Abstract
Background:
Current guidelines recommend normal saline (NS) for fluid resuscitation in the management of patients presenting with diabetic ketoacidosis (DKA). However, previous prospective studies have demonstrated improvement in patient-specific outcomes, including time to DKA resolution, when balanced crystalloid fluids are used.
Methods:
We conducted a single institution, retrospective cohort study of adult patients admitted with DKA before and after a protocol change within our institution, which shifted the default resuscitative and maintenance fluid in our DKA management protocol from NS to lactated Ringer’s solution (LR). The primary outcome was time from DKA clinical presentation until DKA resolution. The secondary outcome was time to discontinuation of DKA protocol insulin drip.
Results:
Of 246 patients meeting inclusion criteria, 119 were in the NS group (preprotocol change, where NS was the default resuscitative fluid) and 127 to the LR group (postprotocol change, where LR was the default resuscitative fluid). Time to DKA resolution was significantly decreased in the LR group (mean = 17.1 hours; standard deviation [SD] = 11.0) relative to the NS group (mean = 20.6 hours; SD = 12.2; P = .02). Duration of DKA protocol insulin drip was shorter in the LR group (mean = 16.0 hours; SD = 8.7) compared with the NS group (mean = 21.4 hours; SD = 12.5; P < .001).
Conclusions:
In this retrospective cohort study, protocolized DKA intravenous fluid management with LR resulted in shorter time to resolution of DKA and reduced duration of DKA protocol insulin drip.
Keywords: diabetic ketoacidosis, DKA, DKA treatment, fluid selection DKA, lactated ringers
Introduction
Intravenous fluids are first-line treatment in the management of diabetic ketoacidosis (DKA) to restore intravascular volume, reduce counter-regulatory hormone production, and ensure adequate tissue perfusion.1-4 Current guidelines from the American Diabetes Association (ADA) and Joint British Diabetes Societies for Inpatient Care (JBDS-IP) recommend 0.9% normal saline (NS) as the resuscitative fluid of choice, followed by the utilization of 0.45% NS or NS, respectively, for maintenance fluid therapy.1,3 However, this approach increases the risk of hyperchloremic metabolic acidosis (HMA) and there is growing evidence of additional detrimental metabolic sequelae that occur with the use of NS in the management of DKA.5-11 Development of iatrogenic HMA in the setting of DKA management may delay resolution of acidosis, delay transfer to lower level of hospital care, and increase length of stay (LOS).6,8-10
Utilization of balanced crystalloids for fluid resuscitation in the setting of other indications, such as sepsis, has demonstrated reduced adverse renal events in critically ill and noncritically ill patients, as well as decreased all-cause mortality rate in critically ill patients.12,13 A recent secondary analysis of two cluster randomized controlled trials demonstrated that balanced crystalloids, compared with NS, resulted in more rapid DKA resolution. 6 In contrast, other studies comparing balanced crystalloids with NS for the treatment of DKA have demonstrated no significant difference between groups with regards to resolution of acidosis.14-17 Furthermore, there is limited real-world data analyzing the efficacy of using a balanced crystalloid fluid, such as lactated Ringer’s solution (LR), on patient outcomes when used as the resuscitative and maintenance fluid of choice in the management of patients presenting with DKA.
Following a quality improvement intervention at our institution where the default intravenous fluid in our DKA management protocol was changed from NS to LR, we aimed to directly compare patient-specific outcomes between the two protocol groups. Outcomes of interest included time to DKA resolution, time to discontinuation of DKA protocol insulin drip, LOS, chloride, anion gap, bicarbonate trends, and potassium supplementation administered. We hypothesized that the use of LR compared with NS for volume management in DKA would shorten time to DKA resolution, reduce time to DKA protocol insulin drip discontinuation, and result in lower postintravenous fluid resuscitation chloride values.
