ABSTRACT
Rationale
Intense exercise promotes fatigue and can impair cognitive function, particularly toward the end of competition when decision-making is often critical for success. For this reason, athletes often ingest caffeinated energy drinks prior to or during exercise to help them maintain focus, reaction time, and cognitive function during competition. However, caffeine habituation and genetic sensitivity to caffeine (CA) limit efficacy. Paraxanthine (PX) is a metabolite of caffeine reported to possess nootropic properties. This study examined whether ingestion of PX with and without CA affects pre- or post-exercise cognitive function.
Methods
12 trained runners were randomly assigned to consume in a double-blind, randomized, and crossover manner 400 mg of a placebo (PL); 200 mg of PL + 200 mg of CA; 200 mg of PL + 200 mg of PX (ENFINITY®, Ingenious Ingredients); or 200 mg PX + 200 mg of CA (PX+CA) with a 7–14-day washout between treatments. Participants donated fasting blood samples and completed pre-supplementation (PRE) side effects questionnaires, the Berg-Wisconsin Card Sorting Test (BCST), and the Psychomotor Vigilance Task Test (PVTT). Participants then ingested the assigned treatment and rested for 60 minutes, repeated tests (PRE-EX), performed a 10-km run on a treadmill at a competition pace, and then repeated tests (POST-EX). Data were analyzed using General Linear Model (GLM) univariate analyses with repeated measures and percent changes from baseline with 95% confidence intervals.
Results
BCST correct responses in the PX treatment increased from PRE-EX to POST-EX (6.8% [1.5, 12.1], p = 0.012). The error rate in the PL (23.5 [−2.8, 49.8] %, p = 0.078) and CA treatment (31.5 [5.2, 57.8] %, p = 0.02) increased from PRE-EX values with POST-EX errors tending to be lower with PX treatment compared to CA (−35.7 [−72.9, 1.4] %, p = 0.059). POST-EX perseverative errors with PAR rules were significantly lower with PX treatment than with CA (−26.9 [−50.5, −3.4] %, p = 0.026). Vigilance analysis revealed a significant interaction effect in Trial #2 mean reaction time values (p = 0.049, = 0.134, moderate to large effect) with POST-EX reaction times tending to be faster with PX and CA treatment. POST-EX mean reaction time of all trials with PX treatment was significantly faster than PL (−23.2 [−43.4, −2.4] %, p = 0.029) and PX+CA (−29.6 [−50.3, −8.80] %, p = 0.006) treatments. There was no evidence that PX ingestion adversely affected ratings of side effects associated with stimulant intake or clinical blood markers.
Conclusions
Results provide some evidence that pre-exercise PX ingestion improves prefrontal cortex function, attenuates attentional decline, mitigates cognitive fatigue, and improves reaction time and vigilance. Adding CA to PX did not provide additional benefits. Therefore, PX ingestion may serve as a nootropic alternative to CA.
KEYWORDS: Nootropic, caffeine alternative, ergogenic aid, sports nutrition
1. Introduction
Prolonged exercise promotes mental fatigue and can impair performance, particularly toward the end of competition when decision-making is often critical for success [1,2]. For this reason, athletes often consume energy drinks containing nootropic nutrients before and/or during exercise to help them maintain focus, cognitive function, and performance [3–8]. Caffeine is one of the most common naturally occurring nootropic nutrients in beverages that help maintain alertness, mental function, and exercise performance [7–9]. According to the International Society of Sports Nutrition, ingesting 3–6 mg/kg of caffeine about 60 minutes before exercise can improve cognition, attention, vigilance, and exercise performance [8,10]. However, the effects of ingesting caffeine before exercise vary depending on the type, amount, and length of exercise. Additionally, individuals with a homogenous A allele of the CYP1A2 gene tend to produce more cytochrome P450, an enzyme responsible for about 95% of caffeine metabolism and consequently metabolize caffeine more quickly [11]. Fast metabolizers of caffeine experience more significant ergogenic outcomes in some [11,12] but not all studies [13]. In addition, habituation to ingesting daily consumption of caffeinated foods and beverages can reduce its efficacy.
In humans, about 70% of CA is metabolized into 1,7-dimethylxanthine or paraxanthine (PX), with the remainder metabolized into 3,7-dimethylxanthine or theobromine (TB) and 1,3-dimethylxanthine or theophylline (TP) [14]. Compared to caffeine, paraxanthine has a shorter half-life and faster clearance from the blood than caffeine, theobromine, and theophylline [15]. Studies also indicate that paraxanthine has less toxic [16], anxiogenic [17], cardiovascular, and gastrointestinal side effects than caffeine [18]. Additionally, paraxanthine has a higher binding potency for adenosine A1 and A2a receptors and more substantial locomotor activation effects [19]. Paraxanthine has also been reported to inhibit phosphodiesterase 9 (PDE9), which terminates nitric oxide (NO) neurotransmission by metabolizing cyclic guanosine monophosphate (cGMP) back to GMP. Through PDE9 inhibition, paraxanthine potentiates NO neurotransmission, while caffeine does not affect this pathway [20]. Paraxanthine has been shown to have protective effects for dopaminergic neurons and has been reported to reduce synaptic function-related neurodegeneration, while caffeine provides marginal protection [21]. In addition, the wake-promoting potency of paraxanthine is greater and longer lasting than caffeine [22].
Theoretically, avoiding genetic and/or metabolically related variations in caffeine metabolism by supplementing directly with paraxanthine may provide a more direct way to improve cognitive and/or exercise performance with fewer side effects. In support of this hypothesis, we reported that ingestion of 200 mg of PX enhanced memory, reaction time, and attention for up to 6 hours in healthy adults [23]. Additionally, the ingestion of 50, 100, and 200 mg of PX for up to 7 days enhanced measures of cognition, memory, reasoning, response time, and helped sustain attention with no apparent side effects [24]. However, we are unaware of any study assessing the effects of ingesting paraxanthine prior to intense exercise on cognition following intense exercise. Moreover, we are not aware of any study that assessed the efficacy of paraxanthine supplementation compared to caffeine following exercise. Since staying focused and making quick decisions influences decision-making in sports and prolonged exercise promotes mental fatigue [2,25–27], we hypothesized that ingestion of paraxanthine prior to exercise may serve as an effective nootropic and/or ergogenic aid and potentially reducing exercise-induced mental fatigue. Therefore, the primary aims of this study were to determine: (1) whether acute paraxanthine ingestion affects cognitive function prior to and/or following exercise, (2) whether paraxanthine has measurable benefits in comparison to caffeine, and (3) whether the co-ingestion of paraxanthine and caffeine has additive or synergistic effects. If effective, acute paraxanthine ingestion could serve as a viable nootropic alternative to caffeine ingestion for energy drinks and/or pre-workout supplements designed to promote and/or sustain cognitive function during exercise.
