Abstract
Background and Objectives
We aimed to investigate the association of triglyceride–glucose (TyG) index with hypertension and compare the discriminative power of the TyG index, lipid, glycemic parameters for hypertension using the China Health Examination Collaborative study (CHEC Study).
Methods and Study Design
Data were collected at Ningbo Mingzhou Hospital and Beijing physical examination center from the CHEC Study during 2014 and 2021. Participants with ≥2 medical check-up times were included. The TyG index is the logarithmized product of fasting triglyceride and glucose. Generalised estimation equation (GEE) model was used to evaluate the association between the TyG index, lipid parameters, glycemic parameters and hypertension. Receiver operating characteristic (ROC) analysis was performed to explore the predictive ability of TyG index on hypertension at different years of medical check-up.
Results
112,902 participants with an average age of 42.8 years were recruited in the study, 36,839 participants developed hypertension over the 8-year period. GEE model analysis showed that the ORs with 95% CI of hypertension were 3.35 (3.15-3.57), 1.86 (1.76-1.95), 1.67 (1.58-1.78), 1.45 (1.33-1.58), 1.24 (1.19-1.29), 0.92 (0.86-0.99), and 1.90 (1.83-1.97) in the highest versus lowest quintiles of TyG index, TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG in model 2. The area under the ROC curve of the overall years of medical check-up was significantly higher than a particular year in predicting hypertension (AUC: 0.883, p < 0.05).
Conclusions
TyG index is associated with hypertension and shows the superior discriminative ability for hypertension compared with lipid and glycemic parameters.
Key Words: TyG index, hypertension, lipid parameters, glycemic parameters, medical check-up
Introduction
Hypertension is one of the most prevalent cardiovascular risk factors and a leading cause of premature death worldwide, affecting up to 1 in 4 men and 1 in 5 women – more than a billion people.1 China, with 20% of the global population, accounts for a large part of this burden, where hypertension and blood pressure-related cardiovascular diseases (CVD) are major public health challenges.2 Nearly half of Chinese adults aged 35–75 years are diagnosed with hypertension, the incidence is still rising, and the onset age is getting younger.3 The prevalence of hypertension has increased in recent decades, leading to an increase in blood pressure-related morbidity and mortality.
Glucose and lipid metabolism disorders are a common pathophysiological feature in hypertension patients, and insulin resistance (IR) plays a significant role in this biological process.4 The gold standard for assessing the status of IR is the hyperinsulinaemic-euglycaemic clamp.5
This assessment technique, however, is costly and difficult, making it unsuitable for routine clinical monitoring. Recently, certain innovative and simple markers, such as the triglyceride-glucose (TyG) index and the triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, have been found to be reliable surrogate measures of IR.
The TyG index is the logarithmized product of fasting triglyceride and glucose. Compared with the hyperinsulinaemic-euglycaemic clamp, the TyG index shows a high sensitivity of 96.5% and a specificity of 85.0% for the diagnosis of IR,6 which has also been linked to diabetes in Chinese population.7 Many observational studies have found that these surrogate indexes are independent risk factors for hypertension.8, 9, 10, 11 A meta-analysis of eight observational studies has shown that higher TyG index may be associated with higher odds of hypertension in general adult population.12 However, there are relatively few longitudinal studies on the association between the TyG index and hypertension among Chinese adults. Besides, comparative studies on the discriminative abilities of the TyG index, lipid, and glycemic parameters for the risk of hypertension are also limited. Additionally, repeated measurements of physiological index have not been adequately considered.
Annual medical check-up is an example of positive health behavior, as such preventive measures are linked to earlier detection of disease, improved treatment success and faster recovery from illness.13 As a result, medical data obtained from primary care are valuable sources since they contain information on symptoms and health care utilization, both of which are relevant for predictive analytics. Medical check-up data often comprise a number of diagnostic tests to evaluate health condition in order to detect and prevent disease early. Additionally, data from medical check-up offer valuable information regarding current and past health conditions that are frequently hard to obtain in most population-based data.14 What's more, data from medical check-up are a reliable and objective measure of physiological indicators.
Therefore, the present study intends to explore the association of the TyG index with hypertension and compare the discriminative power of the TyG index, lipid, glycemic parameters for hypertension using the China Health Examination Collaborative Study (CHEC Study) during 2014 and 2021.
