Abstract
Oxidative stress plays a pivotal role in the pathogenesis of several neurodegenerative diseases. Retinal degeneration causes irreversible death of photoreceptor cells, ultimately leading to vision loss. Under oxidative stress, the synthesis of bioactive sphingolipid ceramide increases, triggering apoptosis in photoreceptor cells and leading to their death. This study investigates the effect of L-Cycloserine, a small molecule inhibitor of ceramide biosynthesis, on sphingolipid metabolism and the protection of photoreceptor-derived 661W cells from oxidative stress.
The results demonstrate that treatment with L-Cycloserine, an inhibitor of Serine palmitoyl transferase (SPT), markedly decreases bioactive ceramide and associated sphingolipids in 661W cells. A nontoxic dose of L-Cycloserine can provide substantial protection of 661W cells against H2O2-induced oxidative stress by reversing the increase in ceramide level observed under oxidative stress conditions. Analysis of various antioxidant, apoptotic, and sphingolipid pathway genes and proteins also confirms the ability of L-Cycloserine to modulate these pathways.
Our findings elucidate the generation of sphingolipid mediators of cell death in retinal cells under oxidative stress and the potential of L-Cycloserine as a therapeutic candidate for targeting ceramide-induced degenerative diseases by inhibiting SPT. The promising therapeutic prospect identified in our findings lays the groundwork for further validation in in-vivo and preclinical models of retinal degeneration.
Keywords: Ceramide biosynthesis, L-Cycloserine, oxidative stress, retina, photoreceptors, retinal cell death, Serine palmitoyl transferase (SPT), sphingolipids, therapeutic intervention
1. Introduction
The irreversible loss of vision in various retinal degenerative diseases, such as Retinitis Pigmentosa (RP) and Age-related Macular Degeneration (AMD), is ultimately attributed to the death of retinal photoreceptor cells. Light-sensitive photoreceptor cells die irreversibly in such retinal degenerative diseases, causing blindness or vision loss. These diseases are heterogeneous and involve complex pathophysiological mechanisms influenced by genetic and environmental factors (Wert et al., 2014). Concerning genetic factors, mutations in more than 250 genes have been associated with monogenic forms of retinal degeneration (Daiger, 1996–2022). Consequently, the development of effective therapies for retinal degenerative diseases presents a significant challenge.
Several reports suggest that excessive exposure to white light (Cruickshanks et al., 1993; Cruickshanks et al., 2001; Hafezi et al., 1997), oxidative damage caused by free radicals (Hollyfield et al., 2008; Shen et al., 2007; Totan et al., 2009; Winkler et al., 1999), and inflammatory stress (Donoso et al., 2006; Hollyfield et al., 2008) are among some of the predominant factors involved in the onset and progression of retinal degenerative diseases, like AMD (Beatty et al., 2000; Hollyfield et al., 2008) and RP (Shen et al., 2005). The retina is particularly susceptible to oxidative stress due to its high oxygen consumption, high concentration of polyunsaturated fatty acids in the photoreceptors, and constant exposure to visible light (Masuda et al., 2017). Retinal cells generate reactive oxygen species (ROS) due to oxygen consumption, and elevated levels of ROS induce damage to DNA, RNA, and proteins at the cellular level, which may underlie the death of photoreceptor cells. Retinal pigment epithelial (RPE) cells support photoreceptors; they phogocytose the shed photoreceptor outer segment membrane, absorb scattered light, transport fluid and ions, and help RPE-photoreceptor apposition (Mazzoni et al., 2014). Therefore, the primary dysfunction of the RPE cells can also contribute to photoreceptor cell death resulting in vision loss.
Numerous reports have highlighted ceramide as the central mediator of apoptosis in retinal photoreceptor cells (German et al., 2006; Prado Spalm et al., 2019; Sanvicens and Cotter, 2006; Sugano et al., 2019). Ceramide is the simplest form of sphingolipid that is a critical component of membrane structure and function. It is also a crucial secondary messenger integral to cellular signaling that regulate cell growth, senescence, and apoptosis (Ohanian and Ohanian, 2001; Yadav and Tiwari, 2014). Although a steady state level of ceramide is necessary for cell proliferation and differentiation, a temporal or consistent elevation have been linked to cellular toxicity, inflammation and cell death (Chen et al., 2012; Garanto et al., 2013; Stiles et al., 2016). Previous studies from our lab demonstrated a link between oxidative stress induced by H2O2 and elevated ceramide and monohexosylceramide (MHC) levels, that resulted into cell death, using human RPE-derived ARPE-19 cells (Sugano et al., 2019). In rat retinal neuronal cultures, an increase in ceramide formation due to paraquat (a well known oxidant) treatment induced photoreceptor cell death (German et al., 2006). In Drosophila mutants, reversing photoreceptor degeneration through targeted over expression of ceramidase correlated with decreased ceramide levels, emphasizing ceramide as a potential death-mediator for photoreceptor cells and the potential of targeting sphingolipid metabolic enzymes for therapeutic development against retinal degenerative disaeses (Acharya et al., 2003). Further development in that area have tested myriocin as an inhibitor of ceramide biosynthesis to prevent retinal degeneration in rd10 mice (Strettoi et al., 2010b). Also, our previous studies demonstrated systemic administration of FTY720, a Ceramide synthase (CerS) inhibitor, could prevent the elevation of retinal ceramide and protect photoreceptor cell death in light-induced retinal degeneration (LIRD) model of Sprauge Dawley (SD) rats, and transgenic Rhodopsin mutant P23H1 rats (Chen et al., 2013; Stiles et al., 2016). Hence, targeting ceramide as a secondary messenger presents a promising therapeutic strategy for protecting retinal cells against apoptosis and may have potential implications for the treatment of retinal degenerative diseases.
Current therapeutic options for retinal degenerative disease are limited to gene therapy, stem cell therapy, optogenetics, and retinal prostheses (Scholl et al., 2016). RPE65 gene therapy (Luxturna, voretigene neparvovec-rzyl, Spark Therapeutics, Inc.) was recently approved to improve functional vision in patients with biallelic RPE65 mutations (Gardiner et al., 2020). Ongoing developments involve the subretinal delivery of genes like LRAT or REP1 using adeno-associated virus (AAV) vectors, which aim to target photoreceptor cells in the fovea (Scholl et al., 2016). Retinal prostheses have also received regulatory approval in Europe and the United States (Scholl et al., 2016). However, it is important to acknowledge that these approaches are invasive and may not always align with patient convenience and adherence, particularly in chronic diseases. Therefore, by targeting ceramide, a common factor in the pathophysiology of various retinal degenerative disease, there is potential for a more comprehensive pharmacotherapeutic impact, providing a broadly applicable approach to managing these conditions.
Multiple ceramide biosynthesis inhibitors are commercially available that include Myriocin, Fumonisin B1, and Fingolimod. In this study, we tested a small molecule ceramide biosynthesis inhibitor L-Cycloserine. Since L-Cycloserine is a well-established ceramide biosynthesis inhibitor that has been employed in various prior investigations (Geekiyanage et al., 2013; Granzotto et al., 2019; Meyer and de Groot, 2003a), we hypothesize that L-Cycloserine will protect retinal photoreceptor cells against oxidative stress-induced damage by inhibiting de-novo ceramide synthesis and can be used as a promising therapeutic candidate for preventing or delaying the onset or progression of RD. This study utilizes a mouse photoreceptor-derived cell line, 661W, which has been immortalized through the expression of simian virus (SV)40 T antigen (T-ag) regulated by the human interphotoreceptor retinol-binding protein (IRBP) promoter (Huang et al., 2021). This promoter is specific to rod and cone photoreceptors. Through comprehensive cellular and molecular analyses, it has been determined that these cells predominantly express cone photoreceptor markers and not rod photoreceptor markers, indicating their origin from a cone photoreceptor lineage (Tan et al., 2004a). Additionally, the expression of rod precursor genes, the ability to grow as a monolayer, and the potential for induction of differentiation through specific treatments or genetic modification make the 661W cell line particularly promising for investigating mechanisms underlying photoreceptor cell death and development of in vitro drug screeeing system for various retinal degenerative diseases.
In our study, we tested how L-Cycloserine influences sphingolipid metabolism and the viability of mouse photoreceptor-derived 661W cells in culture. We further tested the possible protective effect of L-Cycloserine in 661W cells in an in-vitro setting by using an H2O2-mediated oxidative stress-induced cell death model. We also utilized various biochemical and molecular assays to gain deeper insights into the potential mechanism underlying the protective action of L-Cycloserine. Our study demonstrates that L-Cycloserine can protect H2O2-mediated oxidative stress-induced retinal cell death in-vitro.
2. Materials and Methods
2.1. Cell culture:
Mouse photoreceptor-derived 661W cells (Tan et al., 2004b) were generously provided by Dr. Muayyad Al-Ubaidi (University of Houston, Department of Biomedical Engineering). The 661W cells were maintained in Dulbecco’s Modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA). The medium contained 10% fetal bovine serum, 1 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were grown in 5% CO2, and 95% humidity at 37 °C.
2.2. Mass Spectrometry Analysis of Sphingolipids:
For sphingolipid analysis, 661W cells from different treatments were scrapped from 6 well plates, washed twice with ice-cold phosphate-buffered saline (PBS), and collected by centrifugation. The cell pellets were stored at −80°C and later sent for analysis to the lipidomic core facility at the University of Virginia, Charlottesville, VA, USA. Sphingolipids in 661W cells were quantified and analyzed following previously published procedures (Galor et al., 2022; MacKnight et al., 2019; Maus et al., 2022; Maus et al., 2023; Mondal et al., 2022; Mondal et al., 2021; Qi et al., 2017; Stephenson et al., 2023; Stiles et al., 2016; Vu et al., 2022; Wijesinghe et al., 2010; Wijesinghe et al., 2014). Sphingolipid analysis involved the utilization of UPLC ESI-MS/MS. To separate the sphingolipids, a Shimadzu Nexera X2 LC-30AD with an Acentis Express C18 column (5 cm x 2.1 mm, 2.7 in μm) was employed at a flow rate of 0.5 ml/min at 60°C. The process began with equilibration of the column with 100% Solvent A [methanol:water:formic acid (58:44:1, v/v/v) with 5mM ammonium formate] for 5 minutes, followed by injecting 10 μl of the sample. The elution began with 100% Solvent A for the first 0.5 minutes, transitioning to Solvent B [methanol:formic acid (99:1, v/v) with 5mM ammonium formate] with a linear gradient to reach 100% Solvent B from 0.5 to 3.5 minutes. The transition was held constant at 100% Solvent B from 3.5 to 6 minutes, then from 6 to 6.1 minutes, Solvent B reduced to 0%, followed by maintaining Solvent A at 100% from 6.1 to 8 minutes. Sphingolipids underwent analysis through a targeted assay using an AB Sciex Triple Quad 5500 Mass Spectrometer. Detection parameters were established to target specific precursor and product ion pairs solely for sphingolipids. Using positive-ion mode and multiple-reaction monitoring, distinct species of sphingolipids were identified based on their retention time and m/z ratio. The semi-quantitative determination of each species was based on peak area measurements of internal standards spiked in the samples. Resulting values were reported in total picomoles and as mole percentages to standardize the data for comparison (Galor et al., 2022; Mondal et al., 2022; Mondal et al., 2021; Qi et al., 2017; Stiles et al., 2016).
