Abstract
Background:
Continuous glucose monitors (CGMs) are widely used for individuals with diabetes mellitus, particularly those with type 1 diabetes (T1D). Advancements in CGM technology allow for glycemic assessment without capillary glucose measurements as many come factory calibrated. However, exercise, an essential component of diabetes care, has been reported to alter accuracy of earlier generation CGM. Considering the importance of physical activity for individuals with T1D and the progression of CGM technology, we aimed to investigate the accuracy of the Dexcom G6 during physical activity.
Methods:
Adolescents (ages 13-20 years) exercised on a treadmill for 40 minutes, with a 10-minute break at minute 20. We obtained paired CGM and glucometer measurements before and every 10 minutes during and after exercise. Accuracy analysis was determined by mean absolute relative difference (MARD), mean absolute difference (MAD), and Clarke Error Grid Analyses.
Results:
Mean absolute relative difference and MAD increased during exercise (14%-33% and 24.3-34 mg/dL) but improved after exercise. We noted certain CGM locations produced greater changes in accuracy as MARD and MAD increased markedly when the CGM was on the buttocks (18%-46% and 30-41 mg/dL). We also noted decreased odds of Zone A in the Clarke error grid when the CGM was on the buttocks compared to the abdomen (odds ratio [OR]: 0.146; P = 0.0003; 95% CI = 0.052-0.415).
Conclusions:
This CGM system showed alterations in accuracy during exercise. Our findings additionally suggest interstitial fluid changes in muscles during exercise alter accuracy of CGM; however, additional research is required.
Keywords: exercise, continuous glucose monitoring, physical activity, accuracy, youth, type 1 diabetes
Introduction
Real-time continuous glucose monitors (CGMs) are widely used for individuals with diabetes mellitus, particularly those with type 1 diabetes (T1D). The use of CGMs is associated with improved glycosylated hemoglobin A1c (HbA1c) and improved time in range without increased risk of hypoglycemia. 1 Advancement in technology has continually improved CGM devices; however, accuracy in youth when participating in sports and other activities has not been readily reported. Exercise can induce rapid fluctuations in blood glucose and lead to both hyperglycemia and hypoglycemia. Therefore, careful monitoring of glucose is needed before, during, and after exercise.
Several studies investigated the impact of physical exercise on the accuracy of CGM devices with varying results, but, overall, lower accuracy was a significant concern.2-5 These studies were done in the adult population using older generation CGM devices which required calibration. However, to the best of our knowledge, there have been no studies that have examined the accuracy of the Dexcom G6 during extended exercise in youth with T1D. Thus, we investigated the accuracy of the CGM while youth with T1D exercised.
Methods
This study was conducted in the Christenson Family Sports and Physical Activity Center in the Wendy Novak Diabetes Center at Norton Children’s and the University of Louisville, and it was reviewed by the University of Louisville Institutional Review Board. A total of 30 participants, whom were already using the CGM, were recruited from the Wendy Novak Diabetes Center during visits for routine diabetes care. Participant inclusion criteria consisted of being 13 years of age or older and a diagnosis of T1D. In addition, participants had to be capable of safely performing physical activity. Patients were excluded from the study if they were diagnosed with T1D for <2 years or had a HbA1c ≥10%.
Prior to exercise, a body composition measurement was obtained using the InBody 770 analyzer. This instrument was simultaneously used as a scale. The measured parameters of body composition include: body fat and its segmental analysis; total body water and its components: extracellular water and intracellular water; fat-free mass and its components: body cell mass, extracellular mass, and skeletal muscle mass.
For the study, participants placed their CGM in their preferred location, carbohydrate intake and timing was not controlled for to help emulate a real-life scenario. Participants engaged in moderate intensity aerobic physical activity which was defined as 45% to 50% of heart rate reserve. This was calculated using the Karvonen formula using the heart rate reserve method to assess intensity. Each participant performed a 40-minute exercise session on a motorized treadmill with a 10-minute break occurring midway at minute 20 during exercise. The speed and incline of the treadmill was adjusted accordingly to illicit the desired heart rate response. The heart rate reserve of 45% to 50% was used as the target heart rate for all participants throughout the 40 minutes of aerobic exercise.
