Abstract
The true global burden of paediatric critical illness remains unknown. Studies on children with life-threatening conditions are hindered by the absence of a common definition for acute paediatric critical illness (DEFCRIT) that outlines components and attributes of critical illness and does not depend on local capacity to provide critical care. We present an evidence-informed consensus definition and framework for acute paediatric critical illness. DEFCRIT was developed following a scoping review of 29 studies and key concepts identified by an interdisciplinary, international core expert panel (n=24). A modified Delphi process was then done with a panel of multidisciplinary health-care global experts (n=109) until consensus was reached on eight essential attributes and 28 statements as the basis of DEFCRIT. Consensus was reached in two Delphi rounds with an expert retention rate of 89%. The final consensus definition for acute paediatric critical illness is: an infant, child, or adolescent with an illness, injury, or post-operative state that increases the risk for or results in acute physiological instability (abnormal physiological parameters or vital organ dysfunction or failure) or a clinical support requirement (such as frequent or continuous monitoring or time-sensitive interventions) to prevent further deterioration or death. The proposed definition and framework provide the conceptual clarity needed for a unified approach for global research across resource-variable settings. Future work will centre on validating DEFCRIT and determining high priority measures and guidelines for data collection and analysis that will promote its use in research.
Introduction
Critical illness in children is often unexpected and results from the rapid development of acute, life-threatening conditions. Paediatric acute critical illness is considered a substantial global problem; however, its true burden remains unknown because of multiple challenges. One major challenge is the absence of a consensus framework and definition for acute paediatric critical illness that can be used for research.
Although acute paediatric critical illness is generally understood as a serious disease process that can lead to death, there is no universally accepted specific research definition. In its emergency triage assessment and treatment guidelines, WHO defines paediatric critical illness as “any severe problem with the airway, breathing, or circulation, or acute deterioration of conscious state”.1 This definition was formulated to provide guidance in the management of the most common life-threatening conditions in children presenting to hospitals in resource-limited countries—namely, hypoxaemia, circulatory impairment, and seizures.1 Alternatively, the Centers for Medicare and Medicaid Services define paediatric critical illness as “an illness or injury impairing one or more vital organ systems such that there is a high probability of imminent or life-threatening deterioration”,2 primarily for the purpose of billing critical care services in the USA. Existing literature has also considered the complexity of illness and severity of organ dysfunction in working definitions.3 However, these definitions have low specificity and do not include the necessary components to define a patient with critical illness for research studies.
Because of the absence of a standardised definition, acute paediatric critical illness is defined by location of care or admission to an intensive care unit (ICU).4–6 This approach is overly restrictive as patients can present with critical illness in multiple settings,7 such as community clinics and first-response centres, including field hospitals and emergency rooms. Moreover, in resource-limited hospitals, critically ill children are commonly managed in settings other than formal ICUs. Thus, the location-specific approach of defining paediatric critical illness is impractical and risks increasing research disparities by excluding settings with fewer critical care resources. Furthermore, definitions to date do not differentiate between attributes or components of critical illness and therefore cannot aid understanding of and classification of critically ill populations for research purposes. Together, these challenges have hindered comparative epidemiological studies across regions and countries, confounded an understanding of the true global burden of acute paediatric critical illness, and restricted the design of targeted interventions to improve outcomes.7,8
The Paediatric Acute Lung Injury and Sepsis Investigators (PALISI) Global Health Subgroup is dedicated to studying the burden of acute paediatric critical illness globally, including in low-income and low-middle-income countries that might not have formal intensive care services.9,10 We developed a definition and framework for paediatric acute critical illness that can aid research globally, irrespective of resources or the presence of a formal ICU.
Methods and Results
The consensus paediatric cute critical illness definition and framework were developed in three phases (appendix p 2) in accordance with the Conducting and Reporting Delphi Studies Guidelines.11
Phase 1—development of the foundational framework
The foundational framework for acute paediatric critical illness was developed through two scoping reviews of the literature and key concepts identified by a core expert panel. The scoping reviews were done by an experienced medical librarian (AGS) and were guided by the PRISMA guidelines extension for scoping reviews.12 Both search strategies are summarised in the appendix (p 3). The first search was done in April, 2021, by use of Ovid MEDLINE, Embase, and Scopus to look for definitions, key concepts, and characteristics of acute critical illness in paediatric and adult patients published between Jan 1, 1980, and March 31, 2021. Search results were required to have a term related to critical illness (eg, “organ dysfunction,” “life-threatening deterioration,” “organ support,” or “critical care”) and a term related to definition (eg, “definition”, “defined”, and “consensus definition”).
The second literature search was restricted to paediatric patients and was done in May, 2021, by use of Embase. The purpose of this search was to identify criteria used to descibe children at risk of acute critical illness or clinical deterioration that might not have been captured in the first search, and to identify severity of illness scales used in low-income and low-middle-income countries from Jan 1, 2000, to Dec 31, 2021. Search results included terms for “severity of illness”, “scales or indices”, “scores”, and developing countries (eg, “low-middle income countries” and “resource-limited settings”). This search used the list of low-income and low-middle-income socio-demographic index (SDI) nations from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019.13
These reviews yielded 1989 articles that were screened by at least two of three authors (AVA, ML-R, and AIA) with the online systematic review program Covidence. We excluded studies not written in English, studies in which the main study population were preterm infants or neonates, studies that did not discuss criteria or a definition for acute critical illness or severity of illness scales in resource-limited settings, and those not available in full-text forms (eg, conference abstracts). Next, full-text articles were reviewed and concepts extracted independently by two authors (AVA and ML-R) via a standardised data collection form created with the Arksey and O’Malley methodological framework for scoping reviews.14 Data collection included study authors, year of publication, country or countries involved, data collection periods, hospital settings, and study population characteristics. Additionally, we charted or synthesised qualitative data on how researchers defined or described acute paediatric critical illness and severity of illness or any arguments surrounding these concepts by sorting key themes.12,14 Disagreements were resolved by a senior author (AA). 29 publications met inclusion criteria (appendix p 4) for developing the definition and framework of acute paediatric critical illness (DEFCRIT). References and details of the included studies can be found in the appendix (pp 5–7).
