Abstract
Background:
It has been shown that insulin acceleration by itself might not be sufficient to see clear improvements in glycemic metrics, and insulin therapy may need to be adjusted to fully leverage the extra safety margin provided by faster pharmacokinetic (PK) and pharmacodynamic (PD) profiles. The objective of this work is to explore how to perform such adjustments on a commercially available automated insulin delivery (AID) system.
Methods:
Ultra-rapid lispro (URLi) is modeled within the UVA/Padova simulation platform using data from previously published clamp studies. The Control-IQ AID algorithm is selected as it leverages carbohydrate-to-insulin ratio (CR in g/U), correction factor (CF in mg/dL/U), and basal rate (BR in U/h) daily profiles that are fully customizable. An experiment roadmap is proposed to understand how to safely modify these profiles when switching from lispro to URLi.
Results:
Simulations show that a 7% decrease in CR (approximately an 8% increase in prandial insulin) and a 7.5% increase in BR lead to cumulative improvements in glucose control with URLi. Comparing with baseline metrics using lispro, a clinically significant increase in time in the range of 70 to 180 mg/dL (overall: 70.2%-75.2%, P < .001; 6 am-12 am: 62.4%-68.5%, P < .001) and a reduction in time below 70 mg/dL (overall: 1.8%-1.2%, P < .001; 6 am-12 am: 1.8%-1.3%, P < .001) were observed.
Conclusion:
Properly adjusting therapy parameters allows to fully leverage glucose control benefits provided by faster insulin analogues, opening opportunities to take another step forward into a next generation of more effective AID solutions.
Keywords: automated insulin delivery, glucose control, insulin therapy parameters, type 1 diabetes, ultra-rapid insulin analogues
Introduction
Design limitations have been extensively studied in control theory and reflect performance goals that cannot be met due to inherent characteristics of the system to be controlled. A comprehensive analysis on this subject in the time and frequency domains can be found in the work of Seron et al. 1 For instance, bicycles with rear-wheel steering are extremely difficult to control, because they are not self-stabilizing (requiring rapid corrective actions) and their steering is counterintuitive (they turn in the opposite direction first, meaning that rapid corrective actions should be avoided). 2
Delays are arguably the main source of structural limitations and impose constraints on maximum achievable closed-loop bandwidth or how fast the controller can respond. 3 In other words, more conservative controllers are needed in the presence of delays to avoid performance degradation caused by the controller responding to outdated output measurements.
Automated insulin delivery (AID) systems are not exempt from these limitations. They regulate insulin infusion in people with diabetes and who depend on exogenous insulin to maintain normoglycemia. Unfortunately, delays in absorption and action of current insulin analogues increase the risk of controller-induced hypoglycemia due to insulin overdose. The usual solution to this problem is to detune the control law, typically leading to more elevated average glucose values. Thus, insulin acceleration represents an appealing means to allow for more aggressive therapy approaches without additional risk for hypoglycemia.
Substantial effort has been devoted to developing subcutaneous (SC) insulin formulations with faster pharmacokinetic (PK) and pharmacodynamic (PD) profiles that more closely resemble endogenous insulin secretion and action.4,5 Several studies have demonstrated potential glycemic benefits of using faster insulins like fast-acting insulin Aspart (Fiasp)6,7 or ultra-rapid lispro (URLi).8,9 However, there is still no clear indication that combining ultra-rapid analogues with available AID systems leads to superior glucose control. 10 For instance, in the work of Boughton et al, 11 the hybrid CamAPS FX closed-loop system was tested with Aspart and Fiasp in a double-blind, multinational, randomized, crossover study involving 25 adults with type 1 diabetes (T1D). While a significant change in time < 70 mg/dL (time below range [TBR]) was observed in favor of Fiasp, mean percentage of time in 70 to 180 mg/dL (time in range [TIR]) did not differ for both insulins. Performance of Aspart and Fiasp use in a closed-loop setting was also evaluated in the work of Hsu et al, 12 but in this case using the Medtronic MiniMed 670G AID system. Results from a pilot study with 19 adults with T1D revealed an increase in TIR of 3.1% and a decrease in TBR of 0.8% when using Fiasp, but all changes were statistically nonsignificant. The same combinations were tested again in a single-center, randomized, active-controlled, crossover trial in 37 adults with T1D. 13 Results showed a significant but small increase in TIR of 1.81% accompanied by a 0.4% reduction in TBR when comparing Fiasp with Aspart. The Medtronic system was also used to compare URLi and lispro in hybrid closed-loop control. 14 In that study, authors found nonsignificant changes in the percentage of TIR (URLi: 77.0%, lispro: 77.8%, P = .339) and a reduction in TBR (URLi: 1.5%, lispro: 2.2%, P = .009) but at the possible expense of greater time >180 mg/dL (URLi: 21.5%, lispro: 19.9%, P = .088). It is important to remark that in all these clinical studies there was no explicit adaptation of the AID systems or their initialization to the studied insulin analogues.
The idea of adjusting the control strategy when switching to faster analogues was explored by our group in a previous work, 15 where the controller’s aggressiveness was adapted to changes in the insulin PK profile. Similarly, in the work of Lachal et al, 16 performance of the Diabeloop DBLG1 system was tested in an in-house virtual patient simulator with and without adaptation to the insulin type. In the work of Russell et al, 17 a single-center, single-blinded, crossover escalation trial was conducted with the iLet bionic pancreas and faster Aspart, observing improvements in glycemic control when the time to maximal insulin concentration setting was properly reduced.
