Abstract
Ketone bodies are an energy substrate produced by the liver and used during states of low carbohydrate availability, such as fasting or prolonged exercise. High ketone concentrations can be present with insulin insufficiency and are a key finding in diabetic ketoacidosis (DKA). During states of insulin deficiency, lipolysis increases and a flood of circulating free fatty acids is converted in the liver into ketone bodies—mainly beta-hydroxybutyrate and acetoacetate. During DKA, beta-hydroxybutyrate is the predominant ketone in blood. As DKA resolves, beta-hydroxybutyrate is oxidized to acetoacetate, which is the predominant ketone in the urine. Because of this lag, a urine ketone test might be increasing even as DKA is resolving. Point-of-care tests are available for self-testing of blood ketones and urine ketones through measurement of beta-hydroxybutyrate and acetoacetate and are cleared by the US Food and Drug Administration (FDA). Acetone forms through spontaneous decarboxylation of acetoacetate and can be measured in exhaled breath, but currently no device is FDA-cleared for this purpose. Recently, technology has been announced for measuring beta-hydroxybutyrate in interstitial fluid. Measurement of ketones can be helpful to assess compliance with low carbohydrate diets; assessment of acidosis associated with alcohol use, in conjunction with SGLT2 inhibitors and immune checkpoint inhibitor therapy, both of which can increase the risk of DKA; and to identify DKA due to insulin deficiency. This article reviews the challenges and shortcomings of ketone testing in diabetes treatment and summarizes emerging trends in the measurement of ketones in the blood, urine, breath, and interstitial fluid.
Keywords: diabetes, ketones, insulin, diabetic ketoacidosis, continuous ketone monitor, SGLT2 inhibitors
Introduction
Ketones or ketoacids in the body are formed in the liver from fatty acids liberated by lipolysis during periods of insulin deficiency because insulin suppresses lipolysis. 1 Ketones are also produced when glucose concentrations fall during starvation or during very low carbohydrate diets, resulting in decreased insulin concentrations. 2 Mildly increased ketone concentrations due to low carbohydrate intake (but with some insulin still being present) can lead to mild to moderate elevations of ketone concentrations in the blood. This condition is known as hyperketonemia. In these states, the blood can buffer the ketoacids and the blood pH remains normal so that ketonemia is generally not a hazard. However, people with diabetes (PWD) who can experience severe insulin deficiency are at risk of massive ketoacid production. In those cases, the buffering capacity of the blood is overwhelmed, and pH and bicarbonate fall leading to metabolic acidosis. This condition is known as diabetic ketoacidosis (DKA).
The main ketoacid found in the blood during ketoacidosis is beta-hydroxybutyrate. This substance can be measured by point-of-care (POC) blood testing devices. The main ketoacid in urine is acetoacetic acid, which is a conversion product from beta-hydroxybutyrate. This substance is measured by POC urine ketone tests. Urine ketone tests of acetoacetate are acceptable for documenting states of increased ketone production due to a low carbohydrate diet or exercising, but they are not necessarily accurate for diagnosing or following the course of DKA. This is because, in DKA, the ratio of beta-hydroxybutyrate to acetoacetate increase from 1:1 to as much as 10:1. 3 The delayed increase in acetoacetate early in DKA can underestimate the severity of the disease. Later as DKA is evolving, beta-hydroxybutyrate is converted to acetoacetate, and urine concentrations of acetoacetate rise. At this point, the elevated urine result is a lagging indicator of the severity of ketoacidosis and can create a false impression that the condition is persisting or worsening when it is actually resolving. Although urine ketones have been used to screen for impending DKA, this test can underestimate or overestimate the severity of the disease, making blood testing of beta-hydroxybutyrate the preferred test for monitoring ketones. 4 Measurements of acetone in the breath, formed from spontaneous decarboxylation of acetoacetate can be made. Although no breath ketone tests have been cleared by the US Food and Drug Administration (FDA), several small studies have shown a positive correlation between breath acetone and blood beta-hydroxybutyrate.5 -9 Monitoring of ketones is important for individuals with diabetes to detect and treat DKA. Clinicians should educate PWD and their families at least annually, and opportunistically, per the most recent International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines. No comparable guidelines for adults currently exist.
Newer methods of ketone measurement are being developed, including the continuous ketone monitor (CKM), which measures beta-hydroxybutyrate levels in the interstitial fluid of subcutaneous tissue. CKM has the potential to improve the management and monitoring of diabetes in both the inpatient and outpatient settings. 10
People with type 1 diabetes (T1D) are at increased risk of developing DKA during acute illnesses and/or when using sodium-glucose cotransporter-2 inhibitors (SGLT2i) off label. 11 Other people who are at increased risk of DKA include people with type 2 diabetes (T2D) who are using SGLT2i on label or who have experienced an episode of antibody-negative ketosis-prone diabetes (also known as Flatbush Diabetes), which is the reason for DKA in nearly 50% of African Americans and approximately 50% of African Caribbeans.12 -14 A combination of starvation and an alcoholic binge can lead to ketoacidosis in the absence of diabetes, which is known as alcoholic ketoacidosis (AKA). Also, ketoacidosis in the absence of diabetes may rarely occur after a short period of vomiting and is then referred to as starvation ketoacidosis. 15 Ketoacidosis may be due to a mixed acid-base disorder known as diabetic ketoalkalosis, which can rarely occur in a setting of alkalosis due to severe vomiting and volume contraction.16,17 If the vomiting is due to cannabis hyperemesis, then this condition is known as hyperglycemic ketosis due to cannabis hyperemesis syndrome (HK-CHS). 18 Blood ketone monitoring is useful to diagnose early cases of all these conditions. Plans are underway to integrate CKMs into automated insulin delivery (AID) systems that can respond by delivering more insulin if the interstitial fluid ketone concentrations rise above a triggering concentration. In addition, an alarm for an elevated ketone concentration can alert the patient to look for an occluded or dislodged insulin delivery catheter.
Measuring Ketones in Adult Patients in an Ambulatory Setting
Production of ketones is an evolutionary adaptation to prolonged starvation.
The presence of ketones can be beneficial or harmful depending on their rate of appearance in the circulation.
Measurement of plasma ketones is preferable to urine ketones despite limitations in both methods.
Physiology
What are ketones, and why are they important? From an evolutionary point of view, ketones are extremely important. They evolved as a form of alternative energy substrate for when carbohydrate intake was at a minimum. It is important to note that insulin has different physiological effects at varying levels of plasma concentration. At low concentrations, insulin suppresses lipolysis and ketogenesis, but when there is no carbohydrate in the diet, such as in periods of prolonged starvation, insulin concentrations can drop to extremely low concentrations to where lipolysis and ketogenesis are no longer suppressed. This was shown by Cahill back in the 1960s and 1970s that during periods of prolonged starvation of over three or four weeks, plasma ketone concentrations, in particular beta-hydroxybutyrate, can rise to as high as 6 mmol/L or greater.19,20 He also showed that during prolonged starvation, ketone bodies make up more than 60% of the energy substrate used by the brain. The vast majority of these ketones are beta-hydroxybutyrate. 21 The reason that there is no accompanying metabolic acidosis is because of renal and respiratory buffering compensation which can handle the increased amount of ketones produced in these situations. Hyperketonemia occurs with the Keto diet or the Atkins diet where there is very little carbohydrate ingested, which results in high ketone concentrations in the blood because of the breakdown of lipids. When the body uses ketoacids as a fuel source, then the person is said to be in a state of ketosis.