A core expert panel (n=24) was established to develop the foundational framework. The core panel included internationally recognised experts in paediatric critical care medicine from the PALISI Global Health Network to ensure multidisciplinary representation (including paediatric intensivists, nurses, and allied specialties), clinical and research expertise (at least 5 years of clinical practice caring for children with critical illness or involvement with research on paediatric critical illness in low-income and low-middle-income countries), and multiregional representation (table). Six authors acted as the advisory committee (AVA, ML-R, AA, TBK, ATB, and NIS) for the study methodology, survey data analysis, and drafting and harmonising the consensus statements.
Table:
Demographics and characteristics of the core experts (n=24) and expert panel (N=109)
| Core experts | Expert panel | |
|---|---|---|
| Gender | ||
| Female | 9 (38%) | 48 (44%) |
| Male | 15 (63%) | 61 (56%) |
| Profession | ||
| Physicians | 20 (83%) | 96 (88%) |
| Nurses | 3 (13%) | 8 (7%) |
| Physiotherapists | 1 (4%) | 3 (3%) |
| Respiratory therapists | ·· | 2 (2%) |
| Specialty | ||
| Paediatric critical care medicine | 14 (58%) | 75 (69%) |
| Paediatric emergency medicine | 3 (13%) | 12 (11%) |
| Paediatric surgery | 1 (2%) | 6 (6%) |
| Paediatric haematology-oncology | 1 (4%) | 3 (3) |
| Paediatric hospitalists or paediatricians | 5 (21%) | 11 (10%) |
| Prehospital or retrieval | ·· | 2 (2%) |
| Years of medical experience | ||
| 5–10 years | 2 (8%) | 14 (13%) |
| 11–15 years | 10 (42%) | 38 (35%) |
| 16–19 years | 3 (13%) | 23 (21%) |
| ≥20 years | 9 (38%) | 34 (31%) |
| Region of primary practice | ||
| East Asia and Pacific | 2 (8%) | 11 (10%) |
| Europe and central Asia | ·· | 12 (11%) |
| Latin America and the Caribbean | 5 (21%) | 32 (29%) |
| Middle East and North Africa | 1 (4%) | 8 (7%) |
| North America | 9 (38%) | 14 (13%) |
| South Asia | 3 (13%) | 13 (12%) |
| Sub-Saharan Africa | 4 (17) | 19 (17%) |
| Country’s socio-demographic index * | ||
| Low | 5 (21%) | 21 (19%) |
| Low-middle | 2 (8%) | 13 (12%) |
| Middle | 2 (8%) | 27 (25%) |
| High-middle | 4 (17%) | 26 (24%) |
| High | 11 (46%) | 22 (20%) |
Data are n (%).
According to the Global Burden of Diseases, Injuries, and Risk Factors Study (2019).13
The core panel identified the main categories and key concepts (termed attributes) for acute paediatric critical illness by participating in an English-language electronic survey (appendix p 8) by use of Qualtrics (Qualtrics, Provo, UT, USA). The advisory committee extracted key concepts mentioned more than ten times by the core expert panel respondents. These, along with themes extracted from the scoping review, were grouped into seven initial attributes and two foundational domains, each with two subdomains, for the definition and foundational framework (appendix pp 9–10).
The core panel was then divided into four small working groups of six members from different disciplines to provide uniformity and decrease the possibility of bias. All groups participated in meetings in English via Zoom. The groups first agreed upon inclusion of the selected articles, then reviewed the preliminary arrangement of attributes, domains, and subdomains. The first domain—acute physiological instability—encompasses the subdomains of abnormal physiological parameters and vital organ dysfunction or failure. The second domain—clinical support requirement—encompasses the subdomains of the need for frequent or continuous monitoring and the need for time-sensitive intervention (appendix p 11). The working groups also formulated position statements for specific aspects of the attributes, domains, and subdomains to be agreed on in the consensus rounds. Additionally, the groups also categorised these statements into tiers for at risk for acute critical illness or acute critical illness.
Phase 2—modified Delphi consensus process
The consensus statement and proposed definition for acute paediatric critical illness were developed with a two-round modified Delphi process between August, 2022, and November, 2022.11,15 Previously identified attributes, domains, subdomains, and statements by the core panel were adapted into an online survey that consisted of seven potential attributes and 43 statements for round one. The survey was pilot tested for readability, interpretability, and user experience by the advisory committee. Participants for the consensus rounds were recruited from the PALISI Global Health Network and selected on the basis of the same parameters as the core expert panel. 109 international participants formed the expert panel for the consensus rounds and came from a broad range of disciplines (table) across 40 countries (appendix p 12) with varying incomes according to the SDI nations from GBD 2019.13
During the two rounds, the experts were asked to rate—with a Likert scale ranging from 1 to 9—the relevance of each consensus statement and attribute to a global definition of paediatric critical illness.16 The scale was divided into terciles, with the lowest tercile (scores 1–3) indicating strongly disagree, the middle tercile (scores 4–6) indicating uncertainty, and the upper tercile (scores 7–9) indicating strongly agree. Participants could select an additional score of 10 if they felt they were unable to score the statement—ie, if felt they did not have sufficient experience or knowledge to provide a rating. Experts were also given the option to provide narrative feedback on statements and attributes. Responses were collected over a period of 3–4 weeks and non-responders received up to two reminders before the closure of each round.