In case of a heart rate monitor failure, the rate of perceived exertion (RPE) was used in lieu of a measured heart rate. Rate of perceived exertion is based on a scale of 1 to 10. An RPE of 5 to 6 is consistent with moderate intensity activity. This was provided by the participant on a regular basis during exercise. Modification to treadmill speed and incline was made accordingly to ensure RPE of 5 to 6 was maintained.
The CGM device’s measured value was compared to a capillary blood glucose level obtained via a contour next glucometer, provided by the study site, acting as the reference. Measurements were obtained 25 ± 10 minutes prior to exercise, at the beginning of exercise, and then every 10 ± 1 minutes thereafter, both during and for 20 minutes after cessation of physical activity. The contour next glucometer was chosen as it has been shown to be one of the most accurate glucometers on the market assessed using mean absolute relative difference (MARD). 6
If, prior to beginning exercise, the participant had a blood glucose <90 mg/dL, or >250 mg/dL in the presence of moderate or large ketones, they were instructed to treat per their individualized medical regimen prescribed by their diabetes provider.
During exercise, if the Self-Monitoring Blood Glucometer (SMBG) blood glucose was found to be <70 mg/dL, the participant immediately ceased activity and treated the hypoglycemia with approximately 15 g of simple rapid-acting carbohydrates. Blood glucose was re-checked every 10 minutes with repeat treatment of a simple carbohydrate until glucose was greater than 70 mg/dL. The patient then received an additional treatment of a complex carbohydrate. Blood glucose was re-checked every 10 minutes until blood glucose was >90 mg/dL at which point exercise was resumed.
Collected data included age, sex, height, weight, body mass index (BMI), body fat, lean body mass, skeletal muscle mass, resting heart rate, HbA1c, insulin regimen (pump vs multiple daily injections), type of pump (if applicable) (Table 1), CGM location and date/time of last CGM change (Table 2), pump location and date/time of last pump change, last meal consumed (including number of carbohydrates), as well as time and amount of last insulin bolus.
Table 1.
Participant Demographic Characteristics.
| Mean ± SD (range) | |
|---|---|
| Age (years) | 14.6 ± 1.7 (13-20) |
| Sex | 48.3% male |
| Height (cm) | 167 ± 9.4 (150-186.5) |
| Weight (kg) | 66.8 ± 13.3 (41.7-94.4) |
| BMI | 23.6 ± 3.7 (17.4-30.6) |
| Body fat % | 24.1 ± 10.7 (7.5-48.8) |
| Lean body mass (kg) | 50.7 ± 12.8 (34.6-84.8) |
| Skeletal muscle mass (kg) | 28 ± 7.8 (18.3-48.5) |
| Resting heart rate (bpm) | 76.5 ± 9.9 (52-92) |
| Heart rate reserve (bpm) | 169.5 ± 10.2 (142-185) |
| A1c (%) | 7.6 ± 0.7 (6.5-9.3) |
| Using pump (%) | 86% |
| Tandem (%) | 48% |
| Control-IQ (%) | 45% |
| Basal-IQ (%) | 3% |
| Omnipod (%) | 38% |
Abbreviations: SD, standard deviation; BMI, body mass index.
Table 2.
CGM Location on the Participant’s Bodies.
| CGM location | Count (%) | Days since CGM change ± SD |
|---|---|---|
| Abdomen | 8 (27.6%) | 4.6 ± 2.7 |
| Arm | 8 (27.6%) | 5.3 ± 2.5 |
| Buttocks | 11 (37.9%) | 3.7 ± 3.1 |
| Thigh | 2 (6.9%) | 5.5 ± 3.5 |
Abbreviations: CGM, continuous glucose monitor; SD, standard deviation.
Descriptive summaries were performed to characterize the sample and assess differences among age, sex, BMI, body fat, and other medical characteristics. To assess the accuracy of the CGM during exercise, the overall MARD and mean absolute difference (MAD) were calculated, as well as both for each CGM location over time. Similarly, Clarke Error Grid Analyses (EGAs) were performed for each CGM location and overall, along with assessing the percentages of participants over time in each zone of the Clarke EGA.