Methods
Study design and subjects
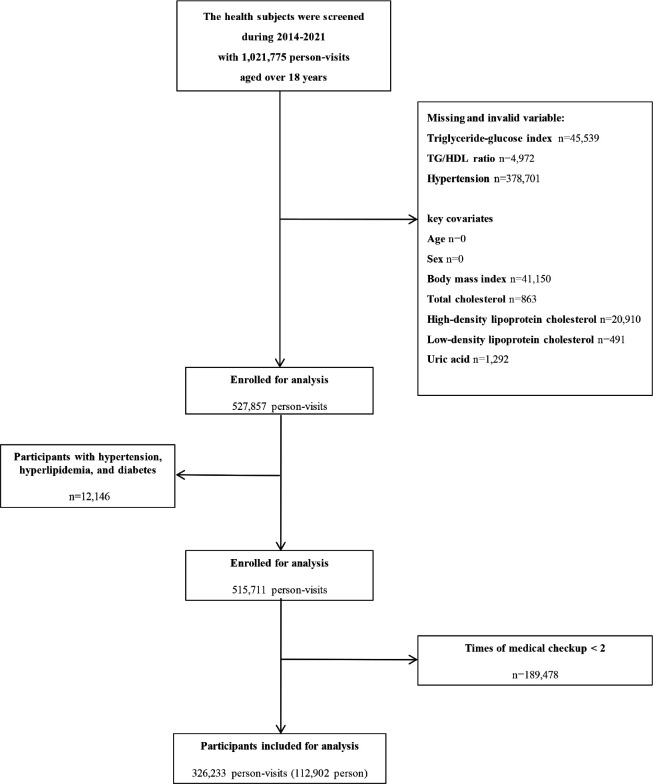
The China Health Examination Collaborative (CHEC) Study is a centralized health examination database formed in conjunction with medical examination centers of hospitals in China. Participants over 18 years old at Mingzhou hospital in Ningbo City and Beijing physical examination center during 2014 and 2021 were included in our study. The subjects were excluded according to the following criteria: (1) Subjects who had absence of blood biochemical examination. (2) Subjects who had a history of hypertension, hyperlipidemia, and diabetes. (3) Subjects who had less than two medical checkups. In total, 112,902 subjects including 55,312 males and 57,590 females were evaluated for the study (Figure 1).
Figure 1.
Flow diagram showing selection process of participants in our study.
Ethical approval
This study was approved by biomedical ethics committee of Peking University (IRB00001052-22186).
Measurements
All participants were invited to join an in-person evaluation that included physical examination and laboratory testing. Physical examinations were conducted following a standardised protocol, including weight, height, systolic blood pressure (SBP), and diastolic blood pressure (DBP). Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Sitting blood pressure was measured from the right arm three times with a 1-min interval between the measurements after the rest for 20 min by trained members. The quality of anthropometric data was confirmed by repeated measurements in the presence of researchers. Venous blood samples were obtained from the subjects in the morning after at least 12 h prior to the examination. Routine biochemical data included total cholesterol (TC), triglycerides (TG), fasting plasma glucose (FPG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and uric acid.
Outcomes and definitions
Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and diastolic blood pressure (DBP) ≥ 90 mmHg.15 The TyG index was calculated with established formulas according to the previous studies:6, 16 TyG = Ln [TG (mg/mL) * FPG (mg/mL) /2]. The TG/HDL-C ratio was calculated as TG (mm/L) divided by HDL-C (mm/L). TyG index, TG/HDL-C ratio, TG, TC, HDL-C, LDL-C, and FPG were divided into five groups according to quintile division of the subjects, respectively.
Statistical analysis
We conducted descriptive analysis to present the characteristics of baselines participants. Continuous variables were reported as mean ± SD and categorical variables as frequency and percentage. Comparisons between two groups (hypertension and non-hypertension) were performed using Student's t-tests for continuous variables and χ2 analyses for categorical variables. Medical check-up records from the first physical examination served as baseline data. Generalised estimating equations (GEE) model with unstructured correlation structures was used to quantify their longitudinal association between physiological indicators and hypertension, given the data on physiological indicators and hypertension were repeatedly measured over the 8-year study period.17 Model 0 was not adjusted for any confounding factors. Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, BMI, TC, TG, HDL-C, LDL-C, FPG and uric acid in each group. We performed subgroup analyses using GEE models by: (1) gender (male vs female); (2) age group (youth <65 years vs elderly ≥65 years); and (3) BMI group (BMI <28 kg/m2 vs BMI ≥28 kg/m2) based on the Asia-Pacific criteria set by the WHO.18, 19 Receiver operating characteristic (ROC) analysis was performed to explore the predictive ability of TyG index on hypertension at different years of medical check-up. The TyG index was fitted into the logistic regression model as a continuous variable. Age, gender, BMI, TC, HDL, LDL and uric acid were included in the model during the calculation of the area under the curve (AUC) of the ROC. We further compared the statistical difference in AUC between different years by using DeLong test.20
For all analyses, a two-tailed p-value <0.05 was considered to be statistically significant. All statistical analyses were performed using Stata version 14.2 (Stata Corp, College Station, TX) and R version 3.5.3 (R foundation).
