Abstract
Several formulations of a recombinant chimeric respiratory syncytial virus (RSV) vaccine consisting of the extramembrane domains of the F and G glycoproteins (FG) were tested in cotton rats to evaluate efficacy and safety. The FG vaccine was highly immunogenic, providing nearly complete resistance to pulmonary infection at doses as low as 25 ng in spite of inducing relatively low levels of serum neutralizing antibody at low vaccine doses. Upon RSV challenge animals primed with FG vaccine showed quite mild alveolitis and interstitial pneumonitis, which were eliminated by the addition of monophosphoryl lipid A to the formulation.
The quest for a safe and effective vaccine against respiratory syncytial virus (RSV), now in its fourth decade, has alternately focused on nonreplicating and replicating candidate vaccines (reviewed in reference 12). The first vaccine to enter clinical trials contained formalin-inactivated RSV (FI-RSV). The vaccine was moderately antigenic, but the unexpected development of enhanced pulmonary disease in vaccinees subsequently undergoing natural RSV infection brought the trials to an abrupt halt (6, 13, 17, 19) and raised general questions concerning the safety of nonreplicating RSV vaccines that persist to this day. Indeed, since 1965 no nonreplicating RSV candidate vaccine has been permitted to be tested in immunologically naive infants, a likely target population. By contrast, replicating virus RSV vaccines have been plagued by genetic instability (10, 18, 36), residual virulence (18), inadequate antigenicity (35, 36), and the blocking effect of maternal antibody (3, 26). Even if such obstacles could be overcome, a heat-stable vaccine would still have the advantage of applicability in developing countries (and even some areas of developed countries) where the “cold chain” essential for replicating viral vaccines cannot be assured. Several nonviral RSV vaccines have also been evaluated, including a DNA plasmid that expresses gene products intracellularly (20) and a live Staphylococcus displaying G glycoprotein peptides (5), which replicates extracellularly.
A number of nonreplicating RSV vaccines are under development. These include chromatographically purified F glycoprotein (15, 24, 31), recombinant chimeric F and G glycoproteins (23), recombinant chimeric RSV-F–parainfluenza virus–HN glycoproteins (11), recombinant F glycoprotein (14) and recombinant G glycoprotein (25), or a synthetic G glycoprotein peptide (1).
We tested a recombinant chimeric vaccine consisting of the extramembrane domains of the F and G viral glycoproteins. Various formulations, differing in expression systems and adjuvants, have been tested using cotton rats, and questions of efficacy and safety are addressed. A similar but not identical vaccine has been tested previously (4) but was shown to cause enhanced pulmonary disease upon live virus challenge (7). The same vaccine was previously tested in mice (23), which do not develop lesions typical of enhanced disease as do cotton rats.
MATERIALS AND METHODS
FG antigen.
The FG fusion protein used in this study is a chimeric construct comprising the amino acid sequence from position 1 to position 526 of RSV F protein and the amino acid sequence from position 69 to position 298 of RSV G protein. It starts at the N-terminal signal sequence of F glycoprotein, followed by the extracellular region of G glycoprotein, without the amino-terminal region that contains the signal and/or anchor domain of G glycoprotein. The sequences are from the A strains of RSV, the F glycoprotein from strain RSS-2 (2), and the G glycoprotein from strain A2 (34). The construct is different than one previously tested in cotton rats (33) but is the same as that tested in mice (23). The fusion protein was expressed in Chinese hamster ovary (CHO) K1 cells (no designation) or in baculovirus (designated FG/1) and purified to near homogeneity from cell culture supernatant by chromatographic methods.
Vaccine formulation.
The FG protein was adsorbed onto aluminum hydroxide gel with or without the addition of 3-deacylated monophosphoryl lipid A (MPL) (Ribi ImmunoChem Research, Inc., Hamilton, Mont.). The formulation that includes both aluminum hydroxide and MPL is also known as adjuvant system SBAS4 (32).
Animals.
