of Li2Q. In situ UV-vis spectra (color-mapped profiles) of Li2Q batteries during charge and discharge processes by using (a) a pristine separator and (b) a ZIF-7-modified separator. (c) CV curves at different scan rates (0.2, 0.5, 1, 2 and 3 mV s−1) and (d) the corresponding observed and fitting plots of log(|ip|) versus log(ν) at the four peak currents. (e) Typical charge/discharge curves at different current rates (0.1, 0.5, 1, 2 and 5 C) and (f) the corresponding differential capacitance dQ/dV plot of the charge/discharge curves at 1 C. (g) Cycling performance (discharge capacity and Coulombic efficiency) at 1 C with pristine and ZIF-7-modified separators. (h) Comparison of capacity, voltage and corresponding energy density of Li2Q, commercial inorganic cathode materials and other representative lithiated organic cathode materials prepared by chemical methods.
To address the dissolution problem, we used a metal-organic framework (zeolitic imidazolate framework-7, ZIF-7) as the modification layer on the Celgard separator because ZIF-7 shows a small pore size of only ∼2.9 Å, which can block active materials [35]. The ZIF-7 material was prepared in aqueous solution and its XRD pattern in Fig. S15 is in agreement with the result of previous reports [35,36]. Moreover, the obtained ZIF-7 exists in the form of micrometer-level cuboid particles, as seen in the SEM images (Fig. S16). The SEM images of the ZIF-7-modified separator in Fig. S17 show that ZIF-7 particles are distributed uniformly on the separator. In addition, the cross-sectional SEM image indicates that the thickness of the ZIF-7-based layer on the separator is ∼10 μm (Fig. S18). The in situ UV-vis spectra of a Li2Q battery using the ZIF-7-modified separator to fully wrap the Li2Q electrode during charge and discharge processes are shown in Fig. 5b and Fig. S14b. The results suggest that the dissolution of the electrode in electrolyte during charge and discharge processes can be suppressed effectively when compared with a pristine separator. Thus, we select the ZIF-7-modified Celgard as the separator for electrochemical performance studies.
As shown in Fig. 5c, cyclic voltammetry (CV) at different scan rates (0.2, 0.5, 1, 2 and 3 mV s−1) of Li2Q indicates that there are two distinct redox couples (O1/R1, O2/R2). The fitting results of the four redox peak currents, log(|ip|), versus scan rate, log(ν), are shown in Fig. 5d. The four b values are confirmed to be 0.56, 0.51, 0.55 and 0.79 for the peaks of O2, R2, O1 and R1, respectively. The results suggest that the electrochemical redox process of Li2Q is primarily controlled by Li+ ion diffusion.
Subsequently, we further investigated the electrochemical performance of Li2Q. Figure 5e shows the charge/discharge curves at different current rates (0.1, 0.5, 1, 2 and 5 C). The corresponding differential capacitance dQ/dV plot of the charge/discharge curves at 1 C is provided in Fig. 5f, which is consistent with the CV results in Fig. 5c. The discharge capacities of Li2Q are 323, 255, 204, 162 and 120 mAh g−1 at 0.1, 0.5, 1, 2 and 5 C, respectively (Fig. 5e). Moreover, the battery with a ZIF-7-modified separator exhibits improved cycling performance with a capacity retention of 61% after 30 cycles at 1 C, which is much higher than with a pristine separator under the same test conditions (32%), demonstrating the positive effect of a ZIF-7-modified separator with regard to the stability of Li2Q during cycles (Fig. 5g). Compared with other reported lithiated organic cathode materials, Li2Q in this work exhibits an outstanding capacity of 323 mAh g−1 and a high energy density of ∼900 Wh kg−1 at 0.1 C. The capacity and energy density of Li2Q are higher than those of not only commercial inorganic cathode materials [37–40], but also most reported lithiated organic cathode materials prepared by chemical methods (Fig. 5h) [17,19–26,41,42]. Together with the developed facile synthesis method under mild conditions that has been demonstrated in this work, Li2Q shows great promise for battery applications. However, although progress has been made in this work, the cycling stability of Li2Q still needs to be further improved through more elaborate efforts in the future, with the aim of achieving the practical application of Li2Q.
