Abstract
Background & Aims:
Corticosteroids are the only effective therapy for severe alcohol-associated hepatitis (AH), defined by a model for end-stage liver disease (MELD) score >20. However, there are patients who may be too sick to benefit from therapy. Herein, we aimed to identify the range of MELD scores within which steroids are effective for AH.
Methods:
We performed a retrospective, international multicenter cohort study across 4 continents, including 3,380 adults with a clinical and/or histological diagnosis of AH. The main outcome was mortality at 30 days. We used a discrete-time survival analysis model, and MELD cut-offs were established using the transform-the-endpoints method.
Results:
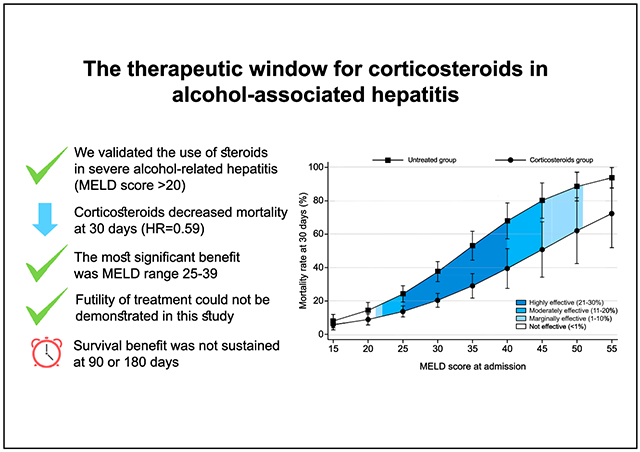
In our cohort, median age was 49 (40–56) years, 76.5% were male, and 79% had underlying cirrhosis. Median MELD at admission was 24 (19–29). Survival was 88% (87–89) at 30 days, 77% (76–78) at 90 days, and 72% (72–74) at 180 days. A total of 1,225 patients received corticosteroids. In an adjusted-survival-model, corticosteroid use decreased 30-day mortality by 41% (hazard ratio [HR] 0.59; 0.47–0.74; p <0.001). Steroids only improved survival in patients with MELD scores between 21 (HR 0.61; 0.39–0.95; p = 0.027) and 51 (HR 0.72; 0.52–0.99; p = 0.041). The maximum effect of corticosteroid treatment (21–30% survival benefit) was observed with MELD scores between 25 (HR 0.58; 0.42–0.77; p <0.001) and 39 (HR 0.57; 0.41–0.79; p <0.001). No corticosteroid benefit was seen in patients with MELD >51. The type of corticosteroids used (prednisone, prednisolone, or methylprednisolone) was not associated with survival benefit (p = 0.247).
Conclusion:
Corticosteroids improve 30-day survival only among patients with severe AH, especially with MELD scores between 25 and 39.
Keywords: alcohol, alcoholic hepatitis, alcohol-associated liver disease, alcoholic liver disease, cirrhosis, steroids, corticosteroids, MELD, Maddrey discriminant function
Graphical Abstract
Lay summary:
Alcohol-associated hepatitis is a condition where the liver is severely inflamed as a result of excess alcohol use. It is associated with high mortality and it is not clear whether the most commonly used treatments (corticosteroids) are effective, particularly in patients with very severe liver disease. In this worldwide study, the use of corticosteroids was associated with increased 30-day, but not 90- or 180-day, survival. The maximal benefit was observed in patients with an MELD score (a marker of severity of liver disease; higher scores signify worse disease) between 25-39. However, this benefit was lost in patients with the most severe liver disease (MELD score higher than 51).
Introduction
Alcohol use disorder (AUD) is one of the leading risk factors for disability and death worldwide1 and constitutes the seventh leading risk factor for premature death and disability.2 Every year, 2.8 million people die as a result of alcohol consumption. Although excessive alcohol consumption is frequent, AUD is usually underdiagnosed. A total of 5.1% of adults have AUD, affecting 8.6% of men and 1.7% of women.3 Alcohol consumption explains half of the cirrhosis cases worldwide, and approximately 35% of patients with AUD will develop chronic liver disease.4 Alcohol-associated hepatitis (AH) constitutes an acute and severe form of alcohol-associated liver disease (ALD) and its global incidence is increasing. Evidence from the Caucasian and Hispanic population suggests an AH incidence from 10% to 35% in patients with ALD.5-8 The mortality rate associated with an AH episode is approximately 30–40% at 90 days.5 Consequently, several efforts have been made to predict disease severity and identify patients who will benefit from corticosteroids. The current models used to predict short-term mortality include the Maddrey’s modified discriminant function (mDF),9 the model for end-stage liver disease (MELD) score,10-12 the ABIC score,13 and the Glasgow AH score.14 The Lille score helps to reassess prognosis and identify corticosteroid non-responders.15 The use of the MELD score at baseline along with the Lille score on day 7 has demonstrated the best performance to predict 2-month and 6-month mortality.16 However, the best predictor of survival at 90 days is the ability to maintain alcohol abstinence.17 There is currently no model that can be used to determine futility of treatment, i.e. the characteristics of a patient in whom the outcome will be poor despite treatment.
