Summary
Catecholamine signaling is thought to modulate cognition in an inverted-U relationship, but the mechanisms are unclear. We measured norepinephrine and dopamine release, postsynaptic calcium responses, and interactions between tonic and phasic firing modes under various stimuli and conditions. High tonic activity in vivo depleted catecholamine stores, desensitized postsynaptic responses, and decreased phasic transmission. Together this provides a clearer understanding of the inverted-U relationship, offering insights into psychiatric disorders and neurodegenerative diseases with impaired catecholamine signaling.
Graphical Abstract
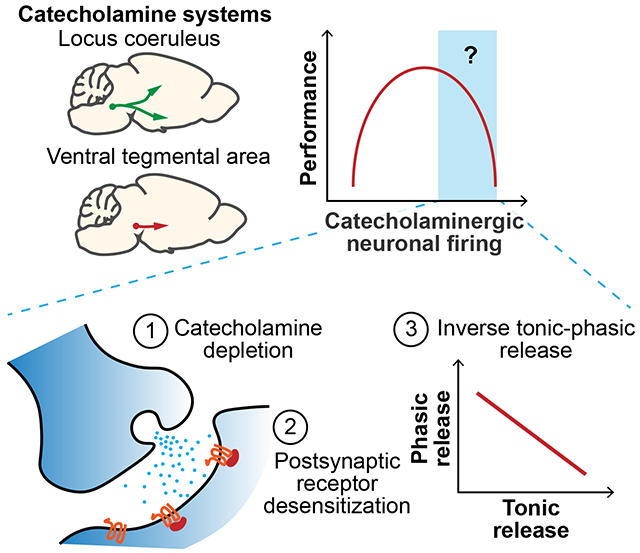
Introduction
Norepinephrine (NE) and dopamine (DA) act as major catecholaminergic neuromodulators to regulate arousal states1–3, attention4–6, learning & memory 5,7 and sensory-motor functions8–10. Interestingly, these catecholaminergic systems exhibit an inverted-U response, between NE neuronal activity in the locus coeruleus (LC) and task performance8; between dopamine D1 receptor activation in the prefrontal cortex (PFC) and working memory7; and between DA neuronal activity in the ventral tegmental area (VTA) and short-term memory performance11. The mechanisms for performance decline at higher neuronal firing remain incompletely understood. It has been hypothesized from observations at glutamatergic synapses that NE and DA may similarly deplete at higher firing of the respective catecholaminergic neurons, but this hypothesis has not been rigorously tested in vivo. It is also unclear whether the phasic transmission mode is affected by higher tonic firing. The recent development of genetically encoded, fluorescent NE15 and DA16,17 sensors can address these questions by overcoming the sensitivity, speed, and spatial limitations of cyclic voltammetry18
Here, we report that high tonic LCNE activity quickly depletes NE, decreases the postsynaptic excitability response, and reduces NE release to subsequent phasic firing in vivo. Similarly, high tonic VTA activity can also deplete DA, and reduce DA release to phasic firing in vivo. Moreover, acute stress affects primarily the postsynaptic response and likewise reduces phasic NE release. Together, these findings implicate a possible synaptic mechanism in which catecholamine signaling becomes progressively impaired at higher neuronal firing due to depleted catecholamine stores and desensitized postsynaptic responses, thus providing a more complete understanding of the nonlinear behavioral effects in catecholamine neuromodulation.
Results
To examine how noradrenergic signaling modulates arousal states via downstream effector regions, we chose two LCNE projections to well-known wake-promoting structures, one to the posterior basal forebrain (BF) and the other to the medial thalamus (Thai)19,20. As shown in Fig 1A, we virally expressed a cre-inducible yellow fluorescent protein (AAV5-EF1α-DIO-EYFP) in the LCNE neurons of Dbh-cre mice, and visualized robust labeling of LC axons in the BF and Thai. Next, we measured axonal NE release in vivo by expressing a genetically encoded fluorescent NE sensor (GRAB_NE2m) in BF neurons, and a cre-inducible optogenetic actuator (ChrimsonR) in the LCNE neurons of Dbh-cre mice; and implanted an optical fiber in the BF for both photometric recording and photostimulation (Fig 1B).
Figure 1. in vivo constraints on norepinephrine signaling.
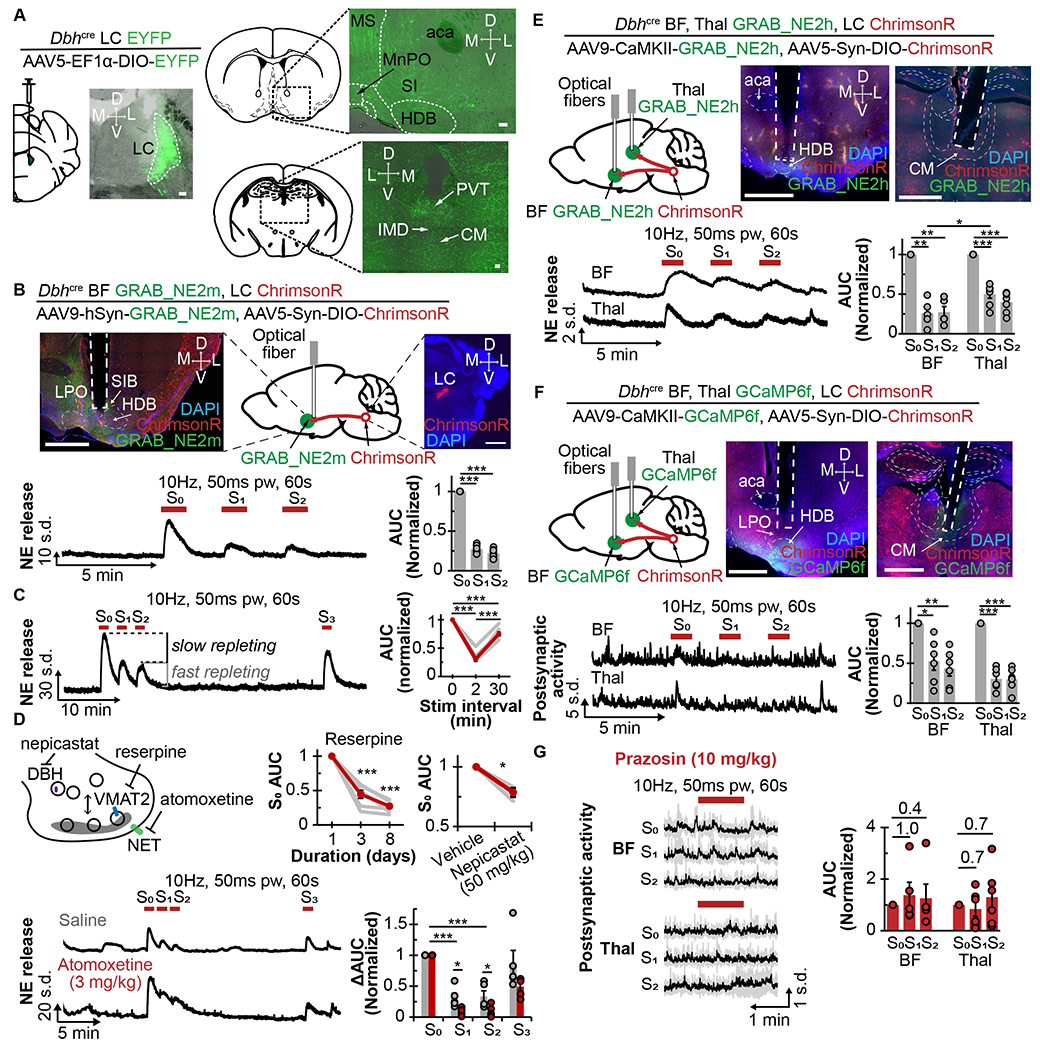
A. Left, schematic shows cre-inducible EYFP virus injected into the LCNE of Dbh-cre mice, accompanied by coronal sections showing EYFP expression in the LC. Right, schematic and coronal sections show the basal forebrain (top) and medial thalamus (bottom). Scale bars are 100μm. aca, anterior. CM, centromedian. HDB, horizontal limb of the diagonal band of Broca. IMD, intermediodorsal nucleus. MS, medial septum. MnPO, median preoptic nucleus. PVT, paraventricular thalamus. SI, substantia innominata.
