Abstract
In this Viewpoint, Holt, Fettiplace, and Müller weigh the evidence supporting a role for PIEZO and TMC channels in mechanosensory transduction in inner ear hair cells.
The molecular identity of the components of the hair cell mechanosensory transduction apparatus, which transforms information carried by sound into electrical signals, has been the focus of intensive research over the past 40 years. In recent years, pieces of this molecular puzzle have come to light, with a handful of components now firmly established (reviewed in Zheng and Holt, 2021; Qiu and Müller, 2022). The tip link, extending from the top of one hair cell stereocilium to the side of an adjacent taller neighbor is formed by CDH23 and PCDH15. At the upper end of the tip link, CDH23 interacts with USH1C (harmonin), USH1G (sans), and MYO7A to anchor the tip link and regulate tip–link tension, respectively (Fig. 1). At the lower end, PCDH15 binds to LHFPL5 and conveys tension to the channel complex which includes TMIE (transmembrane inner ear protein), the pore-forming subunits TMC1 and/or TMC2, and CIB2. All of these molecular components satisfy essential criteria that must be met to claim a rightful place in nature’s most exquisite mechanosensor (Qiu and Müller, 2022), which is capable of detecting movements as small as ∼1 Å (Denk and Webb, 1992).
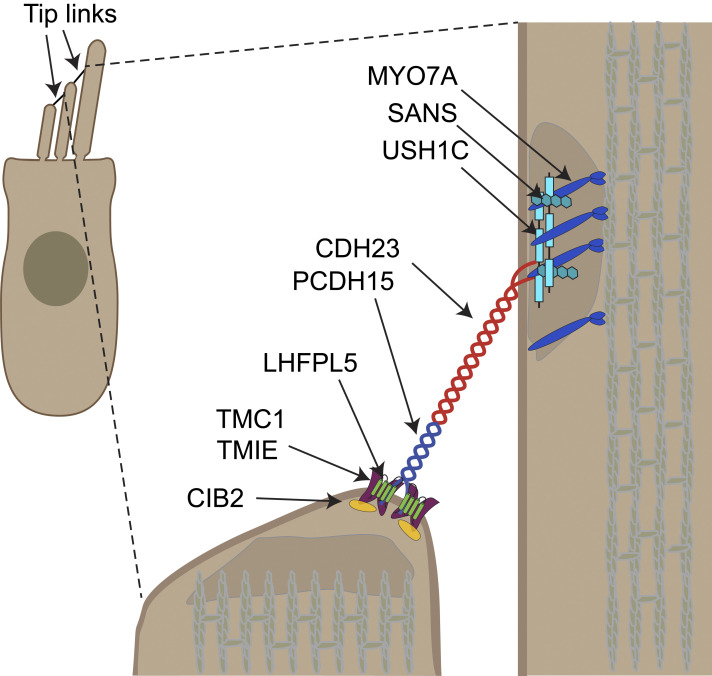
Figure 1.
Schematic diagram of an auditory hair cell (inset) with an expanded view of a single pair of stereocilia (right). Reprinted from Qiu and Müller (2022). Essential and well-established components of the sensory transduction apparatus are labelled, including myosin 7a (MYO7A), harmonin (USH1C), sans (USH1G), cadherin 23 (CDH23), protocadherin 15 (PCDH15), lipoma HMGIC fusion partner-like 5 (LHFPL5), transmembrane inner ear protein (TMIE), transmembrane channel-like 1 (TMC1), and calcium and integrin binding protein 2 (CIB2).
In a recent manuscript, Lee et al. (2024) propose a direct role for PIEZO proteins in hair cell sensory transduction. PIEZOs 1 and 2 form bona fide ion channels and are involved in the sense of touch and other forms of mechanotransduction throughout the body (Wu et al., 2017a). Thus, at first glance, the notion that PIEZO proteins might contribute to mechanotransduction in sensory hair cells does not seem far-fetched. However, as we discuss below, PIEZOs have not satisfied essential criteria to be considered components of the hair cell transduction complex, while TMCs 1 and 2 and other transduction apparatus components do meet these criteria.