Methods
This study was a retrospective cohort review evaluating the impact of a quality improvement project revising our institution’s protocol for the management of patients presenting with DKA. Components of the quality improvement process included: changing the protocolized fluid to LR from NS, linking a separate maintenance fluid order within the initial intravenous fluid (IVF) resuscitation order, educating nursing staff on the changes, and modifying nursing instructions for when to reach out to providers for consideration of transition of IVF formulation. Following our institution’s implementation of the revised protocol in October 2021, we evaluated differences in outcomes between the NS group (preprotocol change) and LR group (postprotocol change). Our study protocol was submitted to the institutional review board (IRB) for review and deemed to be quality improvement, thus IRB approval was not required.
Population
Patients aged 19 years and older meeting diagnostic criteria for DKA presenting to a Nebraska Medicine emergency department and admitted between July 28, 2019 and May 20, 2023 were identified using the diagnosis of ketoacidosis; International Classification of Diseases, Tenth Revision (ICD-10) code: E10.10. Individual medical record review was conducted to confirm diagnosis of DKA and screen for exclusion criteria. In instances where a patient had multiple hospital encounters during the study period, only the first chronological encounter that met study inclusion criteria was included in data analysis. Diabetic ketoacidosis was defined according to the current ADA diagnostic criteria: plasma glucose concentration greater than 250 mg/dL (13.875 mmol/L), plasma bicarbonate concentration less than or equal to 18 mEq/L (18 mmol/L), and calculated anion gap (calculated as sodium concentration – [chloride concentration + bicarbonate concentration]) greater than 10 mEq/L (10 mmol/L)1. Diabetic ketoacidosis severity was defined based on initial serum bicarbonate (15-18 mEq/L mild; 10-14 mEq/L moderate; < 10 mEq/L severe). 1 Exclusion criteria included: (1) DKA not present based on ADA definition, (2) presence of end-stage renal disease (ESRD) at presentation, (3) patients presenting to an outside hospital prior to transfer to our institution (these patients may have received DKA management prior to transfer), (4) LOS less than 24 hours, and (5) DKA protocol insulin drip not used.
Once inclusion was confirmed upon chart review, additional data associated with primary and secondary outcomes were obtained, including demographics, laboratory data, hospital level of care, and therapeutics administered. Patients were grouped according to their date of hospitalization encounter within the context of the DKA protocol revision; those patient encounters with admissions occurring before October 11, 2021 were placed in the NS group (preprotocol change, where NS was the default resuscitative fluid) and those on or after October 11, 2021 were placed in the LR group (postprotocol change, where LR was the default resuscitative fluid).
Intervention
Our institution’s historical DKA protocol used an NS bolus for initial resuscitation, followed by maintenance 0.45% NS as hypovolemia resolved. The protocol relied upon nursing judgment with regards to intravenous fluid rate and formulation of treatment decisions based on urinary output. Once the blood glucose decreased to less than 300 mg/dL, dextrose containing NS, or 0.45% NS was recommended. Further intravenous fluid orders were at the individual provider’s discretion and ordered outside of the DKA protocol order set.
On October 11, 2021, our institution implemented an LR-based DKA protocol. The revised protocol used an LR bolus for initial resuscitation, followed by maintenance LR. Once blood glucose decreased to less than 250 mg/dL, dextrose containing LR was recommended. Orders relying on nursing judgment to make fluid-related treatment decisions were removed, bolus and maintenance fluid orders were separated and defaulted within the protocol order set, and a default end time was introduced for dextrose containing fluids. The same insulin infusion protocol, algorithms, and target glucose levels were used in the preprotocol and postprotocol change groups. Potassium replacement guidance and provider order options were the same preprotocol and postprotocol change.
Outcomes
The primary outcome was time to DKA resolution as defined by the time elapsed between first laboratory evidence of DKA and resolution of DKA using the following criteria: plasma glucose less than 200 mg/dL and two of the following: (1) plasma bicarbonate greater than or equal to 15 mEq/L, (2) venous pH greater than 7.3, and/or (3) anion gap less than or equal to 12 mEq/L. 1 Time to DKA resolution was not determined for patients who discharged prior to laboratory evidence of DKA resolution, and thus, they were excluded from the basic comparison of this outcome between protocol groups. However, they were included in all other analyses, including the survival analysis of time to resolution where they were censored at discharge (see the “Statistical Analysis” section).