2.3. Cytotoxicity assay:
The effect of L-Cycloserine on the viability of 661W cells was detected by MTT assay using a commercial kit (Vybrant® MTT Cell Proliferation Assay Kit V-13154, Thermo Fisher Scientific, Eugene, Oregon). Cells were seeded into 96-well plates and incubated for 24 hours. Next, they were treated with a range of concentrations (0–400 μM) of L-Cycloserine (Cat# C-1159; Millipore Sigma, St. Louis, MO) and incubated for 24 hours again. Untreated 661W cells were used as controls. The old medium was then replaced with 100 μL of fresh medium containing 10 μL of 12 mM MTT solution according to the kit protocol. After 4 hours of incubation at 37°C, formazan crystals were solubilized by adding 100 μL SDS-HCl in each well and incubated for 18 hours. The absorbance levels for each sample were measured using a plate reader (Bio Tek Instruments, Inc) at wavelength 570 nm according to the protocol.
2.4. Cell viability assays:
The 661W cells were cultured for 4 days in 10 ml medium in 10-cm plates to reach 80% confluency. To test the dose-dependent oxidative stress induced by H2O2, 661W cells were treated with different doses of H2O2 (100–750 μM) for 3 hours and a suitable dose was chosen that provided the optimum cytotoxicity or oxidative stress. To test the cytoprotective effect of L-Cycloserine against H2O2-mediated cell death, the cells were at first pretreated with 10 and 25 μM L-Cycloserine (Cat# C-1159; Millipore Sigma, St. Louis, MO) for 24 hours, followed by 3 hours of treatment with 300 μM H2O2 (Sigma, St. Louis, MO), a dose that was chosen based on the previous dose-dependent study of H2O2. The 661W cells were also subjected to a co-treatment plan of 3 hours with 5 to 50 μM of L-Cycloserine and 300 μM H2O2 simultaneously. Cell viability, in all cases, was determined indirectly by measuring the released lactate dehydrogenase (LDH) in the medium by using a commercial kit from Promega (CytoTox-ONE Homogenous Membrane Integrity Assay kit; Madison, WI) following the manufacturer’s instructions. For the LDH release assay, the fluorescence measurements of the release of LDH from the cells with a damaged membrane were calculated by subtracting the culture medium background, and the cytotoxicity (%) was calculated from 100% viability (mean value of untreated control cells) to 0% viability (mean value of 2% Triton X-100-treated cells, Sigma, St. Louis, MO). To isolate the RNA and proteins, 661W cells were scrapped from the 10-cm dishes, washed twice with RNase-free ice-cold PBS, and collected by centrifugation. The cell pellets were then stored at −80°C until the RNA and proteins were extracted.
2.5. Quantification of L-Cycloserine:
LC-MS/MS experiments were acquired using Sciex (Framingham, MA) 5500 TripleQuad Mass Spectrometer coupled with Shimadzu (Columbia, MD) LC20ADXR binary pumps, Shimadzu SIL20ACXR autosampler, and Shimadzu CTO20AC column oven. A Supelco Ascentis Express C18, 2.7 mm, 50 × 4.6 mm HPLC column was used. The mobile phase of pure water (A) and acetonitrile (B) was eluted at a combined flow rate of 0.6 mL/min. The HPLC time program started at 15% of mobile phase B from 0 to 1.5 min, and increased from 15% to 95% of mobile phase B from 1.5 to 1.6 min, and kept at 95% of mobile phase B from 1.6 to 2.1 min, and then decreased from 95% to 15% of mobile phase B from 2.1 to 2.2 min. The time program stopped at 2.5 min. The sample output from the column between 0.5 and 1.5 min was diverted to the ion source of the Mass Spectrometer. Before 0.5 min and after 1.5 min, the output was diverted to a waste bottle. There was an equilibration time of 1.5 min at the initial condition before each run.
Multiple Reaction Monitor (MRM) at positive mode was used for the MS/MS detection. The Ionization Spray capillary voltage was at 5.5 kV; the Ion source temperature was at 500 °C; and CUR, CAD, GS1, and GS2 settings were at 20.0, 8.0, 50.0, and 50.0 PSI, respectively. Compound-specific parameters are listed in Supplementary Table 1.
2.6. RNA isolation, cDNA synthesis, and quantitative reverse transcriptase polymerase chain reaction:
RNA was isolated and purified from frozen cultured 661W cells using the Pure Link Micro-to-Midi Total RNA Purification System from Invitrogen (Thermo Fisher Scientific, CA) following the manufacturer’s protocol. Equal quantities (1.0 μg) of total RNA from each cell sample were converted to first-strand cDNA using Superscript IV First-Strand Synthesis System (Invitrogen- Thermo Fisher Scientific, Lithuania) for reverse transcriptase polymerase chain reaction (RT–PCR). First-strand cDNA was used for quantitative reverse-transcriptase PCR (qRT–PCR). Primers for qRT–PCR were designed in such a way that they spanned at least one intron, which eliminated the chance of amplification from residual genomic DNA contamination. The primer sequences are provided in Supplementary Table 2. Quantitative PCR and melt-curve analyses were performed using PowerUp SYBR Green Master mix from applied biosystems by Thermo Fisher Scientific (Lithuania) and an iCycler machine (QuantStudio 3 from Applied Biosystems by Thermo Fisher Scientific). The relative quantities of the expression of the genes of interest in different samples were calculated with the comparative Cq (threshold cycle) value method (Mandal et al., 2006).
2.7. Western blotting:
Whole-cell lysates from the 661W cells were prepared for western blot by sonicating in T-PER reagent (Pierce, Rockford, IL) containing a protease inhibitor cocktail (Roche, Indianapolis, IN) and then centrifuging at 10,000× g for 15 min at 4 °C to collect the supernatants. After the protein concentrations were determined using BCA reagent (Pierce, IL), equal aliquots (30 μg) of protein samples were applied to 10% sodium dodecyl sulfate-polyacrylamide gels (Invitrogen, CA) and electrophoretically separated. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad, CA) and blocked with 5% Bovine serum albumin (BSA) for 2 hours at room temperature. The membranes were incubated with anti-Ho1 (1:1,000; Cell Signaling, Danvers, MA), anti-PARP (1:100; Santa Cruz Biotechnology, CA) and anti-β-actin (1:1,000; Cell Signaling, Danvers, MA) antibodies for 16 hours at 4°C, after which they were incubated with the appropriate peroxidase-linked secondary antibody for 1 hour at room temperature. Chemiluminescence signals were detected by ECL detection reagent (Pierce; Thermo Scientific, IL) and imaged using Odyssey Imaging system (LI-COR). Densitometric analysis was performed using the manufacturer’s analysis software and was normalized to β-actin.
2.8. Statistical analyses:
Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). The quantitative data are expressed as mean ± standard error of the mean (SEM) for each group. One-way or two-way ANOVA, Student’s t-tests (with Welch’s correction), and paired t-tests were performed to assess the differences between means. A significance is considered with a P value <0.05.
3. Results
3.1. L-Cycloserine treatment alters the sphingolipid levels in 661W cells:
To examine how SPL metabolism is affected by L-Cycloserine in 661W cells, the cells were exposed to varying concentrations of L-Cycloserine (10, 25, and 50 μM) for a period of 24 hours. As presented in Figure 1, a significant and dose-dependent reduction in total ceramide (Cer), monohexosylceramide (MHC), sphingomyelin (SM), and total sphingolipid (SPL) levels was observed. However, when considering the relative fractions or percentages of Cer, MHC, and SM, it was noted that the fraction of Cer remained relatively constant at approximately 10%, regardless of the L-Cycloserine dose utilized (Supplementary Figure 1). In contrast, the percentage of SM increased from 39% to 48%, while the percentage of MHC decreased from 51% to 42%, indicating a shift in the composition of these sphingolipids (Supplementary Figure 1). Further analyses revealed that total sphingosine (So) and ceramide-1-phosphate (C1P) levels decreased significantly with increasing doses of L-Cycloserine (Supplementary Figure 2A and C). Sphingosine-1-phosphate (S1P) also exhibited a dose dependent reduction with increasing L-Cycloserine concentrations, although it was not statistically significant (Supplementary Figure 2B). When evaluating individual ceramide species by their levels (pmol/mg of tissue), all species experienced a significant reduction at the 25 and 50 μM L-Cycloserine doses (Table 1). Similar trends were observed in MHC and SM, where all individual species displayed significant reductions at the 25 and 50 μM L-Cycloserine treatment doses (Table 1).
Figure 1: L-Cs reduce ceramide and other sphingolipids in 661W cells.

Analysis of total sphingolipid levels in 661W cells (n = 5/group) treated with different L-Cs doses (10–50 μM) for 24 hours showing levels of total ceramide (Cer), monohexosylceramide (MHC), sphingomyelin (SM) and total sphingolipid (SPL). (Values are represented as mean ± SEM; t-test; *p < 0.05, **p < 0.01, ***p < 0.001). Control: cells having no treatment with L-Cs; L-Cs10: treated with 10 μM L-Cs for 24 h; L-Cs25: treated with 25 μM L-Cs for 24 h; L-Cs50: treated with 50 μM L-Cs for 24 h.
Table 1.
