Abstract
Background and Objectives
Previous literature mostly has demonstrated the efficacy of pulmonary rehabilitation (PR) combined with whole nutrition powder in patients with chronic obstructive pulmonary disease (COPD). However, the benefits of whey protein as an oral nutritional supplement (ONS) during PR are not clear.
Methods and Study Design
It took 12 weeks to complete the trial, we divided 90 elderly patients with stable-stage COPD into a low-intensity exercise group (n= 30, PR group), PR plus whey proteins complex group (n= 30, PRWP group), and a control group (n= 30) randomly, and assessed index such as exercise capacity, mental health status, lung function, and body composition. Eventually, 84 people persisted until the end of the trial.
Results
Compared with the control group, hand grip strength (HGS)(1.4 ± 0.6 kg, and 1.0 ± 0.2 kg respectively, p< 0.05) in the PRWP and PR group, 6 minutes of walking distance (6MWD)(14.1 ± 3.8m, p< 0.05) in PRWP group improved. Furthermore, compared with the PR group, Medical Research Council Dyspnea Scale (MRC)(−0.2 ± 0.1, p< 0.01), anxiety score (−1.2 ± 0.4, p< 0.01), and body weight (2.0 ± 0.8kg, p< 0.05) improved in the PRWP group. There were no inter-group differences in a fat-free mass index or appendicular skeletal muscle mass index.
Conclusions
Muscle strength could be enhanced in both intervention models. Adding whey protein complex was additionally successful in rectifying dyspnea, anxiety, and weight loss caused by exercise. This rehabilitation pattern might be valuable in elderly patients with COPD.
Key Words: COPD, elderly patients, low-intensity exercise, whey proteins complex, muscle strength
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by limited airflow and chronic airway inflammation, along with anxiety and depression. Approximately 20%-50%1, 2 of patients have malnutrition, atrophy, and loss of muscle groups in the diaphragm or limbs. It leads to pulmonary ventilation and cough expectorant dysfunction, progressive dyspnea, fatigue, and poor exercise tolerance, which ultimately affects the quality of life and survival rate.3 According to the latest international guidelines,4 pulmonary rehabilitation (PR) could improve exercise ability (muscle strength and endurance), and cardiopulmonary function, and reduce the number of acute exacerbations or readmission rates. PR is a comprehensive intervention performed after a thorough, holistic assessment of the patient, including but not limited to exercise training, education, and behavior change. Among them, upper and lower limb endurance and resistance exercise had been confirmed as grade A evidence.5, 6
Although high-intensity exercise is more conducive to functional improvement, it is less durable and less adherent than low-intensity exercise for the elderly.7, 8 Further more, Steiner9 stated that patients undergoing PR were often in negative energy expenditure, that is, the daily diet was not enough to meet the high metabolic needs of training, especially for malnourished patients with COPD. Oral nutritional supplements (ONS) could rectify the negative energy expenditure and maintain patients’ weight.10-13 However, there is still no consensus on other benefits of ONS in PR because of the heterogeneity of previous studies.14
Whey protein contains leucine, an essential amino acid (EAA) known to increase insulin secretion and affect molecular regulation. It stimulates muscle protein synthesis through the mTOR pathway and is often recommended for sarcopenia patients. Daily whey protein is effective in correcting lean tissue loss in resistancetraining in the healthy elderly, menopausal women, or patients with sarcopenic obesity, and also in improving tolerance to training in patients with COPD.15, 16 However, there are still no studies in China on the combination of whey protein and PR for COPD patients, only the effect of taking whey protein alone had been reported in China.17 We thus investigated the effect of whey protein complex during low-intensity endurance or resistance exercise on patient outcomes such as improvement of weight, muscle mass, and muscle strength.
Methods
This randomized controlled trial was registered at clinicaltrials.gov (NCT04741373), and ethical approval was granted by the Medical Ethics Committee of Huadong Hospital Affiliated with Fudan University (acceptance number: 2020K204).
Patients
Patients with COPD were consecutively recruited from March to September 2021, and all subjects gave their informed consent. The target sample size (alpha 0.05, power 80%) was calculated based on weight change was about 1.5 kg from the study of Sugawara11 and accounted for a 25% drop-out.
Of the 263 patients screened at the Fourth Rehabilitation Hospital, Huadong Hospital, Hongqiao Community Health Service Center, Zhoujiaqiao Community Health Service Center, and three nursing homes in Shanghai, a final total of 90 were enrolled according to the following inclusion criteria randomly. Participants were assigned to the low-intensity exercise group (n= 30, PR group), PR plus whey proteins group (n= 30, PRWP group), and a control group (n= 30). Patients of both intervention groups received inpatient rehabilitation at the Fourth Rehabilitation Hospital. Patient data about COPD history, smoking history, comorbidity, medication, oxygen absorption, blood oxygen saturation, heart rate, and blood pressure were recorded.
Inclusion criteria
Age > 60 years old.
Patients diagnosis: after inhaling the bronchodilator, the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio was < 0.7 by spirometric testing (Jaeger MasterScreen, Germany).
Disease severity was evaluated according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Guideline. And they were at moderate (predicted FEV1 50-79%) or severe (predicted FEV1 30-49%) levels.
Stable patients who 1) had been admitted less than twice in the past two years due to acute aggravation, 2) had no change of sputum or other respiratory symptoms, and 3) had no drug adjustment in nearly one month.