The secondary outcome was time to discontinuation of DKA protocol insulin drip. This was defined as the time between nursing documentation of DKA protocol insulin infusion start and end time within the medication administration record (MAR).
Statistical Analysis
Descriptive statistics for continuous data are reported as means and standard deviations (SDs). Trends in laboratory values during the first 48 hours following laboratory confirmed DKA were plotted using Loess curves with associated 95% confidence bands for each protocol group. Associations between categorical variables of interest and the time period were assessed using chi-square tests. Independent samples t tests were used to examine differences in continuous variables of interest between the two protocol periods. Time-to-event survival analysis was also used to analyze time to DKA resolution. Patients who did not meet DKA definition of resolution were censored at the time of their discharge. Patients who had DKA resolution or discharged after 72 hours were censored at 72 hours. Time to resolution was plotted with failure curves for each protocol group and the difference between curves was assessed using a log-rank test. Note that “failing” in this survival context is resolution from DKA, and thus, the upper curve of the failure plot indicates the group with a quicker time to resolution. All analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study Sample
During the 46-month study period, 811 hospital encounters contained an ICD-10-CM code for ketoacidosis (E10.10); 359 encounters in the NS group (preprotocol change) and 452 in the LR group (postprotocol change). Following application of exclusion criteria and screening for the first chronological encounter for each unique patient that met eligibility requirements and excluding subsequent encounters, 246 patients and their associated encounter were included in the analysis: 119 in NS group and 127 in LR group (Figure 1). The most common reason for patient exclusion was not meeting laboratory definition of DKA (115 encounters) and having an LOS less than 24 hours (102 encounters). Among the 246 patients in the study population, the mean age was 38.2 (SD = 16.1) years, 138 were male (56.1%) and most patients were white (71%). The most common cause of DKA was missed medication (42.3%). Baseline characteristics of the two protocol groups were well matched (Table 1).
Figure 1.

Screening and protocol groups.
Table 1.
Baseline Patient Characteristics by Protocol Group.
| Characteristic | Prechange (NS) (n = 119) |
Postchange (LR) (n = 127) |
P value |
|---|---|---|---|
| Age (years), mean (SD) | 37.2 (16.2) | 39.2 (15.9) | .33* |
| Sex, n (%) | .22 | ||
| Female | 57 (47.9%) | 51 (40.2%) | |
| Male | 62 (52.1%) | 76 (59.8%) | |
| Race/ethnicity, n (%) | .59 | ||
| Black | 20 (16.8%) | 30 (23.8%) | |
| Hispanic | 5 (4.2%) | 5 (4.0%) | |
| Other | 6 (5.0%) | 5 (4.0%) | |
| White | 88 (73.9%) | 86 (68.3%) | |
| Missing | 0 | 1 | |
| DKA severity, n (%) | .79 | ||
| Mild | 28 (23.5%) | 26 (20.5%) | |
| Moderate | 31 (26.1%) | 37 (29.1%) | |
| Severe | 60 (50.4%) | 64 (50.4%) | |
| Admitted to ICU, n (%) | .67 | ||
| No | 50 (42.0%) | 50 (39.4%) | |
| Yes | 69 (58.0%) | 77 (60.6%) | |
| DKA as ICU principal diagnosis | .02 | ||
| No | 9 (13.0%) | 2 (2.6%) | |
| Yes | 60 (87.0%) | 75 (97.4%) | |
| Baseline Cr (mg/dL), mean (SD) | 0.8 (0.28) | 0.8 (0.42) | .16* |
| N | 66 | 99 | |
| AKI present on admission | .27 | ||
| No | 46 (69.7%) | 60 (61.2%) | |
| Yes | 20 (30.3%) | 38 (38.8%) | |
| Missing | 53 | 29 | |
| Cause of DKA, n (%) | .004 | ||
| Other | 10 (8.4%) | 19 (15.0%) | |
| New diagnosis of diabetes | 14 (11.8%) | 15 (11.8%) | |
| Missed medications | 55 (46.2%) | 49 (38.6%) | |
| Infection | 14 (11.8%) | 2 (1.6%) | |
| Unknown | 26 (21.8%) | 42 (33.1%) | |
| Initial plasma values (mean, SD) | |||
| Chloride (mEq/L) | 94.2 (7.3) | 92.8 (8.3) | .17* |
| Bicarbonate (mEq/L) | 10.1 (4.4) | 10.2 (4.7) | .86* |
| Anion gap (mEq/L) | 26.7 (6.3) | 26.9 (7.0) | .88* |
| Blood glucose (mg/dL) | 585.0 (260.7) | 562.9 (264.5) | .51* |
P values from chi-square tests unless otherwise indicated.