CONCLUSIONS
In summary, we have successfully developed a thermal intermolecular rearrangement method to prepare the lithiated organic cathode material Li2Q. A combination of theoretical calculations and in situ XRD studies reveals that pristine Li2Q with an orthorhombic structure converts to BQ with a monoclinic structure after charge, and the conversion is reversible during the subsequent discharge process. Further theoretical calculations found that the diffusion of Li+ ion along the c direction is along the channel composed of O atoms, which enables facile Li+ ion diffusion through the Li2Q crystal structure. As a cathode material, Li2Q can deliver a high capacity of 323 mAh g−1 with an average discharge voltage of 2.8 V. In situ UV-vis spectra indicate that the dissolution issue of Li2Q electrodes during charge and discharge processes can be effectively mitigated by the ZIF-7-modified separator, resulting in enhanced cycling stability. This work paves the way for promoting the facile synthesis and battery applications of high-capacity lithiated organic cathode materials.
Supplementary Material
Contributor Information
Yong Lu, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Haoqin Han, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Zhuo Yang, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Youxuan Ni, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Zhicheng Meng, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Qiu Zhang, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Hao Wu, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Weiwei Xie, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Zhenhua Yan, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
Jun Chen, Frontiers Science Center for New Organic Matter, Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), State Key Laboratory of Advanced Chemical Power Sources, College of Chemistry, Nankai University, Tianjin 300071, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (22109075, 22121005 and 22020102002), the National Key R&D Program of China (2022YFB2402200), the Frontiers Science Center for New Organic Matter of Nankai University (63181206) and the Young Elite Scientists Sponsorship Program by China Association for Science and Technology (2023QNRC001).
AUTHOR CONTRIBUTIONS
J.C. and Y.L. conceived the idea. J.C. supervised the research. Y.L., H.H. and Z.M. conducted the synthesis and characterization. Y.L., Z.Y. and H.W. performed the electrochemical performance tests. Y.N. and W.X. carried out the computations. Q.Z. organized the figures. J.C. and Y.L. wrote the manuscript. J.C. and Z.Y. revised the manuscript. All authors contributed to the manuscript preparation.
Conflict of interest statement. None declared.
REFERENCES
- 1. Goodenough JB. How we made the Li-ion rechargeable battery. Nat Electron 2018; 1: 204. 10.1038/s41928-018-0048-6 [DOI] [Google Scholar]
- 2. Lu Y, Zhang Q, Chen J. Recent progress on lithium-ion batteries with high electrochemical performance. Sci China Chem 2019; 62: 533–48. 10.1007/s11426-018-9410-0 [DOI] [Google Scholar]
- 3. Zhang H, Liu H, Piper LFJ et al. Oxygen loss in layered oxide cathodes for Li-ion batteries: mechanisms, effects, and mitigation. Chem Rev 2022; 122: 5641–81. 10.1021/acs.chemrev.1c00327 [DOI] [PubMed] [Google Scholar]
- 4. Wang L, Qiu J, Wang X et al. Insights for understanding multiscale degradation of LiFePO4 cathodes. eScience 2022; 2: 125–37. 10.1016/j.esci.2022.03.006 [DOI] [Google Scholar]
- 5. Shi J-L, Sheng H, Meng X-H et al. Size controllable single-crystalline Ni-rich cathodes for high-energy lithium-ion batteries. Natl Sci Rev 2023; 10: nwac226. 10.1093/nsr/nwac226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turcheniuk K, Bondarev D, Singhal V et al. Ten years left to redesign lithium-ion batteries. Nature 2018; 559: 467–70. 10.1038/d41586-018-05752-3 [DOI] [PubMed] [Google Scholar]