The metabolic processes leading to ketogenesis are shown in Figure 1. The breakdown of lipids leads to the liberation of free fatty acids from adipose tissue. These reach the liver where they are converted into acetyl-CoA, which is condensed further into aceto-acetyl CoA. This substance can either be taken up into the tricarboxylic acid cycle for the generation of energy or it can have a further acetyl-CoA added where it is then converted to beta-hydroxy beta-methylglutaryl-CoA. This molecule then is converted into acetoacetate which itself can be converted into beta-hydroxybutyrate. These two substances are the two main keto acids and circulate in the form of ketone bodies. 23 Therefore, the presence of ketones in the blood represents a state of absolute or relative insulin insufficiency. In summary, ketones are an evolutionary mechanism to allow an alternative energy substrate for the brain and the heart in periods of prolonged starvation and are therefore very useful substances.
Figure 1.
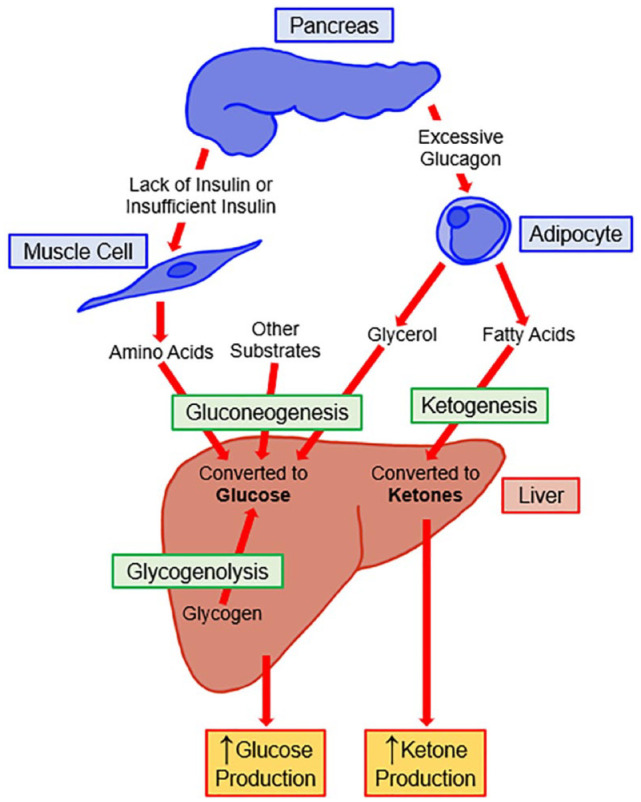
Metabolic processes and hormone imbalances resulting in ketogenesis. Figure modified from Nguyen et al. 22
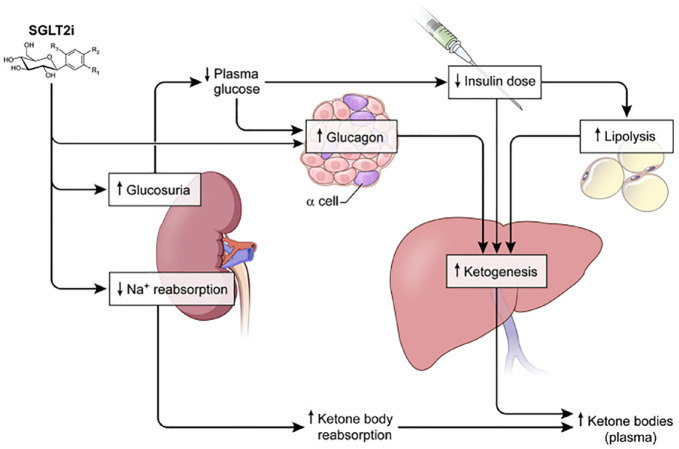
People who are heavy alcohol drinkers often have a degree of carbohydrate reduction in their diet. 23 The enzyme alcohol dehydrogenase metabolizes ethanol to acetaldehyde which itself is metabolized to acetic acid and transported into the mitochondria where it is converted into acetyl-CoA and subsequently condensed into acetoacetate. More recently, the use of SGLT2i has precipitated DKA, through three mechanisms. First, increased ketogenesis in the liver (due to increased glucosuria) is followed by a need for a lower insulin dose. Lower insulin concentrations cause plasma glucagon levels to increase. This increase is due to a paracrine effect induced by diminished inhibition of insulin on glucagon release by the pancreas, which leads to increased ketogenesis by the liver. Second, decreased insulin concentrations lead to increased lipolysis, increased free fatty acid release, and consequently increased ketone production by the liver. Third, SGLT2i decrease sodium reabsorption followed by decreased renal clearance of ketone bodies. 24 These mechanisms are presented in Figure 2. Ketoacidosis as a complication of SGLT2i therapy has been reported in both T1D and T2D. The hyperglycemia in this setting is often less severe than in DKA observed in T1D, which is why the condition is classified as “euglycemic” ketoacidosis. DKA with blood glucose (BG) concentrations of less than 200 mg/dL, is defined as euglycemic DKA. 25 The prevalence of euglycemic DKA in people with T1D using SGLT2i therapy is approximately 4% to 7%,26,27 and the distribution of lower limits of admission serum glucose concentrations for this disease in the United Kingdom is presented in Table 1.1,2 In the United States, cases of euglycemic DKA have been reported to occur with presenting serum glucose concentrations as low as 96 mg/dL 28 or 170 mg/dL. 29
Figure 2.
Potential mechanisms whereby SGLT2i may promote ketosis and increase the risk of ketoacidosis in T1D patients. SGLT2i decrease glucose by an insulin-independent mechanism. To minimize the risk of hypoglycemia, T1D patients may need to decrease their insulin dose, which is predicted to increase the rate of adipose tissue lipolysis, free fatty acid release, and hepatic ketogenesis. In addition, SGLT2i have been demonstrated to increase plasma glucagon levels in T2D patients possibly to compensate for increased urinary excretion of glucose. SGLT2i also reduce renal tubular sodium and glucose reabsorption and increase the reabsorption of ketoacids, resulting in decreased renal clearance of ketone bodies. Figure reproduced from Taylor et al. 24
Abbreviations: SGLT2i, sodium-glucose cotransporter-2 inhibitors; T1D, type 1 diabetes; T2D, type 2 diabetes.
Table 1.
Prevalence of Euglycemic Diabetic Ketoacidosis in People With T1D in the United Kingdom. Data from a national survey 39 and local audit. 40 Data are divided into different thresholds of “euglycemia.”
| Number | Admission glucose < 11.0 mmol/L (200 mg/dL)1 | Admission glucose < 13.9 mmol/L (250 mg/dL)2 | Admission glucose < 16.7 mmol/L (300 mg/dL)3 | |
|---|---|---|---|---|
| National survey (2014) 39 | 277 | 6 | 14 | 23 |
| Local audit (2015) 40 | 57 | 4 | 4 | 6 |
| 334 | 10 | 18 | 29 | |
| 3.0% | 5.4% | 8.7% |
Source: Table reproduced from Macfarlane et al. 26
Abbreviations: T1D, type 1 diabetes; UK, United Kingdom.
Diagnosis of Ketoacidosis
According to a literature review published in 2017 that analyzed 16 years of reports of DKA in T1D adults, the findings included (1) an incidence ranging from 0 to 56 cases per 1000 person-years (except for one outlier study reporting 263 cases per 1000 per patient-years [PYs]), and (2) a prevalence ranging from 0 to 128 per 1000 persons. 30 The increased risk of DKA in users of an SGLT2i compared with a nonuser may be 5 to 17 times higher. 31
The presence of elevated concentrations of blood ketones is the hallmark of a diagnosis of DKA. Patients at risk of this complication of diabetes must receive education on how to operate and interpret home measurement tests of blood ketones. However, for such tests to be effective, they must be used.
