Abstract
Background
Preservation of renal function is an important goal in renal cell carcinoma-related surgery. Although several case-dependent techniques for renal pedicle clamping and hemostasis have been used, their effects on long-term renal function are controversial.
Methods
The clinical records of 114 patients who underwent off-clamp non-renorrhaphy open partial nephrectomy at our hospital were retrospectively reviewed. Perioperative estimated glomerular filtration rate (eGFR) preservation was calculated, and predictors of eGFR decline 12 months post-surgery and overtime deterioration of renal function were identified using a multivariate regression analysis.
Results
The median patient age was 65 years, and the median tumor size was 27 mm. The mean eGFR preservation at 1, 3, and 12 months post-surgery were 90.1%, 89.0%, and 86.9%, respectively. eGFR decline at 1 and 3 months were associated with poor eGFR preservation at 12 months with the odds ratio (95% confidence interval (CI)) of 1.97 and 3.157, respectively. Multivariate regression analyses revealed that tumor size was an independent predictor of eGFR decline at 12 months. Among 65 patients with eGFR preservation over 90% at 1 month post-surgery, eGFR value of 28 patients deteriorated below 90% at 12 months post-surgery compared with preoperative eGFR. Tumor size and eGFR preservation at 1 month were independent predictors of long-term renal function deterioration.
Conclusion
Tumor size predicted eGFR decline 12 months post-surgery. Only a mild decline in eGFR was observed between 3 and 12 months after open partial nephrectomy. Tumor size and eGFR preservation at 1 month predicted the deterioration of renal function over time.
Introduction
As the preservation of renal function is one of the important goals in surgery for RCC, partial nephrectomy (PN) has become the gold standard for renal tumors less than 7 cm in diameter and the means of hemostasis after tumor resection vary among institutes [1–3]. Although cortical renorrhaphy and soft coagulation are the most popular methods of hemostasis, the effect of each method on long-term renal function remains a subject of research [4, 5].
Introduced in PN, renorrhaphy aims to achieve hemostasis and close the urinary collecting system, thereby minimize postoperative complications. However, the pros and cons of renorrhaphy in terms of preserving renal parenchima have been widely debated [4, 6–8]. Renorrhaphy carries the risk of damaging adjacent renal vessels, potentially leading to reduced functioning renal parenchyma after surgery. Additionally, it may contribute to the development of renal artery aneurysms [9].
Techniques such as selective or super-selective clamping at the renal hilum can reduce renal parenchymal ischemia and better preservation of postoperative renal function [10, 11]. However, parenchymal ischemia and reperfusion resulting from hilar clamping could lead to acute kidney injury through production of radical oxygen species. Omitting both renal hilar clamping and renorrhaphy has been reported as an effective approach for preserving renal function during partial nephrectomy in a relatively short period [2, 12–15].
We have been performing off-clamp and non-renorrhaphy open partial nephrectomies for years. While most partial nephrectomies are currently performed laparoscopically or robotically, valuable lessons from open partial nephrectomy can be extrapolated to robotic procedures. In this study, we assessed renal function at 1 year after open off-clamp and non-renorrhaphy partial nephrectomies and investigated the predictors of renal function decline over time.
Materials and methods
Patients
Among 220 patients with renal tumors who underwent off-clamp non-renorrhaphy open partial nephrectomy at our institute between 2013 and 2020, those without follow-up until 12 months after surgery were excluded. Finally, the clinical records of the 114 patients were retrospectively analyzed. The data were accessed for research purpose on 1st December, 2022. The authors did not have access to information that could identify individual participants during or after data collection.
Assessment of renal tumor complexity
The complexity of renal tumors was analyzed according to the R-E-N-A-L nephrometry scoring system. Briefly, (R)adius (tumor size as maximal diameter), (E)xophytic and endophytic properties, (N)earness of the tumor to the collecting system or sinus, and (L)ocation relative to the upper and lower polar lines were scored on a 1-, 2-, or 3-point scale. The RENAL score represents the sum of R, E, N, and L scores [16].
