Abstract
Background:
In clinical practice, females with MS often report menstrually-related symptom fluctuations. Hypothetically, use of oral contraceptives (OCs) could reduce these fluctuations, particularly continuous OCs (11+ weeks of consistent exogenous hormones followed by 1 week placebo).
Objectives:
To prospectively capture (1) whether neurologic and generalized symptoms vary with menstrual cycle phase and (2) whether type of contraception impacts symptom fluctuations.
Methods:
In this two-center pilot study, females with MS and a regular menstrual cycle prospectively tracked their menstrual cycles and completed symptom surveys for up to 6 months. Participants were categorized as 1) users of oral contraceptives, either a) cyclic or b) continuous, or 2) endogenously cycling, either c) hormonal intrauterine device (IUD) users or d) “none users” (e.g. no hormonal contraception; included condoms, copper IUD, tubal ligation, “fertility awareness methods”). There was no correction for multiple analyses.
Results:
Altogether, 47/70 participants (67%) provided >4 weeks of data and were included in the analyses. Mean (SD) age was 35.0 (0.9) years, median (IQR) EDSS was 1.5 (1–2) and mean (SD) SymptoMScreen score was 10.4 (9.6). For endogenously cycling patients (IUD and none users), fatigue (MFIS) was lower in the perimenstrual period than in the luteal period (p < 0.05). For continuous OC users, variability in symptoms was lower than for endogenously cycling females (MFIS: p < 0.01; Daily Hassles, from Uplift & Hassles Survey: p < 0.05) or cyclic OC users (MFIS: p < 0.001).
Conclusions:
In this pilot study, symptom severity did not definitively fluctuate in relationship to the menstrual cycle in endogenously cycling participants. However, fatigue and daily hassles were less variable for participants using continuous OC than for cyclic OC users or no-OC users. Future confirmatory studies are warranted to further examine whether contraceptive choice can be leveraged to manage symptom fluctuation in cycling females with MS. Such studies could enroll larger cohorts over fewer cycles or employ incentivization and hormonal measurements to enhance participant retention and statistical power.
Keywords: Menstrual cycle, Hormone, Estrogen, Contraception, Symptom, Multiple sclerosis
1. Background
MS is an autoimmune, degenerative neurological disease that predominantly affects females, with onset typically between the ages of 20 and 45 years. Previous studies have suggested that marked hormonal fluctuations, such as those seen after menarche and during pregnancy, can modulate both immune regulation (i.e., new demyelinating lesions and relapses), as well as neural activity (e.g., fluctuations in existing symptoms) (Bove and Chitnis, 2014; Lulu et al., 2016) Less studied are the effects of milder endogenous hormonal fluctuations, such as those seen over the course of the menstrual cycle, on either MS inflammatory activity or symptoms. In clinical practice, many females report symptom fluctuation tied to their menstrual cycle, and small studies have similarly described a possible worsening of neurologic symptoms preceding menstruation (Zorgdrager and De Keyser, 2002; Gollenberg et al., 2010). Observational studies have also suggested differences in inflammatory activity across the menstrual cycle (Pozzilli et al., 1999).
Menstrual-associated hormonal fluctuations are a well-known factor that contributes to the experience of a variety of neurologic diseases, including epilepsy and migraine (Herzog et al., 2015; Taubøll et al., 2021; Nappi et al., 2022). Since pharmacologically reducing hormonal fluctuations via oral contraceptives can be used as a strategy to minimize the clinical impact of epilepsy and migraine (De Leo et al., 2011; Nappi et al., 2013; Penovich and Helmers, 2008), we hypothesized that this could also be impactful in MS. Indeed, the application of systemic exogenous hormones such as oral contraceptives (OCs) could play a role in stabilizing inflammatory activity and/or symptom fluctuations (Holmqvist et al., 2006). Supporting this hypothesis, a randomized clinical trial (RCT) of a common OC formulation suggested a reduction in inflammatory activity (Pozzilli et al., 2015), as did a trial of the pregnancy hormone estriol (Voskuhl et al., 2016).