The aim of the current study is to analyze how an AID system that is commercially available, and therefore not easily modifiable, could be tuned to maximize the glycemic benefits of using a faster analogue. The Control-IQ AID algorithm (Tandem Diabetes Care, San Diego, California) is selected for this purpose as it allows for the personalization of pump settings, including the carbohydrate-to-insulin ratio (CR in g/U), correction factor (CF in mg/dL/U), and basal rate (BR in U/h) profiles. The first step to exploring potential adaptations is to modify the SC insulin delivery model within the UVA/Padova simulation platform 18 to accurately reproduce PK/PD differences between lispro and URLi. 19 Once a reliable simulation platform is obtained, we perform a series of experiments to understand how each of those settings could be, if possible, adjusted to define the most aggressive setup without increasing the risk for hypoglycemia. Final settings are then evaluated under time-varying physiological and behavioral conditions that closely resemble real-world glycemic metrics obtained with the AID system under study.
Materials and Methods
SC Insulin Transport Model
To introduce URLi into the UVA/Padova simulation platform, mean glucose infusion rate (GIR) and mean (±SE, standard error) plasma insulin concentration (Ip) profiles were extracted from the work of Linnebjerg et al, 19 where euglycemic clamp studies using lispro and URLi in young and elderly (aged ≥65 years) adults with T1D are reported. Only profiles for the younger adult group (n = 41) were considered in this analysis since its age distribution (mean [range]: 32 [22-45] years) corresponds to the age distribution of the simulator’s in silico adult cohort (mean [range]: 34 [26-40] years). Clamp conditions were replicated in simulation considering the 100 virtual adults of the simulator: (1) glucose concentration was maintained at 100 ± 10 mg/dL, and insulin infusion was stopped prior to dosing; (2) each study participant received a 15-unit SC dose of the study drug; (3) a GIR controller with 100 mg/dL as target was initiated after glucose dropped by 5 mg/dL from baseline (a proportional-derivative controller was used in simulation), and (4) the process was terminated after 10 hours or if glucose >200 mg/dL, whichever happened earlier. Since parameters of the two-compartment PK model of SC insulin delivery were originally identified for lispro, 20 simulation data matched real data for that case, except for expressing a slightly shorter peak and slower tail decay that could have been easily corrected for this study population by slightly accelerating the insulin PK model (by model acceleration, we mean across-the-board scalar transformations of its parameters). However, when the model was further accelerated to match URLi profiles, an unsolvable waterbed effect was observed that prevented to reconstruct both insulin concentration peak and tail simultaneously (if peak errors were reduced, then tail errors got intrinsically larger, and vice versa). To solve this structural limitation, the diffusion term from nonmonomeric to monomeric state was expressed as a Michaelis-Menten saturation curve, updating the PK model presented in the work of Schiavon et al 20 as follows:
| (1) |
| (2) |
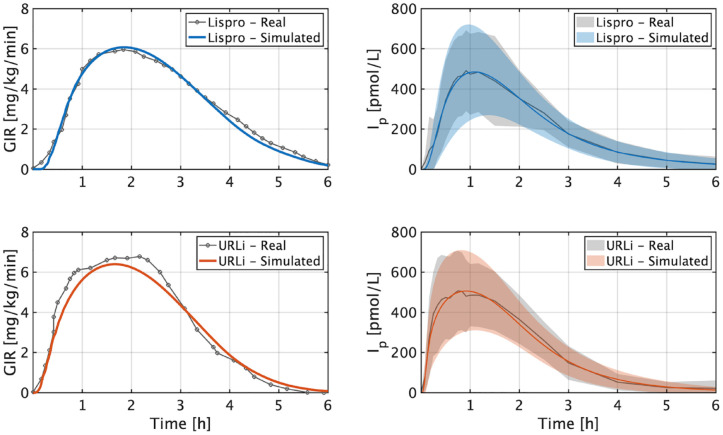
where states and are the amounts of nonmonomeric and monomeric insulin in pmol/kg, is the insulin infusion rate in pmol/kg/min delayed minutes, is the Michaelis constant in pmol/kg, and , , and are rate parameters in 1/min. Note that the insulin on board (IOB) [U] could be estimated as , where is the subject’s body weight in kg. The updated diffusion term limits the maximum inter-compartment flux to , enabling a fast onset of insulin concentration, deceleration to limit and widen the peak, and later acceleration to match the tail decay. Rate parameters, transport delay, and Michaelis constant were modulated to accommodate this new model structure to lispro data and later relatively accelerated to account for URLi’s faster response ( , , , and ). Results from this clamp analysis are presented in Figure 1, where real and simulated GIR and Ip curves are compared. As shown, mean responses and inter-subject variability are well captured in simulation for both lispro and URLi (note that only mean GIR values can be compared from data reported in the work of Linnebjerg et al 19 ).
Figure 1.
Comparison between real and simulated GIR (left) and Ip (right) for lispro (top) and URLi (bottom). The lines are the mean values, and the boundaries of the filled areas represent standard deviation. Real data were extracted from the work of Linnebjerg et al. 19 Abbreviations: GIR, glucose infusion rate; URLi, ultra-rapid lispro.
CR Experiment
Rejecting/mitigating a meal (or any positive disturbance) is the best scenario to leverage a faster insulin analogue. Let us assume that there is a positive driving force, eg, glucose rate of appearance due to a meal, that is pushing up glucose levels. From a control systems viewpoint, an insulin analogue with faster-on and faster-off properties can enable a more aggressive therapy response to further reduce postprandial glucose values without compromising the original risk for hypoglycemia. In this scenario, CR becomes the ideal insulin therapy parameter to be adjusted in a hybrid approach.