Several pharmacological treatments have been assessed for severe AH during the last decades. Despite conflicting evidence, corticosteroids are considered the first-line of pharmacological therapy9 and are recommended by clinical guidelines.18-20 One of the largest randomized clinical trials (STOPAH, 2015)21 demonstrated a non-significant reduction in 30-day mortality in patients with severe AH. However, this benefit was shown in patients predominantly with MELD scores <30 and was lost at 90-day and 1-year follow-up.21 Those results have been consistently observed in 2 systematic reviews.22,23 As a limitation, most of these studies have included only 1 country or region, and ALD is known to be modulated by genetic and environmental factors.18 Studies including multinational cohorts, which could be applicable worldwide, are lacking.
Additionally, corticosteroids have been associated with a higher risk of complications, including bacterial, viral, and fungal infections, gastrointestinal bleeding, and metabolic complications, among others. Currently, more centers are performing early liver transplantation for severe AH and severe infections secondary to the use of corticosteroids may preclude some patients from this possibility. Thus, it becomes even more relevant to define the specific subgroup of patients who will benefit from corticosteroids and those for whom the intervention will not improve outcomes. Moreover, it remains unclear whether there is a ceiling beyond which corticosteroids will cease to confer a benefit. Therefore, we aimed to evaluate the range of MELD scores associated with therapeutic benefit in a multinational cohort of patients with severe AH.
Materials and methods
Study design and participants
We conducted a retrospective registry-based study of patients admitted to the hospital with severe AH. We defined severe AH using the National Institute on Alcohol Abuse and Alcoholism clinical criteria as: i) increase of total bilirubin levels >3 mg/dl (>50 μmol/L), aspartate aminotransferase (AST) >50 IU/ml but <400 IU/L, AST/alanine aminotransferase (ALT) ratio >1.5; ii) absence of other causes of liver disease; iii) consumption of >2 drinks per day (40 g) in women and >3 drinks per day (50–60 g) in men; iv) excessive alcohol consumption for more than 5 years continuously or interrupted; and v) <60 days of abstinence before the onset of jaundice.24 Liver biopsy was obtained when the diagnosis of AH was in question (possible AH) and according to local practice in centers with access to and experience with transjugular liver biopsy. We included all patients meeting the above criteria, clinical (probable AH) or histological (definite AH), independent of the use of steroids during the course of disease. We excluded patients aged <18 year-old, pregnant women and those with AST and/or ALT levels above 400 IU/ml. Patients meeting any of the following criteria were also excluded: i) alcohol abstinence for >60 days before clinical presentation; ii) presence of drug-induced liver injury, ischemic hepatitis, biliary duct obstruction, viral hepatitis, autoimmune hepatitis, or Wilson disease; iii) hepatocellular carcinoma beyond Milan criteria; iv) extrahepatic neoplasia with a life expectancy of less than 6 months; or v) history of severe extrahepatic disease (e.g., chronic kidney failure requiring hemodialysis, heart disease [NYHA class ≥3], and lung disease [mMRC class ≥3] conferring a life expectancy of less than 6 months). We included a total of 53 centers from 17 countries on 4 continents. The median number of patients included per center was 34 [13-80].
Data collection
We retrospectively collected data from the collaborators of each center. We performed a retrospective review of the records of patients hospitalized with the diagnosis of severe AH (from January 2009 to January 2019). The centers were invited through the Engage Platform from ALD special interest group from the American Association for the Study of Liver Diseases. We collected laboratory results at admission, as well as the type of steroids and length of use. We recorded the MELD and mDF scores at admission and during hospitalization, mortality and causes of death at 30 days. The data collected was recorded in a confidential electronic case report form. The electronic database was managed by the main researchers of the study through the RedCap platform. We requested an informed consent waiver at each participating center or leveraging from previous consortia (InTeam, GLOBAL), and de-identified data was analyzed.
Statistical analysis
The primary outcome was 30-day mortality in patients with severe AH, treated or not with corticosteroids, and the MELD therapeutic window that correlates with treatment benefit. The secondary outcomes were complications resulting from the use of corticosteroids in patients with severe AH, and the clinical differences between the steroids used. Categorical variables were summarized using frequencies and percentages. We assessed normality distribution in continuous data using the Kolmogorov-Smirnov test. Continuous variables with normal distribution were described with mean and standard deviation. Variables without a normal distribution were summarized using the median and interquartile ranges. Analyses were completed using the chi-square test for categorical variables, the Student’s t test for normally distributed continuous variables and non-parametric tests in the case of continuous variables that are not normally distributed.
We used discrete-time survival models specified in terms of discrete-time hazard to estimate the risk of death at 30 days. In this model, the beginning and the end of each time analysis interval are the same for all patients.25 This survival model can be estimated through dichotomous response regressions once the database structure has been transformed into a person-period type.26 The estimation of the discrete-time hazard was performed via logistic regression models, obtaining hazards as predicted probabilities. A multivariable logistic regression model was used to adjust for baseline differences in socio-demographics and clinical variables and for potential confounders. To relax a constant effect assumption of the regression models, we added an interaction term between the time and MELD score. Wald’s test was applied to assess the statistical significance of the interaction term added to the logistic regression model. Furthermore, a post-estimation analysis was performed to obtain the predicted probabilities of mortality at 30 days for different MELD scores according to steroid use. Regarding the comparisons between steroid use groups, the hazard ratio (HR) was estimated for different MELD scores, establishing their cut-offs. The transform-the-endpoints method was used for estimation since it produces asymmetric confidence intervals at 95% and guaranteed that they were entirely positive.27 STATA software reports transformed confidence intervals based on the transform-the-endpoints method for standard parametric and semi-parametric survival models.28 All analyses were performed with STATA software version 16 (StataCorp, College Station, Texas).
