Abstract
Background:
ALERTT1 showed that switching from intermittently scanned continuous glucose monitoring (isCGM) without alerts to real-time CGM (rtCGM) with alert functionality improved time in range (TIR; 70-180 mg/dL), glycated hemoglobin (HbA1c), time <54 mg/dL, and Hypoglycemia Fear Survey version II worry subscale (HFS-worry) score after six months in adults with type 1 diabetes (T1D). Moderator analyses aimed to identify certain subgroups that would benefit more from switching to rtCGM than others.
Methods:
Post hoc analyses of ALERTT1 evaluated the impact of 14 baseline characteristics on the difference (delta) in mean TIR, HbA1c, time <54 mg/dL, and HFS-worry score at six months between rtCGM and isCGM. Therefore, the delta was allowed to depend on each of these variables by including interactions in the moderator analysis model. Analyses were performed separately for each variable; variables with P < .10 in the univariable analysis were combined into a single model.
Results:
Univariable analyses showed no dependency of delta TIR, HbA1c, or time <54 mg/dL on variables other than CGM type. Only delta HFS-worry score depended on baseline HbA1c (P = .0059), indicating less worries with rtCGM in people with baseline HbA1c <6.5% or ≥8%. Given P < .10 for dependency of delta TIR on insulin therapy type (favoring multiple daily injections), baseline HbA1c, and baseline TIR, these variables were combined into a multivariable analysis; interactions were not statistically significant.
Conclusions:
Except for HFS-worry score, no interactions between 14 baseline characteristics and the six-month intervention effect of rtCGM on TIR, HbA1c, or time <54 mg/dL were observed, supporting the conclusion of ALERTT1 that switching from isCGM without alerts to rtCGM with alert functionality is beneficial for a wide range of people with T1D.
Keywords: continuous glucose monitoring, intermittently scanned continuous glucose monitoring, moderator analysis, real-time continuous glucose monitoring, type 1 diabetes
Introduction
The use of on-demand continuous glucose monitoring (intermittently scanned continuous glucose monitoring [isCGM]) and in real time (real-time continuous glucose monitoring [rtCGM]) is increasingly accepted in the treatment of people with type 1 diabetes (T1D), given the individual benefit of isCGM and rtCGM on glycemic control and quality of life.1-6 In addition, our previously published ALERTT1 trial showed that switching from isCGM without alerts to rtCGM with alert functionality resulted in an improvement in time in range (TIR; sensor-glucose 70-180 mg/dL) at six months in 254 adults with T1D. 7 This improvement was accompanied by a decrease in glycated hemoglobin (HbA1c) and time <54 mg/dL, and people experienced less hypoglycemia worry with the use of rtCGM.
However, in clinical practice, it is observed that the success of CGM varies from person to person. On the one hand, this may be explained by inter-individual differences in user skills and knowledge of CGM. 8 In addition, there are studies suggesting that there are additional user characteristics associated with the effectiveness of CGM on glycemic control, such as baseline HbA1c9-11 or frequency of CGM use.10-14 Because it is not known whether certain subgroups of people with T1D benefit more from switching to rtCGM with alert functionality than others, we performed a post hoc analysis of ALERTT1 to identify variables associated with the success of switching to rtCGM with alerts, which may be essential from a personalized medicine perspective, as well as from a health economics and scientific perspective.
Methods
Study Design and Participants
This post hoc analysis was based on data of the ALERTT1 trial (ClinicalTrials.gov number NCT03772600). 7 ALERTT1 was a six-month prospective, double arm, parallel-group, nonmasked randomized controlled trial comparing rtCGM with alert functionality (Dexcom G6®, Dexcom, San Diego, California; intervention group, n = 127) with isCGM without alerts (FreeStyle Libre 1®, Abbott Diabetes Care, Alameda, CA, USA; control group, n = 127) (Figure S1). From January 2019 to March 2020, ALERTT1 was conducted in the diabetes clinics of three regional and three university medical centers in Belgium, and included people aged 18 years or older with a diagnosis of T1D for six months or more. Additional inclusion criteria were treatment with multiple daily injections or insulin pump, HbA1c ≤10% (86 mmol/mol), and exclusive isCGM use (FreeStyle Libre 1®) for at least six months. Key exclusion criteria were (planned) pregnancy, severe cognitive impairment limiting CGM usage, use of systemic corticosteroids, or concomitant pathology that could cause edema at anticipated CGM insertion sites.
