Abstract
Background and Objectives
Real-life studies noted that the risk of disease activity in multiple sclerosis (MS) after switching to rituximab (RTX) or ocrelizumab (OCR) may be unequal depending on prior disease-modifying therapy (DMT), with a higher risk associated with fingolimod (FING).
Methods
We performed a retrospective analysis of a structured prospective data collection including all consecutive patients with relapsing MS who were prescribed RTX/OCR in the MS center of Marseille. Cox proportional hazards models were applied to clinical and MRI outcomes.
Results
We included 321 patients with a median (interquartile range [IQR]) follow-up of 3.5 years (1.5–5) after RTX/OCR initiation. At the first RTX/OCR infusion, the mean (SD) age of patients was 37 (10) years, and the median (IQR) disease duration was 8 years (3–15): 68 patients did not receive treatment before RTX/OCR and 108 switched from FING, 47 from low efficacy therapy, and 98 from natalizumab. For statistical analysis, the group “FING” was divided into “short-FING” and “long-FING” groups according to the median value of the group's washout period (27 days). On Cox proportional hazards analysis, for only the “long-FING” group, the risk of relapse within the first 6 months of RTX/OCR was increased as compared with patients without previous DMT (hazard ratio [HR]: 8.78; 95% CI 1.72–44.86; p < 0.01). Previous DMT and washout period duration of FING had no effect on B-cell levels at 6 months. Beyond the first 6 months of RTX/OCR, age <40 years was associated with increased risk of relapse (HR: 3.93; 95% CI 1.30–11.89; p = 0.01), male sex with increased risk of new T2 lesions (HR: 2.26; 95% CI 1.08–4.74; p = 0.03), and EDSS ≥2 with increased risk of disability accumulation (HR: 3.01; 95% CI 1.34–6.74; p < 0.01). Previous DMT had no effect on the effectiveness of RTX/OCR beyond 6 months after initiation.
Discussion
For patients switching from FING to RTX/OCR, the risk of disease reactivation within the first 6 months of treatment was increased as compared with patients with other DMT or no previous DMT only when the washout period exceeded 26 days. Neither FING nor other previous DMT reduced the effectiveness of RTX/OCR beyond the first 6 months of treatment.
Introduction
B-cell–depleting therapy (BCDT), such as rituximab (RTX) or ocrelizumab (OCR), has a high level of effectiveness for patients with relapsing multiple sclerosis (RMS).1-3 BCDT can be used as first-line therapy or for patients who previously received another disease-modifying therapy (DMT).1-8 Pivotal studies clearly demonstrated early and high effectiveness of BCDT in DMT-naive patients, and real-life cohort studies extended these findings to patients who previously received another DMT.1-8 However, real-life studies noted that the risk of disease activity after switching to BCDT may be unequal depending on the prior DMT.4-7,9,10
These real-life studies mostly focused on the effect of previous DMT on the effectiveness of BCDT during the first months after the switch.4-6 They found increased risk of disease reactivation during the first months of treatment in patients who previously received fingolimod, particularly those with a long washout period. However, the studies did not clearly determine the washout period duration for which the risk increased when switching. Moreover, we do not know whether a short washout period associated with incomplete lymphocyte recovery at the time of RTX/OCR infusion could decrease the biological effect of BCDT.
One recent study of the potential effect of previous DMT on the response to BCDT beyond 6 months suggested that the effectiveness of BCDT was diminished in patients who previously received fingolimod.7 This point is of major importance for clinical practice, with implications for counseling and monitoring, because this reduced effectiveness beyond 6 months could not be prevented by any action such as shortening the washout period. Indeed, the authors hypothesized that this long-term reduced effectiveness of BCDT with previous fingolimod treatment could be inherent to a long-lasting effect of fingolimod on the immune system. However, because these results were obtained in a limited number of patients, they should be replicated.7
In this study, we performed a retrospective analysis of a structured prospective data collection including all consecutive patients with RMS who were prescribed RTX/OCR in the MS center of Marseille.11-13 We were first interested to assess more in depth the effect of fingolimod on the response to BCDT during the early phase of treatment. Especially, we aimed to confirm that shortening the washout period in patients switching from fingolimod fully prevented the risk of disease reactivation, thus ruling out a potential inherent effect of a sequestrating agent on the early response to BCDT independent of washout period duration. We also wanted to determine whether a short washout period decreased the biological effect of BCDT. Finally, we focused on the period beyond 6 months to test whether prior treatment with fingolimod has a long-term effect on the effectiveness of BCDT that could be related to a potential inherent effect of a sequestrating agent, as recently suggested.7
Methods
Study Population
In the MS center of Marseille, we started to use RTX in 2015 and OCR in 2019. All consecutive patients under RTX/OCR therapy were prospectively included in an observational cohort study. Patients were seen in the center for a clinical evaluation every 6 months. Brain and spinal cord MRI monitoring was performed after 6 months of treatment and thereafter at least annually. All examinations for a given patient were performed by the same experienced neurologists (A.M., A.R., C.B., S.D., P.D., J.P., and B.A.). The Expanded Disability Status Scale (EDSS) score was collected at each visit. All patients received the phone number of our indoor neuroinflammatory unit, which was open 24 h/d, 7 d/wk. We informed each patient about the need to call the center in case of new neurologic signs. In case of relapse, the patient was admitted to hospital and corticosteroids were administered if necessary. Additional brain and spinal cord MRI monitoring was systematically performed for 3 months after each relapse.
