Abstract
Effective, rigorously evaluated nonpharmacological treatments for chronic pain are needed. This study compared the effectiveness of training in hypnosis (HYP) and mindfulness meditation (MM) to an active education control (ED). Veterans (N=328) were randomly assigned to 8 manualized, group-based, in-person sessions of HYP (n = 110), MM (n = 108), or ED (n = 110). Primary (average pain intensity; API) and secondary outcomes were assessed at pretreatment, posttreatment, and 3- and 6-months posttreatment. Treatment effects were evaluated using linear regression, a generalized estimating equation approach, or a Fisher exact test, depending on the variable. There were no significant omnibus between-group differences in pre- to posttreatment change in API, however pre- to posttreatment improvements in API and several secondary variables were seen for participants in all three conditions. Participation in MM resulted in greater decreases in API and pain interference at 6-months posttreatment relative to ED. Participation in HYP resulted in greater decreases in API, pain interference, and depressive symptoms at 3- and 6-months posttreatment compared to ED. No significant differences on outcomes between HYP and MM were detected at any time point. This study suggests that all three interventions provide posttreatment benefits on a range of outcomes, but the benefits of HYP and MM continue beyond the end of treatment, while the improvements associated with ED dissipate over time. Future research is needed to determine whether the between-group differences that emerged posttreatment are reliable, whether there are benefits of combining treatments, and to explore moderating and mediating factors.
TRIAL REGISTRATION
clinicaltrials.gov identifier: NCT02653664
INTRODUCTION
Recent research estimates that 19% of adults in the US have chronic pain, although rates vary by age, gender, ethnicity, and education level.[45] Veterans of the US Armed Forces are a population of particular importance, as Veterans report greater pain prevalence and greater pain severity than civilians.[53] [31]
Hypnosis (HYP) and mindfulness meditation (MM) are two complementary and integrative health (CIH) treatments that have shown promise for pain management.[3; 9; 16; 33; 42; 55; 57; 59] Both come in manualized form, can be delivered individually, in groups, in person, or via telehealth,[20] have few contraindications or adverse effects,[42] and teach learnable skills.[42] Theoretically, MM for pain management trains individuals to shift their relationship to pain, such that is it non-judgmentally observed and accepted in the present moment.[19; 32] HYP, on the other hand, is theoretically distinguished from MM on the basis that this approach trains individuals to change the nature of the pain sensation.
Veterans report high interest in HYP, MM and similar CIH treatments. [29; 51; 60] There is strong evidence for the use of hypnosis in managing procedural and acute pain[44; 49; 57; 61] and growing evidence for hypnosis for a variety of chronic pain conditions[3; 57; 59]. Similarly, MM and its variants, [43; 47; 54], including Mindfulness-Based Stress Reduction and Mindfulness-Based Cognitive Therapy (MBCT)[19; 20] have demonstrated efficacy for improving pain intensity, pain interference, and pain-related affect.[7; 33] A recent expert panel concluded that there was sufficient evidence for HYP and MM to be used for clinical purposes but called for more rigorous trials before widespread implementation.[10; 24] Specifically, current studies of HYP and MM are limited to specific pain sub-populations,[38] all but two recent studies [16; 41] have been inadequately powered, and no studies to date have compared HYP and MM head-to-head nor included an active control condition.
This randomized controlled trial was designed to address the methodological limitations of prior research by comparing the effectiveness of HYP and MM to an active control (pain education, ED) in a sample of Veterans with chronic pain due to mixed etiology.[63] A mixed etiology sample was selected because many common chronic pain conditions are heterogeneous, with a high degree of overlap or co-prevalence with other common pain conditions, and studies that focus on individuals with a single pain condition may be limited in their generalizability and relevance.[50]
We hypothesized that HYP and MM would result in greater improvements in average pain intensity (API) from pre- to posttreatment (primary endpoint), compared to ED. Secondary analyses examined the effects of the interventions on additional outcomes, between-group differences in all outcome change scores at posttreatment and 3-months and 6-months posttreatment, and the and the proportion of treatment responders at follow-up timepoints.
METHODS
Study Design, Setting and Eligibility Criteria
Participants were recruited from the two Veterans Affairs (VA) medical centers that comprise VA Puget Sound Health Care System (VAPSHCS) into this two-site parallel-group randomized (1:1:1) controlled trial. All study activities occurred in person at the VA sites except optional electroencephalogram (EEG) assessments (not reported here), which were administered at the University of Washington. Study procedures were approved by the VAPSHCS and University of Washington Institutional Review Boards (see published protocol [63]). No substantive changes were made to the methods during the study.
Participants were Veterans who received healthcare at VAPSHCS between 2015 and 2019, and were recruited via clinician referral, medical record review, or self-referral. Eligibility was assessed via medical record review, participant self-report, and psychologist (RMW) screen.[63] Inclusion criteria were: (1) ≥ 18 years of age; (2) self-reported API of ≥ 3 and worst pain intensity (WPI) of ≥ 5 on a 0–10 scale in the last week; (3) duration of pain ≥ 3 months, (4) experiencing pain ≥ 75% of the time, and (5) ability to read, speak and understand English. Exclusion criteria were: (1) severe cognitive impairment defined as ≥ 2 errors on the Six-Item Screener;[14] (2) unstable/severe psychiatric or behavioral symptoms within the past 6 months; (3) psychotic or thought disorder; (4) psychiatric hospitalization for reasons other than suicidal ideation, homicidal ideation, or posttraumatic stress disorder (PTSD) within the past 5 years; (5) behavioral conditions precluding safe or effective group participation; (6) active suicidal ideation or delusional thinking; (7) problems communicating via telephone; and (8) reported average daily use of > 120 mg morphine equivalent dose (MED).
After providing written informed consent, participants were assessed in person at study enrollment (baseline), then by telephone at weeks 0 (pretreatment), 2, 4, and 6 (during treatment; not reported here), 8 (posttreatment), and at 3- and 6-months after treatment. Randomization occurred after the pretreatment assessment. There was no cross-over between arms during the main phase of the study. Participants were compensated up to $250 for completion of study assessments. Upon completion of the 6-month assessment, participants were invited to enroll in an open-label phase in which they could complete a second study intervention of their choice (data not reported here). At the study conclusion it was discovered that 19 subjects were inadvertently enrolled prior to the start date noted on clinicaltrials.gov (NCT02653664). However, no analyses were conducted using data from these subjects until all data were collected.
Randomization
To synchronize timing of assessments and interventions, subjects were recruited in cohorts (3 per study year, 18–34 participants per cohort). Randomization to condition was at the individual level and stratified on self-reported gender and pain type using a spreadsheet of random numbers generated by the data manager. A research staff member unaware of allocation communicated a numeric randomization code to an unmasked staff member, who conveyed randomization assignment to participants and scheduled interventions.
Concealment of treatment condition
Participants were unaware of the study hypotheses. The interventionists were told of the study aims in lay terms at the time of their training (e.g., “see which treatment is most effective for pain intensity and other outcomes, and for whom, and whether gains are maintained”) but were unaware of the specific Aim 1 hypothesis. Additionally, study interventionists were unaware of the research enrollment status of group members (explained below). All outcome assessments and data analyses were conducted by staff who were unaware of treatment allocation.[63] No interim data analyses were conducted.
Interventions
As detailed in the published protocol,[63] all three interventions were offered simultaneously in each of the 12 study cohorts, via eight 90-minute in-person group classes scheduled over 8–10 weeks at the two VA study sites. Group size varied between 3–12 participants and typically included a combination of trial participants (1–9 per class, n = 328 total), open label phase participants (n = 69 total), and non-research participants who met basic inclusion/exclusion criteria and wanted to receive the interventions but declined to enroll in the study (n = 187 total). Participants were asked to continue usual care for pain and other medical conditions during the trial.
Interventions were based on manualized protocols and materials developed by the author team and refined in prior trials (HYP[37; 38]; MM[19–21]; ED[27; 28]); they are available upon request from the corresponding author. Participants received a workbook and audio recordings specific to their assigned treatment condition to facilitate home practice between sessions. The interventions were delivered by a variety of health professionals on staff at VAPSHCS (N=50), including psychologists (n = 10), advanced psychology trainees (n = 25), nurses (n=4), occupational therapists (n=4), speech pathologists (n=3), as well as one of each of the following disciplines: physician, social worker, chiropractor, and physical therapist. Study interventionists led the classes as part of their normal clinical duties, within the clinical infrastructure, and were not paid by the grant. Intervention classes were scheduled as regular clinical appointments with associated encounter documentation requirements and provider workload credit.
All study interventionists completed required reading/self-study and participated in a 2-day in-person training (offered annually in all study years) covering all three interventions. All participated in twice-monthly supervision during treatment delivery to support fidelity. To offset possible bias related to intervention expertise, clinicians were required to participate in the study for three consecutive study cohorts (some clinicians participated for 6 or 9 cohorts) and to facilitate all three interventions in counterbalanced order. Clinicians with psychology training were paired with those in other disciplines to ensure that every class had at least one leader with group facilitation experience.
Hypnosis.
The premise underlying hypnosis pain treatment is that people can be trained to enter a state of focused attention that makes them more open to suggestions for making changes in perceptions, sensations, thoughts, and behaviors.[51] When used as a treatment for chronic pain, patients are taught self-hypnosis, making it possible for them to take advantage of the increased responsivity to suggestions associated with hypnotic inductions.[52] Hypnosis and training in self-hypnosis have been reported to have benefits not just for pain intensity but for pain-related problems with sleep, mood, and fatigue.[53–56] Each HYP session included a scripted hypnosis activity comprised of an induction, hypnotic and post-hypnotic suggestions, and a post-hypnosis alerting process, followed by discussion focused on encouraging home practice. In-session hypnotic inductions ranged from 15–30 minutes in length. All suggestions embedded in the hypnotic sessions related to some combination of increased comfort, increases in adaptive thoughts about or the meaning of pain, or improvement in co-morbid symptoms (e.g., improved mood and optimism, relaxation, sleep quality).[23] The audio recordings provided for home practice included all hypnosis inductions that were provided in the group sessions.