ALERTT1 was divided in a baseline phase of four to seven weeks and a study phase of six months. At different time points, HbA1c samples, CGM data, and (diabetes-related) quality-of-life questionnaires were collected and used for further analysis (Figure S1). Primary outcome was the mean between-group difference at six months in TIR. Key secondary outcomes were mean between-group differences at six months in HbA1c, time <54 mg/dL, and hypoglycemia worry evaluated with the Hypoglycemia Fear Survey version II worry subscale (HFS-worry). For more details on randomization and trial-related procedures, we refer to the original manuscript. 7
Outcomes
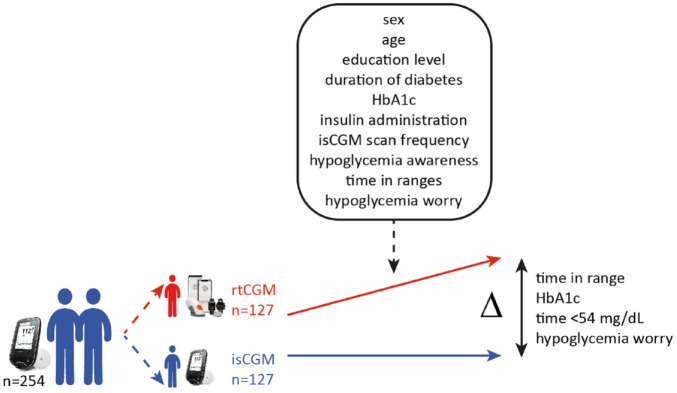
To identify subgroup characteristics associated with the benefits of switching from isCGM without alerts to rtCGM with alert functionality, we evaluated the impact of 14 different baseline user characteristics (so-called moderators) on the difference (delta) in mean TIR, HbA1c, time <54 mg/dL, and HFS-worry score at six months between rtCGM and isCGM (Figure 1). For this evaluation, the delta was allowed to depend on each of these 14 variables by including interactions in a model (moderator analysis).
Figure 1.
Schematic overview of moderator analyses of the ALERTT1 trial. This post hoc analysis of ALERTT1 evaluated the impact of different baseline user characteristics (so-called moderators, as indicated in the box) on the difference (delta [Δ]) in mean time in range, HbA1c, time <54 mg/dL, and hypoglycemia worry at six months between rtCGM and isCGM. For this evaluation, the delta was allowed to depend on each of these variables by including interactions in a model, the moderator analysis. Variables were binary or continuous and selected on the basis of previously published literature. Abbreviations: rtCGM, real-time continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; HbA1c, glycated hemoglobin.
The following variables were considered potential binary moderators: sex (male/female); education level (high [higher education]/low [primary/secondary education]); insulin therapy (multiple daily injections/insulin pump); hypoglycemia awareness reported by the Clarke Hypoglycemia Awareness Survey (aware/unaware). Variables (measured at baseline of ALERTT1) considered as potential continuous moderators were as follows: age (years); diabetes duration (years); HbA1c (%); isCGM scan frequency (scans/day); TIR (%); time <54 mg/dL (%); time <70 mg/dL (%); time >180 mg/dL (%); time >250 mg/dL (%); HFS-worry score (points). Table 1 shows the distribution of the 14 variables as measured at baseline of ALERTT1; baseline characteristics of both groups were similar. All variables were selected based on previous literature.9-15
Table 1.