Inbred cotton rats (Sigmodon hispidus) were obtained from a colony maintained at Virion Systems, Inc., Rockville, Md. The cotton rats were housed in large polycarbonate rat cages with absorbent bedding and fed a diet of rodent chow and water. The cotton rat colony was monitored for antibodies to paramyxoviruses, RSV, and rodent viruses, and no such antibodies were found. The animals were, on average, 5 weeks old and weighed 60 g at the time they were used.
Viruses and cells.
The prototype Long strain of RSV (RSV/Long; group A) was obtained from the American Type Culture Collection (Manassas, Va.). A pool containing 106.6 PFU/ml was prepared in HEp-2 cell monolayers (also from the American Type Culture Collection), grown and maintained in Eagle minimum essential medium supplemented with 10% fetal bovine serum, antibiotics, and glutamine. For quantifying virus by a plaque assay, tissue homogenates were applied to HEp-2 cell monolayers, which were incubated and stained as described previously (29).
Experimental design.
Cotton rats were immunized by intramuscular injection of 200 μl of vaccine, which was repeated 21 days later. Immunization by infection was performed by the intranasal instillation of 100 μl of live virus under light methoxyflurane anesthesia. At 49 days after the initial immunization, the animals were anesthetized and challenged intranasally with 105 PFU of RSV/Long in a volume of 100 μl. At intervals thereafter they were sacrificed by carbon dioxide inhalation. These intervals have, in our experience, been most useful for evaluating kinetics of viral replication (days 1, 2, and 5 postchallenge) and histopathologic changes (days 2, 5, and 10) (30). Lungs and nasal tissues for viral quantitation by a plaque assay were prepared as reported elsewhere (29). Lungs for histologic study were inflated with neutral buffered formalin prior to paraffin embedding, sectioning at a thickness of 4 μm, and staining with hematoxylin and eosin (H&E).
Animals tested for their antibody response to RSV were bled from the retro-orbital venous plexus under methoxyflurane anesthesia. Serum neutralizing antibody was measured using a plaque reduction assay with a 60% endpoint as described earlier (29).
Histologic analysis.
Five parameters of pulmonary inflammatory changes were scored in each lung section: peribronchiolitis, perivasculitis, bronchitis, alveolitis, and interstitial pneumonitis. The scoring was done exactly as in a recent study from our group (30). Prior to scoring, the slides were randomized and then read blindly. The first three components were seen in all types of RSV infection, primary, secondary, or vaccine-enhanced, while alveolitis and interstitial pneumonitis were specific to vaccine-enhanced disease. Each of these parameters was scored separately for each histologic section, on a semiquantitative scale ranging from 0 (no inflammation) to 4 (maximum inflammation). Although each was scored using the same scale, the scores are relative and are valid only for comparing the same parameter in different sections and not for comparing different parameters within a section. Lesion scores were expressed as the arithmetic mean for groups.
Statistical analysis.
Viral titers were expressed as the geometric mean ± the standard error (SE) for all animals in a group at a given time. Histologic findings were expressed as the arithmetic mean ± the SE. Differences between groups were evaluated by using the Student t test of summary data.
RESULTS
A preliminary experiment examined the immunogenicity of graded doses of FG ranging from 0 to 625 ng, with each preparation containing alum. Animals were immunized on days 0 and 21, challenged intranasally on day 49 with 105 PFU of RSV/Long, and sacrificed 5 days thereafter.
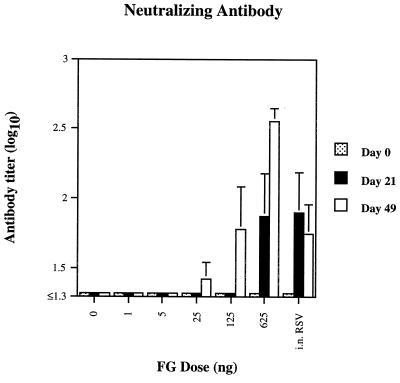
Neutralizing antibody responses (Fig. 1) were dose dependent, with a clear booster effect seen at doses of 25, 125, and 625 ng. Peak antibody titers at the highest dose of FG vaccine approximated those required for passive pulmonary prophylaxis using purified immunoglobulin G (27).
FIG. 1.