Surgical techniques
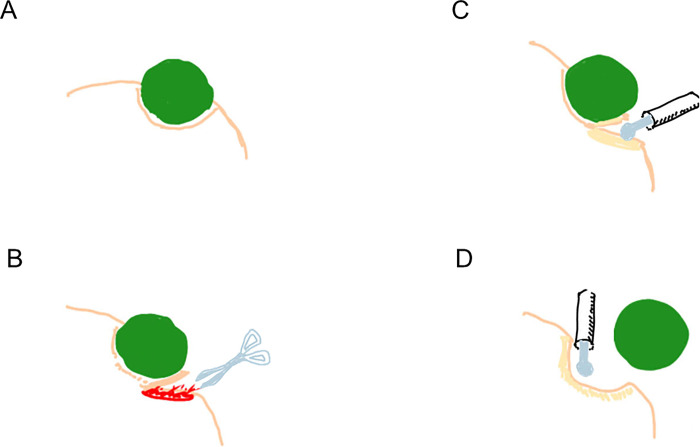
All patients underwent retroperitoneal open partial nephrectomy as described previously [2]. Briefly, a partial nephrectomy was performed by blunt separation and sharp cutting, followed by hemostasis with monopolar SOFT COAG (VIO300D, ERBE, Germany). To minimize the blood loss, tumor resection was performed in millimeters. (Fig 1) The renal pedicle was not secured, and renal pedicle clamping and cortical renorrhaphy were omitted. The resection beds were sutured with 4–0 VICRYL® only when the collecting system was opened. Urine leakage was ruled out by intravenous injection of an indigo carmine solution. TachoSil® was placed on the resection surface to ensure hemostasis.
Fig 1. Surgical technique of partial nephrectomy.
(A) Shema of a renal tumor. (B) Blunt separation and sharp cutting of the renal parenchyma with scissors. (C) Hemostasis using soft coagulation system. (D) Thorough hemostasis of the tumor bed with soft coagulation system.
Assessment of renal function and perioperative reduction in renal function
The estimated glomerular filtration rate (eGFR) was calculated using the equation 186 × (Creatinine/88.4)-1.154 × (age)-0.203 × (0.742, if female). The perioperative eGFR preservation at 5 days, 1 month, 3 months, and 12 months after surgery was calculated as postoperative eGFR/preoperative eGFR × 100 (%). According to the pentafecta definition after partial nephrectomy, we set the eGFR preservation threshold to 90% [3].
Ethics approval and consent to participate
This study was approved by the Ethics Committee of NTT Medical Center, Tokyo (ID: 21–3). Informed consent was obtained in the form of opt-out on the website. Those who were rejected were excluded. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013).
Statistical analysis
The chi-square test was used to analyze categorical variables. Univariate and multivariate regression analyses were performed to identify the predictors of postoperative eGFR preservation 12 months after surgery and overtime eGFR deterioration. The statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 24 (IBM co. ltd., Tokyo, Japan).
Results
The patient characteristics are described in Table 1. The median age and tumor size were 65 years (interquartile range (IQR), 54–69) and 27 mm (IQR, 19.5–40.5), respectively. Among the tumors, 35 tumors (30.7%) were >50% exophytic, 49 tumors (43.0%) were <50% exophytic, and 30 tumors (26.3%) were entirely endophytic. The median RENAL nephrometry score was 7 (IQR, 6–8). The surgical results are presented in Table 2. The median operative time and estimated blood loss were 124 min (IQR, 101–143) and 160 mL (IQR, 68–355), respectively. No major complications (Clavien–Dindo classification grade ≥ 3) occurred perioperatively. The mean eGFR value gradually decreased from 94.3% (standard deviation (SD), 14.0) at 5 days to 86.9% (SD, 12.9) at 12 months after operation.