Distribution of user characteristics (moderators) as measured at baseline of ALERTT1. 7
| Characteristic | rtCGM (n = 127) |
isCGM (n = 127) |
|---|---|---|
| Sex | ||
| Male | 81 (64%) | 76 (60%) |
| Female | 46 (36%) | 51 (40%) |
| Age (years) | 42.8 (19-76) | 43.0 (18-75) |
| Level of education a | ||
| Low | 40 (31%) | 43 (34%) |
| High | 87 (69%) | 84 (66%) |
| Duration of diabetes (years) | 20.2 (1-55) | 19.2 (1-55) |
| HbA1c (%) | 7.4 (5.5-9.8) | 7.4 (5.3-9.9) |
| HbA1c (mmol/mol) | 57.9 (37-84) | 57.6 (35-85) |
| Insulin therapy | ||
| Multiple daily injections | 103 (81%) | 102 (80%) |
| Insulin pump | 24 (19%) | 25 (20%) |
| isCGM scan frequency (number/day) | 11.9 (2-37) | 12.4 (2-42) |
| Participants with hypoglycemia unawareness b | 24 (19%) | 20 (16%) |
| Time in ranges (%) c | ||
| Time >250 mg/dL | 16.5 (0.1-60.4) | 18.3 (0.9-64.7) |
| Time >180 mg/dL | 44.2 (4.7-85.7) | 45.5 (9.2-93.2) |
| Time 70-180 mg/dL | 52.5 (13.1-87.5) | 51.3 (6.7-86.2) |
| Time <70 mg/dL | 3.3 (0.0-23.5) | 3.4 (0.0-19.9) |
| Time <54 mg/dL | 0.9 (0.0-11.2) | 1.0 (0.0-16.3) |
| HFS-worry score (points) | 18.8 (0-62) | 18.7 (0-71) |
Data are n (%) or mean (range). To convert glucose ranges from mg/dL to mmol/L, divide by 18.
Abbreviations: rtCGM, real-time continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; HFS-worry, Hypoglycemia Fear Survey version II worry subscale; HbA1c, glycated hemoglobin.
Low was defined as primary or secondary education; high was defined as higher education.
As reported by the Clarke Hypoglycemia Awareness Survey.
As measured by blinded rtCGM in both groups at baseline.
Moderator analyses were performed for each of these variables separately (univariable moderator analysis). Variables with P < .10 in the univariable analysis were combined into a single model (multivariable moderator analysis). For continuous variables, restricted cubic splines were used to allow a nonlinear relation between the continuous variable and the delta. For time <54 mg/dL, the distribution of the model residuals was right-skewed. Therefore, as a sensitivity analysis, results were additionally reported from a model after applying an inverse hyperbolic sine transformation (a log-transformation which can handle the presence of zero values). Variables that strongly correlated (ie, Spearman’s ρ >0.90) in the univariable model were not combined in the multivariable model. All analyses were performed using SAS software for Windows (version 9.4, SAS Institute, Cary, NC, USA).
Results
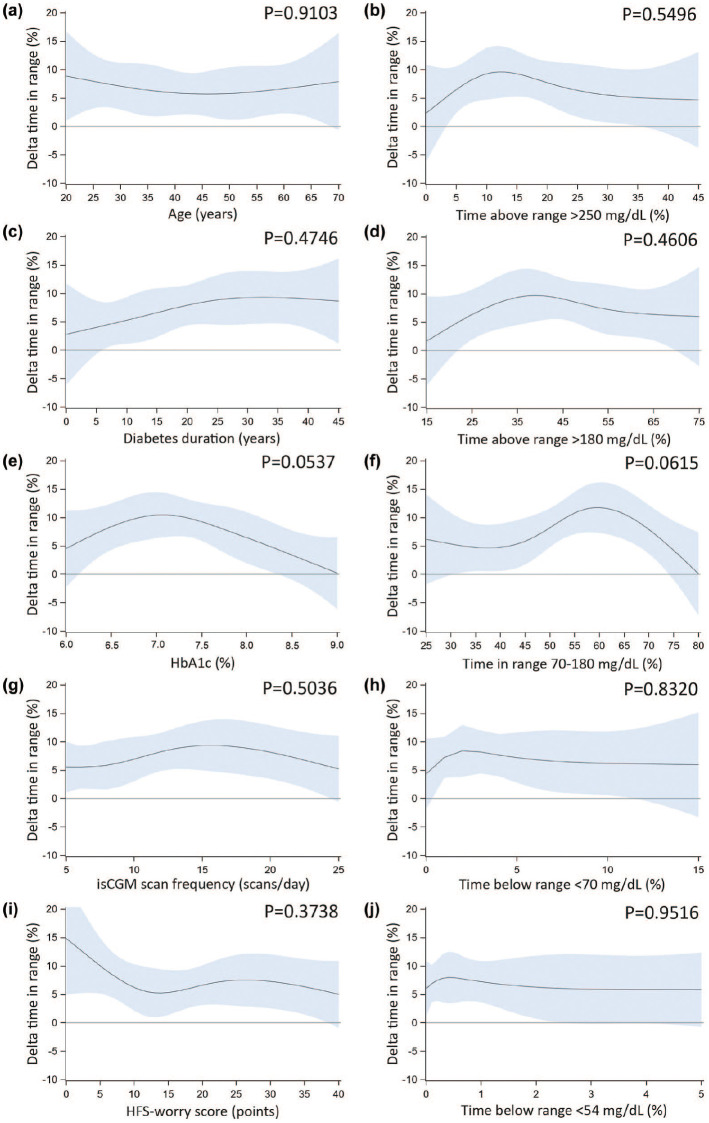
Univariable analyses showed no statistically significant dependency of delta TIR (Figure 2), HbA1c (Figure S2), or time <54 mg/dL (Figure S3) on variables other than CGM type, with sensitivity analyses confirming the finding for delta time <54 mg/dL. Only dependency of delta HFS-worry score on HbA1c was observed (P = .0059; Figure S4), indicating less worries with the use of rtCGM compared with isCGM in people with low (<6.5% [48 mmol/mol]) or high (≥8% [64 mmol/mol]) baseline HbA1c (Figure 3).