OCs consist of exogenous estrogens and/or progestogens that suppress sex hormone fluctuations during the menstrual cycle. OCs act systemically, in contrast to hormonal intrauterine devices (IUDs), which have local effects on endometrial activity but only minor systemic effects (Xiao et al., 1995). Among OCs, cyclic OCs provide consistent hormones for 3 weeks, followed by one week placebo for a withdrawal bleed, while continuous OCs provide 11+ weeks of exogenous hormones followed by the placebo week (Cooper et al., 2022). In an exploratory retrospective study, females on continuous OCs showed a trend towards reduced inflammatory activity on MRI compared to females on cyclic contraceptives (Chen et al., 2020).
The current study expands on these observations by prospectively evaluating the relationship between menstrual cycle phase and day-to-day MS symptoms. We hypothesized that exogenous hormones that reduce endogenous cycling would decrease day-to-day fluctuations in MS symptoms. Moreover, we hypothesized that females on continuous OC would exhibit the least fluctuation in day-to-day symptoms. These hypothetical effects are illustrated in Fig. 1.
Fig. 1. Hypothesized symptom severity changes according to contraceptive group across the menstrual cycle phases.
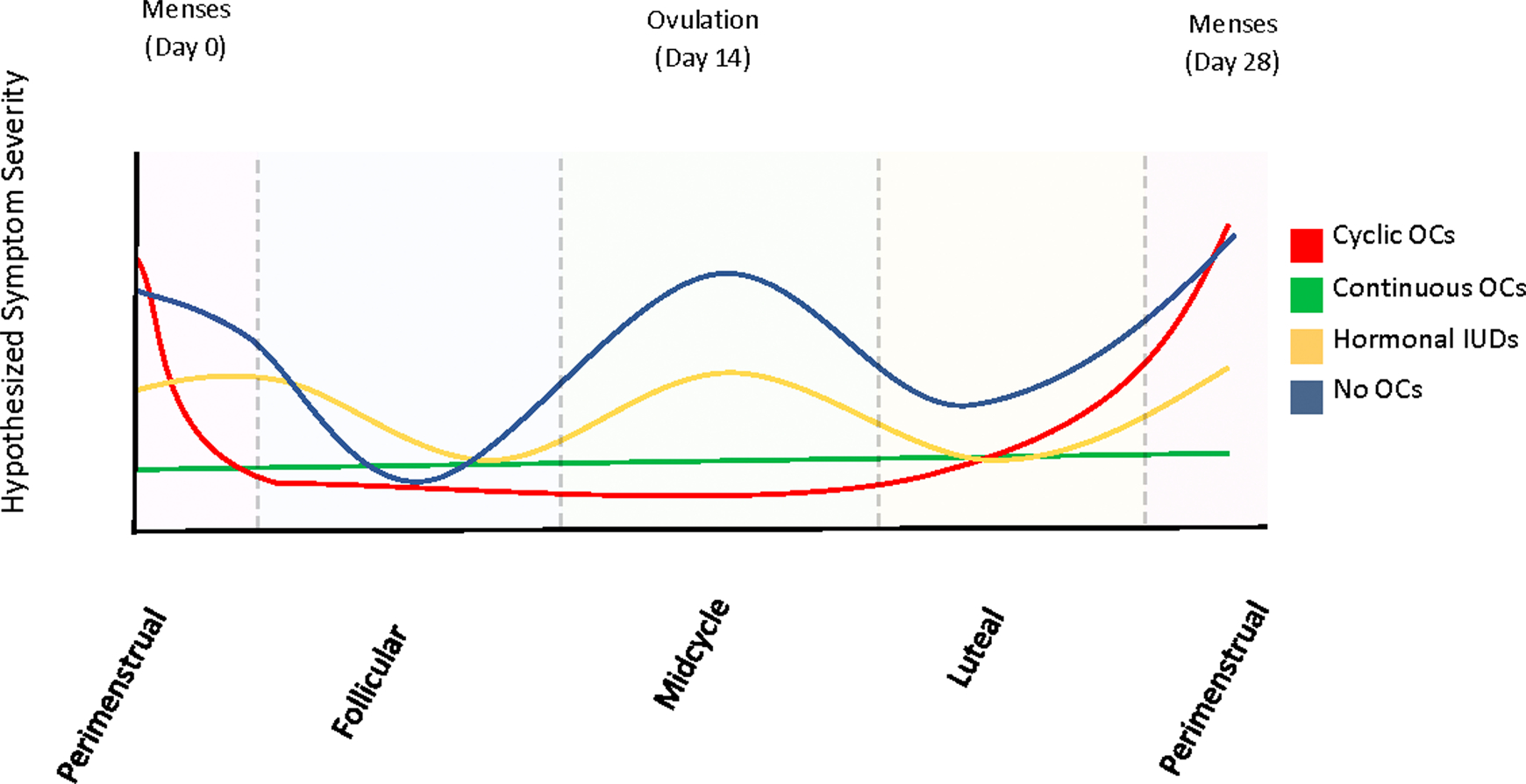
In females on oral contraceptives (OCs), the follicular (day 4–10), midcycle (day 10–17) and luteal (day 17–24) points are collapsed into “ON hormone”, and the perimenstrual (day 25–3) phase as “OFF hormone” (when taking placebo).