Neutralizing antibody response of cotton rats to various doses of recombinant chimeric FG RSV vaccine after one or two doses, with the geometric mean ± the SE and five animals per dosage level.
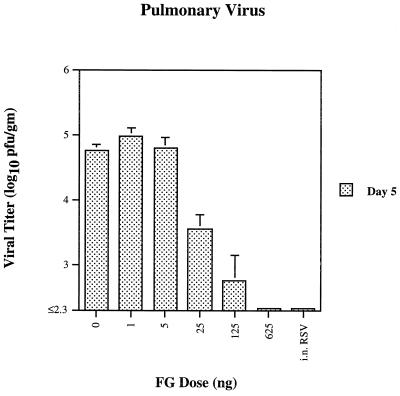
FG vaccine was highly effective in reducing viral replication in the lungs (Fig. 2). A dose of 625 ng resulted in undetectable levels of virus by day 5 postchallenge, the time of peak titers in untreated animals. However, even in these animals, low levels of virus were seen in the lungs on day 1. Doses of 25 and 125 ng effected highly significant reductions in virus (P < 0.005), while doses of 1 and 5 ng had no effect. FG vaccine was less effective in nasal prophylaxis. Day 1 nasal postchallenge viral titers paralleled those of the lungs, with a ≥10-fold decrease in titers from control values at doses of 125 ng or higher (data not shown). By day 5, nasal titers had increased in all dosage groups, although at higher FG doses titers were still reduced in comparison with untreated animals. No animals were virus-free at this site on day 5.
FIG. 2.
Pulmonary viral titers in cotton rats immunized with two doses of various amounts of recombinant chimeric FG RSV vaccine as shown in Fig. 1, challenged with RSV and sacrificed 5 days after challenge, with the geometric mean ± the SE (n = 5 cotton rats per dosage level).
Based upon the results of the preliminary experiment, an intermediate FG dose of 25 ng was used for subsequent experiments. This dose was chosen so that the issue of safety could be addressed under the most stringent experimental conditions, wherein significant viral replication occurred in the milieu of a subprotective response to vaccination. Our prior work showed that enhancement following immunization with FI-RSV was maximal under such conditions (21). We will focus on four vaccine formulations: FG (expressed in Cho cells) with Al(OH)3 with or without MPL and FG/1 (expressed in baculovirus) with Al(OH)3 with or without MPL. These four formulations were compared with four control groups to determine relative efficacy and safety: unimmunized animals undergoing primary RSV infection; previously infected animals receiving a second intranasal challenge; and animals immunized intramuscularly with FI-RSV, either undiluted or at a 1:10 dilution, as in our earlier report (28). Additional animals that received neither pretreatment nor intranasal viral challenge were evaluated for background levels of histopathology, and no significant inflammation of any type was seen in the lungs of these animals (data not shown).
Animals receiving FG formulations or FI-RSV were immunized on days 0 and 21; those pretreated with RSV intranasally received virus on day 0. All groups were bled for serologic study on days 0, 21, and 49. On day 49 all groups were challenged intranasally with live RSV. On days 50, 51, 54, and 59 (1, 2, 5, and 10 days after challenge) animals from each group were sacrificed for virologic and histopathologic studies.
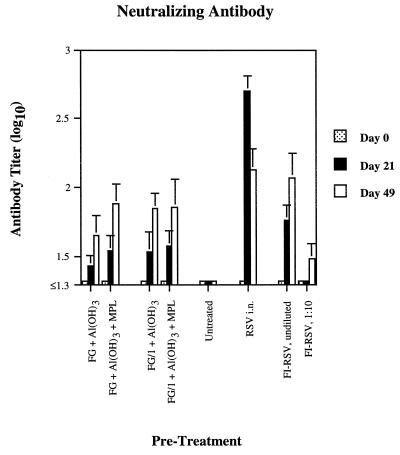
Neutralizing serum antibody responses are depicted (Fig. 3). In comparison to postinfection titers, neither the FG formulations nor FI-RSV was strongly antigenic, with primary (day 21) and boosted (day 49) neutralizing antibody titers 1 order of magnitude lower. There were no significant differences in antibody titers within either FG formulation due to the addition of MPL. In spite of low neutralizing antibody titers, however, all of these groups showed reduced levels of pulmonary viral replication (Fig. 4) but not reduced levels of nasal replication (data not shown). The addition of MPL to either FG formulation did not increase or decrease significantly its effect on the virus titer, although an insignificant trend was seen when MPL was added to FG/1. FG and FG/1 reduced pulmonary viral titers to the same degree at all time points. Similarly, FG + Al(OH)3 + MPL compared favorably with FG/1 + Al(OH)3 + MPL except on day 5, at which the latter was slightly less effective (P < 0.05).