2. Methods
2.1. Design of study
The experimental design was a randomized, crossover, double-blind, placebo-controlled clinical trial. The study was approved by the Human Protection Institutional Review Board (IRB2019–0928F) in accordance with ethical standards for the conduct of human participant research as described in the Declaration of Helsinki. This clinical trial was registered with the International Standard Randomized Control Number registry (ISRCTN14506218). Stimulant ingestion served as the independent. The primary outcome was measures of cognitive function. Secondary outcomes included changes in exercise heart rate, clinical blood chemistry panels, and subjective ratings of symptoms and side effects.
2.2. Study participants
Trained runners from local running and triathlon clubs and races were recruited for this study. Eligibility criteria included healthy trained runners or triathletes between 18–40 years of age, current (≥6 months) history of run training, and documented evidence that they averaged 8 minutes/mile or less running pace during a recent competition (e.g. completing a 5-km road race or marathon). Qualified runners were invited to attend a familiarization session, which provided an overview of the study, and participants informed consent to participate in the study. Participants then completed a medical history and underwent a physical exam. Runners were not eligible to participate in the study if they had (1) a medical condition that hindered the ability to perform the study protocol; (2) a history of cognitive dysfunction; (3) were currently taking prescription medications; (4) a known allergy to wheat flour; (5) a sleep disorder; (6) been/were pregnant or breastfeeding; or (7) a physician’s order to abstain/restrict caffeine or stimulant intake. A Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Figure 1. A total of 32 potential participants responded to study advertisements and were assessed for eligibility. Of these, 28 passed the phone screening and were invited to familiarization sessions. Due to scheduling conflicts, 21 were familiarized and consented to participate in the study. Of these, 15 individuals were able to participate in the study and were randomized into testing sessions. Treatment allocaations are presented by testing rounds with the number (n) of participants tested shown. Due to pandemic-related delays in conducting human participant research, two participants moved during the study. One volunteer was omitted from the analysis because they could not complete testing sessions in a timely manner due to scheduling conflicts. Therefore, 12 runners (11 males, 1 female) were included in the analysis.
Figure 1.
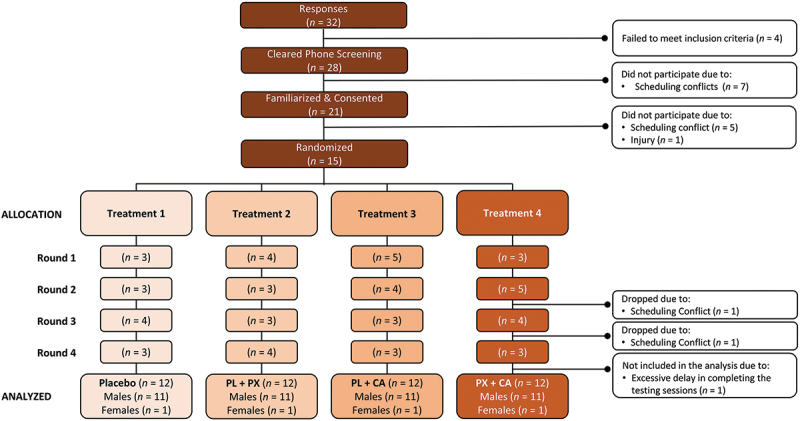
Consolidated standards of reporting trials (CONSORT) illustration for the placebo (PL), paraxanthine (PX), caffeine (CA), and paraxanthine + caffeine (PX+CA) treatments.
2.3. Testing protocol
Volunteers visited the lab five times, including one familiarization session and four experimental sessions. During the familiarization session, participants were explained the study protocol, provided informed consent, answered a medical questionnaire, and had height, weight, resting heart rate, and resting blood pressure. Males then had body composition determined using dual-energy X-ray absorptiometry (DXA), while females took a urine pregnancy test prior to the DXA scan to verify they were not pregnant. Participants then took each cognitive function test three times to familiarize themselves with the tests and establish test reliability. Volunteers practiced running on the treadmill to be used in the study at a competitive pace. Once completed, the participants performed a graded maximal cardiopulmonary (VO2 peak) treadmill test to determine peak heart rate and aerobic capacity. Participants then practiced the anaerobic capacity cycling test.
Participants followed normal eating habits and abstained from ingesting new dietary supplements for the duration of the study. Participants prepared for each testing session as they would a 10-km road race. Additionally, they refrained from vigorous physical activity, alcohol intake, and over-the-counter medications for 24 hours as well as fasted for 8–12 hrs prior to reporting to the lab. Figure 2 shows the timeline of tests performed during each experimental testing session. Upon arriving at the lab, participants had their weight, resting heart rate, and blood pressure determined. Participants then completed a side effects questionnaire, performed cognitive function tests, donated a fasting blood sample, and then ingested 1 of 4 randomly assigned oral supplements (PRE). Participants then rested for 15 minutes and repeated these tests (PRE-EX). Volunteers then performed a 10-km run time trial at their self-determined pace. Performance times, heart rate, and rating of perceived exertion (RPE) were obtained every kilometer during the run. Once completed, participants donated a venous blood sample and then performed a 30-second anaerobic capacity test. Participants then completed the cognitive function tests and reported side effects (POST-EX). After each testing session, participants observed a 7 to 14-day washout period and then identically repeated the protocol while ingesting the remaining treatments in a randomized manner.
Figure 2.
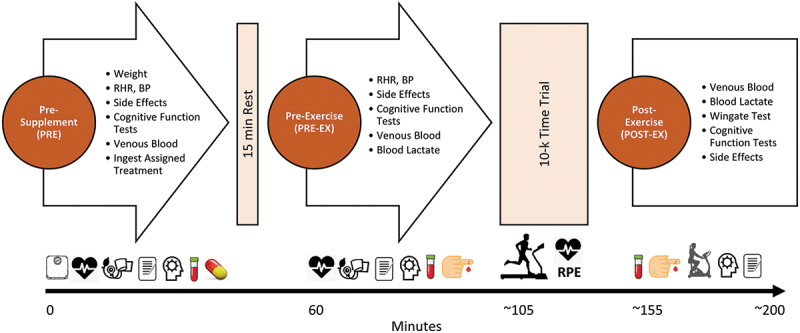
Overview of experiment study timeline. RHR represents resting heart rate, BP represents blood pressure.
2.4. Supplementation protocol
A Balanced Latin Square method was used to counterbalance the order of treatments [28]. Treatments included (1) 400 mg of placebo (PL, wheat flour, Shandong Bailong Chuangyuan Bio-tec Co. Ltd., Dezhou, China); (2) 200 mg of PL +200 mg of CA (CSPC Innovation Pharmaceutical Co. Ltd., Shi Jiazhuang, China); (3) 200 mg of PL +200 mg of PX (ENFINITY®, Ingenious Ingredients, L.P. Lewisville, TX, USA) or (4) 200 mg CA +200 mg of PX (PX+CA). Supplements preparation followed good manufacturing practices and was certified by the manufacturer for content and purity. Supplements were similar in appearance and provided in generically labeled bottles. Supplements were administered after completing all PRE assessments on each testing day. Participants ingested one capsule with eight ounces of water of the assigned treatment, waited 15 minutes, and began post-supplementation assessments.