2. Methods
In this prospective, observational, multicenter study, general and neurologic symptoms were prospectively reported over up to 6 consecutive menstrual cycles, by adult females with MS cared for at two MS Centers: University of California San Francisco (UCSF) MS and Neuroinflammation Center and Yale Multiple Sclerosis Center.
2.1. Participants
Female patients between the ages of 18 and 45 with a diagnosis of MS/Clinically Isolated Syndrome (CIS) per 2017 McDonald Criteria and a history of normal menstrual cycles were included in the study. Normal menstrual cycles were defined as consecutive cycles that are between 21 and 35 days in length, without history of amenorrhea lasting three months or more, or oligomenorrhea (fewer than nine menstrual cycles per year or cycle length greater than 35 days). Participants were neurologically stable with no relapses or steroid use for at least 3 months prior to enrollment and had no changes in disease modifying therapy (DMT) for at least the preceding 6 months. All participants were using a stable contraceptive method for at least 3 months at enrollment; contraceptives were classified as cyclic OC, continuous OC, hormonal intrauterine devices (IUD), or non-hormonal contraception [copper IUD, condoms, tubal ligation, Essure, “fertility awareness method” (FAM)]. While IUDs exert mainly local hormonal effects within the uterus, there is evidence of some systemic hormonal effects as well (Huck et al., 2022). Therefore, hormonal IUD users were grouped separately as this method could result in a unique hormonal pattern. Key exclusion criteria included any gynecologic or metabolic disease affecting menstruation (i. e., polycystic ovarian syndrome), current pregnancy, breastfeeding, or delivery in the prior 6 months. Patients with severe depression (Patient Health Questionnaire 9, PHQ9>15) were also excluded. Full inclusion and exclusion criteria are provided in Supplementary Table 1.
2.2. Study procedures
To minimize health risks and burden to participants, all communication and data collection occurred virtually. After signing the informed consent, participants completed a detailed screening questionnaire using electronic data capture (REDCap) to verify eligibility criteria as outlined in Supplementary Table 1. Demographic (age, race, ethnicity), clinical (body mass index (BMI), smoking status) and MS (most recent EDSS, MS treatment, MS duration) characteristics were extracted from the medical record.