Results
Baseline characteristics of the cohort
We included 3,380 patients from 53 centers in 17 countries on 4 continents. The median age in our cohort was 49 (40–56) years old and 76.5% were male. The most frequent ethnicities were Caucasian (45.3%), Hispanic or Latino (17.1%), Asian (14.3%), and Indian (13.4%). Seventy-nine percent of patients had a prior history of cirrhosis. The median MELD score and mDF at admission were 24 (19–29) and 54 (37–81), respectively. At admission, patients presented with median bilirubin of 12.2 (5.9–22.6) mg/dl, International normalized ratio of 2.0 (1.5–2.0), and albumin 2.6 (2.0–3.0) g/dl. The median creatinine at admission was 0.9 (0.6–1.3) mg/dl and 3.3% of patients required dialysis during hospitalization. Table 1 summarizes the main characteristics of the global cohort, and differences in patients according to use of steroids.
Table 1.
Baseline characteristics of patients according to the use of corticosteroids.
| Characteristics | Global (N = 3,380) |
Non-corticosteroid group (n = 1,592) |
Corticosteroid group (n = 1,225) |
p valueˆ |
|---|---|---|---|---|
| Age (y)† | 49 (40–56) | 49 (42–57) | 47 (39–55) | 0.003 |
| Men (%) | 76.5 | 75.5 | 71.2 | 0.010 |
| Ethnicity (%) | <0.001 | |||
| Caucasian | 45.3 | 43.7 | 59.5 | |
| Hispanic or Latino | 17.1 | 17.1 | 25.0 | |
| Asian | 14.3 | 27.6 | 1.5 | |
| Indian | 13.4 | 3.4 | 3.4 | |
| Black | 4.8 | 2.7 | 4.0 | |
| Mestizo | 3.1 | 3.2 | 4.4 | |
| American-Indian | 0.6 | 0.8 | 0.7 | |
| Other | 1.4 | 1.5 | 1.5 | |
| Cirrhosis (%) | 79.1 | 76.7 | 79.5 | 0.238 |
| MELD at admission† | 24 (19–29) | 22 (18–29) | 25 (21–31) | <0.001 |
| mDF at admission† | 54 (37–81) | 45 (27–68) | 63 (46–90) | <0.001 |
| Laboratory testing: | ||||
| AST (IU/L)† | 142 (96–216) | 142 (94–220) | 148 (110–214) | 0.230 |
| ALT (IU/L)† | 48 (32–80) | 48 (32–78) | 50 (33–79) | 0.323 |
| GGT (IU/L)† | 268 (118–530) | 266 (116–566) | 285 (132–513) | 0.887 |
| Alkaline phosphatase (IU/L)† | 172 (122–260) | 167 (115–249) | 189 (131–292) | 0.015 |
| Total bilirubin (mg/dl)† | 12.2 (5.9–22.6) | 9.4 (4.7–19.3) | 16.4 (8.9–25.9) | <0.001 |
| INR† | 2.0 (1.5–2.0) | 1.8 (1.4–2.0) | 2.0 (1.8–2.1) | <0.001 |
| Creatinine (mg/dl)† | 0.9 (0.6–1.3) | 0.9 (0.7–1.5) | 0.9 (0.6–1.4) | 0.273 |
| Sodium (mEq/L)† | 133 (129–137) | 133 (129–137) | 130 (130–137) | 0.737 |
| Albumin (g/dl)† | 2.6 (2.0–3.0) | 2.7 (2.1–3.0) | 2.7 (2.0–3.0) | 0.371 |
| Dialysis* (%) | 3.4 | 5.7 | 1.4 | <0.001 |
| Liver transplant (%) | 3.3 | 3.0 | 4.1 | 0.266 |
Comparisons were performed using Chi-square test for categorical variables, the Student’s t test for normally distributed continuous variables and non-parametric tests in the case of continuous variables that are not normally distributed. Patients in the global cohort who had missing data were excluded from the multivariable logistic regression model. GGT, gamma-glutamyltransferase; INR, international normalized ratio; mDF, Maddrey’s modified discriminant function; MELD, model for end-stage liver disease.
p value for non-corticosteroid vs. corticosteroid group.
Median and interquartile range [25-75].
At least twice in the last week.
Among patients in whom follow-up information was available, the estimated survival was 88.1% (95% CI 87.2–88.9) at 30 days, 77% (95% CI 75.9–78.1) at 90 days, and 72.4% (95% CI 71.6–73.7) at 180 days. Only 56 of patients underwent liver transplantation during the follow-up period. The main attributed causes of death were multi-organ failure (25.6%), infections (17.4%), liver failure (11.4%), acute kidney injury (9.7%), and gastrointestinal bleeding (9.7%) (Fig. S1), although the majority had more than 1 cause of death.