Levels (pmol/mg of protein) of specific ceramide, monohexosylceramide, and sphingomyelin species among the different groups in 661W cells.
| Ceramide (pmol/mg) | Control | L-Cs10 | L-Cs25 | L-Cs50 |
|---|---|---|---|---|
| C14:0 | 0.45 ± 0.09 | 0.28 ± 0.04 | 0.11 ± 0.01** | 0.08 ± 0.03** |
| C16:0 | 23.84 ± 5.77 | 14.88 ± 1.27 | 6.38 ± 0.92 * | 4.32 ± 1.18 * |
| C18:1 | 5.20 ± 1.10 | 3.74 ± 0.39 | 1.43 ± 0.08 ** | 0.98 ± 0.27** |
| C18:0 | 1.98 ± 0.43 | 1.14 ± 0.09 | 0.47 ± 0.05** | 0.25 ± 0.07** |
| C20:0 | 1.15 ± 0.24 | 0.58 ± 0.08 | 0.23 ± 0.02** | 0.13 ± 0.03** |
| C22:0 | 4.37 ± 0.85 | 2.67 ± 0.2 | 1.11 ± 0.09** | 0.56 ± 0.17** |
| C24:1 | 10.22 ± 2.19 | 6.43 ± 0.77 | 2.64 ± 0.19** | 1.4 ± 0.48** |
| C24:0 | 11.12 ± 2.54 | 6.86 ± 0.93 | 2.66 ± 0.13* | 1.20 ± 0.34** |
| C26:1 | 3.46 ± 0.63 | 2.02 ± 0.21 | 0.88 ± 0.07** | 0.44 ± 0.13** |
| C26:0 | 0.14 ± 0.02 | 0.08 ± 0.01 | 0.03 ± 0.00** | 0.02 ± 0.01** |
| Monohexosylceramide (pmol/mg) | Control | L-Cs10 | L-Cs25 | L-Cs50 |
| C14:0 | 0.41 ± 0.12 | 0.26 ± 0.05 | 0.09 ± 0.01* | 0.05 ± 0.01* |
| C16:0 | 95.14 ± 21.12 | 70.81 ± 6.00 | 26.50 ± 2.44* | 15.76 ± 4.46 |
| C18:1 | 12.77 ± 3.07 | 8.40 ± 1.22 | 3.08 ± 0.26* | 1.52 ± 0.41* |
| C18:0 | 10.44 ± 2.48 | 8.03 ± 0.95 | 2.49 ± 0.35* | 1.38 ± 0.35 |
| C20:0 | 8.57 ± 2.48 | 6.18 ± 0.85 | 1.83 ± 0.18* | 0.93 ± 0.27* |
| C22:0 | 40.76 ± 8.95 | 26.12 ± 3.75 | 9.16 ± 0.84** | 4.23 ± 1.21* |
| C24:1 | 55.18 ± 17.68 | 35.02 ± 4.19 | 11.47 ± 1.3* | 5.35 ± 1.63* |
| C24:0 | 90.10 ± 24.7 | 68.41 ± 8.29 | 23.53 ± 2.46* | 11.70 ± 3.07* |
| C26:1 | 7.78 ± 1.92 | 5.32 ± 0.64 | 1.82 ± 0.22* | 0.93 ± 0.25* |
| C26:0 | 0.75 ± 0.17 | 0.55 ± 0.10 | 0.2 ± 0.02* | 0.085 ± 0.02** |
| Sphingomyelin (pmol/mg) | Control | L-Cs10 | L-Cs25 | L-Cs50 |
| C14:0 | 10.20 ± 2.64 | 6.72 ± 0.67 | 2.63 ± 0.14* | 1.94 ± 0.6* |
| C16:0 | 79.48 ± 23 | 58.44 ±4.23 | 23.10 ± 1.5* | 18.44 ± 3.57* |
| C18:1 | 7.25 ± 1.78 | 5.23 ± 0.5 | 1.97 ± 0.09* | 1.40 ± 0.39* |
| C18:0 | 25.25 ± 6.83 | 17.96 ± 1.7 | 6.62 ± 0.29* | 4.77 ± 1.34* |
| C20:0 | 9.14 ± 2.13 | 6.32 ± 0.66 | 2.41 ± 0.13* | 1.77 ± 0.52** |
| C22:0 | 22.69 ± 5.84 | 15.43 ± 1.57 | 5.61 ± 0.25* | 3.91 ± 1.19* |
| C24:1 | 63.39 ± 16.55 | 44.03 ± 4.01 | 16.09 ± 0.95* | 9.61 ± 1.99* |
| C24:0 | 28.97 ± 7.29 | 20.84 ± 2.34 | 7.63 ± 0.42* | 5.41 ± 1.64* |
| C26:1 | 0.85 ± 0.21 | 0.53 ± 0.06 | 0.20 ± 0.02* | 0.12 ± 0.05** |
| C26:0 | 0.24 ± 0.07 | 0.15 ± 0.02 | 0.06 ± 0.00* | 0.04 ± 0.01* |
Footnote: Semiquantitative levels (pmol/mg of protein) of specific ceramide, monohexosylceramide, and sphingomyelin species among the different groups in 661W cells (n = 5/group) treated with different L-Cycloserine (L-Cs) doses (10–50 μM) for 24 hours. Control: cells having no treatment with L-Cs; L-Cs10: treated with 10 μM L-Cs for 24 h; L-Cs25: treated with 25 μM L-Cs for 24 h; L-Cs50: treated with 50 μM L-Cs for 24 h. (Values are mean ± SEM; t-test;
p < 0.05,
p < 0.01).
3.2. L-Cycloserine treatment for 3 hours shows minimum cytotoxicity in 661W cells:
To determine the effect of L-Cycloserine on 661W cell viability, an MTT assay was performed in which 661W cells were exposed to various L-Cycloserine concentrations ranging from 0 to 400 μM over a 24-hour period. Cell viability was significantly affected at a dose of 75 μM and above (Figure 2A). The results revealed a dose-dependent viability pattern of the cells, with an estimated IC50 (the concentration causing 50% cell death) at approximately 60 μM (Figure 2A). Based on these findings, L-Cycloserine was used at concentrations well below the IC50 value of 10 and 25 μM for further investigation to assess their impact on cell viability using the LDH assay. Treatment of 661W cells with L-Cycloserine at 10 and 25 μM concentrations for 24 hours indicated 2% and 10% toxicity levels, respectively (Figure 2B). To minimize the drug-induced toxic effects, the exposure time of the 661W cells to L-Cycloserine treatment was shortened. Subsequently, when 661W cells were treated with L-Cycloserine for 3 hours, the results revealed negligible toxicity of less than 0.4%, even at the highest dose of 50 μM (Figure 2C).
Figure 2A: Cell viability assay for 661W cells using L-Cs.

661W cells were treated with different doses of L-Cs (0–400 μM) for 24 hours. Cell viability was then determined by thiazolyl blue (MTT) assay (n=3 plate × 3 replication assay). Values are represented as mean ± SEM; One-way ANOVA; *p<0.05, **p < 0.01). Figure 2B: Cell viability assay for 661W cells using L-Cs. 661W cells were treated with different doses (10 and 25 μM) of L-Cs for 24 hours. Cell death was then measured by analyzing the release of lactate dehydrogenase (LDH; n=4 plate × 4 replication assay). Values are represented as mean ± SEM; t-test; **p < 0.01. Figure 2C: Cell viability assay for 661W cells using L-Cs. 661W cells were treated with different doses (5,10, 25 and 50 μM) of L-Cs for 3 hours. Cell death was then measured by analyzing the release of lactate dehydrogenase (LDH; n=4 plate × 4 replication assay). Values are represented as mean ± SEM; no statistical significance was found.
3.3. L-Cycloserine provides cytoprotection in 661W cells against H2O2-mediated oxidative stress:
In order to determine the appropriate level of oxidative stress, 661W cells were exposed to a range of H2O2 doses (100 to 750 μM) for a duration of 3 hours (Figure 3A). 300 μM of H2O2 was selected as the optimal dose since it resulted in approximately 20% cell death (Figure 3A). Subsequently, the cells were pretreated with varying doses of L-Cycloserine (10 and 25 μM) for 24 hours, followed by H2O2 treatment at the chosen 300 μM dose for 3 hours. This strategy revealed that L-Cycloserine significantly protected the cells from oxidative stress in a dose-dependent manner, where 25 μM of L-Cycloserine reduced cytotoxicity in H2O2-stressed cells from around 40% to 20% (Figure 3B). Considering the prior observation that L-Cycloserine treated groups exhibited minimal toxicity at different doses of L-Cycloserine when treated for 3 hours (Figure 2C), a co-treatment plan was developed. In this scenario, 661W cells were exposed to L-Cycloserine at various concentrations (5, 10, 25, and 50 μM) simultaneously with 300 μM H2O2 for a duration of 3 hours. This co-treatment study illustrated that L-Cycloserine, when co-administered with H2O2, significantly protected the cells from cell death in a dose-dependent manner; for instance, 50 μM of L-Cycloserine reduced cytotoxicity in H2O2-stressed cells from approximately 20% to 6% (Figure 3C). Due to the this co-treatment approach, the interaction between L-Cycloserine and H2O2 was also evaluated by quantifying L-Cycloserine both in the presence and absence of H2O2. The results indicated no significant difference between the concentration of L-Cycloserine in the presence or absence of H2O2 (Supplementary Figure 3).
Figure 3A: Dose dependent cytotoxicity of H2O2.

Treatment of 661W cells with different doses of H2O2 (100–750 μM) for 3 hours; 10% Triton was used as positive control. Cell death was then measured by analyzing the release of lactate dehydrogenase (LDH; n=4 plate × 3 replication assay). Figure 3B: L-Cs protects 661W cells from oxidant-induced cell death. 661W cells were pretreated with different doses (10 and 25 μM) of L-Cs for 24 hours followed by 300 μM H2O2 for 3 hours. Cell death was then measured by analyzing the release of lactate dehydrogenase (LDH; n=4 plate × 4 replication assay). Values are represented as mean ± SEM; t-test; **p < 0.01. Figure 3C: L-Cs protects 661W cells from oxidant-induced cell death. 661W cells were cotreated with 300 μM H2O2 and different doses of L-Cs (5,10, 25 and 50 μM) for 3 h. Cell death was then measured by analyzing the release of lactate dehydrogenase (LDH; n=4 plate × 4 replication assay). (Values are represented as mean ± SEM; t-test; *p < 0.05; **p < 0.01; ***p < 0.001).
3.4. L-Cycloserine treatment alters the sphingolipid levels in H2O2-induced oxidative stressed 661W cells:
To investigate the impact of H2O2-induced oxidative stress on the level of sphingolipids in 661W cells and whether L-Cycloserine could modulate these altered levels, total Cer, MHC, SM, and total SPL levels were measured during this 3-hour co-treatment study of L-Cycloserine (10 μM) and H2O2. Although the results revealed no significant differences between the various groups regarding total Cer and SM, co-treatment with L-Cycloserine and H2O2 significantly reduced total MHC and SPL compared to the H2O2-treated cells (Figure 4A). Analysis of the relative percentages or fractions of Cer, MHC, and SM revealed distinct patterns. The percentage of ceramide increased from 12% to 14% upon treatment with H2O2 (Figure 4B2), but with the co-treatment of L-Cycloserine and H2O2, the percentage of ceramide reverted back to 12% (Figure 4B4). The percentage of SM decreased from 42% to 35% upon treatment with H2O2, while co-treatment with L-Cycloserine brought it back to 41% (Figure 4B). The MHC fraction, on the other hand, increased from 46% to 51%, and then reduced back to 47%, approaching the level in the control cells (Figure 4B). In the group with the treatment of L-Cycloserine alone, ceramide percentage decreased from 12% to 10%, SM decreased from 42% to 39%, and MHC increased from 46% to 51% (Figure 4B3). Furthermore, both total S1P and C1P levels were increased in H2O2-treated cells (Supplementary Figure 4A and 4B), and this increase in S1P was reduced when L-Cycloserine and H2O2 were used as co-treatment (Supplementary Figure 4A).
Figure 4A: L-Cs treatment alters level of ceramide and other sphingolipids in H2O2 induced oxidative stressed 661W cells.