Gene expression
First, any gene contributing to auditory transduction must be expressed in sensory hair cells at the right time during development and into adulthood. Rodent auditory hair cells become mechanosensitive during the first postnatal week (Waguespack et al., 2007; Lelli et al., 2009) and auditory function commences during the second postnatal week. Quantitative PCR data demonstrate Tmc2 gene expression transiently during the first postnatal week followed by Tmc1 expression, which rises and is maintained into adulthood (Kurima et al., 2002; Kawashima et al., 2011), preceding the developmental onset of hair cell transduction and auditory function, respectively. Tmc1 and Tmc2 mRNA have also been detected in hair cells using single-cell RNA sequencing (Elkon et al., 2015; Cai et al., 2015; Scheffer et al., 2015; Kolla et al., 2020). Piezo1 was not detected in the same gene expression databases. Piezo2 mRNA expression was detected at low levels but peaks early in the first postnatal week and then declines to near zero by the end of the first postnatal week (Scheffer et al., 2015). Wu et al. (2017b) used in situ hybridization with antisense probes directed against Piezo1 and Piezo2. Piezo1 expression was not detected in the inner ear, while Piezo2 expression was evident in hair cells. The Lee et al. (2024) group used fluorescent in situ hybridization (FISH) and suggested that a few faint puncta are evidence that both Piezo1 and Piezo2 are expressed in auditory hair cells. Confirmation of the specificity of the probes would boost confidence in this result. While gene expression for transduction molecules may be low, it is detectable with modern methods. Currently, there is no compelling evidence for Piezo1 expression in auditory hair cells. Piezo2 does seem to be expressed transiently during the first postnatal week.
Protein localization
Second, protein components of the mechanosensory transduction complex must be localized correctly in the hair bundle, specifically, at the tips of shorter row stereocilia. Using iontophoretic application of transduction blockers or calcium imaging in hair cell stereocilia several groups have demonstrated localization of sensory transduction channels to the tips of hair cell stereocilia (Jaramillo and Hudspeth, 1991; Denk et al, 1995; Lumpkin and Hudspeth, 1995). With higher resolution, Beurg et al. (2009) convincingly showed channel localization at the tips of the second and third rows of stereocilia, at the lower end of tip links. TMC localization is consistent with these patterns. Using viral delivery of TMC coding sequences in frame with coding sequences for fluorescent proteins or a 3xFlag tag, TMCs 1 and 2 were localized to the tips of hair bundles (Kawashima et al., 2011; Askew et al., 2015). Kurima et al. (2015) used a similar approach, but rather than viral delivery, they generated transgenic mice with TMC1 fused to mCherry and TMC2 fused to GFP and observed native fluorescence at the tips of second and third row stereocilia, consistent with the Beurg et al. (2009) data. Two groups have shown anti-TMC1 antibody localization at hair bundle tips and, importantly, a lack of staining in TMC1 knockout controls (Beurg et al., 2015; Giese et al., 2017). Lastly, the Müller group generated transgenic mice with TMC1 fused to an HA tag and TMC2 fused to a MYC tag and reported robust punctate localization at stereocilia tips in two publications (Liang et al., 2021; Qiu et al., 2023). Thus, using four different localization strategies, six different groups have reported localization of TMC proteins in the right place at the right time, greatly boosting confidence in this conclusion. Similarly, several laboratories have localized TMIE and CIB2 to the tips of stereocilia in hair cells (Riazuddin et al., 2012; Wang et al., 2017; Giese et al., 2017; Michel et al., 2017; Liang et al., 2021; Cunnigham et al., 2020; Zhao et al., 2014).
Localization data for PIEZO proteins in hair bundles are limited. Wu et al. (2017b) reported no expression of Piezo1 mRNA and no PIEZO1 protein localization in hair bundles. They did find PIEZO2 protein in hair cells but not in hair bundles. The staining was localized to the apical surface of the hair cell, near the convex side of the hair bundle base (Wu et al., 2017b). Importantly, the staining was absent in Piezo2 knockout controls. Lee et al. (2024) generated knock-in mice with PIEZO1 fused to GFP and PIEZO2 fused to tdTomato. Faint puncta were distributed somewhat randomly throughout the hair bundle but were not concentrated at hair bundle tips, the site of sensory transduction. Control data using the same localization and imaging approach in Piezo knockout mice or wild-type mice that lacked the fusion constructs were not presented but, if available, could boost confidence in the Lee et al. (2024) suggestion that PIEZOs are present in hair bundles. Although detection of faint fluorescence from just a few transduction molecules at stereocilia tips is challenging, the current lack of compelling data indicates this criterion remains unfulfilled for PIEZO proteins.