Mindfulness Meditation.
Mindfulness Meditation (MM) aims to train the mind to observe thoughts, emotions, and bodily sensations intentionally, on a moment-to-moment basis, with a non-judgmental attitude, subsequently fostering perception of these experiences as transient and variable. This combination of regulation of attention decoupled from emotion is hypothesized to be the central mechanism across forms of MM.[18] It has been purported that this, in conjunction with the cultivation of mindfulness and pain acceptance, underlies reductions in pain. The MM intervention taught participants a combination of Shamatha meditation, which involves training the mind for stability and maintaining focus on a specific object, as well as Vipassana, an open monitoring form of MM involving acknowledgement of any sensory, emotional or cognitive event that arises in the mind without evaluation, interpretation, or preference.[64] Each of the MM sessions included an in-session scripted MM practice (which ranged in length from 3 to 45 minutes) and guided inquiry that explored participants’ experiences with the practice and encouraged patient, gentle persistence in daily practice. The audio recordings provided for home practice included all meditation activities that were provided in the group sessions.
Education Control.
ED was used as an active control intervention matched to the HYP and MM interventions on time, attention, and modality. The ED intervention was designed to increase participants’ knowledge about chronic pain and increase perceived efficacy for pain self-management. The ED intervention utilized a combination of facilitated discussion and didactics. ED has been shown to be interesting, credible, and beneficial, but was not expected to have as large of a direct effect on pain intensity as HYP and MM.[28; 39] Sessions included didactic instruction and facilitated discussion about topics such as the biopsychosocial model, sleep hygiene, and mood.[28; 39] The audio recordings provided for home practice ranged from 15–25 minutes and included a review of information provided in the sessions, plus affirming messages for participants’ efforts.
Intervention Fidelity.
All sessions were audio-recorded; 25% of sessions were randomly selected from stratified blocks to ensure equivalent inclusion of each treatment condition and clinician facilitator team. Fidelity was reviewed using standardized therapist adherence rating forms scored and averaged across two raters. The rating forms were tailored for each condition and developed to include required elements, as well as proscribed elements (e.g., participants in the HYP group and only the HYP group would receive hypnotic inductions focused on improved pain experiences, etc.). The forms produced a percent score, with higher scores representing better fidelity. A score of 100% on required elements meant all elements were included, and no proscribed elements were included. Average fidelity adherence ratings were 97% across the clinicians and interventions, and no proscribed elements were detected. All interventions were delivered in accordance with the protocol as published.[63]
Measures
Baseline demographic variables included self-reported gender, race, and ethnicity, consistent with the US National Institutes of Health inclusion policies. All outcomes were assessed via telephone interview as a strategy to minimize missing data. We have used this approach successfully in a number of previous clinical trials.[41]
Primary Outcome
Average Pain Intensity (API) was assessed using a 0 (“No pain”) to 10 (“Worst pain imaginable”) numeric rating scale (NRS) of “…average pain in the past 24 hours.” The NRS was administered up to 4 times within one week at all assessment time points; each administration was separated by at least 24 hours. The goal was to gather four pain ratings if possible; the average API rating was calculated regardless of whether 1–4 pain ratings were gathered.[34–36] The primary outcome was pre- to posttreatment difference in API, which was selected to align with the original request for grant proposals and to facilitate comparison between pain intervention trials. Psychometric theory and research support composite pain measures as more reliable, valid, and sensitive to treatment effects than single ratings.[35] The 0–10 NRS has demonstrated its validity as a measure of pain intensity through its strong association with other pain measures as well as its ability to detect changes in pain with pain treatment. [25] A consensus panel has also recommended the 0–10 NRS as a core outcome measure of pain intensity in clinical trials of pain treatments.[26]
Additional analyses related to pain intensity examined between-group differences in change scores from pretreatment to 3- and 6-months posttreatment, the proportion of subjects reporting clinically meaningfully improvement (“treatment responders”), defined as a ≥2 point reduction in API, which corresponds approximately to a 30% improvement controlling for baseline pain [25; 26] at each time point, and change in API over time.[30]
Secondary Outcomes
Patient Reported Outcomes Measurement Information System® (PROMIS®) measures [4; 15; 17; 56] included Pain Interference SF 6A, Emotional Distress-Depression SF 8A, Emotional Distress -Anxiety SF 8A, and Sleep Disturbance SF 8A, all converted to T-scores. The validity and reliability of the PROMIS measures is well-established. For example, internal consistency of the PROMIS short forms is excellent; alpha coefficients are .95 for depression and .93 for anxiety.[56] The PROMIS Pain Interference SF 6A form has been shown to be reliable and have clinical validity in trials of interventions expected to impact pain.[5] Reliability and validity for the PROMIS Sleep Disturbance SF has also been established in general populations and clinical samples.[48] PTSD symptom severity was assessed using the PTSD Checklist for DSM-5 (PCL-5); possible scores range from 0–80, with higher scores indicating more severe symptoms. [11; 12] The PCL-5 has been shown to have strong internal consistency (α = .94), test-retest reliability (r = .82), and convergent (r = .74 to .85) and discriminant (r = .31 to .60) validity[11]
At posttreatment only, participants were asked how satisfied they were overall with the study treatment, with response options ranging from 0 = Very dissatisfied to 4 = Very satisfied. Three Global Impression of Change scales were assessed at posttreatment only, by asking participants to rate their overall perceived change in pain intensity, pain interference, and ability to manage pain since they began the program. Response options ranged from 1= Very much improved to 7= Very much worse. Psychometric data are not provided for this measure as it is not designed to represent a single underlying construct, but rather several 1-item domains. [58]
Two exploratory outcomes were assessed at each time point: Worst Pain Intensity (WPI) and opioid analgesic medication use. WPI was measured the same way as API, with 0–10 ratings of “Worst pain in the past 24 hours,” administered up to 4 times within one week at each assessment point, and then averaged. To measure opioid use, participants were asked to describe the medication type, dose, and frequency of doses over the past 7 days of any opioid medications taken at each time point. To facilitate recall for these, participants were asked to have their medications physically available at the time of each assessment, and research staff guided them through their personal inventory of medications. Responses were converted to morphine equivalent dose (MED) expressed as daily dose in mg. Because there was a large proportion of non-users (see Table 1), opioid use was converted to a binary variable dichotomized as any/no opioid use.
Table 1:
Descriptive analysis for demographic and clinical characteristics at pretreatment by randomization group
| Self-Reported Characteristic | Intervention Group |
P-valuea | ||
|---|---|---|---|---|
| ED (n=110) | HYP (n=110) | MM (n=108) | ||
|
| ||||
| Demographics | ||||
|
| ||||
| Age in years, mean (SD) | 53.5 (13.5) | 51.0 (12.6) | 55.0 (13.0) | .07 |
| Median (min, max) | 55.5 (24.0, 82.0) | 51.5 (24.0, 73.0) | 56.0 (26.0, 81.0) | |
|
| ||||
| Gender, n (% in category) | ||||
| Male | 80 (73) | 81 (74) | 80 (74) | .36 |
| Female | 27 (24) | 29 (26) | 28 (26) | |
| Transgender | 3 (3) | 0 (0) | 0 (0) | |
|
| ||||
| Race, n (%) in category | ||||
| Caucasian | 68 (62) | 71 (65) | 68 (63) | .72 |
| Black/African American | 19 (17) | 23 (21) | 16 (15) | |
| Asian | 3 (3) | 2 (2) | 4 (4) | |
| Otherb | 19 (17) | 13 (12) | 20 (18) | |
|
| ||||
| Hispanic/Latino, n (%) yes | 6 (6) | 10 (9) | 14 (13) | .16 |
|
| ||||
| Education level, n (%) in category | ||||
| High school or less | 11 (10) | 9 (8) | 6 (6) | .74 |
| Some college/Technical | 48 (44) | 54 (49) | 52 (48) | |
| College degree or higher | 51 (46) | 47 (43) | 50 (46) | |
|
| ||||
| Employment statusc, n (%) in category | ||||
| Unemployed | 54 (49) | 48 (44) | 36 (33) | .06 |
| Retired | 45 (41) | 42 (38) | 47 (44) | .72 |
| Employed full/part time | 26 (24) | 32 (29) | 30 (28) | .63 |
| Student full/part time | 4 (4) | 4 (4) | 9 (8) | .33 |
| Home maker | 6 (6) | 7 (6) | 4 (4) | .74 |
|
| ||||
| Married/Living with partner, n (%) yes | 67 (61) | 60 (55) | 55 (52) | .39 |
|
| ||||
| Homeless in past 6 months, n (%) yes | 3 (3) | 3 (3) | 2 (2) | > .99 |
|
| ||||
| Pain and health characteristics | ||||
|
| ||||
| Type of pain, n (% in each category) d | ||||
| Probable Neuropathic | 58 (53) | 53 (48) | 49 (45) | .78 |
| Probable Non-Neuropathic | 27 (24) | 26 (24) | 27 (25) | |
| Uncertain | 25 (23) | 31 (28) | 32 (30) | |
|
| ||||
| Very good/excellent health, n (% yes)e | 12 (12) | 8 (8) | 12 (12) | .60 |
|
| ||||
| Prior pain education, n (% yes) | 50 (46) | 42 (38) | 36 (33) | .19 |
|
| ||||
| Prior experience in hypnosis, n (% yes) | 17 (16) | 24 (22) | 27 (25) | .20 |
|
| ||||
| Prior experience in meditation, n (% yes) | 64 (58) | 63 (57) | 62 (57) | > .99 |
|
| ||||
| Pretreatment primary & secondary outcomes | ||||
|
| ||||
| Average Pain Intensity, mean (SD) | 5.8 (1.6) | 5.7 (1.8) | 5.9 (1.6) | .63 |
| Median (min, max) | 6.0 (1.5, 9.2) | 5.8 (1.0, 10.0) | 6.0 (2.1, 8.3) | |
|
| ||||
| Worst Pain Intensity, mean (SD) | 7.3 (1.6) | 7.0 (1.8) | 7.2 (1.6) | .42 |
| Median (min, max) | 7.5 (2.6, 10.0) | 7.5 (2.2, 10.0) | 7.5 (3.5, 10.0) | |
|
| ||||
| PROMIS Pain Interference, mean (SD) | 64.6 (5.04) | 63.4 (5.0) | 64.0 (5.8) | .24 |
| Median (min, max) | 64.8 (50.7, 76.3) | 63.8 (50.7, 76.3) | 64.3 (41.1, 76.3) | |
|
| ||||
| PROMIS Sleep Disturbance, mean (SD) | 59.2 (8.5) | 59.0 (8.5) | 57.9 (9.0) | .50 |
| Median (min, max) | 59.4 (28.9, 76.5) | 58.3 (28.9, 76.5) | 57.3 (35.9, 76.5) | |
|
| ||||
| PROMIS Anxiety, mean (SD) | 58.4 (8.9) | 57.0 (9.5) | 57.2 (9.3) | .46 |
| Median (min, max) | 59.4 (37.1, 75.4) | 58.4 (37.1, 75.4) | 57.9 (37.1, 80.0) | |
|
| ||||
| PROMIS Depression, mean (SD) | 55.6 (8.8) | 54.1 (9.3) | 53.4 (8.9) | .18 |
| Median (min, max) | 55.5 (38.2, 81.3) | 54.6 (38.2, 72.8) | 52.6 (38.2, 78.2) | |
|
| ||||
| PCL-5 (PTSD) Score, mean (SD) | 34.3 (17.4) | 32.6 (18.6) | 31.4 (17.3) | .48 |
| Median (min, max) | 36.0 (0.0, 71.0) | 30.0 (0.0, 74.0) | 29.5 (2.0, 73.0) | |
|
| ||||
| Classified as having PTSDf, n (%) yes | 63 (57) | 53 (48) | 49 (45) | .18 |
|
| ||||
| Any opioid analgesic use, n (%) yes | 35 (32) | 19 (17) | 30 (28) | .04 |
|
| ||||
| Morphine-equivalent dose, mean (SD) | 6.0 (15.3) | 5.9 (20.6) | 6.3 (16.1) | .07 |
| Median (min, max) | 0.0 (0.0, 90.0) | 0.0 (0.0, 127.5) | 0.0 (0.0, 90.0) | |
P-value from Kruskall-Wallis test for Motivation; and from Fisher exact test for: gender, race, Hispanic ethnicity, education, employment status, married vs. not, homelessness, type of pain, general health, prior pain education, prior hypnosis, prior meditation, having PTSD, and use of opioids at pretreatment, One-way ANOVA for means for all other variables.