Diagnostic criteria for DKA include either hyperglycemia with a BG >200 mg/dL (>11.1 mmol/L), or a history of diabetes mellitus; the presence of ketones in the blood >3 mmol/L or significant ketonuria >2+ on a standard urine stick; a bicarbonate of <18 mmol/L, and/or a venous pH of <7.3 according to the UK guidelines or a pH of <7.30 with a BG of > 250 mg/dL (13.9 mmol/L) and a bicarbonate of <18 mmol/L with the presence of urine or serum ketones according to the 2023 American Diabetes Association Standards of Care.32 -34 To diagnose DKA, Diabetes Canada specifies similar pH and bicarbonate criteria as in the United Kingdom, similar ketone criteria as the American Diabetes Association, and a plasma glucose that is usually ≥252 mg/dL but can be lower, especially with the use of SGLT2i. 35 In Australia, the Pediatric Clinical Network specifies that a diagnosis of DKA is made if serum glucose is >198 mg/dL, venous pH is <7.3 or bicarbonate is <15 mmol/L, and ketonemia or ketonuria is present. 36 Following treatment of an episode of DKA, although most existing guidelines define DKA resolution as venous pH ≥ 7.3, serum bicarbonate (HCO3) ≥15 mmol/L, and/or anion gap ≤14 mmol/L. An eventual decline of plasma beta-hydroxybutyrate to 1.5 mmol/L or less has been proposed as defining the resolution of the DKA. 37
Technology for Measuring Ketones
Advantages and disadvantages of the measurement of blood versus urine ketones are listed in Table 2. Breath acetone concentrations vary from 1 part per million (ppm) in healthy nonfasting states, to more than 1250 ppm in DKA. In a pilot study of measuring breath acetone in 10 adults and 9 children with T1D, there was a significant association between the breath ketone analyzer and blood ketone meter results in adults (P = .0066), but not in children (P = .4579). 9 A saliva ketone monitoring strip has also been proposed, but little is yet known about the performance of this technology. 41
Table 2.
Comparison of the Advantages and Disadvantages of Ketone Measurement Methods.
| Plasma ketones | Urine ketones | ||
|---|---|---|---|
| Advantage | Disadvantage | Advantage disadvantage | |
| Measures the current plasma ketone concentration, allowing diagnostic certainty, and subsequent management plan | Reading is an average of urine ketone concentration since last void; management may be delayed | ||
| Allows for timely change of treatment as necessary | Length of time to resolution of DKA may be overestimated | ||
| Fast, immediate measurement | Urine sample collection may be delayed due to dehydration | ||
| Greater sensitivity and specificity for DKA | Lower sensitivity and specificity for DKA | ||
| Measures beta-hydroxybutyrate, the predominant ketone in DKA | Measures only acetoacetic acid, not beta-hydroxybutyrate | ||
| Potentially painful | Painless | ||
| Equipment (meter) needed | Readings can be read off the bottle | ||
| Meter needs regular quality assurance testing | No quality assurance needed | ||
| Staff who is able to use the meter required (if in hospital) | No technical skill required to use the equipment | ||
| Individually wrapped ketone strips have a long shelf life | Ketone strips have a relatively short shelf life | ||
| Relatively expensive | Relatively cheap | ||
| Meter may be inaccurate at readings outside the range it is designed for | |||
| Interference caused by other substances (eg, vitamin C), giving inaccurate results | Interference caused by other substances (eg, vitamin C), giving inaccurate results | ||
Source: Reproduced from Dhatariya 38 under the CC BY-NC-ND 4.0 License: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Abbreviation: DKA, diabetic ketoacidosis.
A POC method for measuring beta-hydroxybutyrate has been shown to be precise (with a coefficient of variation <4.5%), and with good correlation compared with an enzyme reference method (r = 0.95). The POC method had a sensitivity of 100% and specificity of 89% for diagnosing DKA (defined as beta-hydroxybutyrate >3 mmol/L), while for excluding DKA (defined as beta-hydroxybutyrate <1 mmol/L), the sensitivity was 100% and specificity was 87.5%. 42 A POC blood ketone result of ≥3.5 mmol/L yielded 100% specificity and sensitivity (compared with a clinical diagnosis combined with venous bicarbonate and urine ketone testing) for the diagnosis of DKA in an emergency department setting. 43 These types of studies indicate that a POC beta-hydroxybutyrate device can be accurate. A review article on the accuracy of capillary beta-hydroxybutyrate measurement for identifying DKA that analyzed nine studies (including the two aforementioned studies) concluded that capillary beta-hydroxybutyrate testing, compared with multiple other types of analytical and clinical tests, has high sensitivity, specificity, positive predictive value, and negative predictive value. 44
An additional and controversial use of blood ketone testing for wellness is monitoring the effect of a ketogenic diet by athletes. Raising blood ketone concentrations may enhance exercise capacity. 45 The hypothesized mechanism is that hyperketonemia may enhance exercise capacity by reducing muscle reliance on carbohydrates. 46 Blood ketone monitors have been advocated for athletes to determine whether a ketogenic diet has resulted in a state of nutritional ketosis, which is a beta-hydroxybutyrate concentration of at least 0.5 mmol/L. A lower concentration would indicate a need for the stricter limiting of carbohydrate intake. 47
Real-World Experience With Ketone Measurements
While there is a preferred ketone measurement modality, what is happening in clinical practice? In a survey of adults with T1D attending two Australian tertiary referral diabetes clinics reported in 2018, 31% of respondents reported not having unexpired ketone test strips at home. 48 Through an online survey completed in 2015 by participants in the T1D Exchange, it was noted that 62% of respondents had urine ketone strips, 18% had blood ketone meters, but a third of the cohort reported having no ketone measurement method at home. 47 When respondents were asked about ketone measurement practices when nauseous/vomiting or when hyperglycemic, a clear pattern emerged: youth with diabetes tended to assess ketones more frequently, while more than 50% of those aged >26 years reported they never assessed ketones in either situation. 49 Furthermore, diabetes awareness campaigns in Italy 50 and the United Kingdom 51 that included information about testing BG and blood ketones have been shown to reduce the number of children presenting in DKA with new onset of T1D.
Measuring Ketones in Pediatric Patients in an Ambulatory Setting
All youth with T1D and their parents must be informed of when and how to check for ketones.
Education regarding the importance of ketone checks must be developmentally appropriate and completed, at least, annually. This will ensure that as youth transition to greater independence with care, they know when to check for ketones, how to interpret ketone levels, and what to do should ketones be present.
Sick day guidelines/management plans should be proactively provided for all youth with diabetes.
ISPAD Clinical Practice Guidelines
In 2022, the ISPAD updated their Clinical Practice Guidelines, which is released every four years. Recognizing that sick day management of youth with diabetes is not a matter of if, it is a matter of when, an entire chapter titled “Sick day Management in Children” is devoted to this topic. 52 Indeed, the guidelines recommend, with grade C evidence, that “People with diabetes, their families and/or caregivers must receive education and be given access to guidelines preparing them for managing diabetes during illness. This education should be delivered at diagnosis, at follow-up at least annually, and opportunistically.” 52 At the heart of sick day management is understanding when to measure ketones, how to measure ketones, and what to do should ketones be present.
Currently, there are two primary methods for ketone measurement used in clinical pediatric practice: (1) assessment of acetoacetate in the urine and (2) capillary measures of blood beta-hydroxybutyrate. In a randomized controlled trial published in 2006, Laffel and colleagues compared these two methods of ketone measurement in 123 youth with T1D who ranged in age from 3 to 22 years. 53 All participants received the same structured sick day education and were advised to check glucose ≥ 3 times a day and check ketones during acute illness or stress, when glucose levels were elevated, or when symptoms of DKA were noted. 53 While the frequency of sick days was similar between groups, engagement with ketone monitoring varied by ketone measurement modality, with those using blood ketone measures checking on 90% of sick days versus only 61% of the time for participants randomized to urine ketone checks. 53 Furthermore, those in the blood ketone group had fewer emergency room visits and hospitalizations. The authors suggested that earlier detection of ketones led to more timely interventions. 53
Translating Guidelines Into Clinical Practice
For youth with diabetes, the transition to greater independence that occurs in adolescence highlights why it is critical to ensure education on the importance of ketone measurement occurs annually or more frequently. Using intake questionnaires to guide the frequency of ketones since the last visit may help broach the conversation and allow time to discuss the how, when, and why of ketone measurement. In addition, data downloads that are reviewed may provide an opportunity to identify a time that it might have been prudent to measure for ketones, for example, sensor glucose >250 mg/dL (>13.9 mmol/L) for more than two hours, and whether it was done at that time. Seamlessly integrating the conversation on ketone measurement is essential and should occur at least annually in a developmentally appropriate manner.