Use of corticosteroids among the cohort
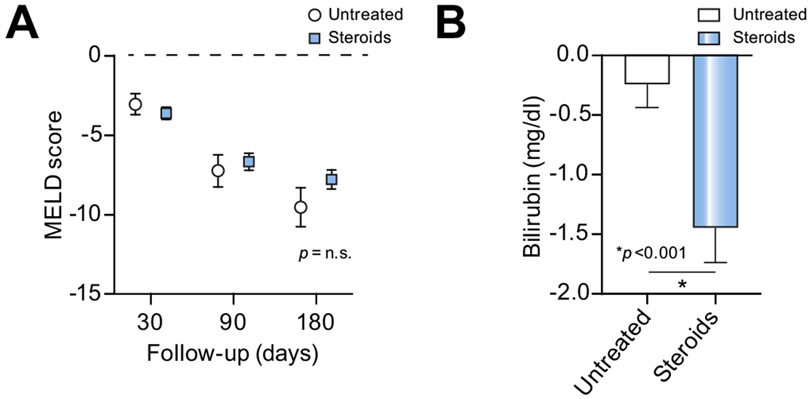
A total of 1,225 patients were treated with corticosteroids (43.5% of the global cohort when patients with missing data were excluded). The median MELD score, mDF, and bilirubin at the onset of corticosteroid treatment were 25 (21–29), 62 (46–85), and 15.2 (8.4–23.9) mg/dl, respectively. The median MELD scores at 30, 90 and 180 days were 20 (15–26), 16 (11–22), and 14 (10–20), respectively. There were no significant differences in MELD score between steroid-treated and untreated groups at 30-, 90-, and 180-day follow-up (Fig. 1A and Fig. S2A). Serum bilirubin was significantly higher in patients treated with corticosteroids; the decrease in serum bilirubin at day 7 was also higher in the steroid-treated group (Fig. 1B and Fig. S2B). The most frequent corticosteroids administered were prednisone (53.2%), prednisolone (31.3%), and methylprednisolone (11.9%). The median time of use of corticosteroids was 20.1 (7–28) days.
Fig. 1. Impact of steroid use in terms of severity at 30, 90 and 180 days.
(A), Changes in MELD score between admission and 30, 90 and 180 days according to steroid use. (B), Change in serum bilirubin between admission and by day 7 according to steroid use. The groups were compared with the Mann-Whitney U test, and a p value <0.05 was considered significant. MELD, model for end-stage liver disease; n.s., not significant.
Impact of corticosteroid use on survival and identification of the optimal therapeutic window
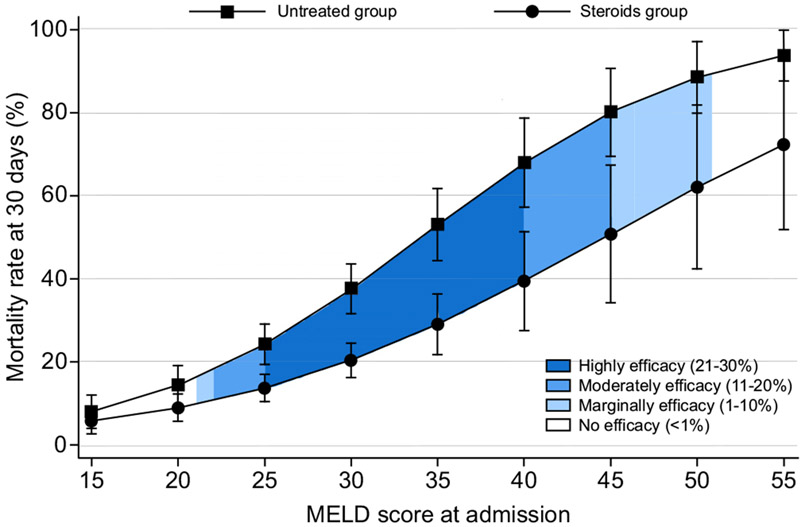
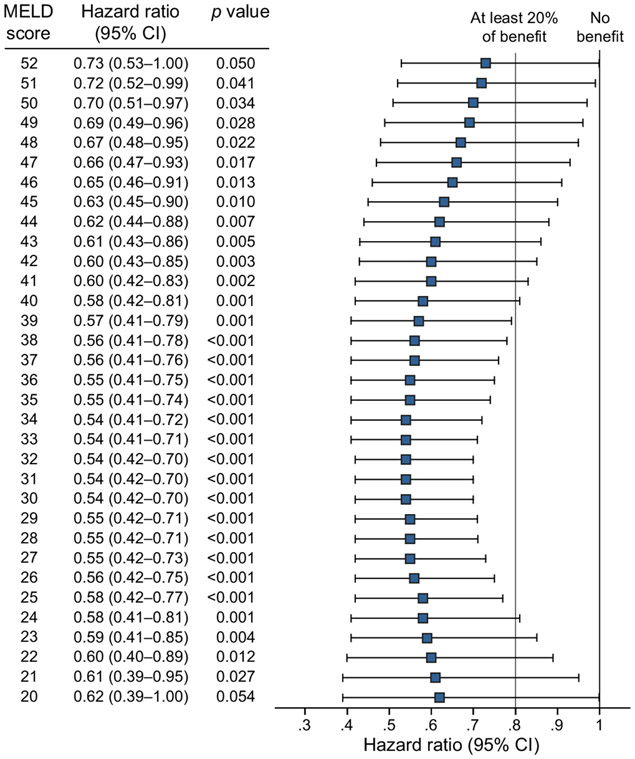
Thirty-day mortality was 10.1% (95% CI 8.9–11.4) in the corticosteroids group vs. 12.8% (11.6–14.1) in the untreated group (p = 0.238). The 90-day mortality was 21.0% (95% CI 19.1–23.1) in the corticosteroids group and 20.1% (95% CI 18.4–21.9) in the untreated group; 180-day mortality was 24.5% (95% CI 22.1–27.1) in the corticosteroids group and 26.4% (95% CI 23.7–29.4) in the untreated group. Due to the baseline differences between both groups, we analyzed the data adjusting for age, gender, ethnicity, cirrhosis, dialysis, and MELD score. On adjusted analysis, the use of corticosteroids decreased the relative risk of 30-day mortality by 41% (HR 0.59; 95% CI 0.47–0.74; p <0.001)(Fig. 2); however, there were no significant differences at 90 (HR 0.92; 95% CI 0.33–2.56; p = 0.871) or 180 days (HR 0.14; 95% CI 0.01–1.48; p = 0.102) (Fig. S3). Additionally, the therapeutic benefit in reducing 30-day mortality was only observed when steroids were used in patients with MELD scores between 21 (HR 0.61; 95% CI 0.39–0.95; p = 0.027) and 51 (HR 0.72; 95% CI 0.52–0.99; p = 0.041) (Fig. 2). Importantly, considering the upper limit of the 95% CI of the HR, the maximum effect of corticosteroids (21–30% survival benefit) was observed in patients with MELD scores between 25 (HR 0.58; 95% CI 0.42–0.77; p <0.001) and 39 (HR 0.57; 95% CI 0.41–0.79; p <0.001). Moderate benefit (11–20% survival benefit) was observed with MELD between 22–24 and 40–44 (Fig. 2). There was no significant association between survival and the type of corticosteroid used (prednisone, prednisolone, or methylprednisolone) (p = 0.247). Different MELD cut-offs and 95% CIs for 30-day patient mortality are described in Fig. 3.