Criteria for acceptance of attributes and statements (consensus) were established a priori by the advisory committee as at least 82 scored responses from 1 to 9 (75% of respondents), reaching a median score for relevance in the upper tercile, at least 80% of participants scoring a statement 7–9 (evaluator agreement), and fewer than 15% of participants scoring a statement 1–3 (strongly disagree).11,16 Results from all rounds were analysed to calculate median relevance and percent agreement among the experts. Scores of 10 were excluded from calculations. An item was rejected when it scored in the lowest tercile or more than 15% of participants scored it 1–3. Consensus items and those classified as uncertain (median scores 4–9 with <80% evaluator agreement) were candidates for round two of the Delphi process. The advisory committee adapted and refined the statements on the basis of aggregated feedback from the experts. To prevent bias, two authors (AVA and ML-R) exclusively coordinated the rounds and did not participate in consensus rounds. Comments and scores provided by the panellists were anonymised before being sent to the advisory committee, so the rest of the authors and the committee did not know the identity of the panellists. After each survey round, results showing central tendencies, percentages, and frequency distributions were presented to all panel experts in graphical and tabular formats for review (appendix p 13).
In round one, the experts refined wording and concepts and added one new attribute and three statements. Additionally, 26 of 43 statements were merged into 13 new statements, and two statements were removed as they did not reach consensus criteria. Removed statements had less than 80% of participants scoring them 7–9 (strongly agree), with more than 15% of experts scoring them in the lower tercile. On the basis of the attributes and subdomain statements accepted by consensus in round one, a proposed definition for acute paediatric critical illness was developed and was entered into expert voting in round two.
In round two, all attributes and statements reached consensus with established criteria, resulting in the acceptance of eight attributes and 28 statements for the final DEFCRIT framework. There were 46 (0·9%) and 25 (0·7%) unable to score responses from a total of 4957 recorded responses in round 1, and 3783 in round 2, respectively. The retention rate among experts between rounds was 89% (97 of 109 participants completed both rounds). Results from both consensus rounds are in the appendix (pp 14–17).
Phase 3—consensus summary recommendations
Once repeated voting showed consistency in expert responses and a consensus was reached, the definition and framework for paediatric acute critical illness were circulated to all core panel members in December, 2022, for final recommendations. All statements, recommendations, and remarks were discussed within the core panel during two teleconference meetings in January, 2023, for final approval. The consensus DEFCRIT definition and framework are summarised below and in the appendix (pp 18–22).
Proposed research definition for acute paediatric critical illness
An infant, child, or adolescent with an illness, injury, or post-operative state that increases the risk for or results in acute physiological instability (abnormal physiological parameters or vital organ dysfunction or failure) or a clinical support requirement (such as frequent or continuous monitoring or time-sensitive intervention) to prevent further deterioration or death. The patient can meet this definition by having physiological instability, support requirements, or both (median score 9 [IQR 0·25]; 99·0% agreement). This definition is—by design—not restricted by available resources or admission to a formal ICU. This definition can also be used in studies aiming to include patients with chronic conditions (eg, cerebral palsy or chronic renal disease) who develop a new acute critical illness.
The eight attributes of acute paediatric critical illness
First, physiological instability: an acute inability to maintain one or more physiological parameters within a normal range for the patient’s age (eg, heart rate, respiratory rate, oxygen saturation, and level of consciousness) in the absence of clinical support (median score 9 [IQR 1]; 94·9% agreement).
Second, one or more vital organ dysfunctions or failures: the acute, severe impairment of one or more vital organs (specifically the heart, lungs, and brain) that require clinical support (median score 9 [0]; 95·9% agreement).
Third, risk of imminent life-threatening deterioration or death in the absence of appropriate recognition and management (median score 9 [0]; 99·0% agreement).
Fourth, acute paediatric critical illness requires appropriate and time-sensitive intervention, monitoring, or both: patients with critical illness (including those with severely abnormal laboratory or imaging results or postsurgical state) need appropriate time-sensitive interventions, monitoring, or both, to support vital organ function (median score 9 [0]; 97·4% agreement). The need, frequency, or type of monitoring or interventions will depend on the provider’s clinical judgement and the disease process (figure).
Figure: Possible illness trajectories over time.
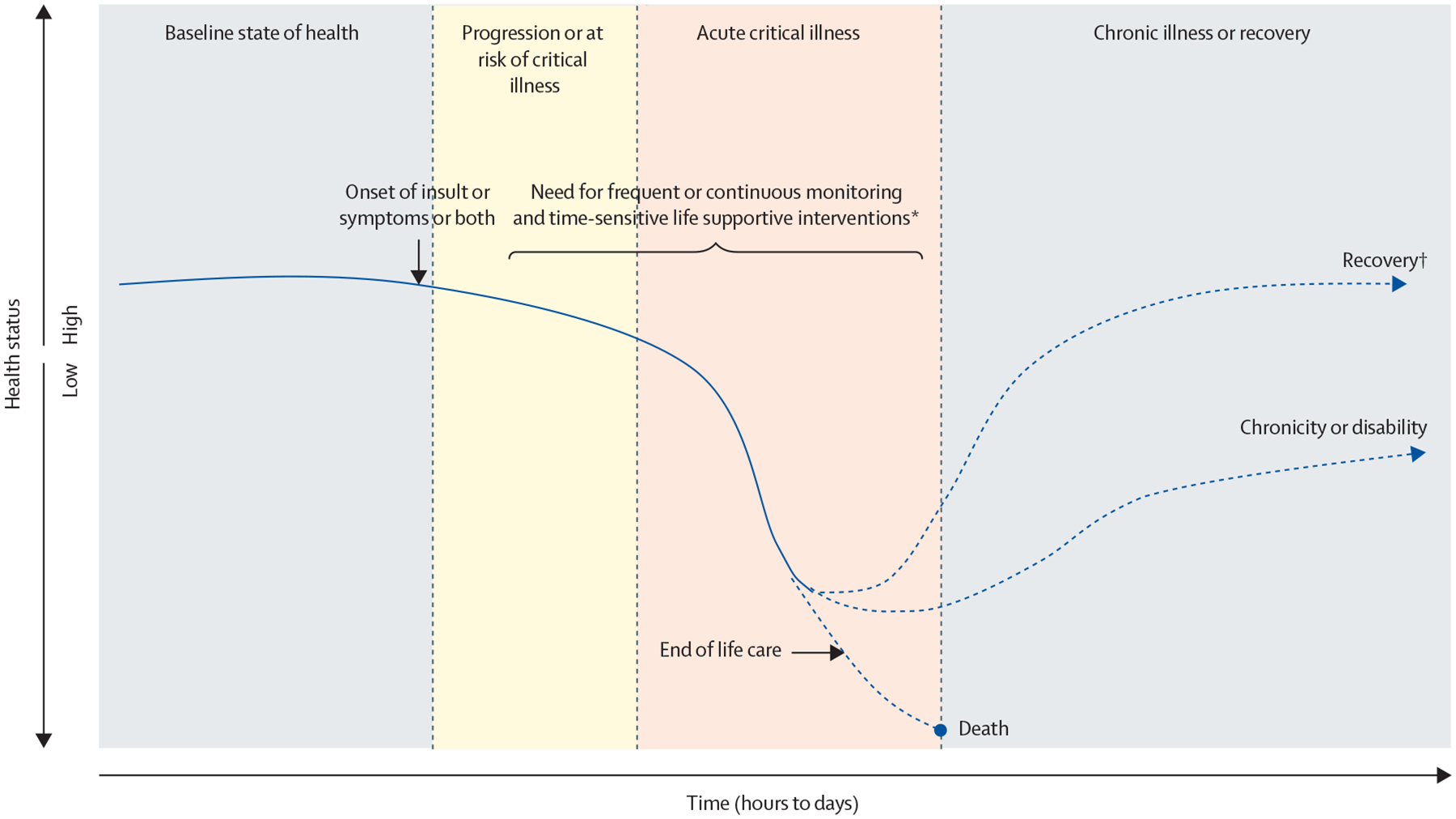