Includes American Indian/Alaskan Native (n=8), Native Hawaiian or Other Pacific Islander (n=3), more than one race (n=29) and other (n=12).
A person may be counted in more than one category.
Pain was classified as “probable neuropathic” if scores on the LANSS[9] were >=12 and scores on the PAINDETECT[30] were ≥19. Pain was classified as “probable nociceptive/non-neuropathic” if scores on the LANSS[9] were < 12 and scores on the PAINDETECT[30] were ≤12. When the combination of scores on the LANSS[9] and PAINDETECT[30] did not meet these conditions, pain was classified as “mixed/undetermined.”
From PROMIS® General Health[16] recoded to “very good or excellent” vs. “poor, fair or good”.
Moderators, Mediators, and Exploratory Outcomes
This manuscript is focused on the primary aims of the study, and it is beyond the scope of this paper to include all the measures included in this trial. A theoretically informed selection of potential moderator (e.g., EEG measures of brain activity, participant factors such as cognitive function) and mediator (e.g., dose/session attendance, home practice) variables was included in this study and will be presented in later manuscripts. A full list of included variables can be found in our published protocol paper.[63]
Adverse Events
Study participants were asked to complete in-person skills practice logs at the start of each intervention session, which asked, “Have you experienced any problems or concerns related to [insert intervention]?” as well as assessments at the end of each intervention class which asked, “Was there anything that was not so helpful about today’s session?”. Study clinicians perused these forms in each session and would follow-up with participants as indicated if they reported a potential adverse event when responding to either of these questions. Potential adverse events were included in the clinical progress notes for each participant. The study coordinator and a study investigator (RMW) were alerted to this for follow-up as indicated. Adverse events were formally assessed after the EEG assessments with a single question, “Have you experienced any negative effects associated with the study procedures during today’s visit?”
Sample Size
Sample size was calculated a priori to ensure adequate power for testing the primary study hypothesis; that is, for detecting between-group differences in a reduction in API from pre- to posttreatment. Based on our prior work comparing similar interventions,[40] assuming decreases in API of 0.3 points (on a 0–10 scale) for ED, between 0.8 to 1.4 points for HYP, and between 0.6 to 1.0 for MM, with standard deviations (SD) ranging from 0.15 to 1.00, a significance level of 0.05, and using ANOVA as the statistical method, we calculated that 80 recruits per condition (total N = 240) at posttreatment would provide at least 80% power to detect between-groups differences, as specified.
Statistical Analyses
Statistical analyses were conducted using STATA[2] as planned and as reported in the published protocol, [63] following an intention-to-treat approach. All tests and confidence intervals (CI) were 2-sided, and statistical significance was set at 0.05. All numeric outcomes were defined as change scores computed from pre- to posttreatment, 3-month and 6-month follow-ups. Patterns of missing values were examined per protocol,[63] and multiple imputation was performed to account for missing values at each follow-up time prior to final data analysis. The imputation module (mi in STATA) was used to create 20 sets of complete data for each outcome. Twenty sets of imputed data for the changes in outcome at each time were modeled using pretreatment values of the outcome, age, gender, employment (yes/no), and treatment group as explanatory variables, using a chained equations approach.[1]
Regression was used to test between-group differences in pre- to posttreatment change in API and explore between-group differences in change scores in the secondary variables and at later time points. For each of the three change scores computed for each outcome variable, a separate linear regression model was fit to the imputed data with pretreatment value as a covariate and treatment group as the main factor. Means and 95% confidence intervals (CI) for outcomes and mean differences between interventions were calculated. When there was a statistically significant (at 0.05 level) omnibus effect of interventions, interventions were considered different if the CI for their mean difference did not include zero.
We used a different approach for the binary outcomes and categorical outcomes. At each follow-up we calculated the proportion of treatment responders and of participants using opioid analgesics, with 95% confidence intervals (Jeffreys method).[13] We used a generalized linear model (binomial family, log link, producing risk ratios [RR]) for these binary outcomes, with treatment group as the factor of interest and pretreatment status as the covariate. Categorical measures of GIC and treatment satisfaction were collected only at posttreatment and were analyzed using Fisher exact tests to compare response distribution among interventions. Imputation was not used for categorical or binary variables, given the challenges of creating robust imputation models with the available data.
Exploratory analyses were conducted with the sets of imputed data to investigate longitudinal changes in outcomes using Generalized Estimating Equations. For each outcome, a longitudinal (population-average) model for change in outcome as the response variable was created, with outcome value at pretreatment as covariate, and treatment group (ED, HYP, and MM), time (pre- to posttreatment, pre- to 3-months, and pre- to 6-month posttreatment), and an interaction between treatment and time as factors of interest. We assumed an unstructured correlation matrix. Statistically significant interactions were interpreted as different outcome paths over time for different interventions. Paths might differ for interventions in magnitude (different rates of improvement over time, for example), or in direction (improvement for one intervention at a certain time and worsening for another, for example).
RESULTS
Participant flow is shown in Figure 1. Among 3,773 potential participants, 2839 were excluded before eligibility was confirmed, because they could not be contacted (n=712) or because they declined before screening (n=2095). Of those screened, 515 were ineligible, and 91 were eligible but not randomized (see Figure 1 for additional details and explanation). Of the 2095 who declined prior to screening, 187 (9%) participated in the interventions as “non-research participants. A total of 328 were randomized. Of these, approximately half had been enrolled via chart reviews and study staff approach, 43% were referred into the study by clinicians, and 9% had self-referred into the study. We met a priori enrollment goals and exceeded retention goals, ensuring adequate statistical power. Proportions of retention were 90% (n=294) at posttreatment, 81% (267) at 3-month and 80% (261) at 6-month follow-up.
Figure 1. Flow of Participants through trial comparing hypnosis, mindfulness meditation, and pain education for Veterans with chronic pain.