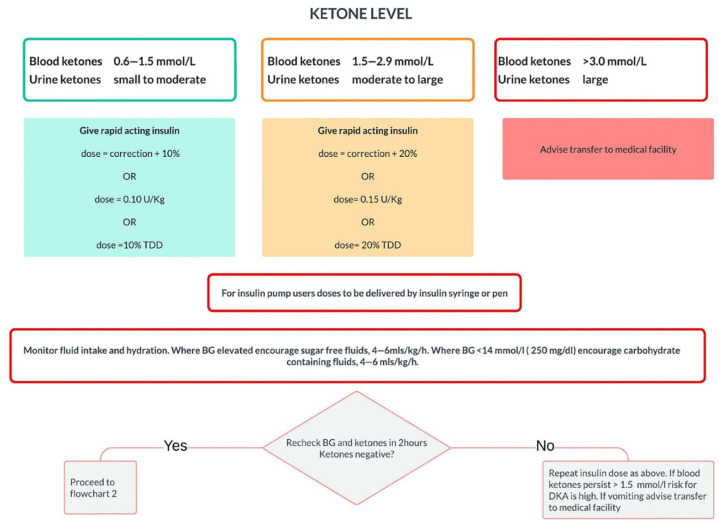
While it is critical to know when to measure for ketones, it is imperative that proactive sick day instructions are provided to families. As shown in Figure 3, the “ISPAD Clinical Practice Guidelines on Sick Day Management in Children” offers a very structured approach. 52 Ensuring families have the tools necessary to try to manage sick days at home is crucial, as well as access to a provider with a 24-hour contact number for support.
Figure 3.
Sick day management in children and adolescents with diabetes for treatment of ketosis in the home. Reproduced from Phelan et al. 52
Abbreviation: BGL, blood glucose level; TDD, total daily dose.
Thus, healthcare professionals should seek to provide education at least annually, and opportunistically, to the PWD they treat; prescribe a method to measure ketones while advocating for reimbursement of this necessary medical equipment; and create a sick day action plan that can be quickly implemented when ketones arise. Future methods to allow for more seamless measurement of ketones, like a CKM, has the potential to transform care delivery, but the foundation of education regarding ketone measures will remain a cornerstone of treatment.
Measuring Ketones in Hospitalized Patients
Laboratory analyzers and bedside point-of-care tests (POCTs) can be used to measure beta-hydroxybutyrate to diagnose DKA.
AKA causes elevated ketone concentrations that are comparable with DKA, while starvation ketoacidosis rarely causes decreased bicarbonate concentration or hyperglycemia.
Maternal ketoacidosis is accelerated during the third trimester, and low carbohydrate diets should be avoided during pregnancy to prevent adverse fetal outcomes.
Ketoacidosis
Clinical forms of ketoacidosis are DKA, AKA, and starvation ketoacidosis. The two main ketone bodies are acetoacetate and beta-hydroxybutyrate, while acetone is the third, and least abundant, ketone body. A normal serum concentration of ketone bodies is defined as ≤0.5 mM; hyperketonemia is defined as being in excess of 1.0 mM, and ketoacidosis as ketone concentrations in excess of 3.0 mM. 54 Assessment of ketonemia is performed by the nitroprusside reaction, which provides a semiquantitative estimation of acetoacetate and acetone levels. The nitroprusside test (both in urine and in serum) is highly sensitive; however, it frequently underestimates the severity of ketoacidosis because this assay does not recognize the presence of beta-hydroxybutyrate, the main ketone body in ketoacidosis. 23 Because of this, if available, measurement of serum beta-hydroxybutyrate is the preferred test for diagnosis, treatment monitoring, and an indication of the resolution of ketoacidosis. 55 As of 2020, more than 30% of laboratories in the United States were measuring blood ketones with a nitroprusside assay that predominantly measures acetoacetate rather than a beta-hydroxybutyrate assay. 56 This practice can lead to the same errors in the estimation of the severity of ketoacidosis as urine ketone testing. Blood ketone beta-hydroxybutyrate measurement can be performed as a laboratory test or with handheld meters such as a POCT. 38 The convenience of testing and rapidity of results from a POCT instrument in a hospital setting are major reasons for implementing blood ketone measurements in some clinical guidelines. In DKA, the use of blood beta-hydroxybutyrate monitoring, compared with urine acetoacetate monitoring, can result in faster resolution of ketoacidosis and shorter stays in the intensive care unit. 50 Some concern has been raised about the reliability of POCT instruments at high concentrations of blood beta-hydroxybutyrate concentrations above 5 mmol/L for following the improvement of treated DKA patients, but this concentration is above the diagnostic threshold and would not likely affect the identification of DKA. 56
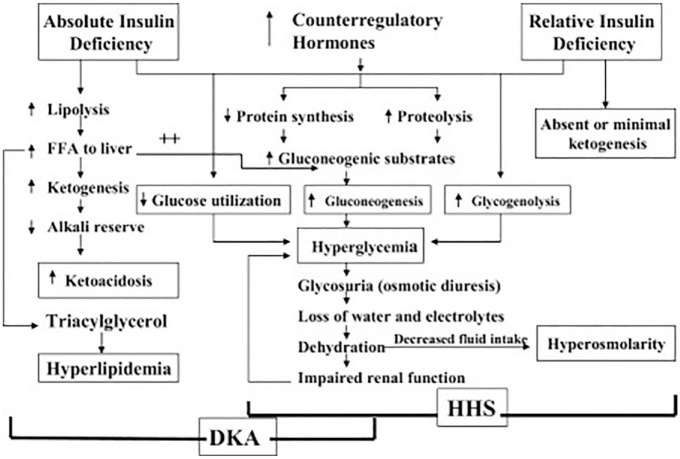
The most serious hyperglycemic emergency in individuals with T1D and T2D is DKA, which is characterized by a triad of hyperglycemia, metabolic acidosis, and elevated serum and urine ketones. The pathogenesis of DKA (which is associated with an absolute insulin deficiency) and of the related condition, hyperosmolar hyperglycemic state (which is associated with a relative insulin deficiency without significant ketoacidosis), 57 is shown in Figure 4. 33
Figure 4.
Pathogenesis of DKA and HHS. Reproduced from Kitabchi et al 33 under the CC BY-NC-ND 4.0 License: https://creativecommons.org/licenses/by-nc-nd/4.0/. Abbreviations: DKA, diabetic ketoacidosis; HHS, hyperosmolar hyperglycemic state.
Other Forms of Ketoacidosis
AKA occurs most often in a setting of chronic liver disease and low glycogen stores due to long-term alcohol abuse. 58 The condition typically presents in alcoholics after a recent binge of drinking followed by the abrupt cessation of alcohol consumption and food intake for at least 24 hours due to abdominal pain and vomiting. The result is dehydration, starvation, and acidosis with elevated production of beta-hydroxybutyrate. In the most quoted series of AKA cases, the elevation of total ketone body concentration to between 7 and 10 mmol/L is comparable with the ketone body concentrations reported in patients with DKA. 58 In this clinical setting, the combination of ketoacidosis in the absence of hyperglycemia confirms the diagnosis of AKA.