3. Procedures
3.1. Demographics
Weight and height were obtained using a Health-O-Meter Professional 500KL scale (Pelstar LLC, Alsip, IL, USA). Sitting resting heart rate and blood pressure were obtained after resting for 5-minutes. Resting heart rate was determined via palpation of the radial artery, while blood pressure was determined using a stethoscope and sphygmomanometer using standard procedures [29]. Body composition (excluding cranium) was determined using DXA (Hologic Inc., Waltham, MA, USA) with APEX Version 3.1 software (APEX Corporation Software, Pittsburg, PA, USA) [30,31] with test-retest and day-to-day variability found mean coefficients of variability (CV) for bone mineral content and lean mass of 0.31–0.45% with a mean intra-class correlation (ICC) of 0.985 [32,33]. Maximal aerobic capacity was determined from an incremental, symptom-limited, maximal cardiopulmonary exercise test using the Bruce treadmill protocol following standard procedures [34]. Aerobic capacity was determined using a ParvoMedics TrueMax 2400 Metabolic Measurement System (ParvoMedics Inc, Sandy, UT). The system volume measurement was calibrated with a series 5530 three-liter volume syringe (Hans Rudolph Inc., Kansas City, MO). Oxygen and carbon dioxide analyzers were calibrated to known medical-grade gases prior to each test following standard procedures.
3.2. Diet control
Participants prepare for testing sessions as they would leading up to a road race. For diet consistency, participants recorded food and beverage intake for 4 days prior to each testing session using the MyFitnessPal Calorie Counter phone application (MyFitnessPal, Inc., Baltimore, MD, USA) or written food logs [35,36]. Participants were asked to maintain caffeine intake (e.g. <200 mg/d) and refrain from ingesting any other stimulants not commonly consumed in their diet for 48 hours prior to each testing session as well as reported to the lab after an 8–12 hour fast to normalize diet and caffeine intake on performance. Participants replicated this diet prior to each testing session. Diet inventories were reviewed for consistency by one research assistant and analyzed using standard nutritional analysis software (Food Processor Version 11.4.412, ESHA Nutrition Research, Salem, OR, USA) [37].
3.3. Running performance assessment
The 10-km run was performed on a TrackMaster tmx425c treadmill (Full Vision INC., Newton, KS). Participants warmed up as they were accustomed to before competitive running and were then asked to perform each run to the best of their ability. Split times and RPE using the Borg 6–20 scale [38] were recorded at 1 km intervals. Heart rate was monitored using a Polar H10 Heart Rate Monitor (Polar Electro, Inc., Bethpage, NY, USA). Participants were offered water ad libitum at 1 km intervals. The volume from the first trial was used as a standard for the remaining trials. After completing the 10-km run and donating a blood sample for lactate determination, participants performed a 30-sec Wingate anaerobic cycling test on a Lode Excalibur Sport 925,900 cycle ergometer (Lode BV, Groningen, The Netherlands) at a standardized work rate of 7.5 J/kg/rev. Test-to-test variability of repeated Wingate anaerobic capacity tests in our laboratory yielded correlation coefficients of r = 0.98 ± 15% for mean power.
3.4. Cognitive assessment
Cognitive function was assessed by having participants perform the Psychology Experiment Building Language (PEBL) test (Version 2.1, http://pebl.sourceforge.net, accessed 19 June 2019) [39]. A more complete description of the PEBL tests employed in our lab were previously described [23]. The assessment battery included the Berg-Wisconsin Card Sorting Task test (BCST) that assesses reaction time and accuracy of sorting cards and thereby assesses reasoning, learning, executive function, attention shifting (i.e. flexibility in responding to changing schedules of reinforcement), and impulsiveness [39–43]; and, the Psychomotor Vigilance Task Test (PVTT) that assesses sustained attention and reaction times by pressing a key on a keyboard when a randomly illuminating light is displayed on a monitor every few seconds [40,41,44–46]. Participants practiced the test sessions during the familiarization session three times to establish test reliability. During each testing session, tests were administered in the same order and took about 30–35 minutes to complete. Participants were allowed to relax between each cognitive function test with no more than 5 minutes between trials.
3.5. Blood analysis
Fasting blood was obtained before treatment ingestion, before performing the run, and after the run. This involved taking about 10 mL of blood from an antecubital vein in the forearm using standard phlebotomy procedures [47,48]. Blood was collected in serum separation (SST) and K2 EDTA BD Vacutainer® tubes (Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The SST tubes were left at room temperature for 15 minutes and then centrifuged for 10 minutes at 3,500 g in a refrigerated (4°C) Thermo Scientific Heraeus MegaFuge 40 R Centrifuge (Thermo Electron North America LLC, West Palm Beach, FL, USA) [49]. Clinical Pathology Labs, Inc. (Austin, TX. CLIA #45D0505003, CAP Accreditation #21525–01) analyzed whole blood and serum samples. Whole blood cell counts were analyzed by an automated multichannel hematology analyzer. A Roche Cobas Gen 2 analyzer (Roche Diagnostics International AG, Rotkreuz, Switzerland) was used to assess serum samples. Test-retest reliability of performing the assays evaluated in this lab ranged from 2% to 6%. Additionally, prior to and following the performance runs, about 0.7 µL of arterialized venous blood was obtained from a clean finger and measured for blood lactate using a calibrated Lactate Plus Meter (Nova Biomedical, Waltham, MA). Intra-analyzer reliability of the device demonstrated a typical error of measurement of 0.4 mM, with CV values at 8.5% [50].
3.6. Side effect questionnaire
The frequency and severity of dizziness, tachycardia, heart palpitations, shortness of breath, blurred vision, and nervousness were assessed using a Likert-type scale where 0 represented none; 1 represented 1–2 per week or minimal; 2 represented 3–4 per week or slight; 3 represented 5–6 per week or moderate; 4 represented 7–8 per week or severe; and 5 represented ≥ 9 per week or very severe. Volunteers were also asked to report any other side effects they may have experienced in response to taking the assigned treatments. Reliability in answering these side effects questions in our lab revealed mean CVs ranging from 1.2–2.6% with a mean ICC ranging between 0.59–0.88 [51].
3.7. Statistical analysis
Data were analyzed by the IBM® Version 29 SPSS® statistical analysis software (IBM Corp., Armonk, NY, USA). The sample size was selected based on our previous work in this area [23,52–54] assuming an expected improvement of 5% with a power of 80% in primary outcome cognitive function-related variables. We previously demonstrated that the sample size was sufficient to assess clinically significant results [52–56]. A balanced Latin Square designer program [28] was used to randomize participants to treatments in a crossover manner. General linear model (GLM) multivariate and univariate analyses with repeated measures were used to analyze the data. The Wilks’ Lambda and Greenhouse-Geisser univariate correction tests assessed Time and Treatment x Time interaction effects. The probability of type I error was p < 0.05. Statistical tendencies were noted when p-values >0.05 to < 0.10 were observed. Pairwise comparisons were assessed using Fisher’s least significant difference statistics. We also analyzed data using relative dose as a covariate. Since the results were similar, we did not report this analysis. Mean changes from baseline with 95% confidence intervals (CIs) were used to assess the clinical significance of findings. Means and 95% CI’s completely above or below baseline were considered clinically significant [57]. Data are presented means and ± standard deviations (SD) or mean changes from baseline with lower and upper confidence intervals (mean [LL, UL]). Partial Eta squared () values were used to assess effect size where values of 0.01 represented a small effect, 0.06 represented a medium effect, and 0.14 represented a large effect size [58]. This statistical approach is consistent with recommendations from Earnest et al. [59] on best practices in reporting sport nutrition-related research.