Results
Characteristics of participants
This study included 112,902 participants (55,312 men) with an average age of 42.8 years (SD: 13.0), of whom 36,839 (32.6%) had hypertension. The average number of health check-up for each participant was 3.94 years. Compared to non-hypertensive participants, the hypertensive ones were older, male, characterized by larger BMI, higher mean SBP and DBP, less favorable metabolic profile (TG, TC, HDL-C, LDL-C, FPG, Uric acid), and higher TyG index and TG/HDL-C ratio (Table 1).
Table 1.
Characteristics of the participants†
| Characteristics | All tharticithants | Hythertension‡ | Non-hythertension§ | th-value¶ |
|---|---|---|---|---|
| Participants, n | 112902 | 36839 | 76063 | |
| Age (year) | 42.8 ± 13.0 | 50.4 ± 14.2 | 39.2 ± 10.7 | <0.001 |
| Sex (%) | <0.001 | |||
| Male | 55312 (49.0) | 27581 (74.9) | 27731 (36.5) | |
| Female | 57590 (51.0) | 9258 (25.1) | 48332 (63.5) | |
| Body mass index (kg/m2) | 23.5 ± 3.7 | 26.1 ± 3.6 | 22.3 ± 3.0 | <0.001 |
| SBP, mmHg | 118 ± 20.1 | 143 ± 14.2 | 106 ± 7.8 | <0.001 |
| DBP, mmHg | 74.5 ± 13.4 | 91.0 ± 9.3 | 66.7 ± 5.9 | <0.001 |
| TG, mmol/L | 1.3 ± 0.8 | 1.7 ± 1.0 | 1.1 ± 0.6 | <0.001 |
| TC, mmol/L | 4.7 ± 0.9 | 5.0 ± 0.9 | 4.6 ± 0.9 | <0.001 |
| HDL-C, mmol/L | 1.4 ± 0.3 | 1.2 ± 0.3 | 1.4 ± 0.3 | <0.001 |
| LDL-C, mmol/L | 2.8 ± 0.8 | 3.0 ± 0.8 | 2.7 ± 0.7 | <0.001 |
| FPG, mmol/L | 5.3 ± 1.1 | 5.8 ± 1.5 | 5.0 ± 0.7 | <0.001 |
| Uric acid, μmol/L | 312 ± 88.7 | 354 ± 88.8 | 293 ± 81.5 | <0.001 |
| TyG index | 8.4 ± 0.6 | 8.8 ± 0.6 | 8.2 ± 0.5 | <0.001 |
| TG/HDL-C ratio | 1.1 ± 0.8 | 1.5 ± 1.0 | 0.9 ± 0.7 | <0.001 |
TyG index: Triglyceride-glucos index; TG/HDL-C ratio: Triglyceride to high-density lipoprotein cholesterol ratio; TG: Triglyceride; TC: Total cholesterol, LDL-C: Low-density lipoprotein cholesterol, HDL-C: High-density lipoprotein cholesterol, FPG: Fasting plasma glucose.
Mean ± SD is presented for continuous variables and numbers (percentage) for categorical variables.
Hypertension was defined as participants who had hypertension at least once during the study period, regardless of whether their blood pressure returned to normal levels.
Non-hypertension was defined as participants who had never had hypertension during the study period.
p value are obtained from the Student's t-tests for continuous variables and the Χ2 test for categorical variables.
Association of the TyG index, lipid parameters, glycemic with hypertension
GEE model that considers separately each index and their individual components as predictors of hypertension was constructed. Table 2 showed OR and 95% CI of hypertension with the groups of TyG index, TG/HDL-C ratio, TG, TC, HDL-C, LDL-C and FPG in the total population. The crude ORs with 95% CI of hypertension were 21.84 (20.91-22.80), 12.82 (12.30-13.37), 12.75 (12.25-13.27), 1.67 (1.58-1.78), 2.02 (1.95-2.08), 0.23 (0.22-0.24), and 4.10 (3.97-4.23) in the highest versus lowest quintiles of TyG index, TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG, respectively. After adjustment for age and gender, every index was still significantly associated with hypertension. After further adjustments in Model 2, the multivariate adjusted ORs with 95% CI of hypertension were 3.35 (3.15-3.57), 1.86 (1.76-1.95), 1.67 (1.58-1.78), 1.45 (1.33-1.58), 1.24 (1.19-1.29), 0.92 (0.86-0.99), and 1.90 (1.83-1.97) in the highest versus lowest quintiles of TyG index, TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG, respectively. Those in the top quintile of TyG index were two times more likely to develop hypertension than those in the bottom quintile. Similarly, the associations between hypertension and continuous values of TyG index, TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG were statistically significant. Moreover, compared with lipid and glycemic parameters, TyG index had stronger association with hypertension.