This study had the following inclusion criteria: diagnosis of RMS at the onset of RTX/OCR, age 18–55 years at the onset of RTX/OCR, at least 6 months' follow-up after the onset of RTX/OCR, and no DMT before RTX/OCR or a previous DMT including beta-interferon formulations, glatiramer acetate, dimethyl fumarate, fingolimod, or natalizumab in case of a previous DMT washout period defined as time from the last prior DMT intake to the first RTX/OCR infusion <3 months.
Statistical Analysis
We divided patients into 5 groups for statistical analysis. The “no therapy” group included patients without DMT before RTX/OCR; the “low efficacy therapy” group patients receiving beta-interferon formulations, glatiramer acetate, or dimethyl fumarate before RTX/OCR; the “NTZ” group patients receiving natalizumab before RTX/OCR; and the “fingolimod (FING)” group patients receiving fingolimod before RTX/OCR. To assess the potential effect of the washout period duration on the risk of disease activity after RTX/OCR initiation in patients who previously received fingolimod, the group “FING” was divided into 2 groups: “short-FING” patients previously received fingolimod with a washout period less than the median value of the washout period duration of the “FING” group and “long-FING” patients previously received fingolimod with a washout period greater than or equal to the median value of the washout period duration of the “FING” group. Baseline demographics and disease characteristics were compared between groups by the Fisher exact test for categorical variables and Kruskal-Wallis test for continuous variables. The Dunn test was used to explore pairwise group differences in case of a significant Kruskal-Wallis result. For analyzing the effectiveness of BCDT, we compared groups with the Kaplan-Meier method and Cox proportional hazards models, with time since RTX/OCR initiation as the time scale. We used the following outcomes: time to first relapse defined as the occurrence of neurologic signs persisting >24 hour in the absence of fever, infection, or other intercurrent phenomena; time to first 6-month confirmed disability worsening based on the EDSS score (+1.5 points [baseline = 0.0], +1.0 point [baseline = 1.0–4.0], and +0.5 points [baseline ≥4.5]); and time to first detection of new T2-weighted or gadolinium-enhanced lesions on MRI as compared with MRI findings after RTX/OCR onset. The time of event was the date of relapse or the clinical visit for the disability and MRI outcomes. We used multivariate Cox proportional hazards models to test the potential effect of previous treatment on the effectiveness of RTX/OCR, estimating hazard ratios (HRs) and 95% CIs. To assess potential factors affecting the early and late effectiveness of BCDT, we performed 2 analyses: one restricted to the evolution of disease during the first 6 months after RTX/OCR onset and one to the evolution of disease after the first 6 months of RTX/OCR. For the first 6-month period, the time to first relapse was the only outcome used because the disability outcome needed at least 6 months' follow-up to be confirmed and the MRI outcome needed a baseline MRI performed within the first 6 months. The models used included the covariates sex, age, disease duration at RTX/OCR onset, disease activity (new T2-weighted lesion or relapse) in the year before RTX/OCR, EDSS score at RTX/OCR onset, and last previous DMT.
Finally, we compared B-cell and T-cell levels (including CD4+ and CD8+ T cells) at baseline and at 6 months after RTX/OCR onset between the patient groups (“no therapy,” “low efficacy therapy,” “NTZ,” “short-FING,” and “long-FING”) using the Kruskal-Wallis test and Dunn test to explore pairwise group differences. We used multivariable linear regression models to test the potential effect of previous DMT on B-cell and T-cell levels 6 months after RTX/OCR onset, including in the models the covariates sex, age, disease duration at BCDT onset, and last previous DMT.
Analyses were conducted with JMP 16.1.0 (SAS Institute Inc). p < 0.05 was considered statistically significant.
Standard Protocol Approvals, Registrations, and Patient Consents
The authors obtained ethical approval from the institutional review board of the university hospital of Marseille, France (approval no.: PADS-21-60) for this study.
Data Availability
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.
Results
Study Population
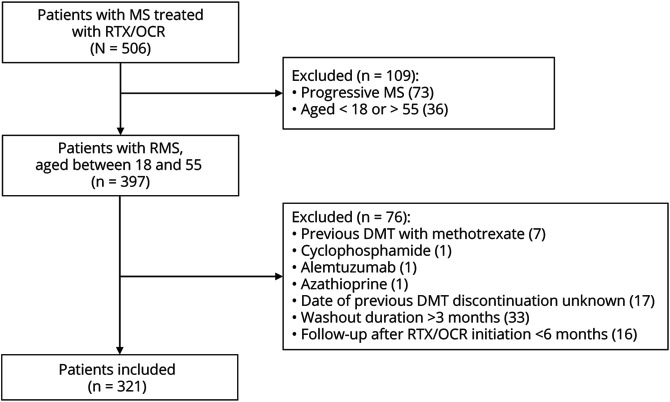
In total, 321 patients who were followed in our department since 2015 after the first infusion of RTX/OCR fulfilled the inclusion criteria (Figure 1, Table 1). At the time of the first RTX/OCR infusion, the mean (SD) age of patients was 37 (10) years, there were 222 women (69%), and the median (IQR) disease duration was 8 years (3–15), median (IQR) EDSS score 2.5 (1–4.5), and median (IQR) washout period 31 days (21–42). The median (IQR) washout period for the “FING” group was 27 days (18–34) and was 16.5 days (14–22) and 33 (29–41) days for the “short-FING” group (washout <27 days) and “long-FING” group (washout ≥27 days), respectively. The median (IQR) follow-up after the first RTX/OCR infusion was 3.5 years (1.5–5).