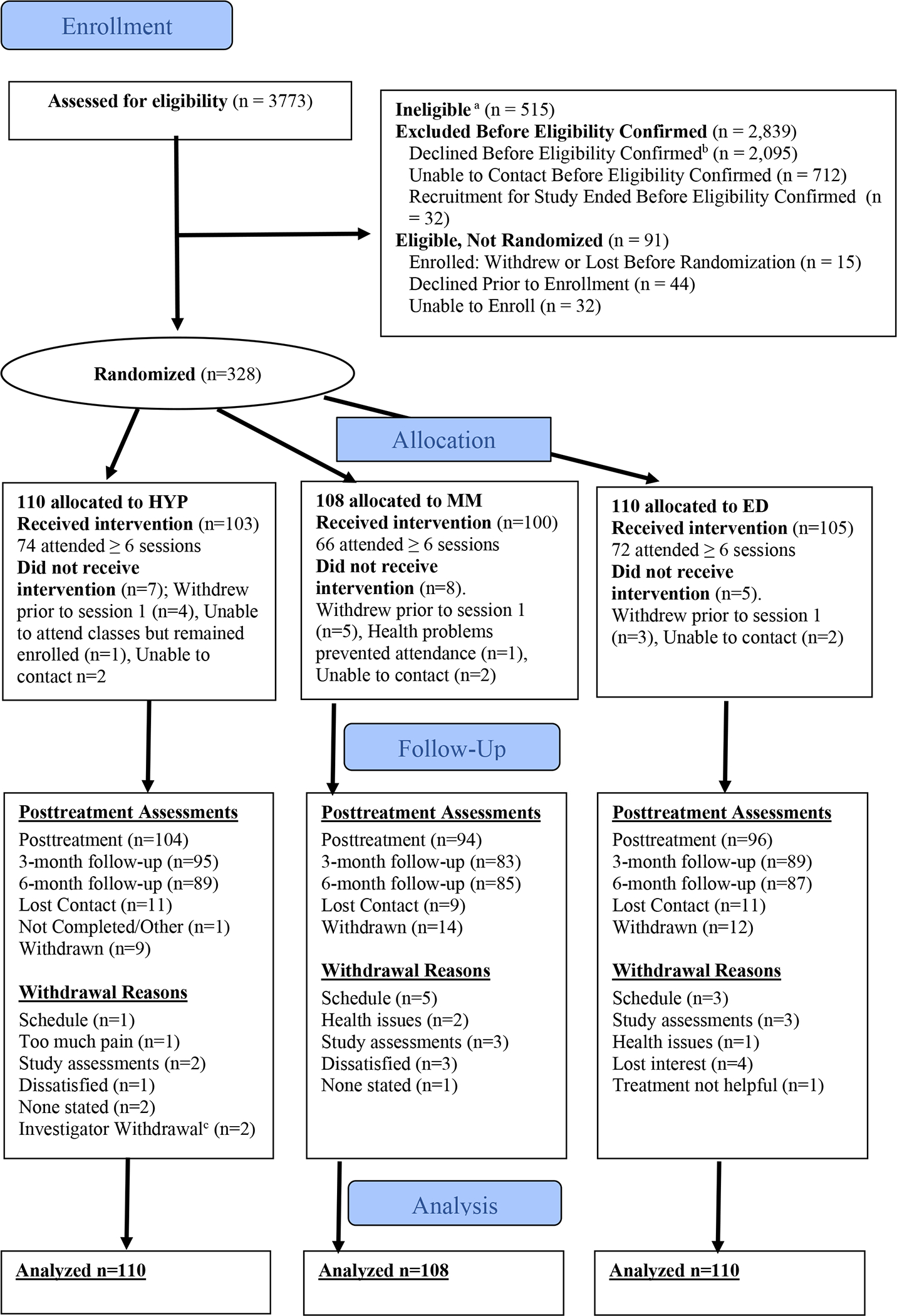
a Reasons for ineligibility and numbers excluded: psychiatric or behavioral conditions (n=352), pain of insufficient intensity or duration (n=122), cognitive or communication limitation (n=23), daily MED exceeded 120 mg (n=7), or other reasons (n=11).
b Reasons for declining to participate and frequency: lack of interest (n=539), too much time involved (n=531), lives too far away (n=274), pain not enough of a concern (n=211), prefer to do interventions as Non-Research participants (n=187), do not think treatment will help (n=129), scheduling conflicts (n=82), lack of transportation (n=65), current medical issues (n=61), not wanting group treatment (n=37) or other (n=23).
c 2 participants in HYP withdrawn by PI team for inappropriate treatment of study staff.
Median number of intervention sessions attended was 7 (out of 8). Individuals who had missing outcomes at all three follow-ups were younger than those without any missing outcomes. Individuals endorsing Hispanic ethnicity had a relatively larger proportion of missing outcomes at posttreatment, but not at other time points. No differences were found for gender, race, education level, employment status, or marital status. There was no evidence that the missing data was systematic; therefore, missing data were considered missing at random. Table 1 shows the pretreatment subject characteristics, by intervention group; no substantial between-group differences were found, suggesting effective randomization. Specific details about missing data by condition and time point are shown in Supplementary Tables 1 and 2. Descriptive data for the outcome measures at all timepoints are shown in Supplementary Table 3.
Pain Intensity
At pretreatment, 95.1% of the sample completed 4 of 4 possible pain ratings and 4.9% completed 1–3 ratings. At posttreatment 81.3% completed 4 of 4 ratings and 18.7% completed 1–3 pain ratings. At the 3- and 6-month follow-ups, 82.4% and 84.3%, respectively, of the sample completed all 4 pain ratings and the remainder completed 1–3 ratings.
Table 2 shows the mean change in API by group at each follow-up time, and the results of analyses using imputed data for all primary and secondary outcomes, expressed as changes. For each outcome, the table presents means and CIs of change scores for each intervention group at each assessment point, p-values for the omnibus comparison of the three means, and mean differences and CIs between combinations of two groups, based on the models for each follow-up time separately.
Table 2:
Mean (95% CI) change in outcomes from baseline by treatment group and mean (95% CI) differences between treatment groups for numeric outcomes at each follow-up up time (using imputed data and adjusting for outcome at pretreatment)
| Results from Analysis of Covariance at Each Follow-up Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Follow-up time | Change from Pretreatment, Mean (95% CI)a | P-valueb | Between Group Differences, Mean (95% Confidence Interval)a | ||||||
| ED | HYP | MM | HYP vs. ED | MM vs. ED | MM vs. HYP | ||||
| Change in Average Pain Intensity (Primary Outcome) | Post-treatment | −0.57 (−0.63, −0.52) | −0.61 (−0.67, −0.56) | −0.85 (−0.90, −0.79) | .39 | −0.07 (−0.43, 0.30) | −0.26 (−0.66, 0.13) | −0.19 (−0.59, 0.20) | |
| 3-month | −0.48 (−0.55, −0.42) | −0.95 (−1.02, −0.88) | −0.67 (−0.74, −0.61) | .05* | −0.50 (−0.91, −0.08)* | −0.17 (−0.59, 0.24) | 0.32 (−0.07, 0.72) | ||
| 6-month | −0.28 (−0.34, −0.22) | −1.04 (−1.11, −0.98) | −0.86 (−0.92, −0.80) | <.001* | −0.79 (−1.21, −0.37)* | −0.56 (−0.98, −0.15)* | 0.23 (−0.22, 0.68) | ||
| Change in Worst Pain Intensity | Post-treatment | −0.81 (−0.86, −0.75) | −0.67 (−0.73, −0.61) | −0.86 (−0.91, −0.81) | .77 | 0.08 (−0.35, 0.51) | −0.08 (−0.50, 0.35) | −0.16 (−0.59, 0.27) | |
| 3-month | −0.59 (−0.66, −0.52) | −1.08 (−1.16, −1.00) | −0.85 (−0.92, −0.79) | .06 | −0.56 (−1.01, −0.11) | −0.30 (−0.76, 0.16) | 0.26 (−0.23, 0.76) | ||
| 6-month | −0.55 (−0.64, −0.46) | −1.20 (−1.30, −1.09) | −0.94 (−1.03, −0.85) | .01* | −0.74 (−1.23, −0.25)* | −0.43 (−0.89, 0.02) | 0.31 (−0.20, 0.81) | ||
| PROMIS Pain Interference | Post-treatment | −2.52 (−2.93, −2.11) | −2.75 (−3.16, −2.35) | −3.32 (−3.79, −2.84) | .31 | −0.76 (−2.20, 0.68) | −1.04 (−2.46, 0.38) | −0.28 (−1.82, 1.26) | |
| 3-month | −2.31 (−2.76, −1.87) | −2.93 (−3.36, −2.49) | −2.62 (−3.13, −2.10) | .44 | −1.18 (−2.99, 0.62) | −0.56 (−2.26, 1.14) | 0.62 (−1.10, 2.34) | ||
| 6-month | −1.03 (−1.44, −0.62) | −2.83 (−3.23, −2.43) | −2.89 (−3.36, −2.41) | .009* | −2.32 (−3.81, −0.82)* | −2.09 (−3.83, −0.35)* | 0.23 (−1.30, 1.76) | ||
| PROMIS Sleep Disturbance | Post-treatment | −4.08 (−4.54, −3.61) | −3.92 (−4.38, −3.45) | −3.27 (−3.77, −2.77) | .90 | 0.10 (−1.74, 1.94) | 0.43 (−1.54, 2.41) | 0.33 (−1.52, 2.18) | |
| 3-month | −4.01 (−4.62, 3.41) | −3.77 (−4.37, −3.16) | −2.29 (−2.93, −1.64) | .47 | .17 (−1.81, 2.16) | 1.24 (−0.96, 3.44) | 1.07 (−0.95, 3.10) | ||
| 6-month | −3.09 (−3.76, −2.42) | −3.50 (−4.17, −2.84) | −3.06 (−3.78, −2.35) | .87 | −0.50 (−2.60, 1.61) | −0.51 (−2.68, 1.67) | −0.01 (−2.08, 2.07) | ||
| Change in PROMIS Anxiety Score | Post-treatment | −1.12 (−1.56, −0.68) | −1.02 (−1.49, −0.54) | −2.06 (−2.53, −1.59) | .38 | −0.28 (−2.07, 1.51) | −1.26 (−3.19, 0.66) | −0.99 (−2.80, 0.83) | |
| 3-month | −0.90 (−1.35, −0.44) | −1.38 (−1.87, −0.89) | −2.51 (−2.99, −2.03) | .18 | −0.87 (−2.83, 1.09) | −1.95 (−3.98, 0.08) | −1.08 (−3.19, 1.03) | ||
| 6-month | −0.49 (−0.90, −0.09) | −1.13 (−1.57, −0.70) | −1.87 (−2.30, −1.44) | .27 | −0.99 (−2.86, 0.91) | −1.68 (−3.80, 0.44) | −0.69 (−2.72, 1.34) | ||
| Change in PROMIS Depression Score | Post-treatment | −1.27 (−1.69, −0.85) | −2.68 (−3.13, −2.24) | −2.17 (−2.60, −1.75) | .09 | −1.81 (−3.48, −0.14) | −1.47 (−3.22, 0.27) | 0.34 (−1.34, 2.02) | |
| 3-month | −1.36 (−1.73, −0.98) | −2.44 (−2.84, −2.05) | −2.49 (−2.87, −2.11) | .18 | −1.44 (−3.22, 0.35) | −1.63 (−3.49, 0.23) | −0.19 (−2.01, 1.63) | ||
| 6-month | −0.28 (−0.71, 0.15) | −2.57 (−3.02, −2.11) | −1.68 (−2.11, −1.24) | .02* | −2.69 (−4.59, −0.80)* | −1.98 (−4.02, 0.07) | 0.72 (−1.18, 2.62) | ||
| Change in PTSD score | Post-treatment | −4.18 (−4.79, −3.56) | −3.25 (−3.90, −2.59) | −3.79 (−4.41, −3.18) | .87 | 0.61 (−2.35, 3.57) | −0.16 (−3.12, 2.80) | −0.77 (−3.74, 2.20) | |
| 3-month | −3.54 (−4.18, −2.90) | −3.93 (−4.61, −3.25) | −5.91 (−6.55, −5.27) | .11 | −0.73 (−3.70, 2.24) | −2.93 (−5.89, 0.03) | −2.20 (−5.05, 0.64) | ||
| 6-month | −2.01 (−2.52, 1.51) | −4.02 (−4.56, −3.48) | −3.80 (−4.31, −3.29) | .27 | −2.28 (−5.47, 0.92) | −2.23 (−5.55, 1.08) | 0.04 (−2.91, 3.00) | ||
Omnibus test was statistically significant, and confidence interval does not include zero.