Genetic deletion
A third criterion is loss of sensory transduction with genetic deletion of the candidate proteins. For TMCs, eight publications report consistent results demonstrating that deletion of Tmc1 and Tmc2 results in complete loss of sensory transduction in auditory and vestibular hair cells throughout the inner ear at all developmental stages tested (Kawashima et al., 2011; Pan et al., 2013, 2018; Kim et al., 2013; Beurg et al., 2014; Askew et al., 2015; Nist-Lund et al., 2019; Cunningham et al., 2020). Similar results have been reported for zebrafish, where mutations in tmcs disrupt sensory transduction in auditory, vestibular, and lateral line hair cells (Chou et al., 2017; Erickson et al., 2017; Smith et al., 2020). The same also holds true for Tmie and Cib2, where several groups have reported that mutations disrupt sensory transduction in mice and zebrafish (Zhao et al., 2014; Cunningham et al., 2020; Pacentine and Nicolson, 2019; Gleason et al., 2009; Wang et al., 2017, 2023; Michel et al., 2017; Giese et al., 2017). There are no publications reporting contrary results. Thus, there is little doubt about the conclusion that expression of either TMC1 or TMC2, as well as TMIE and CIB2, are necessary for sensory transduction in vertebrate hair cells.
Conditional deletion of floxed Piezo1, Piezo2, or both in mice expressing an inner ear–specific Pax2-Cre yielded no deficit in hair cell sensory transduction (Wu et al., 2017b). Interestingly, Piezo2 expression was found to be associated with anomalous mechanotransduction currents evoked by mechanical stimulation of the hair-cell cell-body (Beurg et al., 2016) in a similar location to where Wu et al. (2017b) detected PIEZO2 protein. The current was observed only during the first postnatal week, coincident with the transient expression of Piezo2 during this time frame. Conditional deletion of Piezo2 abolished the anomalous current, solidly linking Piezo2 with this current and confirming Pax2-Cre effectively excised Piezo2 (Beurg and Fettiplace, 2017). Conditional knockout of Piezo2 was necessary because of the lethality of the full knockout due to effects on lung function. However, recordings of transduction currents from Piezo2 full knockouts were possible immediately after birth and resulted in abolition of the anomalous current but no effect on conventional hair cell transduction currents (Beurg and Fettiplace 2017). This laid to rest any concern regarding incomplete efficacy of Pax2-Cre in the Piezo2 conditional knockout. Lee et al. (2024) generated mice conditionally deficient in Piezo1 and Piezo2 but did not present transduction currents recorded from those mice. Thus, there is no evidence suggesting that deletion of either Piezo1, Piezo2, or both results in a deficit in hair cell sensory transduction.
Point mutations
Fourth, while genetic deletions linked to loss of function can establish the necessity of a gene or protein for a biological process, perhaps a stronger criterion is a change in function that results from more subtle genetic manipulations. For TMC1, the point mutation p.M412K, termed Beethoven (Vreugde et al., 2002), was found by three different groups to reduce calcium selectivity (Pan et al., 2013; Beurg et al., 2015; Corns et al., 2016), strongly linking TMC1 with pore properties of hair cell transduction. Beurg et al. (2019), (2021) went on to examine six additional mouse lines, each bearing unique point mutations in TMC1 and reported those substitutions also changed permeation properties.
The strongest evidence linking TMC1 with the pore of the hair cell sensory transduction channel was generated using a chemical-genetic approach (Pan et al., 2018). 17 amino acids within transmembrane domains 4–8 were selected for cysteine substitution. The TMC coding sequence was packaged into AAV vectors, which were introduced into the inner ears of Tmc1/Tmc2 double knockout mice. 16 of the constructs yielded robust transduction currents. Application of cysteine modification reagents reduced current amplitudes for five constructs. 11 constructs led to reduced calcium selectivity. Importantly, the effect of cysteine modification reagents was inhibited by transduction channel closure or by pre-incubation with established open channel blockers, which confirmed the cysteine substitutions were within the pore region of the channel. Three research groups, six publications, and 20 different point mutations have yielded data showing reduced current amplitude, reduced calcium selectivity or both, strongly supporting TMC1 as a pore-forming subunit of the hair cell transduction channel. Notably, a point mutation in TMIE has also been shown to affect conductance and ion selectivity of hair cell transduction channels (Cunningham et al., 2020), which further supports the argument that both TMC1 and TMIE are essential components of the ion channel complex.