Figure 1. Flowchart of Participants in the Study.
Table 1.
Characteristics of All Patients and by Group
| Whole group, N = 321 | “No therapy,” N = 68 | “FING,” N = 108 | “Low efficacy therapy,” N = 47 | “NTZ,” N = 98 | |
| Age at RTX/OCR start, y, mean (SD) | 37 (10) | 34.5 (10) | 37 (9) | 37 (11) | 38 (10) |
| Female, n (%) | 222 (69) | 48 (70) | 73 (67) | 38 (81) | 63 (64) |
| Disease duration at RTX/OCR start, y, median (IQR) | 8 (3–15) | 2 (1–6.5)a,b,c | 10.5 (5.5–16.5) | 9 (3–16) | 8.5 (5–13.5) |
| EDSS score at RTX/OCR start, median (IQR) | 2.5 (1–4.5) | 2 (0.5–3.5)a | 3.5 (1–6) | 2 (1–4.5) | 3.5 (1–4.5) |
| Disease activity within the year before RTX/OCR start, n (%) | 215 (67) | 66 (97)a | 95 (88) | 44 (94)d | 10 (10)a,b,d |
| Duration of washout period, d, median (IQR) | NA | NA | 27 (18–34)c | 18 (13–37)c | 42 (32–53) |
| Follow-up after RTX/OCR start, y, median (IQR) | 3.5 (1.5–5) | 2 (1–3)a,c | 4 (2–5) | 2.5 (1.5–5) | 3.5 (1.5–5) |
Abbreviations: OCR = ocrelizumab; EDSS = Expanded Disability Status Scale; IQR = interquartile range; RTX = rituximab.
“No therapy”: no disease-modifying therapy (DMT) before RTX/OCR, “low efficacy therapy”: beta-interferon formulations, glatiramer acetate, or dimethyl fumarate before RTX/OCR, “NTZ”: natalizumab before RTX/OCR, “FING”: fingolimod before RTX/OCR.
Lower than “FING,” p < 0.05.
Lower than “low efficacy therapy,” p < 0.05.
Lower than “NTZ,” p 0.05.
Lower than “no therapy,” p < 0.05.
Disease Activity and Disability Progression During RTX/OCR Treatment for All Patients
During the follow-up, we observed 40 relapses in 38 of 321 patients (12%): 17 occurred within the first 6 months of RTX/OCR. Overall, 64 of 321 patients (20%) experienced 6-month confirmed worsening of disability and 29 of 321 (9%) had at least one MRI examination with at least one new T2-weighted lesion or contrast-enhancing lesion as compared with an MRI performed after RTX/OCR onset. One tumefactive lesion was observed in the group “NTZ”.
Effect of Previous DMT on Risk of Disease Reactivation Within the First 6 Months After the Switch to RTX/OCR
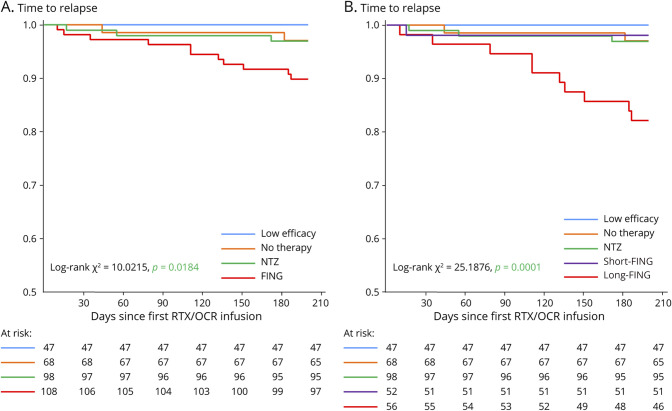
The patient groups “no therapy,” “low efficacy therapy,” “NTZ,” and “FING” significantly differed in survival curve estimates for time to first relapse within the first 6 months after RTX/OCR initiation (log-rank test p = 0.01) (Figure 2A). Within the first 6 months, the proportion of patients with relapse was 3/68 (4.5%) for the “no therapy” group, 0/47 for the “low efficacy therapy” group, 3/98 (3%) for the “NTZ” group, and 11/108 (10%) for the “FING” group. The patient groups “no therapy,” “low efficacy therapy,” “NTZ,” “short-FING,” and “long-FING” significantly differed in survival curve estimates for time to first relapse within the first 6 months after RTX/OCR initiation (log-rank test p < 0.001) (Figure 2B). Within the first 6 months, the proportion of patients with relapse was 3/68 (4.5%) for the “no therapy” group, 0/47 for the “low efficacy therapy” group, 3/98 (3%) for the “NTZ” group, 1/50 (2%) for the “short-FING” group, and 10/58 (17%) for the “long-FING” group. The washout period of the unique patient in the “short-FING” group experiencing a relapse was 25 days. On Cox proportional hazards analysis, as compared with the “no therapy” group, only the “long-FING” group exhibited increased risk of relapse within the first 6 months after RTX/OCR initiation (HR: 8.78; 95% CI 1.72–44.86; p < 0.01) (Table 2).