B. Top, schematic and coronal sections show expression of ChrimsonR in LCNE; and GRAB_NE2m and optical fiber in the basal forebrain (BF). Scale bars are 1mm. HDB, horizontal limb of the diagonal band. LPO, lateral preoptic area. SIB basal portion of substantia innominata. Bottom left, trace shows NE release in response to repeated axonal photostimulation at 10Hz, 50ms pw for 60s at 2min intervals. Bottom right, quantification of AUC normalized to S0 response (N=8).
C. Left, trace shows NE release with repeated photostimulations at 10Hz, 50ms pw for 60s at 2min and 30min intervals. Right, graph shows corresponding AUC normalized to So response (N=8).
D. Top left, diagram shows the drugs used to alter presynaptic NE metabolism. Top right, graphs show normalized AUC S0 after multiple days of reserpine treatment (SFig 1C , N=7) and 2h after intraperitoneal (i.p.) administration of 50mg/kg nepicastat (N=3). Bottom left, traces of NE release 20min after i.p. saline (gray) or atomoxetine (red) with repeated 10Hz photostimulations. Bottom right, graph shows changes to AUC normalized to S0 response (N=3).
E. Top left, schematic shows expression of ChrimsonR in LCNE, and GRAB_NE2h and optical fibers in BF and Thal. Top right, histological coronal brain slices show viral expressions and optical fiber placements in Thal and BF. Scale bars are 1mm. CM, centromedian nucleus. aca, anterior commissure. HDB, horizontal limb of diagonal band. Bottom left, traces show NE release in response to 10Hz photostimulation in the BF and Thal. Bottom right, plot shows corresponding AUC normalized to S0 response in BF (N=5) and Thal (N=7), respectively.
F. Top left, schematic shows expression of ChrimsonR in LCNE, and GCaMP6f and optical fibers in BF and Thal. Top middle and right, histological coronal brain slices show viral expressions and optical fiber placement in Thal and BF. Scale bars are 1mm. CM, centromedian nucleus. aca, anterior commissure. HDB, horizontal limb of diagonal band. Bottom left, traces show postsynaptic activity in response to 10Hz photostimulation in BF and Thal. Bottom right, plot shows AUC normalized to So response in BF (N=6) and Thal (N=6), respectively.
G. Left, average traces show the postsynaptic activity in BF and Thal in response to repeated 10Hz photostimulations 20min after i.p. 10mg/kg prazosin injection. Right, plot shows AUC normalized to S0 response in BF (N=6) and Thal (N=6), respectively.
*p<0.05, **p<0.01, ***p<0.001. Graphs: mean±SEM; photometry traces: mean±std.
LCNE tonic frequency typically ranges from 1-6Hz12,21, but can increase to ~8-10Flz when severely stressed22,23 Given tonic 10Flz somatic LCNE activation for 10min has been previously shown to decrease PFC NE2, we reasoned that 10Flz may provide a suitable starting frequency to examine NE depletion. When LCBF axons in unanesthetized mice were repeatedly photostimulated at 10Hz, 50ms pulse width (pw) for 60s at 2min intervals, the initial stimulation (S0) elicited a robust increase in NE release, but the following stimulations (S1 and S2) elicited only 27±2% and 22±2%, respectively, of the initial evoked release (Fig 1B, Supplemental Table 1), consistent with NE depletion from axonal release sites. Repeating stimulation 30min after the depleting stimulation induced 75±3% of the initial NE release (Fig 1C), consistent with NE repletion. Moreover, the observation that S1 and S2 NE release was similar suggests the existence of a fast- and a slow-repleting NE pool.
Next, we determined whether pharmacologically altering NE metabolism changes the proportions of these fast- and slow-repleting NE pools. Using reserpine to inhibit vesicular monoamine transporter or nepicastat to inhibit dopamine β-hydroxylase, we found that both treatments significantly reduced NE release (Fig 1D), but did not alter the extent of NE depletion and repletion (SFig 1B,C), indicating that the reduced NE is likely distributed equally amongst the pools. Blocking NE reuptake transporter (NET) with atomoxetine (ATOM) when compared to saline control (SAL), produced a significantly slower decay (TATOM 0.006±0.002s versus TSAL 0.022±0.004s), but not rise, of NE fluorescence after stimulation (SFig 1D). Interestingly, we also found a significantly reduced NE release to S1 (SAL: 31 ±7% versus ATOM: 9±3%) and S2 (SAL: 33±9% versus ATOM: 9±4%), indicating that the fast-repleting NE pools rely on NE reuptake; and that the reduction in NE fluorescence to S1 and S2 is not due to sensor internalization, as seen in vitro?5. Furthermore, when compared to SAL, selective activation of α2-adrenergic receptors (α2-ARs) with dexmedetomidine (DEXMED) and inhibition with atipamezole (ATI) significantly altered S1 NE release (SAL: 48±7% DEXMED: 22±4%; SAL 26±3%, ATI: 39±2%) and S2 (SAL: 37±5%, DEXMED: 21 ±3%; SAL 22±3%, ATI: 34±2%, SFig 1E). This result suggests that α2-mediated signaling partially modulates the sizes of fast- and slow-repleting NE pools. As an added control, we showed that 1 mg/kg ATI could antagonize the sedating effect of 50mcg/kg DEXMED (SFig 1F). Combined, these experiments show that although NE depletion cannot be wholly prevented, its extent can be modified by NE metabolism.