P value from independent samples t test.
Fluid Administration and Electrolytes
During both the preprotocol and postprotocol periods, individual providers could order alternative and/or additional intravenous fluids outside the protocol. However, the primary intravenous fluid received correlated with the default fluid of the protocol active at that time and was significantly different between the groups (Table 2): The mean percentage of NS received in the NS group was 53.5% compared with 12.7% in the LR group, P < .001. The mean percentage of LR received in the LR group was 84.0% compared with 25.6% in the NS group, P < .001. Mean volume of intravenous fluid received was significantly less in the LR group (7373 mL) compared with the NS group (9798 mL; P < .0001). Mean potassium supplementation was significantly lower in the LR group (143.3 mEq) compared with the NS group (214.2 mEq; P = .004).
Table 2.
DKA Outcomes and Isotonic Fluids Received by Protocol Group.
| Variable | Prechange (NS) (n = 119) mean (SD) |
Postchange (LR) (n = 127) mean (SD) |
P value |
|---|---|---|---|
| Time to DKA resolution (hours) | 20.6 (12.2) | 17.1 (11.0) | .02 |
| N | 109 | 119 | |
| Time on DKA protocol insulin infusion (hours) | 21.4 (12.5) | 16.0 (8.7) | < .001 |
| ICU LOS (hours) | 28.0 (17.4) | 21.2 (9.4) | .01 |
| n | 60 | 75 | |
| Hospitalization LOS (days) | 4.6 (9.0) | 3.8 (3.9) | .36 |
| Total volume of intravenous fluids received (mL) | 9798.0 (4546.1) | 7372.7 (3372.1) | < .001 |
| Total volume of LR received (mL) | 2694.3 (2930.7) | 6212.2 (3190.3) | < .001 |
| Total volume of NS received (mL) | 5120.9 (2832.5) | 930.1 (1578.5) | < .001 |
| LR received (%) | 25.6 (24.5) | 84.0 (19.6) | < .001 |
| NS received (%) | 53.5 (18.7) | 12.7 (17.4) | < .001 |
| Potassium supplementation (mEq) | 214.2 (216.6) | 143.3 (156.6) | .004 |
| First lab values at least 48 hours after DKA diagnosis | n = 69 | n = 81 | |
| Time from diagnosis to first lab values at least 48 hours after diagnosis (hours) | 55.5 (5.4) | 55.8 (5.5) | .75 |
| 48-hour chloride value (mEq/L) | 105.2 (5.6) | 102.8 (4.7) | .004 |
| 48-hour anion gap (mEq/L) | 8.2 (2.2) | 9.1 (2.8) | .04 |
| 48-hour bicarbonate (mEq/L) | 24.6 (4.5) | 25.6 (4.0) | .13 |
P values from independent samples t tests.