Figure 2. Time to First Relapse Within the First 6 Months After Rituximab/Ocrelizumab (RTX/OCR) Onset.
(A) Stratification according to “no therapy,” “low efficacy therapy,” “NTZ,” and “FING” groups. (B) Stratification according to “no therapy,” “low efficacy therapy,” “NTZ”; “short-FING” (washout period <27 days); and “long-FING” (≥27 days) groups. “No therapy”: no disease-modifying therapy (DMT) before RTX/OCR, “low efficacy therapy”: beta-interferon formulations, glatiramer acetate, or dimethyl fumarate before RTX/OCR, “NTZ”: natalizumab before RTX/OCR, “FING”: fingolimod before RTX/OCR, “short-FING”: previously received fingolimod with a washout period less than the median value of the washout period duration of the “FING” group (27 days), “long-FING”: previously received fingolimod with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 days). RTX, rituximab; OCR, ocrelizumab.
Table 2.
Cox Proportional-Hazards Analysis of Time to First Relapse Within the First 6 Months of RTX/OCR
| Time to first relapse within the first 6 mo of RTX/OCR | ||
| Adjusted HR (95% CI) | p Value | |
| Age (<40 vs ≥ 40 [ref.]) | 2.25 (0.63–8.07) | 0.21 |
| Sex (male vs female [ref.]) | 1.21 (0.43–3.37) | 0.70 |
| Disease duration at RTX/OCR initiation by month | 0.21 (0.01–2.80) | 0.24 |
| Disease activity in the year before RTX/OCR initiation (yes vs no [ref.]) | 1.30 (0.33–5.05) | 0.35 |
| EDSS at RTX/OCR initiation (≥2 vs < 2 [ref.]) | 0.76 (0.19–2.94) | 0.69 |
| Previous DMT | ||
| No therapy [ref.] | ||
| Low efficacy therapy (IFN/GLAT/DMF)a | NA | NA |
| NTZ | 2.38 (0.23–24.64) | 0.46 |
| Short-FINGb | 1.02 (0.08–11.87) | 0.98 |
| Long-FINGb | 8.78 (1.72–44.86) | <0.01 |
Abbreviations: DMF = dimethyl fumarate; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; FING = fingolimod; GLAT = glatiramer acetate; HR = hazard ratio; IFN = interferon beta; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab.
No relapse occurred in this group, which prevented determination of the HR.
The group “FING” was split into 2 groups: “short-FING” patients who previously received FING with a washout period less than the median value of the washout period duration of the “FING” group (27 d) and “long-FING” patients who previously received FING with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 d).
Effect of Previous DMT on Disease Evolution After the First 6 Months of RTX/OCR
After the first 6 months of RTX/OCR, the patient groups “no therapy,” “low efficacy therapy,” “NTZ,” and “FING” did not significantly differ in survival curve estimates for time to first relapse (log-rank test p = 0.3), time to first 6-month confirmed disability accumulation (log-rank test p = 0.4), or time to first development of at least one new T2-weighted lesion on MRI (log-rank test p = 0.6). On Cox proportional hazards analysis, age <40 years was associated with increased risk of new relapse after the first 6 months of RTX/OCR (HR: 4.04; 95% CI 1.33–12.23; p = 0.01) (Table 3), EDSS ≥2 at RTX/OCR initiation with increased risk of 6-month confirmed disability worsening after the first 6 months of RTX/OCR (HR: 3.01; 95% CI 1.34–6.74; p < 0.01) (Table 4), and male sex with increased risk of development of new T2-weighted or gadolinium-enhanced lesions after the first 6 months of RTX/OCR (HR: 2.25; 95% CI 1.07–4.72; p = 0.03) (Table 5).
Table 3.
Cox Proportional-Hazards Analysis of Time to First Relapse After the First 6 Months of RTX/OCR
| Time to first relapse after the first 6 mo of RTX/OCR | ||
| Adjusted HR (95% CI) | p Value | |
| Age (<40 vs ≥ 40 [ref.]) | 4.04 (1.33–12.23) | 0.01 |
| Sex (male vs female [ref.]) | 2.08 (0.90–4.79) | 0.08 |
| Disease duration at RTX/OCR initiation by month | 3.44 (0.34–33.03) | 0.28 |
| Disease activity in the year before RTX/OCR initiation (yes vs no [ref.]) | 1.73 (0.44–6.69) | 0.42 |
| EDSS at RTX/OCR initiation (≥2 vs < 2 [ref.]) | 1.22 (0.45–3.29) | 0.68 |
| Previous DMT | ||
| No therapy [ref.] | ||
| Low efficacy therapy (IFN/GLAT/DMF)a | 0.86 (0.11–6.34) | 0.88 |
| NTZ | 1.86 (0.26–12.94) | 0.52 |
| Short-FINGa | 1.60 (0.29–8.84) | 0.58 |
| Long-FINGa | 2.08 (0.39–10.85) | 0.38 |
Abbreviations: DMF = dimethyl fumarate; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; FING = fingolimod; GLAT = glatiramer acetate; HR = hazard ratio; IFN = interferon beta; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab.