The y–axis represents the health or clinical status of a patient; high indicating good or towards baseline health, and a trend towards low indicating worsening clinical status. Acute critical illness can be identified at any point along this trajectory, depending on when the patient presents to medical care and the provider’s ability to recognise critical illness. The timecourse of acute critical illness progresses on the x–axis. For example, a previously healthy child presents with fever, tachycardia, tachypnoea, and respiratory distress and is diagnosed with pneumonia. The disease progresses to acute respiratory distress syndrome and the patient requires respiratory support. Monitoring and interventions reverse the trajectory and result in recovery, or the course of illness results in ventilatory dependence (chronicity or disability), or disease progression or complications result in death. *The need, frequency, or type of monitoring or interventions depend on the provider’s clinical judgement and the disease process. †Recovery can be variable, either returning to baseline or not. A patient’s trajectory can involve recurrent illness and multiple insults.
Fifth, location independence: paediatric acute critical illness can develop and be managed in any setting and is not location specific (eg, prehospital settings, emergency departments, and wards; median score 9 [0]; 99·0% agreement).
Sixth, independence from resource availability: patients can develop acute critical illness regardless of whether critical care interventions are possible or not, or if resources are present in their clinical setting or not (eg, a patient can develop respiratory failure regardless of the ability to provide mechanical ventilation; median score 9 [0]; 95·9% agreement).
Seventh, potential reversibility: acute changes in a patient’s clinical condition should be potentially reversible with appropriate interventions at the time of assessment (median score 9 [1]; 94·8% agreement). Reversibility might be difficult to establish at the time of initial assessment. Some conditions might not be reversible but still require critical care support (eg, a patient who develops brain death and requires support for organ donation). This attribute does not imply that patients with critical illness should return to their previous baseline health status or level of functioning (figure).
Finally, acute or sudden onset of illness or clinical deterioration: critical illness, injury, or deterioration that develops acutely (median score 9 [1]; 88·9% agreement). How much time constitutes an acute change will vary by the disease process and context of the study, but should be contrasted with a long-lasting or chronic critical illness (often described in days to weeks;17,18 figure).
Consensus DEFCRIT framework and statements
The DEFCRIT framework contains two domains, each of which have two subdomains. Consensus statements 1–25 for these subdomains, and statements 26–28 for other epidemiological considerations are summarised in the panel.
Panel: Definition for acute paediatric critical illness (DEFCRIT) consensus statements for subdomains A–D.
Domain 1: acute physiological instability—subdomain A: abnormal physiological parameters
Statement 1
Paediatric patients at risk for or with acute critical illness might have abnormal physiological parameters in the absence of clinical support (median score 9 [IQR 0]; 97·9% agreement). Patients who do not have abnormal (or different from baseline) physiological parameters or vital signs might still be at risk for or have acute critical illness and require clinical support, monitoring, or both (eg, patients with abnormal laboratory results, such as high lactate, hypoglycaemia, hyperglycaemia, etc; see domain 2 (subdomains C and D) for consensus statements on clinical support requirements).
Statement 2
Tier 1 (at risk for acute critical illness). Paediatric patients at risk for acute critical illness might have one or more abnormal physiological parameters or vital signs (>95th or <5th percentile, or >2 or <2 SD for age) in the absence of clinical support (eg, inotropes; median score 9 [IQR 1]; 96·9% agreement). Accepted references are inconsistent in the normal ranges (eg, The Harriet Lane Handbook19 and the American Heart Association Pediatric Advanced Life Support Provider Manual20) and percentile cutoff points they cite for paediatric vital signs, see statement 4.21,22
Statement 3
Tier 2 (acute critical illness). Paediatric patients with acute critical illness might have persistent (>1 h) or worsening abnormalities of one or more physiological parameters or vital signs (>95th or <5th percentile, or >2 or <2 SD for age) in the absence of or despite clinical support (eg, inotropes; median score 9 [IQR 1]; 92·8% agreement). We recommend defining persistence of abnormal physiological parameters or vital signs as longer than 1 h. However, this timeframe can be modified according to disease process and study context. For example, status epilepticus has been defined as a 30-min seizure.23
Statement 4
Normal range of vital signs can be defined with any accepted reference, including, but not limited to, the PALS guidelines20 and the WHO Pocket Book of Hospital Care for Children24 (median score 9 [IQR 0]; 99·0% agreement). As mentioned earlier, there are inconsistent data on threshold values and normal ranges for vital signs. We acknowledge that there is a need for global standardisation of age-based vital sign ranges, but this is beyond the scope of this study.