At laboratory diagnosis of DKA, both protocol groups had similar baseline chloride, bicarbonate, and anion gap values (Table 1). Mean chloride at 48 hours was significantly lower in the LR group (102.8 mEq/L) compared with the NS group (105.2 mEq/L; P = .004). Mean bicarbonate at 48 hours was not significantly different between LR and NS groups but favored the LR group (LR = 25.6 mEq/L; NS = 24.6 mEq/L; P = .13). Mean anion gap at 48 hours was significantly higher in the LR group (9.1 mEq/L) compared with the NS group (8.2 mEq/L; P = .04).
DKA-Associated Outcomes
Diabetic ketoacidosis severity at presentation and intensive care unit (ICU) level of care utilization were similar between protocol groups. Of patients who were admitted to the ICU, more patients were admitted with a principal diagnosis other than DKA in the NS group, n = 9 (13.0%) compared with the LR group, n = 2 (2.6%); P = .02. Time to DKA resolution was 17.1 hours in the LR group compared with 20.6 hours in the NS group (P = .02). Using survival analysis to assess time to DKA resolution, which includes patients who did not resolve and censors them at discharge, patients in the LR group had a faster time to resolution (Figure 2; P = .03). The mean time to discontinuation of DKA protocol insulin drip in the LR group was 16.0 hours compared with 21.4 hours in the NS group (P < .001). Among patients with an ICU admission, ICU LOS was significantly shorter in the LR group (21.2 hours) compared with the NS group (28.0 hours; P = .01). Hospital LOS was not statistically significant between the groups but did favor the LR group (4.6 days in NS group, 3.8 days in LR group; P = .36).
Figure 2.
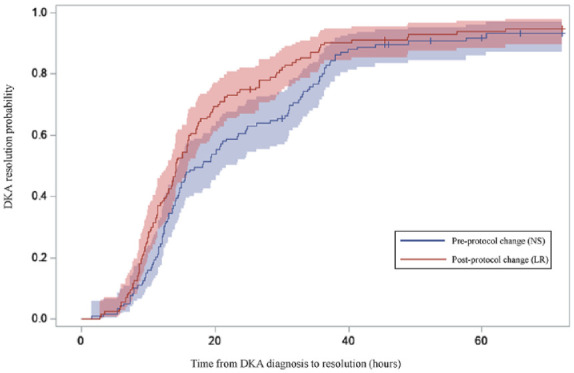
Probability of DKA resolution by protocol group.
Vertical ticks indicate censored observations (ie, patients who had not resolved at discharge or by 72 hours after DKA diagnosis). Shading represents 95% confidence limits. Time 0 is defined as time of laboratory diagnosis of DKA. Log-rank test P value comparing groups was .03.
Discussion
In this retrospective cohort study, a quality improvement process changing the default fluid from NS to LR in a single institution’s DKA management protocol was associated with a faster time to DKA resolution, faster time to discontinuation of DKA protocol insulin drip, and shorter length of ICU stay. The LR (postchange) group also had significantly lower serum chloride and higher (nonsignificant) bicarbonate concentrations at 48 hours (Figure 3). Mean potassium supplementation and total volume of intravenous fluid administered was also lower in the LR group.
Figure 3.
Loess curves of plasma electrolyte concentrations of chloride (A) and bicarbonate (B), and calculated anion gap (C), in the first 48 hours following DKA diagnosis by protocol group.
Time 0 is defined as time of laboratory diagnosis of DKA. Shading represents 95% confidence limits.
Diabetic ketoacidosis is a common diagnosis associated with hospitalization and ICU utilization. 1 Current guidelines from the ADA and JBDS-IP recommend NS directed resuscitative and maintenance fluid in the management of DKA. Our results are consistent with prior literature suggesting balanced crystalloids may be preferred over NS in the acute management of patients presenting with DKA.5-11 Previous studies comparing balanced crystalloids to NS for the treatment of DKA have demonstrated no significant difference between groups with regards to resolution of acidosis; however, these studies were limited by sample size and were powered to detect differences in only one variable of DKA resolution, introducing risk for type II error.14,15 In a healthcare era focused on decreasing hospitalization-associated resource utilization, cost, and LOS, our results are particularly relevant as they demonstrate a significantly significant decreased ICU LOS, decreased volume of fluid administered, decreased potassium supplementation, and a trend toward decreased hospital LOS. Although more patients in the NS group (9 [13.0%]) compared with the LR group (2 [2.6%]) were admitted to the ICU with a primary diagnosis other than DKA, we would not expect this small number of patients, compared with the overall sample size, to be clinically significant. In addition, there were more overall patients that required ICU care in the LR group relative to the NS group and severity of DKA did not differ between groups.