There are no reports of single point mutations in either PIEZO1 or PIEZO2 that alter the pore properties of hair cell transduction. Lee et al. (2024) did report PIEZO constructs that included four mutations in the C-terminal domain of PIEZO1 or 2 which when overexpressed in hair cells disrupted transduction current amplitudes. They argue that the reduced currents provide evidence that the mutant constructs co-assemble with endogenous PIEZOs in the native transduction complex in mouse hair cells. However, they did not examine whether overexpression of the exogenous mutant PIEZO proteins caused developmental consequences, altered the localization or function of TMC1 or TMC2, or affected hair cells in other non-specific ways.
Auditory function in mice and humans
Genetic disruption of Tmc1 causes profound deafness in mice (Kurima et al., 2002; Vreugde et al., 2002; Marcotti et al., 2006) and double knockout of Tmc1 and Tmc2 causes complete loss of auditory and vestibular function (Kawashima et al., 2011). Tmc2 deletion alone does not cause auditory dysfunction, but does yield mild vestibular dysfunction, suggesting a role for Tmc2 in semicircular canal function (Kawashima et al., 2011; Ratzen et al., 2024). 10 unique mouse lines with Tmc1 mutations have been reported which also have complete loss of auditory function (Vreugde et al., 2002; Marcotti et al., 2006; Kawashima et al., 2011; Manji et al., 2012; Beurg et al., 2019, 2021; Marcovich et al., 2022). In addition, restoration of Tmc1 expression via gene replacement therapy for recessive mutations (Askew et al., 2015; Nist-Lund et al., 2019; Wu et al., 2021; Marcovich et al., 2022) or genome editing for dominant mutations (Gao et al., 2018; Gyorgy et al., 2019; Yeh et al., 2020; Wu et al., 2021) restores auditory function in deaf Tmc1 mutant mice.
Conditional deletion of Piezo1 in the inner ear does not cause auditory dysfunction (Wu et al., 2017b). Wu et al. (2017b) do report mild elevation (10–20 dB) of auditory brainstem response (ABR) thresholds at three of seven frequencies tested in mice with conditional inner ear deletion of Piezo2. However, this result was not investigated by Lee et al. (2024) who instead opted to generate transgenic mice overexpressing a mutant form of Piezo1 or Piezo2. The Piezo mutants included an AAAA substitution, previously reported as nonfunctional (Zhao et al., 2016). When overexpressed in the inner ear via a constitutively active CAG promoter, Lee et al. (2024) reported elevated ABR thresholds in response to broadband click stimulation. However, the elevation of ABR thresholds progressed overtime from 4 to 12 wk of age and paralleled degeneration of sensory hair cells in the transgenic mice, raising the possibility that the loss of auditory function was a consequence of a non-specific degenerative effect of overexpression of the mutant constructs. The level of overexpression was not quantified despite the inclusion of a GFP tag on the C-terminus of the mutant constructs. Potential consequences of these Piezo mutants, such as developmental effects, on the expression of TMC1, TMIE, and CIB2 were not examined.
In humans, over 100 genetic mutations in TMC1 have been identified that cause hereditary hearing loss (reviewed in Jung and Müller [2023]; genome variation database: http://dgv.tcag.ca/dgv/app/home). Both dominant and recessive mutations have been identified. Recessive loss of function mutations typically cause profound, congenital deafness, while dominant TMC1 mutations tend to cause progressive hearing loss. In addition, numerous genetic mutations have been identified in each of the other components of the hair cell transduction apparatus, MYO7A, USH1C, CDH23, PCDH15, LHFPL5, TMIE, and CIB2, all of which cause deafness in mice and humans (reviewed in Jung and Müller [2023]; genome variation database: http://dgv.tcag.ca/dgv/app/home). However, while humans with homozygous loss-of-function mutations in PIEZO1 and PIEZO2 have been identified with a range of hereditary disorders, hearing loss is not associated with any of these genetic mutations, suggesting that PIEZOs are not essential for human hearing.
Summary and conclusions
PIEZO proteins have been extensively studied in other systems and play critical roles in the sense of touch and other forms of mechanotransduction, yet there is little evidence suggesting PIEZOs play an essential role in the auditory system. Thus far, neither PIEZO1 nor PIEZO2 have met basic criteria required for other bona fide components of the hair cell mechanosensory transduction apparatus (Table 1). As outlined above, there is now abundant and rigorous evidence supporting the molecular identity of 8 to 10 different protein components of the hair cell mechanosensory transduction apparatus. While the identity of these components remained a mystery for decades, recent advances, fueled by genetic studies in humans and functional investigations in mouse and zebrafish models, have propelled the field forward. These studies have led to the identification of core-components of the sensory transduction apparatus in auditory hair cells, including molecular components of the tip–link complex (PCDH15, CDH23, LHFPL5) and of the sensory transduction channel (TMC1/2, TMIE, CIB2; Fig. 1). The genetic studies are well supported by functional studies and biochemical studies, which have demonstrated that the proteins of the transduction apparatus directly bind to each other (reviewed in Qiu and Müller [2022]; Holt et al. [2021]).