All 100 in silico adults of the UVA/Padova simulator were stabilized at 110 mg/dL (operating point). Two hours afterward, each subject received a meal of 0.8 g/kg of carbohydrates assuming time-invariant parameters and open-loop conditions. The reason for not conducting a closed-loop experiment is to isolate the effect of CR on mitigating the meal-related glucose excursion that otherwise could be also compensated by basal adjustments and/or automatic correction doses. For each subject, an insulin bolus delivered at mealtime was initially set to 0.05 U/kg for each analogue (lispro and URLi), and then progressively adjusted by means of an integral controller as follows:
| (3) |
where = {lispro, URLi} represents the study drug, is the iteration number, is the integral gain, and is the error signal with being the minimum glucose value and , the reference. The iteration process ceased when . It is important to clarify that is an arbitrary design choice and that therefore a different reference value could have been defined instead. The only requirement is that , ie, the setpoint should be lower than the operating point for the meal to be covered mainly by MB instead of background insulin. In this way, this method allows to determine how much the meal dose MB can be adjusted (potentially increased) for the faster analogue, URLi, guaranteeing that each subject’s hypoglycemia risk will remain identical between both analogues.
BR Experiment
Control-IQ operates around a user-defined BR profile, modulating insulin infusion above or below this reference to respond to predicted deviations from a predetermined (and time varying) glucose reference. Ideally, the BR profile is used to define an operating point, ie, the BR that is needed to maintain glucose flat at a certain value in absence of any disturbance. It is well known that such condition is virtually impossible to observe in real life, and the existence of a steady state is at least called in question in T1D. 21 It can also be assumed insulin acceleration does not affect bioavailability, ie, if units per hour (U/h) of lispro maintain the glucose level at mg/dL, then U/h of URLi will do the same work. Despite this theoretical discussion, increasing the BR profile will effectively make the AID controller deliver more insulin and a faster insulin analogue may offer a safety net to do so. However, it is important to remind that the controller will not respond faster and/or more aggressively but will just work on a theoretical lower operating point. In the following experiment, it is assumed that the BR profile has been already optimized to focus differences on the analogue properties instead of on therapy optimization, which is out of the scope of this work.
This experiment consisted of one-day simulations with three meals (0.8 g/kg at 7 am, 0.6 g/kg at 1 pm, and 1.0 g/kg at 7 pm) treated with an analogue-appropriate meal bolus (see prior section), considering an operating point of 110 mg/dL and a factor γ that multiplies the BR profile. The experiment was repeated for different values of γ ranging from 1 to 1.25 in 0.025 increments, and glucose metrics (from 6 am to 12 am) were computed to compare URLi with to lispro with γ = 1. An optimal γ was defined as the maximum factor for which the time <70 mg/dL (TBR) obtained with URLi and remains lower or equal than the TBR obtained with lispro and γ = 1. Our focus on daytime outcomes is explained by the differential effect of BR changes between daytime and overnight: while an increased BR profile can be smoothly attenuated in absence of disturbances, it can also occur concurrently to postprandial basal corrections (from the feedback control), potentially leading to late hypoglycemia. As URLi allows γ to be increased in both fasting and postprandial states but with different magnitudes, we decided to be conservative and focus this analysis on the daytime interval, so that, both fasting TBR and postprandial TBR remain below the lispro levels.
CF Experiment
Correction factor is typically defined as the glucose drop in mg/dL per unit of insulin. In our selected AID controller, it is also used to deliver automatic correction doses in case of prevailing hyperglycemia, to add a correction dose to a meal bolus when needed or to compute a manual correction, and to determine positive micro-corrections on the BR profile that would be needed to bring glucose back to target. While the first two use the user-defined CF value directly, the BR modulation uses a saturated CF (active CF). This means that the effect of adjusting CF will propagate across multiple modules differently. Let us discuss possible situations.
Bolus calculator
When CF is used to compute a correction dose that is added to a meal bolus, having a more aggressive CF for URLi could be beneficial, because, as discussed before, there is a positive driving force that is pushing up glucose levels.
BR modulation
Since an active CF is used instead of CF, it is more difficult to assess the actual impact of CF adjustments on BR modulation. However, if the system tries to compensate for a BR profile that is rather conservative, it can be assumed that having a more aggressive CF for URLi could be beneficial under fasting conditions.
Autocorrection bolus
Decreasing CF does not necessarily lead to bigger automatic corrections. The reason is that the estimated IOB is subtracted from the proposed correction dose. This means that a lower CF could fire an automatic dose earlier but not necessarily bigger, because it will depend on how the estimated IOB is changing over time. More importantly, an U correction dose with a faster insulin analogue under steady-state conditions will lead to a bigger glucose drop. The experiment that follows explores this case.
All in silico subjects were stabilized at various glucose concentrations ranging from 100 to 180 mg/dL in 10 mg/dL increments. At hour, a manual correction dose was administered to each subject under open-loop conditions using lispro, and five minutes afterward Control-IQ was activated. The initial correction dose was defined as , and then iteratively adjusted using an integral controller:
| (4) |
where is the iteration number, is the integral gain, and e = min(G) – r is the error signal with r min(G) being the minimum glucose value and r = 70 mg/dL, the reference. Once this process was completed, the estimated correction doses for lispro were tested using URLi, and differences in min (G) were computed for each operating point. As in the CR analysis, the reference value was an arbitrary design choice. In fact, any other reference value lower than the minimum operating point (100 mg/dL) could have been used instead without loss of generality. In this case, reference was set to 70 mg/dL so any negative glucose deviation observed when comparing URLi with lispro can have a direct interpretation as increased risk for hypoglycemia.