Starvation ketoacidosis occurs after the body is deprived of glucose (<500 Kcal/day) as its primary source of energy for a prolonged time, causing fatty acids to replace glucose as the major metabolic fuel. 59 During food deprivation, starvation ketosis is defined as occurring when the blood/plasma concentration of acetoacetate is about 1.0 mmol/L and beta-hydroxybutyrate concentration is >2-5 mM. Such values are usually present after two to three days of total starvation with maximal blood/plasma concentrations developing after several weeks of total fasting. If physiological stress accompanies starvation, then elevated counterregulatory hormones, including catecholamines, cortisol, and glucagon, further promote lipolysis, which in turn results in increased ketoacid production. 60 A healthy individual is able to adapt to prolonged fasting by increasing the clearance of ketone bodies in peripheral tissues (brain and muscle) and by enhancing the kidneys’ ability to excrete ammonium to compensate for the increased ketoacid production. 61 Thus, people with starvation ketosis rarely present with a serum bicarbonate concentration <18 mmol/L and do not exhibit hyperglycemia.
Maternal ketogenesis is accelerated in pregnancy, particularly in the third trimester. In women admitted to the hospital, fasting serum beta-hydroxybutyrate and acetoacetate concentrations are up to three times higher in pregnant women in the third trimester compared with nonpregnant women serving as controls. 62 The increase in maternal ketogenesis is secondary to multiple metabolic changes including maternal insulin resistance which develops in the second trimester largely because of the secretion of placental growth hormone as well as increased maternal lipolysis and ketogenesis. In addition, low-carbohydrate diets result in elevated ketone levels because of the reduction in available glucose. 63 Elevated ketones are a key concern with low carbohydrate diets and are cited as a reason to avoid such diets during pregnancy. Some studies have reported a correlation between maternal blood ketone concentrations and poor fetal and childhood outcomes, including reduced childhood intelligence quota, oligohydramnios, fetal heart decelerations, and nonreactive nonstress tests; however, studies do not consistently find such associations with these outcomes. 62
Diabetic ketoalkalosis is a rare, atypical form of DKA presenting with severe vomiting. In diabetic ketoalkalosis, a metabolic alkalosis occurs because of (1) hydrogen ion loss from the gastrointestinal tract due to vomiting, (2) a shift of hydrogen into cells due to potassium deficiency from decreased food intake and loss through vomiting, and (3) increased hydrogen ion secretion into the urine due to volume contraction. For these reasons, alkalemia occurs instead of acidemia, which would typically occur in DKA because of ketoacid formation.16,17 One example of diabetic ketoalkalosis has recently been reported, which is hyperglycemic ketosis due HK-CHS. Whereas typical DKA is characterized by hyperglycemia, followed by acidosis, in HK-CHS, ketosis and alkalosis due to severe vomiting occur first and are followed by hyperglycemia. A typical presentation of HK-CHS (like with DKA) includes BG >250 mg/dL and beta-hydroxybutyrate >0.6 mmol/L; however, HK-CHS presents with pH >7.4 with bicarbonate of >15 mmol/L (which are both higher than typically seen in DKA) urine drug screen positive for marijuana. 18
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies used in cancer chemotherapy to block upregulated tumor surface proteins that induce pathological immune tolerance. This type of treatment results in favorable anti-tumor stimulation of the immune system but can also result in pathological states of increased autoimmunity whereby T cells destroy islet beta cells.63(p1) ICI–associated autoimmune diabetes mellitus is a recently recognized form of autoimmune diabetes that occurs in 0.2% to 1.4% of patients treated with programmed cell death protein 1 (PD-1) or programmed cell death ligand 1 (PD-L1) ICIs. 64 It is a quickly developing (sudden onset) form of diabetes with marked hyperglycemia (in contrast to milder hyperglycemia or a euglycemic state in DKA due to SGLT2i) that often presents initially with DKA. The median time from initiation of ICI therapy to the onset of diabetes is approximately 1.5 to 2 months.65,66 A typical presentation of this syndrome is a new onset of DKA with low c-peptide concentrations, and normal HbA1c concentrations (indicating rapid islet failure) in a setting of ICI therapy.66 -69
Continuous Ketone Monitoring: The Future of Ketone Testing
The most clinically relevant ketone to measure is beta-hydroxybutyrate.
Measurement of interstitial beta-hydroxybutyrate using a factory-calibrated CKM sensor may be useful in ambulatory and hospital settings.
CKMs can identify changing ketone levels and flag significant changes—offering a new tool for the prevention and management of DKA in PWD.
The Principle of Continuous Ketone Monitoring
CKMs are under development. This type of device can identify changing ketone levels and flag significant changes, offering a new tool for the prevention and management of DKA in PWD. CKMs could measure interstitial beta-hydroxybutyrate using a similar electrochemical technology and form factor as existing glucose monitoring devices. 70 An implanted fluorescent sensor has been proposed but no clinical data on this method have been reported. 71 Theoretically, a combined continuous glucose monitor (CGM) and CKM system, offering both continuous glucose and ketone data within a single system, may be feasible. 72 Another method under development would be with a microneedle sampling method with a sensor array, which could measure interstitial fluid ketone levels and potentially also measure other analytes, such as glucose and lactate.73,74 As ketone concentrations in blood and interstitial fluid are normally low, a factory calibration feature for nonadjunctive use will greatly increase the adoption of these products. A combined CGM and CKM could be incorporated into AID systems and provide alarms and additional safety measures related to these two analytes.
CKM offers a paradigm shift from diabetes management focused solely on glucose as an analyte of interest to a multianalyte approach. A CKM, particularly if combined with a CGM, may provide DKA risk mitigation for several scenarios where the risk of DKA is high, including SGLT-2 inhibitor use in T2D and especially in T1D; in people with a history or risk of repeated episodes of DKA (such as ketosis-prone T2D); in people maintaining low carbohydrate or ketogenic diets; during ICI therapy; in cases of pancreoprivic diabetes (due to pancreatectomy or cystic fibrosis); or during significant, such as can occur with illness or surgery, intense exercise; and other activities potentially accelerating DKA risk. 22
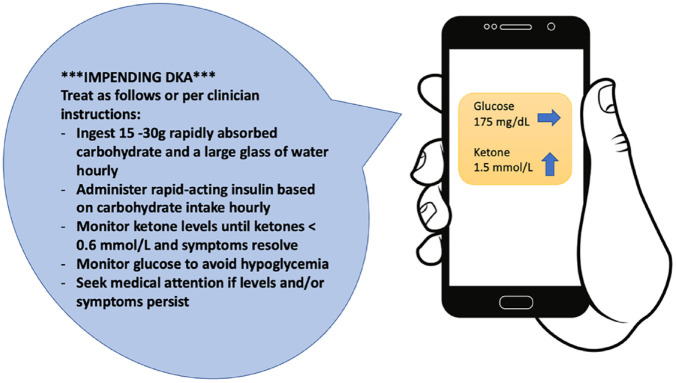
Insulin-requiring PWD are using SGLT2i with increasing frequency because of their renal and cardiovascular risk reduction profiles, despite their association with euglycemic DKA. 75 A CKM may help identify rising ketone levels and allow people with impeding euglycemic DKA to take corrective action, such as hydration and insulin treatment, with or without carbohydrate intake, to prevent ketoacidosis.75 -77 For SGLT2i users with diabetes, ketone measurements are indicated for symptoms suggesting DKA, such as malaise, fatigue, nausea, or vomiting. These SGLT2i users should also measure ketones in a setting of infection, dehydration, injury, or an insulin pump malfunction. 31 An example of an alert for impending ketoacidosis is shown in Figure 5.
Figure 5.
An example of an alert based on continuous ketone monitor data with behavioral suggestions to prevent ketoacidosis. Figure courtesy of Kristin Castorino. Abbreviation: DKA, diabetic ketoacidosis.