Fig. 2. Predictive model of 30-day survival adjusted by age, gender, ethnicity, cirrhosis, dialysis, and MELD score.
The curves represent mortality per use of steroids and severity (MELD score). A discrete-time hazard was estimated at 30 days using an adjusted multivariable logistic regression. We added an interaction term between the time and the MELD score. Efficacy was defined based on the upper limit of the 95% CI of the hazard ratio. The hazard ratio was 0.59, 95% CI 0.47–0.74, p <0.001. MELD, model for end-stage liver disease.
Fig. 3. Forest plot of the predictive model of 30-day survival adjusted by age, gender, ethnicity, cirrhosis, dialysis, and MELD score.
A hazard ratio less than 1 represents a survival benefit with corticosteroids treatment. The transform-the-endpoints method was used to estimate the hazard ratio for different MELD scores. A p value <0.05 was considered statistically significant. MELD, model for end-stage liver disease.
We evaluated the response to treatment with the Lille model at day 7 (according to the validated cut-off value <0.45). We stratified patients into 3 groups according to MELD score at admission (less than 25, between 25–39, and 40 or more)(Fig. S4). In patients with MELD scores of 25 or less, 70.9% of the group without corticosteroids had response criteria, compared to 62.8% of treated patients from the corticosteroids group (p = 0.006). Inversely, in patients with MELD scores over 40, the treatment response was higher in the corticosteroids group (44.2% vs. 21.8%, p = 0.018). There were no differences in response to treatment in patients with MELD scores between 25–39 (36.4% in the group without corticosteroids and 39% in the corticosteroids group; p = 0.482).
Complications of corticosteroid use
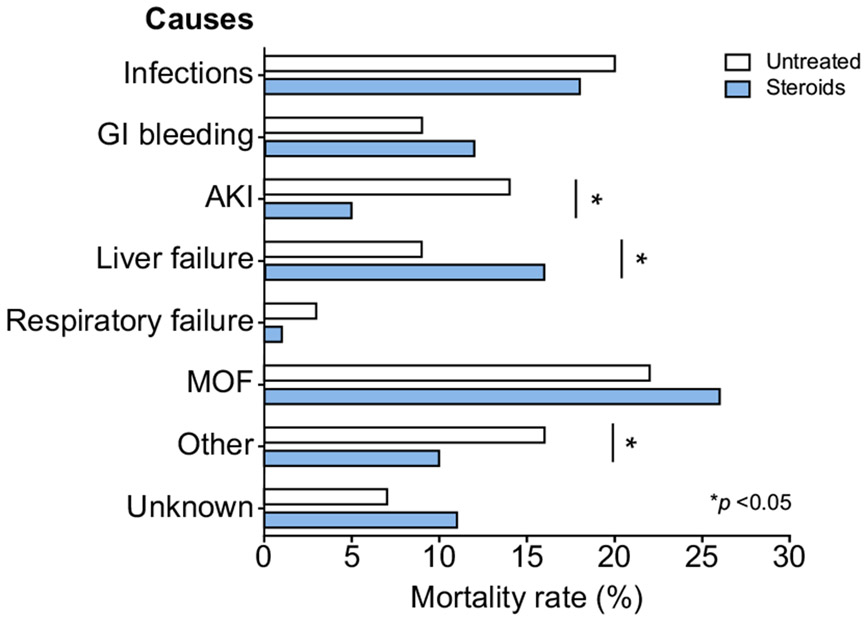
The most frequent reason for discontinuing corticosteroid treatment was non-variceal gastrointestinal bleeding (78%), infections (15.7%), variceal bleeding (3.4%), and acute kidney injury (3%). At the end of follow-up, there were no differences in mortality rate due to documented infections between corticosteroids and untreated groups (18.4% vs. 19.5%, respectively; p = 0.709). The observed mortality due to acute kidney injury was higher in the untreated group than the corticosteroids group (14.2% vs. 5.4%, respectively; p <0.001)(Fig. 4).
Fig. 4. Principal causes of death according to corticosteroid use at 180 days of follow-up.
Comparisons were performed using the chi-square test. A p value <0.05 was considered statistically significant. AKI, acute kidney injury; GI bleeding, gastrointestinal bleeding; MOF, multiple organ failure.