Experiment Mimicking Real-Life Conditions
A final evaluation of the proposed therapy parameter changes was conducted on the in silico adult cohort of the UVA/Padova simulator in 14-day simulations encompassing physiological variations and behavioral uncertainty that mimic observed real-life variability. To introduce changes in insulin sensitivity (IS), nominal IS parameters were multiplied daily by a factor , and allowed to fluctuate during the day within a certain band as described in the work of Visentin et al. 22 Each in silico subject received three main meals and three snacks per day. Meal sizes were based on the subject’s body weight, and mealtimes were drawn from uniform distributions with two- and three-hour widths for snacks and main meals, respectively. Hypoglycemia treatments were administered when the glucose level dropped below 70 mg/dL. Carb-counting errors up to 30%, bolus-meal misalignments from −15 to 60 minutes, and chances of unannounced snacks were also considered.
Data Presentation
Simulation results were processed and analyzed in MATLAB R2021b (MathWorks, Natick, Massachusetts). Performance metrics include average glucose concentration (mg/dL), coefficient of variation (%), percentages of time spent below 54 mg/dL, below 70 mg/dL, between 70 and 140 mg/dL, between 70 and 180 mg/dL, above 180 mg/dL, and above 250 mg/dL, total daily insulin (TDI) in U (basal, prandial, and correction doses), and number of hypo-treatments. Unless otherwise stated, across-subject mean or mean ± standard deviation values are reported. Comparison between different therapy adjustments were performed using paired t-tests with significance level at .001.
Results
CR Experiment
This clamp-like experiment is illustrated in Figure 2. Average results across all subjects indicate that can be 7.7% higher than without increasing the hypoglycemia risk. Considering that in this scenario , where M represents the grams of carbohydrates, it is estimated that CR could be safely decreased approximately 7% on average when switching from lispro to URLi.
Figure 2.
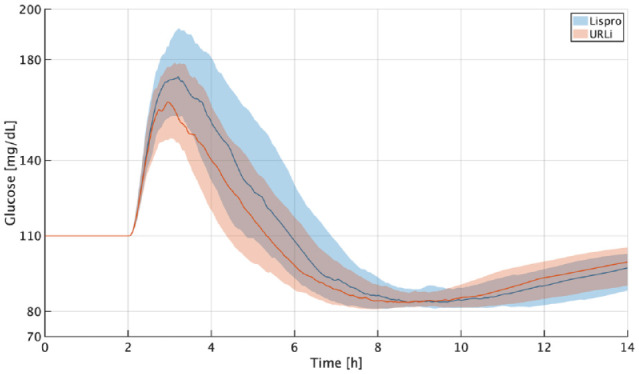
Results of clamp-based method to estimate MB for lispro (blue) and URLi (orange). Glucose responses for all the in silico adults. The thick lines are the median values, and the boundaries of the filled areas are the 25th and 75th percentiles.Abbreviations: MB, meal bolus; URLi, ultra-rapid lispro.
As mentioned in the “CR Experiment” section, parameter was an arbitrary design choice and the only requirement for it is that it should be lower than 110 mg/dL, the operating point. In fact, if this procedure is repeated but with , it is obtained that CR could be decreased 6.65% when switching from lispro to URLi, which, in practical terms, represents virtually the same change as the approximately 7% decrease reported above.
BR Experiment
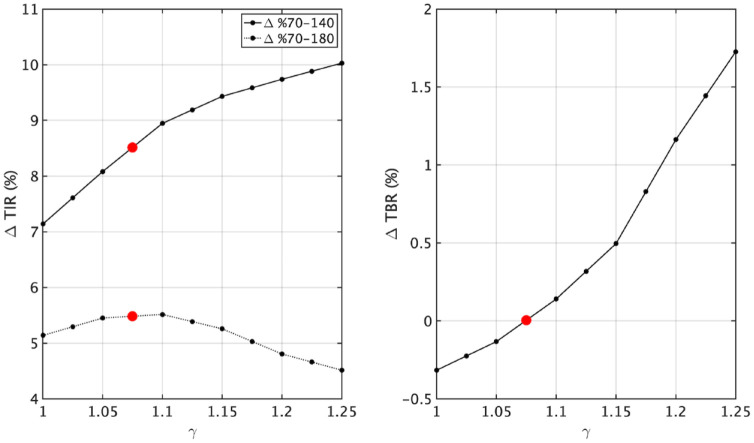
This experiment was conducted with the optimal CR values obtained above. Results are illustrated in Figure 3, where it is shown that the desired condition (identical risk for hypoglycemia) is reached for γ = 1.075, or in other words, when the BR profile is increased 7.5%. In this way, proper modulation of the BR profile allows to further tighten glucose control (Δ%70–140: +7.1% to +8.5%; Δ%70–180: +5.1% to +5.5%) without compromising the risk for hypoglycemia obtained using lispro with original settings (γ = 1).
Figure 3.
Estimation of basal profile factor γ for URLi. Differences in time in range (ΔTIR, left) and below range (ΔTBR, right) obtained using URLi with γ ≥ 1 and lispro with γ = 1. Red dots indicate the optimal case. Abbreviations: URLi, ultra-rapid lispro; TIR, time in range; TBR, time below range.