Estimates from a linear regression for change in outcome, adjusting for outcome value at pretreatment. Negative values indicate a reduction in the outcome at follow-up compared to pretreatment.
P-value from omnibus test for treatment group effect on change in outcome, while adjusting for pretreatment outcome in a linear regression
Participants in all three intervention conditions reported significant within-group mean decreases in API from pre- to posttreatment (see Figure 2). Mean API dropped from 5.8 to 5.3 for those in ED, from 5.7 to 5.2 in HYP, and from 5.9 to 5.1 in MM. Changes in API were not statistically different between groups at posttreatment (F2,290= 0.93, p=.39), thus refuting the study’s primary hypothesis.
Figure 2. Changes in mean average pain intensity and 95% confidence intervals from pretreatment to follow-up assessments by interventions.
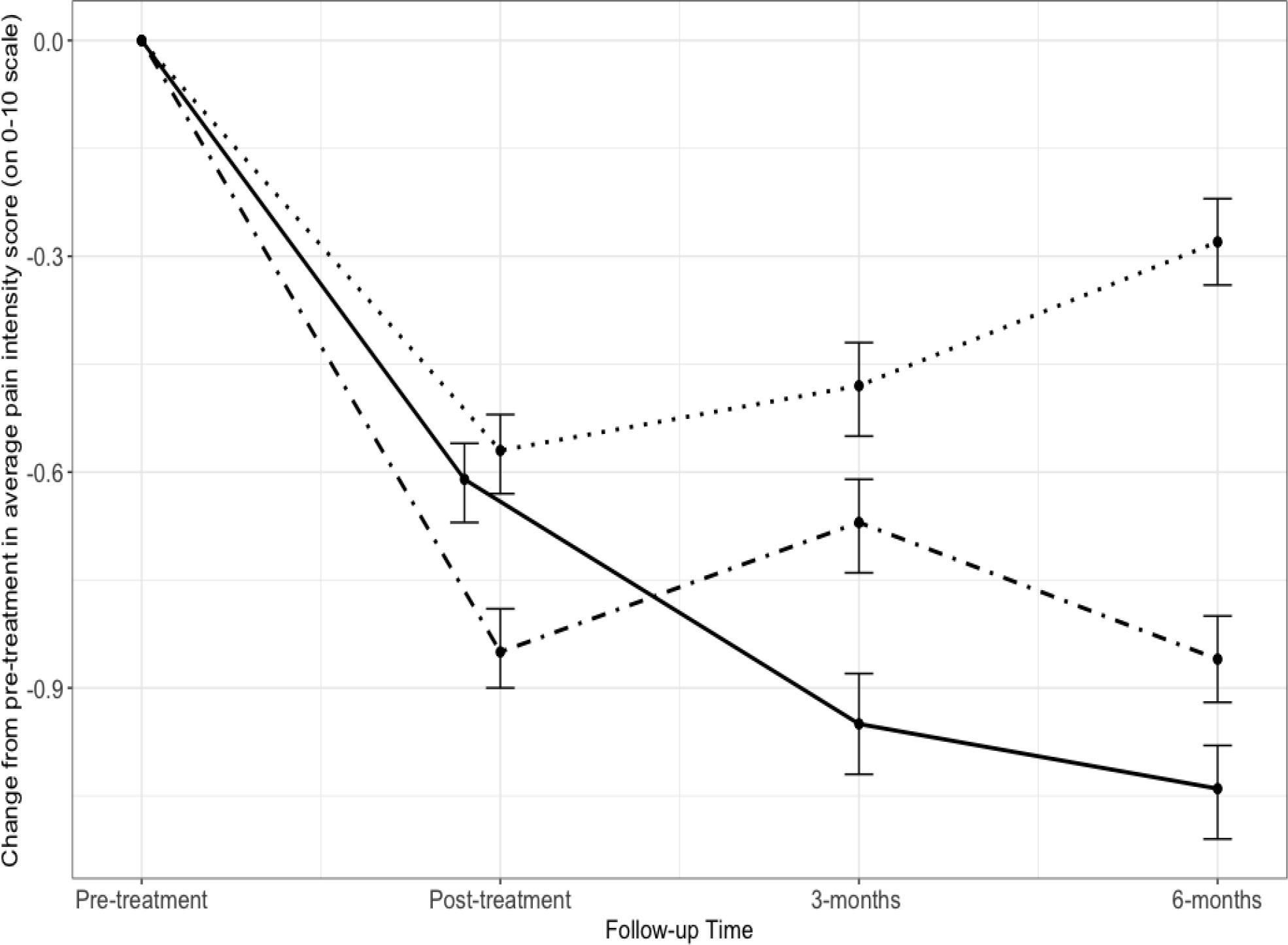
•••• ED • – • MM ––– HYP
Note: Figure 2 shows means and 95% CIs from Table 4 (adjusted analysis with imputed data). Data for post-treatment in HYP group is slightly offset to the left to avoid overlap with data from ED group.
Between-group differences in decreases in API from pretreatment to 3- (F2,271= 2.96, p < .05) and 6-month follow-up (F2,264.2= 7.29, p < .001) were found. At 3 months, HYP participants reported greater reductions in API than those in ED (−0.50, [95% CI: −0.91, −0.08]). At 6 months, both HYP and MM participants reported greater reductions in API than those in ED (HYP: −0.79 [95%CI: −1.21, −0.37]; MM: −0.56 [95% CI: −.98, −0.15]). HYP and MM changes in API were not statistically different from each other at any assessment point. Participants randomized to HYP or MM were also more likely to have a clinically meaningful response to treatment (change of −2 or more points on the 0–10 numeric rating scale in API) compared to ED, but only at 6-month posttreatment (for HYP: RR = 2.85, 95% CI: 1.35, 6.00; for MM: RR = 2.35, 95% CI: 1.09, 5.08) (Table 3).
Table 3:
Proportion of participants with clinically meaningful improvement in average pain intensity and proportion endorsing any opioid analgesic medication use at each follow-up time by treatment group (no imputed data), and risk ratios comparing treatment groups
| Proportion (95% Confidence Interval)a | P-valueb | Risk Ratio (95% Confidence Interval)e | ||||||
|---|---|---|---|---|---|---|---|---|
| ED | HYP | MM | HYP vs. ED | MM vs. ED | MM vs. HYP | |||
| Clinically Meaningful Responder (yes) | Post-treatment | 0.16 (0.09, 0.24) | 0.12 (0.06, 0.19) | 0.20 (0.13, 0.29) | .29 | 0.75 (0.37, 1.51) | 1.27 (0.69, 2.35) | 1.70 (0.57, 2.83) |
| 3-months | 0.17 (0.10, 0.26) | 0.20 (0.13, 0.29) | 0.18 (0.11, 0.27) | .80 | 1.20 (0.65, 2.19) | 1.01 (0.52, 1.93) | 0.84 (0.33, 1.36) | |
| 6-months | 0.09 (0.04, 0.17) | 0.26 (0.18, 0.36) | 0.22 (0.15, 0.32) | .02* | 2.85 (1.35, 6.00)* | 2.35 (1.09, 5.08)* | 0.83 (0.39, 1.26) | |
| Using any opioid analgesic Medications (yes) | Post-treatment | 0.24 (0.16, 0.33) | 0.20 (0.13, 0.29) | 0.22 (0.15, 0.32) | .003* | 1.40 (1.11, 1.78)* | 0.97 (0.69, 1.37) | 0.69 (0.52, 0.86)* |
| 3-months | 0.23 (0.15, 0.33) | 0.20 (0.13, 0.29) | 0.26 (0.17, 0.36) | .05* | 1.28 (0.97, 1.68) | 0.96 (0.67, 1.36) | 0.75 (0.54, 0.96)* | |
| 6-months | 0.25 (0.16, 0.35) | 0.21 (0.14, 0.31) | 0.24 (0.16, 0.34) | .18 | 1.17 (0.87, 1.57) | 0.85 (0.58, 1.24) | 0.73 (0.47, 0.98) | |
Denotes significance at p < .05
Proportions based on complete dataset, and Jeffreys 95% confidence intervals
P-value from omnibus test for treatment group effect on being a responder to treatment (adjusting for pretreatment average pain intensity) or using opioid analgesic medications (adjusting for use at pretreatment) in a logistic regression.