4. Results
4.1. Demographic data
Table S1 shows participant demographic data. Twelve trained runners completed this study (11 males and 1 female). Participants were 26.4 ± 5.1 years old, 1.76 ± 0.1 m tall, weighed 68.6 ± 9.6 kg, had a body mass index (BMI) of 22.2 ± 2.8 kg/m2, 16.2 ± 5.2 percent body fat, had a peak oxygen uptake of 52.4 ± 10.6 ml/kg/min (15.0 ± 3.0 metabolic equivalents or METS), and peak heart rate observed of 185 ± 9.9 bpm. Participants also had a resting heart rate of 59.6 ± 10.7 bpm, a systolic blood pressure of 115.4 ± 8.3 mmHg, and a diastolic blood pressure of 68.9 ± 6.1 mmHg.
4.2. Performance analysis
Table S2 in the Supplemental Materials file presents split times, heart rate, RPE, and pre-and post-run blood lactate values observed during the 10 km runs. An overall GLM time effect was observed among split times, heart rate, and RPE (p < 0.001, = 0.902, large effect) with no significant treatment by interaction effects observed (p = 1.000, = 0.025, small effect). Comparable results were observed in univariate analyses. Blood lactate increased from pre- to post-exercise by 5.34 [4.57, 6.10] mmol/L, p < 0.001). However, no significant differences were observed among treatments (p = 0.738, = 0.028, small effect). Participants completed the runs in an average of 48.37 ± 6.75 min with an average heart rate of 185.7 ± 11.4 bpm, an RPE of 18.4 ± 1.7 on the 6–20 scale, and a blood lactate of 7.38 ± 2.5 mmol/L. No significant differences were observed between treatments with 1 km data. Similarly, no significant differences were observed among treatments in post-run anaerobic capacity (Table S3).
4.3. Cognitive function assessment
4.3.1. Berg-Wisconsin card sorting test
Table S4 in the Supplemental Materials file presents the BCST results observed, while Figure S1a in the Supplemental Materials file presents individual and mean responses observed. Analysis of BCST data results revealed no significant multivariate or univariate treatment x time interaction effects in correct responses, errors, PEBL calculated perseverative errors, or perseverative errors with PAR rules (revised scoring method). Correct responses increased from PRE to POST-EX with PL treatment (p = 0.011) and from PRE-EX to POST-EX in the PX treatment (p = 0.028). A moderate treatment x time effect size was seen in errors ( = 0.072, medium effect) with pairwise comparison analysis indicating that PRE-EX values between the PX and PX+CA treatments tended to differ (3.30 [−0.4, 6.6], p = 0.077). No significant time or treatment x time effects were seen in PEBL perseverative errors. However, a moderate effect size was observed in perseverative errors with PAR rules ( = 0.077, medium effect). Treatment with CA increased preservative errors with PAR rules from PRE-EX to POST-EX (p = 0.032). Analysis of percent changes from PRE-EX to POST-EX is presented in Figure 3 below, while Figure S1b (shown in the Supplemental Materials file) shows mean changes from baseline with individual data points. Results revealed that correct responses in the PX treatment increased from PRE to POST-EX (6.8 [1.5, 12.1] %, p = 0.012). Errors in the PL (23.5 [−2.8, 49.8] %, p = 0.078) and CA treatment (31.5 [5.2, 57.8] %, p = 0.02) increased from PRE-EX values with POST-EX errors tending to be lower with PX treatment compared to CA (−35.7 [−72.9, 1.4] %, p = 0.059). Perseverative errors increased from PRE-EX to POST-EX values with CA treatment (20.0 [2.6, 37.4] %, p = 0.025). Finally, perseverative errors with PAR rules increased from PRE-EX to POST-EX with CA treatment (25.7% [9.0, 42.3], p = 0.003) with POST-EX values with PX treatment significantly lower than CA (−26.9% [−50.5, −3.4], p = 0.026). These findings provide evidence that PX ingestion significantly improves correct responses over time while significantly reducing errors in comparison to CA ingestion. However, PX treatment did not improve BCST performance measures compared to PL treatment.
Figure 3.
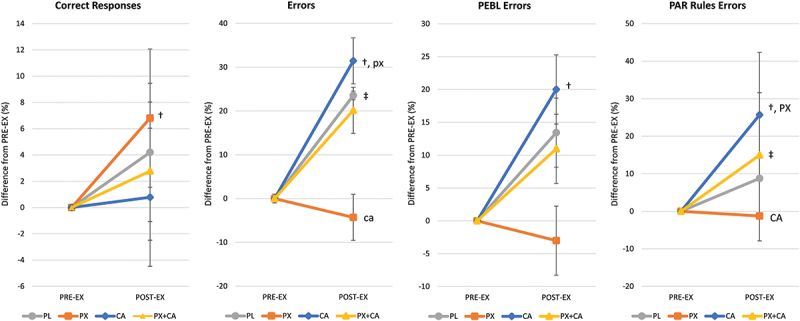
Berg-Wisconsin Card Sorting test mean percent changes from pre-exercise (PRE-EX) values with 95% confidence intervals. † represents p < 0.05 from PRE-EX values while ‡ represents p > 0.05 to p < 0.10 effect. Treatment differences (p < 0.05) are shown as differences from placebo (PL), paraxanthine (PX), caffeine (CA) and PX + CA. Statistical trends (p > 0.05 to p < 0.10) are shown as small case (pl, px, ca, px+ca).
4.3.2. Psychomotor vigilance task test
Table S3 in the Supplemental Materials file shows PVTT-related variables while Figure S2a in the Supplemental Materials file presents individual and mean responses observed. A significant univariate treatment x time effect with a large effect size was observed in trial #2 mean reaction time values (p = 0.049, = 0.134, medium to large effect). Pairwise comparisons revealed that reaction time increased with PL from PRE to POST-EX (1057 [365, 1750] ms, p = 0.004) while CA values tended to increase over time (618 [−74, 1310] ms, p = 0.079) in Trial #2. Post-EX trial #2 reaction times tended to be faster with PX and CA compared to PX+CA treatment. There was also evidence that mean reaction time increased over time with PL and PX+CA treatment while tending to decrease from PRE-EX to POST-EX with PX ingestion. Analysis of mean changes from PRE-EX values with 95% CI’s (Figure 4) revealed that reaction times increased in the PL and PX+CA treatments while PX and CA ingestion promoted faster reaction times to perform the PVTT. This was more evident when comparing POST-EX mean reaction times for all twenty trials. POST-EX mean reaction times in the PX treatment were significantly faster than PL (−23.2 [−43.4, −2.4] %, p = 0.029) and PX + CA (−29.6 [−50.3, −8.80] %, p = 0.006) while not significantly different than CA (−5.1 [−25.5, 15.7] %, p = 0.626). However, PX + CA ingestion promoted a greater improvement in mean reaction times compared to CA (−24.5 [−45.3, −3.7] %, p = 0.022). Figure S2b in the Supplemental Materials file shows the mean changes from baseline with individual data points. Results provide evidence that PX supplementation increases early and overall attention and prevents exercise-induced attention lapses compared to PL and in a similar manner as CA. However, ingesting PX+CA did not provide additive benefits.