Figure 2.
Univariable moderator analyses with continuous moderators—time in range. Results from univariable moderator analyses, evaluating whether the difference in means of rtCGM versus isCGM at six months (delta) for time in range (sensor-glucose 70-180 mg/dL; plotted on the y-axis), depend on the level of different baseline variables (plotted on the x-axis): age (panel a); diabetes duration (panel c); HbA1c (panel e); isCGM scan frequency (panel g); HFS-worry score (panel i); and different time in ranges (panels b, d, f, h, j). Dark blue lines represent the predicted mean delta, blue shaded areas represent the 95% confidence interval. A flatter line indicates less interaction, while a more curved line indicates more interaction. For each considered moderator, the P value for interaction is reported in the graph. Note that only continuous moderators are reported here. To convert glucose ranges from mg/dL to mmol/L, divide by 18. To convert HbA1c from % to mmol/mol: (10.93 × [HbA1c %]) − 23.5 mmol/mol. Abbreviations: rtCGM, real-time continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; HFS-worry, Hypoglycemia Fear Survey version II worry subscale; HbA1c, glycated hemoglobin.
Figure 3.
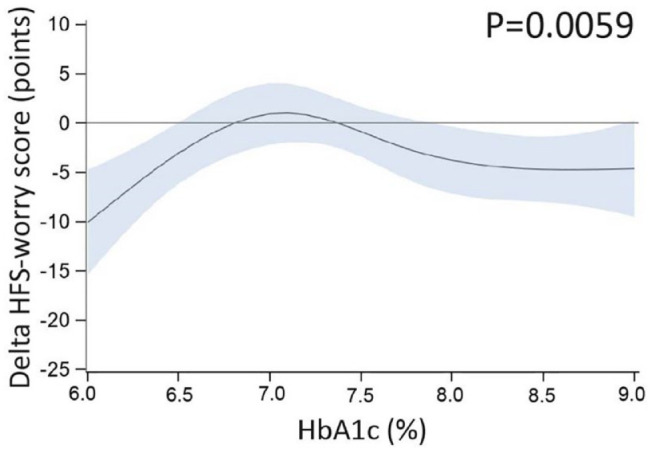
Univariable moderator analysis—HFS-worry score and baseline HbA1c. Result from a univariable moderator analysis, evaluating whether the difference in means of rtCGM versus isCGM at six months (delta) for HFS-worry score (plotted on the y-axis), depends on the level of baseline HbA1c (plotted on the x-axis). Dark blue lines represent the predicted mean delta, blue shaded areas represent the 95% confidence interval. A flatter line indicates less interaction, while a more curved line indicates more interaction. The P value for interaction is reported in the graph. Results show that there was dependency of delta HFS-worry score on HbA1c, indicating less worries with the use of rtCGM compared with isCGM in people with low (<6.5% [48 mmol/mol]) or high (≥8% [64 mmol/mol]) baseline HbA1c. To convert HbA1c from % to mmol/mol: (10.93 × [HbA1c %]) − 23.5 mmol/mol. Abbreviations: HFS-worry, Hypoglycemia Fear Survey version II worry subscale; rtCGM, real-time continuous glucose monitoring; isCGM, intermittently scanned continuous glucose monitoring; HbA1c, glycated hemoglobin.