Analysis of total fatty acid composition of ceramide (Cer), sphingomyelin (SM), monohexosylceramide (MHC) and all sphingolipids (SPL) in 661W cells (n = 6/group). Control: cells having no treatment with L-Cs or H2O2; L-Cs: treated with 10 μM L-Cs for 3 h; H2O2: treated with 300 μM of H2O2 for 3 h; L-Cs + H2O2: cotreated with 10 μM L-Cs and 300 μM H2O2 for 3 h. Values are presented as mean ± SEM; t-test; # represents significance between H2O2 and L-Cs + H2O2 treatment; #p < 0.05. Figure 4B: L-Cs treatment alters ceramide and other sphingolipids in H2O2 induced oxidative stressed cells. Analysis of major sphingolipid levels represented in a pie chart by absolute value (pmol/ mg of protein) showing total composition of ceramide (Cer), sphingomyelin (SM) and monohexosylceramide (MHC) percent in 661W cells (n = 6/group). B1 represents control or cells having no treatment with L-Cs or H2O2; B2 represents cells treated with 300 μM of H2O2 for 3 h; B3 represents cells treated with 10 μM L-Cs for 3 h; B4 represents cells cotreated with 10 μM L-Cs and 300 μM H2O2 for 3 h.
Different species of ceramide and its metabolites play diverse roles in cellular processes, including stress response (Stith et al., 2019). Therefore, the effects of oxidative stress induced by H2O2 treatment on specific SPL species in 661W cells were evaluated by their absolute values (pmol/mg of protein). Following oxidative stress, there was a significant increase in dihydro ceramide C16:0 DH (P < 0.01) compared to control (Table 2). Conversely, following oxidative stress, there was a significant decrease in long-chain ceramide species like C24:0 (P < 0.05), C26:1 (P < 0.01), C26:0 (P < 0.05), and the shortest SM species C14:0 (P < 0.001) compared to control cells (Table 2). Co-treatment with L-Cycloserine and H2O2 significantly reduced ceramide species C16 DH (P < 0.05), C26:0 (P < 0.05), MHC species C18:0 (P < 0.05), C20:0 (P < 0.05), C22:0 (P < 0.01), C24:0 (P < 0.05), C26:1 (P < 0.05), C26:0 (P < 0.05) compared to H2O2-induced oxidative stressed cells (Table 2). Among all the species from different sphingolipid classes, no significant differences existed between the control cells and the L-Cycloserine-only treated group (Table 2).
Table 2.
Levels (pmol/mg of protein) of specific ceramide, monohexosylceramide, sphingomyelin and ceramide-1-phosphate species among the different groups in 661W cells.
| Ceramide (pmol/mg) | Control | L-Cs only | H2O2 only | L-Cs + H2O2 |
|---|---|---|---|---|
| C14:0 | 0.63 ± 0.09 | 0.81 ± 0.14 | 0.57 ± 0.11 | 0.59 ± 0.14 |
| C16:0 | 25.36 ± 2.77 | 25.50 ± 4.25 | 31.05 ± 9.33 | 21.51 ± 3.15 |
| C16 DH | 1.67 ± 0.19 | 1.66 ± 0.18 | 15.25 ± 4.31b | 4.93 ± 0.65 f |
| C18:1 | 13.67 ± 4.08 | 16.00 ± 3.67 | 25.21 ± 8.75 | 19.54 ± 4.82 |
| C18:0 | 4.76 ± 1.05 | 4.67 ± 0.8 | 5.40 ± 1.79 | 4.89 ± 0.7 |
| C20:0 | 4.31 ± 0.38 | 3.68 ± 0.43 | 4.56 ± 1.52 | 2.66 ± 0.23 |
| C22:0 | 27.10 ± 2.15 | 26.49 ± 2.10 | 22.27 ± 6.9 | 14.42 ± 1.32 |
| C24:1 | 53.50 ± 5.01 | 47.69 ± 4.78 | 39.12 ± 9.81 | 31.22 ± 3.73 |
| C24:0 | 56.43 ± 6.13 | 51.59 ± 5.64 | 29.35 ± 8.14 c | 21.54 ± 2.51 |
| C26:1 | 21.60 ± 2.5 | 18.00 ± 2.22 | 10.60 ± 3.09 b | 8.01 ± 0.65 |
| C26:0 | 0.93 ± 0.12 | 0.70 ± 0.15 | 0.48 ± 0.11 c | 0.26 ± 0.02 f |
| Monohexosylceramide (pmol/mg) | Control | L-Cs only | H2O2 only | L-Cs + H 2 O 2 |
| C14:0 | 1.20 ± 0.15 | 1.73 ± 0.21 | 1.15 ± 0.15 | 1.05 ± 0.21 |
| C16:0 | 216.71 ± 44.32 | 228.93 ± 31.8 | 125.61 ± 44.32 | 101.75 ± 31.8 |
| C16 DH | 2.45 ± 1.38 | 0.42 ± 0.09 | 0.72 ± 1.38 | 0.57 ± 0.09 |
| C18:1 | 32.53 ± 9.07 | 39.00 ± 5.92 | 25.98 ± 9.07 | 21.06 ± 5.92 |
| C18:0 | 19.58 ± 3.02 | 23.85 ± 3.8 | 16.81 ± 3.02 | 13.06 ± 3.8 f |
| C20:0 | 11.52 ± 1.36 | 12.98 ± 1.55 | 11.68 ± 1.36 | 8.04 ± 1.55 f |
| C22:0 | 119.15 ± 5.94 | 130.37 ± 13.14 | 107.95 ± 5.94 | 65.36 ± 13.14 e |
| C24:1 | 133.63 ± 7.84 | 146.68 ± 14.74 | 121.48 ± 7.84 | 90.82 ± 14.74 |
| C24:0 | 272.46 ± 17.6 | 359.24 ± 34.20 | 245.00 ± 17.6 | 179.88 ± 34.2 f |
| C26:1 | 22.06 ± 1.72 | 23.80 ± 2.18 | 19.78 ± 1.72 | 14.79 ± 2.18 f |
| C26:0 | 3.60 ± 0.26 | 3.57 ± 0.33 | 2.74 ± 0.26 | 1.76 ± 0.33 f |
| Sphingomyelin (pmol/mg) | Control | L-Cs only | H2O2 only | L-Cs + H 2 O 2 |
| C14:0 | 29.53 ± 2.49 | 24.63 ± 1.88 | 13.24 ± 2.01 a | 12.85 ± 0.79 |
| C16:0 | 275.04 ± 34.24 | 298.71 ± 27.41 | 188.19 ± 148.51 | 180.11 ± 26.18 |
| C16 DH | 24.75 ± 4.55 | 24.18 ± 4.20 | 27.90 ± 23.81 | 24.88 ± 3.92 |
| C18:1 | 6.82 ± 1.71 | 7.074 ± 1.4 | 2.68 ± 3.27 | 3.07 ± 0.83 |
| C18:0 | 59.06 ± 7.60 | 49.77 ± 8.18 | 34.09 ± 24.68 | 27.29 ± 4.51 |
| C20:0 | 17.13 ± 0.99 | 15.23 ± 1.12 | 9.36 ± 5.7 | 9.19 ± 0.5 |
| C22:0 | 67.63 ± 3.88 | 61.08 ± 4.96 | 36.41 ± 23.49 | 31.59 ± 2.31 |
| C24:1 | 178.86 ± 11.73 | 170.84 ± 8.93 | 107.17 ± 64.13 | 95.65 ± 5.53 |
| C24:0 | 90.12 ± 7.77 | 87.99 ± 6.42 | 48.73 ± 33.27 | 40.87 ± 3.23 |
| C26:1 | 2.77 ± 0.3 | 2.83 ± 0.19 | 1.56 ± 0.95 | 1.30 ± 0.09 |
| C26:0 | 0.81 ± 0.09 | 0.79 ± 0.07 | 0.43 ± 0.25 | 0.35 ± 0.03 |
| Ceramide-1-phosphate (pmol/mg) | Control | L-Cs only | H2O2 only | L-Cs + H2O2 |
| C14:0 | 4.50 ± 0.35 | 4.22 ± 0.26 | 4.52 ± 0.97 c | 4.76 ± 0.24 |
| C16:0 | 0.50 ± 0.12 | 0.33 ± 0.07 | 0.68 ± 0.59 | 0.46 ± 0.08 |
Footnote: Semiquantitative levels (pmol/mg of protein) of specific ceramide, monohexosylceramide, sphingomyelin and ceramide-1-phosphate species among the different groups in 661W cells (n = 6/group). Control: cells having no treatment with L-Cs or H2O2; L-Cs only: treated with 10 μM L-Cs for 3 h; H2O2 only: treated with 300 μM of H2O2 for 3 h; L-Cs + H2O2: cotreated with 10 μM L-Cs and 300 μM H2O2 for 3 h. Values are represented as mean ± SEM; t-test;
P < 0.001 between H2O2 only treated group and control;
P < 0.01 between H2O2 only treated group and control;
P < 0.05 between H2O2 only treated group and control;
P < 0.01 between L-Cs + H2O2 treated group and H2O2 only treated group;
P < 0.05 between L-Cs + H2O2 treated group and H2O2 only treated group;
An assessment of the relative composition or mole percent of specific sphingolipid species revealed that following oxidative stress, there were significant increases in shorter-chain ceramide species like C16:0 (P < 0.01), C16 DH (P < 0.001), C18:1 (P < 0.05), while significant decrease in the longer-chain ceramides, C24:1 (P < 0.05), C24:0 (P < 0.001), C26:1 (P < 0.001), C26:0 (P < 0.05) compared to control cells (Supplementary Table 3). Co-treatment with L-Cycloserine and H2O2 significantly reduced C16 DH (P < 0.001) and C26:0 (P < 0.05) compared to H2O2 induced stressed cells (Supplementary Table 3). Among the SM species, following oxidative stress, we observed significant increases in species like C16 DH (P < 0.001), while a significant decrease in species like C14:0 (P < 0.05), C20:0 (P < 0.05), C26:0 (P < 0.05) (Supplementary Table 3).
3.5. L-Cycloserine alters expression of specific genes in H2O2-induced oxidative stressed 661W cells:
The expression of a series of genes related to the antioxidant, apoptotic, and sphingolipid pathways was investigated in the 661W cells co-treated with L-Cycloserine (10 μM) and H2O2 for 3 hours using qRT–PCR (Figure 5). The expression data was analyzed utilizing the comparative Cq value method, with normalization against the housekeeping genes, Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) and Ribosomal Protein L19 (Rpl19). Compared to the untreated cells, cells treated with 300 μM H2O2 for 3 hours exhibited a significant upregulation in the expression of the heme oxygenase 1 (Ho1) gene (Figure 5A, 7.26-fold increase, P < 0.01). H2O2-treated cells also displayed a significant increase in the expression of Mt2 (Figure 5A, 2.87-fold; P < 0.05). Co-treatment with L-Cycloserine and H2O2 for 3 hours restored the expression of these genes to normal levels (Figure 5). Furthermore, cells treated with H2O2 for 3 hours exhibited a significant decrease in the expression of Trx1 ( Figure 5A, 1.33 folds; P < 0.05), Cers2 ( Figure 5B, 1.23 folds ; P < 0.05), and Cers4 (Figure 5B, 3.44 folds ; P < 0.001) when compared to the untreated or control group. Co-treatment with L-Cycloserine and H2O2 for 3 hours restored the expression of these genes to their normal levels (Figure 5).