The group “FING” was split into 2 groups: “short-FING” patients who previously received FING with a washout period less than the median value of the washout period duration of the “FING” group (27 d) and “long-FING” patients who previously received FING with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 d).
Table 4.
Cox Proportional Hazards Analysis of Time to First 6-Month Confirmed Worsening of Disability After the First 6 Months of RTX/OCR
| Time to first 6-mo confirmed worsening of disability after the first 6 mo of RTX/OCR | ||
| Adjusted HR (95% CI) | p Value | |
| Age (<40 vs ≥ 40 [ref.]) | 0.91 (0.49–1.69) | 0.77 |
| Sex (male vs female [ref.]) | 0.96 (0.54–1.69) | 0.89 |
| Disease duration at RTX/OCR initiation by month | 1.63 (0.50–5.18) | 0.40 |
| Disease activity in the year before RTX/OCR initiation (yes vs no [ref.]) | 0.95 (0.37–2.45) | 0.92 |
| EDSS at RTX/OCR initiation (≥2 vs < 2 [ref.]) | 3.01 (1.34–6.75) | <0.01 |
| Previous DMT | ||
| No therapy [ref.] | ||
| Low efficacy therapy (IFN/GLAT/DMF)a | 1.09 (0.42–2.79) | 0.85 |
| NTZ | 0.81 (0.25–2.58) | 0.73 |
| Short-FINGa | 0.58 (0.20–1.61) | 0.29 |
| Long-FINGa | 0.59 (0.24–1.44) | 0.25 |
Abbreviations: DMF = dimethyl fumarate; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; FING = fingolimod; GLAT = glatiramer acetate; HR = hazard ratio; IFN = interferon beta; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab.
The group “FING” was split into 2 groups: “short-FING” patients who previously received FING with a washout period less than the median value of the washout period duration of the “FING” group (27 d) and “long-FING” patients who previously received FING with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 d).
Table 5.
Cox Proportional Hazards Analysis of Time to First New T2-Weighted or Gadolinium-Enhanced Lesions After the First 6 Months of RTX/OCR
| Time to first new T2-weighted or gadolinium-enhanced lesions after the first 6 mo of RTX/OCR | ||
| Adjusted HR (95% CI) | p Value | |
| Age (<40 vs ≥ 40 [ref.]) | 1.31 (0.50–3.40) | 0.57 |
| Sex (male vs female [ref.]) | 2.25 (1.07–4.72) | 0.03 |
| Disease duration at RTX/OCR initiation by month | 0.45 (0.05–3.32) | 0.44 |
| Disease activity in the year before RTX/OCR initiation (yes vs no [ref.]) | 0.93 (0.30–2.87) | 0.90 |
| EDSS at RTX/OCR initiation (≥2 vs < 2 [ref.]) | 0.68 (0.27–1.67) | 0.40 |
| Previous DMT | ||
| No therapy [ref.] | ||
| Low efficacy therapy (IFN/GLAT/DMF) | 1.20 (0.23–6.17) | 0.82 |
| NTZ | 1.26 (0.23–6.77) | 0.78 |
| Short-FINGa | 2.09 (0.50–8.76) | 0.31 |
| Long-FINGa | 2.33 (0.57–9.44) | 0.23 |
Abbreviations: DMF = dimethyl fumarate; DMT = disease-modifying therapy; EDSS = Expanded Disability Status Scale; FING = fingolimod; GLAT = glatiramer acetate; HR = hazard ratio; IFN = interferon beta; NTZ = natalizumab; OCR = ocrelizumab; RTX = rituximab.
The group “FING” was split into 2 groups: “short-FING” patients who previously received FING with a washout period less than the median value of the washout period duration of the “FING” group (=27 d) and “long-FING” patients who previously received FING with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 d).
Effect of Previous DMT on B-Cell and T-Cell Levels 6 Months After RTX/OCR Initiation
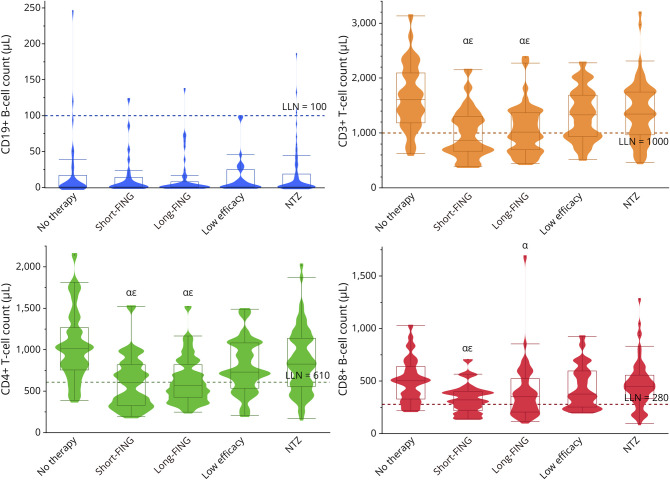
Measures of B and T cells were available for 114 patients on the day of the first RTX/OCR infusion and for 195 patients at 6 months. On the day of the first RTX/OCR infusion, B-cell levels were lower in the “short-FING” and “long-FING” groups than in all other groups except the “low efficacy therapy” group (Table 6, Figure 3). B-cell levels were lower in all groups than the “no therapy” group. At 6 months after RTX/OCR infusion, B-cell levels did not differ between all groups (Table 6 and Figure 3). At 6 months, the proportions of patients with B cells <10/mm3 were 71%, 74%, 72%, 76%, and 67% for the “no therapy,” “low efficacy therapy,” “short-FING,” “long-FING,” and “NTZ” groups. Linear regression analysis revealed no predictor of B-cell levels at 6 months. For patients who previously received fingolimod and with B-cell measures available at 6 months (N = 25 for “short-FING” and N = 42 for “long-FING” groups), the mean (SD) washout period was 18 (1.15) and 40 (15) days for the “short-FING” and “long-FING” groups, and the mean (SD) B-cell levels at the time of the first RTX/OCR infusion were 117 (148) and 129 (59) cells/mm3, respectively.