To determine if the NE depletion-repletion properties observed in the BF are similar in other brain regions, we next examined NE release in LCThal projections using the GRAB_NE2h sensor (which has higher NE affinity than, but comparable on-off kinetics to GRAB_NE2m) and LCNE-expressed ChrimsonR (Fig 1E). Using repeated photostimulation in BF elicited significantly decreased NE release (S1 31 ±6% and S2 30±6% of S0), suggesting that GRAB_NE2h also consistently detect this NE depletion. Interestingly, NE depletion in the Thal (S1 53±11% and S2 43±9% of S0) was significantly less than in the BF, suggesting brain region-specific variations in fast- and slow-repleting NE pools.
We next determined whether local NE depletion affects local postsynaptic activity in vivo. Similar to the above strategy, ChrimsonR was expressed in the LCNE neurons of Dbh-cre mice, but the calcium sensor GCaMP6f was expressed in the BF and Thal (Fig 1F). Repeatedly photostimulating LCNE axons at 10Hz (50ms pw) for 60s either in the BF or Thal significantly reduced the postsynaptic calcium rise, as shown from S1 (BF: 53±11%, Thal: 31±6% of S0) and S2 (BF: 43±9%, Thal: 30±6% of S0) stimulations, mirroring the axonal NE depletion. This increased postsynaptic calcium rise was blocked by prazosin (10mg/kg), a selective α1-AR antagonist, but not by propranolol (10mg/kg), a β-AR antagonist (Fig 1G, SFig 1G,H), consistent with the α1/Gq-mediated signaling cascade that leads to increased intracellular calcium25. It also demonstrates that the postsynaptic calcium rise is indeed primarily mediated by NE release and not by other co-released transmitters.
We next examined whether NE depletes at the higher frequencies of tonic LCNE firing. Using unanesthetized mice with LCNE expressing ChrimsonR and BF expressing GRAB_NE2m, we measured NE release during 30min of no stimulation versus axonal photostimulations at 3, 5, and 10Hz (10ms pw). As shown in Fig 2A, the stimulations elicited an initial, frequency-dependent increase in NE release, followed by a slow decline, consistent with NE depletion. When GRAB_NE2m was expressed in the Thal, a similar pattern of frequency-dependent NE release kinetics was observed (Fig 2B). In a similar manner, we also investigated postsynaptic activity in the BF and Thal during the 30min axonal stimulations using GCaMP6f to measure calcium dynamics. Despite the expected higher variability, we observed a transient increase in postsynaptic activity in a frequency-dependent manner in both the BF and Thal (SFig 2A, Fig 2C,D), again mirroring the corresponding patterns of axonal NE release. These experiments, therefore, indicate that NE depletion occurs with prolonged, elevated tonic activity.
Figure 2. Norepinephrine depletion and postsynaptic response to varying stimulation frequency and stress.
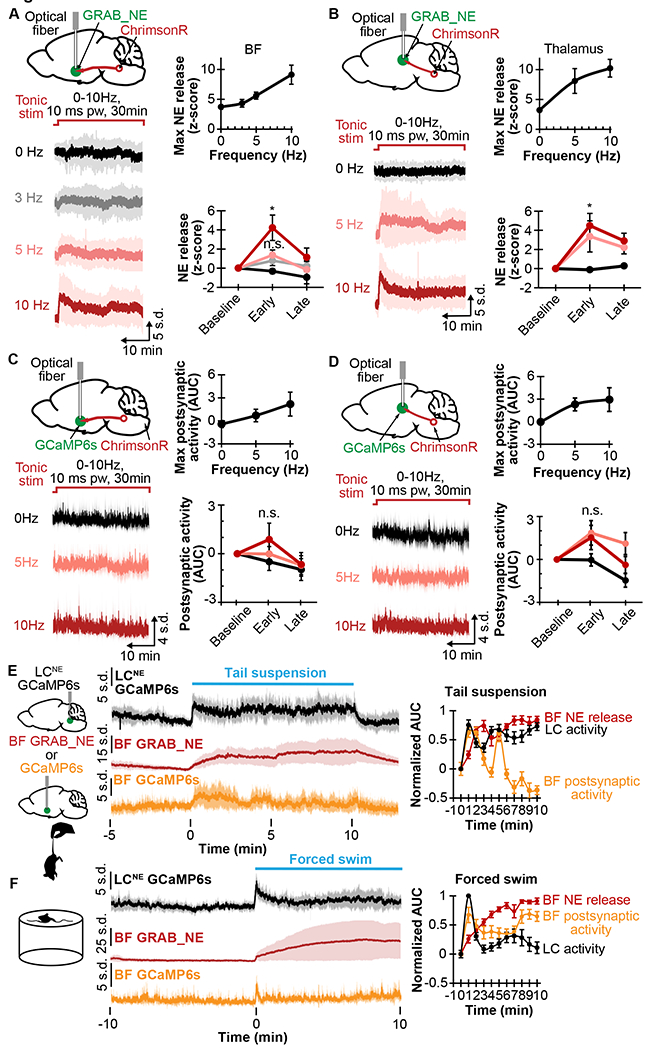
A. Left top, schematic shows ChrimsonR in LCNE, and GRAB_NE and optical fiber in BF. Left bottom, average traces show NE release with 30min photostimulation at 0, 3, 5, and 10Hz 10ms pw. Right top, plot shows maximum NE release in relation to stimulation frequency (N=8). Right bottom, plot shows NE release at baseline (1min before stimulation), early stimulation (1-5min), and late stimulation (25-30min) at different stimulation frequencies (N=8).
B. Left top, schematic shows ChrimsonR in LCNE, and GRAB_NE and optical fibers in Thal. Left bottom, average traces show NE release with 30min photostimulation at 0, 5, and 10Hz 10ms pw. Right top, plot shows maximum NE release in relation to stimulation frequency (N=7). Right bottom, plot shows NE release at the baseline (1min before stimulation), early stimulation (1-5min), and late stimulation (25-30min) at different stimulation frequencies (N=7).