Figure 5: Gene expression in 661W cells measured by quantitative reverse transcriptase PCR (qRT–PCR).

Gene expression was analyzed with the comparative Cq value method after normalizing against the housekeeping genes, Gapdh and Rpl19. Expression values (±SEM) are presented against fold change over control value, which was set to 1.0 (n=3 samples ×3 replication assay per sample). Control: cells having no treatment with L-Cs or H2O2; L-Cs: treated with 10 μM L-Cs for 3 h; H2O2: treated with 300 μM of H2O2 for 3 h; L-Cs + H2O2: cotreated with 10 μM L-Cs and 300 μM H2O2 for 3 h (* represents significance between control and H2O2; *p < 0.05, **p < 0.01; ***p < 0.001, by the student t test; $ represents significance between H2O2 and L-Cs + H2O2 treatment; $p < 0.05, $ $p < 0.01, $ $ $p < 0.001, by the student t test; unmarked bars implies no significance between the comparative groups).
3.6. L-Cycloserine alters expression of Ho1 and poly-ADP ribose polymerase (PARP) protein in H2O2-induced oxidative stressed 661W cells:
The expression of specific proteins involved in cellular antioxidant and cell death pathways were also assessed. Western blot analysis was performed to evaluate protein expression, and subsequent quantification was carried out through densitometric analysis. Treatment with L-Cycloserine significantly upregulated Ho1 expression (P < 0.05). Treatment with 300 μM H2O2 for 3 hours resulted in a significant upregulation of both Ho1 and PARP protein expression, showing increases of 2-fold and 15-fold, respectively (P < 0.001) (Figure 6B and C). However, when co-treated with 10 μM L-Cycloserine and 300 μM H2O2 for 3 hours, the expression of both proteins was significantly reduced (Ho1: P < 0.05, PARP: P < 0.001) (Figure 6B and C).
Figure 6: Expression and quantification of selected proteins in 661W cells cotreated with L-Cs and H2O2.
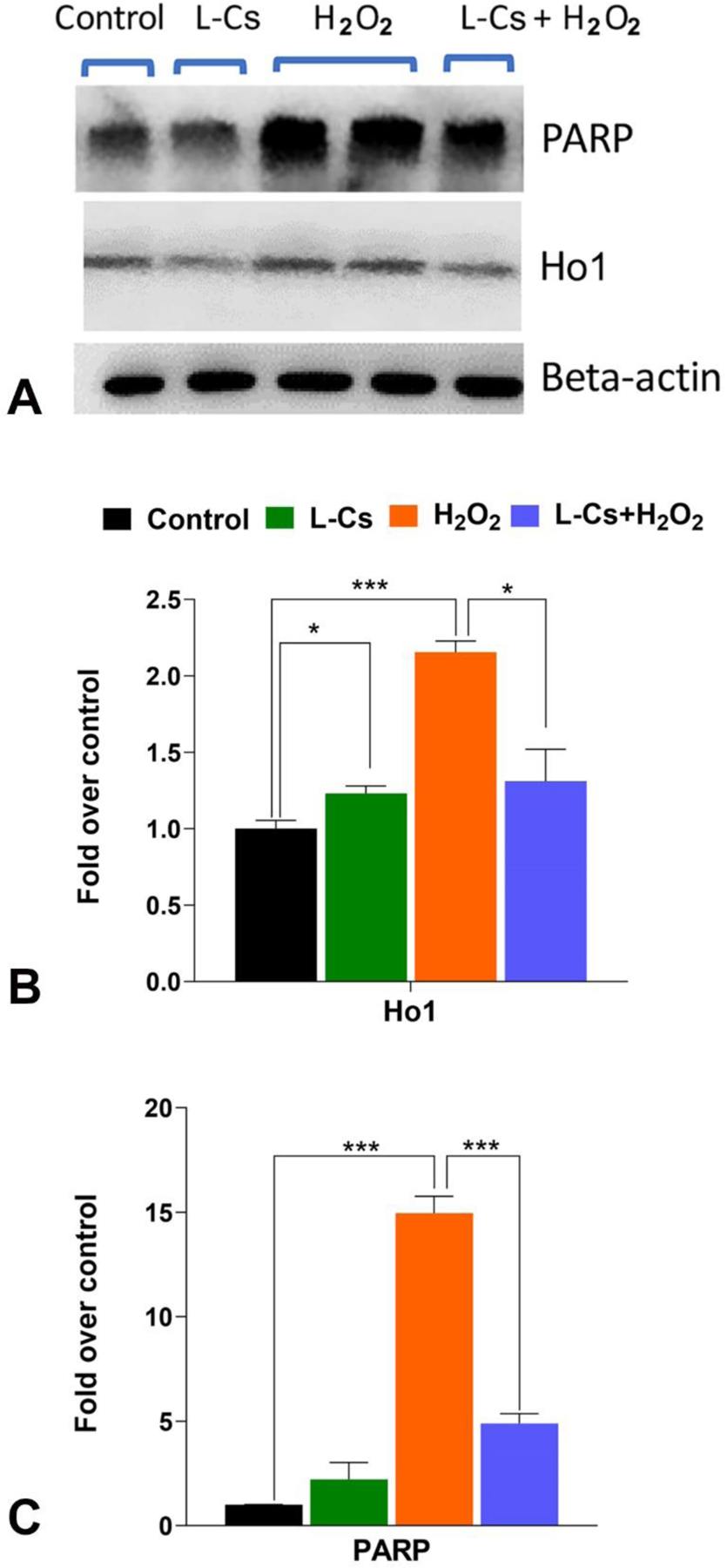
A: Expression and quantification of heme oxygenase 1 (Ho1) and Poly (ADP-ribose) polymerase (PARP) proteins in 661W cells was measured by western blot analysis. Proteins were extracted and subjected to western blot with anti-Ho1 and anti-PARP antibodies. Lane 1 (control): no treatment; lane 2 (L-Cs): 10 μM L-Cs treated cells; lanes 3 and 4 (H2O2): 300 μM H2O2 treated cells; lane 5 (L-Cs+ H2O2): cotreated with 10 μM L-Cs and 300 μM H2O2. B: Quantification of Ho1 in 661W cells with western blot. C: Quantification of PARP in 661W cells with western blot. Quantification of Ho1 and PARP was obtained with densitometric analysis and normalized with β-actin. (n=3; values are represented as mean ± SEM; t-test; *p < 0.05, ***p < 0.001).
4. Discussion
The present study investigated how inhibition of ceramide biosynthesis by small molecule inhibitor L-Cycloserine affects oxidative stress-mediated retinal cell death in vitro. Oxidative stress is reported to be a key contributor of the pathogenesis of many neurodegenerative diseases, including retinal degenerative diseases (Beatty et al., 2000; Campochiaro et al., 2015; Kowluru and Chan, 2007). Factors like exposure to UV light and smoking can trigger the generation of ROS in the RPE cells of AMD-affected eyes, initiating oxidative stress and ultimately contributing to the disease pathology (Jarrett and Boulton, 2012). One of the primary byproducts of ROS is the superoxide anion, which can generate hydroxyl radicals directly or in conjunction with its secondary product, H2O2, leading to lipid peroxidation in the retina and, ultimately, cell death (Rao, 1990). Multiple studies, including our own, have demonstrated that oxidative stress induced by various sources, such as light, chemical, and physical factors, leads to increased ceramide biosynthesis (Chen et al., 2013; Chimin et al., 2017; Hannun and Obeid, 2008; Sanvicens and Cotter, 2006). Furthermore, multiple reports have highlighted the association between ceramide accumulation and retinal cell death (Chen et al., 2012; Chen et al., 2013; Garanto et al., 2013; Stiles et al., 2016). Myriocin, a potent inhibitor of ceramide de-novo biosynthesis, exhibited promising therapeutic potential in retinal degenerative diseases. In Tvrm4 mice, a model of autosomal dominant RP, Myriocin administration through various routes correlated with reduced retinal ceramides, preserving electroretinogram (ERG) responses, and alleviated oxidative damage, suggesting its efficacy independent of the underlying mutation(Piano et al., 2020). Myriocin demonstrated the ability to rescue photoreceptors from apoptotic death in the rd10 mouse model of RP, through intraocular injections and noninvasive eye drop treatments using solid-lipid nanoparticle(Strettoi et al., 2010a). Moreover, Myriocin loaded solid-lipid nanoparticle eye-drops in another study using this rd10 mouse model of RP, demonstrated the rescue of rods from degeneration and subsequent preservation of cones, inner retinal layers, and overall visual performance (Piano et al., 2013). As part of our ongoing efforts to target ceramide biosynthesis pathways in order to mitigate ceramide accumulation and thereby protect retinal photoreceptor cells against damage induced by oxidative stress (Chen et al., 2013; Stiles et al., 2016; Sugano et al., 2019), we hypothesized that L-Cycloserine, another potent inhibitor of de-novo ceramide biosynthesis, would protect photoreceptor cells from oxidative stress-mediated damage by reducing ceramide level through inhibiting its biosynthesis. The results of this study can help in the assessment and subsequent development of L-Cycloserine as a therapeutic candidate for the treatment and prevention of retinal degenerative diseases.
Cycloserine exists in two stereochemical configurations: D and L. D-Cycloserine, an FDA-approved drug, was initially introduced to the market in 1952 as ‘Seromycin’ (Eli Lilly). This compound is a broad-spectrum antibiotic that can be derived from a strain of Streptomyces orchidaceus or synthesized. D-cycloserine has been used as a second-line treatment for tuberculosis (2008). Structurally similar to alanine, D-Cycloserine functions by inhibiting bacterial cell wall biosynthesis through two mechanisms: L-alanine racemase and D-alanyl-D-alanine synthetase (Jack, 1990). Both enantiomers can be viewed as cyclic analogs of serine and/or alanine and have been proven to be irreversible inhibitors of a variety of pyridoxal 5’-phosphate (PLP)-dependent enzymes, such as transaminases, racemases, and decarboxylases (Lowther et al., 2010). Ceramide synthesis in a cell typically occurs through three main pathways: de-novo biosynthesis, hydrolysis from complex sphingolipids or sphingomyelinase (SMase) pathway, and the salvage pathway (Chen et al., 2013). The initial and rate-limiting step in de-novo ceramide biosynthesis involves the condensation of L-serine with palmitoyl CoA to produce 3-ketodihydrosphingosine (Lowther et al., 2010). This reaction is catalyzed by the PLP-dependent enzyme Srine-palmitoyl transferase (SPT), which belongs to the α-oxoamine synthase (AOS) subfamily (Chang et al., 2022). It has been reported that both L- and D-Cycloserine inactivate SPT by transamination, resulting in the formation of free pyridoxamine 5′-phosphate (PMP) and β-aminooxy acetaldehyde. These products remain bound at the enzyme’s active site, a process that involves the opening of the cycloserine ring followed by decarboxylation (Lowther et al., 2010). L-Cycloserine is reportedly a highly effective SPT inhibitor, displaying a 100-fold greater inhibition compared to D-Cycloserine (Sundaram and Lev, 1984). Consequently, it is reasonable to expect that L-Cycloserine, as a potent SPT inhibitor, can reduce ceramide levels and other complex sphingolipids in the cell.