CF Experiment
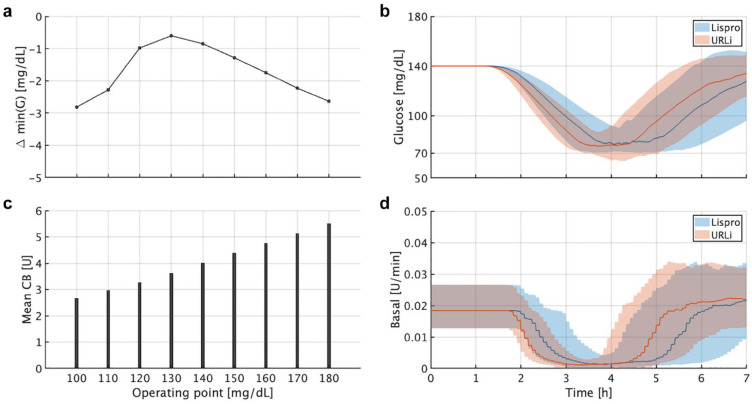
Results are illustrated in Figure 4. Note that the average Δmin(G) across subjects was relatively small but lower than 0 for all study cases. This means that although the system was able to safely handle the more pronounced glucose drop with URLi due in part to its faster response, decreasing CF might be risky since Δmin(G) is already slightly negative and there is no margin left. Based on this and the previous analysis, it is understood that altering CF for a faster analogue using Control-IQ can impact on multiple functionalities of the controller and does not lead to definite conclusions. While reducing CF can be beneficial, for instance, when it is used to add a correction dose to a meal bolus, it can also be counter-productive when it is used to compute a correction dose under other circumstances. Therefore, no CF adjustments will be proposed when switching from lispro to URLi.
Figure 4.
Clamp-like experiment for the correction factor analysis. (a) Mean Δmin(G) between URLi and lispro. (b) Mean values for each operating point. (c) Average glucose responses for all in silico subjects stabilized at 140 mg/dL. (d) Average commanded basal rate for all in silico subjects stabilized at 140 mg/dL. (c and d) The thick lines are the median values, and the boundaries of the filled areas are the 25th and 75th percentiles. Abbreviations: URLi, ultra-rapid lispro; CB, correction bolus.
Experiment Mimicking Real-Life Conditions
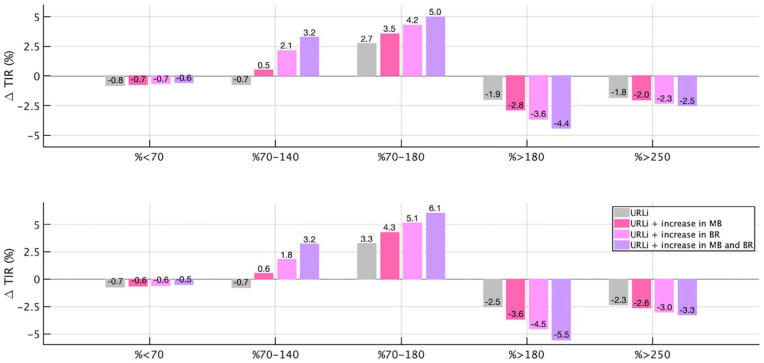
Six different setups using Control-IQ were evaluated for comparison purposes: lispro + original therapy parameters (baseline), URLi + original therapy parameters, URLi + decrease in CR (or increase in MB), URLi + increase in BR, URLi + increase in MB and BR, and lispro + CR and BR altered to match the TIR obtained in the preceding case. Numerical results are tabulated in Table 1, and a bar chart comparing relative changes in main glycemic metrics between gradual therapy adjustments using URLi and baseline is illustrated in Figure 5.
Table 1.
Comparison Between Average Performance Metrics With Control-IQ for Each Study Case.
| Overall | Lispro | URLi | URLi ↑ MB |
URLi ↑ BR |
URLi ↑ MB, BR | Lispro ↑ MB, BR |
|---|---|---|---|---|---|---|
| Average glucose (mg/dL) | 157.0 ± 8.6 | 156.1 ± 8.3 | 154.8 ± 8.5 | 153.2 ± 8.3 | 152.0 ± 8.5 | 146.2 ± 8.8 |
| Coefficient of variation (%) | 31.6 ± 6.4 | 28.4 ± 6.0 | 28.5 ± 6.0 | 28.6 ± 6.0 | 28.7 ± 5.9 | 32.3 ± 6.1 |
| % time <54 mg/dL | 0.5 ± 0.6 | 0.2 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.9 ± 0.9 |
| % time <70 mg/dL | 1.8 ± 1.5 | 1.0 ± 1.0 | 1.1 ± 1.1 | 1.1 ± 1.2 | 1.2 ± 1.2 | 2.9 ± 2.0 |
| % time in 70 to 140 mg/dL | 41.9 ± 6.9 | 41.2 ± 7.6 | 42.4 ± 7.6 | 44.0 ± 7.4 | 45.1 ± 7.5 | 50.2 ± 7.9 |
| % time in 70 to 180 mg/dL | 70.2 ± 8.0 | 72.9 ± 7.9 | 73.7 ± 8.0 | 74.4 ± 8.2 | 75.2 ± 8.2 | 75.2 ± 8.6 |
| % time >180 mg/dL | 28.0 ± 7.2 | 26.1 ± 7.5 | 25.2 ± 7.6 | 24.4 ± 7.7 | 23.6 ± 7.8 | 22.0 ± 7.5 |
| % time >250 mg/dL | 5.9 ± 5.5 | 4.1 ± 4.8 | 3.9 ± 4.7 | 3.6 ± 4.6 | 3.4 ± 4.4 | 3.7 ± 4.3 |
| Total daily insulin (U) | 46.0 ± 14.9 | 45.5 ± 14.7 | 45.9 ± 14.9 | 46.2 ± 15.0 | 46.5 ± 15.1 | 48.9 ± 15.9 |
| Total daily basal (U) | 23.3 ± 7.3 | 23.6 ± 7.4 | 23.5 ± 7.3 | 24.8 ± 7.7 | 24.6 ± 7.6 | 25.6 ± 8.0 |
| Total daily meal bolus (U) | 16.0 ± 6.4 | 16.0 ± 6.3 | 16.9 ± 6.7 | 15.9 ± 6.4 | 16.8 ± 6.7 | 18.4 ± 7.4 |
| Total daily corrections (U) | 6.8 ± 3.7 | 5.8 ± 3.4 | 5.5 ± 3.3 | 5.5 ± 3.3 | 5.2 ± 3.2 | 4.9 ± 3.2 |
| Total # hypo-treatments | 11.0 ± 10.0 | 6.0 ± 6.0 | 7.0 ± 7.0 | 7.0 ± 7.0 | 8.0 ± 7.0 | 18.0 ± 13.0 |