The NS group received a significantly higher volume of intravenous fluid compared with the LR group. This could be due to multiple factors including: (1) development of HMA in the NS group may delay resolution of laboratory evidence of acidosis leading to provider continuation of intravenous fluids and (2) other components of the DKA protocol quality improvement intervention led to earlier discontinuation of fluids.
While HMA in the setting of resuscitation of DKA can be prevented by switching to 0.45% NS after initial resuscitation with NS, consistent and optimally timed transition of intravenous fluid types is difficult to achieve in clinical practice. Utilization of LR for resuscitation and maintenance reduces risk of HMA and minimizes the opportunity for human error of needing to switch fluids.6-8 We believe that the feasibility of using a single intravenous fluid formulation is clinically less cumbersome to operationalize and manage by providers. However, similar to any other medical intervention, the use of balanced crystalloids for fluid resuscitation in DKA must be considered within the context of the patient’s unique clinical situation and morbidities.
Strengths
The retrospective nature of this study is reflective of clinical practice as fluid choice, fluid infusion rates, electrolyte supplementation, and transition off DKA protocol insulin drip were not controlled and were entirely up to the providers caring for the patient. Importantly, the predominant intravenous fluid type each group received matched the protocol’s default fluid at the time of treatment (Table 2). Our study adds to the paucity of real-life data surrounding this topic and to our knowledge is the largest retrospective study conducted to evaluate DKA-specific outcomes. In addition, LR was used as the balanced crystalloid in this study, which is applicable to many other healthcare institutions given its wide availability and low cost.
Limitations
While the main institutional DKA management protocol change was the conversion to LR as the default fluid, there were other simultaneous changes that may have impacted the results. These include: (1) education of nursing and hospital providers about the protocol change; (2) linking a maintenance fluid order with the initial bolus fluid order, as opposed to relying upon nursing staff judgment to modify the fluid rate based on urine output; and (3) adding a recommendation for nursing to contact primary providers when blood glucose fell under 250 mg/dL to expedite conversion to dextrose containing fluids. However, quality improvement programs are common and implementing the above processes as part of a quality improvement process requires minimal additional resources.
Conclusion
A quality improvement intervention changing NS to LR as the default fluid of choice in the treatment of DKA was associated with faster time to DKA resolution, faster time to discontinuation of DKA protocol insulin drip, and shorter length of ICU stay. This suggests that LR may be preferred over NS for intravenous fluid management of adults presenting with DKA.
Acknowledgments
The authors would like to thank Jana Wardian, PhD, MSW, Whitney Goldner, MD, Molly Johnson, CPA, Jason Shiffermiller, MD, Jennifer Larson, MD, and Cyrus Desouza, MD for their contributions toward this study.