Table 1. Patient and tumor characteristics.
| No. patients | 114 |
| Sex (%) | |
| Male | 80 (70.2) |
| Female | 34 (29.8) |
| Median age (IQR) | 65 (54–69) |
| Median BMI (IQR) | 23. (21–26) |
| Median tumor size, mm (IQR) | 27.0 (19.5–40.5) |
| Median RENAL nephrometry score (IQR) | 7 (6–8) |
| Exophytic/Endophytic properties (%) | |
| > 50% exophytic | 35 (30.7) |
| < 50% exophytic | 49 (43.0) |
| Entirely endophytic | 30 (26.3) |
| Location relative to the polar lines (%) | |
| Entirely above the upper or below the lower polar line | 39 (34.2) |
| Lesion crosses polar line | 56 (49.1) |
| > 50% of mass is across polar line or mass crosses the axial renal midline or mass is entirely between the polar lines | 19 (16.7) |
| Comorbidity, n (%) | |
| Hypertension | 43 (37.7) |
| Diabetes mellitus | 16 (14.0) |
| Dyslipidemia | 12 (10.5) |
Table 2. Surgical results and complications.
| Median operative time, min (IQR) | 124 (101–143) |
| Median volume of estimated blood loss, mL (IQR) | 160 (68.8 − 355) |
| No. additional resections | 0 |
| No. conversion to nephrectomy | 0 |
| Intraoperative blood transfusion | 0 |
| Postoperative blood transfusion | 0 |
| Pathology (%) | |
| Clear cell RCC | 79 (69.3) |
| Papillary RCC | 11 (9.6) |
| Chromophobe RCC | 9 (7.9) |
| Angiomyolipoma | 11 (9.6) |
| Others | 4 (3.5) |
| Mean eGFR preservation, % (SD) | |
| Five days after surgery | 94.3 (14.0) |
| One month after surgery | 90.1 (11.5) |
| Three months after surgery | 89.0 (13.7) |
| Twelve months after surgery | 86.9 (12.9) |
eGFR estimated glomerular filtration ratio, RCC renal cell carcinoma, IQR interquartile range, SD standard deviation
The tumor size and estimated blood loss were identified as predictors of eGFR preservation 12 months postoperatively. Multivariate analyses revealed only tumor size was an independent predictor of eGFR preservation at 12 months, with unstandardized regression coefficient B (95% confidence interval (CI)) of -0.183 (-0.316, -0.005) (P = 0007). (Table 3)
Table 3. Predictors of perioperative decline in eGFR at 12 months after surgery.
Univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Unstandardized regression coefficient B (95% CI) | Standardized regression coefficient β | p -value | Unstandardized regression coefficient B (95% CI) | Standardized regression coefficient β | p -value | |
| Age† | -0.123 (-0.323, 0.077) | -0.114 | 0.225 | -0.174 (-0.364, 0.016) | -0.162 | 0.072 |
| BMI | -0.157 (-0.753, 0.439) | -0.05 | 0.602 | |||
| Tumour size† | -0.213 (-0.335, -0.091) | -0.31 | 0.01* | -0.183 (-0.316, -0.05) | -0.267 | 0.007* |
| Preoperative eGFR | -0.070 (-0.217, 0.077) | -0.089 | 0.345 | |||
| R | -6.827 (-11.068, -2.585) | -0.289 | 0.002* | |||
| E | 1.761 (-1.405, 4.927) | 0.104 | 0.273 | |||
| N | -1.635 (-4.398, 1.128) | -0.11 | 0.224 | |||
| L | -2.073 (-5.522, 1.376) | -0.112 | 0.236 | |||
| RENAL score | -1.113 (-2.511, -0.284) | -0.148 | 0.117 | |||
| eBlood loss† | -0.014 (-0.023, -0.005) | -0.269 | 0.004* | -0.009 (-0.018, -0.001) | -0.165 | 0.091 |
| HTN | -0.053 (-5.004, 4.898) | -0.002 | 0.983 | |||
| DM | -4.051 (-10.917, 2.816) | -0.11 | 0.245 | |||
| DL | -2.122 (-9.931, 5.687) | -0.051 | 0.591 | |||
eGFR estimated glomerular filtration rate, BMI body mass index, eBlood loss estimated blood loss, R Radius, E exophytic and endophytic properties, N Nearness of the tumor to the collecting system or sinus, L location relative to the upper and lower polar lines, (R,E,N, and L were scored according to RENAL nephrometry scoring system), HTN hypertension, DM diabetes mellitus, DL dyslypidemia, CI confidence interval, † These factors were put in multivariate regression analysis, * p-value < 0.05 was considered statistically significant.