Table 6.
B-Cell and T-Cell Counts at First RTX/OCR Infusion and after 6 Months by Patient Group
| “No therapy” | “Short-FING” | “Long-FING” | “Low efficacy therapy” | “NTZ” | |
| B-cell count at the first RTX/OCR infusion | 283 (149)a | 106 (88)a,b,c | 145 (83)a,b,c | 335 (170) | 583 (314) |
| T-cell count at the first RTX/OCR infusion | 1,688 (640) | 754 (494)a,b,c | 913 (429)a,b,c | 1,458 (706) | 2,142 (1,006) |
| CD4+ T-cell count at the first RTX/OCR infusion | 1,074 (502) | 399 (322)a,b,c | 550 (309)a,b | 884 (499) | 1,309 (652) |
| CD8+ T-cell count at the first RTX/OCR infusion | 544 (167) | 325 (208)a,b | 328 (157)a,b | 451 (313)a | 767 (406) |
| B-cell count 6 mo after RTX/OCR infusion | 21 (48) | 15 (30) | 13 (28) | 15 (27) | 17 (34) |
| T-cell count 6 mo after RTX/OCR infusion | 1,655 (591) | 994 (457)a,b | 1,066 (449)a,b | 1,319 (474) | 1,381 (544) |
| CD4+ T-cell count 6 mo after RTX/OCR infusion | 1,048 (419) | 600 (317)a,b | 632 (275)a,b | 793 (324) | 863 (383) |
| CD8+ T-cell count 6 mo after RTX/OCR infusion | 507 (207) | 334 (141)a,b | 397 (272)b | 440 (206) | 470 (221) |
Data are mean (SD).
RTX, rituximab; OCR, ocrelizumab; “no therapy”: no disease-modifying therapy before RTX/OCR; “low efficacy therapy”: beta-interferon formulations, glatiramer acetate, or dimethyl fumarate before RTX/OCR; “NTZ”: natalizumab before RTX/OCR; “short-FING”: previously received fingolimod with a washout period less than the median value of the washout period duration of the “FING” group (27 d); “long-FING”: previously received fingolimod with a washout period greater than or equal to the median value of the washout period duration of the “FING” group (27 d).
Lower cell count than “NTZ,” p < 0.05.
Lower cell count than “no therapy,” p < 0.05.
Lower cell count than “BASIC,” p < 0.05.
Figure 3. B-Cell and T-Cell Counts 6 Months After RTX/OCR Initiation.
α lower cell count than “no therapy,” p < 0.05; ε lower cell count than “NTZ,” p < 0.05.
On the day of the first RTX/OCR infusion, T-cell levels were lower in the “short-FING” and “long-FING” groups than all other groups (Table 6 and Figure 3). T-cell levels were lower in all groups except the “low efficacy therapy” vs the “no therapy” group. At 6 months, T-cell levels were lower in the “short-FING” and “long-FING” groups than all other groups and in all groups than the “no therapy” group. At 6 months, the proportion of patients with T-cell levels <1,000 per mm3 was 12%, 33%, 52%, 50%, and 27% in the “no therapy,” “low efficacy therapy,” “short-FING,” “long-FING,” and “NTZ” groups, respectively. On linear regression analysis, the only predictor of T-cell levels at 6 months was the last previous DMT.
Discussion
This study found that previous DMT could alter early but not late effectiveness of BCDT in patients with RMS. Risk of relapse within the first 6 months after switching to RTX/OCR was associated with prior DMT with fingolimod but only in patients with a washout period exceeding 26 days. After 6 months, the effectiveness of RTX/OCR was not affected by any previous DMT. Finally, previous DMT and washout period duration had no effect on B-cell levels 6 months after RTX/OCR initiation.
Previous studies clearly demonstrated that MS disease reactivation is frequent and could be acute after fingolimod withdrawal.14-16 Recent studies specifically assessed the switch from fingolimod to BCDT4-6,17 and found a long washout period in patients switching from fingolimod to BCDT associated with risk of relapse. Zhong et al.6 reported a higher risk of relapse within the first 3 months of OCR for patients switching from fingolimod with a mean washout period of 48.5 days than for patients who previously received another DMT. Of note, another study including patients with a very long washout period (median 2.4 months) found a higher risk of relapse associated with fingolimod than another DMT but only within the washout period.4 Ferraro et al. restricted the analysis to a large group of patients switching from fingolimod to cell-depleting therapy and found that the duration of the washout period was the most important factor predicting the risk of relapse after switching from fingolimod.5 In a population with a long washout period (median 11 week), the authors found a washout period >6 weeks as an independent predictor of relapse after the onset of cell-depleting therapy, but the risk of relapse for patients with a washout period <6 weeks remained significant.