In order to estimate the impact of CGM location on the MARD and Clarke EGA, generalized linear mixed effects models (GLMMs) were calculated, accounting for repeated measurements of subjects over time. For the regression analysis, CGM location was categorized as abdomen, arm, buttocks, and thigh, with abdomen serving as the reference category. A log-linked, gamma GLMM was used to estimate the impact on MARD of the CGM location and time, while a binomial logistic GLMM was used to assess the impact on whether each measurement was within Zone A on the Clarke EGA. The study ID was included as a random effect in the model to adjust for the dependent nature of observations repeated for each subject over time. Time was additionally included as a continuous fixed effect in each of the regression analyses.
Results
Thirty participants enrolled in the study. One participant withdrew prior to completion of study procedures, and their data were excluded from the final study results. Of the 29 participants who completed the study, 28 had full data sets collected and 1 had all but 3 measurements collected due to sensor reading failures. Therefore, a total of 258 paired CGM and SMBG values were collected for analysis.
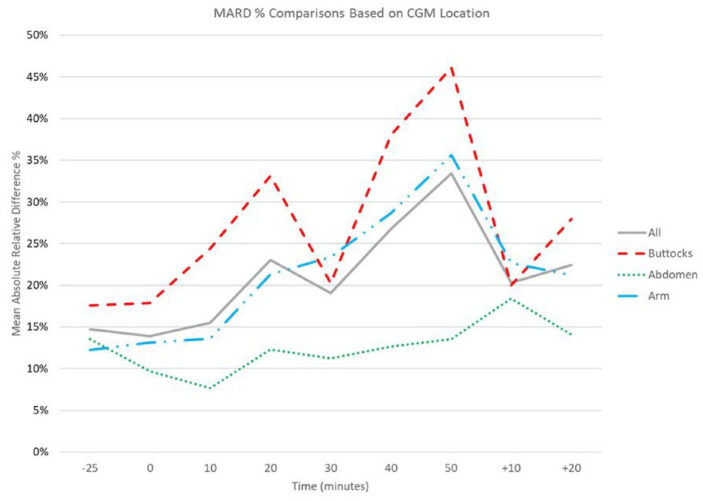
Analysis of the complete data set revealed that there was an increase in the MARD and MAD over time during exercise. The MARD and MAD 25 minutes prior to exercise were 15% and 26.6 mg/dL respectively, and at initiation of exercise were 14% and 24.3 mg/dL. The MARD and MAD rose during exercise until the 10-minute break occurred, at which point they both improved. Once exercise was reinitiated, they continued to increase, to a maximum of 33% and 34 mg/dL at the end of the exercise session. After exercise cessation, both MARD and MAD improved dramatically (Figures 1 and 2).
Figure 1.
MARD over time, before, during, and after exercise. Time 0 represents beginning of exercise session. +10/+20 represent 10 and 20 minutes after exercise cessation. All participants represented by solid line. Subset of participants with CGM only on buttocks represented by dashed line. Subset of participants with CGM on only abdomen represented by dotted line. Subset of participants with CGM on only arm represented by dash-dot line. Abbreviations: CGM, continuous glucose monitor; MARD, mean absolute relative difference.
Figure 2.
MAD over time, before, during, and after exercise. Time 0 represents beginning of exercise session. +10/+20 represent 10 and 20 minutes after exercise cessation. All participants represented by solid line. Subset of participants with CGM only on buttocks represented by dashed line. Subset of participants with CGM on only abdomen represented by dotted line. Subset of participants with CGM on only arm represented by dash-dot line. Abbreviations: CGM, continuous glucose monitor; MAD, mean absolute difference.
Subset analyses were performed based on CGM location, BMI, and body fat percentage. There were no statistically significant findings when substratifying based on BMI or body fat percentage. However, subcategorizing based on CGM location provided statistically significant results.
This subset analysis demonstrated a more pronounced increase in MARD and MAD when the CGM device was worn on the buttocks. The opposite was found when the CGM was worn on the abdomen. When worn of the arm, the CGM performed better than the buttocks but worse than the abdomen (Figures 1 and 2).