Discussion
Severe AH is a life-threatening condition, with high short-term mortality.18 Corticosteroids constitute the first-line therapy for patients with severe AH (MELD >20), despite conflicting data on their benefit.18-20 However, it is unclear whether there is an upper limit of MELD score beyond which corticosteroids will cease to confer a benefit. In this large retrospective, multicenter cohort study, we demonstrated that: i) the use of corticosteroids decreases 30-day mortality in severe AH by 41%, but does not reduce mortality at 90 or 180 days; ii) using an MELD score >20 to initiate treatment with corticosteroids for severe AH is valid; iii) steroid therapy is beneficial in patients with severe AH with MELD scores between 21 and 51 points. Interestingly, corticosteroids confer their maximum survival benefit (of at least 20–30%) in patients with MELD scores between ≥25 and 39; and vi) there is a moderate benefit (10–20% survival benefit) with steroid therapy in patients with MELD scores between 22–24 and 40–44.
One of the main strengths of our study is its global nature. We included 3,380 patients from 17 countries and 4 continents, including 7 different ethnicities (especially Caucasian, Hispanic, Asian-Pacific Islander, and South Asian). This is arguably the largest and ethnically most heterogenous cohort in this field. Nearly half of the cohort included in the multivariable analysis (43.5%) were treated with corticosteroids; treated patients had more severe liver disease at baseline, evidenced by a higher MELD score and mDF. Thus, only after appropriate adjustment for disease severity and baseline characteristics could the real benefit of corticosteroids be assessed. Prednisone was the most frequently used therapy, and the median time of therapy was 3 weeks (20.1 days). Our cohort is quite different from previous studies. First, a large percentage of prior studies were carried out in the 70s and 80s, most had a low number of patients, and the intensive care support for patients with severe AH was not what we currently have today.9,29-38 In these studies, there were important differences in corticosteroid dosage, with doses up to 3 grams of methylprednisolone in 1 study.36 Therefore, most of the initial systematic reviews yielded contradictory conclusions.39-42 Three prior studies with 131, 101, and 61 patients demonstrated a short-term benefit with corticosteroid use.43,44 In 2015, the STOPAH study demonstrated that the use of prednisolone in patients with severe AH was associated with a non-significant decrease in mortality at 28 days; however, there was no significant effect on mortality at 90-day or 1-year follow-up. That study included 1,103 patients from the UK, with a mean MELD lower than our cohort (21.2 ± 6.2).21 These results have also been supported by recent systematic reviews.22,45 In 2019, a Cochrane systematic review that included 16 studies (from 1977 to 2015) with a total of 1,884 participants concluded that corticosteroids confer no clear benefit over placebo with respect to all-cause mortality at 3 months in patients with severe AH. However, there is great heterogeneity in the included studies (severity of AH, corticosteroid dose, presence of cirrhosis), many of them with a high-risk of bias or unclear risk of bias.23 Although the STOPAH trial suggested short-term benefits, it raised concerns regarding the benefit of corticosteroids in the most severe stages of AH and in different ethnicities. That is, it is unclear from current studies whether there is a level of disease severity beyond which steroids are ineffective or futile in patients with AH.
Based on previous data, there is no robust evidence of a possible disease severity window in which steroids are most effective. In fact, the STOPAH study suggested a narrow therapeutic window (mean MELD score of 21.2 ± 6.2).21 In the current study, we demonstrated the short-term benefit of corticosteroids even with higher MELD scores, and the highest effect was observed in patients with MELD scores between 25 (HR 0.58; 95% CI 0.42–0.77; p <0.001) and 39 (HR 0.57; 95% CI 0.41–0.79; p <0.001), expanding the therapeutic window suggested by the STOPAH study. Different preparations of corticosteroids are used in various countries, depending on availability. We demonstrated in this study that prednisone was as effective as prednisolone, confirming its benefit in patients with severe AH.
Corticosteroid therapy has several adverse effects, including an increased risk of severe infections, upper gastrointestinal bleeding, hyperglycemia, or decompensation of diabetes mellitus, psychological disturbances, and adrenal insufficiency, among others.46 All of these conditions increase morbidity, mortality, length of hospital stay, and healthcare costs. Thus, it becomes even more relevant to abstain or suspend corticosteroid treatment when the risks outweigh the benefits. Infections are the most important adverse effect in severe AH. Two previous studies reported a higher risk of severe infections with the use of prednisolone.21,46 A recent systematic review showed that corticosteroid use does not increase mortality from bacterial infections, but it can increase the risk of fungal infections.47 Other systematic reviews did not show that corticosteroids increase severe adverse effects; however, the evidence is not strong.48 In our study, even with prolonged use of corticosteroids, only 17.4% of the cohort died due to infections, with no differences in the infection rate between treated and untreated patients.
Our retrospective cohort study includes a vast number of patients, ethnicities, and centers. However, our study suffers from the limitations of any retrospective cohort study, including the extensive variability regarding the indication of steroid use and the absence of all the desired variables for all patients. Further, of the 3,380 patients included, only 45% completed follow-up to 180 days, reducing the sample size for long-term outcome analysis. Also, identifying cut-offs based on repeated confidence intervals has limitations since this could disadvantage groups with smaller sample size. The causes of death and the numbers of organ failures could not be clearly ascertained from the data. It was also unclear whether all infections were captured in the database, given the lower prevalence than in other studies. Based on these limitations, the indication for steroids in patients with high MELD (over 40) must be analyzed individually, balancing the benefit and the risk of infections.