Statement 5
Examples of physiological parameters or vital signs can include, but are not limited to (median score 9 [IQR 0]; 95·9% agreement):
CNS: level of consciousness (by use of the GCS or the AVPU scale), pupil size and reactivity, etc
Respiratory system: signs of airway obstruction and respiratory distress, respiratory rate and effort, oxygen saturation, etc
Cardiovascular system: heart rate, blood pressure, capillary refill time, quality of central and peripheral pulses, skin (colour and perfusion), urine output, etc
Statement 6
Other parameters to consider (often associated, but on their own might not qualify as critical illness): temperature, fontanel fullness, hepatomegaly, signs of dehydration (eg, sunken eyes and dry mucosa), skin turgor, nutritional status (eg, weight-for-age and mid-upper arm circumferences), pain scores (established by an age-appropriate scale), parental or caregiver concern, etc (median score 8·5 [IQR 1]; 95·9% agreement).
Statement 7
Physiological parameters and vital signs can be monitored using non-invasive methods (eg, pulse oximeter) and, if available, by invasive monitoring (eg, arterial line to measure blood pressure; median score 9 [IQR 0]; 99·0% agreement).
Domain 1: acute physiological instability—subdomain B: vital organ dysfunction or failure
Statement 8
Paediatric patients at risk for or with acute critical illness might have new or acute vital organ dysfunction or failure requiring clinical support (median score 9 [IQR 1]; 94·9% agreement). Patients who do not have acute vital organ dysfunction or failure might still be at risk of or have critical illness if they have abnormal physiological parameters (see subdomain A) or if they require clinical support, monitoring, or both (see domain 2 [subdomains C and D] for consensus statements on clinical support requirement).
Statement 9
Tier 1 (at risk for acute critical illness). Paediatric patients at risk for acute critical illness might be suspected to have or be at risk for new or acute vital organ dysfunction or failure requiring clinical support (median score 9 [IQR 1]; 94·9% agreement).
Statement 10
Tier 2 (acute critical illness). Paediatric patients with acute critical illness have confirmed new or acute vital organ dysfunction or failure requiring clinical support (median score 9 [IQR 1]; 95·9% agreement).
Statement 11
Clinical features of CNS dysfunction or failure can include, but are not limited to, an altered level of consciousness (V, P, or U on the AVPU scale or GCS <12 or ≥3 points from baseline in the absence of sedatives), focal deficits, miosis or mydriasis not explained by medications, seizures that do not respond to antiepileptics or status epilepticus, new onset paralysis, etc (median score 8 [IQR 1]; 95·9% agreement).
Statement 12
Clinical features of respiratory dysfunction or failure can include, but are not limited to, an inability to protect the airway, moderate–severe respiratory distress (established by an illness-appropriate clinical scale), depressed respiratory effort, abnormal airway sounds (eg, wheezing, stridor, or grunting), poor to absent air movement, signs of poor gas exchange (hypercarbia and hypoxia), etc (median score 9 [IQR 0]; 97·9% agreement).
Statement 13
Clinical features of cardiovascular dysfunction or failure can include, but are not limited to, delayed or brisk capillary refill, signs of shock or poor perfusion (eg, cold extremities, weak, absent, or bunding pulses, mottled skin, or pallor), persistent or worsening tachycardia or bradycardia, signs of severe dehydration (eg, lethargy, thready pulses, and sunken eyes), uncontrolled bleeding or haemorrhage, cardiac arrest, arrhythmias causing haemodynamic instability, oliguria, anuria, etc (median score 9 [IQR 0]; 97·9% agreement).
Statement 14
In general, other organ dysfunction alone does not qualify as critical illness, as dysfunction in other organs becomes critical when it affects one of the three major organs listed above (eg, hepatic dysfunction causing confusion and bleeding, acute abdomen with peritoneal signs or severe abdominal distension causing respiratory or cardiovascular dysfunction, renal dysfunction with elevated potassium concentrations increasing the risk of developing or causing cardiovascular dysfunction, etc; median score 9 [IQR 1]; 93·8% agreement).
Statement 15
Vital organ dysfunction or failure can also be defined using any accepted references, including, but not limited to, the paediatric acute respiratory distress syndrome guidelines by PALICC,25 the acute kidney injury guidelines by KDIGO,26 and the PODIUM criteria for organ dysfunction27 (median score 9 [IQR 1]; 97·9% agreement). We acknowledge that there is a need for global standardisation of definitions for vital organ dysfunction or failure, but this is beyond the scope of this study.
Domain 2: clinical support requirement—subdomain C: need for frequent or continuous monitoring
Statement 16
Paediatric patients at risk for or with acute critical illness might need frequent or continuous monitoring (median score 9 [IQR 0]; 96·9% agreement).
Statement 17
Tier 1 (at risk for acute critical illness). Paediatric patients at risk for acute critical illness might need frequent (at least every 2 h) human-dependent monitoring or assessment (eg, by trained health-care staff or caregivers; median score 9 [IQR 1]; 94·9% agreement).
Statement 18
Tier 2 (acute critical illness). Paediatric patients with acute critical illness might need continuous human-dependent monitoring or assessment (eg, by trained health-care staff or caregivers; median score 9 [IQR 0]; 96·9% agreement).
Statement 19
Examples of human-dependent monitoring and assessment can include, but are not limited to, vital signs, work of breathing, capillary refill, perfusion and pulse checks, serial neurological examinations, progression of skin lesions, pain, urine output (eg, diaper count and weight), fluid loss (eg, diarrhoea and bleeding) assessments, and signs of clinical deterioration (eg, using the scoring tool from PEWS;28 median score 9 [IQR 0]; 99·0% agreement).
Statement 20
If resources are available at the centre or hospital and are indicated for the patient, then device-dependent (eg, non-invasive or invasive respiratory support), laboratory or imaging-based monitoring can be used in addition to human assessment (median score 9 [IQR 0]; 99·0% agreement).