FIG. 3.
Neutralizing antibody titers in cotton rats after one or two 25-ng doses of various formulations of RSV recombinant chimeric FG vaccine, formalin-inactivated whole-virus RSV vaccine, or infection with live virus, with the geometric mean ± the standard deviation (n = 10 cotton rats per treatment).
FIG. 4.
Pulmonary virus titers in cotton rats after two 25-ng doses of various formulations of RSV recombinant chimeric FG vaccine, formalin-inactivated whole-virus RSV vaccine, or infection with live virus, followed by live-virus challenge, with the geometric mean ± the SE (n = 3 to 4 cotton rats per treatment and day of sacrifice).
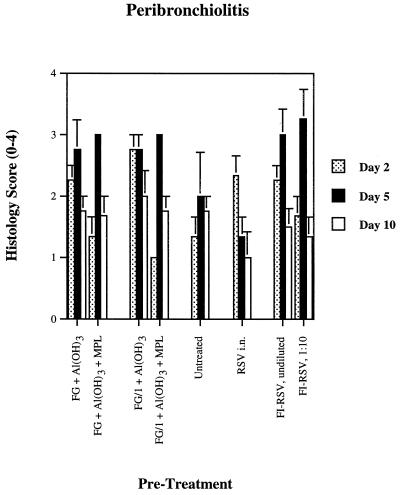
Peribronchiolitis, seen in all eight groups of animals, was the most prominent of the histologic lesions and was not related to the magnitude of viral replication (Fig. 5). Indeed, the animals with the highest viral titers (previously untreated) had slightly lower levels of peribronchiolitis than other groups, a finding consistent with our earlier report (30). There were no statistically significant differences in the magnitude of peribronchiolitis between any of the groups. Perivasculitis and bronchitis, though less prominent, were similarly seen in all groups (data not shown).
FIG. 5.
Peribronchiolitis in cotton rats after two 25-ng doses of various formulations of RSV recombinant chimeric FG vaccine, formalin-inactivated whole-virus RSV vaccine, or infection with live virus, followed by live-virus challenge, with the arithmetic mean ± the SE (n = 3 to 4 cotton rats per treatment and day of sacrifice).
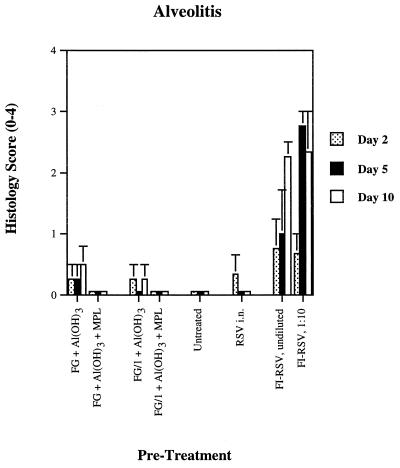
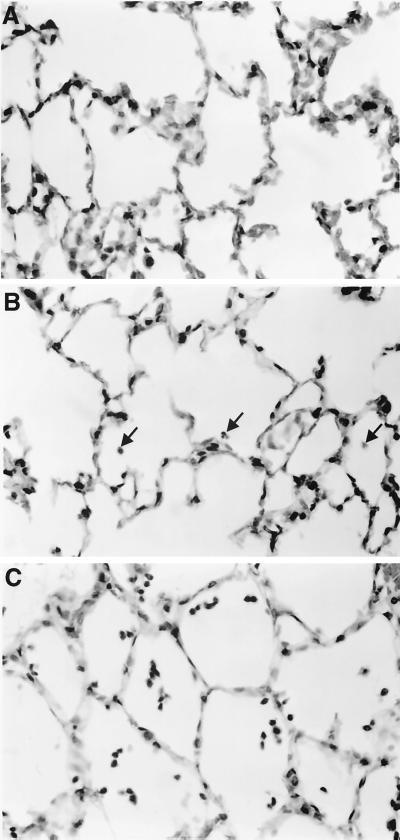
In sharp contrast were alveolitis (Fig. 6) and, though less prominent, interstitial pneumonitis (data not shown). These lesions were seen primarily in animals immunized with FI-RSV and, as shown in our prior report, appear to be the histologic markers of vaccine-enhanced RSV disease (30). Alveolitis was also seen, though to a much smaller extent (P < 0.05 to P < 0.001), in animals immunized with the FG formulations not containing MPL. Addition of MPL to either of these formulations completely eliminated alveolitis but had no significant effect on peribronchiolitis (Fig. 5). The lack of alveolitis in an animal immunized with a FG vaccine containing MPL and challenged with RSV is illustrated (Fig. 7A). This can be contrasted with the extremely mild alveolitis in an animal immunized with FG vaccine not containing MPL and challenged with RSV (Fig. 7B) and the marked alveolitis and mild interstitial pneumonitis seen in an animal immunized with FI-RSV vaccine and challenged with live virus (Fig. 7C).
FIG. 6.
Alveolitis in cotton rats after two 25-ng doses of various formulations of RSV recombinant chimeric FG vaccine, formalin-inactivated whole-virus RSV vaccine, or infection with live virus, followed by live-virus challenge, with the arithmetic mean ± the SE (n = 3 to 4 cotton rats per treatment and day of sacrifice).