C. Left top, schematic shows ChrimsonR in LCNE, GCaMP6f in BF, and implanted optical fiber in BF. Left bottom, average traces show postsynaptic activity with 30min photostimulation at 0, 3, 5, and 10Hz 10ms pw. Right top, plot shows maximum postsynaptic activity in relation to stimulation frequency (N=6). Right bottom, plot shows postsynaptic activity at baseline (1min before stimulation), early stimulation (1-5min), and late stimulation (25-30min) at different frequencies (N=6).
D. Left top, schematic shows ChrimsonR in LCNE, GCaMP6f in Thal, and implanted optical fiber in Thal. Left bottom, average traces show postsynaptic activity with 30min photostimulation at 0, 5, and 10Hz 10ms pw. Right top, plot shows maximum postsynaptic activity in relation to stimulation frequency (N=6). Right bottom, plot shows postsynaptic activity at the baseline (1min before stimulation), early stimulation (15min), and late stimulation (25-30min) at different frequencies (N=6).
E. Left, schematic shows GCaMP6s in LCNE, GRAB_NE or GCaMP6f in BF, and tail suspension. Right, plots show corresponding traces of the LCNE activity (N=6), BF NE release (N=8), and BF postsynaptic activity (N=6), and corresponding AUC in 1-min binning normalized to 1min before 10min tail suspension.
F. Left, schematic shows forced swim. Right, plots show traces of LCNE activity (N=7), BF NE release (N=6), and BF postsynaptic activity (N=7); and corresponding AUC in 1min binning normalized to 1min before forced swim.
*p<0.05. n.s. not significant. Graphs: mean±SEM; photometry traces: mean±std.
Given the observed NE depletion and postsynaptic desensitization in BF with photostimulation, we then asked whether these effects occur under naturalistic stimulations such as during acute stress. Cohorts expressing either GRAB_NE2m/h or GCaMP6f in the BF were used to determine axonal release of NE and postsynaptic activity, respectively, while another cohort expressing GCaMP6s in LCNE neurons of Dbh-cre mice was used to determine LCNE activity. We first tested tail suspension; and observed that the LCNE activity remained continuously elevated during the suspension, with the NE release gradually plateaued, and the postsynaptic activity initially increased then steadily decreased (Fig 2E). Pre-treating with 10mg/kg prazosin, but not 10mg/kg propranolol or saline, caused a faster decay in postsynaptic activity (SFig 2B). Together, these results indicate that although tail suspension does not deplete axonal NE, the postsynaptic activity diminishes over time, likely due to well-established AR desensitization26. We then tested forced swim, another acute stressor in rodents27, and observed that LCNE activity initially increased, then decreased sharply after 1min. Interestingly, NE release again steadily increased, while the postsynaptic activity reflected LCNE activity with an initial increase, followed by a decrease after 1min (Fig 2F). These results show that NE release does not acutely deplete; rather, a decreased postsynaptic response occurred on the order of minutes.
We then sought to determine whether NE depletion from increased tonic LCNE activity affects the phasic mode of LCNE transmission. Using ChrimsonR expressed in the LCNE and GRAB_NE2m/h in the BF, we simulated a range of tonic LCNE activity by continuously photostimulating LCBF terminals at 0, 3, 5, and 10Hz for 30min. Concurrently, we simulated phasic activity (typically ~10-20Hz, 200-500ms for LC12) by photostimulating at 20Hz, 10ms pw for 200ms every 20s. The progressive increased NE release from higher tonic stimulation frequencies was associated with a progressive decreased release from phasic stimulations (Fig 3A). This inverse relationship suggests that both transmission modes draw from the same NE pools. Additionally, the decreased phasic response is not due to α2-mediated inhibition of release, since ATI pre-treatment did not prevent this decrease (Fig 3B). In a complementary experiment, we further examined simulated phasic NE release during changes in tonic activity from acute stressors. During a 10min tail suspension, for instance, tonic NE release remained continually elevated, resulting in a significant decrease in phasic NE release as compared to baseline (Fig 1C). However, during restraint stress, NE release was only transiently elevated, and the simulated phasic NE release during restraint did not significantly differ from before (SFig 2C). We also stressed the animals with repeated footshocks to elicit strong phasic NE release as shown in Fig 3D. After the initial footshock, the basal NE release increased and remained elevated for the duration of the session. The phasic NE release from repeated footshocks, on the other hand, diminished over the course of the session, indicating that increased NE release from tonic activity limits the release from phasic activity. This decreased phasic NE release was not due to decreasing LCNE activity over the session (Fig 3D), nor due α2-mediated inhibition of LCNE activity or NE release (SFig 2D,E). Together, these experiments demonstrate that phasic NE release can be directly influenced by the extent of tonic NE release depending on stressor.
Figure 3. Phasic norepinephrine release constrained by tonic activity.
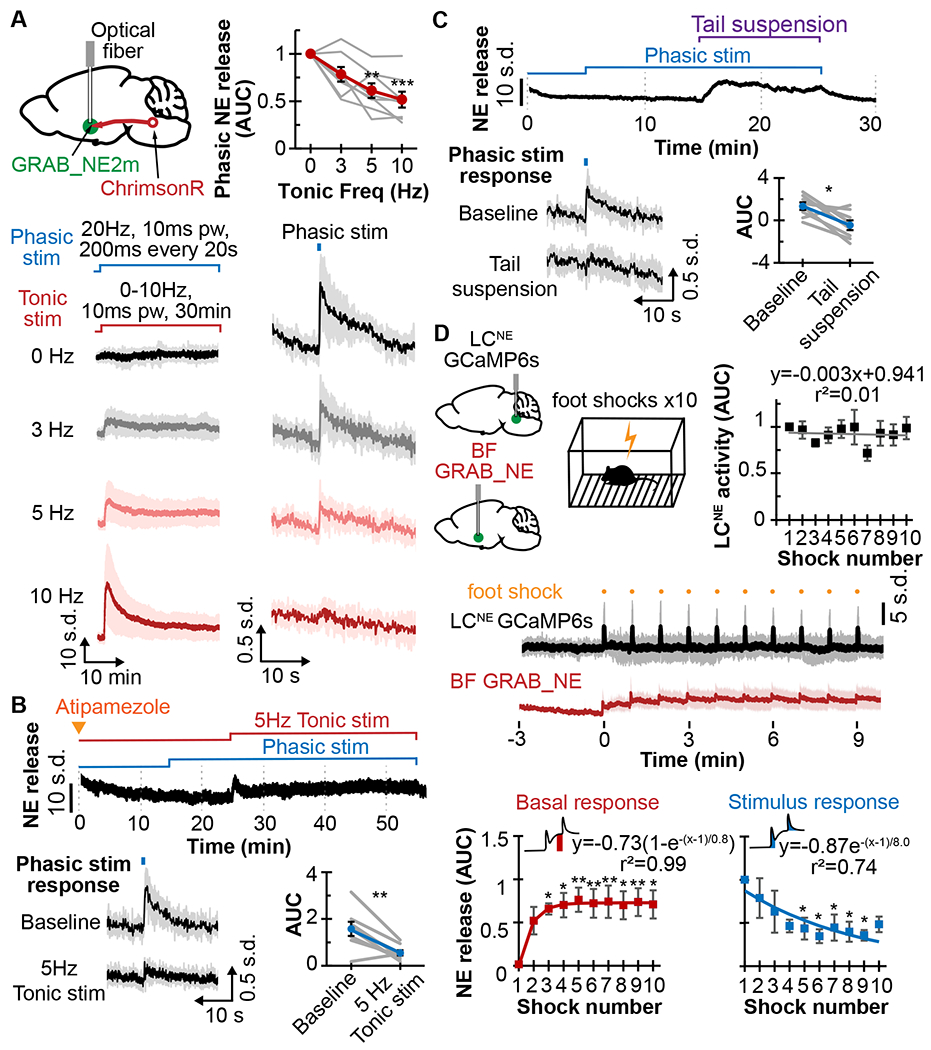
A. Top left, schematic shows ChrimsonR expressed in LCNE, GRAB_NE2m in BF, and an implanted optical fiber in BF. Bottom, traces of NE release for 30min of 0, 3, 5, and 10Hz tonic photostimulation (left) with 20Hz, 10ms pw, 200ms phasic stimulation every 20s (right). Top right, plot shows the relation between AUC of phasic NE release and tonic stimulation frequency (N=8).