Table 2.
Association of the TyG index, lipid parameters, and glycemic with hypertension in total subjects using generalised estimation equation model (GEE)†
| Variable | Model 0‡ OR (95%CI) | p-value | Model 1§ OR (95%CI) | p-value | Model 2¶ OR (95%CI) | p-value |
|---|---|---|---|---|---|---|
| TyG index | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 2.28 (2.19-2.37) | <0.001 | 1.56 (1.50-1.63) | <0.001 | 1.34 (1.28-1.40) | <0.001 |
| Q3 | 4.64 (4.45-4.83) | <0.001 | 2.35 (2.25-2.45) | <0.001 | 1.69 (1.61-1.78) | <0.001 |
| Q4 | 9.61 (9.22-10.02) | <0.001 | 3.96 (3.79-4.14) | <0.001 | 2.30 (2.19-2.43) | <0.001 |
| Q5 | 21.8 (20.91-22.80) | <0.001 | 7.88 (7.52-8.26) | <0.001 | 3.35 (3.15-3.57) | <0.001 |
| Per-1 | 6.11 (5.97-6.26) | <0.001 | 3.54 (3.45-3.63) | <0.001 | 2.64 (2.53-2.75) | <0.001 |
| TG/HDL-C ratio | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 1.99 (1.92-2.07) | <0.001 | 1.41 (1.36-1.47) | <0.001 | 1.24 (1.19-1.30) | <0.001 |
| Q3 | 3.72 (3.58-3.87) | <0.001 | 2.01 (1.93-2.09) | <0.001 | 1.45 (1.38-1.51) | <0.001 |
| Q4 | 6.84 (6.57-7.12) | <0.001 | 3.05 (2.92-3.19) | <0.001 | 1.68 (1.60-1.76) | <0.001 |
| Q5 | 12.8 (12.3-13.4) | <0.001 | 5.27 (5.03-5.52) | <0.001 | 1.86 (1.76-1.95) | <0.001 |
| Per-1 | 2.54 (2.50-2.59) | <0.001 | 1.84 (1.80-1.87) | <0.001 | 1.19 (1.16-1.21) | <0.001 |
| TG, mmol/L | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 1.96 (1.89-2.03) | <0.001 | 1.37 (1.32-1.42) | <0.001 | 1.12 (1.07-1.17) | <0.001 |
| Q3 | 3.60 (3.46-3.73) | <0.001 | 1.95 (1.87-2.03) | <0.001 | 1.28 (1.22-1.33) | <0.001 |
| Q4 | 6.59 (6.34-6.84) | <0.001 | 3.01 (2.89-3.14) | <0.001 | 1.50 (1.42-1.57) | <0.001 |
| Q5 | 12.8 (12.3-13.3) | <0.001 | 5.36 (5.13-5.60) | <0.001 | 1.67 (1.58-1.78) | <0.001 |
| Per-1 | 2.84 (2.79-2.89) | <0.001 | 2.02 (1.98-2.06) | <0.001 | 1.23 (1.19-1.27) | <0.001 |
| TC, mmol/L | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 1.12 (1.07-1.17) | <0.001 | 1.22 (1.18-1.26) | <0.001 | 1.09 (1.05-1.14) | <0.001 |
| Q3 | 1.28 (1.22-1.33) | <0.001 | 1.46 (1.41-1.52) | <0.001 | 1.20 (1.14-1.26) | <0.001 |
| Q4 | 1.50 (1.42-1.57) | <0.001 | 1.72 (1.66-1.79) | <0.001 | 1.28 (1.20-1.36) | <0.001 |
| Q5 | 1.67 (1.58-1.78) | <0.001 | 2.27 (2.18-2.36) | <0.001 | 1.45 (1.33-1.58) | <0.001 |
| Per-1 | 1.49 (1.47-1.51) | <0.001 | 1.37 (1.35-1.39) | <0.001 | 1.76 (1.65-1.88) | <0.001 |
| LDL-C, mmol/L | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 0.90 (0.87-0.92) | <0.001 | 0.96 (0.93-1.00) | 0.04 | 0.98 (0.94-1.02) | 0.27 |
| Q3 | 1.10 (1.07-1.13) | <0.001 | 1.07 (1.03-1.11) | <0.001 | 1.03 (0.99-1.07) | 0.15 |
| Q4 | 1.43 (1.38-1.47) | <0.001 | 1.20 (1.16-1.25) | <0.001 | 1.08 (1.04-1.13) | <0.001 |
| Q5 | 2.02 (1.95-2.08) | <0.001 | 1.49 (1.43-1.54) | <0.001 | 1.24 (1.19-1.29) | <0.001 |
| Per-1 | 1.40 (1.38-1.42) | <0.001 | 1.21 (1.19-1.23) | <0.001 | 1.12 (1.10-1.14) | <0.001 |
| HDL-C, mmol/L | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 0.70 (0.68-0.72) | <0.001 | 0.84 (0.81-0.87) | <0.001 | 1.08 (1.04-1.12) | <0.001 |