The eGFR preservation at 1 and 3 months was associated with poor eGFR preservation at 12 months. The calculated odds ratios (95%CI) were 1.97 (1.43, 2.71) and 3.157 (2.03, 4.90), respectively (Table 4). Among 65 patients with eGFR preservation over 90% at 1 month after surgery, the eGFR preservation of 28 patients deteriorated below 90% at 12 months after surgery. To further clarify the predictor of overtime eGFR deterioration from 1 month to 12 months, we performed univariate and multivariate analyses on patients with an eGFR preservation > 90% at one month after surgery. Tumor size and eGFR preservation at 1 month were the independent predictors of further deterioration in renal function, with unstandardized regression coefficients B (95% CI) of -0.189 (-0.378, -0.001) and 0.425 (0.096, 0.755), respectively (P-value, 0.048 and 0.012, respectively) (Table 5).
Table 4. The comparison of eGFR preservation ratio between 1, 3, and 12 months after operation.
| eGFR at 12 months ≥ 90% | eGFR at 12 months < 90% | OR | 95% CI | *p value | |
|---|---|---|---|---|---|
| eGFR at 1 month ≥ 90% | 36 | 28 | ref | ||
| eGFR at 1 month < 90% | 9 | 41 | 1.971 | 1.431–2.717 | < 0.001 |
| eGFR at 3 months ≥ 90% | 35 | 17 | ref | ||
| eGFR at 3 months < 90% | 10 | 52 | 3.157 | 2.031–4.908 | < 0.001 |
CI confidence interval, eGFR estimated glomerular filtration ratio, OR odds ratio, * pearson’s chi-square test
Table 5. Predictors of overtime decline in eGFR (from 1 month to 12 months after surgery).
Univariate and multivariate analysis.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Unstandardized regression coefficient B (95% CI) | Standardized regression coefficient β | p -value | Unstandardized regression coefficient B (95% CI) | Standardized regression coefficient β | p -value | |
| Age† | -0. 006 (-0.171, 0.184) | 0.009 | 0.945 | -0.053(-0.233, 0.127) | -0.076 | 0.558 |
| Tumour size† | -0.173 (-0.327, -0.02) | -0.276 | 0.027* | -0.189 (-0.378, -0.001) | -0.303 | 0.048* |
| Preoperative eGFR | 0.052 (-0.106, 0.209) | 0.083 | 0.514 | |||
| RENAL score | -0.793(-2.104, -0.518) | -0.152 | 0.231 | |||
| eBlood loss† | -0.005 (-0.016, -0.007) | -0.098 | 0.441 | 0.004 (-0.009, 0.017) | 0.085 | 0.547 |
| HTN | -0.053 (-5.004, 4.898) | -0.002 | 0.983 | |||
| eGFR preservation at one month† | 0.482 (0.155, 0.808) | 0.353 | 0.005* | 0.425 (0.096, 0.755) | 0.312 | 0.012* |
eGFR estimated glomerular filtration rate, BMI body mass index, eBlood loss estimated blood loss, R Radius, E exophytic and endophytic properties, N Nearness of the tumor to the collecting system or sinus, L location relative to the upper and lower polar lines, (R,E,N, and L were scored according to RENAL nephrometry scoring system), HTN hypertension, CI confidence interval, † These factors were put in multivariate regression analysis, * p-value < 0.05 was considered statistically significant.