Over the 6-month study, participants recorded their menstrual cycle symptoms daily, using the Fitbit cycle tracker mobile app accessed by using an anonymized study-specific email login and password. They also completed weekly questionnaires that assessed key symptoms of interest. The SymptoMScreen is a battery of 7-point Likert scales for 12 distinct domains commonly affected by MS: mobility, dexterity, body pain, sensation, bladder function, fatigue, vision, dizziness, cognition, depression, and anxiety (Green et al., 2017). Depression was evaluated weekly using the Patient Health Questionnaire 9 (PHQ9), a validated survey that evaluates depressive symptoms over the preceding two weeks; with 9 items each allowing four response options for the frequency of symptom experience (Patrick and Connick, 2019). Fatigue was evaluated using the Modified Fatigue Impact Scale (MFIS) (Larson, 2013). The Perceived Stress Scale (PSS10) (Wu and Amtmann, 2013) was used to evaluate fluctuations in subjective stress. To capture daily hassles and uplifts experienced by patients, two questions were selected from the Uplift & Hassles survey (DeLongis et al., 1982): one measures the magnitude of hassles, the other one the uplifts experienced; answers are selected from a 6-item Likert scale. Finally, overall quality of life (MSQOL-54) (Vickrey et al., 1995) was assessed at baseline, month 3 and end of follow-up. The data acquisition timeline is summarized in Supplementary Table 2. Patients with monthly menses were asked to complete questionnaires on the first day of their menstrual period and weekly thereafter. Participants without monthly menses (e.g., continuous OCs) began completing weekly questionnaires at the time of enrollment.
At study completion, each data collection timepoint was categorized based on the participants’ menstrual period dates: perimenstrual phase (3 days before and 3 days after the last menstrual period (LMP)); follicular phase (4 to 10 days after the LMP), midcycle (10–17 days after the LMP) and luteal phase (17–24 day after the LMP).
3. Statistical analyses
To describe participants’ characteristics, descriptive statistics were utilized, and then the groups were compared using analysis of variance (ANOVA).
To assess changes in severity in general and neurological symptoms across the menstrual cycle phases for endogenously cycling versus non-cycling females, we categorized females into “endogenously cycling” (those using hormonal IUDs and non-hormonal contraception) or “not endogenously cycling” (those using OCs). We generated linear mixed effects models with repeated measures, using survey responses as the dependent variable. Time was considered in two ways: considering all four menstrual phases separately, and categorizing into either perimenstrual or non-perimenstrual (combined: follicular, midcycle, luteal timepoints). Here, patients on hormonal IUDs were considered to be “endogenously cycling”, because the hormones exert local effects (on endometrial lining) without exerting marked systemic effects (that would be expected to influence neurological symptoms).
To determine whether females on OCs exhibit a net increase in symptom severity in the perimenstrual, OFF timepoint, as compared to the non-perimenstrual, ON timepoints, a linear mixed model for repeated measures was employed.
To determine whether symptom variability was lower among females using continuous OCs, the standard deviation of survey scores was compared between the continuous OC users and the cyclic OC users, and then between the continuous OC users and the endogenously cycling (hormonal IUDs or no hormonal contraception) participants using ANOVA and the difference in variability was assessed with Barlett’s test for equal variances.
Finally, to test whether there was a difference in overall symptom burden experienced based on the contraceptive method used, the week-to-week score means were compared between the four contraceptive groups using repeated measures ANOVA.
4. Results
Between March and October 2021 70 participants meeting eligibility criteria were enrolled (Fig. 2). All were cis-women assigned female at birth. Participants who were lost to follow-up or provided less than 4 weeks of data were excluded from analysis. A total of 47 participants provided at least 4 weeks of follow-up data; their clinical, demographic and study participation details are summarized in Table 1. Overall, this was a low-disability cohort. Forty-four (93.6%) participants had RRMS (relapsing remitting MS). The median (IQR) EDSS was 1.5 (1–2) and most participants were treated with infusible disease modifying therapies. When comparing across groups, the continuous OC group had the fewest participants, slightly older age and longer mean time on study than their counterparts, but these differences were not statistically significant (p > 0.10 for each).
Fig. 2. STROBE Diagram detailing study participation.