| Q3 | 0.47 (0.45-0.48) | <0.001 | 0.68 (0.65-0.70) | <0.001 | 1.04 (1.00-1.09) | 0.07 |
| Q4 | 0.32 (0.31-0.33) | <0.001 | 0.55 (0.53-0.58) | <0.001 | 0.99 (0.94-1.04) | 0.64 |
| Q5 | 0.23 (0.22-0.24) | <0.001 | 0.47 (0.45-0.49) | <0.001 | 0.92 (0.86-0.99) | 0.02 |
| Per-1 | 0.18 (0.17-0.18) | <0.001 | 0.41 (0.39-0.43) | <0.001 | 0.88 (0.81-0.96) | <0.001 |
| FPG, mmol/L | ||||||
| Q1 | 1.0 | 1.0 | 1.0 | |||
| Q2 | 0.53 (0.52-0.55) | <0.001 | 0.71 (0.68-0.73) | <0.001 | 0.77 (0.74-0.80) | <0.001 |
| Q3 | 0.86 (0.83-0.89) | <0.001 | 0.98 (0.95-1.01) | 0.25 | 0.96 (0.93-1.00) | 0.03 |
| Q4 | 1.52 (1.47-1.57) | <0.001 | 1.44 (1.39-1.49) | <0.001 | 1.27 (1.22-1.31) | <0.001 |
| Q5 | 4.10 (3.97-4.23) | <0.001 | 2.65 (2.56-2.74) | <0.001 | 1.90 (1.83-1.97) | <0.001 |
| Per-1 | 1.16 (1.14-1.18) | <0.001 | 1.12 (1.10-1.13) | <0.001 | 1.07 (1.06-1.08) | <0.001 |
Model was adjusted for the variables of repeated years of medical check-up based on all medical check-up participants by using GEE.
Model 0: adjusted for nothing.
Model 1: adjusted for age and sex.
Model 2: adjusted for age; sex; center; body mass index; total cholesterol; total triglyceride; high-density lipoprotein cholesterol; low-density lipoprotein cholesterol; fasting plasma glucose; uric acid.
TyG index: Triglyceride-glucos index; TG/HDL-C ratio: Triglyceride to high-density lipoprotein cholesterol ratio; TG: Triglyceride; TC: Total cholesterol, LDL-C: Low-density lipoprotein cholesterol, HDL-C: High-density lipoprotein cholesterol, FPG: Fasting plasma glucose.
To calculate the discrimination ability of TyG index on hypertension at different years of medical check-up (2014 to 2021), ROC curves were calculated. Table 3 summarized the AUC of TyG index on hypertension. The sample sizes for the year-stratified ROC analysis from 2014 to 2021 were 29992, 33346, 36987, 45152, 46305, 49498, 44877, and 40076, respectively. The AUC of the overall years of medical check-up in predicting hypertension was 0.883. The p values for comparing AUC difference between different years were all < 0.001. The difference in AUC between the overall years and any individual year was minimal, indicating that the association between TyG index and hypertension was very stable.
Table 3.
The Area Under Curve of TyG index on hypertension by sex and different times of medical checkups.
| Years | Area Under Curve | ||
|---|---|---|---|
| All participants | Male | Female | |
| Overall | 0.883 | 0.808 | 0.899 |
| Year 2014 | 0.880 | 0.802 | 0.897 |
| Year 2015 | 0.878 | 0.801 | 0.897 |
| Year 2016 | 0.880 | 0.802 | 0.898 |
| Year 2017 | 0.882 | 0.806 | 0.899 |
| Year 2018 | 0.882 | 0.806 | 0.899 |
| Year 2019 | 0.882 | 0.806 | 0.899 |
| Year 2020 | 0.882 | 0.807 | 0.898 |
| Year 2021 | 0.881 | 0.805 | 0.898 |
TyG index: Triglyceride-glucos index; TG/HDL-C ratio: Triglyceride to high-density lipoprotein cholesterol ratio; TG: Triglyceride; TC: Total cholesterol, LDL-C: Low-density lipoprotein cholesterol, HDL-C: High-density lipoprotein cholesterol, FPG: Fasting plasma glucose.