B. Top, trace shows NE release in response to phasic photostimulations (20Hz, 10ms pw for 200ms every 20s) after 1mg/kg atipamezole treatment, with or without 5Hz tonic photostimulation. Bottom left, averaged traces are shown in response to phasic stimulation at baseline and during 5Hz tonic stimulation. Bottom right, corresponding AUC of phasic NE release is shown (N=8).
C. Top, trace shows NE release in response to 10min tail suspension with 20Hz, 10ms pw, 200ms phasic stimulation. Bottom left, averaged traces are shown in response to phasic stimulation at baseline and during tail suspension. Bottom right, corresponding AUC of phasic NE release is shown (N=8).
D. Top left, schematic shows GCaMP6s in LCNE, GRAB_NE in BF, and mouse experiencing 10 footshocks. Middle, average traces are shown of LCNE activity (black) and GRAB_NE in the BF (red) in response to footshocks. Top right, plot shows the AUC of LCNE activity in response to footshocks (N=7). Bottom, plots show the basal changes to NE release (left, red) and AUC of NE release (right, blue) in response to footshocks (N=8), along with exponential fitting curves and equations.
*p<0.05, **p<0.01, ***p<0.001. Graphs: mean±SEM; photometry traces: mean±std.
Next, we asked whether DA, another catecholamine, has similar constraints on the release and modes of transmission (tonic ~4-5Hz, phasic ~20Hz, <200ms)13,14 as observed in the LCNE projections. ChrimsonR was expressed in DA neurons in the VTA of DAT-cre mice; a fluorescent DA sensor GRAB_DA2m was expressed in the nucleus accumbens core (NAc), with an optical fiber placed there (SFig 3A). When repeated axonal photostimulations in unanesthetized mice were applied for 1min at 10Hz, 50ms pw, DA release was robustly elicited with S0, but subsequently decreased with S1 (72±7%) and S2 (69±6%). Increasing stimulation frequency to 20Hz, 10ms pw, showed similar decrease in S1 (77±6%) and S2 (75±4%). Photostimulating for 30min at 0, 5, and 10Hz 5ms pw (SFig 3B) also produced increased DA release with increased stimulation frequency, without significant decay over the course of stimulation. DA release using 10ms pw instead as in the LCNE stimulation did not differ significantly from using 5ms pw at 10Hz (SFig 3C). Furthermore, when we simulated phasic stimulations (20Hz 5ms pw for 200ms every 20s) during various tonic stimulation frequencies, we found that phasic DA release decreased as tonic frequencies increased (SFig 3D), similar to the inverse relation observed for NE release. These experiments also show that although NAc DA depletes at the high tonic activity, the DA pools replete more rapidly than the NE pools we examined. Taken together, these results demonstrate an optimal activity window for catecholamine signaling.
Discussion
In this study, we used the latest catecholamine biosensors to unveil two distinct pools of the respective NE and DA in vivo based on fast and slow repletion kinetics (Fig 1C). The fast-repleting pool constitutes ~25-50% of NE transmission in the brain regions examined from our axonal photoactivation, but constitutes a much larger proportion, ~70-75%, of DA transmission. For NE, the slow repletion takes place on the order of tens of minutes, suggesting that these different pools may translate to differing time scales on which these neuromodulators can act to regulate behavior.
Interestingly, the NE fast repleting pool strongly depends on NET (Fig 1D), shown previously to colocalize with Rab11, a marker of recycling endosomes28, suggesting a potential source for this NE pool. Flowever, it is important to consider several other possibilities for these different pools. NE and DA are reported to reside in both small synaptic vesicles and large dense core vesicles29–31, and the differing molecular compositions of these vesicles that are thought to explain exocytic differences32, may explain endocytic differences as well in repletion. These pools may also differ in their synaptic localization as readily releasable versus reserve pools33–35. They may also represent differing release from different axons, as seen before with NE release36, or represent (synaptic) point-to-point versus (non-synaptic) volumetric transmission37,38. Thus, the molecular identities of these pools remain to be determined. NE depletion from the same stimulus also differed depending on the brain region, likely due to variations in NET and α2-AR levels as observed across LCNE subpopulations from single-nucleus RNA sequencing24, and may explain the non-homogenous effect that increased LCNE activity has across the brain39. Previous modeling of striatal DA release also showed three short-term processes regulating release40, suggesting possible additional regulations of catecholamine pools. Moreover, given increased evidence of NE and DA co-release from the LC41–43, together with our observation of more pronounced NE depletion in BF and Thal (Fig 1E) as compared to DA depletion in NAc (SFig 3B), it would be important to determine whether co-released NE depletes more rapidly than DA within the same brain regions.