To comprehend the impact of L-Cycloserine on sphingolipid metabolism in 661W cells, we treated these cells to varying doses of L-Cycloserine for 24 hours. Our findings reveal that L-Cycloserine treatment significantly reduces the levels of bioactive ceramide and other associated sphingolipids from the MHC and SM class (Figure 1). This reduction is indicative of the role of L-Cycloserine as an effective SPT inhibitor, as established, interfering with the de novo pathway of ceramide biosynthesis. Consequently, the reduction in ceramide levels due to increased L-Cycloserine dosage results in a significant decrease in total sphingosine (So) and ceramide-1-phosphate (C1P) levels, as they are directly derived from ceramide (Supplementary Figure 2). While sphingosine-1-phosphate (S1P) also exhibits a reduction with increasing L-Cycloserine concentration (S1P being directly phosphorylated from So), the decrease is not statistically significant (Supplementary Figure 2).
The effect of L-Cycloserine on sphingolipid metabolism in 661W cells can explain the effect of L-Cycloserine on cell viability. The cell viability (MTT) assay, with 24 hours treatment of L-Cycloserine to the 661W cells, revealed a clear dose-dependent cytotoxicity pattern (Figure 2A) that can be attributed to the essential role of ceramide in membrane structure and function, which is crucial for cell integrity and differentiation (Jarvis and Grant, 1998; Merrill et al., 1997; Spiegel and Merrill Jr, 1996). Therefore, two L-Cycloserine doses, 10 and 25 μM, which are well below the IC50 value (~60 μM), were used for further investigation of cell viability in a 24-hour treatment using the LDH assay. The cytotoxicity of the drug as measured by the MTT assay is also reflected in this LDH assay result, which demonstrates a cytotoxicity of around 10% at a dose of 25 μM (Figure 2B). In an effort to minimize L-Cycloserine-induced toxic effects, the exposure time was reduced to 3 hours while still maintaining doses below the IC50 value. Under these conditions, we observed negligible toxicity, even at the maximum dose of 50 μM of L-Cycloserine (Figure 2C).
To examine our hypothesis that L-Cycloserine can protect 661W cells from H2O2-induced oxidative stress, we conducted a dose-response study with different doses of H2O2. This was integral in choosing a suitable dose of H2O2, causing an optimum level of cytotoxicity (Figure 3A). In both the 24-hour pretreatment and the 3-hour co-treatment studies, L-Cycloserine significantly protected the cells from oxidative stress in a dose-dependent manner (Figure 3B and 3C, respectively). Additionally, the 3-hour co-treatment showed minimal drug-induced cytotoxicity, suggesting that a shorter drug exposure significantly protected the cells from H2O2-induced oxidative stress with minimal side effects. Furthermore, quantitative analysis using LC-MS/MS revealed that L-Cycloserine and H2O2 had no interaction during co-treatment, indicating that the observed protection is primarily due to L-Cycloserine and not due to a byproduct of L-Cycloserine and H2O2 interaction (Supplementary Figure 3).
Further evaluation of L-Cycloserine-induced protection in 661W cells against H2O2-induced oxidative stress involved a detailed sphingolipid analysis of the groups subjected to the 3-hour co-treatment study. 10 μM L-Cycloserine dose was chosen, as it was the lowest dose to provide the most significant protection against H2O2-induced oxidative stress (~6-fold less than the IC50 value of L-Cycloserine obtained from the MTT assay, Figure 2A). Although the results revealed no significant differences between the various groups in terms of total Cer and SM, co-treatment with L-Cycloserine and H2O2 significantly reduced total MHC and total SPL compared to the H2O2-treated cells (Figure 4A), supporting L-Cycloserine’s role as ceramide biosynthesis inhibitor. No significant difference between the various groups can be explained as the 3-hour co-treatment study did not provide sufficient time to observe significant differences between the sphingolipids, contrary to what was seen in the study with L-Cycloserine treatment for 24-hour. However, there was an increase in the relative percentage of ceramide in the H2O2-treated cells compared to the control (Figure 4B2). This upregulation of ceramide under oxidative stress supports our initial hypothesis. Additionally, as expected, the co-treatment of L-Cycloserine and H2O2 successfully reduced the ceramide fraction back to control levels, which is also consistent with the L-Cycloserine-mediated ceramide biosynthesis inhibition (Figure 4B4). Additionally, the cells treated with H2O2 exhibited increased levels of S1P and C1P, both of which have anti-apoptotic properties (Newton et al., 2015), indicating a stress-induced activation of the salvage pathway to counteract H2O2-induced apoptosis (Supplementary Figure 4). On exposure of the cells to 3 hours of oxidative stress, dihydroceramide species C16DH (P < 0.01 and P <0.001, respectively) significantly increased compared to the control, suggesting the activation of the de-novo ceramide biosynthesis pathway (Table 2 and Supplementary Table 3). C16DH ceramide is an integral intermediate in the de-novo ceramide biosynthesis, further supporting the notion that oxidative stress induces de-novo ceramide biosynthesis, which aligns with previous research findings (Chen et al., 2013; Chimin et al., 2017; Hannun and Obeid, 2008; Sanvicens and Cotter, 2006). Co-treatment with L-Cycloserine and H2O2 significantly reduced C16DH (P < 0.05 and P <0.001, respectively) compared to H2O2-induced oxidative stress cells (Table 2 and Supplementary Table 3), underscoring the role of L-Cycloserine as an inhibitor of the de-novo ceramide biosynthesis pathway. Additionally, the overall trend from the relative composition or mol percent analysis of sphingolipids (Supplementary Table 3) is that oxidative stress increases the short-chain ceramides and decreases the long-chain ceramides. Elevated short-chain ceramide levels are often associated with various inflammatory conditions (Uche et al., 2021), aligning with the induction of an inflammatory response by oxidative stress conditions (Hussain et al., 2016). However, this trend is reversed, with the altered sphingolipids returning to normal levels when co-treated with L-Cycloserine and H2O2. Interestingly, the group treated with L-Cycloserine alone for this 3 hour co-treatment study showed no significant differences in individual species of Cer, MHC, and SM when compared to control cells (Table 2). This outcome explains the negligible cytotoxicity observed at a dose of 10 μM L-Cycloserine in our LDH assay for the co-treatment plan (Figure 2C). In summary, our findings suggest the following: 1) H2O2-induced oxidative stress results in changes in sphingolipid levels, particularly an increased level of Cer, which contributes to cell death in 661W cells; and 2) L-Cycloserine inhibition of SPT and thereby the de-novo ceramide biosynthesis pathway can prevent cell death by reducing the elevated levels of ceramide and other associated sphingolipids.
We also evaluated how the relevant antioxidant, apoptotic, and sphingolipid pathway genes (Figure 5) and selective proteins (Figure 6) are affected in this co-treatment study. 661W cells under oxidative stress conditions demonstrate higher expression of Ho1 gene as well as the protein (Figure 5A, 6B). Heme oxygenase 1 is an intracellular antioxidant representing the stress-induced isoform of heme oxygenases (Motterlini et al., 2000). It is a widely distributed and redox-sensitive stress protein (Motterlini et al., 2000). It catalyzes the degradation of heme to generate carbon monoxide, free ferrous iron, and biliverdin (Choi and Alam, 1996). It is induced rapidly after oxidative stress to act as a potent endogenous factor for the resolution of stress-induced inflammatory injury (Chatterjee, 2021). Treatment with L-Cycloserine significantly reduces Ho1 gene back to a normal control cellular level, indicating mitigation of the H2O2-mediated stress (Figure 5). Oxidative stress condition also significantly induces expression of Mt2 and reduces Trx1 expression (Figure 5), which are all involved in antioxidant and/or apoptotic pathways (Nishinaka et al., 2001; Ruttkay-Nedecky et al., 2013). Thus, L-Cycloserine helps in cell survival against oxidative stress through the modulation of apoptotic and antioxidant pathways. However, the precise mechanisms behind these actions and the exact role of ceramide in these processes are yet to be fully established. The expression of genes like Cers2 and Cers4 is significantly induced in the presence of oxidative stress (Figure 5), indicating a potential stimulation of increased ceramide synthesis as a cellular response to stress. Induction of Sphk1 gene (Figure 5) may reflect a genomic response aimed at enhancing the synthesis of S1P, a molecule that promotes cell proliferation and migration through signaling via one of five S1P receptors (Gómez-Muñoz, 2006; Maceyka et al., 2002; Tani et al., 2007) but also to counter the increase in ceramide level. This delicate balance between ceramide and S1P, often referred to as ‘sphingolipid rheostat’, ultimately determines whether the cell proceeds toward apoptosis or proliferation (Huwiler and Pfeilschifter, 2006).
In 661W cells exposed to oxidative stress, PARP expression is found to be significantly induced (Figure 6). PARP is an enzyme naturally occurring in cells, stimulated in response to cell death, and is a marker of the parthanatos-mediated cell death pathway (David et al., 2009). This observation is significant, given that PARP is involved in DNA excision repair, and elevation in PARP activation has been associated with apoptosis triggered by conditions such as cerebral ischemia, inflammation, and oxidative stress injuries (David et al., 2009). PARP has also been implicated in ceramide-induced apoptosis (Prado Spalm et al., 2019). Previous studies have reported that C2-Cer induces photoreceptor cell death through a novel, caspase-independent mechanism involving the activation of PARP (Prado Spalm et al., 2019). This pathway leads to a decline in mitochondrial membrane potential, calpain activation, and the translocation of apoptosis-inducing factor (AIF) (David et al., 2009). Co-treatment of 661W cells with L-Cycloserine and H2O2 significantly reduces PARP levels back to normal (Figure 6). These biochemical findings support the hypothesis that H2O2 is capable of activating the cellular antioxidative defense mechanism by upregulating the relevant genes and proteins in 661W cells. Importantly, this effect is mitigated in the presence of L-Cycloserine, as observed during co-treatment with H2O2 for 3 hours, highlighting its protective role against H2O2-mediated oxidative stress-induced ceramide-mediated cell death.