Most of these studies found the duration of the washout period critical for risk of relapse but did not clearly determine the optimal washout duration after fingolimod withdrawal because the durations of the washout period for most patients were long. Rowles et al. described evolution of patients switching from fingolimod to BCDT with a shorter washout period (median 28 days) and evidenced no relapse within the first 6 months of treatment in patients with a washout period <1 month.17 We evidenced very similar results in this study in a group of patients with a short washout duration. We also compared here the potential risk of reactivation between fingolimod and another prior DMT. The analysis confirmed the increased risk of disease reactivation for patients switching from fingolimod but only when the washout period was >26 days. Importantly, no patients with a washout period <25 days experienced relapse within the first 6 months of RTX/OCR as previously evidenced.17 Hence, the risk of early disease reactivation after switching from fingolimod to BCDT may be driven by only the washout period duration and not a potential inherent effect of fingolimod on the future response to BCDT.
We found no effect of previous DMT on B-cell depletion after 6 months of BCDT initiation. Crucially, we found no pejorative effect of shortening the washout period for patients switching from fingolimod on B-cell depletion at 6 months. Indeed, patients switching from fingolimod with a short washout period (median [IQR] 16.5 days [14–22]) showed similar depletion of B cells at 6 months as other patients despite a significantly lower B-cell level at the first RTX/OCR infusion. Thus, the full biological response of BCDT may be obtained when full lymphocyte recovery has not yet occurred at the first infusion after fingolimod discontinuation. Hence, shortening the washout period to <3 weeks for patients switching from fingolimod to prevent the risk of early disease reactivation does not seem to decrease the biological effect of BCDT.
As recently reported, we found lower T-cell levels at 6 months for patients who previously received DMT than DMT-naive patients, especially those who had received fingolimod.18 Importantly, the low proportion of patients with T-cell lymphopenia at 6 months in DMT-naive patients suggests that the effect of BCDT on T-cell levels is marginal and that previous DMT mostly drives T-cell lymphopenia as previously reported. The potential impact of long-lasting T-cell lymphopenia in patients receiving BCDT on the risk of infection should be assessed in future studies.
We also wanted to assess the potential effect of previous DMT on the late response to BCDT. To do this, we compared the evolution after 6 months of BCDT treatment between patients who previously received different DMT and with a median follow-up of 3.5 years. We found no effect of previous DMT on time to first relapse after 6 months of RTX/OCR. Similarly, we found no effect on the development of new T2-weighted or gadolinium-enhanced lesions and time to confirmed disability worsening after 6 months of treatment. Especially, we found no pejorative evolution of disease in patients who previously received fingolimod, as recently suggested.7 The large sample of patients switching from fingolimod we included (108 vs 38 in the Pfeuffer et al. study) and the longer follow-up (3.5 vs 2.3 years) could explain in part the discrepancy with the previous study. In addition, the longer washout period of patients switching from fingolimod in the study by Pfeuffer et al. (48 vs 27 days in our study) might explain why some patients in the previous study showed persistent disease reactivation beyond 6 months.7
For patients switching from fingolimod to RTX/OCR, the risk of disease reactivation during the first 6 months of RTX/OCR treatment increased greatly when the washout duration exceeded 26 days. Fingolimod treatment had no effect on the response to RTX/OCR independent of the washout period duration even after 6 months.
Glossary
- BCDT
B-cell–depleting therapy
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- FING
fingolimod
- HR
hazard ratio
- IQR
interquartile range
- MS
multiple sclerosis
- OCR
ocrelizumab
- RMS
relapsing multiple sclerosis
- RTX
rituximab
Appendix. Authors
| Name | Location | Contribution |
| Lisa Graille-Avy, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Major role in the acquisition of data; analysis or interpretation of data |
| Clemence Boutiere, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Camille Rigollet, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Major role in the acquisition of data |
| Marine Perriguey, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Major role in the acquisition of data |
| Audrey Rico, MD, PhD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie; Aix-Marseille University, CNRS, CRMBM, Marseille, France | Major role in the acquisition of data; study concept or design |
| Sarah Demortiere, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Major role in the acquisition of data; study concept or design |
| Pierre Durozard, MD | Centre hospitalier d’Ajaccio, Service de Neurologie, France | Major role in the acquisition of data; study concept or design |
| Frederic Hilezian, MD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie, Marseille, France | Major role in the acquisition of data; study concept or design |
| Frederic Vely, PharmD, PhD | APHM, Hôpital de la Timone, Service d’immunologie, Marseille Immunopôle; Aix Marseille University, CNRS, INSERM, CIML, Marseille, Franc | Major role in the acquisition of data |
| Pierre Bertault-Peres, MD | APHM, Hôpital de la Timone, Service Pharmacie, Marseille, France | Major role in the acquisition of data |
| Jean Pelletier, MD, PhD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie; Aix-Marseille University, CNRS, CRMBM, Marseille, France | Major role in the acquisition of data; study concept or design |
| Adil Maarouf, MD, PhD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie; Aix-Marseille University, CNRS, CRMBM, Marseille, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
| Bertrand Audoin, MD, PhD | APHM, Hôpital de la Timone, Pôle de Neurosciences Cliniques, Service de Neurologie; Aix-Marseille University, CNRS, CRMBM, Marseille, France | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/NN for full disclosures.