- 7. Ciez RE, Whitacre JF. Examining different recycling processes for lithium-ion batteries. Nat Sustain 2019; 2: 148–56. 10.1038/s41893-019-0222-5 [DOI] [Google Scholar]
- 8. Su Y, Johannessen B, Zhang S et al. Soft−rigid heterostructures with functional cation vacancies for fast-charging and high-capacity sodium storage. Adv Mater 2023; 35: 2305149. 10.1002/adma.202305149 [DOI] [PubMed] [Google Scholar]
- 9. Kim J, Kim Y, Yoo J et al. Organic batteries for a greener rechargeable world. Nat Rev Mater 2023; 8: 54−70. 10.1038/s41578-022-00478-1 [DOI] [Google Scholar]
- 10. Poizot P, Gaubicher J, Renault S et al. Opportunities and challenges for organic electrodes in electrochemical energy storage. Chem Rev 2020; 120: 6490–557. 10.1021/acs.chemrev.9b00482 [DOI] [PubMed] [Google Scholar]
- 11. Xie J, Zhang Q. Recent progress in aqueous monovalent-ion batteries with organic materials as promising electrodes. Mater Today Energy 2020; 18: 100547. 10.1016/j.mtener.2020.100547 [DOI] [Google Scholar]
- 12. Lu Y, Chen J. Prospects of organic electrode materials for practical lithium batteries. Nat Rev Chem 2020; 4: 127–42. 10.1038/s41570-020-0160-9 [DOI] [PubMed] [Google Scholar]
- 13. Huang W, Liu S, Li C et al. Calix[8]quinone: a new promising macrocyclic molecule as an efficient organic cathode in lithium ion batteries with a highly-concentrated electrolyte. EcoMat 2022; 4: e12214. 10.1002/eom2.12214 [DOI] [Google Scholar]
- 14. Xiong P, Zhang S, Wang R et al. Covalent triazine frameworks for advanced energy storage: challenges and new opportunities. Energy Environ Sci 2023; 16: 3181–213. 10.1039/D3EE01360J [DOI] [Google Scholar]
- 15. Sun J, Xu Y, Lv Y et al. Recent advances in covalent organic framework electrode materials for alkali metal-ion batteries. CCS Chem 2023; 5: 1259–76. 10.31635/ccschem.023.202302808 [DOI] [Google Scholar]
- 16. Lu Y, Zhang Q, Li F et al. Emerging lithiated organic cathode materials for lithium-ion full batteries. Angew Chem Int Ed 2023; 62: e202216047. 10.1002/anie.202216047 [DOI] [PubMed] [Google Scholar]
- 17. Wang S, Wang L, Zhang K et al. Organic Li4C8H2O6 nanosheets for lithium-ion batteries. Nano Lett 2013; 13: 4404–9. 10.1021/nl402239p [DOI] [PubMed] [Google Scholar]
- 18. Renault S, Gottis S, Barrès A-L et al. A green Li—organic battery working as a fuel cell in case of emergency. Energy Environ Sci 2013; 6: 2124–33. 10.1039/c3ee40878g [DOI] [Google Scholar]
- 19. Jouhara A, Dupré N, Gaillot A-C et al. Raising the redox potential in carboxyphenolate-based positive organic materials via cation substitution. Nat Commun 2018; 9: 4401. 10.1038/s41467-018-06708-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rambabu D, Lakraychi AE, Wang J et al. An electrically conducting Li-ion metal-organic framework. J Am Chem Soc 2021; 143: 11641−50. 10.1021/jacs.1c04591 [DOI] [PubMed] [Google Scholar]
- 21. Gottis S, Barrès A-L, Dolhem F et al. Voltage gain in lithiated enolate-based organic cathode materials by isomeric effect. ACS Appl Mater Interfaces 2014; 6: 10870−6. 10.1021/am405470p [DOI] [PubMed] [Google Scholar]
- 22. Sieuw L, Lakraychi AE, Rambabu D et al. Through-space charge modulation overriding substituent effect: rise of the redox potential at 3.35 V in a lithium-phenolate stereoelectronic isomer. Chem Mater 2020; 32: 9996−10006. 10.1021/acs.chemmater.0c02989 [DOI] [Google Scholar]
- 23. Wang J, Lakraychi AE, Liu X et al. Conjugated sulfonamides as a class of organic lithium-ion positive electrodes. Nat Mater 2021; 20: 665–73. 10.1038/s41563-020-00869-1 [DOI] [PubMed] [Google Scholar]
- 24. Wang J, Guo X, Apostol P et al. High performance Li-, Na-, and K-ion storage in electrically conducting coordination polymers. Energy Environ Sci 2022; 15: 3923–32. 10.1039/D2EE00566B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo X, Apostol P, Zhou X et al. Towards the 4 V-class n-type organic lithium-ion positive electrode materials: the case of conjugated triflimides and cyanamides. Energy Environ Sci 2024; 17: 173–82. 10.1039/D3EE02897F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deng W, Shi W, Li P et al. A Li-contained air-stable cathode for high-performance all-organic lithium-ion batteries. Energy Storage Mater 2022; 46: 535–41. 10.1016/j.ensm.2022.01.039 [DOI] [Google Scholar]