Patients could complete lung function, 6-min walking distance, and grip strength assessments. During the past year, patients had not been enrolled in any form of PR and had not taken any kind of ONS.
Patients agreed to participate in the trial and gave their informed consent and had good compliance.
Exclusion criteria
Patients with heart disease (e.g., ischemic heart disease with a history of angina).
Patients with cognitive impairment, kidney failure, and malabsorption syndrome.
Patients who could not exercise or complete the testing program due to intermittent claudication caused by peripheral artery disease or osteoporosis.
Patients whose arterial SpO2 oxygen saturation was < 88% were in a quiet state.
Patients who received systemic steroids.
Intervention protocol
Only those in the PRWP group received whey protein supplements enriched with leucine.
The patients in both intervention groups underwent 12-week supervised low-intensity exercise. All subjects received usual care including routine education brochures, and medication including anticholinergic, β2-agonist, or bronchodilators such as LAMAs, LABAs, or ICS.
Low-intensity exercise program
Exercise training included aerobic, resistance, and respiratory training. Aerobic exercise mode was upper and lower limb linkage via equipment named MOTOmed viva2 (Jiangsu Tianrui Medical Device Co., Ltd). According to the maximum oxygen intake from the cardiopulmonary exercise test, the exercise intensity range was low to moderate, and the exercise duration was 20 min, 4 times/week. After a 10-minute warm-up, electrocardiogram (ECG), and oxygen saturation were monitored simultaneously during exercises. If oxygen saturation was < 85%, blood pressure > 200/100 mmHg, or heart rate reached 85% of the maximum heart rate, and training was stopped.
Before resistance training, a rehabilitation therapist tested the single repeated maximum load (1RM) and guided the correct use of weight-bearing tools (range 1-10 kg). The load was gradually increased to 70-85% of 1RM. The upper limb was exercised 4 times/week using a dumbbell, and 5 sets of movements each time. Respiratory training was processed 6 times/ week including lip contraction and abdominal breathing. About a 10-min break was allowed between the two training methods each time.
Oral nutritional supplement (ONS)
The ONS was a packet of powder containing 10g of whey protein with 0.2g leucine and 0.2g beta-hydroxy-beta-methylbutyrate (HMB) (Beijing Qiwang Nutrition Technology Co., Ltd).
HMB is an intermediate metabolite of leucine which plays an anabolic role in muscle, where it acts as a signaling molecule to stimulate protein synthesis. It reduces proteasome expression and proteasome enzyme activity, thereby reducing myocyte death. Patients in the PRWP group consumed ONS twice a day. They also were encouraged to eat the daily meals provided by the hospital.
Education
Hospitalized patients in all groups received health education brochures that emphasized correct methods of medication intake, oxygen absorption, self-assessment of nutritional status, diet, and a video about respiratory exercises. The PR and control groups did not have extra activities and ONS outside of the intervention as a result of health education under the supervision of medical staff.
Outcome assessments
Exercise capacity
Isometric handgrip strength (HGS) was measured using a handgrip dynamometer (Jamar® Hand Evaluation, USA) to represent upper limb muscle strength. Strength was measured twice in each hand, and the mean values were used. Meanwhile, 6 min walking distance (6MWD) was measured in a corridor to represent muscular endurance.18
Mental health status
The Chinese version of the Hospital Anxiety and Depression Scale (HADS) translated by Ye19 had good reliability and validity20 and was used in our study. It comprised 14 items to evaluate anxiety and depression symptoms, with a score ≥ 8 denoting anxiety and depression.
Pulmonary function
Post-bronchodilator FEV1, FVC, maximal inspiratory pressure (PImax, which represents inspiratory muscle strength), and maximal expiratory pressure (PEmax, which represents expiratory muscle strength) were assessed by standardized COPD diagnosis equipment (Jaeger MasterScreen, Germany), to evaluate lung function grade and respiratory muscle forces. Dyspnea at rest was evaluated by Medical Research Council Dyspnea Scale (MRC)21 and the cough sputum was assessed by COPD assessment test (CAT) score.22 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) assessment consisted of the number of lung infections, MRC, and CAT scores.
Energy intake and body composition
Patients were asked to register their food intakes over 3 consecutive days at baseline. The information was analyzed by computer nutrient software (ver2.05 on DRIs 2018, SY, UIC, Shanghai, China). First, height and body weight (BW) were measured with the shoes off and light clothing using digital scales (Inbody BSM370, Korea), and body mass index BMI (kg/m2) was calculated automatically. Body composition including fat-free mass (FFM), fat mass (FM), and appendicular skeletal muscle mass (ASM) was measured using multi-frequency bioelectrical impedance analyzers23 (Inbody S10, Korea) on an empty stomach in the early morning when no exercise was done, in a sitting position. Finally, the indexes of FMI(FM(kg)/height(m)2), FFMI(FFM(kg)/ height(m)2), and ASMI(ASM(kg)/height(m)2) were calculated.
Statistical analyses
Results were presented as mean ± standard error (X ± SE), median and interquartile range (IQR) (25-75th percentile). Intra-group changes were analyzed by paired samples T-test for continuous normally-distributed data, or Wilcoxon rank-sum test for continuous non-normally distributed data. Among the three groups, parametric data were compared by one-way ANOVA-, and nonparametric data were compared by the Kruskal-Wallis H test taking the difference between the baseline and end of the test as a compare factor. Bonferroni tests were used to compare the two groups. Two-sided p−values < 0.05 were considered statistically significant. SPSS 24.0 for Windows (IBM Statistics SPSS, Chicago, IL, USA) was used for statistical analysis.