Associations of the TyG index, lipid parameters, glycemic with hypertension for stratified subgroups of gender, age and BMI.
As shown in Supplementary Table 1, similar results for GEE model analyses were observed in subgroups of gender: (1) female: the multivariate adjusted ORs with 95% CI of hypertension were 4.08 (3.65-4.57), 2.69 (2.47-2.93), 2.02 (1.81-2.26), 1.30 (1.12-1.50), 1.19 (1.11-1.28), 0.80 (0.71-0.91), and 1.89 (1.77-2.03) in the highest versus lowest quintiles of TyG index, TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG in Model 2, respectively. (2) male: the multivariate adjusted ORs with 95% CI of hypertension were 3.04 (2.83-3.27), 1.22 (1.16-1.29), 1.48 (1.37-1.59), 1.51 (1.36-1.67), 1.16 (1.10-1.22), and 2.00 (1.91-2.10) in the highest versus lowest quintiles of TyG index, TG/HDL-C ratio, TG, TC, LDL-C and FPG in Model 2, respectively. A slight positive association between HDL-C and hypertension was revealed (fifth quintile: OR 1.10, 95% CI 1.02–1.18, p =0.01). The results of subgroup analyses stratified by age and BMI were shown in Supplementary Tables 2 and 3. The associations were not statistically significant in TG, TC, HDL-C and FPG in the older age (≥ 65 years). When compared to lipid and glycemic parameters, TyG index had stronger association with hypertension.
Discussion
We explored the association of the TyG index, lipid, and glycemic parameters with risk of hypertension using the 8-year repeated medical check-up data among Chinese adults from 2014 to 2021. The TyG index was significantly associated with hypertension and remained significant after LDL-C or HDL-C and obesity were well-controlled using GEE model, and the association of the TyG index with hypertension was stronger than lipid or glycemic parameters. TG/HDL-C ratio, TG, TC, LDL-C, HDL-C and FPG were also associated with hypertension but were inferior to the TyG index. ROC analysis showed that the association between TyG index and hypertension was very stable, given the minimal difference in AUC between the overall years and any individual year.
Previous observational studies have discussed the association between TyG and hypertension, but limited longitudinal studies have been conducted among Chinese population.21, 22, 23 A Korean cohort study involving 15,721 adults aged over 40 years indicated that TyG index was an independent hazard indicator for hypertension.11 A Spanish cohort study including 3,637 participants aged over 40 years suggested that those in the top quintile of TyG index were two times more likely to develop hypertension than those in the bottom quintile in men but not for women, and this association independently of obesity.24 A meta-analysis including eight observational studies showed that the risk of hypertension increased by 53% in the highest versus lowest categories of TyG index.12 In the present study, higher TyG index was associated with higher risk of hypertension, and this positive association remained significant after additional controlling for confounding by obesity, suggesting that higher TyG index in individuals are associated with an increased risk of hypertension whenever they are obese or not.
Our study suggested that the TyG index outperformed other lipid or glycemic parameters in terms of its ability to discriminate hypertension. Our study was in agreement with a previous study,25 although the participants in Zhu et al.'s study were over 40 years, and the follow-up period was only seven months. Although American College of Cardiology (ACC)/American Heart Association (AHA) and European Society of Cardiology (ESC)/European Society of Hypertension (ESH) blood pressure guidelines have recommended LDL-C to be the most crucial lipid risk factor and treatment goal for CVD,26 dyslipidemia remains as a conventional risk factor for CVD including atherosclerosis. In this study, higher TyG index was still significantly associated with hypertension even after controlling for LDL-C or HDL-C. In addition, previous studies have shown that patients with CVD may benefit from better glycemic control.27, 28 However, this study found only a slight association between FPG and hypertension. Moreover, there was a slight negative association between FPG and hypertension in the older population. Although elevated glucose concentration has been recognized as a more robust predictor of diabetes than the TyG index,29 FPG is only a less effective predictor of cardiovascular outcomes.30
Due to the close correlation between TG and FPG, TyG index was applied to evaluate their joint value. Hypertriglyceridemia remains one of the most common abnormalities in patients with Type 2 diabetes, and its association with an increased risk of CVD has been well established.31, 32 TG might contribute to the formation of atherosclerotic plaque, while blood glucose might be involved in endothelial cell and platelet dysfunction.33, 34 Their values in relation to hypertension might be better interpreted when they are considered as a whole. Our study shows that the TyG index helps identify potential risks in individuals who might otherwise be overlooked. Clinicians usually focus merely on individuals with high FPG or TG. Such traditional clinical practice might miss some potential risk groups whose FPG and TG are in the normal or marginal range.