Discussion
In our previous report, we examined perioperative renal function within 3 months after off-clamp, non-renorrhaphy open partial nephrectomy [12]. Notably, the renal function at 1 year in the current follow-up study remained nearly comparable to that at 3 months after surgery. Specifically, the eGFR decreased 2.4%, from 89.0% at 3 months to 86.9% at 12 months after surgery. Considering the spontaneous eGFR decline of 0.89–1.49 ml/min/1.73 m2/year associated with aging, the impact of surgery on the eGFR decline during this period appears to be mild [17–20]. Understandably, an eGFR preservation less than 90% at 1 month and 3 months was associated with eGFR preservation less than 90% at 12 months after surgery. Several groups reported predictive factors of decline in renal function after partial nephrectomy, including patient age, preoperative eGFR, tumor size and depth, warm ischemia time, and BMI [21–23]. In the current study, we also identified tumor size as a predictor for postoperative renal function decline.
The advantages of the off-clamp procedure for long-term renal function remain a topic of debate. Over a short period, no clear advantages of the off-clamp surgery were observed compared to on-clamp surgery in the robot-assisted setting [5, 24]. One meta-analysis indicated no difference in the eGFR preservation between both procedures within 6 months. However, it is essential to consider the selection bias as tumor size was significantly smaller in the off-clamp group [25]. Conversely, another meta-analysis demonstrated less decline in both short- and long-term (6 months) renal function in the off-clamp group [26]. Furthermore, off-clamp surgery was associated with a 7.3-fold decreased risk of developing stage ≥3b chronic kidney disease during a follow-up period of 2–8 years after surgery [27].
Regarding renorrhaphy, omitting cortical renorrhaphy led to eGFR preservation during the early postoperative period [7, 8, 28]. Renorrhaphy may damage the intraparenchymal vessels or cause renal artery pseudoaneurysms, both of which contribute to a reduction in vascularized renal volume. In a propensity score-matched analysis of open partial nephrectomy, renal artery pseudoaneurysms tended to be more frequent in the renorrhaphy group [9]. These findings underscore the importance of considering surgical techniques and their impact on long-term renal function when managing renal tumors.
Hemostasis using soft coagulation may also negatively impact renal function. In our previous study, the estimated blood loss served as a predictor for perioperative decline in renal function within the first three months after surgery. We hypothesized that intense use of soft coagulation to control bleeding might damage the renal parenchyma, leading to the loss of renal function [12]. Over the long term, estimated blood loss remained a predictor of renal function. However, tumor size and low eGFR preservation at 1 month emerged as notable independent predictors of renal function deterioration of renal function from 1 to 12 months. Although compensation by the contralateral kidney cannot be ignored one year after surgery, the broad use of soft coagulation may cause persistent damage to the renal parenchyma. Further studies, including assessment of divided kidney function, may clarify the effect of soft coagulation on the affected kidney.
The retrospective nature of this study and its relatively small number size are limitations of this study. In the current follow-up study, only a mild decline in eGFR was observed between three and 12 months after open partial nephrectomy. Tumor size and eGFR preservation at one month could be predictors of long-term renal function deterioration.