In conclusion, our study confirms that corticosteroid use increases 30-day, but not 90- or 180-day, survival in patients with severe AH. The maximum benefit of corticosteroid therapy was observed in patients with MELD scores between 25 and 39; futility of corticosteroid treatment was observed in patients with MELD scores >51. The benefit of multiple investigational agents is currently being investigated in clinical trials. Until the benefit of these agents is demonstrated, it seems reasonable to use corticosteroids in patients with severe AH and MELD scores between 21–51 in the absence of contraindications.
Supplementary Material
Highlights.
We validated the use of corticosteroids for patients with severe alcohol-associated hepatitis defined by an MELD score >20.
The use of corticosteroids was associated with increased 30-day survival.
The maximum benefit of corticosteroids was seen in patients with MELD scores between 25-39.
A MELD score >51 can be used to define futility of corticosteroid treatment in patients with severe AH.
The survival benefit was not sustained at 90 or 180 days.
Financial support
Juan Pablo Arab and Marco Arrese receive support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227 to JPA and 1191145 to MA) and the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT, AFB170005, CARE Chile UC). Ramón Bataller is recipient of NIAAA U01AA021908 and U01AA020821. Dalia Morales Arraez, Meritxell Ventura Cots, and Ana Clemente-Sanchez are recipients of a scholarship grant for study extension abroad, sponsored by the Spanish Association for the Study of the Liver (AEEH). Vijay Shah is supported by NIH AA26974-01 grant. Patrick S. Kamath has received grant support through NIH AA26974-01. Manuel Mendizabal received support from the National Cancer Institute, Argentina (DI-2018-19-APN-INC#MS). Andreea Bumbu, Adelina Horhat and Horia Stefanescu are supported by the Romanian Executive Unit for Scientific Research (UEFISCDI) through the PN-III-P1-1.1-TE-2016-1196 grant awarded to HS.
Abbreviations
- AH
alcohol-associated hepatitis
- ALD
alcohol-associated liver disease
- AST
aspartate aminotransferase
- AUD
alcohol use disorder
- HR
hazard ratio
- mDF
Maddrey’s modified discriminant function
- MELD
model for end-stage liver disease
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.06.019.
Data availability statement
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- [1].Lopez AD, Williams TN, Levin A, Tonelli M, Singh JA, Burney PGJ, et al. Remembering the forgotten non-communicable diseases. BMC Med 2014;12:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. Global Status Report on Alcohol and Health 2018. World Health Organization; 2019. [Google Scholar]
- [4].Arab JP, Bataller R, Roblero JP. Are we really taking care of alcohol-related liver disease in Latin America? Clin Liver Dis 2020;16:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jmelnitzky A. Alcoholic hepatitis: epidemiologic nature and severity of the clinical course in Argentina. Acta Gastroenterol Latinoam 1987;17:287–297. [PubMed] [Google Scholar]
- [6].Christoffersen P, Nielsen K. Histological changes in human liver biopsies from chronic alcoholics. Acta Pathol Microbiol Scand A 1972;80:557–565. [DOI] [PubMed] [Google Scholar]
- [7].Trabut J-B, Plat A, Thepot V, Fontaine H, Vallet-Pichard A, Nalpas B, et al. Influence of liver biopsy on abstinence in alcohol-dependent patients. Alcohol Alcohol 2008;43:559–563. [DOI] [PubMed] [Google Scholar]
- [8].Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology 1997;25:108–111. [DOI] [PubMed] [Google Scholar]
- [9].Maddrey WC, Boitnott JK, Bedine MS, Weber FL Jr, Mezey E, White RI Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology 1978;75:193–199. [PubMed] [Google Scholar]
- [10].Dunn W, Jamil LH, Brown LS, Wiesner RH, Kim WR, Menon KVN, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology 2005;41:353–358. [DOI] [PubMed] [Google Scholar]
- [11].Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl 2005;11:336–343. [DOI] [PubMed] [Google Scholar]
- [12].Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464–470. [DOI] [PubMed] [Google Scholar]
- [13].Dominguez M, Rincón D, Abraldes JG, Miquel R, Colmenero J, Bellot P, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol 2008;103:2747–2756. [DOI] [PubMed] [Google Scholar]
- [14].Forrest EH, Evans CDJ, Stewart S, Phillips M, Oo YH, McAvoy NC, et al. Analysis of factors predictive of mortality in alcoholic hepatitis and derivation and validation of the Glasgow alcoholic hepatitis score. Gut 2005;54:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Louvet A, Naveau S, Abdelnour M, Ramond M-J, Diaz E, Fartoux L, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007;45:1348–1354. [DOI] [PubMed] [Google Scholar]
- [16].Louvet A, Labreuche J, Artru F, Boursier J, Kim DJ, O’Grady J, et al. Combining data from liver disease scoring Systems better predicts outcomes of patients with alcoholic hepatitis. Gastroenterology 2015;149. 398–406.e8; quiz e16–7. [DOI] [PubMed] [Google Scholar]
- [17].Altamirano J, López-Pelayo H, Michelena J, Jones PD, Ortega L, Ginès P, et al. Alcohol abstinence in patients surviving an episode of alcoholic hepatitis: prediction and impact on long-term survival. Hepatology 2017;66:1842–1853. [DOI] [PubMed] [Google Scholar]