Our study has some strengths. First, this study filled current gaps in literature by analysing the relationship of TyG index, lipid, and glycemic parameters with risk of hypertension using medical check-up data. The medical check-up data used in this study can help provide information that will facilitate intervention development and adoption at the individual level. The utility of medical check-up data can potentially reach beyond predictive power alone in the near future. Additionally, the study analysis was based on the GEE model with high quality data by controlling for confounding factors, which can increase the accuracy of the prediction. Moreover, participants were representative of the general population with regard to clinical check-up and hypertension status, enhancing the generalisability of our findings.
Our study has some limitations. First, we were not able to directly measure IR in our study population and further compare the surrogate indices with direct markers of IR. Second, there are some confounding factors that have not been considered, which can be studied together with questionnaires in the future. Third, because the data of this research were from Chinese individuals, it remains uncertain whether these findings can be applicable to other ethnic groups.
Conclusion
In conclusion, our study reveals a significant association between the TyG index and hypertension in the 8-year medical check-up study in Chinese adults, and it is superior to other lipid profiles and FPG. Therefore, we propose that the TyG index could be a more efficient, useful and simple index for screening and managing hypertension. More evidence from well-designed studies is needed to confirm our findings.
Acknowledgements
We thank all study participants and the members of the research teams at the 2 hospitals of the China Health Examination Collaborative Study.
Conflict of Interest and Funding Disclosures
The authors declare that they have no competing interests.
Tao Huang received grants (2020YFC2003401) from the National Key R&D Program of China. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
Supplementary data
Funding Statement
Tao Huang received grants (2020YFC2003401) from the National Key R&D Program of China. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization Hypertension Overview. 2023/5/19. [cited 2023/6/1]; Available from: https://www.who.int/health-topics/hypertension#tab=tab_1.
- 2.He J, Gu D, Chen J, Wu X, Kelly TN, Huang JF, Chen JC, Chen CS, Bazzano LA, Reynolds K, Whelton PK, Klag MJ. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet. 2009;374:1765–1772. doi: 10.1016/s0140-6736(09)61199-5. [DOI] [PubMed] [Google Scholar]
- 3.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in China: data from 1·7 million adults in a population-based screening study (China PEACE Million Persons Project) Lancet. 2017;390:2549–2558. doi: 10.1016/s0140-6736(17)32478-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Chen Y, Zeng Q, Huang X, Cai J. Reduction of leukocyte-derived H(2)S linked to abnormal glycolipid metabolism in hypertensive subjects. Clin Exp Hypertens. 2017;39:427–434. doi: 10.1080/10641963.2016.1267193. [DOI] [PubMed] [Google Scholar]
- 5.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 6.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: The Rural Chinese Cohort Study. Cardiovasc Diabetol. 2017;16:30. doi: 10.1186/s12933-017-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20:134. doi: 10.1186/s12933-021-01330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Yang X, Wu Y, Huang H, Hu F, Zhang M, Sun L, Hu D. Association of triglyceride-glucose index and its 6-year change with risk of hypertension: A prospective cohort study. Nutr Metab Cardiovasc Dis. 2023;33:568–576. doi: 10.1016/j.numecd.2022.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Zhang F, Zhang Y, Guo Z, Yang H, Ren M, Xing X, Cong H. The association of triglyceride and glucose index, and triglyceride to high-density lipoprotein cholesterol ratio with prehypertension and hypertension in normoglycemic subjects: A large cross-sectional population study. J Clin Hypertens. 2021;23:1405–1412. doi: 10.1111/jch.14305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DH, Park JE, Kim SY, Jeon HJ, Park JH. Association between the triglyceride-glucose (TyG) index and increased blood pressure in normotensive subjects: a population-based study. Diabetol Metab Syndr. 2022;14:161. doi: 10.1186/s13098-022-00927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Yang W, Jiang X. Association Between Triglyceride-Glucose Index and Hypertension: A Meta-Analysis. Front Cardiovasc Med. 2021;8:644035. doi: 10.3389/fcvm.2021.644035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagahama S, Kashino I, Hu H, Nanri A, Kurotani K, Kuwahara K, et al. Haemoglobin A1c and hearing impairment: longitudinal analysis using a large occupational health check-up data of Japan. BMJ open. 2018;8:e023220. doi: 10.1136/bmjopen-2018-023220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Park H. Improving Prediction of High-Cost Health Care Users with Medical Check-Up Data. Big data. 2019;7:163–175. doi: 10.1089/big.2018.0096. [DOI] [PubMed] [Google Scholar]