Furthermore, our observation that NE and DA gradually exhaust their release pools at higher stimulation frequencies, mirrored by a gradual decrease in postsynaptic calcium, provides an overdue plausible mechanistic explanation for the inverted-U relation. Specifically, as phasic activity of LCNE is associated with focused task performance8, our finding that higher tonic NE release from increased tonic activity causes a proportional reduction in phasic-mediated NE release, provides a natural explanation for the decreased behavioral performance at higher LCNE activity. Interestingly, tail suspension and forced swim, two highly stressful, acute interventions known to increase LCNE firing34 did not deplete NE, but exhibited instead a gradual decrease in postsynaptic calcium following an acute rise, suggesting that acute stressors do not exhaust brain NE, likely due to negative feedback or asynchronous release kinetics. The postsynaptic calcium response, on the other hand, diminishes in minutes, likely due to desensitization of α1-ARs44,45, though downstream signaling may still persist46. The temporal pattern of β-AR-mediated signaling, however, remains to be explored. Thus, postsynaptic AR-desensitization appears to be the predominant mechanism in acute stress, and catecholamine depletion may play a more important role in prolonged, elevated neuronal firing.
Moreover, we show that the tonic and phasic LC and DA firing, as two modes of transmission, exhibit an inverse relationship (Fig 3A, SFig 3D), suggesting they draw from the same respective stores. Specifically, in situations where LC tonic activity is high (e.g. acutely stressful events), the NE signal-to-noise from phasic firing is greatly diminished, and thus, contribute to increased exploratory behaviors and decreased task performance8,47. NE levels from prolonged, elevated LC activity from chronic stress48 remains to be explored, and may have implications in psychiatric disorders and treatments. Additionally, the study corroborates the hypothesized impaired phasic activity from increased tonic activity that may help explain neurodegeneration in Alzheimer’s and Parkinson’s diseases49, especially as LC likely modulates brain’s waste clearance50,51.
Limitations of the Study
There are several study limitations to consider. First, although photostimulation was performed at axonal terminals to specify LC projections, the response at terminals may differ from soma-generated action potentials. Second, tonic photostimulation generates synchronized release, whereas tonic LCNE activity is asynchronous, which may buffer against catecholamine depletion. Third, we used ChrimsonR for photostimulation, an optimized version of the red-shifted excitatory opsin Chrimson that has not been tested for opsin fatigue during prolonged stimulations. However, unlike Chrimson, its faster recovery and its reported ability to support 40Hz photostimulation52 suggest that ChrimsonR likely does not suffer from the same degree of opsin fatigue as Chrimson.
In summary, this study demonstrates in vivo constraints on catecholamine signaling at the higher end of neuronal activity, showing axonal catecholamine depletion, postsynaptic desensitization, and overall decreased signal-to-noise in the phasic mode of transmission. These constraints introduce a form of non-linearity in neuromodulation occurring at level of synaptic transmission that may explain the upper end of the inverted-U relationships between catecholaminergic neural activity and cognitive performance7,8,11, and provide insights into catecholamine-associated psychiatric disorders48,53–56 and neurodegenerative diseases57,58.
STAR Methods
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Michael Bruchas (mbruchas@uw.edu).
Materials availability
There are restrictions to the availability of the novel genetically encoded norepinephrine sensors, GRABNE2m and GRABNE2h, due to Material Transfer Agreement.
Data and code availability
All photometry data reported in this paper will be shared by the lead contact upon reasonable request.
All original code is available in the paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and subject details
Mice.
All animal experiments were conducted in accordance with the guidelines and regulation of the National Institutes of Health and the University of Washington under IACUC protocol 4452-01. Adult male and female Dbh-cre C57BL/6J mice (Dbhtm3.2(cre)Pjen, Jackson Laboratory #033951) were group housed on a 12:12 h reverse light-dark cycle and given food and water ad libitum.
Method details
Surgery
Surgeries were performed on mice ages >8 weeks under 1.5-2.0% isoflurane. Mice were injected in the LC (AP −5.45mm, ML +1.1 mm, DV −4.2 to −3.7mm DV to bregma) using a medial-facing beveled 1 μl Hamilton syringe. A blunt tip 1 μl Hamilton syringe was used for injections to the posterior basal forebrain (BF) (AP +0.1 mm, ML +1.3mm,. DV −5.3 to −5.15mm), the medial Thal (AP −1.7mm, ML +1.0mm at 15° angle, DV −3.95 to −3.8mm), the ventral tegmental area (VTA) (AP −3.4mm , ML +0.6mm, DV −4.7 to −4.4mm), and nucleus accumbens core (NAc) (AP +1.4mm, ML +1.5mm at 10° angle, DV −4.1 to −3.8mm). Respective 400 μm optical fibers (Doric Inc., MFC_400/430-0.48_MF2.5_FLT) were placed along the same AP and ML coordinates except DV is −3.8mm for LC, −5.2mm for BF, −3.8mm for Thal, −3.85mm for NAc and secured using Metabond (Parkell #S380). Mice recovered for at least 5 weeks before experiments to allow optimal viral expression.
Viruses
| AAV5-EF1α-DIO-EYFP | Addgene #20756 |
| AAV5-Syn-FLEX-rc[ChrimsonR-tdTomato] | Addgene #62723 |
| AAV-DJ-EF1α-DIO-GCaMP6s | Stanford Gene Vector and Viral Core |
| AAV-DJ-EF1α-DIO-GCaMP6f | Stanford Gene Vector and Viral Core |
| AAV-hSyn-GRAB_NE2m | Yulong Li (Peking University) |
| AAV-hSyn-GRAB_NE2h | Yulong Li (Peking University) |
| AAV9-hSyn-GRAB_DA2m | Addgene #140553 |
Histology
Mice were euthanized with sodium pentobarbital and transcardially perfused with 4% paraformaldehyde (PFA), post-fixed for 1-3 days in 4% PFA, and then cryo-protected in 30% sucrose. Brain sections (30-100μm) were collected and kept in 0.1M phosphate buffer at 4°C. Then, the sections were mounted with VectaShield Vibrance Hardset mounting medium (Vector Laboratories) with DAPI, and coverslips placed. Images were acquired on an epifluorescent microscope (Leica DFC700T).
Fiber photometry and photostimulation
Fiber photometry recordings of the genetically encoded fluorescent sensors were performed as previously described59. Briefly, the implanted fiber was connected to the patch cable via a ferrule sleeve. A real-time processor (TDT RZ5P, sampling rate of 1017.25Hz) recorded the filtered emission (Doric FMC4 filter cube) from the fluorescent sensor at upon excitation at 470nm and 405nm using the accompanied TDT Synapse software. For terminal stimulations, the same photometry fiber served as conduit for 625nm LED photostimulation adjusted for light intensity ~2.5mW/mm2 at the optical fiber tip. All photostimulation experiments were done in unanesthetized, freely behaving mice.