Supporting information
(XLSX)
Data Availability
The data underlying the results presented in the study are available from Supporting information.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Ficarra V, Rossanese M, Gnech M, Novara G, Mottrie A. Outcomes and limitations of laparoscopic and robotic partial nephrectomy. Curr Opin Urol. 2014;24(5):441–7. doi: 10.1097/MOU.0000000000000095 [DOI] [PubMed] [Google Scholar]
- 2.Nakamura M, Ambe Y, Teshima T, Shirakawa N, Inatsu H, Amakawa R, et al. Assessment of surgical outcomes of off-clamp open partial nephrectomy without renorrhaphy for ≥T1b renal tumours. Int J Clin Oncol. 2021. [DOI] [PubMed] [Google Scholar]
- 3.Zargar H, Allaf ME, Bhayani S, Stifelman M, Rogers C, Ball MW, et al. Trifecta and optimal perioperative outcomes of robotic and laparoscopic partial nephrectomy in surgical treatment of small renal masses: a multi-institutional study. BJU Int. 2015;116(3):407–14. doi: 10.1111/bju.12933 [DOI] [PubMed] [Google Scholar]
- 4.Alrishan Alzouebi I, Williams A, Thiagarjan NR, Kumar M. Omitting Cortical Renorrhaphy in Robot-Assisted Partial Nephrectomy: Is it Safe? A Single Center Large Case Series. J Endourol. 2020;34(8):840–6. doi: 10.1089/end.2020.0121 [DOI] [PubMed] [Google Scholar]
- 5.Anderson BG, Potretzke AM, Du K, Vetter JM, Bergeron K, Paradis AG, et al. Comparing Off-clamp and On-clamp Robot-assisted Partial Nephrectomy: A Prospective Randomized Trial. Urology. 2019;126:102–9. doi: 10.1016/j.urology.2018.11.053 [DOI] [PubMed] [Google Scholar]
- 6.Bahler CD, Cary KC, Garg S, DeRoo EM, Tabib CH, Kansal JK, et al. Differentiating reconstructive techniques in partial nephrectomy: a propensity score analysis. Can J Urol. 2015;22(3):7788–96. [PubMed] [Google Scholar]
- 7.Bahler CD, Sundaram CP. Effect of Renal Reconstruction on Renal Function After Partial Nephrectomy. J Endourol. 2016;30 Suppl 1:S37-41. [DOI] [PubMed] [Google Scholar]
- 8.Bertolo R, Campi R, Mir MC, Klatte T, Kriegmair MC, Salagierski M, et al. Systematic Review and Pooled Analysis of the Impact of Renorrhaphy Techniques on Renal Functional Outcome After Partial Nephrectomy. Eur Urol Oncol. 2019;2(5):572–5. doi: 10.1016/j.euo.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 9.Tachibana H, Takagi T, Kondo T, Ishida H, Tanabe K. Comparison of perioperative outcomes with or without renorrhaphy during open partial nephrectomy: A propensity score-matched analysis. Int Braz J Urol. 2018;44(3):467–74. doi: 10.1590/S1677-5538.IBJU.2016.0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa J, Miyake H, Hinata N, Muramaki M, Tanaka K, Fujisawa M. Renal Functional and Perioperative Outcomes of Selective Versus Complete Renal Arterial Clamping During Robot-Assisted Partial Nephrectomy: Early Single-Center Experience With 39 Cases. Surg Innov. 2016;23(3):242–8. doi: 10.1177/1553350615610648 [DOI] [PubMed] [Google Scholar]
- 11.Mattevi D, Luciani LG, Mantovani W, Cai T, Chiodini S, Vattovani V, et al. Fluorescence-guided selective arterial clamping during RAPN provides better early functional outcomes based on renal scan compared to standard clamping. J Robot Surg. 2019;13(3):391–6. doi: 10.1007/s11701-018-0862-x [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Kameyama S, Ambe Y, Teshima T, Izumi T, Tsuru I, et al. Predictive factors for postoperative renal function after off-clamp, non-renorrhaphy partial nephrectomy. Transl Androl Urol. 2022;11(9):1226–33. doi: 10.21037/tau-22-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hongo F, Kawauchi A, Ueda T, Fujihara-Iwata A, Nakamura T, Naya Y, et al. Laparoscopic off-clamp partial nephrectomy using soft coagulation. Int J Urol. 2015;22(8):731–4. doi: 10.1111/iju.12808 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Imamura Y, Yamamoto S, Sazuka T, Sakamoto S, Ichikawa T. Soft coagulation in robot-assisted partial nephrectomy without renorrhaphy: Comparison with standard suture. Int J Urol. 2020;27(4):352–4. doi: 10.1111/iju.14195 [DOI] [PubMed] [Google Scholar]