We also performed a Clarke EGA (Figure 3) and calculated percentages of Clarke EGA zones for all participants (Figure 4). The analysis showed a steady increase in Zone “B” (from 21% to 45%) and a decrease in Zone “A” (from 79% to 34%). Zones A and B are the clinically “safe” zones. 7 However, there were also values which corresponded to Zone “D” that occurred at the end of the exercise session (0%-21%). Zone “D” corresponds to a missed treatment opportunity and are not considered to be in the clinically “safe” zones. 7 Similar to the MARD, there was an increase in values in Zone “A” after short periods of rest.
Figure 3.

Clarke error grid analysis showing paired continuous glucose monitoring values and glucometer reading for all participants and times. Abbreviation: CGM, continuous glucose monitors.
Figure 4.
Clark error grid zone percentages from all participants over time. Zone A represents glucose values that deviate from the reference by no more than 20% or are <70 mg/dl when the reference is also <70 mg/dl. Zone B represents values that deviate from the reference by >20% but lead to benign or no erroneous treatment. Zone A and B are both clinically safe zones. Zone D represents dangerous failure to detect and treat errors.
We again performed subset analyses. Based on CGM location, there were differences in Clarke error grids (Figure 5). All values that corresponded to Zone D on the Clarke Error Grid were from participants that were wearing the CGM on their buttocks (n = 7) or thigh (n = 1). There was an increased number of values in Zone “A” when the CGM was being worn on the abdomen in comparison to other CGM locations.
Figure 5.
Clarke error grid analyses showing paired continuous glucose monitoring values and glucometer reading for subset analyses based on CGM location. The top left represents a Clarke EGA for participants wearing the CGM on their buttocks, top right wearing CGM on their thigh, bottom left wearing CGM on abdomen, bottom right wearing CGM on arms. Abbreviations: CGM, continuous glucose monitors; EGA, error grid analyses.
A log-linked, gamma GLMM was used to estimate the impact on MARD of the CGM location and time. The results show a statistically significant increase in MARD by a ratio of 1.6 when the CGM was placed on the arm (P = 0.038; 95% CI = 1.02-2.39), and a ratio of 2.15 when placed on the buttocks (P = 0.0001; 95% CI = 1.45-3.18), compared to abdominal placement. Thigh placement tended to show a MARD 1.68 times higher compared to abdominal placement (P = 0.127; 95% CI = 0.86-3.27). However, this finding did not reach statistical significance. Similarly, results from the logistic regression analysis show decreased odds of attaining Zone “A” under the Clarke error grid when the CGM was placed on the arm (odds ratio [OR]: 0.237; P = 0.011; 95% CI = 0.078-0.718), buttocks (OR: 0.146; P = 0.0003; 95% CI = 0.052-0.415), and thigh (OR: 0.172; P = 0.037; 95% CI = 0.033-0.895), compared to abdominal placement.
Discussion
Our study results found significant divergence of the blood glucose readings on the CGM when compared to SMBG during moderate physical activity. In addition, we found higher levels of discrepancy when the CGM was placed on the buttocks, compared to other sites, with the least difference seen between SMBG and abdominally placed CGM. This is in contrast to what was reported by Shah et al 8 who found that there was similar accuracy between CGM placed on the abdomen and buttocks; however, this study was not performed during exercise.
Exercise and physical activity are fundamentally important and recommended for most individuals with T1D. However, concerns for erratic blood glucose levels, including hypoglycemia, can create apprehension to engage in exercise. Having accurate and readily available glucose information allows patients to feel comfortable and safe while undertaking the recommended daily allotment of physical activity.
Prior investigations into the accuracy of CGM devices have raised concerns about clinical accuracy during activity.2-5 In 2016, Taleb et al 3 found that MARD increased from 13.77% to 22.53% in the Dexcom G4 Platinum during exercise, and the Medtronic Paradigm Veo increased from 12.38% to 20.44%. In addition, in 2018, Biagi et al 2 noted an increase in MARD from 9.5% to 16.5% for the Paradigm Veo sensor during aerobic exercise. Subsequently, an investigation of the Dexcom G4 Platinum and Dexcom G5 during aerobic exercise in adults was performed by Zaharieva et al 4 in 2019 which reported CGM lag times and increased in MARD from 8% to 13%. However, most recently one study published in 2020 investigated the Dexcom G6 in adults showing acceptable clinical accuracy with MARD increasing from 9.5% to 13.3% for aerobic exercise limited to 30 minutes. 9
However, these studies were done in adults and little research has investigated accuracy in youth. Thus, our study was designed to mimic that of a youth sports match consisting of 40 minutes of exercise divided into two 20-minute sessions with a 10-minute break at the halfway point. This would be similar to a secondary school basketball game or soccer match in terms of length and inclusion of a halftime.