FIG. 7.
(A) Lack of alveolitis in a cotton rat immunized twice with 25 ng of recombinant chimeric FG RSV vaccine, to which MPL was added, and challenged with RSV 5 days previously. The alveoli are indistinguishable from those in unmanipulated animals or those with a primary RSV infection. (B) Very mild alveolitis in a cotton rat immunized twice with 25 ng of recombinant chimeric FG RSV vaccine without MPL and challenged with RSV 5 days previously. (C) Alveolitis in a cotton rat immunized with formalin-inactivated RSV vaccine and challenged with RSV 5 days previously. Cells, predominantly polymorphonuclear leukocytes, are free in the alveolar space, and the alveolar walls are thickened. Samples were H&E stained. Magnification, ×140.
DISCUSSION
This study addresses two separate issues regarding a nonreplicating RSV vaccine: its efficacy and its safety. The separation between these issues is highlighted by the fact that in this and previous reports (21, 22, 28) immunization with FI-RSV, although leading to enhanced histopathology, actually reduced viral titers upon subsequent challenge by >90%.
The present preparation of FG, a chimeric recombinant glycoprotein incorporating the extramembrane domains of the F and G glycoproteins of RSV, is highly immunogenic. Doses of as low as 25 ng elicit a measurable serum neutralizing antibody response and reduce pulmonary virus, upon subsequent intranasal challenge, by >90%. Doses of as low as 625 ng result in undetectable levels of pulmonary virus on day 5 postchallenge, the time of peak titers in control animals, a reduction of >1,000-fold. The mechanisms underlying FG-induced protection are not yet defined but probably involve both humoral and cellular effectors. This assumption is sustained both by the fact that lungs can be protected completely even though serum antibody levels fall somewhat below those required for protection by antibody alone (27) and by the finding that groups which are virus-free at the time of peak infectivity in control animals (day 5) have measurable virus at day 1, a pattern consistent with cell-mediated viral clearance.
We previously demonstrated that a dose of FI-RSV that protected fully against pulmonary viral replication did not lead to enhanced disease, while smaller doses of FI-RSV in the same experiment did lead to enhancement (21). Therefore, in order to apply stringent conditions to the testing of the safety of the FG formulations, we employed a relatively low dose of FG vaccine of 25 ng, which elicited a low to moderate antibody response and reduced pulmonary viral titers approximately 10-fold but allowed for the same level of viral replication that resulted in enhanced disease in the FI-RSV-immunized groups.