Risk ratio estimates from a logistic regression with log-link for a responder to treatment (adjusting for pretreatment average pain intensity) or using opioid analgesic medication at posttreatment (adjusting for use at pretreatment)
Secondary Outcomes
No significant between-treatment effects on any secondary outcomes were found at posttreatment or 3-months. However, significant between-treatment effects were found at 6-months follow-up for WPI (F2,270= 4.62, p < .01), pain interference (F2,229.6= 4.84, p < .01), and depressive symptom severity (F2,224.9= 3.95, p < .05) (Table 2). For pain interference, participants receiving HYP reported a mean decrease of 2.32 points more than those in ED (95% CI: −3.81, −0.82) and those receiving MM reported a mean decrease of 2.09 points more than ED (95% CI: −3.83, −0.35). Participants receiving HYP, but not MM, also reported a larger mean decrease in depressive symptoms (−2.69 [95% CI: −4.59, −0.80]) and WPI (−0.74, [95% CI: −1.23, −0.25]) compared to ED.
The proportion of individuals reporting any use of opioid analgesic medication was different by condition at pretreatment (Fisher exact test p = .04); only 17% of those in HYP reported any opioid analgesic medication use, compared to 32% of those in ED and 28% of those in MM (see Table 1). At all follow-up time points, the portion reporting any opioid analgesic medication use was 23–25% of those in ED, 20–21% of those in HYP, and 24–26% of those in MM. However, when controlling for baseline use, it appeared that those in HYP were more likely than those in MM and ED to report any use at posttreatment (χ22 for omnibus test of equality of group effect = 16.22, p < .01) and 3-month follow-up (χ22 for omnibus test of equality of group effect = 6.08, p < .05) (Table 3). This finding should be interpreted with caution, however, given the significant difference in baseline rates.
Most participants in each treatment reported at least minimally improved pain on the Global Impression of Change measure at posttreatment (Table 4). Treatment satisfaction was high for participants in all conditions; 78% of those in ED, 84% of those in HYP and 80% of in MM indicated they were “Satisfied” or “Very satisfied” with treatment. Only 5–6% of participants were “Dissatisfied” or “Very dissatisfied” with treatment, and there were no differences in satisfaction by treatment condition. Self-reported improvements in pain intensity were congruent with the proportion demonstrating clinically meaningful improvement on the API for ED and MM (about 16% and 20%, respectively), but not for HYP, where 29% of those in HYP self-reported “Very much” or “Much” improvement in pain intensity, exceeding the 12% who demonstrated a clinically meaningful response in API at posttest. There was a significant difference between groups in reported change in ability to manage pain (Fisher exact test, p = 0.02), such that 30% of those in the ED group reported a “Much” or “Very much” improved ability to manage pain, while 52% of those in the HYP group and 45% of those in the MM group reported this level of improvement.
Table 4:
Global Impression of Change and Treatment Satisfaction at posttreatment.
| Participant Perceptions | Intervention Group | P-valuea | ||
|---|---|---|---|---|
| ED | HYP | MM | ||
|
| ||||
| Change in pain intensity since treatment began, n (%) in categoryb | ||||
| Very much improved | 1 (1) | 4 (4) | 2 (2) | .22 |
| Much improved | 16 (17) | 26 (25) | 20 (22) | |
| Minimally improved | 38 (41) | 49 (47) | 43 (47) | |
| No Change | 34 (37) | 20 (19) | 18 (20) | |
| Minimally worse | 3 (3) | 4 (4) | 5 (5) | |
| Much worse | 1 (1) | 0 (0) | 2 (2) | |
| Very much worse | 0 (0) | 1 (1) | 1 (1) | |
| Change in ability to manage pain, n (%) in categoryb | ||||
| Very much improved | 1 (1) | 9 (9) | 7 (8) | .02 |
| Much improved | 27 (29) | 45 (43) | 34 (37) | |
| Minimally improved | 42 (45) | 36 (35) | 33 (36) | |
| No Change | 23 (25) | 12 (12) | 16 (18) | |
| Minimally worse | 0 (0) | 1 (1) | 1 (1) | |
| Very much worse | 0 (0) | 1 (1) | 0 (0) | |
| Change in pain interference, n (%) in categoryb | ||||
| Very much improved | 2 (2) | 2 (2) | 1 (1) | .38 |
| Much improved | 9 (10) | 21 (20) | 10 (11) | |
| Minimally improved | 33 (35) | 39 (38) | 39 (43) | |
| No Change | 44 (47) | 33 (32) | 37 (41) | |
| Minimally worse | 4 (4) | 6 (6) | 3 (3) | |
| Much worse | 1 (1) | 3 (3) | 1 (1) | |
| Satisfaction with study treatment n (%) in categoryc | ||||
| Very satisfied | 24 (26) | 35 (34) | 24 (27) | .89 |
| Satisfied | 48 (52) | 51 (50) | 47 (53) | |
| No preference | 15 (16) | 11 (11) | 13 (15) | |
| Dissatisfied | 5 (5) | 3 (3) | 4 (4) | |
| Very Dissatisfied | 1 (1) | 2 (2) | 1 (1) | |
P-value for Fisher exact test
17 missing in ED, 6 missing in HYP, and 17 missing in MM at posttreatment
17 missing in ED, 8 missing in HYP, and 19 missing in MM at posttreatment
Exploration of Group x Time Interactions
Results of the exploratory longitudinal analysis (not shown) were generally confirmatory, most noticeably for HYP; there were significant overall group by time interactions for API (F4,1465.4)= 3.20, p < .01) and WPI (F4,1545.4)= 2.57, p <.05). Specifically, compared to ED, average and worst pain continued to decrease overtime in HYP (coefficients for the interaction with 3 months were −.42 [95% CI: −0.83, −0.01] for API and −.63 [95%CI: −1.10, −.15] for WPI; and at 6 months were −.72 [95% CI: −1.14, −0.30] for API and −.78 [95%CI: −1.27, −.29] for WPI), but these interactions were not significant for MM.
Adverse Events
Of the 328 randomized participants, only one participant reported a SAE (involving a visit to the emergency room for increased pain, migraine, and vomiting) that was conservatively deemed at least possibly related to study procedures.
Of the 328 randomized participants, 79 (24%) subjects reported at least one non-serious AE at least possibly related to study procedures. The most common non-serious AEs at least possibly related to study procedures were: (1) new, unusual, or worsened pain or physical discomfort (42 participants, 13%); and (2) new, unusual, or worsened psychological discomfort (37 participants, 11%). Twenty-eight participants (9%) reported an AE associated with the EEG assessments; 26 (8%) reported an AE related to treatment procedures; 25 (8%) reported an AE associated with the baseline hypnotizability assessment[62]; 12 (4%) reported an AE associated with the study self-report measures or consent session.
Of the 110 and 108 randomized to HYP and MM, respectively, 13 (12%) and 10 (9%) respectively, reported an AE at least possibly associated with treatment procedures. All the treatment-related AEs reported by participants in HYP and MM were related to new, unusual, or worsened pain/physical or psychological discomfort. Three of the 110 (3%) participants assigned to the ED intervention reported an AE related to treatment procedures.
Data Transparency
Requests for access to limited, fully deidentified data will be considered on a case-by-case basis; please contact the corresponding author. Access is limited because open access to data was not included as part of informed consent.
DISCUSSION
In this trial, all three interventions (HYP, MM and ED) resulted in decreased average pain intensity from pre- to posttreatment. Neither HYP nor MM resulted in significantly larger reductions in average pain intensity than ED at posttreatment, consequently the primary study hypothesis was not supported. The changes in pain intensity reported here are consistent with reviews of HYP and MM [3; 9; 33] and with two recent, well-powered trials of HYP[41] and Mindfulness-Based Stress Reduction (MBSR),[16] respectively. With respect to HYP and ED specifically, decreases in average pain intensity at posttreatment were comparable to those in a recent well-powered trial using comparable interventions, which found mean decreases of −.78 (on a 0–10 numeric rating scale of pain intensity) for HYP and −.76 for ED.[41] Decreases in average pain intensity in this study were larger than those found in a study of adults with low back pain (LBP) that used a similar MM intervention (which found mean decrease of .29 points on a 0–10 numeric rating scale), [22] but smaller than those in trials using Mindfulness Based Stress Reduction (1.0 point decrease) [16] and Mindfulness Based Cognitive Therapy (1.07 point decrease on 0–10 scale), respectively.[22] However, the present sample differs from those in other pain trials, which tend to be comprised of primarily Caucasian women with at least a college education.[16; 41] The present sample was 74% male, with 63% of participants identifying as Caucasian, and only 45% having a college degree. Additionally, the present study focused on Veterans, who, as a result of their military experience, may have been exposed to higher rates of injury, trauma, psychological stressors, and social risk factors that can amplify the impact of chronic pain.[53] [8; 31] Last, the present study included participants with a broad range of pain conditions, including neuropathic pain, in contrast to studies that include only a participants with a single type of pain (e.g., low back pain).[16; 22]
Although the primary aim of the study was to examine the pre- to posttreatment effects of the interventions on average pain intensity, several additional findings warrant comment. Secondary analyses identified between-group differences in the months following treatment completion. These findings suggested that the benefits of HYP and MM extended beyond pain intensity and endure and even increase in the months following treatment; this did not occur for those randomized to ED, where benefits appeared to dissipate over time. Specifically, consistent with a recent trial of Mindfulness Based Stress Reduction [16] but not a HYP trial, [41] in the present study those in HYP and MM reported significantly larger decreases in average pain intensity, pain interference, depressive symptoms and worst pain intensity, compared to those in ED at later time points. The decreases in pain interference and depressive symptoms seen among those randomized to HYP and MM are particularly important to note; although these were secondary outcomes in this particular trial, it may be that these or other outcomes that we did not assess are more functionally relevant for those with chronic pain than average pain intensity. This is an important finding given that relapse following a variety of interventions is common. Further, given the high rates of comorbidity between chronic pain and mental health symptoms such as those associated with depression[6] and PTSD,[46] these findings highlight the ways that HYP and MM can target multiple interrelated symptoms simultaneously over the long term. Also, more HYP (26%) and MM (22%) participants were classified as treatment responders at 6-months, relative to ED (9%). While no significant differences between HYP and MM on any outcome measures were detected, HYP was noted to have significant benefits compared to ED on more outcomes than MM. We speculate that ongoing use and development of HYP and MM skills may account for the improvement in outcomes over time. Research to determine the role that ongoing skill practice plays in producing continued improvements in outcome post-treatment is warranted.