Results
Baseline characteristics of patients
Mean age 82.5 years, height 162.8 cm, BMI 22.8 kg/m2, FFMI 14.8 kg/m2, FFMI was lower than normal in about 78.5% of cases (based on less than 18 kg/m2 for males and less than 15 kg/m2 for female).24 Pulmonary function grade was moderate (69.1%) and patients in group D (GOLD subgroup) accounted for about 75%. Patients all received hospital-supplied meals and ate essentially the same amount of energy and protein on PR and non-PR days. Data from food intakes over 3 consecutive days showed that the actual baseline energy intakes for the three groups were 1599.8 kcal, 1626.5 kcal, and 1603.9 kcal while protein intakes were 62.9 g, 65.6 g, and 63.8 g respectively.
Indicators, such as age, gender, history of COPD, smoking history, type of comorbidity, oxygen flow, oxygen saturation, pulmonary function grade, and GOLD subgroup, were not significantly different at baseline. But there were differences in weight, BMI, FMI, PImax, and PEmax among the three groups at baseline (Table 1). To avoid bias from baseline data, differences before and after the intervention was used for comparison among the three groups.
Table 1.
The study procedure
| Parameters | Mean (SE) |
p | ||
|---|---|---|---|---|
| PRWP (n = 30) | PR (n = 30) | Control (n = 30) | ||
| General | ||||
| Gender (M/F), n | 13/14 | 15/14 | 10/18 | NS |
| Age, years | 80.5 (1.4) | 81.1 (2.2) | 85.9 (0.9) | NS |
| Height, cm | 163 (1.7) | 161(1.8) | 162 (1.5) | NS |
| Weight, kg | 57.9 (1.7) | 64.8 (2.3) | 58.6 (1.8) | 0.035** |
| COPD history, year | 9.6 (1.5) | 7.8 (1.3) | 6.7 (0.3) | NS |
| Smoke history, pack-year | 13.7 (3.4) | 12.8 (3.2) | 6.4 (2.4) | NS |
| Self-report co-morbidities, n† | 2 (1) | 1 (2) | 1 (0) | NS |
| Dietary intake | ||||
| Energy intake, kcal | 1599 (57.3) | 1626 (54.4) | 1603 (51.9) | NS |
| Protein intake, g | 62.9 (2.4) | 65.6 (2.3) | 63.8 (2.9) | NS |
| Lung function and symptom scores | ||||
| FEV1, L, % predicted** | 71.7 (1.4) | 67.2 (2.3) | 68.6 (1.2) | NS |
| FEV1/FVC, % | 63.3 (4.1) | 61.8 (5.6) | 62.4 (4.8) | NS |
| Frequency of acute exacerbation† | 1 (2) | 1 (1) | 1 (1) | NS |
| MRC dyspnea scale | 2.1 (0.1) | 2.1 (0.2) | 2.5 (0.2) | NS |
| CAT | 17.0 (0.8) | 17.9 (1.0) | 16.6 (0.6) | NS |
| GOLD groups, A: B: C: D | 0:10:0:17 | 2:6:0:21 | 0:10:0:18 | NS |
| Nutritional and emotional status | ||||
| MNA-SF | 7.2 (0.5) | 7.2 (0.4) | 11.7 (0.2) | 0.0001** |
| Serum Albumin, g/L | 37.9 (0.7) | 37.7 (0.7) | 36.5 (0.7) | NS |
| HADS-Anxiety | 6.4 (0.5) | 7.6 (0.4) | 7.7 (0.4) | NS |
| HADS-Depression | 12.2 (0.7) | 13.3 (0.7) | 13.4 (0.8) | NS |
| Muscle strength | ||||
| HGS, kg | 14.3 (1.3) | 13.4 (1.9) | 15.1 (1.0) | NS |
| 6MWD, m | 138 (8.1) | 135(8.0) | 166 (12.5) | NS |
| PImax, cmH2O† | 33 (10) | 32 (16) | 51 (19) | 0.003* |
| PEmax, cmH2O† | 40 (11) | 37 (18) | 67.5 (28) | 0.004* |
| Body composition | ||||
| BMI, kg/m2 | 21.6 (0.6) | 24.6 (0.7) | 22.1 (0.6) | 0.003** |
| FMI, kg/m2 | 7.4 (0.5) | 9.7 (0.5) | 7.6 (0.7) | 0.013** |
| FFMI, kg/m2 | 14.2 (0.3) | 14.9 (0.5) | 15.2 (0.4) | NS |
| ASMI, kg/m2 | 6.1 (0.2) | 5.9 (0.3) | 6.4 (0.4) | NS |
MNA-SF, Mini Nutritional Assessment-Short Form; HADS, Hospital Anxiety and Depression; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; MRC, Medical Research Council; CAT, COPD assessment test; BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index; ASMI, appendicular skeletal muscle index; HGS, hand grip strength; 6MWD, 6 min walking distance; PImax, maximum inspiratory mouth pressure; PEmax, maximum expiratory mouth pressure.