Given P < .10 for the dependency of delta TIR on insulin therapy (P = .0851; favoring multiple daily injections [data not shown]), baseline HbA1c (P = .0537; Figure 2e), and baseline TIR (P = .0615; Figure 2f), these variables were combined into a multivariable model. None of the interactions were statistically significant.
Discussion
To our knowledge, this is the first study evaluating subgroup characteristics associated with the effect of switching from isCGM without alerts to rtCGM with alert functionality. We only observed a greater improvement in HFS-worry score in people with low or high baseline HbA1c. In contrast, we found no statistically significant interaction between 14 different variables and the six-month intervention effect of rtCGM on TIR, HbA1c, or time <54 mg/dL, supporting the conclusion of our original manuscript that the benefit of switching from isCGM without alerts to rtCGM with alert functionality applies to a wide range of people with T1D.
To date, only a few trials have been published on the association between user characteristics and the effectiveness of CGM on glycemic control. A previous prospective observational study showed that an HbA1c reduction of ≥0.5% (5 mmol/mol) after start of isCGM was associated with a higher baseline HbA1c, male gender, and early commencement of isCGM after introduction of reimbursement. 9 In contrast, higher socioeconomic deprivation and the collection of less than two sensors per month were associated with no response in HbA1c. 9 The finding of a greater isCGM benefit on glycemic control in people with higher HbA1c at baseline was also demonstrated in another trial. 10 Also, isCGM scan frequency is found to be inversely related to glycemic control.10,12-14 With regard to rtCGM, frequency of sensor use and higher baseline HbA1c are associated with a greater reduction in HbA1c. 11 A systematic review and meta-analysis evaluating the effects of CGM (both isCGM and rtCGM) on glycemic control concluded from a subgroup analysis that TIR improvement was independent of diabetes type, method of insulin administration, and reason for CGM use. 15 However, as these trials evaluated the impact of user characteristics on isCGM or rtCGM individually, it is difficult to compare these findings with our outcomes. In addition, there are a few caveats to be made about the current sub-analysis. Although statistically significant interactions were generally absent, we did observe some trends in the univariable analyses, pointing to a possible dependency of delta TIR on insulin administration method, baseline HbA1c, and baseline TIR. However, as the performed post hoc analysis was exploratory and not prespecified in the original study design, the original sample size calculation did not account for these moderator analyses, thus lacking statistical power. In addition, P values were not corrected for multiple testing, so all outcomes and possible trends need to be interpreted with caution. Therefore, further studies in a larger T1D population would be needed to confirm the findings of our current sub-analysis. But until then, we would advise clinicians to consider rtCGM with alert functionality instead of isCGM without alerts to improve the health and quality of life to a broad population of adults with T1D.
Conclusions
ALERTT1 showed that switching from isCGM without alerts to rtCGM with alert functionality improved TIR, HbA1c, time <54 mg/dL, and hypoglycemia worry after six months in adults with T1D. Post hoc analyses of ALERTT1 aimed to identify subgroups of people with T1D who would benefit more from switching to rtCGM than others. The impact of 14 baseline user characteristics on the difference in mean TIR, HbA1c, time <54 mg/dL, and hypoglycemia worry at six months between rtCGM and isCGM was evaluated by univariable and multivariable moderator analyses. Except for hypoglycemia worry, the difference in mean TIR, HbA1c, and time <54 mg/dL at six months did not depend on variables other than CGM type, supporting the conclusion of ALERTT1 that the benefit of switching from isCGM without alerts to rtCGM with alerts applies to a wide range of people with T1D.
Supplemental Material
Supplemental material, sj-doc-1-dst-10.1177_19322968221128315 for The Impact of Baseline User Characteristics on the Benefits of Real-Time Versus Intermittently Scanned Continuous Glucose Monitoring in Adults With Type 1 Diabetes: Moderator Analyses of the ALERTT1 Trial by Margaretha Martha Visser, Sara Charleer, Steffen Fieuws, Christophe De Block, Robert Hilbrands, Liesbeth Van Huffel, Toon Maes, Gerd Vanhaverbeke, Eveline Dirinck, Nele Myngheer, Chris Vercammen, Frank Nobels, Bart Keymeulen, Chantal Mathieu and Pieter Gillard in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968221128315 for The Impact of Baseline User Characteristics on the Benefits of Real-Time Versus Intermittently Scanned Continuous Glucose Monitoring in Adults With Type 1 Diabetes: Moderator Analyses of the ALERTT1 Trial by Margaretha Martha Visser, Sara Charleer, Steffen Fieuws, Christophe De Block, Robert Hilbrands, Liesbeth Van Huffel, Toon Maes, Gerd Vanhaverbeke, Eveline Dirinck, Nele Myngheer, Chris Vercammen, Frank Nobels, Bart Keymeulen, Chantal Mathieu and Pieter Gillard in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: HbA1c, glycated hemoglobin; HFS-worry, Hypoglycemia Fear Survey version II worry subscale; isCGM, intermittently scanned continuous glucose monitoring; rtCGM, real-time continuous glucose monitoring; TIR, time in range (sensor-glucose 70-180 mg/dL); T1D, type 1 diabetes.