| 6 am to 12 am | Lispro | URLi | URLi ↑ MB |
URLi ↑ BR |
URLi ↑ MB, BR | Lispro ↑ MB, BR |
| Average glucose (mg/dL) | 166.8 ± 10.8 | 164.8 ± 10.2 | 163.3 ± 10.5 | 161.9 ± 10.3 | 160.4 ± 10.5 | 154.9 ± 11.2 |
| Coefficient of variation (%) | 30.2 ± 5.9 | 27.3 ± 5.4 | 27.5 ± 5.4 | 27.5 ± 5.4 | 27.6 ± 5.4 | 31.1 ± 5.6 |
| % time <54 mg/dL | 0.5 ± 0.7 | 0.3 ± 0.4 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.9 ± 1.0 |
| % time <70 mg/dL | 1.8 ± 1.5 | 1.1 ± 1.1 | 1.2 ± 1.2 | 1.2 ± 1.2 | 1.3 ± 1.2 | 2.8 ± 2.0 |
| % time in 70 to 140 mg/dL | 32.3 ± 5.8 | 31.6 ± 5.8 | 32.9 ± 6.1 | 34.1 ± 6.2 | 35.5 ± 6.7 | 41.0 ± 8.2 |
| % time in 70 to 180 mg/dL | 62.4 ± 9.5 | 65.7 ± 9.8 | 66.7 ± 9.9 | 67.5 ± 10.1 | 68.5 ± 10.2 | 68.9 ± 10.4 |
| % time >180 mg/dL | 35.8 ± 8.9 | 33.3 ± 9.4 | 32.2 ± 9.5 | 31.3 ± 9.7 | 30.3 ± 9.8 | 28.3 ± 9.5 |
| % time >250 mg/dL | 7.8 ± 7.3 | 5.5 ± 6.3 | 5.2 ± 6.2 | 4.8 ± 6.0 | 4.5 ± 5.8 | 4.9 ± 5.7 |
| #Hypo-treatments | 8.0 ± 7.0 | 5.0 ± 5.0 | 5.0 ± 6.0 | 5.0 ± 6.0 | 6.0 ± 6.0 | 13.0 ± 9.0 |
Mean and standard deviation values are reported.
Abbreviations: URLi, ultra-rapid lispro; MB, meal bolus; BR, basal rate.
Figure 5.
Relative changes in overall (top) and diurnal (bottom) glycemic metrics for URLi with original therapy parameters, URLi + increase in MB (↑ MB), URLi + increase in BR (↑ BR), and URLi + increase in both MB and BR (↑ MB, BR) with respect to lispro with original therapy parameters. Abbreviations: URLi, ultra-rapid lispro; MB, meal bolus; BR, basal rate; TIR, time in range; TBR, time below range.
Baseline metrics (lispro + original settings) closely match glycemic outcomes reported in the work of Brown et al 23 with average glucose: 157 vs 156 mg/dL, percentage of time in 70 to 180 mg/dL: 70.2% vs 71.0%, and percentage of time <70 mg/dL: 1.8% vs 1.6%. Note that setting adjustments using URLi lead to cumulative improvements in TIR (no adjustment 72.9%, ↑ MB 73.7%, ↑ BR 74.4%, and ↑ MB, BR 75.2%), maintaining TBR below the baseline level (ΔTBR %). As indicated in Table 1, trying to match the TIR obtained with URLi by adjusting CR and BR settings using lispro (URLi ↑ MB, BR: 75.2% vs lispro ↑ MB, BR: 75.2%, P = .89) comes in tandem with a marked increase in risk for hypoglycemia (TBR: 1.2 to 2.9%, P < .001).
Discussion
Most of previous works where new faster insulins were combined with AID systems only showed small improvements in glucose control with respect to standard analogues.10,11,14 The hypothesis of the current work is that pump settings need to be adjusted to fully leverage the benefits of faster insulin PK profiles, considering how insulin dynamics can impose fundamental limitations in designing or tuning an AID system. When switching to a faster analogue, it is assumed that the more control adaptability and less user intervention, the better glycemia-wise. Therefore, from a control design standpoint, using a commercial hybrid AID system is somehow limiting, since the core algorithm should remain unaltered, leaving only room for changes to user-defined profiles (CR, CF, and BR) that the controller uses to command insulin. For instance, in the work of Colmegna et al, 15 it is shown that having the freedom to change the cost function of an model predictive control (MPC)-based AID allows to match glycemic performances between hybrid and fully automated approaches when insulin is accelerated. To the best of the authors’ knowledge, the only previous works where the control strategy was explicitly adapted to the new insulin type were presented in the works of Lachal et al 16 and Russell et al, 17 showing that adaptation increases differences in favor of the faster analogue.