Figure 4.
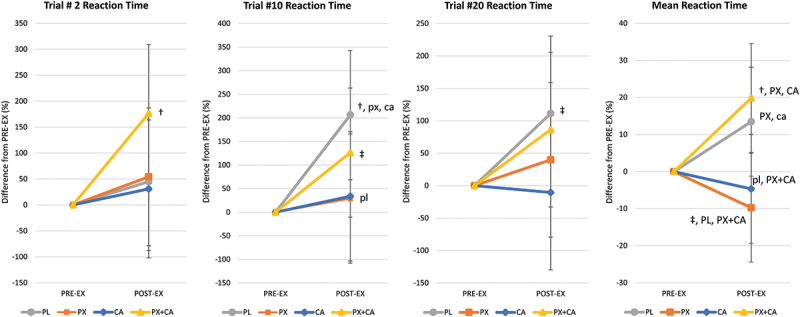
Psychomotor vigilance task mean percent changes from PRE-EX values with 95% confidence interval data. Mean reaction time is the mean for all 10 trials performed. † represents p < 0.05 from PRE-EX values while ‡ represents p > 0.05 to p < 0.10 trend. Treatment differences (p < 0.05) are shown as differences from placebo (PL), paraxanthine (PX), caffeine (CA) and PX + CA. Statistical trends (p > 0.05 to p < 0.10) are shown as small case (pl, px, ca, px+ca).
4.4. Safety assessment
Tables S6 - S9 in the Supplemental Materials file show blood-related data. No significant multivariate or univariate interaction effects were observed in whole blood cell counts (Table S6), blood lipids (Table S7), markers of liver function (Table S8), and most markers of renal function (Table S9). Significant differences were observed among treatments in serum potassium and carbon dioxide, with PRE-EX and POST-EX values in the CA and PX+CA lower than PL values. Observed changes between PRE-EX and POST-EX were deemed exercise-induced and unrelated to supplementation. Tables S10 and S11 in the Supplemental Materials present the frequency and severity of common side effects associated with taking supplements, respectively. Results revealed no significant interactions among treatments. Several pairwise differences were observed among treatments, particularly in POST-EX responses. However, the mean rating values were typically less than 1.0, indicating that the side effects were infrequent and of minimal severity and associated with CA ingestion. There was no evidence that PX alone promoted any side effects. These findings suggest that acute ingestion of PX was well tolerated.
5. Discussion
Athletes often consume caffeinated beverages to increase energy, maintain alertness and/or provide cognitive or ergogenic benefits [1,4,9,10,60,61]. While caffeine’s pharmacokinetics and ergogenic value have been well documented [7,8,15,60,62–64], less is known about paraxanthine. We recently reported evidence that acute ingestion of 200 mg of paraxanthine ingestion influenced memory, cognition, and attention in healthy male and female participants [54]. A dose-response study confirmed the nootropic effects of paraxanthine in acute doses as little as 50 mg [24]. Moreover, Jäger and coworkers [65] reported that paraxanthine supplementation increased muscle mass, strength, and endurance in mice. While preclinical and mechanistic data suggest some advantages of paraxanthine over caffeine, no study has compared the nootropic effects of ingesting paraxanthine with or without caffeine on cognition after intense exercise. Demonstrating that paraxanthine supplementation has independent and/or synergistic effects on cognition after intense exercise compared to caffeine could provide evidence to support use of paraxanthine as an alternative to caffeine in pre-workout supplements and energy drinks.
Present findings add to initial findings that acute paraxanthine has nootropic properties. In this regard, analysis of the Berg Card Sorting Test results revealed that correct responses increased from PRE to POST-EX by 6.8% (p = 0.012) with paraxanthine ingestion, while POST-EX errors with paraxanthine ingestion tended to be lower than CA (−35.7%, p = 0.059). POST-EX perseverative errors with PAR rules were also significantly lower with PX treatment compared to CA (−26.9%, p = 0.026) while not significantly different than PL (−10.0 [−33.6, 13.5] %, p = 0.40). The BCST assesses thought, reasoning, learning, executive control, attention shifting, and impulsiveness [42,43]. Increases in perseverative errors are indicative of greater mental fatigue [66]. These findings provide evidence that paraxanthine ingestion can help sustain attention and improve accuracy over time. However, it should be noted that PX treatment did not improve BCST performance measures compared to PL treatment. Participants also performed the PVTT that assesses sustained attention reaction times to visual stimuli [44–46]. Our previous studies [54,56] reported that paraxanthine helped sustain attention over time. In the current study, paraxanthine ingestion prior to exercise improved reaction times during trial #2 of the PVTT while promoting faster POST-EX reaction times than observed after placebo ingestion PL (−23.2 [−43.4, −2.4] %, p = 0.029) and PX+CA (−29.6 [−50.3, −8.80] %, p = 0.006) while not significantly different than CA (−5.1 [−25.5, 15.7] %, p = 0.626). Nitric oxide neurotransmission plays a vital role in both the learning process and memory of the learned task [67]. Paraxanthine inhibits PDE9 and thereby potentiates NO neurotransmission, while caffeine does not have that effect. In addition, paraxanthine releases neurotransmitters (e.g. dopamine and glutamine) to a greater extent than caffeine. Although we did not assess neurotransmitter levels in the current study, the differences in neurotransmission between paraxanthine and caffeine treatments could explain measurable differences observed in cognitive performance (i.e. faster responses with fewer mistakes). The BCST was used to assess learning, and in our study, paraxanthine ingestion significantly reduced total errors POST-EX in comparison to caffeine (−35.8% (−72.9, 1.4), p = 0.059) and PAR rule errors (−26.9 (−50.5, −3.4), p = 0.026) while significantly increasing the number of correct responses from PRE-EX to POST-EX (6.8% (1.5, 12.1), p = 0.012). These findings provide evidence that acute ingestion paraxanthine significantly improves correct responses over time while significantly reducing errors compared to caffeine ingestion.