Author Contributions: M.M.V. analyzed and discussed the data, wrote the manuscript, and made figures. S.F. performed statistical analyses, discussed the data, made figures, and edited the manuscript. P.G., C.M., and S.C. analyzed and discussed the data, and wrote the manuscript. C.D.B., R.H., L.V.H., T.M., G.V., E.D., N.M., C.V., F.N., and B.K. discussed the data, and edited the manuscript. The submitted version of this manuscript was approved by all authors. M.M.V. and P.G. are the guarantors of this work and, as such had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: UZ Leuven received nonfinancial support for travel from Novo Nordisk, and Boehringer-Ingelheim for M.M.V. M.M.V. serves or has served on the speakers bureau for Dexcom—financial compensation for these activities has been received by KU Leuven. KU Leuven received nonfinancial support for travel from Medtronic, and financial support for travel from Roche for S.C. C.D.B. reports consulting fees and honoraria for speaking for Abbott, AstraZeneca, Boehringer-Ingelheim, A. Menarini Diagnostics, Eli Lilly, Medtronic, Novo Nordisk, and Roche. R.H. serves or has served on the advisory panel for Merck Sharp and Dohme, Boehringer-Ingelheim, and Eli Lilly. L.V.H. reports consulting fees and honoraria for speaking for Abbott, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Medtronic, Merck Sharp and Dohme, Novo Nordisk, and Sanofi-Aventis. G.V. serves or has served on the advisory panel for Merck Sharp and Dohme, Boehringer-Ingelheim, and Eli Lilly. G.V. reports consulting fees and honoraria for speaking from Merck Sharp and Dohme, Boehringer-Ingelheim, AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Eli Lilly. E.D. has served on the advisory panel for Novo Nordisk. E.D. reports speaking fees from Novo Nordisk, Boehringer-Ingelheim, Eli Lilly, and AstraZeneca. N.M. serves or has served on the advisory panel for Boehringer-Ingelheim. N.M. reports speaking fees from Merck Sharp and Dohme, Boehringer-Ingelheim, AstraZeneca, Sanofi-Aventis, Novo Nordisk, and Eli Lilly. C.V. reports consulting and speaking fees from Medtronic, Boehringer-Ingelheim, AstraZeneca, and Sanofi-Aventis. F.N. reports consulting fees and honoraria for speaking from Abbott, AstraZeneca, Boehringer-Ingelheim, Eli Lilly, Johnson and Johnson, Medtronic, Merck Sharp and Dohme, Novo Nordisk, Roche, and Sanofi-Aventis. C.M. serves or has served on the advisory panel for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme, Eli Lilly, Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, and Zealand Pharma. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for C.M. from Medtronic, Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme, Eli Lilly, Roche, Abbott, ActoBio Therapeutics, and Novartis; C.M. serves or has served on the speakers bureau for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme, Eli Lilly, Boehringer-Ingelheim, AstraZeneca, and Novartis. Financial compensation for these activities has been received by KU Leuven. P.G. serves or has served on the advisory panel for Novo Nordisk, Sanofi-Aventis, Boehringer-Ingelheim, Janssen Pharmaceuticals, Roche, Medtronic, and Bayer. Financial compensation for these activities has been received by KU Leuven. P.G. serves or has served on the speakers bureau for Merck Sharp and Dohme, Boehringer-Ingelheim, Bayer, Medtronic, Insulet, Novo Nordisk, Abbott, Roche, and Dexcom. Financial compensation for these activities has been received by KU Leuven. KU Leuven received nonfinancial support for travel from Sanofi-Aventis, A. Menarini Diagnostics, Medtronic, and Roche for P.G. All disclosures were unrelated to the present work. S.F., T.M., and B.K. have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: University Hospitals Leuven acted as sponsor of the original randomized controlled ALERTT1 trial and received a research grant from Dexcom.