†Median (IQR) values, using Kruskal-Wallis H test, and *p<0.05; the rest were normal data, using One-way ANCOVA, and **p<0.05
Outcome assessment
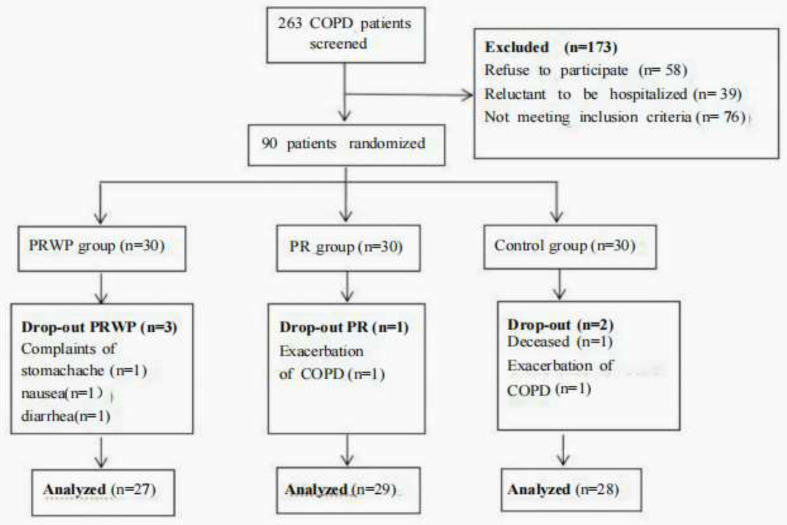
During the trial, the drop-out rate in the PRWP, PR, and control groups was 10%, 3.3%, and 6.6% respectively because of death and other side effects. All other patients had good compliance. Mean whey protein intake reached 1.89 ± 0.2 bags/day, and just 3 subjects reported side effects including stomachache, nausea, and diarrhea. A patient flowchart was shown in Figure 1. Mean changes after the interventions in PRWP, PR, and the control groups were shown in Table 2.
Figure 1.
Patients flow diagram based on completion of body composition testing
Table 2.
Outcome of the intervention
| Parameters | PRWP (n=27) Mean (SE) |
PR (n=29) Mean (SE) |
Control (n=28) Mean (SE) |
Among group differences |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ΔPre-post | Pre | Post | ΔPre-post | Pre | Post | ΔPre-post | p-value | |
| Muscle strength and endurance | ||||||||||
| HGS, kg | 14.3 (1.4) | 15.8 (1.4)** | 1.4 (0.5) | 13.4 (1.5) | 14.5 (1.9)* | 1.1 (0.6) | 15.1 (1.1) | 15.2 (1.1) | −0.02 (0.2) | 0.031 |
| 6MWD, m | 139 (8.6) | 155 (8.3)** | 16.1 (3.6) | 135 (8.2) | 140 (9.5) | 4.9 (4.3) | 167 (11.0) | 168 (12.7) | 1.9 (2.2) | 0.023 |
| PImax, cmH2O† | 33 (10) | 34 (10)* | 1 (4) | 32 (16) | 33 (20) | 1 (3) | 51 (19) | 52.5 (19)* | 2 (4) | 0.52 |
| PEmax, cmH2O† | 40 (11) | 43 (10) | 2 (5) | 37 (18) | 38 (26)* | 2 (5) | 67.5 (28) | 68.5 (32) | 2 (6) | 0.85 |
| Mental health status | ||||||||||
| HADS-A | 6.4 (0.5) | 6.4 (0.4) | −0.04 (0.3) | 7.6 (0.4) | 8.5 (0.3)** | 0.9 (0.3) | 7.6 (0.4) | 7.8 (0.4) | 0.2 (0.2) | 0.022 |
| HADS-D | 12.2 (0.8) | 11.8 (0.9) | −0.4 (0.7) | 13.3 (0.7) | 13.0 (0.7) | 0.3 (0.03) | 13.4 (0.8) | 12.9 (0.8) | −0.4 (0.4) | 0.865 |
| Lung function and symptom scores | ||||||||||
| %FEV1, pred | 71.7 (1.4) | 71.9 (1.4) | 0.3 (0.5) | 67.2 (2.3) | 66.8 (2.3) | 0.4 (0.6) | 68.6 (1.2) | 67.5 (1.2)* | −1.1 (0.5) | 0.242 |
| MRC | 2.1 (0.2) | 1.8 (0.2) | −0.3 (0.1)* | 2.1 (0.2) | 2.3(0.2) | 0.2(0.1) | 2.5 (0.2) | 2.5 (0.1) | 0.04 (0.05) | 0.003 |
| CAT scores | 17.0 (0.8) | 16.4 (1.1) | −0.7 (0.7) | 17.9 (1.0) | 17.9 (1.0) | 0.0 (0.35) | 16.6 (0.6) | 18.0 (0.9) | 1.4 (0.9) | 0.009 |
| Body composition | ||||||||||
| Weight, kg | 57.9 (1.8) | 59.2 (1.8) | 1.2 (0.7) | 64.8 (2.3) | 63.5 (2.1) | −1.4 (0.7) | 58.6 (1.9) | 58.4 (1.9) | −0.1 (0.2) | 0.03 |
| BMI, kg/m2 | 21.6 (0.7) | 22.1 (0.6) | 0.5 (0.3) | 24.6 (0.7) | 24.1 (0.6) | −0.5 (0.3) | 22.1 (0.6) | 22.1 (0.6) | −0.05 (0.07) | 0.051 |
| FMI, kg/m2 | 7.4 (0.6) | 8.3 (0.6) | 0.9 (0.5) | 9.7 (0.5) | 8.9 (0.7) | −0.8 (0.5) | 7.6 (0.7) | 7.3 (0.7) | −0.3 (0.9) | 0.137 |
| FFMI, kg/m2 | 14.2 (0.3) | 13.7 (0.4) | −0.5 (0.4) | 14.9 (0.5) | 15.3 (0.6) | 0.4 (0.4) | 15.2 (0.4) | 14.8 (0.6) | −0.4 (0.7) | 0.419 |
| ASMI, kg/m2 | 6.1 (0.3) | 5.6 (0.2) | −0.1 (0.3) | 5.9 (0.3) | 6.4 (0.3)* | 0.5 (0.05) | 6.4 (0.5) | 6.1 (0.3) | −0.3 (0.5) | 0.153 |