As noted previously, some types of inflammation were seen in all groups of animals, whether immunized parenterally with FG or FI-RSV, rechallenged following prior intranasal infection, or undergoing primary RSV infection. This was true for peribronchiolitis, perivasculitis, and bronchitis and is demonstrated most dramatically by comparing primary infection with rechallenge of previously infected animals. Animals undergoing primary infection had the highest levels of viral replication of any group, yet the same level of peribronchiolitis as in animals undergoing rechallenge, from which no infectious virus could be recovered. We presume the latter group to be analogous to humans undergoing secondary RSV infection, which uniformly results in milder clinical disease than primary infection (16). We further infer that exposure of pulmonary tissues to viral antigens, whether or not it leads to productive infection, results in inflammatory changes that, though likely subclinical, are nonetheless visible on histologic sections. In the absence of any known data concerning the histopathology of human lungs in nonfatal RSV infection, we speculate that the difference in clinical disease in primary versus secondary RSV infection is related more to the pattern and magnitude of the acellular response of the host (cytokines and chemokines) than to the histopathology of the lungs.
In sharp contrast to peribronchiolitis, perivasculitis, and bronchitis are alveolitis and interstitial pneumonitis, the hallmarks of FI-RSV-enhanced disease (30). These were absent in primary infection and nearly so in secondary infection and yet prominent in animals receiving either dose of FI-RSV. It is significant that a small degree of alveolitis (but not interstitial pneumonitis [data not shown]) was seen with both FG formulations not containing MPL. The demonstration that MPL completely eliminated alveolitis suggests a profile of safety of the vaccine. Indeed, MPL can eliminate alveolitis in animals immunized with FI-RSV (G. A. Prince, unpublished data).
Preliminary evidence suggests that the enhanced disease caused by FI-RSV is associated with a Th2-type immune response in mice (8). RSV vaccine formulations containing MPL have shown a tendency to shift the immune response toward a Th1 profile in mice (23) and presumably away from a profile associated with enhanced disease. Full evaluation of the mechanisms underlying FI-RSV-enhanced disease is not likely to occur using the mouse model, as it lacks important correlates of the enhanced disease process that were seen in humans (9), while the cotton rat exhibits such correlates. Therefore, we are preparing cotton rat-specific reagents which will allow us to quantitate a variety of Th1- and Th2-associated cytokines in order to characterize the immune response to FI-RSV and FG formulations and the change in immunologic profile induced by the addition of MPL to such formulations.
ACKNOWLEDGMENTS
This work was funded by Region Wallonne (contract number 3278:01/03/96-02/02/98) and by SmithKline Beecham Biologicals, Belgium.
We thank Miriam E. R. Darnell, Leilani L. Denk, Susan A. Johnson, Sara Mortensen, and M. Natalia Zimmerman for technical assistance and Charles Smith and Victor Tineo for assistance with the animals.