- 27. Wang J, Apostol P, Rambabu D et al. Revealing the reversible solid-state electrochemistry of lithium-containing conjugated oximates for organic batteries. Sci Adv 2023; 9: eadg6079. 10.1126/sciadv.adg6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Howie RT, Narygina O, Guillaume CL et al. High-pressure synthesis of lithium hydride. Phys Rev B 2012; 86: 064108. 10.1103/PhysRevB.86.064108 [DOI] [Google Scholar]
- 29. Bishop CA, Tong LKJ. Equilibria of substituted semiquinones at high pH. J Am Chem Soc 1965; 87: 501–5. 10.1021/ja01081a018 [DOI] [Google Scholar]
- 30. Ou X, Liang G, Wang L et al. Effects of magnesium doping on electronic conductivity and electrochemical properties of LiFePO4 prepared via hydrothermal route. J Power Sources 2008; 184: 543–7. 10.1016/j.jpowsour.2008.02.077 [DOI] [Google Scholar]
- 31. Ball B, Chakravarty C, Sarkar P. Two-dimensional covalent triazine framework as a promising anode material for Li-ion batteries. J Phys Chem C 2019; 123: 30155−64. 10.1021/acs.jpcc.9b09268 [DOI] [Google Scholar]
- 32. Wang J, Li F, Qu Y et al. PNTCDA: a promising versatile organic electrode material for alkali-metal ion batteries. J Mater Chem A 2018; 6: 24869−76. 10.1039/C8TA08621D [DOI] [Google Scholar]
- 33. Li M, Hicks RP, Chen Z et al. Electrolytes in organic batteries. Chem Rev 2023; 123: 1712–73. 10.1021/acs.chemrev.2c00374 [DOI] [PubMed] [Google Scholar]
- 34. Lu Y, Yang Z, Zhang Q et al. Regulating electrostatic interaction between hydrofluoroethers and carbonyl cathodes toward highly stable lithium−organic batteries. J Am Chem Soc 2024; 146: 1100–8. 10.1021/jacs.3c12358 [DOI] [PubMed] [Google Scholar]
- 35. Chang Z, Qiao Y, Deng H et al. A liquid electrolyte with de-solvated lithium ions for lithium-metal battery. Joule 2020; 4: 1776–89. 10.1016/j.joule.2020.06.011 [DOI] [Google Scholar]
- 36. Cai W, Lee T, Lee M et al. Thermal structural transitions and carbon dioxide adsorption properties of zeolitic imidazolate framework-7 (ZIF-7). J Am Chem Soc 2014; 136: 7961–71. 10.1021/ja5016298 [DOI] [PubMed] [Google Scholar]
- 37. Cai Y, Huang Y, Wang X et al. Facile synthesis of LiMn2O4 octahedral nanoparticles as cathode materials for high capacity lithium ion batteries with long cycle life. J Power Sources 2015; 278: 574–81. 10.1016/j.jpowsour.2014.12.082 [DOI] [Google Scholar]
- 38. Cho J, Kim YJ, Park B. Novel LiCoO2 cathode material with Al2O3 coating for a Li ion cell. Chem Mater 2000; 12: 3788–91. 10.1021/cm000511k [DOI] [Google Scholar]
- 39. Ju SH, Kang I-S, Lee Y-S et al. Improvement of the cycling performance of LiNi0.6Co0.2Mn0.2O2 cathode active materials by a dual-conductive polymer coating. ACS Appl Mater Interfaces 2014; 6: 2546–52. 10.1021/am404965p [DOI] [PubMed] [Google Scholar]
- 40. Huang X, Yao Y, Liang F et al. Concentration-controlled morphology of LiFePO4 crystals with an exposed (100) facet and their enhanced performance for use in lithium-ion batteries. J Alloys Compd 2018; 743: 763–72. 10.1016/j.jallcom.2018.02.048 [DOI] [Google Scholar]
- 41. Barrès A-L, Geng J, Bonnard G et al. High-potential reversible Li deintercalation in a substituted tetrahydroxy-p-benzoquinone dilithium salt: an experimental and theoretical study. Chem Eur J 2012; 18: 8800–12. 10.1002/chem.201103820 [DOI] [PubMed] [Google Scholar]
- 42. Yao M, Taguchi N, Ando H et al. Improved gravimetric energy density and cycle life in organic lithium-ion batteries with naphthazarin-based electrode materials. Commun Mater 2020; 1: 70. 10.1038/s43246-020-00071-5 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