Similar to research performed on previous iterations of CGM devices,2-5 our research continues to raise concern for discrepancies between CGM and SMBG readings during times of exercise. Our study demonstrated an increase in MARD and MAD from 14% to 33% and 24.3 to 34 mg/dL by the end of the exercise session. In addition, there was a change in Clarke EGA over time. At the initiation of exercise 76% of participants were in “Zone A” with the remaining 24% in “Zone B.” Zone A represents glucose values that deviate from the reference by no more than 20% or are <70 mg/dl when the reference is also <70 mg/dl. 7 Values falling within this range are clinically accurate in that they would lead to clinically correct treatment decisions. Zone B represents values that deviate from the reference by >20% but would lead to benign or no erroneous treatment. Zone A and B are both clinically safe zones. 7 In our study, by the end of the exercise period, only 34% of participants were in “Zone A,” with 45% in “Zone B,” and 21% in “Zone D.” Zone D represents “dangerous failure to detect and treat” errors. 7 These results raise concerns for adolescents who may solely rely on CGM while participating in extended periods of exercise.
Interestingly, we also noted accuracy differences between different CGM locations. The Dexcom G6 has 2 approved locations, the abdomen and buttocks, for children and adolescents ages 2 to 17 years in the United States. 10 It is approved to also be worn on the upper arm for ages >2 years of age in the United Kingdom. 11 When the CGM was being worn on an area of the body that was not actively being used, such as the abdomen, the MARD was improved in comparison to other locations, as was the Clarke error grid zone analysis. However, when the CGM was being worn on the buttocks, which is more actively used during a treadmill exercise, the MARD/MAD demonstrated a larger discrepancy. There was an increase in the MARD by a ratio of 2.15 when wearing the CGM on the buttocks in comparison to the abdomen. In addition, there is a decreased odds ratio of having a Zone A when the CGM was worn on the buttocks in comparison to the abdomen. Furthermore, all values that corresponded to Zone D on the Clarke error grid were from participants that were wearing the CGM on their buttocks or thigh, which were being more actively used.
We hypothesize that these differences may be due to interstitial fluid changes in actively exercising muscles which alter the accuracy during exercise. This would provide support and an explanation for some of the differences seen at different site locations. The differences may also have been due to lag time which has been noted in prior studies.4,9 However, further studies and data would be required, particularly in children and adolescents to substantiate this theory.
Nevertheless, although our study raises concern of CGM performance during physical activity, there are other factors to consider when using a CGM for management during physical activity. Continuous glucose monitoring improves time in range and A1c compared to SMBG alone. 12 By using a CGM, patients can follow the trend of their glucose. By following trends, patients and families could intervene if hypoglycemia was anticipated as suggested by Moser et al. 13 These anticipatory trend data also help reduce the aforementioned issue of potential lag time. For these reasons, the use of CGM to identify impending hypoglycemia is an advantage over intermittent blood glucose monitoring techniques. Earlier identification would allow for earlier treatment and potentially prevent more pronounced hypoglycemia from occurring. Thus, despite our findings, we continue to advocate for CGM use in all scenarios, including physical activity. However, due to the influence on accuracy, we recommend caution and counseling patients about their use of CGM during physical activity and advocate confirmatory SMBG testing be used.