The Psychomotor Vigilance Task Test assesses sustained attention and reaction times over time by performing twenty test trials. Delays in response are considered lapses in attention. We previously reported that acute ingestion of paraxanthine sustained attention over time. In the present study, we found that paraxanthine ingestion before exercise promoted faster overall mean response times than the placebo (−23.2 [−43.4, −2.4] %, p = 0.029) and paraxanthine + caffeine treatment (−29.6 [−50.3, −8.80] %, p = 0.006). while not significantly different than CA (−5.1 [−25.5, 15.7] %, p = 0.626). However, paraxanthine + caffeine ingestion promoted a greater improvement in mean reaction times compared to caffeine alone (−24.5 [−45.3, −3.7] %, p = 0.022). Results provide some evidence that paraxanthine supplementation increased early and overall attention and prevents exercise-induced attention lapses compared to ingesting a placebo and in an analogous manner as caffeine. Interestingly, co-ingesting paraxanthine and caffeine did not provide additive benefits. These findings suggest that paraxanthine may have independent nootropic effects than caffeine.
Participants in our initial studies did not report any side effects from paraxanthine supplementation [23,24]. Conversely, caffeine and/or other stimulants have been reported to experience unwanted side effects in some individuals [60,68]. In the current study, caffeine intake increased perceptions of the frequency of tachycardia and shortness of breath over time while increasing perceptions of the severity of tachycardia, shortness of breath, and nervousness. Conversely, paraxanthine ingestion did not affect perceptions of the frequency or severity of these side effects. In fact, perceptions of the frequency of nervousness were significantly lower after exercise. Additionally, the paraxanthine treatment was associated with lower frequency and severity of monitored side effects than when ingesting caffeine or PX+CA before exercise. These findings support contentions that paraxanthine may be better tolerated than caffeine while still providing nootropic benefits [15,22].
A strength of this investigation is that it represents the first double-blind, placebo-controlled, crossover study in healthy younger individuals that directly compared the effects of pre-exercise paraxanthine ingestion with and without caffeine on cognitive function and psychomotor vigilance. Results add to accumulating evidence that paraxanthine may serve as a safe and effective nootropic nutrient. However, the study is limited by sample size, particularly in terms of the number of women studied. Additionally, although we asked participants to refrain from excessive stimulant intake during the study, differences in habitual diets and stimulant sensitivity to caffeine and/or paraxanthine may have varied, thereby influencing results. Further, although participants practiced the cognitive tests, natural day-to-day variability in cognitive function, mood, and/or motivation to perform cognitive function tests may have influenced the results. Further research is warranted to corroborate findings and explore whether individual variability, sensitivity in response to acute paraxanthine ingestion, caffeine restriction prior to use of paraxanthine, sex differences, and/or length of supplementation protocols may affect responsiveness to paraxanthine ingestion for cognitive and psychomotor enhancement.
6. Conclusion
Acute paraxanthine ingestion is safe and improved some measures of executive function, attenuate attentional degradation, and mitigate cognitive fatigue before and after exercise. We also observed some evidence that paraxanthine ingestion promoted more significant improvements than caffeine independently while co-ingesting paraxanthine with caffeine did not provide any additional benefit. These findings indicate that paraxanthine may serve as a viable alternative to caffeine in helping maintain cognitive function during prolonged exercise.
Supplementary Material
Acknowledgments
We thank the study participants, Dr. J.P. Bramhall for providing medical oversight, Dr. Peter Murano, and who served as an external safety and quality assurance monitor for this study.
Funding Statement
Ingenious Ingredients, LP (Lewisville, TX, USA) provided funding for this study through a fee-for-service contract to the Human Clinical Research Facility at Texas A&M University. The study was conducted by the Exercise & Sport Nutrition Laboratory. The sponsor did not participate in data collection or statistical analysis of data.
Disclosure statement
R.J. M.P., and S.D.W. are researchers, scholars, and principals for the study’s sponsor. They are investors of several patent applications, including paraxanthine. However, they were not involved in data collection or analysis. R.B.K. has conducted sponsored research through grants and contracts awarded to the universities he has been affiliated with, received an honorarium for presenting research, served as an expert on cases related to exercise and nutrition, and consulted with industry on product development unrelated to the nutrient studied in the present investigation. Other authors report no conflicts.
Author contributions
Conceptualization, R.J., M.P., S.D.W., K.L., R.B.K.; project management, R.B.K., C.J.R., R.S., C.Y.; D.X., data collection C.Y., D.X., D.E.G. V.J., K.N., B.D., M.L., J.K., M.H.L.; data analysis R.B.K., R.S., M.F., W.K.; writing – original draft preparation, R.B.K.; writing – review and editing, R.B.K., R.S., M.F., W.K., R.J.; funding acquisition, R.B.K. All authors have read and approved publication of this paper.
Consent for publication
The sponsor approved the publication of this paper.
Data availability statement
Data and statistical analyses are available upon request on a case-by-case basis for noncommercial scientific inquiry and/or educational use if IRB restrictions and research agreement terms are not violated.
Institutional review board statement
This study was conducted with authorization by Texas A&M University’s Institutional Review Board (IRB2019–0928F). This study was registered with the ISRCTN registry (ISRCTN14506218).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2024.2352779.
References
- 1.Kerksick CM, Wilborn CD, Roberts MD, et al. ISSN exercise & sports nutrition review update: research & recommendations. J Int Soc Sports Nutr. 2018;15(1):38. doi: 10.1186/s12970-018-0242-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan R, Sun H, Soh KG, et al. The effects of mental fatigue on sport-specific motor performance among team sport athletes: a systematic scoping review. Front Psychol. 2023;14:1143618. doi: 10.3389/fpsyg.2023.1143618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez DE, McAllister MJ, Waldman HS, et al. International society of sports nutrition position stand: tactical athlete nutrition. J Int Soc Sports Nutr. 2022;19(1):267–399. doi: 10.1080/15502783.2022.2086017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jagim AR, Harty PS, Tinsley GM, et al. International society of sports nutrition position stand: energy drinks and energy shots. J Int Soc Sports Nutr. 2023;20(1):2171314. doi: 10.1080/15502783.2023.2171314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowery LM, Anderson DE, Scanlon KF, et al. International society of sports nutrition position stand: coffee and sports performance. J Int Soc Sports Nutr. 2023;20(1):2237952. doi: 10.1080/15502783.2023.2237952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sims ST, Kerksick CM, Smith-Ryan AE, et al. International society of sports nutrition position stand: nutritional concerns of the female athlete. J Int Soc Sports Nutr. 2023;20(1):2204066. doi: 10.1080/15502783.2023.2204066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders B, da Costa LR, de Souza RAS, et al. Caffeine and sport. Adv Food Nutr Res. 2023;106:95–127. [DOI] [PubMed] [Google Scholar]