In summary, L-Cycloserine, previously identified as an SPT inhibitor in multiple studies, (Geekiyanage et al., 2013; Astarita et al., 2015; Geekiyanage et al., 2013; German et al., 2006; Hinkovska-Galcheva et al., 2003; Lewandowski et al., 2022; Meyer and de Groot, 2003b) was examined in our investigation for its impact on ceramide levels and subsequent protection of 661W cells from H2O2-mediated oxidative stress. The study revealed a significant reduction in ceramide levels and protection of 661W cells following L-Cycloserine treatment. We conclude that this observed protection demonstrated by L-Cycloserine might be attributed to SPT inhibition. However, further experiments are necessary to prove SPT inhibition by L-Cycloserine in this oxidative stress induced 661W cell model. Nevertheless, our in vitro study demonstrated that bioactive ceramide can be targeted to prevent oxidative stress induced cell death by using L-Cycloserine.
Supplementary Material
Highlights:
L-Cycloserine treatment can decrease bioactive ceramide and associated sphingolipids in 661W cells by inhibiting de-novo ceramide biosynthesis via the Serine palmitoyl transferase (SPT) enzyme.
A nontoxic dose of L-Cycloserine can provide significant protection of 661W cells against H2O2-induced oxidative stress.
Comprehensive analysis of various antioxidant, apoptotic, and sphingolipid pathway genes and proteins affirms the ability of L-Cycloserine to modulate these pathways.
Our research generates valuable insights into the promising therapeutic use of L-Cycloserine for degenerative retinal diseases associated with ceramide-induced pathways.
Acknowledgments:
Dr. Soumyajit Majumdar (Professor, Department of Pharmaceutics and Drug Delivery, University of Mississippi) is gratefully acknowledged by the authors for his support.
Funding:
This research was supported by the funding from National Institutes of Health (National Eye Institute grants [EY022071, R01 EY031316] (N.M.), R01 AI139072 (C.E.C.), P01 CA171983-Project 1 (C.E.C.), and GM137578 (to C.E.C.)), US Department of Defense Office of the Congressionally Directed Medical Research Programs (CDMRP), Vision Research Program grant (W81XWH-20-1-0900) (N.M.), US Dept of Veterans’ Administration (VA Merit Review Award I01BX004893) (N.M.), VA Merit Review award, BX001792 (C.E.C.), VA Merit Review award, BX 006063 (C.E.C.), and a Senior Research Career Scientist Award, IK6BX004603 (C.E.C.)), and Research to Prevent Blindness Inc., USA (N.M.). This work was also supported by a center grant to the UVA Comprehensive Cancer Center from the National Cancer Institute (2P30CA044579-26). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Abbreviations:
- RP
Retinitis Pigmentosa
- DR
Diabetic Retinopathy
- AMD
Age-Related Macular Degeneration
- SPT
Serine palmitoyl transferase
- ROS
Reactive oxygen species
- RPE
Retinal pigment epithelial
- MHC
Monohexosylceramide
- CerS
Ceramide synthase
- LIRD
light-induced retinal degeneration
- SD
Sprauge Dawley
- AAV
adeno-associated virus
- LDH
lactate dehydrogenase
- DMEM
Dulbecco’s modified Eagle’s medium
- PBS
phosphate-buffered saline
- MRM
Multiple Reaction Monitor
- RT-PCR
reverse transcriptase polymerase chain reaction
- qRT-PCR
quantitative reverse transcriptase polymerase chain reaction
- BSA
Bovine serum albumin
- Cer
ceramide
- MHC
monohexosylceramide
- SM
sphingomyelin
- SPL
sphingolipid
- So
sphingosine
- C1P
ceramide-1-phosphate
- S1P
Sphingosine-1-phosphate
- Gapdh
Glyceraldehyde 3-phosphate dehydrogenase
- Rpl19
Ribosomal Protein L19
- Ho1
heme oxygenase 1
- PARP
poly-ADP ribose polymerase
- PLP
pyridoxal 5’-phosphate
- SMase
sphingomyelinase
- AOS
α-oxoamine synthase
- PMP
pyridoxamine 5′-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that they have no conflicts of interest with the contents of this article.
References
- 2008. Cycloserine. Tuberculosis 88, 100–101. [DOI] [PubMed] [Google Scholar]
- Acharya U, Patel S, Koundakjian E, Nagashima K, Han X, Acharya JK, 2003. Modulating sphingolipid biosynthetic pathway rescues photoreceptor degeneration. Science (New York, N.Y.) 299, 1740–1743. [DOI] [PubMed] [Google Scholar]
- Astarita G, Avanesian A, Grimaldi B, Realini N, Justinova Z, Panlilio LV, Basit A, Goldberg SR, Piomelli D, 2015. Methamphetamine accelerates cellular senescence through stimulation of de novo ceramide biosynthesis. PloS one 10, e0116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty S, Koh H, Phil M, Henson D, Boulton M, 2000. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Survey of ophthalmology 45, 115–134. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA, Strauss RW, Lu L, Hafiz G, Wolfson Y, Shah SM, Sophie R, Mir TA, Scholl HP, 2015. Is There Excess Oxidative Stress and Damage in Eyes of Patients with Retinitis Pigmentosa? Antioxidants & redox signaling 23, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H-Y, Lo L-H, Lan Y-H, Hong M-X, Chan YT, Ko T-P, Huang Y-R, Cheng T-H, Liaw C-C, 2022. Structural insights into the substrate selectivity of α-oxoamine synthases from marine Vibrio sp. QWI-06. Colloids and Surfaces B: Biointerfaces 210, 112224. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, 2021. Endothelial Signaling in Vascular Dysfunction and Disease, Elsevier. [Google Scholar]
- Chen H, Tran J-TA, Brush RS, Saadi A, Rahman AK, Yu M, Yasumura D, Matthes MT, Ahern K, Yang HJRDD, 2012. Ceramide signaling in retinal degeneration. Advances in Experimental Medicine and Biology, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Tran JA, Eckerd A, Huynh TP, Elliott MH, Brush RS, Mandal NA, 2013. Inhibition of de novo ceramide biosynthesis by FTY720 protects rat retina from light-induced degeneration. J Lipid Res 54, 1616–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimin P, Andrade ML, Belchior T, Paschoal VA, Magdalon J, Yamashita AS, Castro É, Castoldi A, Chaves-Filho AB, Yoshinaga MY, Miyamoto S, Câmara NO, Festuccia WT, 2017. Adipocyte mTORC1 deficiency promotes adipose tissue inflammation and NLRP3 inflammasome activation via oxidative stress and de novo ceramide synthesis. J Lipid Res 58, 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi AM, Alam J, 1996. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. American journal of respiratory cell and molecular biology 15, 9–19. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein R, Klein BE, 1993. Sunlight and age-related macular degeneration. The Beaver Dam Eye Study. Archives of ophthalmology (Chicago, Ill. : 1960) 111, 514–518. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein R, Klein BE, Nondahl DM, 2001. Sunlight and the 5-year incidence of early age-related maculopathy: the beaver dam eye study. Archives of ophthalmology (Chicago, Ill. : 1960) 119, 246–250. [PubMed] [Google Scholar]
- Daiger SP, 1996–2022. Retinal Information Network. [Google Scholar]
- David KK, Andrabi SA, Dawson TM, Dawson VL, 2009. Parthanatos, a messenger of death. Frontiers in bioscience (Landmark edition) 14, 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso LA, Kim D, Frost A, Callahan A, Hageman G, 2006. The role of inflammation in the pathogenesis of age-related macular degeneration. Survey of ophthalmology 51, 137–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galor A, Sanchez V, Jensen A, Burton M, Maus K, Stephenson D, Chalfant C, Mandal N, 2022. Meibum sphingolipid composition is altered in individuals with meibomian gland dysfunction-a side by side comparison of Meibum and Tear Sphingolipids. The ocular surface 23, 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garanto A, Mandal NA, Egido-Gabas M, Marfany G, Fabrias G, Anderson RE, Casas J, Gonzalez-Duarte RJEe.r., 2013. Specific sphingolipid content decrease in Cerkl knockdown mouse retinas. Experimental eye research 110, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner KL, Cideciyan AV, Swider M, Dufour VL, Sumaroka A, Komáromy AM, Hauswirth WW, Iwabe S, Jacobson SG, Beltran WA, Aguirre GD, 2020. Long-Term Structural Outcomes of Late-Stage RPE65 Gene Therapy. Molecular therapy : the journal of the American Society of Gene Therapy 28, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geekiyanage H, Upadhye A, Chan C, 2013. Inhibition of serine palmitoyltransferase reduces Aβ and tau hyperphosphorylation in a murine model: a safe therapeutic strategy for Alzheimer’s disease. Neurobiology of aging 34, 2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German OL, Miranda GE, Abrahan CE, Rotstein NP, 2006. Ceramide is a Mediator of Apoptosis in Retina Photoreceptors. Investigative Ophthalmology & Visual Science 47, 1658–1668. [DOI] [PubMed] [Google Scholar]
- Gómez-Muñoz A, 2006. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochimica et biophysica acta 1758, 2049–2056. [DOI] [PubMed] [Google Scholar]
- Granzotto A, Bomba M, Castelli V, Navarra R, Massetti N, d’Aurora M, Onofrj M, Cicalini I, Del Boccio P, Gatta V, Cimini A, Piomelli D, Sensi SL, 2019. Inhibition of de novo ceramide biosynthesis affects aging phenotype in an in vitro model of neuronal senescence. Aging 11, 6336–6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi F, Marti A, Munz K, Remé CE, 1997. Light-induced apoptosis: differential timing in the retina and pigment epithelium. Experimental eye research 64, 963–970. [DOI] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM, 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature Reviews Molecular Cell Biology 9, 139–150. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva V, Boxer L, Mansfield PJ, Schreiber AD, Shayman JA, 2003. Enhanced phagocytosis through inhibition of de novo ceramide synthesis. The Journal of biological chemistry 278, 974–982. [DOI] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL, 2008. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature medicine 14, 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kutluer M, Adani E, Comitato A, Marigo V, 2021. New In Vitro Cellular Model for Molecular Studies of Retinitis Pigmentosa. International journal of molecular sciences 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N, 2016. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative medicine and cellular longevity 2016, 7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huwiler A, Pfeilschifter J, 2006. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Current pharmaceutical design 12, 4625–4635. [DOI] [PubMed] [Google Scholar]
- Jack DB, 1990. Biochemistry of antimicrobial action, 4th edn, by Franklin TJ and Snow GA, Chapman and Hall, 1989, 216 pages. ISBN 0-412-30260-8, paperback, price £12.95. 11, 553–553. [Google Scholar]