Photometry recordings were analyzed using custom python script. Baseline drift from photobleaching artifact was corrected with an exponential decay curve. For GRAB_NE and GCaMP signals, dF/F was calculated as the linear least-squares fit of 405nm signals subtracted from the 470nm signals. The 405nm signals were not used in GRAB_DA as it does not appear to serve as an appropriate isobestic channel. Z-scores were calculated using the mean and standard deviation of a 5-10 min baseline before photostimulation.
Drugs
All drugs were administered intraperitoneally (i.p.). These include reserpine (Sigma 83580) dissolved in 0.2% glacial acetic acid to 0.5 mg/ml; nepicastat (Sigma-Aldrich SML0940) suspended as 5mg/ml in saline mixed with 1.5% DMSO, 1.5% Kolliphor; dexmedetomidine (100mcg/ml Piramal Critical Care, PSLAB-020872-00) diluted to 5mcg/ml in saline, atipazemole made to 0.1mg/ml in saline, atomoxetine hycrochloride (thermoscientific, Cat 467680010) make to 0.3mg/ml in saline, propranolol hydrochloride (Tocris Bioscience Cat. 0624) made to 1mg/ml in sterile water, prazosin hydrochloride (Tocris Bioscience Cat. 0623) made to 1mg/ml in distilled water. Drugs were administered at least 15-20min prior to experiments unless otherwise noted.
Stress behavioral assays
Tail suspension
Mice were suspended by an experimenter holding the tail for 10min ~10in above the floor.
Forced swim
Mice were transferred from a holding chamber into a 5L cylindrical container containing 4L of water (~24°C) and allowed to swim up to 15min. Mice were removed from water if there was evidence of drowning. Mice were subsequently dried on a heating pad for recovery.
Physical restraint
Mice were placed in a conical tube with a strip cut open on top to allow photometry patch cable attachment and the front tapered end cut open to allow nose access. The other end has a lid with a center hole for the tail.
Foot shock
Mice were placed in a Med Associates Fear Conditioning Chamber (NIR-022MD, 29.53cm L x 23.5cm W x 20.96cm H) with a conductive grid floor, a soundproof barrier, and an infrared lighting. They were habituated to the box for 3min before experiencing 1s 0.5mA shocks every min for 10 minutes.
Quantification and statistical analysis
All summary data are expressed as mean±SEM. Statistical significance was denoted as *p<0.05, **p<0.01, ***p<0.001, ****p<0.001 as determined by Student’s t-test, one-way or two-way repeated measure analysis of variance (ANOVA), followed by Dunnett’s post-hoc test. Statistical tests were performed in Excel and in R, and a summary of the statistical tests performed and p-values are shown in Supplemental Table 1
Supplementary Material
Supplemental Table 1
Statistical summary is shown for experiments in the main and supplemental figures.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| AAV5-EF1α-DIO-EYFP | Addgene | #20756 |
| AAV5-Syn-FLEX-rc[ChrimsonR-tdTomato] | Addgene | #62723 |
| AAV-DJ-EF1α-DIO-GCaMP6s | Stanford Gene Vector and Viral Core | N/A |
| AAV-DJ-EF1α-DIO-GCaMP6f | Stanford Gene Vector and Viral Core | N/A |
| AAV-hSyn-GRAB_NE2m | Yulong Li (Peking University) | N/A |
| AAV-hSyn-GRAB_NE2h | Yulong Li (Peking University) | N/A |
| AAV9-hSyn-GRAB_DA2m | Addgene | #140553 |
| Chemicals, peptides, and recombinant proteins | ||
| VECTASHIELD Hardset Antifade Mounting Medium with DAPI | Vector Laboratories | CAT#H-1800 |
| Reserpine | Sigma | 83580 |
| Nepicastat | Sigma-Aldrich | SML0940 |
| Dexmedetomidine | Piramal Critical Care | PSLAB-020872-00 |
| Atipamezole | Sigma-Aldrich | A9611 |
| Atomoxetine hydrochloride | Thermoscientifc | 467680010 |
| Propranolol hydrochloride | Tocris Bioscience | 0624 |
| Prazosin hydrochloride | Tocris Bioscience | 0623 |
| Experimental models: Organisms/strains | ||
| C57BL/6J | Jackson Laboratory | 000664 |
| Dbh-cre | Jackson Labarotory | 033951 |
| Software and algorithms | ||
| FIJI/ImageJ | NIH | https://fiji.sc/ |
| Python 3.0 | Python Software Foundation |
https://www.python.org/ |
| Illustrator CS8 | Adobe | https://www.adobe.com/products/illustrator.html |
| Excel | Microsoft | N/A |
| R | GNU project | https://www.r-project.org/ |
| Other | ||
| RZ5P fiber photometry system | Tucker-Davis Technologies | N/A |
| 5mm or 6mm 200μm diameter optical fibers | Doric | N/A |
Acknowledgements
We thank the Bruchas lab and UW NAPE Center colleagues for their insights and manuscripts discussions; and our funding: FAER MRTG (L.L.), K99DA053336 (L.L.), Mary Gates Research Scholarship (E.M.L.), National Natural Science Foundation of China grants 31925017 and 31871087 (Y.L.), and R01MH112355 (M.R.B).
Footnotes
Declaration of interests
The authors declare no competing interest.