Table 1.
Essential criteria for components of the hair cell sensory transduction channel complex are satisfied for TMC proteins (+) but not for PIEZO proteins (−)
| Criteria | TMC1 | TMC2 | PIEZO1 | PIEZO2 |
|---|---|---|---|---|
| Gene expression in neonatal hair cells | + | + | − | + |
| Gene expression in adult hair cells | + | − | − | − |
| Protein localization at hair bundle tips | + | + | − | − |
| Loss of sensory transduction in KOs | + | + | − | − |
| Point mutations alter channel properties | + | + | ? | ? |
| Loss of auditory function in mice | + | − | − | − |
| Loss of auditory function in humans | + | − | − | − |
Remarkably, TMC-dependent ion channel complexes are also expressed in invertebrates. The structures of the native ion channel complexes, purified from Caenorhabditis elegans, were solved by cryo-electron microscopy (Jeong et al., 2022; Clark et al., 2024). The native channels consist of TMCs, TMIE, and a CIB2 homolog, CALM-1, which assemble into an ion channel complex with striking twofold symmetry. A similar twofold symmetry is predicted for the sensory transduction complex in mammalian hair cells (Pan et al., 2018; Ballesteros et al., 2018). Collectively, these findings provide strong evidence that this ion channel complex is an evolutionary invention that predates the emergence of mammals but is ideally suited for mechanosensory transduction enabling the sense of hearing.
Acknowledgments
Christopher J. Lingle served as editor.
The work of these authors is supported by National Institutes of Health/National Institute on Deafness and Other Communication Disorders grants R01DC013521 (to J.R. Holt), R01DC001362 (to R. Fettiplace), and R01DC005965 (to U. Müller).
References
- Askew, C., Rochat C., Pan B., Asai Y., Ahmed H., Child E., Schneider B.L., Aebischer P., and Holt J.R.. 2015. Tmc gene therapy restores auditory function in deaf mice. Sci. Transl. Med. 7:295ra108. 10.1126/scitranslmed.aab1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros, A., Fenollar-Ferrer C., and Swartz K.J.. 2018. Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. eLife. 7. e38433. 10.7554/eLife.38433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Barlow A., Furness D.N., and Fettiplace R.. 2019. A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc. Natl. Acad. Sci. USA. 116:20743–20749. 10.1073/pnas.1908058116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., and Fettiplace R.. 2017. PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells. J. Physiol. 595:7039–7048. 10.1113/JP274996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Fettiplace R., Nam J.H., and Ricci A.J.. 2009. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat. Neurosci. 12:553–558. 10.1038/nn.2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Goldring A.C., and Fettiplace R.. 2015. The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J. Gen. Physiol. 146:233–243. 10.1085/jgp.201511458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Goldring A.C., Ricci A.J., and Fettiplace R.. 2016. Development and localization of reverse-polarity mechanotransducer channels in cochlear hair cells. Proc. Natl. Acad. Sci. USA. 113:6767–6772. 10.1073/pnas.1601067113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Kim K.X., and Fettiplace R.. 2014. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J. Gen. Physiol. 144:55–69. 10.1085/jgp.201411173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg, M., Schimmenti L.A., Koleilat A., Amr S.S., Oza A., Barlow A.J., Ballesteros A., and Fettiplace R.. 2021. New Tmc1 deafness mutations impact mechanotransduction in auditory hair cells. J. Neurosci. 41:4378–4391. 10.1523/JNEUROSCI.2537-20.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai, T., Jen H.I., Kang H., Klisch T.J., Zoghbi H.Y., and Groves A.K.. 2015. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. J. Neurosci. 35:5870–5883. 10.1523/JNEUROSCI.5083-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, S.W., Chen Z., Zhu S., Davis R.W., Hu J., Liu L., Fernando C.A., Kindig K., Brown W.C., Stepanyan R., and McDermott B.M. Jr.. 2017. A molecular basis for water motion detection by the mechanosensory lateral line of zebrafish. Nat. Commun. 8:2234. 