Future Research
CKMs should be integrated into AID systems to flag possible ketonemia and prevent DKA. 78 The AID systems generally have two algorithms that run in parallel, one which is aimed at keeping glucose in the target range, and a second, which is a safety algorithm aimed at risk mitigation, such as hypoglycemia prevention. Future safety algorithms can also be developed for dynamic ketone levels to flag ketonemia and identify impending ketoacidosis from several causes, such as interruption of insulin infusion or intercurrent illness. The data from CKMs will be maximally useful if they can be automatically integrated into the electronic health record (EHR) the way wearable CGMs can now be integrated through the application of the Integration of Continuous Glucose Monitor Data Into the Electronic Health Record (iCoDE) standard. 79 In addition, incorporating CGM and CKM data into a standardized report format familiar to clinicians like the ambulatory glucose profile report, which may then be the ambulatory glucose ketone profile report, would likely be needed to facilitate a good dialogue between patient and clinician.
Future CKM research should assess clinically relevant endpoints such as this technology’s effect on emergency room visits and hospitalizations as well as the economics of this type of wearable continuous monitoring device. Such clinical and economic outcomes data will be needed to provide sufficient justification to support reimbursement for making the technology accessible for PWD. 22 Ultimately, diabetes technology should reduce the burden of disease management. CKMs hold the promise to simplify the prevention and treatment of DKA.
Conclusion
Ketone bodies are an alternative energy substrate used during low carbohydrate states such as starvation. Pathological states such as DKA can occur with the rapid development of high levels of ketone bodies; therefore, monitoring ketone levels is essential for the prevention and treatment of DKA. Ketones have traditionally been measured via urine or blood. Blood ketone measurement is a valuable tool to prevent DKA, given that the rise in blood ketones may precede the rise in urine ketones. CKMs have been introduced as an innovative investigational device to address some of the challenges and shortcomings of conventional ketone testing in diabetes treatment. By monitoring changes in beta-hydroxybutyrate levels in real time, CKM may help decrease the risk of DKA in PWD during illness, surgery, and while on SGLT2i or ketogenic diets. There is also the potential for CKM to integrate with CGM and AID systems for fault detection by determining inadequate insulin delivery from an insulin infusion site. Future research studies will be essential to understand the feasibility, acceptability, usability, efficacy, and safety of CKM devices before widespread use and adoption.
Acknowledgments
The authors thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AID, automated insulin delivery; AKA, alcoholic ketoacidosis; CGM, continuous glucose monitor; CKM, continuous ketone monitor; DKA, diabetic ketoacidosis; EHR, electronic health record; FDA, US Food and Drug Administration; HK-CHS, hyperglycemic ketosis due to cannabis hyperemesis syndrome; ICI, immune checkpoint inhibitor; iCoDE, Integration of Continuous Glucose Monitor Data Into the Electronic Health Record; ISPAD, International Society for Pediatric and Adolescent Diabetes; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; POC, point-of-care; POCT, point-of-care test; PWD, people with diabetes; SGLT2i, sodium-glucose cotransporter-2 inhibitors; TDD, total daily dose; T1D, type 1 diabetes; T2D, type 2 diabetes.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.M.B. has received research support, consulted, or has been on a scientific advisory board for Abbott Diabetes Care, Ascensia, Bigfoot Biomedical, CeQur, Dexcom, Hygieia, Insulet, Lilly, Medtronic, Novo Nordisk, Onduo, Roche Diabetes Care, Sanofi, Tandem Diabetes Care, United Healthcare, Vertex Pharmaceutical and Zealand Pharma. His technology research is funded in part by NIH/NIDDK and Helmsley Charitable Trust. R.M.B.’s employer, nonprofit HealthPartners Institute, contracts for his services, and no personal income goes to him. K.C. receives research support provided to her institution from Dexcom, Abbott, Medtronic, Altimmune, Lilly, Novo Nordisk, and Insulet and has received consulting fees from Dexcom. E.C. is an advisory board member and consultant for Novo Nordisk, Eli Lilly, Adocia, MannKind, Lexicon, Arecor. E.C. was also a speaker for Novo Nordisk. K.D. is the chair of the Joint British Diabetes Societies for Inpatient Care and has received speaker fees, travel, or taken part in advisory boards for AstraZeneca, Sanofi Diabetes, Boehringer Ingelheim, Lilly, and Novo Nordisk. J.L.S. has conducted clinical trials for Eli Lilly, Insulet, and Medtronic and has received in-kind support for research studies from Dexcom and Medtronic. She has consulted for Eli Lilly, Lexicon, Medtronic, and Sanofi. She has been a member of advisory boards for Bigfoot Biomedical, Cecelia Health, Eli Lilly, Insulet, the T1D Fund, and Vertex. G.E.U. reports research funds to Emory University from Astra Zeneca, Abbott, and Dexcom. The support from Astra Zeneca ended in 2022. D.C.K. is a consultant to EOFlow, Fractyl Health, Integrity, Lifecare, Rockley Photonics, and Thirdwayv. J.H., A.M.Y., and I.N. have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was supported by a grant from Abbott Diabetes Care.
ORCID iDs: Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
Richard M. Bergenstal  https://orcid.org/0000-0002-9050-5584
https://orcid.org/0000-0002-9050-5584
Kristin Castorino  https://orcid.org/0000-0001-6386-9203
https://orcid.org/0000-0001-6386-9203
Eda Cengiz  https://orcid.org/0000-0001-7992-9506
https://orcid.org/0000-0001-7992-9506
Ketan Dhatariya  https://orcid.org/0000-0003-3619-9579
https://orcid.org/0000-0003-3619-9579
Isabella Niu  https://orcid.org/0000-0002-7190-079X
https://orcid.org/0000-0002-7190-079X
Jennifer L. Sherr  https://orcid.org/0000-0001-9301-3043
https://orcid.org/0000-0001-9301-3043
Guillermo E. Umpierrez  https://orcid.org/0000-0002-3252-5026
https://orcid.org/0000-0002-3252-5026
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Møller N. Ketone body, 3—hydroxybutyrate: minor metabolite—major medical manifestations. J Clin Endocrinol Metab. 2020;105(9):dgaa370. doi: 10.1210/clinem/dgaa370. [DOI] [PubMed] [Google Scholar]