Several limitations should be considered for this study. As part of our study design, we did not control for insulin use or carbohydrate intake prior to exercise in order to simulate real-world conditions. In addition, having participants continuously run on a treadmill as opposed to having them participate in real-life exercise routines (playing soccer, basketball, cheerleading, and generalized play) may limit the generalizability to mixed activity sports. Our study also used an SMBG device for comparison instead of plasma glucose or a yellow springs instrument glucose values. However, SMBG devices are currently the standard for confirmatory glucose values for all patients in real-life practice. Therefore, to emulate a real-life scenario, we used this as a comparison. Nevertheless, as with all glucometers, there is potential for inconsistency which can lead to glucose-level measurement inaccuracies. We did attempt to limit this by using the Contour Next glucometer which has been shown to be a top-performing glucometer regarding accuracy and performance. 6
Finally, our study had a limited number of participants (n = 29) with an almost exclusively homogenous demographic profile. We limited enrollment of our participants to those with HbA1c < 10% and those 13 years and older. Owing to this limitation, these results may not be generalizable to other groups. Additional research should be pursued with a more heterogeneous group of participants. Furthermore, only one CGM was used for this study. Thus, these results should be interpreted in this context as this only reflects one of several available CGM.
Conclusion
The Dexcom G6 showed changes in the MARD and MAD during exercise which improved quickly after exercise termination. Thus, for adolescents participating in physical activity, it is important to keep this in mind when using a CGM. In addition, the location of the CGM device may alter accuracy. This is suggestive of interstitial fluid changes in actively exercising muscles which may alter the accuracy of CGM during exercise and is important for patients to consider when participating in specific activities. However, further investigation is needed to better understand this influence.
Footnotes
Abbreviations: CGM, continuous glucose monitor; EGA, error grid analysis; GLMM, generalized linear mixed effects model; HbA1c, glycosylated hemoglobin A1c; MAD, mean absolute difference; MARD, mean absolute relative difference; RPE, rate of perceived exertion; SMBG, self-monitoring blood glucometer; T1D, type 1 diabetes.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was generously supported by funding from the University of Louisville Department of Pediatrics Office of Medical Education and the Norton Children’s Hospital Foundation.
ORCID iD: Ryan J. Dyess  https://orcid.org/0000-0002-6858-9355
https://orcid.org/0000-0002-6858-9355
References
- 1. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323:2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biagi L, Bertachi A, Quiros C, et al. Accuracy of continuous glucose monitoring before, during, and after aerobic and anaerobic exercise in patients with type 1 diabetes mellitus. Biosensors. 2018;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taleb N, Emami A, Suppere C, et al. Comparison of two continuous glucose monitoring systems, Dexcom G4 platinum and medtronic paradigm veo enlite system, at rest and during exercise. Diabetes Technol Ther. 2016;18(9):561-567. [DOI] [PubMed] [Google Scholar]
- 4. Zaharieva DP, Turksoy K, McGaugh SM, et al. Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diabetes Technol Ther. 2019;21(6):313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herrington SJ, Gee DL, Dow SD, Monosky KA, Davis E, Pritchett KL. Comparison of glucose monitoring methods during steady-state exercise in women. Nutrients. 2012;4(9):1282-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klonoff DC, Parkes JL, Kovatchev BP, et al. Investigation of the accuracy of 18 marketed blood glucose monitors. Diabetes Care. 2018;41(8):1681-1688. [DOI] [PubMed] [Google Scholar]
- 7. Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl S. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622-628. [DOI] [PubMed] [Google Scholar]
- 8. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428-433. doi: 10.1089/dia.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guillot FH, Jacobs PG, Wilson LM, et al. Accuracy of the Dexcom G6 glucose sensor during aerobic, resistance, and interval exercise in adults with type 1 diabetes. Biosensors. 2020;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Where can I insert my Dexcom G6 sensor? https://www.dexcom.com/faqs/where-can-i-insert-my-dexcom-g6-sensor. Accessed March 25, 2022
- 11. Where can I insert my G6 sensor? https://www.dexcom.com/en-GB/faqs/where-can-i-insert-my-g6-sensor. Accessed September 8, 2022.
- 12. Thabit H, Prabhu JN, Mubita W, et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care. 2020;43(10):2537-2543. doi: 10.2337/dc20-0736. [DOI] [PubMed] [Google Scholar]
- 13. Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia. 2020;63(12):2501-2520. doi: 10.1007/s00125-020-05263-9. [DOI] [PubMed] [Google Scholar]