ORCID iDs: Sara Charleer  https://orcid.org/0000-0003-2100-4927
https://orcid.org/0000-0003-2100-4927
Pieter Gillard  https://orcid.org/0000-0001-9111-4561
https://orcid.org/0000-0001-9111-4561
Data Availability: This post hoc analysis is based on data from the original randomized controlled ALERTT1 trial. Please refer to the original manuscript for information on data availability.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, nonmasked, randomised controlled trial. Lancet. 2016;388: 2254-2263. [DOI] [PubMed] [Google Scholar]
- 2. Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015;31(1):61-68. [DOI] [PubMed] [Google Scholar]
- 3. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893-902. [DOI] [PubMed] [Google Scholar]
- 4. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections the GOLD randomized clinical trial. JAMA. 2017;317:379-387. [DOI] [PubMed] [Google Scholar]
- 5. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections the DIAMOND randomized clinical trial. JAMA. 2017;317:371-378. [DOI] [PubMed] [Google Scholar]
- 6. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391:1367-1377. [DOI] [PubMed] [Google Scholar]
- 7. Visser MM, Charleer S, Fieuws S, et al. Comparing real-time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6-month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397:2275-2283. [DOI] [PubMed] [Google Scholar]
- 8. Peters AL, Ahmann AJ, Battelino T, et al. Diabetes technology-continuous subcutaneous insulin infusion therapy and continuous glucose monitoring in adults: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3922-3937. [DOI] [PubMed] [Google Scholar]
- 9. Stimson RH, Dover AR, Ritchie SA, et al. HbA1c response and hospital admissions following commencement of flash glucose monitoring in adults with type 1 diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Broos B, Charleer S, Bolsens N, et al. Diabetes knowledge and metabolic control in type 1 diabetes starting with continuous glucose monitoring: FUTURE-PEAK. J Clin Endocrinol Metab. 2021;106:e3037-e3048. [DOI] [PubMed] [Google Scholar]
- 11. Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. BMJ. 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between self-monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37-46. [DOI] [PubMed] [Google Scholar]
- 13. Lameijer A, Lommerde N, Dunn TC, et al. Flash glucose monitoring in The Netherlands: increased monitoring frequency is associated with improvement of glycemic parameters. Diabetes Res Clin Pract. 2021;177:108897. [DOI] [PubMed] [Google Scholar]
- 14. Hohendorff J, Gumprecht J, Mysliwiec M, Zozulinska-Ziolkiewicz D, Malecki MT. Intermittently scanned continuous glucose monitoring data of polish patients from real-life conditions: more scanning and better glycemic control compared to worldwide data. Diabetes Technol Ther. 2021;23(8):577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020;43(5):1146-1156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-dst-10.1177_19322968221128315 for The Impact of Baseline User Characteristics on the Benefits of Real-Time Versus Intermittently Scanned Continuous Glucose Monitoring in Adults With Type 1 Diabetes: Moderator Analyses of the ALERTT1 Trial by Margaretha Martha Visser, Sara Charleer, Steffen Fieuws, Christophe De Block, Robert Hilbrands, Liesbeth Van Huffel, Toon Maes, Gerd Vanhaverbeke, Eveline Dirinck, Nele Myngheer, Chris Vercammen, Frank Nobels, Bart Keymeulen, Chantal Mathieu and Pieter Gillard in Journal of Diabetes Science and Technology
Supplemental material, sj-docx-2-dst-10.1177_19322968221128315 for The Impact of Baseline User Characteristics on the Benefits of Real-Time Versus Intermittently Scanned Continuous Glucose Monitoring in Adults With Type 1 Diabetes: Moderator Analyses of the ALERTT1 Trial by Margaretha Martha Visser, Sara Charleer, Steffen Fieuws, Christophe De Block, Robert Hilbrands, Liesbeth Van Huffel, Toon Maes, Gerd Vanhaverbeke, Eveline Dirinck, Nele Myngheer, Chris Vercammen, Frank Nobels, Bart Keymeulen, Chantal Mathieu and Pieter Gillard in Journal of Diabetes Science and Technology