- Jarrett SG, Boulton ME, 2012. Consequences of oxidative stress in age-related macular degeneration. Molecular aspects of medicine 33, 399–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis WD, Grant S, 1998. The role of ceramide in the cellular response to cytotoxic agents. Current opinion in oncology 10, 552–559. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chan PS, 2007. Oxidative stress and diabetic retinopathy. Experimental diabetes research 2007, 43603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski D, Foik AT, Smidak R, Choi EH, Zhang J, Hoang T, Tworak A, Suh S, Leinonen H, Dong Z, Pinto AFM, Tom E, Luu J, Lee J, Ma X, Bieberich E, Blackshaw S, Saghatelian A, Lyon DC, Skowronska-Krawczyk D, Tabaka M, Palczewski K, 2022. Inhibition of ceramide accumulation in AdipoR1–/– mice increases photoreceptor survival and improves vision. JCI Insight 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther J, Yard BA, Johnson KA, Carter LG, Bhat VT, Raman MC, Clarke DJ, Ramakers B, McMahon SA, Naismith JH, Campopiano DJ, 2010. Inhibition of the PLP-dependent enzyme serine palmitoyltransferase by cycloserine: evidence for a novel decarboxylative mechanism of inactivation. Molecular bioSystems 6, 1682–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Payne SG, Milstien S, Spiegel S, 2002. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochimica et biophysica acta 1585, 193–201. [DOI] [PubMed] [Google Scholar]
- MacKnight HP, Stephenson DJ, Hoeferlin LA, Benusa SD, DeLigio JT, Maus KD, Ali AN, Wayne JS, Park MA, Hinchcliffe EH, Brown RE, Ryan JJ, Diegelmann RF, Chalfant CE, 2019. The interaction of ceramide 1-phosphate with group IVA cytosolic phospholipase A(2) coordinates acute wound healing and repair. Science signaling 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal MN, Vasireddy V, Jablonski MM, Wang X, Heckenlively JR, Hughes BA, Reddy GB, Ayyagari R, 2006. Spatial and temporal expression of MFRP and its interaction with CTRP5. Invest Ophthalmol Vis Sci 47, 5514–5521. [DOI] [PubMed] [Google Scholar]
- Masuda T, Shimazawa M, Hara H, 2017. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxidative medicine and cellular longevity 2017, 9208489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus KD, Stephenson DJ, Ali AN, MacKnight HP, Huang HJ, Serrats J, Kim M, Diegelmann RF, Chalfant CE, 2022. Ceramide kinase regulates acute wound healing by suppressing 5-oxo-ETE biosynthesis and signaling via its receptor OXER1. J Lipid Res 63, 100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus KD, Stephenson DJ, Macknight HP, Vu NT, Hoeferlin LA, Kim M, Diegelmann RF, Xie X, Chalfant CE, 2023. Skewing cPLA(2)α activity toward oxoeicosanoid production promotes neutrophil N2 polarization, wound healing, and the response to sepsis. Science signaling 16, eadd6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni F, Safa H, Finnemann SC, 2014. Understanding photoreceptor outer segment phagocytosis: Use and utility of RPE cells in culture. Experimental eye research 126, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH Jr., Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E, 1997. Sphingolipids--the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicology and applied pharmacology 142, 208–225. [DOI] [PubMed] [Google Scholar]
- Meyer SG, de Groot H, 2003a. Cycloserine and threo-dihydrosphingosine inhibit TNF-alpha-induced cytotoxicity: evidence for the importance of de novo ceramide synthesis in TNF-alpha signaling. Biochimica et biophysica acta 1643, 1–4. [DOI] [PubMed] [Google Scholar]
- Meyer SGE, de Groot H, 2003b. Cycloserine and threo-dihydrosphingosine inhibit TNF-α-induced cytotoxicity: evidence for the importance of de novo ceramide synthesis in TNF-α signaling. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1643, 1–4. [DOI] [PubMed] [Google Scholar]
- Mondal K, Porter H, Cole J, Pandya HK, Basu SK, Khanam S, Chiu C-Y, Shah V, Stephenson DJ, Chalfant CE, Mandal N, 2022. Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome–Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Molecular Neurobiology 59, 3873–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal K, Takahashi H, Cole J, Del Mar NA, Li C, Stephenson DJ, Allegood J, Cowart LA, Chalfant CE, Reiner A, Mandal N, 2021. Systemic Elevation of n-3 Polyunsaturated Fatty Acids (n-3-PUFA) Is Associated with Protection against Visual, Motor, and Emotional Deficits in Mice following Closed-Head Mild Traumatic Brain Injury. Molecular Neurobiology 58, 5564–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ, 2000. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. The Journal of biological chemistry 275, 13613–13620. [DOI] [PubMed] [Google Scholar]
- Newton J, Lima S, Maceyka M, Spiegel S, 2015. Revisiting the sphingolipid rheostat: Evolving concepts in cancer therapy. Experimental cell research 333, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinaka Y, Masutani H, Nakamura H, Yodoi J, 2001. Regulatory roles of thioredoxin in oxidative stress-induced cellular responses. Redox report : communications in free radical research 6, 289–295. [DOI] [PubMed] [Google Scholar]
- Ohanian J, Ohanian V, 2001. Sphingolipids in mammalian cell signalling. Cellular and Molecular Life Sciences CMLS 58, 2053–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano I, D’Antongiovanni V, Novelli E, Biagioni M, Dei Cas M, Paroni RC, Ghidoni R, Strettoi E, Gargini C, 2020. Myriocin Effect on Tvrm4 Retina, an Autosomal Dominant Pattern of Retinitis Pigmentosa. Frontiers in neuroscience 14, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piano I, Novelli E, Gasco P, Ghidoni R, Strettoi E, Gargini C, 2013. Cone survival and preservation of visual acuity in an animal model of retinal degeneration. 37, 1853–1862. [DOI] [PubMed] [Google Scholar]
- Prado Spalm FH, Vera MS, Dibo MJ, Simón MV, Politi LE, Rotstein NP, 2019. Ceramide Induces the Death of Retina Photoreceptors Through Activation of Parthanatos. Molecular Neurobiology 56, 4760–4777. [DOI] [PubMed] [Google Scholar]
- Qi H, Priyadarsini S, Nicholas SE, Sarker-Nag A, Allegood J, Chalfant CE, Mandal NA, Karamichos D, 2017. Analysis of sphingolipids in human corneal fibroblasts from normal and keratoconus patients. Journal of Lipid Research 58, 636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, 1990. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Transactions of the American Ophthalmological Society 88, 797–850. [PMC free article] [PubMed] [Google Scholar]
- Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R, 2013. The role of metallothionein in oxidative stress. International journal of molecular sciences 14, 6044–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvicens N, Cotter TG, 2006. Ceramide is the key mediator of oxidative stress-induced apoptosis in retinal photoreceptor cells. Journal of Neurochemistry 98, 1432–1444. [DOI] [PubMed] [Google Scholar]
- Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel JA, 2016. Emerging therapies for inherited retinal degeneration. Science translational medicine 8, 368rv366. [DOI] [PubMed] [Google Scholar]
- Shen J, Yang X, Dong A, Petters RM, Peng YW, Wong F, Campochiaro PA, 2005. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. Journal of cellular physiology 203, 457–464. [DOI] [PubMed] [Google Scholar]
- Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA, 2007. Oxidative damage in age-related macular degeneration. Histology and histopathology 22, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Merrill AH Jr, 1996. Sphingolipid metabolism and cell growth regulation. The FASEB Journal 10, 1388–1397. [DOI] [PubMed] [Google Scholar]
- Stephenson DJ, MacKnight HP, Hoeferlin LA, Washington SL, Sawyers C, Archer KJ, Strauss JF 3rd, Walsh SW, Chalfant CE, 2023. Bioactive lipid mediators in plasma are predictors of preeclampsia irrespective of aspirin therapy. J Lipid Res 64, 100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles M, Qi H, Sun E, Tan J, Porter H, Allegood J, Chalfant CE, Yasumura D, Matthes MT, LaVail MM, Mandal NA, 2016. Sphingolipid profile alters in retinal dystrophic P23H-1 rats and systemic FTY720 can delay retinal degeneration [S]. Journal of Lipid Research 57, 818–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stith JL, Velazquez FN, Obeid LM, 2019. Advances in determining signaling mechanisms of ceramide and role in disease. J Lipid Res 60, 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R, 2010a. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. 107, 18706–18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strettoi E, Gargini C, Novelli E, Sala G, Piano I, Gasco P, Ghidoni R, 2010b. Inhibition of ceramide biosynthesis preserves photoreceptor structure and function in a mouse model of retinitis pigmentosa. Proceedings of the National Academy of Sciences 107, 18706–18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugano E, Edwards G, Saha S, Wilmott LA, Grambergs RC, Mondal K, Qi H, Stiles M, Tomita H, Mandal N, 2019. Overexpression of acid ceramidase (ASAH1) protects retinal cells (ARPE19) from oxidative stress [S]. Journal of Lipid Research 60, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram KS, Lev M, 1984. Inhibition of sphingolipid synthesis by cycloserine in vitro and in vivo. J Neurochem 42, 577–581. [DOI] [PubMed] [Google Scholar]
- Tan E, Ding X-Q, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR, 2004a. Expression of Cone-Photoreceptor–Specific Antigens in a Cell Line Derived from Retinal Tumors in Transgenic Mice. Investigative Ophthalmology & Visual Science 45, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR, 2004b. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest Ophthalmol Vis Sci 45, 764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani M, Ito M, Igarashi Y, 2007. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cellular signalling 19, 229–237. [DOI] [PubMed] [Google Scholar]
- Totan Y, Yağci R, Bardak Y, Ozyurt H, Kendir F, Yilmaz G, Sahin S, Sahin Tiğ U, 2009. Oxidative macromolecular damage in age-related macular degeneration. Current eye research 34, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Uche LE, Gooris GS, Bouwstra JA, Beddoes CM, 2021. Increased Levels of Short-Chain Ceramides Modify the Lipid Organization and Reduce the Lipid Barrier of Skin Model Membranes. Langmuir : the ACS journal of surfaces and colloids 37, 9478–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu NT, Kim M, Stephenson DJ, MacKnight HP, Chalfant CE, 2022. Ceramide Kinase Inhibition Drives Ferroptosis and Sensitivity to Cisplatin in Mutant KRAS Lung Cancer by Dysregulating VDAC-Mediated Mitochondria Function. Molecular cancer research : MCR 20, 1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wert KJ, Lin JH, Tsang SH, 2014. General pathophysiology in retinal degeneration. Developments in ophthalmology 53, 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe DS, Allegood JC, Gentile LB, Fox TE, Kester M, Chalfant CE, 2010. Use of high performance liquid chromatography-electrospray ionization-tandem mass spectrometry for the analysis of ceramide-1-phosphate levels. J Lipid Res 51, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesinghe DS, Brentnall M, Mietla JA, Hoeferlin LA, Diegelmann RF, Boise LH, Chalfant CE, 2014. Ceramide kinase is required for a normal eicosanoid response and the subsequent orderly migration of fibroblasts. J Lipid Res 55, 1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler BS, Boulton ME, Gottsch JD, Sternberg P, 1999. Oxidative damage and age-related macular degeneration. Molecular vision 5, 32. [PMC free article] [PubMed] [Google Scholar]
- Yadav RS, Tiwari NK, 2014. Lipid Integration in Neurodegeneration: An Overview of Alzheimer’s Disease. Molecular Neurobiology 50, 168–176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