HADS, Hospital Anxiety and Depression; FEV1, forced expiratory volume in 1s; MRC, Medical Research Council; HGS, hand grip strength; 6MWD, 6 min walking distance; PImax, maximum inspiratory mouth pressure; PEmax, maximum expiratory mouth pressure; CAT, COPD assessment test; BMI, body mass index; FMI, fat mass index; FFMI, fat-free mass index; ASMI, appendicular skeletal muscle index
†Median (IQR) values, and within group changes compared using Wilcoxon signed ranks test, among group differences compared using Kruskal-Wallis test. and Bonferroni tests were further used for comparing the differences between the two groups.
*p< 0.05, **p< 0.01.
Exercise capacity
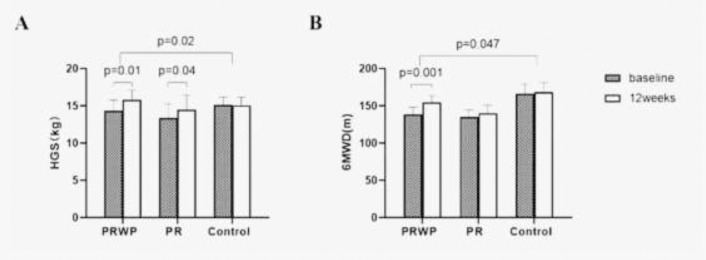
HGS (+1.4 ± 0.5 kg, and +1.1 ± 0.6 kg, p< 0.05) were significantly increased in both the PRWP and PR groups after the intervention, resulting in the improvement of HGS in both intervention groups when compared with the controls (+1.4 ± 0.6 kg, and +1.0 ± 0.2 kg, p< 0.05). Meanwhile, 6MWD improved from baseline in the PRWP group (+16.1 ± 3.6 m, p< 0.01), resulting in a significant difference compared to the controls (+14.1 ± 3.8 m, p< 0.05) (Figure 2). PImax in the PRWP group and PEmax in the PR group at 12 weeks both increased, but there was no difference among the three groups (p> 0.05) (Table 2).
Figure 2.
Mean baseline and 12 weeks values of A: Handgrip strengths (HGS)t; B: 6 min walking distance (6MWD). Data are presented as mean (SE). Within group changes were tested with the paired t test, between groups by linear regression (p<0.05).
Mental health status
At baseline, eight (26.6%) patients had anxiety, and twenty-five (83.3%) patients were depressive in the PRWP group, whereas seventeen (56.6%) patients had anxiety, and twenty-seven (90%) patients were depressive in the PR group. After the intervention, anxiety scores remained unchanged in the PRWP group but worsen in the PR group (0.9 ± 0.3, p= 0.01). So anxiety was significantly improved in the PRWP group compared to the PR group (−1.2 ± 0.4, p<0.01). There was no statistically significant change in depression in any of the three groups before and after the intervention (p> 0.05).
Pulmonary function
In the PRWP group, the MRC score (dyspnea) decreased after intervention (−0.3 ± 0.1, p< 0.05), so MRC and CAT scores (cough, sputum) were statistically improved (−0.2 ± 0.1, and −2.1 ± 1.2, p< 0.05) when compared with that of the PR group. There were also differences in the GOLD groupings determined by the frequency of respiratory infections, MRC, and CAT scores: 11 patients in the PRWP group changed from GOLD D to B, and 18 patients in the PR group. This resulted in a significant improvement compared to the control group (p< 0.05). There was no statistically significant difference in pulmonary function classification among the three groups (p> 0.05).
Body composition
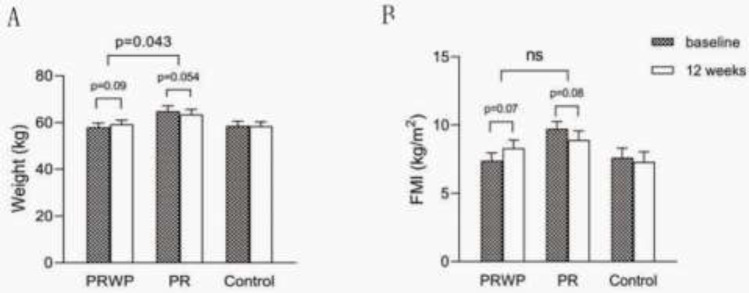
From baseline to the end of the trial, BW (−1.4 ± 0.7 kg, p= 0.054) and FMI (−0.8 ± 0.5 kg, p= 0.08) had a decreased tendency in the PR group whereas BW (+1.2 ± 0.7 kg, p= 0.09) and FMI (+0.9 ± 0.5 kg, p= 0.07) had an increased tendency in PRWP group. These resulted in an improvement of BW (+2.0 ± 0.8 kg, p< 0.05) in the PRWP group, compared with the PR group (Figure 3). There were no significant inter-group differences in FFMI and ASMI (Table 2).