- 8.Guest NS, VanDusseldorp TA, Nelson MT, et al. International society of sports nutrition position stand: caffeine and exercise performance. J Int Soc Sports Nutr. 2021;18(1):1. doi: 10.1186/s12970-020-00383-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nawrot P, Jordan S, Eastwood J, et al. Effects of caffeine on human health. Food Addit Contam. 2003;20(1):1–30. doi: 10.1080/0265203021000007840 [DOI] [PubMed] [Google Scholar]
- 10.Campbell B, Wilborn C, La Bounty P, et al. International Society of Sports Nutrition position stand: energy drinks. J Int Soc Sports Nutr. 2013;10(1):1. doi: 10.1186/1550-2783-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minaei S, Rahimi MR, Mohammadi H, et al. CYP1A2 genotype polymorphism influences the effect of caffeine on anaerobic performance in trained males. Int J Sport Nutr Exerc Metab. 2022;32(1):16–21. doi: 10.1123/ijsnem.2021-0090 [DOI] [PubMed] [Google Scholar]
- 12.Womack CJ, Saunders MJ, Bechtel MK, et al. The influence of a CYP1A2 polymorphism on the ergogenic effects of caffeine. J Int Soc Sports Nutr. 2012;9(1):7. doi: 10.1186/1550-2783-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pataky MW, Womack CJ, Saunders MJ, et al. Caffeine and 3-km cycling performance: effects of mouth rinsing, genotype, and time of day. Scand J Med Sci Sports. 2016;26(6):613–619. doi: 10.1111/sms.12501 [DOI] [PubMed] [Google Scholar]
- 14.Stavric B. Methylxanthines: toxicity to humans. 3. Theobromine, paraxanthine and the combined effects of methylxanthines. Food Chem Toxicol Int J Pub Br Ind Biol Res Assoc. 1988;26(8):725–733. doi: 10.1016/0278-6915(88)90073-7 [DOI] [PubMed] [Google Scholar]
- 15.Lelo A, Birkett DJ, Robson RA, et al. Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man. Br J Clin Pharmacol. 1986;22(2):177–182. doi: 10.1111/j.1365-2125.1986.tb05246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purpura M, Jager R, Falk M.. An assessment of mutagenicity, genotoxicity, acute-, subacute and subchronic oral toxicity of paraxanthine (1,7-dimethylxanthine). Food Chem Toxicol Int J Pub Br Ind Biol Res Assoc. 2021;158:112579. doi: 10.1016/j.fct.2021.112579 [DOI] [PubMed] [Google Scholar]
- 17.Benowitz NL, Jacob P, Mayan H, et al. Sympathomimetic effects of paraxanthine and caffeine in humans. Clin Pharmacol Ther. 1995;58(6):684–691. doi: 10.1016/0009-9236(95)90025-X [DOI] [PubMed] [Google Scholar]
- 18.Undem BJ. Pharmacotherapy of asthma. In: Brunton L; Lazo J, and Parker K, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill Professional; 2005. p. 717–736. [Google Scholar]
- 19.Orrú M, Guitart X, Karcz-Kubicha M, et al. Psychostimulant pharmacological profile of paraxanthine, the main metabolite of caffeine in humans. Neuropharmacology. 2013;67:476–484. doi: 10.1016/j.neuropharm.2012.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferre S, Orru M, Guitart X. Paraxanthine: connecting caffeine to Nitric oxide neurotransmission. J Caffeine Res. 2013;3(2):72–78. doi: 10.1089/jcr.2013.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guerreiro S, Toulorge D, Hirsch E, et al. Paraxanthine, the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol Pharmacol. 2008;74(4):980–989. doi: 10.1124/mol.108.048207 [DOI] [PubMed] [Google Scholar]
- 22.Okuro M, Fujiki N, Kotorii N, et al. Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33(7):930–942. doi: 10.1093/sleep/33.7.930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo C, Xing D, Gonzalez D, et al. Acute paraxanthine ingestion improves cognition and short-term memory and helps sustain attention in a double-blind, placebo-controlled, crossover trial. Nutrients. 2021;13(11):3980. doi: 10.3390/nu13113980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing D, Yoo C, Gonzalez D, et al. Dose-response of Paraxanthine on cognitive function: a double blind, placebo controlled, crossover trial. Nutrients. 2021;13(12):4478. doi: 10.3390/nu13124478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chacko SC, Quinzi F, De Fano A, et al. A single bout of vigorous-intensity aerobic exercise affects reactive, but not proactive cognitive brain functions. Int J Psychophysiol. 2020;147:233–243. doi: 10.1016/j.ijpsycho.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 26.Hwang J, Brothers RM, Castelli DM, et al. Acute high-intensity exercise-induced cognitive enhancement and brain-derived neurotrophic factor in young, healthy adults. Neurosci Lett. 2016;630:247–253. doi: 10.1016/j.neulet.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 27.Stimpson NJ, Davison G, Javadi AH. Joggin’ the noggin: towards a physiological understanding of exercise-induced cognitive benefits. Neurosci Biobehav Rev. 2018;88:177–186. doi: 10.1016/j.neubiorev.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 28.Kim B, Stein H. A spreadsheet program for making a balanced Latin Square design. Revista Colombiana de Ciencias Pecuarias. 2009;22:591–596. [Google Scholar]
- 29.Ferguson B. ACSM’s guidelines for exercise testing and prescription 9th ed. 2014. J Can Chiropr Assoc. 2014;58(3):328–328. [Google Scholar]
- 30.Klesges RC, Ward KD, Shelton ML, et al. Changes in bone mineral content in male athletes. Mechanisms of action and intervention effects. JAMA. 1996;276(3):226–230. doi: 10.1001/jama.1996.03540030060033 [DOI] [PubMed] [Google Scholar]
- 31.Lohman TG, Harris M, Teixeira PJ, et al. Assessing body composition and changes in body composition. Another look at dual-energy X-ray absorptiometry. Annals Of The New York Academy Of Sciences. 2000;904(1):45–54. doi: 10.1111/j.1749-6632.2000.tb06420.x [DOI] [PubMed] [Google Scholar]
- 32.Magrans-Courtney T, Wilborn C, Rasmussen C, et al. Effects of diet type and supplementation of glucosamine, chondroitin, and MSM on body composition, functional status, and markers of health in women with knee osteoarthritis initiating a resistance-based exercise and weight loss program. J Int Soc Sports Nutr. 2011;8(1):8. doi: 10.1186/1550-2783-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowinski R, Grubic T, Dalton R, et al. Impact of omega-3 fatty acids among other nonpharmacological interventions on behavior and quality of life in children with compromised conduct in Spain. J Diet Suppl. 2020;17(1):1–12. doi: 10.1080/19390211.2018.1481165 [DOI] [PubMed] [Google Scholar]
- 34.Bruce RA. Methods of exercise testing. Step test, bicycle, treadmill, isometrics. Am J Cardiol. 1974;33(6):715–720. doi: 10.1016/0002-9149(74)90211-2 [DOI] [PubMed] [Google Scholar]
- 35.Teixeira V, Voci SM, Mendes-Netto RS, et al. The relative validity of a food record using the smartphone application MyFitnessPal. Nutr Diet. 2018;75(2):219–225. doi: 10.1111/1747-0080.12401 [DOI] [PubMed] [Google Scholar]