- 2. Dilliraj LN, Schiuma G, Lara D, et al. The evolution of ketosis: potential impact on clinical conditions. Nutrients. 2022;14(17):3613. doi: 10.3390/nu14173613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999;15(6):412-426. doi: 10.1002/(sici)1520-7560(199911/12)15:6<412::aid-dmrr72>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4. Lohano PD, Ibrahim M, Raza SJ, Gowa M, Baloch SH. Comparing finger-stick βeta-hydroxybutyrate with dipstick urine tests in the detection of ketone bodies in the diagnosis of children with diabetic ketoacidosis. J Coll Physicians Surg Pak. 2022;32(4):483-486. doi: 10.29271/jcpsp.2022.04.483. [DOI] [PubMed] [Google Scholar]
- 5. Musa-Veloso K, Likhodii SS, Cunnane SC. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr. 2002;76(1):65-70. doi: 10.1093/ajcn/76.1.65. [DOI] [PubMed] [Google Scholar]
- 6. Musa-Veloso K, Likhodii SS, Rarama E, et al. Breath acetone predicts plasma ketone bodies in children with epilepsy on a ketogenic diet. Nutrition. 2006;22(1):1-8. doi: 10.1016/j.nut.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 7. Saasa V, Beukes M, Lemmer Y, Mwakikunga B. Blood ketone bodies and breath acetone analysis and their correlations in type 2 diabetes mellitus. Diagnostics (Basel). 2019;9(4):224. doi: 10.3390/diagnostics9040224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Güntner AT, Kompalla JF, Landis H, et al. Guiding ketogenic diet with breath acetone sensors. Sensors. 2018;18(11):3655. doi: 10.3390/s18113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akturk HK, Snell-Bergeon J, Pyle L, Fivekiller E, Garg S, Cobry E. Accuracy of a breath ketone analyzer to detect ketosis in adults and children with type 1 diabetes. J Diabetes Complications. 2021;35(11):108030. doi: 10.1016/j.jdiacomp.2021.108030. [DOI] [PubMed] [Google Scholar]
- 10. Zhang JY, Shang T, Koliwad SK, Klonoff DC. Continuous ketone monitoring: a new paradigm for physiologic monitoring. J Diabetes Sci Technol. 2021;15(4):775-780. doi: 10.1177/19322968211009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lupsa BC, Kibbey RG, Inzucchi SE. Ketones: the double-edged sword of SGLT2 inhibitors. Diabetologia. 2023;66(1):23-32. doi: 10.1007/s00125-022-05815-1. [DOI] [PubMed] [Google Scholar]
- 12. Sjöholm Å. Ketosis-prone type 2 diabetes: a case series. Front Endocrinol (Lausanne). 2019;10:684. doi: 10.3389/fendo.2019.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang Y, Lu C, Augusto Monteiro Cardoso Lopes M, et al. A cross-sectional study of atherosclerosis in newly diagnosed patients with ketosis-prone type 2 diabetes. Diabetes Metab Syndr Obes. 2022;15:933-941. doi: 10.2147/DMSO.S349467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shankar M, Chowdhury T, Gousy N, Parthasarathi A. An insight into flatbush diabetes: a rare form of diabetes. Cureus. 2022;14(1):e21567. doi: 10.7759/cureus.21567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iwata H, Tsuzuki S, Iwata M, Terasawa T. Ketoacidosis due to a low-carbohydrate diet in an elderly woman with dementia and abnormal eating behavior. Intern Med. 2017;56(19):2671-2675. doi: 10.2169/internalmedicine.8689-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nanavati S, Kumar V, Melki G, Singhal M. Diabetic ketoalkalosis: misnomer or undiagnosed variant of diabetic ketoacidosis. BMJ Case Rep. 2018;2018:bcr2018226092. doi: 10.1136/bcr-2018-226092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaramillo J, Joseph M, Aldubayan M, Sinert R. New test, old disease: a case series of diabetic ketoalkalosis [published online ahead of print November 18, 2019]. J Emerg Med. doi: 10.1016/j.jemermed.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 18. Akturk HK, Snell- Bergeon J, Kinney GL, Champakanath A, Monte A, Shah VN. Differentiating diabetic ketoacidosis and hyperglycemic ketosis due to cannabis hyperemesis syndrome in adults with type 1 diabetes. Diabetes Care. 2022;45(2):481-483. doi: 10.2337/dc21-1730. [DOI] [PubMed] [Google Scholar]
- 19. Cahill GF. Starvation in man. N Engl J Med. 1970;282(12):668-675. doi: 10.1056/NEJM197003192821209. [DOI] [PubMed] [Google Scholar]
- 20. Cahill GF. The Banting Memorial lecture 1971. Physiology of insulin in man. Diabetes. 1971;20(12):785-799. doi: 10.2337/diab.20.12.785. [DOI] [PubMed] [Google Scholar]
- 21. Cahill GF. Fuel metabolism in starvation. Annu Rev Nutr. 2006;26:1-22. doi: 10.1146/annurev.nutr.26.061505.111258. [DOI] [PubMed] [Google Scholar]
- 22. Nguyen KT, Xu NY, Zhang JY, et al. Continuous ketone monitoring consensus report 2021. J Diabetes Sci Technol. 2022;16(3):689-715. doi: 10.1177/19322968211042656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers. 2020;6(1):40. doi: 10.1038/s41572-020-0165-1. [DOI] [PubMed] [Google Scholar]
- 24. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852. doi: 10.1210/jc.2015-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Modi A, Agrawal A, Morgan F. Euglycemic diabetic ketoacidosis: a review. Curr Diabetes Rev. 2017;13(3):315-321. doi: 10.2174/1573399812666160421121307. [DOI] [PubMed] [Google Scholar]
- 26. Macfarlane J, Dhatariya K. Incidence of euglycemic diabetic ketoacidosis in adults with type 1 diabetes in the United Kingdom before the widespread use of sodium glucose cotransporter 2 inhibitors. Mayo Clin Proc. 2019;94(9):1909-1910. doi: 10.1016/j.mayocp.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 27. Horii T, Oikawa Y, Atsuda K, Shimada A. On-label use of sodium-glucose cotransporter 2 inhibitors might increase the risk of diabetic ketoacidosis in patients with type 1 diabetes. J Diabetes Investig. 2021;12(9):1586-1593. doi: 10.1111/jdi.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687-1693. doi: 10.2337/dc15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015;38(12):2258-2265. doi: 10.2337/dc15-1730. [DOI] [PubMed] [Google Scholar]
- 30. Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. doi: 10.1136/bmjopen-2017-016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolfsdorf JI, Ratner RE. SGLT inhibitors for type 1 diabetes: proceed with extreme caution. Diabetes Care. 2019;42(6):991-993. doi: 10.2337/dci19-0008. [DOI] [PubMed] [Google Scholar]
- 32. Dhatariya KK; Joint British Diabetes Societies for Inpatient Care. The management of diabetic ketoacidosis in adults—an updated guideline from the Joint British Diabetes Society for Inpatient Care. Diabet Med. 2022;39(6):e14788. doi: 10.1111/dme.14788. [DOI] [PubMed] [Google Scholar]
- 33. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335-1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. ElSayed NA, Aleppo G, Aroda VR, et al. 6. Glycemic targets: standards of care in diabetes—2023. Diabetes Care. 2022;46(suppl 1):S97-S110. doi: 10.2337/dc23-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Can J Diabetes. 2018;42:S109-S114. doi: 10.1016/j.jcjd.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 36. Clinical Practice Guidelines. Diabetic ketoacidosis. Date unknown. https://www.rch.org.au/clinicalguide/guideline_index/Diabetic_Ketoacidosis/. Accessed January 3, 2023.