Figure 3.
Mean baseline and 12 weeks values of A: Weight; B: Fat Mass Index (FMI). Data are presented as mean (SE). Within groupchanges were tested with the paired t test, between groups by linear regression (p˂0.05).
Discussion
High-intensity exercise, despite its ability to improve exercise capacity, cardiopulmonary function, and quality of life, is difficult to adhere to over the long term, limiting both the target subjects (hospitalized patients in the acute infection phase) and the duration of benefit (9-12 months).28, 29 The reason might be the negative energy expenditure caused by high-intensity exercise. Low-intensity exercise, which not only can have similar efficacy30 to high-intensity, but also with better compliance and sustainability, is becoming a PR modality to change the daily activity patterns of patients, especially the elderly.31 In our trial, the benefit was only one person dropping out of the test and an increase in grip strength.
In addition to low-intensity PR, lots of studies investigated the influence of ONS during PR. The types of ONS were being explored, ranging from energy-only to those containing the functional ingredient Vit D-3, n-3 unsaturated fatty acids. The current consensus was weight gain, but the other outcomes such as lean tissue, muscle strength, or muscular endurance remain controversial.10, 11, 12, 13 Whey protein and its functional components were used and improvement in both HGS (representing muscle strength) and 6MWD (representing muscular endurance) were observed in our study. The outcomes coincided with previous literature. Deutz's26 found that supplementation with a high-protein oral nutritional supplement (ONS) containing HMB (HP-HMB) was associated with improved handgrip strength, and the body weight of malnourished, hospitalized patients with COPD. Laviolette16 stated pressurized whey supplementation, by its antioxidant and nutritional properties, may improve exercise tolerance and potentiate the effects of exercise training in patients with COPD. A significant increase in 6MWD was a marker for maintenance of daily activity after PR and distance differences greater than 30-50 m were highly correlated with 5-year survival.7
In addition to that, the improvement in dyspnea and anxiety in the PRWP group compared to the PR group was also similar to the previous findings. Korkmaz27 showed that nutritional support with integrated PR significantly improved physical performance, dyspnea, and body composition in COPD patients. Jaatinen32 found that intake of yogurt enriched with a-lac, casein tripeptides may contribute beneficially to stress coping in stress-prone but healthy subjects. Its mechanism might be as follows: Individuals with high trait anxiety were characterized by functional shortages of 5-hydroxytryptophan (5-HT) during stress, which is a neurotransmitter that regulates mood, appetite, behavior, and sleep. Whereas tryptophan is a precursor to 5-HT, whey proteins that provide high tryptophan concentrations may bring benefits by modulating the body's acute stress response.33, 34
Finally, changes in weight and FMI in the PRWP group were mentioned at the time of this trial, the weight gain in the PRWP group was caused by a rise in fat mass within the group, where the cause could be the supplement itself, or the supplement might promote appetite. Unfortunately, no difference in muscle mass gain between groups was bserved. Depletion of muscle tissue was also considered an independent risk factor for a poor COPD prognosis and was positively associated with higher mortality. Muscle consumption was caused not only by an energy imbalance, but also by protein metabolism disorders due to muscle disuse, hypoxia, or drugs. Constantin35 stated that protein supplementation after exercise in COPD patients was unresponsive to candidate genes and proteins regulating muscle protein breakdown and synthesis. Recent literature stated that antioxidant and anti-inflammatory markers inhibited muscle catabolism through functional component supplements, such as n-3PUFA, vitamin C, and creatine,11, 25 which later experiments could focus on.
Limitations
Some limitations of our study were the small sample size and the lack of a non-nutritional placebo for the PR group. Adequate COPD clinical trials are needed to explore nutritional strategies to maintain the effect of exercise training after PR.
Conclusions
HGS improved in both intervention groups while 6MWD improved only in the PRWP group. Furthermore, the combination of whey proteins complex and exercise improved dyspnea, a symptom of cough, anxiety, and body weight loss caused by PR in stable elderly patients with COPD. In summary, whey protein complex combined with low-intensity exercise had good compliance in elderly patients to improve muscle function and pulmonary symptoms.
Acknowledgements
We would like to thank Shaowu Wang, and Ling Mao for assistance with supervising the resistance training, Yiru Zhao for assistance with dietary assessment, and Huili Zhu, Yinan Zhou, Weiping Jiang for counseling on the respiratory knowledge, Also we would like to thank Rufeng Jin for statistical knowledge guidance.
Conflict of Interest and Funding Disclosure
The authors have declared that no competing interests exist. Shanghai Municipal Health System Important and Weak Discipline Project (Clinical Nutrition) whose funding number was No.2019ZB0102.
Funding Statement
Shanghai Municipal Health System Important and Weak Discipline Project (Clinical Nutrition) whose funding number was No.2019ZB0102.