- 15.Ooi A. Advances in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) research. Semin Cancer Biol. 2020;61:158–66. doi: 10.1016/j.semcancer.2019.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844–53. doi: 10.1016/j.juro.2009.05.035 [DOI] [PubMed] [Google Scholar]
- 17.Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, et al. Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J. 2017;10(4):545–51. doi: 10.1093/ckj/sfx026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, et al. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75(10):1079–87. doi: 10.1038/ki.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noronha IL, Santa-Catharina GP, Andrade L, Coelho VA, Jacob-Filho W, Elias RM. Glomerular filtration in the aging population. Front Med (Lausanne). 2022;9:769329. doi: 10.3389/fmed.2022.769329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottel H, Hoste L, Yayo E, Delanaye P. Glomerular Filtration Rate in Healthy Living Potential Kidney Donors: A Meta-Analysis Supporting the Construction of the Full Age Spectrum Equation. Nephron. 2017;135(2):105–19. doi: 10.1159/000450893 [DOI] [PubMed] [Google Scholar]
- 21.Yu YD, Nguyen NH, Ryu HY, Hong SK, Byun SS, Lee S. Predictors of renal function after open and robot-assisted partial nephrectomy: A propensity score-matched study. Int J Urol. 2019;26(3):377–84. doi: 10.1111/iju.13879 [DOI] [PubMed] [Google Scholar]
- 22.Takagi T, Kondo T, Iizuka J, Tomita E, Kobayashi H, Hashimoto Y, et al. Predictors for postoperative renal function after open partial nephrectomy: including postoperative biomarkers. Int J Urol. 2012;19(9):823–8. doi: 10.1111/j.1442-2042.2012.03037.x [DOI] [PubMed] [Google Scholar]
- 23.Chan AA, Wood CG, Caicedo J, Munsell MF, Matin SF. Predictors of unilateral renal function after open and laparoscopic partial nephrectomy. Urology. 2010;75(2):295–302. doi: 10.1016/j.urology.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 24.Anderson BG, Potretzke AM, Du K, Vetter J, Figenshau RS. Off-clamp robot-assisted partial nephrectomy does not benefit short-term renal function: a matched cohort analysis. J Robot Surg. 2018;12(3):401–7. doi: 10.1007/s11701-017-0745-6 [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A, Veccia A, Francavilla S, Bertolo R, Bove P, Hampton LJ, et al. On-clamp versus off-clamp robotic partial nephrectomy: A systematic review and meta-analysis. Urologia. 2019;86(2):52–62. doi: 10.1177/0391560319847847 [DOI] [PubMed] [Google Scholar]
- 26.Deng W, Liu X, Hu J, Chen L, Fu B. Off-clamp partial nephrectomy has a positive impact on short- and long-term renal function: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):188. doi: 10.1186/s12882-018-0993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simone G, Capitanio U, Tuderti G, Presicce F, Leonardo C, Ferriero M, et al. On-clamp versus off-clamp partial nephrectomy: Propensity score-matched comparison of long-term functional outcomes. Int J Urol. 2019;26(10):985–91. doi: 10.1111/iju.14079 [DOI] [PubMed] [Google Scholar]
- 28.Bahler CD, Dube HT, Flynn KJ, Garg S, Monn MF, Gutwein LG, et al. Feasibility of omitting cortical renorrhaphy during robot-assisted partial nephrectomy: a matched analysis. J Endourol. 2015;29(5):548–55. doi: 10.1089/end.2014.0763 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The data underlying the results presented in the study are available from Supporting information.