- 37. Tremblay ES, Millington K, Wu Y, et al. Utility of plasma beta-hydroxybutyrate to define resolution of diabetic ketoacidosis. Pediatr Diabetes. 2022;23(8):1621-1627. doi: 10.1111/pedi.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dhatariya K. Blood ketones: measurement, interpretation, limitations and utility in the management of diabetic ketoacidosis. Rev Diabet Stud. 2016;13(4):217-225. doi: 10.1900/RDS.2016.13.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dhatariya KK, Nunney I, Higgins K, Sampson MJ, Iceton G. A national survey of the management of diabetic ketoacidosis in the UK in 2014. Diabetic Med. 2016;33(2):252-260. [DOI] [PubMed] [Google Scholar]
- 40. Varadarajan M, Patel M, Kakkar N, et al. Are the results from the 2014 UK national survey on the management of diabetic ketoacidosis applicable to individual centres? Diabetes Res Clin Pract. 2017;127:140-146. [DOI] [PubMed] [Google Scholar]
- 41. Moonla C, Del Caño R, Sakdaphetsiri K, et al. Disposable screen-printed electrochemical sensing strips for rapid decentralized measurements of salivary ketone bodies: towards therapeutic and wellness applications. Biosens Bioelectron. 2022;220:114891. doi: 10.1016/j.bios.2022.114891. [DOI] [PubMed] [Google Scholar]
- 42. Coetzee A, Hoffmann M, Ascott -Evans BH. The role of point-of-care blood testing for ketones in the diagnosis of diabetic ketoacidosis. S Afr Med J. 2015;105(9):756-759. doi: 10.7196/SAMJnew.7889. [DOI] [PubMed] [Google Scholar]
- 43. Charles RA, Bee YM, Eng PHK, Goh SY. Point-of-care blood ketone testing: screening for diabetic ketoacidosis at the emergency department. Singapore Med J. 2007;48(11):986-989. [PubMed] [Google Scholar]
- 44. Brooke J, Stiell M, Ojo O. Evaluation of the accuracy of capillary hydroxybutyrate measurement compared with other measurements in the diagnosis of diabetic ketoacidosis: a systematic review. Int J Environ Res Public Health. 2016;13(9):837. doi: 10.3390/ijerph13090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dearlove DJ, Soto Mota A, Hauton D, et al. The effects of endogenously- and exogenously-induced hyperketonemia on exercise performance and adaptation. Physiol Rep. 2022;10(10):e15309. doi: 10.14814/phy2.15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cox PJ, Kirk T, Ashmore T, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256-268. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 47. Moore AR, Holland-Winkler AM, Ansley JK, Boone EDH, Schulte MKO. Reliability and diagnostic performance of a new blood ketone and glucose meter in humans. J Int Soc Sports Nutr. 2021;18(1):6. doi: 10.1186/s12970-020-00404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Larsson CR, Januszewski AS, McGrath RT, et al. Suboptimal behaviour and knowledge regarding overnight glycaemia in adults with type 1 diabetes is common. Intern Med J. 2018;48(9):1080-1086. doi: 10.1111/imj.13798. [DOI] [PubMed] [Google Scholar]
- 49. Albanese-O’Neill A, Wu M, Miller KM, et al. Poor adherence to ketone testing in patients with type 1 diabetes. Diabetes Care. 2017;40(4):e38-e39. doi: 10.2337/dc16-2620. [DOI] [PubMed] [Google Scholar]
- 50. Vanelli M, Chiari G, Capuano C, Iovane B, Bernardini A, Giacalone T. The direct measurement of 3-beta-hydroxy butyrate enhances the management of diabetic ketoacidosis in children and reduces time and costs of treatment. Diabetes Nutr Metab. 2003;16(5-6):312-316. [PubMed] [Google Scholar]
- 51. King BR, Howard NJ, Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13(8):647-651. doi: 10.1111/j.1399-5448.2012.00896.x. [DOI] [PubMed] [Google Scholar]
- 52. Phelan H, Hanas R, Hofer SE, et al. Sick day management in children and adolescents with diabetes. Pediatric Diabetes. 2022;23(7):912-925. doi: 10.1111/pedi.13415. [DOI] [PubMed] [Google Scholar]
- 53. Laffel LMB, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23(3):278-284. doi: 10.1111/j.1464-5491.2005.01771.x. [DOI] [PubMed] [Google Scholar]
- 54. Umpierrez G, Korytkowski M. Diabetic emergencies-ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12(4):222-232. doi: 10.1038/nrendo.2016.15. [DOI] [PubMed] [Google Scholar]
- 55. Kitabchi AE, Umpierrez GE, Murphy MB, et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24(1):131-153. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 56. Kilpatrick ES, Butler AE, Ostlundh L, Atkin SL, Sacks DB. Controversies around the measurement of blood ketones to diagnose and manage diabetic ketoacidosis. Diabetes Care. 2022;45(2):267-272. doi: 10.2337/dc21-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care. 2014;37(11):3124-3131. doi: 10.2337/dc14-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Umpierrez GE, DiGirolamo M, Tuvlin JA, Isaacs SD, Bhoola SM, Kokko JP. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care. 2000;15(2):52-59. doi: 10.1053/jcrc.2000.7900. [DOI] [PubMed] [Google Scholar]
- 59. Owen OE, Caprio S, Reichard GA, Mozzoli MA, Boden G, Owen RS. Ketosis of starvation: a revisit and new perspectives. Clin Endocrinol Metab. 1983;12(2):359-379. doi: 10.1016/s0300-595x(83)80046-2. [DOI] [PubMed] [Google Scholar]
- 60. Boal AH, Panarelli M, Millar C. Starvation ketoacidosis and refeeding syndrome. BMJ Case Rep. 2021;14(12):e245065. doi: 10.1136/bcr-2021-245065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palmer BF, Clegg DJ. Starvation ketosis and the kidney. Am J Nephrol. 2021;52(6):467-478. doi: 10.1159/000517305. [DOI] [PubMed] [Google Scholar]
- 62. Tanner HL, Dekker Nitert M, Callaway LK, Barrett HL. Ketones in pregnancy: why is it considered necessary to avoid them and what is the evidence behind their perceived risk? Diabetes Care. 2021;44(1):280-289. doi: 10.2337/dc20-2008. [DOI] [PubMed] [Google Scholar]
- 63. Metzger BE, Freinkel N. Accelerated starvation in pregnancy: implications for dietary treatment of obesity and gestational diabetes mellitus. Biol Neonate. 1987;51(2):78-85. doi: 10.1159/000242636. [DOI] [PubMed] [Google Scholar]
- 64. Rahman W, Conley A, Silver KD. Atezolizumab-induced type 1 diabetes mellitus in a patient with metastatic renal cell carcinoma. BMJ Case Reports CP. 2020;13(7):e233842. doi: 10.1136/bcr-2019-233842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morton A. Review article: ketoacidosis in the emergency department. Emerg Med Australas. 2020;32(3):371-376. doi: 10.1111/1742-6723.13503. [DOI] [PubMed] [Google Scholar]
- 66. Wu L, Tsang VHM, Sasson SC, et al. Unravelling checkpoint inhibitor associated autoimmune diabetes: from bench to bedside. Front Endocrinol (Lausanne). 2021;12:764138. doi: 10.3389/fendo.2021.764138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lin C, Li X, Qiu Y, Chen Z, Liu J. PD-1 inhibitor-associated type 1 diabetes: a case report and systematic review. Front Public Health. 2022;10:885001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. doi: 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Akturk HK, Kahramangil D, Sarwal A, Hoffecker L, Murad MH, Michels AW. Immune checkpoint inhibitor-induced type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2019;36(9):1075-1081. doi: 10.1111/dme.14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alva S, Castorino K, Cho H, Ou J. Feasibility of continuous ketone monitoring in subcutaneous tissue using a ketone sensor. J Diabetes Sci Technol. 2021;15(4):768-774. doi: 10.1177/19322968211008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Li N, Ni R, et al. A highly selective turn-on Schiff base fluorescent sensor for diabetic biomarker beta-hydroxybutyrate (β-HB). Dyes and Pigments. 2022;207:110765. doi: 10.1016/j.dyepig.2022.110765. [DOI] [Google Scholar]
- 72. Abbott MediaRoom. Abbott announces development of novel continuous glucose-ketone monitoring system. Date unknown. https://abbott.mediaroom.com/2022-06-03-Abbott-Announces-Development-of-Novel-Continuous-Glucose-Ketone-Monitoring-System. Accessed November 17, 2022.
- 73. Teymourian H, Moonla C, Tehrani F, et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal Chem. 2020;92(2):2291-2300. doi: 10.1021/acs.analchem.9b05109. [DOI] [PubMed] [Google Scholar]
- 74. Taylor RM, Baca JT. Feasibility of interstitial fluid ketone monitoring with microneedles. Metabolites. 2022;12(5):424. doi: 10.3390/metabo12050424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Danne T, Garg S, Peters AL, et al. International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care. 2019;42(6):1147-1154. doi: 10.2337/dc18-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther. 2018;20(9):571-575. doi: 10.1089/dia.2018.0246. [DOI] [PubMed] [Google Scholar]
- 77. Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA protocol. Diabetes Obes Metab. 2019;21(10):2192-2202. doi: 10.1111/dom.13811. [DOI] [PubMed] [Google Scholar]
- 78. Lee MH, Paldus B, Krishnamurthy B, et al. The clinical case for the integration of a ketone sensor as part of a closed loop insulin pump system. J Diabetes Sci Technol. 2019;13(5):967-973. doi: 10.1177/1932296818822986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. iCoDE. Diabetes Technology Society press release. https://www.diabetestechnology.org/icode/iCoDE-Press-Release-11-7-2022.pdf?ver=5. Published 2022. Accessed November 17, 2022.