References
- 1.Nguyen HT, Collins PF, Pavey TG, Nguyen NV, Pham TD, Gallegos DL. Nutritional status, dietary intake, and health-related quality of life in outpatients with COPD. Int J Chronic Obstruct Pulmon Dis. 2019;14:215–226. doi: 10.2147/COPD.S181322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walter SL, Christian O, Steven P, Andrea AM, Gustavo D, Vanessa SP. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:1164–1176. doi: 10.1002/jcsm.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallin R, Koivisto-Hursti UK, Lindberg E, Janson C. Nutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2006;100:561–567. doi: 10.1016/j.rmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Martijn AS, Sally JS, Chris G, Richard Z, Linda N, Carolyn R, et al. An Official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 5.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigare R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/ccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters JB, Boer LM, Molema J, Heijdra YF, Prins JB, Vercoulen JH. Integral Health Status-Based Cluster Analysis in Moderate-Severe COPD Patients Identifies Three Clinical Phenotypes: Relevant for Treatment As Usual and Pulmonary Rehabilitation. Int J Behav Med. 2017;24:571–583. doi: 10.1007/s12529-016-9622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christian RO, Matthias L, Zafeiris L, Heleen D, Daniel L, Fernanda MR, Wim J, Loannis V,Thierry T. The likelihood of improving physical activity after pulmonary rehabilitation is increased in patients with COPD who have better exercise tolerance. Int J Chronic Obstruct Pulmon Dis. 2018;13:3515–3527. doi: 10.2147/COPD.S174827. 10.2147/ COPD.S174827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gianjoppe-Santos J, Barusso-Grüninger M, Lorenzo VA. Effects of low and high resistance training intensities on clinical outcomes in patients with COPD - a randomized trial. Physiother Theory Pract. 2022;38:2471–2482. doi: 10.1080/09593985.2021.1929616. [DOI] [PubMed] [Google Scholar]
- 9.Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2003;58:745–751. doi: 10.1136/thorax.58.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van de Bool C, Rutten, EPA, Van Helvoort A, Franssen FME, Wouters EF, Schols A. A randomized clinical trial investigating the efficacy of targeted nutrition as an adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle. 2017;8:748–758. doi: 10.1002/jcsm.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugawara K, Takahashi H, Kasa C, Kiyokawa N, Watanabe T, Fujii S, Kashiwagura T, Honma M, Satake M, Shioya T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med. 2010;104:1883–1889. doi: 10.1016/j.rmed.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Van Beers M, Rutten-Molken M, Van de Bool C, Boland M, Kremers SP, Franssen F, Helvoort A, Gosker HR, Wouters EF, Schols AM. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention program in COPD patients with low muscle mass: The randomized controlled NUTRAIN trial. Clin Nutr. 2020;39:405–413. doi: 10.1016/j.clnu.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Ahnfeldt-Mollerup P, Hey H, Johansen C, Kristensen S, Lindskov JB, Jensahnfeldt-Mollerupen C. The effect of protein supplementation on quality of life, physical function, and muscle strength in patients with chronic obstructive pulmonary disease. Eur J Phys Rehabil Med. 2015;51:447–456. doi: 10.1097/PHM.0b013e31818dff86. [DOI] [PubMed] [Google Scholar]
- 14.Aldhahir AM, Rajeh AM, Aldabayan YS, Drammeh S, Subbu V, Alqahtani JS, Hurst JR, Mandal S. Nutritional supplementation during pulmonary rehabilitation in COPD: A systematic review. Chron Respir Dis. 2020;17:1–21. doi: 10.1177/1479973120904953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volek JS, Volk BM, Gómez AL, Kunces LJ, Kupchak BR, Freidenreich DJ, et al. Whey protein supplementation during resistance training augments lean body mass. J Am Coll Nutr. 2013;32:122–135. doi: 10.1080/07315724.2013.793580. [DOI] [PubMed] [Google Scholar]
- 16.Laviolette L, Lands LC, Dauletbaev N, Saey D, Milot J, Provencher S, LeBlanc P, Maltais F. Combined Effect of Dietary Supplementation with Pressurized Whey and Exercise Training in Chronic Obstructive Pulmonary Disease: A Randomized, Controlled, Double-Blind Pilot Study. J Med Food. 2010;13:589–598. doi: 10.1089/jmf.2009.0142. [DOI] [PubMed] [Google Scholar]
- 17.Bo Y, Liu C, Ji Z, Yang RH, An QQ, Zhang XY, et al. A high whey protein, vitamin D and E supplement preserves muscle mass, strength, and quality of life in sarcopenic older adults: A double-blind randomized controlled trial. Clin Nutr. 2019;38:159–164. doi: 10.1016/j.clnu.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 18.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/rccm.166/1/111. [DOI] [PubMed] [Google Scholar]
- 19.Ye WF, Xu JG. Application and evaluation of the “General Hospital Anxiety and Depression Inventory” in general hospital patients. Chinese Journal of Behavioral Medicine. 1993;2:17–19. [Google Scholar]