10.1038/s41467-017-01604-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, S., Jeong H., Posert R., Goehring A., and Gouaux E.. 2024. The structure of the Caenorhabditis elegans TMC-2 complex suggests roles of lipid-mediated subunit contacts in mechanosensory transduction. Proc. Natl. Acad. Sci. USA. 121:e2314096121. 10.1073/pnas.2314096121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns, L.F., Johnson S.L., Kros C.J., and Marcotti W.. 2016. Tmc1 point mutation affects Ca2+ sensitivity and block by dihydrostreptomycin of the mechanoelectrical transducer current of mouse outer hair cells. J. Neurosci. 36:336–349. 10.1523/JNEUROSCI.2439-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, C.L., Qiu X., Wu Z., Zhao B., Peng G., Kim Y.H., Lauer A., and Müller U.. 2020. TMIE defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron. 107:126–143.e8. 10.1016/j.neuron.2020.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk, W., and Webb W.W.. 1992. Forward and reverse transduction at the limit of sensitivity studied by correlating electrical and mechanical fluctuations in frog saccular hair cells. Hear. Res. 60:89–102. 10.1016/0378-5955(92)90062-R [DOI] [PubMed] [Google Scholar]
- Denk, W., Holt J.R., Shepherd G.M., and Corey D.P.. 1995. Calcium imaging of single stereocilia in hair cells: Localization of transduction channels at both ends of tip links. Neuron. 15:1311–1321. 10.1016/0896-6273(95)90010-1 [DOI] [PubMed] [Google Scholar]
- Elkon, R., Milon B., Morrison L., Shah M., Vijayakumar S., Racherla M., Leitch C.C., Silipino L., Hadi S., Weiss-Gayet M., et al. 2015. RFX transcription factors are essential for hearing in mice. Nat. Commun. 6:8549. 10.1038/ncomms9549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, T., Morgan C.P., Olt J., Hardy K., Busch-Nentwich E., Maeda R., Clemens R., Krey J.F., Nechiporuk A., Barr-Gillespie P.G., et al. 2017. Integration of Tmc1/2 into the mechanotransduction complex in zebrafish hair cells is regulated by Transmembrane O-methyltransferase (Tomt). eLife. 6. e28474. 10.7554/eLife.28474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, X., Tao Y., Lamas V., Huang M., Yeh W.H., Pan B., Hu Y.J., Hu J.H., Thompson D.B., Shu Y., et al. 2018. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature. 553:217–221. 10.1038/nature25164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese, A.P.J., Tang Y.Q., Sinha G.P., Bowl M.R., Goldring A.C., Parker A., Freeman M.J., Brown S.D.M., Riazuddin S., Fettiplace R., et al. 2017. CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat. Commun. 8:43. 10.1038/s41467-017-00061-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, M.R., Nagiel A., Jamet S., Vologodskaia M., López-Schier H., and Hudspeth A.J.. 2009. The transmembrane inner ear (Tmie) protein is essential for normal hearing and balance in the zebrafish. Proc. Natl. Acad. Sci. USA. 106:21347–21352. 10.1073/pnas.0911632106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- György, B., Nist-Lund C., Pan B., Asai Y., Karavitaki K.D., Kleinstiver B.P., Garcia S.P., Zaborowski M.P., Solanes P., Spataro S., et al. 2019. Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 25:1123–1130. 10.1038/s41591-019-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt, J.R., Tobin M., Elferich J., Gouaux E., Ballesteros A., Yan Z., Ahmed Z.M., and Nicolson T.. 2021. Putting the pieces together: The hair cell transduction complex. J. Assoc. Res. Otolaryngol. 22:601–608. 10.1007/s10162-021-00808-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo, F., and Hudspeth A.J.. 1991. Localization of the hair cell’s transduction channels at the hair bundle’s top by iontophoretic application of a channel blocker. Neuron. 7:409–420. 10.1016/0896-6273(91)90293-9 [DOI] [PubMed] [Google Scholar]