Footnotes
Abbreviations: DKA, diabetic ketoacidosis; ADA, American Diabetes Association; JBDS-IP, Joint British Diabetes Societies for Inpatient Care; NS, normal saline; HMA, hyperchloremic metabolic acidosis; LOS, length of stay; LR, lactated ringers; IRB, Institutional Review Board; MAR, medication administration record; SD, standard deviation; ICU, intensive care unit.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jake Johnson  https://orcid.org/0009-0003-9821-0654
https://orcid.org/0009-0003-9821-0654
Andjela Drincic  https://orcid.org/0000-0001-8365-7662
https://orcid.org/0000-0001-8365-7662
References
- 1. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabet Care. 2009;32(7):1335-1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nyenwe EA, Kitabchi AE. Evidence-based management of hyperglycemic emergencies in diabetes mellitus. Diabetes Res Clin Pract. 2011;94(3):340-351. doi: 10.1016/j.diabres.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 3. Mustafa OG, Haq M, Dashora U, Castro E, Dhatariya KK; Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Management of hyperosmolar hyperglycaemic state (HHS) in adults: an updated guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Diabet Med. 2023;40(3):e15005. doi: 10.1111/dme.15005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Martin HE, Smith K, Wilson ML. The Fluid and Electrolyte Therapy of Severe Diabetic Acidosis and Ketosis. Tucson, AZ: American Journal of Medicine; 1958. [DOI] [PubMed] [Google Scholar]
- 5. Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29(6):670-674. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6. Self WH, Evans CS, Jenkins CA, et al. Clinical effects of balanced crystalloids vs saline in adults with diabetic ketoacidosis: a subgroup analysis of cluster randomized clinical trials. JAMA Netw Open. 2020;3(11):e2024596. doi: 10.1001/jamanetworkopen.2020.24596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrillo AR, Elwood K, Werth C, Mitchell J, Sarangarm P. Balanced crystalloid versus normal saline as resuscitative fluid in diabetic ketoacidosis. Ann Pharmacother. 2022;56(9):998-1006. doi: 10.1177/10600280211063651. [DOI] [PubMed] [Google Scholar]
- 8. Goad NT, Bakhru RN, Pirkle JL, Kenes MT. Association of hyperchloremia with unfavorable clinical outcomes in adults with diabetic ketoacidosis. J Intensive Care Med. 2020;35(11):1307-1313. doi: 10.1177/0885066619865469. [DOI] [PubMed] [Google Scholar]
- 9. Alghamdi NA, Major P, Chaudhuri D, et al. Saline compared to balanced crystalloid in patients with diabetic ketoacidosis: a systematic review and meta-analysis of randomized controlled trials. Crit Care Explor. 2022;4(1):e0613. doi: 10.1097/CCE.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catahay JA, Polintan ET, Casimiro M, et al. Balanced electrolyte solutions versus isotonic saline in adult patients with diabetic ketoacidosis: a systematic review and meta-analysis. Heart Lung. 2022;54:74-79. doi: 10.1016/j.hrtlng.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 11. Chua HR, Venkatesh B, Stachowski E, et al. Plasma-Lyte 148 vs 0.9% saline for fluid resuscitation in diabetic ketoacidosis. J Crit Care. 2012;27(2):138-145. doi: 10.1016/j.jcrc.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 12. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. doi: 10.1056/NEJMoa1711586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Zyl DG, Rheeder P, Delport E. Fluid management in diabetic-acidosis–Ringer’s lactate versus normal saline: a randomized controlled trial. QJM. 2012;105(4):337-343. doi: 10.1093/qjmed/hcr226. [DOI] [PubMed] [Google Scholar]
- 15. Yung M, Letton G, Keeley S. Controlled trial of Hartmann’s solution versus 0.9% saline for diabetic ketoacidosis. J Paediatr Child Health. 2017;53(1):12-17. doi: 10.1111/jpc.13436. [DOI] [PubMed] [Google Scholar]
- 16. Williams V, Jayashree M, Nallasamy K, Dayal D, Rawat A. 0.9% saline versus Plasma-Lyte as initial fluid in children with diabetic ketoacidosis (SPinK trial): a double-blind randomized controlled trial. Crit Care. 2020;24(1):1. doi: 10.1186/s13054-019-2683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long B, Willis GC, Lentz S, Koyfman A, Gottlieb M. Evaluation and management of the critically ill adult with diabetic ketoacidosis. J Emerg Med. 2020;59(3):371-383. doi: 10.1016/j.jemermed.2020.06.059. [DOI] [PubMed] [Google Scholar]