References
- 1.Aston-Jones G & Bloom FE Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1, 876–86 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter ME et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13, 1526–33 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eban-Rothschild A, Rothschild G, Giardino WJ, Jones JR & de Lecea L VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat. Neurosci 19, 1356–1366 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aston-Jones G, Rajkowski J, Kubiak P & Alexinsky T Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. Off. J. Soc. Neurosci 14, 4467–4480 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge CW & Waterhouse BD The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42, 33–84 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Noudoost B & Moore T Control of visual cortical signals by prefrontal dopamine. Nature 474, 372–375 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayraghavan S, Wang M, Birnbaum SG, Williams GV & Arnsten AFT Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci 10, 376–384 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Aston-Jones G & Cohen JD An integrative theory of locus coeruleusnorepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Sara SJ The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci 10, 211–223 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Engelhard B et al. Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570, 509–513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JY et al. A Comparison of Dopaminergic and Cholinergic Populations Reveals Unique Contributions of VTA Dopamine Neurons to Short-Term Memory. Cell Rep. 33, 108492 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devilbiss DM & Waterhouse BD Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J. Neurophysiol 105, 69–87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinelli M & McCutcheon JE Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 282, 176–197 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz W Predictive Reward Signal of Dopamine Neurons. J. Neurophysiol 80, 1–27 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Feng J et al. A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron 102, 745–761 e8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patriarchi T et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun F et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat. Methods 17, 1156–1166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leopold AV, Shcherbakova DM & Verkhusha VV Fluorescent Biosensors for Neurotransmission and Neuromodulation: Engineering and Applications. Front. Cell. Neurosci 13, 474 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuels ER & Szabadi E Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part II: physiological and pharmacological manipulations and pathological alterations of locus coeruleus activity in humans. Curr Neuropharmacol 6, 254–85 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulaman BA, Wang S, Tyan J & Eban-Rothschild A Neuro-orchestration of sleep and wakefulness. Nat. Neurosci 26, 196–212 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Vazey EM, Moorman DE & Aston-Jones G Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc. Natl. Acad. Sci 115, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentino R & Foote S Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J. Neurosci 8, 1016–1025 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCall JG et al. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron 87, 605–20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luskin AT et al. A diverse network of pericoerulear neurons control arousal states. 10.1101/2022.06.30.498327 (2022) doi: 10.1101/2022.06.30.498327. [DOI] [Google Scholar]
- 25.Brodde ΟE & Michel MC Adrenergic and muscarinic receptors in the human heart. Pharmacol. Rev 51, 651–690 (1999). [PubMed] [Google Scholar]
- 26.Kelly E, Bailey CP & Henderson G Agonist-selective mechanisms of GPCR desensitization. Br. J. Pharmacol 153 Suppl 1, S379–388 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Commons KG, Cholanians AB, Babb JA & Ehlinger DG The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem. Neurosci 8, 955–960 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vuorenpää A et al. Differential Internalization Rates and Postendocytic Sorting of the Norepinephrine and Dopamine Transporters Are Controlled by Structural Elements in the N Termini. J. Biol. Chem 291, 5634–5651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thureson-Klein A Exocytosis from large and small dense cored vesicles in noradrenergic nerve terminals. Neuroscience 10, 245–259 (1983). [DOI] [PubMed] [Google Scholar]
- 30.Nirenberg MJ, Liu Y, Peter D, Edwards RH & Pickel VM The vesicular monoamine transporter 2 is present in small synaptic vesicles and preferentially localizes to large dense core vesicles in rat solitary tract nuclei. Proc. Natl. Acad. Sci 92, 8773–8777 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nirenberg MJ, Chan J, Liu Y, Edwards RH & Pickel VM Ultrastructural Localization of the Vesicular Monoamine Transporter-2 in Midbrain Dopaminergic Neurons: Potential Sites for Somatodendritic Storage and Release of Dopamine. J. Neurosci 16, 4135–4145 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards RH Neurotransmitter release: Variations on a theme. Curr. Biol 8, R883–R885 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Rizzoli S. o. & Betz WJ The structural organization of the readily releasable pool of synaptic vesicles. Science 303, 2037–9 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Kaeser PS & Regehr WG The readily releasable pool of synaptic vesicles. Curr. Opin. Neurobiol 43, 63–70 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patzke C et al. Neuromodulator Signaling Bidirectionally Controls Vesicle Numbers in Human Synapses. Cell 179, 498–513.e22 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunn M et al. Designing a norepinephrine optical tracer for imaging individual noradrenergic synapses and their activity in vivo. Nat. Commun 9, 2838 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuxe K, Agnati LF, Marcoli M & Borroto-Escuela DO Volume Transmission in Central Dopamine and Noradrenaline Neurons and Its Astroglial Targets. Neurochem. Res 40, 2600–2614 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Liu C, Goel P & Kaeser PS Spatial and temporal scales of dopamine transmission. Nat. Rev. Neurosci 22, 345–358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zerbi V et al. Rapid Reconfiguration of the Functional Connectome after Chemogenetic Locus Coeruleus Activation. Neuron 103, 702–718.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Montague PR et al. Dynamic Gain Control of Dopamine Delivery in Freely Moving Animals. J. Neurosci 24, 1754–1759 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devoto P, Flore G, Pani L & Gessa GL Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatry 6, 657–664 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D & Kandel ER Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci 113, 14835–14840 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi T et al. Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leeb-Lundberg LM, Cotecchia S, DeBlasi A, Caron MG & Lefkowitz RJ Regulation of adrenergic receptor function by phosphorylation. I. Agonist-promoted desensitization and phosphorylation of alpha 1-adrenergic receptors coupled to inositol phospholipid metabolism in DDT1 MF-2 smooth muscle cells. J. Biol. Chem 262, 3098–3105 (1987). [PubMed] [Google Scholar]
- 45.Perez DM α1-Adrenergic Receptors in Neurotransmission, Synaptic Plasticity, and Cognition. Front. Pharmacol 11, 581098 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calebiro D, Nikolaev VO, Persani L & Lohse MJ Signaling by internalized G-protein-coupled receptors. Trends Pharmacol. Sci 31, 221–228 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Clayton EC, Rajkowski J, Cohen JD & Aston-Jones G Phasic activation of monkey locus ceruleus neurons by simple decisions in a forced-choice task. J. Neurosci. Off. J. Soc. Neurosci 24, 9914–9920 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross JA & Van Bockstaele EJ The Locus Coeruleus-Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 11, 601519 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janitzky K Impaired Phasic Discharge of Locus Coeruleus Neurons Based on Persistent High Tonic Discharge—A New Hypothesis With Potential Implications for Neurodegenerative Diseases. Front. Neurol 11, 371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie L et al. Sleep drives metabolite clearance from the adult brain. Science 342, 373–377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mather M & Harley CW The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci 20, 214–226 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klapoetke NC et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davis MT, Holmes SE, Pietrzak RH & Esterlis I Neurobiology of Chronic Stress-Related Psychiatric Disorders: Evidence from Molecular Imaging Studies. Chronic Stress 1, 247054701771091 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briley M & Chantal M The importance of norepinephrine in depression. Neuropsychiatr. Dis. Treat 9 (2011) doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koob GF A Role for Brain Stress Systems in Addiction. Neuron 59, 11–34 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wise RA & Robbie MA Dopamine and Addiction. Annu. Rev. Psychol 71, 79–106 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Holland N, Robbins TW & Rowe JB The role of noradrenaline in cognition and cognitive disorders. Brain J. Neurol 144, 2243–2256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rangel-Barajas C, Coronel I & Florán B Dopamine Receptors and Neurodegeneration. Aging Dis. 6, 349–368 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parker KE et al. A Paranigral VTA Nociceptin Circuit that Constrains Motivation for Reward. Cell 178, 653–671 e19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1
Statistical summary is shown for experiments in the main and supplemental figures.
Data Availability Statement
All photometry data reported in this paper will be shared by the lead contact upon reasonable request.
All original code is available in the paper’s supplemental information.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