- [18].Arab JP, Roblero JP, Altamirano J, Bessone F, Chaves Araujo R, Higuera-De la Tijera F, et al. Alcohol-related liver disease: clinical practice guidelines by the Latin American association for the study of the liver (ALEH). Ann Hepatol 2019;18:518–535. [DOI] [PubMed] [Google Scholar]
- [19].Crabb DW, Im GY, Szabo G, Mellinger JL. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American association for the study of liver diseases. Hepatology [Internet] 2020. Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/abs/10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- [20].European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol 2018;69:154–181. [DOI] [PubMed] [Google Scholar]
- [21].Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015;372:1619–1628. [DOI] [PubMed] [Google Scholar]
- [22].Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology 2015;149. 958–70.e12. [DOI] [PubMed] [Google Scholar]
- [23].Pavlov CS, Varganova DL, Casazza G, Tsochatzis E, Nikolova D, Gluud C. Glucocorticosteroids for people with alcoholic hepatitis. Cochr Database Syst Rev 2019;4:CD001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Crabb DW, Bataller R, Chalasani NP, Kamath PS, Lucey M, Mathurin P, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology 2016;150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. Second Edition. Stata Press; 2008. [Google Scholar]
- [26].Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev 2017;85:185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cummings P. Analysis of Incidence Rates. CRC Press; 2019. [Google Scholar]
- [28].Cummings P. Estimating adjusted risk ratios for matched and unmatched data: an update. Stata J 2011;11:290–298. [Google Scholar]
- [29].Helman RA, Temko MH, Nye SW, Fallon HJ. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med 1971;74:311–321. [DOI] [PubMed] [Google Scholar]
- [30].Porter HP, Simon FR, Pope CE 2nd, Volwiler W, Fenster LF. Corticosteroid therapy in severe alcoholic hepatitis. A double-blind drug trial. N Engl J Med 1971;284:1350–1355. [DOI] [PubMed] [Google Scholar]
- [31].Campra JL, Hamlin EM Jr, Kirshbaum RJ, Olivier M, Redeker AG, Reynolds TB. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Ann Intern Med 1973;79:625–631. [DOI] [PubMed] [Google Scholar]
- [32].Blitzer BL, Mutchnick MG, Joshi PH, Phillips MM, Fessel JM, Conn HO. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double-blind randomized study. Am J Dig Dis 1977;22:477–484. [DOI] [PubMed] [Google Scholar]
- [33].Lesesne HR, Bozymski EM, Fallon HJ. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology 1978;74:169–173. [PubMed] [Google Scholar]
- [34].Shumaker JB, Resnick RH, Galambos JT, Makopour H, Iber FL. A controlled trial of 6-methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. Am J Gastroenterol 1978;69:443–449. [PubMed] [Google Scholar]
- [35].Depew W, Boyer T, Omata M, Redeker A, Reynolds T. Double-blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology 1980;78:524–529. [PubMed] [Google Scholar]
- [36].Theodossi A, Eddleston AL, Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut 1982;23:75–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bories P, Guedj JY, Mirouze D, Yousfi A, Michel H. Treatment of acute alcoholic hepatitis with prednisolone. 45 patients. Presse Med 1987;16:769–772. [PubMed] [Google Scholar]
- [38].Carithers RL Jr, Herlong HF, Diehl AM, Shaw EW, Combes B, Fallon HJ, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med 1989;110:685–690. [DOI] [PubMed] [Google Scholar]
- [39].Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomized trials. Ann Intern Med 1990;113:299–30 . [DOI] [PubMed] [Google Scholar]
- [40].Hofer T, McMahon L. Corticosteroids and alcoholic hepatitis. Hepatology 1991;13:199–201. [DOI] [PubMed] [Google Scholar]
- [41].Daures JP, Peray P, Bories P, Blanc P, Yousfi A, Michel H, et al. [Corticoid therapy in the treatment of acute alcoholic hepatitis. Results of a meta-analysis]. Gastroenterol Clin Biol 1991;15:223–228. [PubMed] [Google Scholar]
- [42].Christensen E, Gluud C. Glucocorticoids are ineffective in alcoholic hepatitis: a meta-analysis adjusting for confounding variables. Gut 1995;37:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mendenhall CL, Anderson S, Garcia-Pont P, Goldberg S, Kiernan T, Seeff LB, et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med 1984;311:1464–1470. [DOI] [PubMed] [Google Scholar]
- [44].Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis–a randomised clinical trial. J Hepatol 2006;44:784–790. [DOI] [PubMed] [Google Scholar]
- [45].Louvet A, Thursz MR, Kim DJ, Labreuche J, Atkinson SR, Sidhu SS, et al. Corticosteroids reduce risk of death within 28 Days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology 2018;155. 458–468.e8. [DOI] [PubMed] [Google Scholar]
- [46].Vergis N, Atkinson SR, Knapp S, Maurice J, Allison M, Austin A, et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology 2017;152. 1068–1077.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hmoud BS, Patel K, Bataller R, Singal AK. Corticosteroids and occurrence of and mortality from infections in severe alcoholic hepatitis: a meta-analysis of randomized trials. Liver Int 2016;36:721–728. [DOI] [PubMed] [Google Scholar]
- [48].Pavlov CS, Varganova DL, Casazza G, Tsochatzis E, Nikolova D, Gluud C. Glucocorticosteroids for people with alcoholic hepatitis (Cochrane review). Cochrane Database Syst Rev 2017. Nov 2;11(11):CD001511. 10.1002/14651858.CD001511.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.