References
- 1.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358(7):676-688. doi: 10.1056/NEJMoa0706383 [DOI] [PubMed] [Google Scholar]
- 2.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221-234. doi: 10.1056/NEJMoa1601277 [DOI] [PubMed] [Google Scholar]
- 3.Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320-327. doi: 10.1001/jamaneurol.2017.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Signoriello E, Lus G, Bonavita S, et al. Switch from sequestering to anti-CD20 depleting treatment: disease activity outcomes during wash-out and in the first 6 months of ocrelizumab therapy. Mult Scler. 2022;28(1):93-101. doi: 10.1177/13524585211005657 [DOI] [PubMed] [Google Scholar]
- 5.Ferraro D, Iaffaldano P, Guerra T, et al. Risk of multiple sclerosis relapses when switching from fingolimod to cell-depleting agents: the role of washout duration. J Neurol. 2022;269(3):1463-1469. doi: 10.1007/s00415-021-10708-1 [DOI] [PubMed] [Google Scholar]
- 6.Zhong M, van der Walt A, Stankovich J, et al. Prediction of multiple sclerosis outcomes when switching to ocrelizumab. Mult Scler. 2022;28(6):958-969. doi: 10.1177/13524585211049986 [DOI] [PubMed] [Google Scholar]
- 7.Pfeuffer S, Rolfes L, Ingwersen J, et al. Effect of previous disease-modifying therapy on treatment effectiveness for patients treated with ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2023;10(3):e200104. doi: 10.1212/NXI.0000000000200104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu C, Zhou Z, Roos I, et al. Comparing switch to ocrelizumab, cladribine or natalizumab after fingolimod treatment cessation in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(12):1330-1337. doi: 10.1136/jnnp-2022-330104 [DOI] [PubMed] [Google Scholar]
- 9.Nygaard GO, Torgauten H, Skattebøl L, et al. Risk of fingolimod rebound after switching to cladribine or rituximab in multiple sclerosis. Mult Scler Relat Disord. 2022;62:103812. doi: 10.1016/j.msard.2022.103812 [DOI] [PubMed] [Google Scholar]
- 10.Rauma I, Viitala M, Kuusisto H, et al. Finnish multiple sclerosis patients treated with cladribine tablets: a nationwide registry study. Mult Scler Relat Disord. 2022;61:103755. doi: 10.1016/j.msard.2022.103755 [DOI] [PubMed] [Google Scholar]
- 11.Perriguey M, Maarouf A, Stellmann JP, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1115. doi: 10.1212/NXI.0000000000001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claverie R, Perriguey M, Rico A, et al. Efficacy of rituximab outlasts B-cell repopulation in multiple sclerosis: time to rethink dosing?. Neurol Neuroimmunol Neuroinflamm. 2023;10(5):e200152. doi: 10.1212/NXI.0000000000200152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demortiere S, Maarouf A, Rico A, et al. Disease evolution in women with highly active MS who suspended natalizumab during pregnancy vs rituximab/ocrelizumab before conception. Neurol Neuroimmunol Neuroinflamm. 2023;10(5):e200161. doi: 10.1212/NXI.0000000000200161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatcher SE, Waubant E, Nourbakhsh B, Crabtree-Hartman E, Graves JS. Rebound Syndrome in patients with multiple sclerosis after cessation of fingolimod treatment. JAMA Neurol. 2016;73(7):790-794. doi: 10.1001/jamaneurol.2016.0826 [DOI] [PubMed] [Google Scholar]
- 15.Bernard-Valnet R, Pignolet B, Biotti D, et al. Unexpected high multiple sclerosis activity after switching from fingolimod to alemtuzumab. Mult Scler Relat Disord. 2018;25:216-218. doi: 10.1016/j.msard.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Cellerino M, Bonavita S, Ferrero M, Inglese M, Boffa G. Severe disease activity in MS patients treated with cladribine after fingolimod withdrawal. J Neurol Sci. 2020;418:117156. doi: 10.1016/j.jns.2020.117156 [DOI] [PubMed] [Google Scholar]
- 17.Rowles WM, Hsu WY, McPolin K, et al. Transitioning from S1P receptor modulators to B cell-depleting therapies in multiple sclerosis: clinical, radiographic, and laboratory data. Neurol Neuroimmunol Neuroinflamm. 2022;9(4):e1183. doi: 10.1212/NXI.0000000000001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landi D, Grimaldi A, Bovis F, et al. Influence of previous disease-modifying drug exposure on T-lymphocyte dynamic in patients with multiple sclerosis treated with ocrelizumab. Neurol Neuroimmunol Neuroinflamm. 2022;9(3):e1157. doi: 10.1212/NXI.0000000000001157 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study will be shared anonymized by reasonable request of a qualified investigator to the corresponding author.