- Jeong, H., Clark S., Goehring A., Dehghani-Ghahnaviyeh S., Rasouli A., Tajkhorshid E., and Gouaux E.. 2022. Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature. 610:796–803. 10.1038/s41586-022-05314-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, J., and Müller U.. 2023. Mechanoelectrical transduction-related genetic forms of hearing loss. Curr. Opin. Physiol. 32:100632. 10.1016/j.cophys.2023.100632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima, Y., Géléoc G.S., Kurima K., Labay V., Lelli A., Asai Y., Makishima T., Wu D.K., Della Santina C.C., Holt J.R., and Griffith A.J.. 2011. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J. Clin. Invest. 121:4796–4809. 10.1172/JCI60405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.X., Beurg M., Hackney C.M., Furness D.N., Mahendrasingam S., and Fettiplace R.. 2013. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. J. Gen. Physiol. 142:493–505. 10.1085/jgp.201311068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla, L., Kelly M.C., Mann Z.F., Anaya-Rocha A., Ellis K., Lemons A., Palermo A.T., So K.S., Mays J.C., Orvis J., et al. 2020. Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat. Commun. 11:2389. 10.1038/s41467-020-16113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima, K., Peters L.M., Yang Y., Riazuddin S., Ahmed Z.M., Naz S., Arnaud D., Drury S., Mo J., Makishima T., et al. 2002. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat. Genet. 30:277–284. 10.1038/ng842 [DOI] [PubMed] [Google Scholar]
- Kurima, K., Ebrahim S., Pan B., Sedlacek M., Sengupta P., Mills B.A., Cui R., Nakanishi H., Fujikawa T., Kawashima Y., et al. 2015. TMC1 and TMC2 localize at the site of mechanotransduction in mammalian inner ear hair cell stereocilia. Cell Rep. 12:1606–1617. 10.1016/j.celrep.2015.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.H., Perez-Flores M.C., Park S., Kim H.J., Chen Y., Kang M., Kersigo J., Choi J., Thai P.N., Woltz R.L., et al. 2024. The Piezo channel is a mechano-sensitive complex component in the mammalian inner ear hair cell. Nat. Commun. 15:526. 10.1038/s41467-023-44230-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli, A., Asai Y., Forge A., Holt J.R., and Géléoc G.S.. 2009. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J. Neurophysiol. 101:2961–2973. 10.1152/jn.00136.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X., Qiu X., Dionne G., Cunningham C.L., Pucak M.L., Peng G., Kim Y.H., Lauer A., Shapiro L., and Müller U.. 2021. CIB2 and CIB3 are auxiliary subunits of the mechanotransduction channel of hair cells. Neuron. 109:2131–2149.e15. 10.1016/j.neuron.2021.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin, E.A., and Hudspeth A.J.. 1995. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proc. Natl. Acad. Sci. USA. 92:10297–10301. 10.1073/pnas.92.22.10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji, S.S., Miller K.A., Williams L.H., and Dahl H.H.. 2012. Identification of three novel hearing loss mouse strains with mutations in the Tmc1 gene. Am. J. Pathol. 180:1560–1569. 10.1016/j.ajpath.2011.12.034 [DOI] [PubMed] [Google Scholar]
- Marcotti, W., Erven A., Johnson S.L., Steel K.P., and Kros C.J.. 2006. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J. Physiol. 574:677–698. 10.1113/jphysiol.2005.095661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcovich, I., Baer N.K., Shubina-Oleinik O., Eclov R., Beard C.W., and Holt J.R.. 2022. Optimized AAV vectors for TMC1 gene therapy in a humanized mouse model of DFNB7/11. Biomolecules. 12:914. 10.3390/biom12070914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, V., Booth K.T., Patni P., Cortese M., Azaiez H., Bahloul A., Kahrizi K., Labbé M., Emptoz A., Lelli A., et al. 2017. CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol. Med. 9:1711–1731. 10.15252/emmm.201708087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nist-Lund, C.A., Pan B., Patterson A., Asai Y., Chen T., Zhou W., Zhu H., Romero S., Resnik J., Polley D.B., et al. 2019. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 10:236. 10.1038/s41467-018-08264-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacentine, I.V., and Nicolson T.. 2019. Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells. PLoS Genet. 15:e1007635. 10.1371/journal.pgen.1007635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B., Akyuz N., Liu X.P., Asai Y., Nist-Lund C., Kurima K., Derfler B.H., György B., Limapichat W., Walujkar S., et al. 2018. TMC1 forms the pore of mechanosensory transduction channels in vertebrate inner ear hair cells. Neuron. 99:736–753.e6. 10.1016/j.neuron.2018.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, B., Géléoc G.S., Asai Y., Horwitz G.C., Kurima K., Ishikawa K., Kawashima Y., Griffith A.J., and Holt J.R.. 2013. TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron. 79:504–515. 10.1016/j.neuron.