Treatment adherence and satisfaction were high for all conditions, suggesting that even for those who do not experience large improvements in pain intensity or interference, there is perceived benefit associated with treatment. That said, it is also important to note that a small portion (i.e., < 6%) of participants in each condition reported dissatisfaction and/or worsening with treatment, which not differ significantly by condition.
The present study was designed as an explanatory trial to ensure a rigorous treatment comparison with statistically credible results. However, the study also featured some important aspects of a pragmatic comparative effectiveness trial to increase generalizability. First, the study utilized a range of healthcare professionals to deliver the interventions; comparable studies have used only a few clinicians with demonstrated mastery in the specific interventions.[16; 41] Second, the study intervention classes allowed for the participation of non-research participants. Interestingly, among the 187 Veterans who participated in the intervention classes but did not enroll in the study, and could choose whichever treatment they preferred, all treatments were selected equally. The high levels of treatment satisfaction among study participants and equivalent treatment preferences among the non-research participants speak to the potential clinical value of having a range of available treatment options and the participants’ perceived relevance and value of the interventions included in this study.
The study has several limitations. First, it was not powered a priori to detect between-group differences at the follow-up timepoints, where larger effects appeared to have emerged. Second, pain etiology and locations were not assessed, making it difficult to characterize the sample in a nuanced way. Third, although randomization was effective, as indicated by a lack of significant differences between groups in terms of demographic characteristics, opioid analgesic medication use data differed between conditions at pretreatment. In addition, opioid use data were heavily skewed, making it difficult to meaningfully discern whether there were treatment effects on this outcome. The findings related to opioid analgesic use should be interpreted cautiously. Fourth, the degree to which these findings generalize outside of Veteran populations is unknown. Fifth, more than half of those approached (57%) declined to enroll in the research study, although of these 10% participated in the intervention classes as non-research participants. Of those who declined, important barriers to access were noted, which could be addressed in future studies by using telehealth or increasing the flexibility of the scheduling. It should also be noted that a significant portion declined due to the time-consuming nature of the study. Thus, these findings are best characterized as reflective of Veterans with pain that was sufficient to motivate them to engage in treatment, with sufficient time and motivation to engage in assessments, and with fewer access barriers or health issues than those who declined. Sixth, we did not systematically include stakeholders (such as Veterans with chronic pain) in the design, conduct, nor dissemination of results for this study. Had Veteran stakeholders been included in the study design, it is possible that more relevant and clinically important outcomes would be included; our findings highlight that pain intensity may not be the most important subjective outcome. Last, the study did not include a usual care comparison group, thus making it impossible to control for time effects or the potential clinical effects of the ED treatment control group.
Study strengths include the better-than-expected retention rates, a diverse sample, excellent intervention fidelity, high ecological validity at the participant and clinician level, logistical innovation in including non-research participants, and details about adverse events, a gap in prior literature.[10] The inclusion of non-research participants demonstrated that a significant portion of Veterans were interested in the interventions, but preferred to not enroll in the study, a well-known concern related to clinical trials.
The study also highlights promising areas for future study. These include examination of moderating factors (to identify participants who are most likely to respond to each intervention), and mediating factors (to better understand mechanisms of change) which may provide a foundation to refine and optimize these interventions. Planned analyses that are outside of the scope of this paper and the primary aims will allow us to examine some of these questions. For example, one of the most important mechanism questions raised by the findings of this study pertains to the degree to which skills acquisition may account for maintenance of treatment benefits. This study included multiple measures of home practice and treatment engagement, which will be evaluated as part of secondary analysis that will systematically explore treatment moderators and mediators. This study also included both planned (e.g., sleep, substance use) and exploratory (e.g., positive affect) outcomes to be described in future papers. Future papers are also planned to explore the additive benefit of engaging in a second treatment as part of the open label study phase.
Conclusions
In this first randomized controlled trial to directly compare HYP and MM to ED, no significant between-treatment effects were found at immediate posttreatment, contrary to our primary hypothesis. All three treatments resulted in modest decreases in average pain intensity as well as other outcome measures at posttreatment. However, through secondary analysis, HYP and MM appeared to show superior benefits compared to ED on pain intensity and multiple outcome measures at later time points.
These findings demonstrate that all three interventions can feasibly be delivered to a diverse, complex population, in a manualized form, by a variety of healthcare professionals with brief training, in a clinical setting. Emerging evidence [52] has already demonstrated the viability of integrating the HYP protocol used in this study in a clinical environment; further studies are needed to examine the parallel implementation of MM and ED and to find ways to make these interventions more accessible and convenient.
Supplementary Material
Acknowledgements:
This work was supported by the National Center for Complementary and Integrative Health (Grant # 1R01AT008336-01, awarded to Co-Principal Investigators Mark Jensen and Rhonda Williams). The study sponsor did not participate in study design, implementation of any part of the study, nor any dissemination decisions or activities. This work was also supported in part with resources and facilities at VA Puget Sound Health Care System and the University of Washington. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. None of the authors have conflicts of interest to declare. This trial is registered in Clinical Trials.gov (NCT02653664). The analyses were conducted in accordance with the prespecified analysis plan. The authors acknowledge an administrative error in trial registration; n=19 subjects were inadvertently enrolled prior to the start date noted on clinicaltrials.gov. There were no separate analyses conducted with these subjects nor substantive changes in procedure after these subjects were enrolled; this error was not detected until the entire study was completed. This error does not impact the scientific integrity of the study.
The authors would like to acknowledge the invaluable contributions of our VAPSHCS and UW research staff: Carrie Kincaid, BA, who coordinated regulatory activities, conveyed randomization assignment to participants and scheduled treatments, oversaw treatment fidelity and clinician support, Alisha H. McCall, BA, who oversaw all blinded study assessments and coordinated annual study clinician trainings; Genevra Levinson, MA, Ellen Cambron, MSW, Nicole Hodgkinson, BA, Emma Herbeck, BS, Makena Kaylor, BA, Emily Cary, MS, Emily Stensland, BS, and Ben Korman, MS, all of whom assisted with the study assessments. We acknowledge UW employee Joy F. Chan, BS, who randomized participants, and Mark Pettet, PhD, who oversaw the completion of the EEG assessments at the UW Integrated Brain Imaging Center (IBIC). We also gratefully acknowledge the participation of the clinicians at VAPSHCS who conducted these interventions.
REFERENCES
- [1].Stata 15 Base Reference Manual. College Station, TX: Stata Press, 2017. [Google Scholar]
- [2].Stata Statistical Software: Release 15. College Station, TX: StataCorp.LLC, 2017. [Google Scholar]
- [3].Adachi T, Fujino H, Nakae A, Mashimo T, Sasaki J. A meta-analysis of hypnosis for chronic pain problems: a comparison between hypnosis, standard care, and other psychological interventions. Int J Clin Exp Hypn 2014;62(1):1–28. [DOI] [PubMed] [Google Scholar]
- [4].Amtmann DA, Cook KF, Jensen MP, Chen W-H, Choi SW, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai J-S. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Askew RL CK, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol 2016;73:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163(20):2433–2445. [DOI] [PubMed] [Google Scholar]
- [7].Ball EF, Nur Shafina Muhammad Sharizan E, Franklin G, Rogozinska E. Does mindfulness meditation improve chronic pain? A systematic review. Curr Opin Obstet Gynecol 2017;29(6):359–366. [DOI] [PubMed] [Google Scholar]
- [8].Baria AM, Pangarkar S, Abrams G, Miaskowski C. Adaption of the biopsychosocial model of chronic noncancer pain in Veterans. Pain Med 2019;20(1):14–27. [DOI] [PubMed] [Google Scholar]
- [9].Bawa FL, Mercer SW, Atherton RJ, Clague F, Keen A, Scott NW, Bond CM. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. Br J Gen Pract 2015;65(635):e387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Becker WC, DeBar LL, Heapy AA, Higgins D, Krein SL, Lisi A, Makris UE, Allen KD. A Research Agenda for advancing non-pharmacological management of chronic musculoskeletal pain: findings from a VHA State-of-the-art Conference. J Gen Intern Med 2018;33(Suppl 1):11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Blevins CA, Weathers FW, Davis MT, Witte TK, Domino JL. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. J Trauma Stress 2015;28(6):489–498. [DOI] [PubMed] [Google Scholar]
- [12].Bovin MJ, Marx BP, Weathers FW, Gallagher MW, Rodriguez P, Schnurr PP, Keane TM. Psychometric properties of the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (PCL-5) in veterans. Psychol Assess 2016;28(11):1379–1391. [DOI] [PubMed] [Google Scholar]
- [13].Brown LD, Cai, Tony T, DasGupta A. Interval Estimation for a Binomial Proportion. Statistical Science 2001;16(2):32. [Google Scholar]