REFERENCES
- 1.Bastien N, Trudel M, Simard C. Complete protection of mice from respiratory syncytial virus infection following mucosal delivery of synthetic peptide vaccine. Vaccine. 1999;17:832–836. doi: 10.1016/s0264-410x(98)00267-9. [DOI] [PubMed] [Google Scholar]
- 2.Baybutt H N, Pringle C R. Molecular cloning and sequencing of the F and 22K membrane protein genes of the RSS-2 strain of respiratory syncytial virus. J Gen Virol. 1987;68:2789–2796. doi: 10.1099/0022-1317-68-11-2789. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R B, Van Voris L P, Mufson M A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982;145:311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- 4.Brideau R J, Walters R R, Stier M A, Wathen M W. Protection of cotton rats against human respiratory syncytial virus by vaccination with a novel chimeric FG glycoprotein. J Gen Virol. 1989;70:2637–2644. doi: 10.1099/0022-1317-70-10-2637. [DOI] [PubMed] [Google Scholar]
- 5.Cano F, Plotnicky-Gilquin H, Nguyen T N, Liljeqvist S, Samuelson P, Bonnefoy J-Y, Ståhl S, Robert A. Partial protection to respiratory syncytial virus (RSV) elicited in mice by intranasal immunization using live staphylococci with surface-displayed RSV-peptides. Vaccine. 2000;18:2743–2752. doi: 10.1016/s0264-410x(00)00063-3. [DOI] [PubMed] [Google Scholar]
- 6.Chin J, Magoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 7.Connors M, Collins P L, Firestone C-Y, Sotnikov A V, Waitze A, Davis A R, Hung P P, Chanock R M, Murphy B R. Cotton rats previously immunized with a chimeric RSV FG glycoprotein develop enhanced pulmonary pathology when infected with RSV, a phenomenon not encountered following immunization with vaccinia-RSV recombinants or RSV. Vaccine. 1992;10:475–484. doi: 10.1016/0264-410x(92)90397-3. [DOI] [PubMed] [Google Scholar]
- 8.Connors M, Giese N A, Kulkarni A B, Firestone C-Y, Morse H C, Murphy B R. Enhanced pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of interleukin-4 (IL-4) and IL-10. J Virol. 1994;68:5321–5325. doi: 10.1128/jvi.68.8.5321-5325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connors M, Kulkarni A B, Firestone C-Y, Holmes K L, Morse H C, Sotnikov A V, Murphy B R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992;66:7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe J E. Current approaches to the development of vaccines against disease caused by respiratory syncytial virus (RSV) and parainfluenza virus (PIV). A meeting report of the WHO programme for vaccine development. Vaccine. 1995;13:415–421. doi: 10.1016/0264-410x(95)98266-d. [DOI] [PubMed] [Google Scholar]
- 11.Du R-P, Jackson G E D, Wyde P R, Yan W-Y, Wang Q, Gisonni L, Sanhueza S E, Klein M H, Ewasyshyn M E. A prototype recombinant vaccine against respiratory syncytial virus and parainfluenza virus type 3. Bio/Technology. 1994;12:813–818. doi: 10.1038/nbt0894-813. [DOI] [PubMed] [Google Scholar]
- 12.Dudas R A, Karron R A. Respiratory syncytial virus vaccines. Clin Microbiol Rev. 1998;11:430–439. doi: 10.1128/cmr.11.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulginiti V A, Eller J J, Sieber O F, Joyner J W, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- 14.Goetsch L, Plotnicky-Gilquin H, Champion T, Beck A, Corvaïa N, Ståhl S, Bonnefoy J-Y, Nguyen T N, Power U F. Influence of administration dose and route on the immunogenicity and protective efficacy of BBG2Na, a recombinant respiratory syncytial virus subunit vaccine candidate. Vaccine. 2000;18:2735–2742. doi: 10.1016/s0264-410x(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 15.Hancock G E, Smith J D, Heers K M. The immunogenicity of subunit vaccines for respiratory syncytial virus after co-formulation with aluminum hydroxide adjuvant and recombinant interleukin-12. Viral Immunol. 2000;13:57–72. doi: 10.1089/vim.2000.13.57. [DOI] [PubMed] [Google Scholar]
- 16.Henderson F W, Collier A M, Clyde W A, Denny F W. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Eng J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 17.Kapikian A Z, Mitchell R H, Chanock R M, Shvedoff R A, Stewart C E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 18.Kim H W, Arrobio J O, Brandt C D, Wright P, Hodes D, Chanock R M, Parrott R H. Safety and antigenicity of temperature sensitive (is) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973;52:56–63. [PubMed] [Google Scholar]