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, X., Liang X., Llongueras J.P., Cunningham C., and Müller U.. 2023. The tetraspan LHFPL5 is critical to establish maximal force sensitivity of the mechanotransduction channel of cochlear hair cells. Cell Rep. 42:112245. 10.1016/j.celrep.2023.112245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, X., and Müller U.. 2022. Sensing sound: Cellular specializations and molecular force sensors. Neuron. 110:3667–3687. 10.1016/j.neuron.2022.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan E.M., Lee J., Madison M.A., Zhu H., Zhou W., Géléoc G.S.G., Holt J.R.. (2024). TMC function, dysfunction, and restoration in mouse vestibular organs. Front Neurol. 15:1356614. 10.3389/fneur.2024.1356614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin, S., Belyantseva I.A., Giese A.P., Lee K., Indzhykulian A.A., Nandamuri S.P., Yousaf R., Sinha G.P., Lee S., Terrell D., et al. 2012. Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat. Genet. 44:1265–1271. 10.1038/ng.2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer, D.I., Shen J., Corey D.P., and Chen Z.Y.. 2015. Gene expression by mouse inner ear hair cells during development. J. Neurosci. 35:6366–6380. 10.1523/JNEUROSCI.5126-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E.T., Pacentine I., Shipman A., Hill M., and Nicolson T.. 2020. Disruption of tmc1/2a/2b genes in zebrafish reveals subunit requirements in subtypes of inner ear hair cells. J. Neurosci. 40:4457–4468. 10.1523/JNEUROSCI.0163-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugde, S., Erven A., Kros C.J., Marcotti W., Fuchs H., Kurima K., Wilcox E.R., Friedman T.B., Griffith A.J., Balling R., et al. 2002. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat. Genet. 30:257–258. 10.1038/ng848 [DOI] [PubMed] [Google Scholar]
- Waguespack, J., Salles F.T., Kachar B., and Ricci A.J.. 2007. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J. Neurosci. 27:13890–13902. 10.1523/JNEUROSCI.2159-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Liu S., Cheng Q., Qu C., Ren R., Du H., Li N., Yan K., Wang Y., Xiong W., and Xu Z.. 2023. CIB2 and CIB3 regulate stereocilia maintenance and mechanoelectrical transduction in mouse vestibular hair cells. J. Neurosci. 43:3219–3231. 10.1523/JNEUROSCI.1807-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Li J., Yao X., Li W., Du H., Tang M., Xiong W., Chai R., and Xu Z.. 2017. Loss of CIB2 causes profound hearing loss and abolishes mechanoelectrical transduction in mice. Front. Mol. Neurosci. 10:401. 10.3389/fnmol.2017.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Solanes P., Nist-Lund C., Spataro S., Shubina-Oleinik O., Marcovich I., Goldberg H., Schneider B.L., and Holt J.R.. 2021. Single and dual vector gene therapy with AAV9-PHP.B rescues hearing in Tmc1 mutant mice. Mol. Ther. 29:973–988. 10.1016/j.ymthe.2020.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J., Lewis A.H., and Grandl J.. 2017a. Touch, tension, and transduction - the function and regulation of Piezo ion channels. Trends Biochem. Sci. 42:57–71. 10.1016/j.tibs.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Grillet N., Zhao B., Cunningham C., Harkins-Perry S., Coste B., Ranade S., Zebarjadi N., Beurg M., Fettiplace R., et al. 2017b. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nat. Neurosci. 20:24–33. 10.1038/nn.4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, W.H., Shubina-Oleinik O., Levy J.M., Pan B., Newby G.A., Wornow M., Burt R., Chen J.C., Holt J.R., and Liu D.R.. 2020. In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci. Transl. Med. 12:eaay9101. 10.1126/scitranslmed.aay9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B., Wu Z., Grillet N., Yan L., Xiong W., Harkins-Perry S., and Müller U.. 2014. TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron. 84:954–967. 10.1016/j.neuron.2014.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q., Wu K., Geng J., Chi S., Wang Y., Zhi P., Zhang M., and Xiao B.. 2016. Ion permeation and mechanotransduction mechanisms of mechanosensitive Piezo channels. Neuron. 89:1248–1263. 10.1016/j.neuron.2016.01.046 [DOI] [PubMed] [Google Scholar]
- Zheng, W., and Holt J.R.. 2021. The mechanosensory transduction machinery in inner ear hair cells. Annu. Rev. Biophys. 50:31–51. 10.1146/annurev-biophys-062420-081842 [DOI] [PMC free article] [PubMed] [Google Scholar]