- [14].Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical care 2002;40(9):771–781. [DOI] [PubMed] [Google Scholar]
- [15].Cella D, Choi SW, Condon DM, Schalet B, Hayes RD, Rothrock NE, Yount S. Cook KF, Gerson RC, Amtnamm D, DeWalt DA, Pilkonis PA, Stone AA. Weinfurt K, Reeve BB. PROMIS Adult Health Profiles: Efficient Short-form measures of seven health domains. Value Health 2019;22(5):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cherkin DC, Sherman KJ, Balderson BH, Cook AJ, Anderson ML, Hawkes RJ, Hansen KE, Turner JA. Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA 2016;315(12):1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cook KF JS, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, Dewalt DA, Fries JF, Pilkonis PA, Reeve BB, Stone AA, Weinfurt KP, Cella D. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstraetd clinical validity accross a range of chronic conditions. J Clin Epidemiol 2016;73(May):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Davidson RJ, Goleman DJ. The role of attention in meditation and hypnosis: a psychobiological perspective on transformations of consciousness. Int J Clin Exp Hypn 1977;25(4):291–308. [DOI] [PubMed] [Google Scholar]
- [19].Day MA. Mindfulness-based Cognitive Therapy for Chronic Pain: a Clinical Manual and Guide. Chichester, West Sussex; Malden, MA: John Wiley & Sons Inc., 2017. [Google Scholar]
- [20].Day MA, Ehde DM, Burns J, Ward LC, Friedly JL, Thorn BE, Ciol MA, Mendoza E, Chan JF, Battalio S, Borckardt J, Jensen MP. A randomized trial to examine the mechanisms of cognitive, behavioral and mindfulness-based psychosocial treatments for chronic pain: Study protocol. Contemp Clin Trials 2020;93:106000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Day MA, Thorn BE, Ward LC, Rubin N, Hickman SD, Scogin F, Kilgo GR. Mindfulness-based Cognitive Therapy for the treatment of headache pain: a pilot study. Clin J Pain 2014; 30(2)152–61. [DOI] [PubMed] [Google Scholar]
- [22].Day MA, Ward LC, Ehde DM, Thorn BE, Burns J, Barnier A, Mattingley JB, Jensen MP. A Pilot Randomized Controlled Trial Comparing Mindfulness Meditation, Cognitive Therapy, and Mindfulness-Based Cognitive Therapy for Chronic Low Back Pain. Pain Med 2019;20(11):2134–2148. [DOI] [PubMed] [Google Scholar]
- [23].Dillworth T, Jensen MP. The Role of Suggestions in Hypnosis for Chronic Pain: A Review of the Literature. Open Pain J 2010;3(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Driscoll MA, Edwards RR, Becker WC, Kaptchuk TJ, Kerns RD. Psychological Interventions for the Treatment of Chronic Pain in Adults. Psychol Sci Public Interest 2021;22(2):52–95. [DOI] [PubMed] [Google Scholar]
- [25].Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin M, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [26].Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008;9(2):105–121. [DOI] [PubMed] [Google Scholar]
- [27].Ehde DM, Alschuler KN, Day MA, Ciol MA, Kaylor ML, Altman JK, Jensen MP. Mindfulness-based cognitive therapy and cognitive behavioral therapy for chronic pain in multiple sclerosis: a randomized controlled trial protocol. Trials 2019;20(1):774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ehde DM, Jensen MP. Feasibility of a cognitive restructuring intervention for treatment of chronic pain in persons with disabilites. Rehab Psych 2004;49:254–258. [Google Scholar]
- [29].Elwy AR, Johnston JM, Bormann JE, Hull A, Taylor SL. A systematic scoping review of complementary and alternative medicine mind and body practices to improve the health of veterans and military personnel. Med Care 2014;52(12 Suppl 5):S70–82. [DOI] [PubMed] [Google Scholar]
- [30].Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, Poole MR. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001;94(2):149–158. [DOI] [PubMed] [Google Scholar]
- [31].Gallagher RM. Advancing the Pain Agenda in the Veteran Population. Anesthesiol Clin 2016;34(2):357–378. [DOI] [PubMed] [Google Scholar]
- [32].Grant JA. Meditative analgesia: the current state of the field. Ann N Y Acad Sci 2014;1307:55–63. [DOI] [PubMed] [Google Scholar]
- [33].Hilton L, Hempel S, Ewing BA, Apaydin E, Xenakis L, Newberry S, Colaiaco B, Maher AR, Shanman RM, Sorbero ME, Maglione MA. Mindfulness Meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med 2017;51(2):199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jensen M, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain 1993;55:195–203. [DOI] [PubMed] [Google Scholar]
- [35].Jensen M, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994;58:387–392. [DOI] [PubMed] [Google Scholar]
- [36].Jensen MP. Measurement of pain. In: Fishman SM, Ballantyne JC, Rathmell JP, Editors. Bonica’s management of pain. Media, PA: Williams & Wilkins, 2010. pp. 251–270. [Google Scholar]
- [37].Jensen MP, Barber J, Romano JM, Hanley MA, Raichle KA, Molton IR, Engel JM, Osborne TL, Stoelb BL, Cardenas DD, Patterson DR. Effects of self-hypnosis training and EMG biofeedback relaxation training on chronic pain in persons with spinal-cord injury. Int J Clin Exp Hypn 2009;57(3):239–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jensen MP, Barber J, Romano JM, Molton IR, Raichle KA, Osborne TL, Engel JM, Stoelb BL, Kraft GH, Patterson DR. A comparison of self-hypnosis versus progressive muscle relaxation in patients with multiple sclerosis and chronic pain. Int J Clin Exp Hypn 2009;57(2):198–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jensen MP, Ehde DM, Gertz KJ, Stoelb BL, Dillworth TM, Hirsh AT, Molton IR, Kraft GH. Effects of self-hypnosis training and cognitive restructuring on daily pain intensity and catastrophizing in individuals with multiple sclerosis and chronic pain. Int J Clin Exp Hypn 2011;59(1):45–63. [DOI] [PubMed] [Google Scholar]
- [40].Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, Kraft GH, Hoffman AJ, Patterson DR. Hypnotic analgesia for chronic pain in persons with disabilities: a case series. Int J Clin Exp Hypn 2005;53(2):198–228. [DOI] [PubMed] [Google Scholar]
- [41].Jensen MP, Mendoza ME, Ehde DM, Patterson DR, Molton IR, Dillworth TM, Gertz KJ, Chan J, Hakimian S, Battalio SL, Ciol MA. Effects of hypnosis, cognitive therapy, hypnotic cognitive therapy, and pain education in adults with chronic pain: a randomized clinical trial. Pain 2020;161(10):2284–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Jensen MP, Patterson DR. Hypnotic approaches for chronic pain management: clinical implications of recent research findings. Am Psychol 2014;69(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med 1985;8(2):163–190. [DOI] [PubMed] [Google Scholar]
- [44].Kendrick C, Sliwinski J, Yu Y, Johnson A, Fisher W, Kekecs Z, Elkins G. Hypnosis for acute procedural pain: A Critical Review. Int J Clin Exp Hypn 2016;64(1):75–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J Pain 2014;15(10):979–984. [DOI] [PubMed] [Google Scholar]
- [46].Kind S, Otis JD. The Interaction between chronic pain and PTSD. Curr Pain Headache Rep 2019;23(12):1. [DOI] [PubMed] [Google Scholar]
- [47].la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: a randomized controlled trial. Pain Med 2015;16(4):641–652. [DOI] [PubMed] [Google Scholar]
- [48].Lan Y, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE. Development of Short Forms from the PROMIS Sleep Disturbance and Sleep-Related Impairment Item Banks. Behavioral Sleep Med 2012;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liossi C, White P, Hatira P. A randomized clinical trial of a brief hypnosis intervention to control venepuncture-related pain of paediatric cancer patients. Pain 2009;142(3):255–263. [DOI] [PubMed] [Google Scholar]
- [50].Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain 2016;17(9 Suppl):T93–T107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McEachrane-Gross FP, Liebschutz JM, Berlowitz D. Use of selected complementary and alternative medicine (CAM) treatments in veterans with cancer or chronic pain: a cross-sectional survey. BMC Complement Altern Med 2006;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].McKernan LC, Finn MTM, Patterson DR, Williams RM, Jensen MP. Clinical hypnosis for chronic pain in outpatient integrative medicine: an implementation and training model. J Altern Complement Med 2020;26(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain 2017;18(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Patil SG. Effectiveness of mindfulness meditation (Vipassana) in the management of chronic low back pain. Indian J Anaesth 2009;53(2):158–163. [PMC free article] [PubMed] [Google Scholar]
- [55].Patterson DR, Jensen MP. Hypnosis and clinical pain. Psychol Bull 2003;129(4):495–521. [DOI] [PubMed] [Google Scholar]
- [56].Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D, Group PC. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rizzo RRN, Medeiros FC, Pires LG, Pimenta RM, McAuley JH, Jensen MP, Costa LOP. Hypnosis enhances the effects of pain education in patients with chronic nonspecific low back pain: a randomized controlled trial. J Pain 2018;19(10):1103 e1101–1103 e1109. [DOI] [PubMed] [Google Scholar]
- [58].Scott W, McCracken LM. Patients’ impression of change following treatment for chronic pain: global, specific, a single dimension, or many? J Pain 2015;16(6):518–526. [DOI] [PubMed] [Google Scholar]
- [59].Tan G, Rintala DH, Jensen MP, Fukui T, Smith D, Williams W. A randomized controlled trial of hypnosis compared with biofeedback for adults with chronic low back pain. Eur J Pain 2015;19(2):271–280. [DOI] [PubMed] [Google Scholar]
- [60].Taylor SL, Elwy AR. Complementary and alternative medicine for US veterans and active duty military personnel: promising steps to improve their health. Med Care 2014;52(12 Suppl 5):S1–4. [DOI] [PubMed] [Google Scholar]
- [61].Thompson T, Terhune DB, Oram C, Sharangparni J, Rouf R, Solmi M, Veronese N, Stubbs B. The effectiveness of hypnosis for pain relief: A systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev 2019;99:298–310. [DOI] [PubMed] [Google Scholar]
- [62].Weitzenhoffer A, Hilgard ER Stanford Hypnotic Susceptibility Scale, Form C. Palo Alto, CA: Consulting Psychologists Press, 1963. [Google Scholar]
- [63].Williams RM, Ehde DM, Day M, Turner AP, Hakimian S, Gertz K, Ciol M, McCall A, Kincaid C, Pettet MW, Patterson D, Suri P, Jensen MP. The chronic pain skills study: protocol for a randomized controlled trial comparing hypnosis, mindfulness meditation and pain education in Veterans. Contemp Clin Trials 2020;90:105935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zeidan F, Grant JA, Brown CA, McHaffie JG, Coghill RC. Mindfulness meditation-related pain relief: evidence for unique brain mechanisms in the regulation of pain. Neurosci Lett 2012;520(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