- 19.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Sambhara S, Li C X, Ettorre L, Switzer I, Cates G, James O, Parrington M, Oomen R, Du R-P, Klein M. Plasmid DNA encoding the respiratory syncytial virus G protein is a promising vaccine candidate. Virology. 2000;269:54–65. doi: 10.1006/viro.2000.0186. [DOI] [PubMed] [Google Scholar]
- 21.Murphy B R, Prince G A, Lawrence L A, Croen K D, Collins P L. Detection of respiratory syncytial virus (RSV) infected cells by in situ hybridization in the lungs of cotton rats immunized with formalin-inactivated virus or purified RSV F and G glycoprotein subunit vaccine and challenged with RSV. Virus Res. 1990;16:153–162. doi: 10.1016/0168-1702(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 22.Murphy B R, Sotnikov A V, Lawrence L A, Banks S M, Prince G A. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 23.Neuzil K M, Johnson J E, Tang Y-W, Prieels J-P, Slaoui M, Gar N, Graham B S. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine. 1997;15:525–532. doi: 10.1016/s0264-410x(97)00218-1. [DOI] [PubMed] [Google Scholar]
- 24.Piedra P A, Grace S, Jewell A, Spinelli S, Bunting D, Hogerman D A, Malinoski F, Hiatt P W. Purified fusion protein vaccine protects against lower respiratory tract illness during respiratory syncytial virus season in children with cystic fibrosis. Pediatr Infect Dis J. 1996;15:23–31. doi: 10.1097/00006454-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Plotnicky-Gilquin H, Huss T, Aubry J-P, Haeuw J-F, Beck A, Bonnefoy J-Y, Nguyen T N, Power U F. Absence of lung immunopathology following respiratory syncytial virus (RSV) challenge in mice immunized with a recombinant RSV G protein fragment. Virology. 1999;258:128–140. doi: 10.1006/viro.1999.9702. [DOI] [PubMed] [Google Scholar]
- 26.Prince G A, Horswood R L, Camargo E, Suffin S C, Chanock R M. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect Immun. 1982;37:1074–1078. doi: 10.1128/iai.37.3.1074-1078.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince G A, Horswood R L, Chanock R M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince G A, Jenson A B, Hemming V G, Murphy B R, Walsh E E, Horswood R L, Chanock R M. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–728. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prince G A, Jenson A B, Horswood R L, Camargo E, Chanock R M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771–791. [PMC free article] [PubMed] [Google Scholar]
- 30.Prince G A, Prieels J-P, Slaoui M, Porter D D. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Investig. 1999;79:1385–1392. [PubMed] [Google Scholar]
- 31.Tebbey P W, Scheuer C A, Peek J A, Zhu D, LaPierre N A, Green B A, Phillips E D, Ibraghimov A R, Eldridge J H, Hancock G E. Effective mucosal immunization against respiratory syncytial virus using purified F protein and a genetically detoxified cholera holotoxin, CT-E29H. Vaccine. 2000;18:2723–2734. doi: 10.1016/s0264-410x(00)00058-x. [DOI] [PubMed] [Google Scholar]
- 32.Thoelen S, Van Damme P, Mathei C, Leroux-Roels G, Desombere I, Safary A, Vandepapeliere P, Slaoui M, Meheus A. Safety and immunogenicity of a hepatitis B vaccine formulated with a novel adjuvant system. Vaccine. 1998;16:708–714. doi: 10.1016/s0264-410x(97)00254-5. [DOI] [PubMed] [Google Scholar]
- 33.Wathen M W, Brideau R J, Thomsen D R, Murphy B R. Characterization of a novel human respiratory syncytial virus chimeric FG glycoprotein expressed using a baculovirus vector. J Gen Virol. 1989;70:2625–2635. doi: 10.1099/0022-1317-70-10-2625. [DOI] [PubMed] [Google Scholar]
- 34.Wertz G W, Collins P L, Huang Y, Gruber C, Levine S, Ball L A. Nucleotide sequence of the G protein gene of human respiratory syncytial virus reveals an unusual type of viral membrane protein. Proc Natl Acad Sci USA. 1985;82:4075–4079. doi: 10.1073/pnas.82.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright P F, Belshe R B, Kim H W, Van Voris L P, Chanock R M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982;37:397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright P F, Shinozaki T, Fleet W, Sell S H, Thompson J, Karzon D T. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. J Pediatr. 1976;88:931–936. doi: 10.1016/s0022-3476(76)81044-x. [DOI] [PubMed] [Google Scholar]