In this work, we conducted a series of experiments aimed at understanding how to adjust user-defined settings of Control-IQ when switching to a faster insulin analogue without compromising user safety. It was adjusting CR and BR profiles to make therapy more aggressive using URLi that allowed for safe improvements in glycemic control. Changing the CF profile, however, did not seem to improve performances.
Results from simulations under multiple meals and variability in both IS and subject behavior revealed that if insulin therapy profiles are properly adjusted when switching from lispro to URLi, positive cumulative effects lead to clinically significant changes in glucose control (ΔTIR ≥5%) 24 without compromising the original risk for hypoglycemia. Of note, trying to match performance using lispro leads to an unavoidable increase in risk for hypoglycemia due to phenomenological constraints.
It is also noteworthy that not only TBR is less with URLi than with lispro, but fewer hypo-treatments are needed per subject (lispro: 11, URLi ↑ MB, BR: 8, P < .001), leading to a two-fold benefit for glucose control and user burden. In terms of insulin use, changes in TDI are not proportional to changes in therapy settings (about 1 U increase when BR and MB changes are combined using URLi). One reason could be that fewer automatic corrections are needed when therapy parameters are more aggressive (see total daily corrections), another one that increasing BR x% does not mean that the controller will command x% more basal insulin since adjustments are regulated via feedback control, and finally, meal insulin doses will also depend on the estimated IOB. This indicates that adjusting therapy settings under these conditions should not be considered as simply increasing or decreasing insulin delivery overall but as rebalancing it, providing the ability for the controller to be more aggressive when required, even when the control law remains unchanged. Ideally, the IOB curve used by the pump’s bolus calculator should be updated when switching among analogues to account for changes in SC kinetics. If mean IOB curves are computed from Equations (1) and (2) as described in the “SC Insulin Transport Model” section, average settling times of approximately 5.5 and 4 hours are obtained for lispro and URLi, respectively. The effect of accelerating the IOB curve when switching to URLi was not tested in this work, because when the Control-IQ technology is enabled on a t: slim X2 insulin pump, a five-hour IOB curve is selected and cannot be changed.
It is important to acknowledge that achievable glucose control with a hybrid AID system like Control-IQ will depend on the subject’s meal bolus behavior. For example, suboptimal glycemic control arises when carbs are underestimated and the insulin dose is given earlier than or at mealtime, or when carbs are overestimated, and the insulin dose is delayed. On the other hand, although delaying the insulin dose will increase hypoglycemia for both analogues compared with an on-time bolus, URLi will offer additional protection due to its faster absorption and action even when the therapy strategy is more aggressive than for lispro.
The main limitation of this study is the current lack of clinical validation. Safety and efficacy of the proposed strategy will be evaluated in an upcoming randomized controlled trial in 20 Control-IQ users during two weeks under normal conditions at home. Another limitation is the lack of direct access to URLi data that led us to extract mean GIR and mean (±SE) plasma insulin concentration-time profiles from the work of Linnebjerg et al. 19
Conclusion
A methodological procedure driven by a series of experiments was followed to propose therapy parameter adjustments in a commercial AID system to safely increase TIR when switching from lispro to URLi. As discussed throughout this article, despite potentially large inter-subject variability, faster insulin analogues offer opportunities for more aggressive therapy strategies that should be leveraged by the AID system to maximize glycemic control benefits.
Footnotes
Abbreviations: AID, automated insulin delivery; BR, basal rate; CF, correction factor; CR, carbohydrate-to-insulin ratio; GIR, glucose infusion rate; IOB, insulin on board; IS, insulin sensitivity; PD, pharmacodynamic; PK, pharmacokinetic; SC, subcutaneous; T1D, type 1 diabetes; TBR, time below range; TDI, total daily insulin; TIR, time in range; URLi, ultra-rapid lispro.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: PC, JD, and JG receive research support and royalties from Dexcom handled by the University of Virginia’s Licensing and Ventures Group. MDD has received research support from Tandem, Dexcom, and Medtronic. MDB consults for Roche Diagnostics, Dexcom, Adocia, and Arecor; receives research support from Dexcom, Tandem, and Novo Nordisk.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the UVA LaunchPad for Diabetes Program.