Statement 21
Examples of device-dependent monitoring can include, but are not limited to, frequent (at least every 2 h) or continuous non-invasive monitoring, invasive monitoring, or both: cardiorespiratory (eg, heart rate, blood pressure, and oxygen saturation), temperature, end-tidal carbon dioxide, continuous electroencephalogram, intracranial pressure, urine output or bladder pressure via indwelling catheter, laboratory results (eg, glucose, haemoglobin, and lactate), and point-of-care ultrasound (median score 9 [IQR 0]; 99·0% agreement).
Domain 2: clinical support requirement—subdomain D: need for time-sensitive interventions
Statement 22
Paediatric patients at risk for or with acute critical illness might need time-sensitive interventions to support vital organs and avoid risk of further deterioration or death (median score 9 [IQR 0]; 97·9% agreement).
Statement 23
Paediatric patients at risk for or with acute critical illness might need frequent (at least every 2 h) time-sensitive hands-on interventions. Examples can include, but are not limited to, suctioning, oral care, repositioning, tracheostomy care, cleaning and dressing of wounds and burns, and cold sponge bathing for fever (median score 9 [IQR 1]; 91·8% agreement).
Statement 24
Paediatric patients at risk for or with acute critical illness might need time-sensitive life-supporting interventions (eg, resuscitation, medications, and surgical procedures) depending on available resources and clinical judgement (median score 9 [IQR 0]; 96·9% agreement).
Statement 25
Examples of life-supporting interventions often associated with acute critical illness can include, but are not limited to, (median score 9 [IQR 0]; 96·9% agreement):
CNS: rewarming or cooling (targeting normothermia), antidotes (eg, naloxone), anticonvulsants, hyperosmolar therapy, cerebrospinal fluid drainage for raised intracranial pressure, and decompressive surgery
Respiratory: improving airway patency, continuous nebulisers, non-invasive or invasive ventilatory support (eg, HFNC, BIPAP, CPAP, intubation, and mechanical ventilation), thoracostomy (needle or tube), heliox (helium–oxygen gas mixture), and inhaled nitrous oxide
Cardiovascular: inotropes, vasopressors, vasodilators, cardiopulmonary resuscitation, pericardiocentesis, extracorporeal life support, and control of life-threatening bleeding and haemorrhage (eg, surgery and massive transfusion)
Other interventions to consider: antibiotics, insulin drip, renal replacement therapy, urgent surgical procedures (eg, correction of intestinal perforation), and peritoneal drain for abdominal compartment syndrome
The statements in subdomains C and D could apply to patients with severely abnormal laboratory or imaging results, postoperative patients, and those requiring timely surgical interventions and critical interventions to support other organs not listed above (eg, kidney dysfunction requiring renal replacement therapy).
Other epidemiological considerations in study designs
Statement 26
Studies in paediatric critical illness can include children aged 1 month to 18 years (median score 9 [IQR 0]; 95·9% agreement). Experts acknowledge that age ranges (eg, paediatric patients) can vary by facility, country, and individual study. For instance, a term infant aged 2 weeks with respiratory failure due to respiratory syncytial virus infection could potentially be included if they do not have perinatal or birth-related conditions. Similarly, some paediatric facilities might extend care provision to patients older than 18 years.
Statement 27
Paediatric populations can be categorised or divided into subgroups by age—eg, with the WHO age classification29 or other subclassification schema30 (median score 9 [IQR 0]; 97·9% agreement). We recommend categorising age according to the WHO age classification (appendix p 21).
Statement 28
Comorbidities and pre-existing or high-risk conditions should be considered and documented when studying acute paediatric critical illness. Patients with these conditions have higher risk of complications, support requirements, and death. These conditions include, but are not restricted to, communicable or chronic infections (eg, HIV and tuberculosis) and non-communicable diseases, such as neurological or developmental conditions (eg, neurodisability and prematurity), respiratory conditions (eg, asthma), and cardiovascular conditions (eg, congenital heart disease and hypertension; median score 9 [IQR 0]; 99% agreement; appendix p 21).
PALS=Paediatric Advanced Life Support. GCS=Glasgow Coma Scale. AVPU=Alert, Voice, Pain, Unresponsive. PALICC=Pediatric Acute Lung Injury Consensus Conference. KDIGO=Kidney Disease Improving Global Outcomes. PODIUM=Pediatric Organ Dysfunction Information Update Mandate. PEWs=Paediatric Early Warning Score. HFNC=high-flow nasal cannula. BIPAP=bilevel positive airway pressure. CPAP=continuous positive airway pressure.
Discussion
We have described the development of a consensus, evidence-informed definition and framework for acute paediatric critical illness. Although others have previously defined critical illness,1–3,31 to our knowledge, this is the first definition that is specifically designed for use in research across resource-variable settings. The DEFCRIT definition with two principal domains—acute physiological instability and a clinical support requirement—emerged from eight attributes and 28 statements identified by a scoping review of relevant paediatric and adult literature, consultation meetings with a core expert panel, and a consensus process by 109 international experts with diverse backgrounds. Our proposed definition achieved a median score of 9 of 9 with 99% expert agreement.
A strength of the DEFCRIT definition is that it is patient-centred and resource and location independent—it describes critical illness rather than the delivery of critical care. DEFCRIT addresses existing global challenges in paediatric research by being applicable in all settings where children can present as critically ill (especially outside of formal ICU environments). As in the 2022 review by Kayambankadzanja and colleagues,31 we prioritised the need for critical care support regardless of patient location or resource availability over the selection of patients based solely on admission to an ICU. In doing so, the DEFCRIT definition allows previously ineligible children with acute critical illness in resource-limited hospitals without formal ICUs to be included in studies.