- 36.Lin AW, Morgan N, Ward D, et al. Comparative validity of mostly unprocessed and minimally processed food items differs among popular commercial nutrition apps compared with a research food database. J Acad Nutr Diet. 2022;122(4):825–832 e1. doi: 10.1016/j.jand.2021.10.015 [DOI] [PubMed] [Google Scholar]
- 37.Bazzano LA, He J, Ogden LG, et al. Agreement on nutrient intake between the databases of the first national health and nutrition examination survey and the ESHA food processor. Am J Epidemiol. 2002;156(1):78–85. doi: 10.1093/aje/kwf003 [DOI] [PubMed] [Google Scholar]
- 38.Borg GA, Noble BJ. Perceived exertion. Exercise And Sport Sciences Reviews. 1974;2(1):131–153. doi: 10.1249/00003677-197400020-00006 [DOI] [PubMed] [Google Scholar]
- 39.Piper BJ, Mueller ST, Geerken AR, et al. Reliability and validity of neurobehavioral function on the psychology experimental building language test battery in young adults. PeerJ. 2015;3:e1460. doi: 10.7717/peerj.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mueller ST, Piper BJ. The psychology experiment building language (PEBL) and PEBL test battery. J Neurosci Methods. 2014;222:250–259. doi: 10.1016/j.jneumeth.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller ST. Assessment of motor planning and inhibition performance in non-clinical sample-reliability and factor structure of the tower of London and go/no go computerized tasks. Brain Sci. 2021;11(11). doi: 10.3390/brainsci11111420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39(1):15–22. doi: 10.1080/00221309.1948.9918159 [DOI] [PubMed] [Google Scholar]
- 43.Verbruggen F, Logan GD. Automatic and controlled response inhibition: associative learning in the go/no-go and stop-signal paradigms. J Exp Psychol Gen. 2008;137(4):649–672. doi: 10.1037/a0013170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leark RA, Greenberg LK, Kindschi CL, et al. Test of variables of attention: professional manual. Langley (WA): The TOVA Company; 2008. [Google Scholar]
- 45.Greenberg LK, Kindschi CL, Dupuy TR, et al. Test of variables of attention: clinical manual. Langley (WA): The TOVA Company; 2020. [Google Scholar]
- 46.Piper BJ, Mueller ST, Geerken AR, et al. Reliability and validity of neurobehavioral function on the psychology experimental building language test battery in young adults. PeerJ. 2015;3:e1460–e1460. doi: 10.7717/peerj.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowling JL, Katayev A. An evaluation of the Roche Cobas c 111. Lab Med. 2010;41(7):398–402. doi: 10.1309/LM6T8D1LKQXVNCAC [DOI] [Google Scholar]
- 48.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34(3):362–366. doi: 10.1093/ajcn/34.3.362 [DOI] [PubMed] [Google Scholar]
- 49.Bush VJ, Janu MR, Bathur F, et al. Comparison of BD vacutainer SST plus tubes with BD SST II plus tubes for common analytes. Clin Chim Acta. 2001;306(1–2):139–143. doi: 10.1016/S0009-8981(01)00396-5 [DOI] [PubMed] [Google Scholar]
- 50.Tanner RK, Fuller KL, Ross ML. Evaluation of three portable blood lactate analysers: lactate pro, lactate Scout and lactate plus. Eur J Appl Physiol. 2010;109(3):551–559. doi: 10.1007/s00421-010-1379-9 [DOI] [PubMed] [Google Scholar]
- 51.Grubic TJ, Sowinski RJ, Nevares BE, et al. Comparison of ingesting a food bar containing whey protein and isomalto-oligosaccharides to carbohydrate on performance and recovery from an acute bout of resistance-exercise and sprint conditioning: an open label, randomized, counterbalanced, crossover pilot study. J Int Soc Sports Nutr. 2019;16(1):34. doi: 10.1186/s12970-019-0301-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sowinski R, Gonzalez D, Xing D, et al. Effects of inositol-enhanced bonded arginine silicate ingestion on cognitive and executive function in gamers. Nutrients. 2021;13(11):3758. doi: 10.3390/nu13113758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jung YP, Earnest CP, Koozehchian M, et al. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J Int Soc Sports Nutr. 2017;14(1):3. doi: 10.1186/s12970-016-0159-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins PB, Earnest CP, Dalton RL, et al. Short-term effects of a ready-to-drink pre-workout beverage on exercise performance and recovery. Nutrients. 2017;9(8):823. doi: 10.3390/nu9080823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalton RL, Sowinski RJ, Grubic TJ, et al. Hematological and hemodynamic responses to acute and short-term creatine nitrate supplementation. Nutrients. 2017;9(12):1359. doi: 10.3390/nu9121359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galvan E, Walker DK, Simbo SY, et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J Int Soc Sports Nutr. 2016;13(1):12. doi: 10.1186/s12970-016-0124-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Page P. Beyond statistical significance: clinical interpretation of rehabilitation research literature. Int J Sports Phys Ther. 2014;9(5):726–736. [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen J. Statistical power analysis for the social sciences. 2nd ed. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 59.Earnest CP, Roberts BM, Harnish CR, et al. Reporting characteristics in sports nutrition. Sports. 2018;6(4):139. doi: 10.3390/sports6040139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magkos F, Kavouras SA. Caffeine use in sports, pharmacokinetics in man, and cellular mechanisms of action. Crit Rev Food Sci Nutr. 2005;45(7–8):535–562. doi: 10.1080/1040-830491379245 [DOI] [PubMed] [Google Scholar]
- 61.Chester N, Wojek N. Caffeine consumption amongst British athletes following changes to the 2004 WADA prohibited list. Int J Sports Med. 2008;29(6):524–528. doi: 10.1055/s-2007-989231 [DOI] [PubMed] [Google Scholar]
- 62.Brachtel D, Richter E. Absolute bioavailability of caffeine from a tablet formulation. J Hepatol. 1992;16(3):385. doi: 10.1016/S0168-8278(05)80676-2 [DOI] [PubMed] [Google Scholar]
- 63.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37(8):693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x [DOI] [PubMed] [Google Scholar]
- 64.Graham TE. Caffeine and exercise: metabolism, endurance and performance. Sports Med. 2001;31(11):785–807. doi: 10.2165/00007256-200131110-00002 [DOI] [PubMed] [Google Scholar]
- 65.Jager R, Purpura M, Wells SD, et al. Paraxanthine supplementation increases muscle mass, strength, and endurance in mice. Nutrients. 2022;14(4):893. doi: 10.3390/nu14040893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Landry O, Mitchell P, Marno H. An examination of perseverative errors and cognitive flexibility in autism. PLOS ONE. 2021;16(1):e0223160. doi: 10.1371/journal.pone.0223160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. Indian J Med Res. 2011;133(5):471–478. [PMC free article] [PubMed] [Google Scholar]
- 68.Bell DG, Jacobs I, McLellan TM, et al. Reducing the dose of combined caffeine and ephedrine preserves the ergogenic effect. Aviat Space Environ Med. 2000;71(4):415–419. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and statistical analyses are available upon request on a case-by-case basis for noncommercial scientific inquiry and/or educational use if IRB restrictions and research agreement terms are not violated.