ORCID iDs: Patricio Colmegna  https://orcid.org/0000-0001-9074-8634
https://orcid.org/0000-0001-9074-8634
Jose Garcia-Tirado  https://orcid.org/0000-0002-9970-2162
https://orcid.org/0000-0002-9970-2162
Marc D. Breton  https://orcid.org/0000-0001-7645-2693
https://orcid.org/0000-0001-7645-2693
References
- 1. Seron MM, Braslavsky JH, Goodwin GC. Fundamental Limitations in Filtering and Control. London, England: Springer; 1997. doi: 10.1007/978-1-4471-0965-5. [DOI] [Google Scholar]
- 2. Astrom KJ, Klein RE, Lennartsson A. Bicycle dynamics and control: adapted bicycles for education and research. IEEE Control Syst. 2005;25(4):26-47. doi: 10.1109/MCS.2005.1499389. [DOI] [Google Scholar]
- 3. Richard JP. Time-delay systems: an overview of some recent advances and open problems. Automatica. 2003;39(10):1667-1694. doi: 10.1016/S0005-1098(03)00167-5. [DOI] [Google Scholar]
- 4. Wong EY, Kroon L. Ultra-rapid-acting insulins: how fast is really needed. Clin Diabetes. 2021;39(4):415-423. doi: 10.2337/cd20-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirsch IB, Juneja R, Beals JM, et al. The evolution of insulin and how it informs therapy and treatment choices. Endocrine Reviews. 2020;41(5):733-755. doi: 10.1210/ENDREV/BNAA015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haahr H, Heise T. Fast-acting insulin aspart: a review of its pharmacokinetic and pharmacodynamic properties and the clinical consequences. Clin Pharmacokinet. 2020;59(2):155-172. doi: 10.1007/s40262-019-00834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fath M, Danne T, Biester T, Erichsen L, Kordonouri O, Haahr H. Faster-acting insulin aspart provides faster onset and greater early exposure vs insulin aspart in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18(8):903-910. doi: 10.1111/pedi.12506. [DOI] [PubMed] [Google Scholar]
- 8. Leohr J, Kazda C, Liu R, et al. Ultra rapid lispro (URLi) shows faster pharmacokinetics and reduces postprandial glucose excursions versus Humalog® in patients with type 2 diabetes mellitus in a randomized, controlled crossover meal test early phase study. Diabetes Obes Metab. 2022;24(2):187-195. doi: 10.1111/DOM.14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heise T, Linnebjerg H, Coutant D, et al. Ultra rapid lispro lowers postprandial glucose and more closely matches normal physiological glucose response compared to other rapid insulin analogues: a phase 1 randomized, crossover study. Diabetes Obes Metab. 2020;22(10):1789-1798. doi: 10.1111/dom.14094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boughton CK. Fully closed-loop insulin delivery—are we nearly there yet? Lancet Digit Health. 2021;3(11):e689-e690. doi: 10.1016/S2589-7500(21)00218-1. [DOI] [PubMed] [Google Scholar]
- 11. Boughton CK, Hartnell S, Thabit H, et al. Hybrid closed-loop glucose control with faster insulin aspart compared with standard insulin aspart in adults with type 1 diabetes: a double-blind, multicentre, multinational, randomized, crossover study. Diabetes Obes Metab. 2021;23(6):1389-1396. doi: 10.1111/dom.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu L, Buckingham B, Basina M, et al. Fast-acting insulin aspart use with the MiniMedTM 670G system. Diabetes Technol Ther. 2021;23(1):1-7. doi: 10.1089/dia.2020.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozer K, Cooper AM, Ahn LP, Waggonner CR, Blevins TC. Fast acting insulin aspart compared with insulin aspart in the Medtronic 670G hybrid closed loop system in type 1 diabetes: an open label crossover study. Diabetes Technol Ther. 2021;23(4):286-292. doi: 10.1089/dia.2020.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bode B, Carlson A, Liu R, et al. Ultrarapid lispro demonstrates similar time in target range to lispro with a hybrid closed-loop system. Diabetes Technol Ther. 2021;23(12):828-836. doi: 10.1089/dia.2021.0184. [DOI] [PubMed] [Google Scholar]
- 15. Colmegna P, Cengiz E, Garcia-Tirado J, Kraemer K, Breton MD. Impact of accelerating insulin on an artificial pancreas system without meal announcement: an in silico examination. J Diabetes Sci Technol. 2020;15(4):833-841. doi: 10.1177/1932296820928067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lachal S, Tourki Y, Franc S, Huneker E, Charpentier G, Benhamou PY. Hybrid closed-loop control with ultrarapid lispro compared with standard insulin aspart and faster insulin aspart: an in silico study [published online ahead of print October 17, 2021]. J Diabetes Sci Technol. doi: 10.1177/19322968211046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Russell SJ, Balliro C, Ekelund M, et al. Improvements in glycemic control achieved by altering the tmax setting in the iLet® bionic pancreas when using fast-acting insulin aspart: a randomized trial. Diabetes Ther. 2021;12(7):2019-2033. doi: 10.1007/S13300-021-01087-X/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Visentin R, Campos-Náñez E, Schiavon M, et al. The UVA/Padova Type 1 Diabetes Simulator goes from single meal to single day. J Diabetes Sci Technol. 2018;12(2):273-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Linnebjerg H, Zhang Q, LaBell E, et al. Pharmacokinetics and glucodynamics of ultra rapid lispro (URLi) versus Humalog® (lispro) in younger adults and elderly patients with type 1 diabetes mellitus: a randomised controlled trial. Clin Pharmacokinet. 2020;59(12):1589-1599. doi: 10.1007/S40262-020-00903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schiavon M, Dalla Man C, Cobelli C. Modeling subcutaneous absorption of fast-acting insulin in type 1 diabetes. IEEE Trans Biomed Eng. 2018;65(9):2079-2086. [DOI] [PubMed] [Google Scholar]
- 21. Kovatchev B. Glycemic variability: risk factors, assessment, and control. J Diabetes Sci Technol. 2019;13(4):627-635. doi: 10.1177/1932296819826111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Visentin R, Dalla Man C, Kudva YC, Basu A, Cobelli C. Circadian variability of insulin sensitivity: physiological input for in silico artificial pancreas. Diabetes Technol Ther. 2015;17(1):1-7. doi: 10.1089/dia.2014.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381(18):1707-1717. doi: 10.1056/NEJMOA1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]