- 20.Xie NH, Yan S, Ding J, Wang X. Reliability and validity analysis of the Hospital Anxiety and Depression Scale in HIV/AIDS patients. Chinese Journal of AIDS STD. 2020;26:1328–1331. doi: 10.13419/j.cnki.aids.2020.12.15. [DOI] [Google Scholar]
- 21.Nici L, Donner C, Wouters E, Zuwallack R, Ambrosino N, Bourbear J, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med. 2006;173:1390–1413. doi: 10.1164/rccm.200508-1211ST. [DOI] [PubMed] [Google Scholar]
- 22.Gil HI, Zo S, Jones PW, Kim BG, Kang N, Choi Y, et al. Clinical Characteristics of COPD Patients According to COPD Assessment Test (CAT) Score Level: Cross-Sectional Study. Int J Chronic Obstruct Pulmon Dis. 2021;16:1509–1517. doi: 10.2147/COPD.S297089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurt RT, Ebbert JO, Croghan I, Nanda S, Schroeder DR, Teigen LM, Velapati SR, Mundi MS. The Comparison of Segmental Multifrequency Bioelectrical Impedance Analysis and Dual-Energy X-ray Absorptiometry for Estimating Fat-Free Mass and Percentage Body Fat in an Ambulatory Population. J Parenter Enteral Nutr. 2021;45:1231–1238. doi: 10.1002/jpen.1994. [DOI] [PubMed] [Google Scholar]
- 24.Amercan Association of Cardiovascular and Pulmonary Rehabilitation . Trans Jianing Xi. Beijing, China: Beijing Science and Technology Press; 2020. Guidelines for Pulmonary Rehabilitation Programs: Assessment, Strategy, and Management 5th Edition; p. p70. [Google Scholar]
- 25.Calder PC, Laviano A, Lonnqvist F, Muscaritoli M, Öhlander M, Schols A. Targeted medical nutrition for cachexia in chronic obstructive pulmonary disease: a randomized, controlled trial. J Cachexia Sarcopenia Muscle. 2018;9:28–40. doi: 10.1002/jcsm.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deutz NE, Ziegler TR, Matheson EM, Matarese LE, Tappenden KA, Baggs GE, et al. Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin Nutr. 2021;40:1388–1395. doi: 10.1016/j.clnu.2020.08.031. [DOI] [PubMed] [Google Scholar]
- 27.Korkmaz C, Demirbas S, Vatansev H, Yildirim E, Teke T, Zamani A. Effects of comprehensive and intensive pulmonary rehabilitation and nutritional support on quality of life and functional status in patients with chronic obstructive pulmonary disease. J Int Med Res. 2020;48:300060520919567. doi: 10.1177/0300060520919567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jacome C, Marques A. Short- and Long-term effects of Pulmonary Rehabilitation in patients with mild COPD: a comparison with patients with moderate to severe COPD. J Cardiopulm Rehabil Prev. 2016;36:445–453. doi: 10.1097/HCR.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 29.Geidl W, Semrau J, Streber R, Lehbert N, Wingart S, Tallner A, Wittmann M, Wagner R, Schultz K, Pfeifer K. Effects of a brief, pedometer-based behavioral intervention for individuals with COPD during inpatient pulmonary rehabilitation on 6-week and 6-month objectively measured physical activity: study protocol for a randomized controlled trial. Trials. 2017;18:396. doi: 10.1186/s13063-017-2124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esteve Simo V, Junqué Jiménez A, Moreno Guzmán F, Carneiro Oliveira J, Fulquet Nicolas M, Pou Potau M, Saurina Sole A, Duarte Gallego V, Tapia Gonzalez I, Ramirez de Arellano M. Benefits of a low-intensity exercise program during hemodialysis sessions in elderly patients. Nefrologia. 2015;35:385–394. doi: 10.1016/j.nefro.2015.03.006. 10.1016/j.nefro.2015.03. 006 . [DOI] [PubMed] [Google Scholar]
- 31.Mesquita R, Meijer K, Pitta F, Azcuna H, Goërtz YM, Essers JM, Wouters EF, Spruit MA. Changes in physical activity and sedentary behavior following pulmonary rehabilitation in patients with COPD. Respir Med. 2017;126:122–129. doi: 10.1016/j.rmed.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Jaatinen N, Korpela R, Poussa T, Turpeinen A, Mustonen S, Merilahti J, Peuhkuri K. Effects of daily intake of yogurt enriched with bioactive components on chronic stress responses: a double-blinded randomized controlled trial. Int J Food Sci Nutr. 2014;65:507–514. doi: 10.3109/09637486.2014.880669. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt, JA, Jorissen BL, Dye L, Markus CR, Deutz NE, Riedel WJ. Memory function in women with premenstrual complaints and the effect of serotonergic stimulation by acute administration of an alpha-lactalbumin protein. J Psychopharmacol. 2005;19:375–384. doi: 10.1177/0269881105053288. 10.1177/02698811 05053288 . [DOI] [PubMed] [Google Scholar]
- 34.Markus CR, Olivier B, de Haan EH, Haan E. Whey protein rich in alpha-lactalbumin increases the ratio of plasma tryptophan to the sum of the other large neutral amino acids and improves cognitive performance in stress-vulnerable subjects. Am J Clin Nutr. 2002;75:1051–1056. doi: 10.1093/ajcn/75.6.1051. [DOI] [PubMed] [Google Scholar]
- 35.Constantin D, Menon MK, Houchen-Wolloff L, Morgan MD, Singh SJ, Greenhaff P, Steiner MC. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax. 2013;68:625–633. doi: 10.1136/thoraxjnl-2012-202764. [DOI] [PubMed] [Google Scholar]