The four defined DEFCRIT subdomains in the framework can inform study design and help to elucidate differences across populations by facilitating patient categorisation. For example, a population that consists predominantly of patients with abnormal physiological parameters (class 1A patients—eg, with systemic inflammatory response syndrome) could be compared with those who have organ dysfunction (class 1A–1B patients—eg, with septic shock). A population of patients who only need frequent or continuous monitoring (class 2C patients—eg, with subdural haematoma) could be compared with patients with abnormal vital signs who need frequent monitoring while waiting for a time-sensitive intervention (class 1A–2C–2D patients—eg, with moderate, symptomatic hydrocephalus requiring a ventricular shunt placement). Such categorisations create a foundation that allows for comparisons across studies and allow researchers doing epidemiological studies on the burden of paediatric critical illness to pool data across settings, therefore increasing their effective sample size and statistical power.
By their nature, Delphi processes elicit differing opinions. The DEFCRIT framework is organ system oriented, and experts expressed differing views about the need to include other organ systems (eg, renal or gastrointestinal systems) in the vital organ dysfunction or failure subdomain. Over the two consensus rounds, opinions coalesced around the view that other organ dysfunction or failure (aside from cardiovascular, respiratory, and neurological systems) leads to acute critical illness principally by causing disturbances to the cardiovascular, respiratory, or neurological systems (statement 14), aligning this aspect of DEFCRIT with the WHO definition.1
We acknowledge that our study has limitations. The literature search included only three major biomedical literature databases and excluded non-English language articles. Despite these limitations, we identified almost 2000 articles, 21 of which included definitions of acute paediatric critical illness and eight with indices to recognise children at risk of acute critical illness in low-income and low-middle-income countries. To overcome the language limitation and decrease the risk of missing potentially relevant key concepts, we convened an international multidisciplinary core expert panel to develop the foundational framework. Another limitation of our study is that we had no representation from Europe and central Asia in our core expert panel, and participants were skewed towards physicians. Although considerable efforts were made to increase participation from other regions and disciplines, we still had no representation in specific disciplines, such as pharmacy and nutrition. Despite this limitation, our panel included more than 100 diverse international experts from 40 countries, representing all seven world regions and multiple disciplines, economic settings, and geographical regions. Thus, we are confident that our definition represents a global and multidisciplinary perspective.
Our proposed framework does not prescribe specific scoring scales or systems for the subdomains. Although they are valuable, there are multiple standards for normal and abnormal paediatric vital signs19,20 and several scoring systems for vital organ dysfunction and failure.25–27,32 Similarly, we did not incorporate severity of illness or mortality prediction measures. Although these measures have proven effective in high-resource settings, previous studies have shown that they are not widely used globally, especially in LMICs.33,34 Reasons for their inconsistent use include that they often rely on laboratory data that might be challenging to obtain in low-resource environments (eg, arterial blood gases, creatinine, and coagulation parameters), and barriers in measure implementation due to deficiencies in staff training and buy-in from local stakeholders.28 In addition, evidence of the validity and reliability of these measures in this heterogeneous diverse population in low-resource settings is insufficient.33,34 Furthermore, we believe that a more adaptable framework creates space for investigators to use tools that are best suited for their research objectives. Finally, although our Delphi process drew from an evidence base, Delphi processes are a convergence of expert opinion by design. As such, the DEFCRIT definition for paediatric critical illness must be empirically tested, and we expect it to evolve as the body of relevant evidence grows.
The DEFCRIT definition and framework have relevance for researchers and policy makers who wish to understand the burden of, and outcomes associated with childhood acute critical illness globally. Similar to the Brighton Collaboration on the Global Alignment on Immunisation Safety Assessment in Pregnancy, we developed a common definition and framework for acute paediatric critical illness to improve comparability of data across settings.35 Future work will include assessments of feasibility, acceptability, and validity of the proposed definition for use in research across resource-variable settings. A key next step will be the development of common data elements with a minimal set of high priority measures within subdomains to promote their use as a clinical research tool and to record differences across the age spectrum. Likewise, as with the Brighton collaboration, we will develop case definitions with guidelines for data collection, analysis, and levels of certainty to account for differences in diagnostic and regional capacity for research and the scientific evidence available.35
Conclusion
In this Health Policy, we propose an inclusive research definition and framework for acute paediatric critical illness through a modified Delphi methodology with an international, multidisciplinary expert panel. This work provides conceptual clarity and a unified approach to define, characterise, and select populations of children with acute critical illness for global studies. In addition, it allows for the comparison of outcomes across settings, regardless of resource availability. Future research using this definition to understand the burden of acute paediatric critical illness can help to optimise critical care service availability, guide policy making, and improve outcomes for acute critically ill paediatric patients worldwide.
Supplementary Material
Acknowledgments
We thank Andrea Goldstein Shipper, Temple University, Philadelphia, PA, USA, for assisting with the literature search. We thank Andrew Pappas and Kolanda Ackey, St Jude Global Informatics, Memphis, TN, USA, for helping develop the electronic consensus surveys. We thank our international panel of experts who made this study possible. This study has been endorsed by the World Federation of Pediatric Intensive and Critical Care Societies and the Society of Critical Care Medicine. This study was supported by the American Lebanese Syrian Associated Charities. The sponsor had no role in study design, data collection, data analysis, data interpretation, or the writing of the report.
Declaration of interests
AA reports funding from the National Institutes of Health National Cancer Institute (R37CA276215-01) outside of this Health Policy. BMM reports funding from Eurogol and the National Research Foundation; reports royalties from the Society of Critical Care Medicine as Senior Associate Editor of Pediatric Critical Care Medicine; receives support for travel and meetings from the European Cystic Fibrosis Society, Critical Care Society of Southern Africa, and the South Africa Thoracic Society, all outside of this Health Policy; and is the unpaid President for the World Federation of Pediatric Intensive and Critical Care Society and the Critical Care Society of Southern Africa. JHL reports funding from the National Research Medical Council of Singapore and the Thrasher Foundation in the USA, outside of this Health Policy. VMN is the President of the Society of Critical Care Medicine. The views expressed in this Health Policy do not necessarily reflect those of the institutions to which the authors are affiliated. All other authors declare no competing interests.