- 15.1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension Guidelines Subcommittee. J Hypertens. 1999;17:151–183. doi: 10.3109/10641969909061028. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60:1673–1676. doi: 10.1016/j.metabol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Bůzková P, Brown ER, John-Stewart GC. Longitudinal data analysis for generalized linear models under participant-driven informative follow-up: an application in maternal health epidemiology. Am J Epidemiol. 2010;171:189–197. doi: 10.1093/aje/kwp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies Lancet. 2004;363:157–163. doi: 10.1016/s0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 19.Li MF, Ren Y, Zhao CC, Zhang R, Li LX, Liu F, et al. Prevalence and clinical characteristics of lower limb atherosclerotic lesions in newly diagnosed patients with ketosis-onset diabetes: a cross-sectional study. Diabetol Metab Syndr. 2014;6:71. doi: 10.1186/1758-5996-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Xuan H, Yin J, Wang L, Wang C, Xu X, Chen J, Li D, Xu T. Triglyceride Glucose Index Increases Significantly Risk of Hypertension Development in Chinese Individuals Aged ≥45 Years Old: Analysis from the China Health and Retirement Longitudinal Study. J Multidiscip Healthc. 2023;16:63–73. doi: 10.2147/jmdh.s391905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales-Gurrola G, Simental-Mendía LE, Castellanos-Juárez FX, Salas-Pacheco JM, Guerrero-Romero F. The triglycerides and glucose index is associated with cardiovascular risk factors in metabolically obese normal-weight subjects. J Endocrinol Invest. 2020;43:995–1000. doi: 10.1007/s40618-020-01184-x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16:175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Íñigo L, Navarro-González D, Pastrana-Delgado J, Fernández-Montero A, Martínez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34:1257–1265. doi: 10.1097/hjh.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 25.Zhu B, Wang J, Chen K, Yan W, Wang A, Wang W, et al. A high triglyceride glucose index is more closely associated with hypertension than lipid or glycemic parameters in elderly individuals: a cross-sectional survey from the Reaction Study. Cardiovasc Diabetol. 2020;19:112. doi: 10.1186/s12933-020-01077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Tinsley LJ, Kupelian V, D'Eon SA, Pober D, Sun JK, King GL, Keenan HA. Association of Glycemic Control With Reduced Risk for Large-Vessel Disease After More Than 50 Years of Type 1 Diabetes. J Clin Endocrinol Metab. 2017;102:3704–3711. doi: 10.1210/jc.2017-00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paneni F, Lüscher TF. Cardiovascular Protection in the Treatment of Type 2 Diabetes: A Review of Clinical Trial Results Across Drug Classes. Am J Cardiol. 2017;120:S17–S27. doi: 10.1016/j.amjcard.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: A retrospective cohort study. Prim Care Diabetes. 2020;14:161–167. doi: 10.1016/j.pcd.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Borg R, Kuenen JC, Carstensen B, Zheng H, Nathan DM, Heine RJ, Nerup J, Borch-Johnsen K, Witte DR. HbA₁(c) and mean blood glucose show stronger associations with cardiovascular disease risk factors than do postprandial glycaemia or glucose variability in persons with diabetes: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2011;54:69–72. doi: 10.1007/s00125-010-1918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167:1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 32.Ren Y, Ren Q, Lu J, Guo X, Huo X, Ji L, Yang X. Low triglyceride as a marker for increased risk of cardiovascular diseases in patients with long-term type 2 diabetes: A cross-sectional survey in China. Diabetes Metab Res Rev. 2018;34 doi: 10.1002/dmrr.2960. [DOI] [PubMed] [Google Scholar]
- 33.Tenenbaum A, Klempfner R, Fisman EZ. Hypertriglyceridemia: a too long unfairly neglected major cardiovascular risk factor. Cardiovasc Diabetol. 2014;13:159. doi: 10.1186/s12933-014-0159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farhangkhoee H, Khan ZA, Barbin Y, Chakrabarti S. Glucose-induced up-regulation of CD36 mediates oxidative stress and microvascular endothelial cell dysfunction. Diabetologia. 2005;48:1401–1410. doi: 10.1007/s00125-005-1801-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data