Footnotes
See Online for appendix
For more on Covidence see https://www.covidence.org
References
- 1.WHO. Paediatric emergency triage, assessment and treatment: care of critically-ill children. Geneva: World Health Organization, 2016 [PubMed] [Google Scholar]
- 2.Department of Health and Human Services, Centers for Medicare and Medicaid Services. CMS Manual System: Pub 100–04 Medicare Claims Processing. 2014. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R2997CP.pdf (accessed Oct 2, 2023).
- 3.Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet 2010; 376: 1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019; 7: 115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fink EL, Kochanek PM, Tasker RC, et al. International survey of critically ill children with acute neurologic insults: the prevalence of acute critical neurological disease in children: a global epidemiological assessment study. Pediatr Crit Care Med 2017; 18: 330–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191: 1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argent AC, Ranjit S, Peters MJ, et al. Factors to be considered in advancing pediatric critical care across the world. Crit Care Clin 2022; 38: 707–20. [DOI] [PubMed] [Google Scholar]
- 8.Prin M, Wunsch H. International comparisons of intensive care: informing outcomes and improving standards. Curr Opin Crit Care 2012; 18: 700–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kortz TB, Nielsen KR, Mediratta RP, et al. The burden of critical illness in hospitalized children in low- and middle-income countries: protocol for a systematic review and meta-analysis. Front Pediatr 2022; 10: 756643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbas Q, Holloway A, Caporal P, et al. Global PARITY: study design for a multi-centered, international point prevalence study to estimate the burden of pediatric acute critical illness in resource-limited settings. Front Pediatr 2022; 9: 793326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on conducting and reporting Delphi studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017; 31: 684–706. [DOI] [PubMed] [Google Scholar]
- 12.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–73. [DOI] [PubMed] [Google Scholar]
- 13.Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2019 (GBD 2019) Socio-Demographic Index (SDI) 1950–2019. 2020. https://ghdx.healthdata.org/record/ihme-data/gbd-2019-socio-demographic-index-sdi-1950-2019 (accessed March 20, 2023).
- 14.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005; 8: 19–32. [Google Scholar]
- 15.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000; 32: 1008–15. [PubMed] [Google Scholar]
- 16.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA appropriateness method user’s manual. Santa Monica, CA: RAND Corporation, 2001. [Google Scholar]
- 17.Shapiro MC, Henderson CM, Hutton N, Boss RD. Defining pediatric chronic critical illness for clinical care, research, and policy. Hosp Pediatr 2017; 7: 236–44. [DOI] [PubMed] [Google Scholar]
- 18.Zorko DJ, Mcnally JD, Rochwerg B, et al. Defining pediatric chronic critical illness: a scoping review. 2023; 24: e91–103. [DOI] [PubMed] [Google Scholar]
- 19.Johns Hopkins Hospital, Kleinman K, McDaniel L, Molloy M. The Harriet Lane handbook, 22nd edition. Amsterdam: Elsevier, 2021. [Google Scholar]
- 20.American Heart Association. Pediatric advanced life support: provider manual. Dallas, TX: American Heart Association, 2016. [Google Scholar]
- 21.Fleming S, Thompson M, Stevens R, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet 2011; 377: 1011–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haque IU, Zaritsky AL. Analysis of the evidence for the lower limit of systolic and mean arterial pressure in children. Pediatr Crit Care Med 2007; 8: 138–44. [DOI] [PubMed] [Google Scholar]
- 23.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015; 56: 1515–23. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses, 2nd edn. Geneva: World Health Organization, 2013. [PubMed] [Google Scholar]
- 25.Emeriaud G, López-Fernández YM, Iyer NP, et al. Executive summary of the second International Guidelines for the Diagnosis and Management of Pediatric Acute Respiratory Distress Syndrome (PALICC-2). Pediatr Crit Care Med 2023; 24: 143–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bembea MM, Agus M, Akcan-Arikan A, et al. Pediatric Organ Dysfunction Information Update Mandate (PODIUM) contemporary organ dysfunction criteria: executive summary. Pediatrics 2022; 149 (suppl 1): S1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agulnik A, Ferrara G, Puerto-Torres M, et al. Assessment of barriers and enablers to implementation of a pediatric early warning system in resource-limited settings. JAMA Netw Open 2022; 5: e221547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Age Group Codelist. World Health Organisation. 2013. https://apps.who.int/gho/data/node.searo-metadata.AGEGROUP?lang=en (accessed Jan 9, 2023). [Google Scholar]
- 30.National Institutes of Health. NIH style guide: age. 2023. https://www.nih.gov/nih-style-guide/age (accessed Jan 9, 2023).
- 31.Kayambankadzanja RK, Schell CO, Gerdin Wärnberg M, et al. Towards definitions of critical illness and critical care using concept analysis. BMJ Open 2022; 12: e060972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlapbach LJ, Weiss SL, Bembea MM, et al. Scoring systems for organ dysfunction and multiple organ dysfunction: the PODIUM consensus conference. Pediatrics 2022; 149 (suppl 1): S23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muttalib F, Clavel V, Yaeger LH, Shah V, Adhikari NKJ. Performance of pediatric mortality prediction models in low- and middle-income countries: a systematic review and meta-analysis. J Pediatr 2020; 225: 182–92.e2. [DOI] [PubMed] [Google Scholar]
- 34.van den Brink DA, de Vries ISA, Datema M, et al. Predicting clinical deterioration and mortality at differing stages during hospitalization: a systematic review of risk prediction models in children in low- and middle-income countries. J Pediatr 2023; 260: 113448. [DOI] [PubMed] [Google Scholar]
- 35.Kohl KS, Bonhoeffer J, Braun MM, et al. The Brighton Collaboration: creating a global standard for case definitions (and guidelines) for adverse events following immunization. In: Henrikson K, Battles JB, Marks ES, Lewin DI, eds. Advances in patient safety: from research to implementation (volume 2: concepts and methodology). Rockville, MD: Agency for Healthcare Research and Quality, 2005: 87–102. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