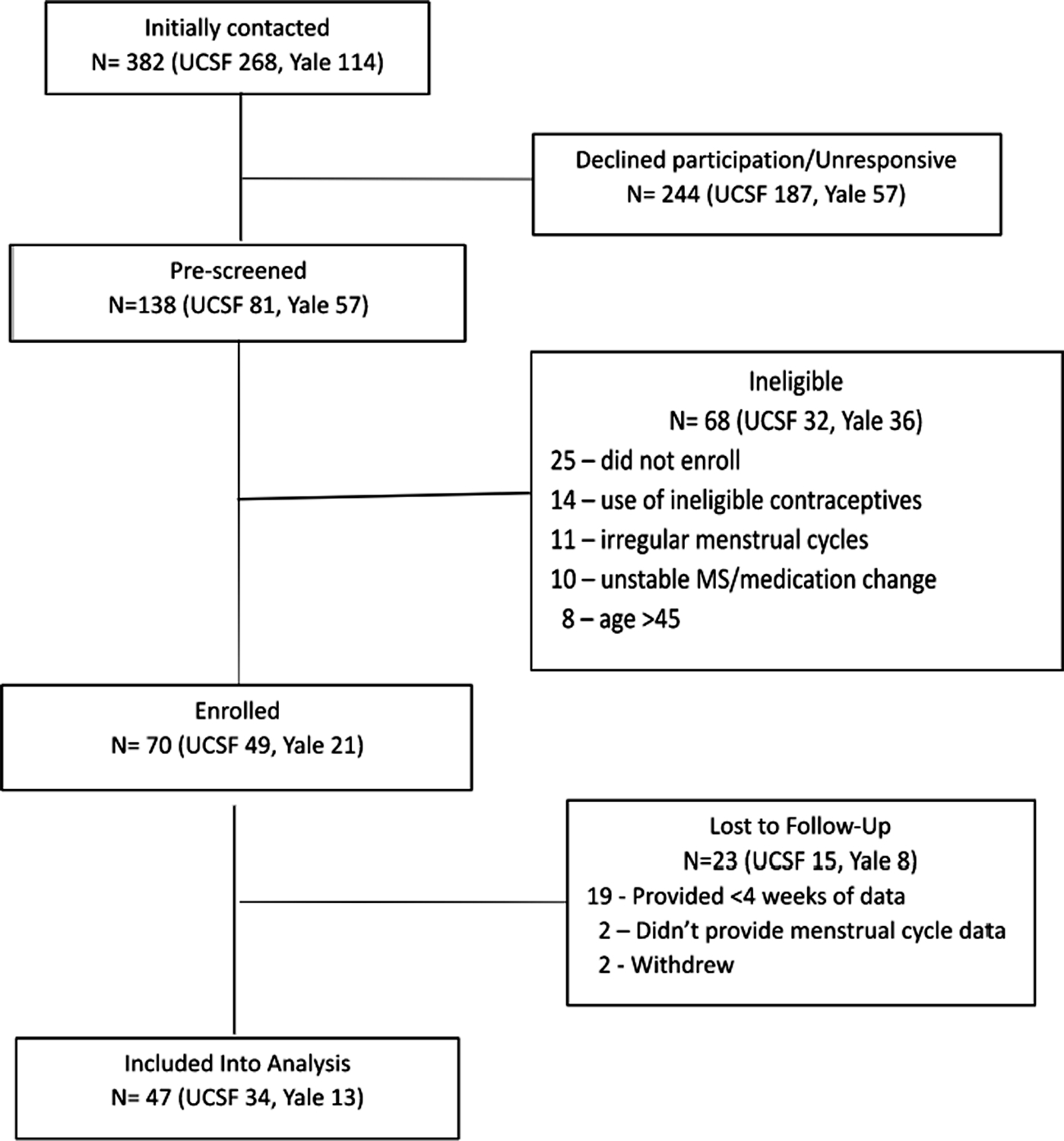
Table 1.
Demographic and clinical characteristics of study participants.
| Oral contraceptives (OC) | No OC | ||||
|---|---|---|---|---|---|
| Cyclic OC (n = 7) | Continuous OC (n = 6) | IUD (n = 11) | No hormones (n = 23)* | Total (n = 47) | |
|
| |||||
| Age | |||||
| Mean ± SD | 32 ± 5.2 | 38.2 ± 5.4 | 34.5 ± 4.1 | 35.3 ± 7.3 | 35 ± 6.2 |
| Race (%) | |||||
| White/Caucasian | 6 (85.7) | 5 (83.3) | 7 (63.6) | 20 (87.0) | 38 (80.8) |
| Asian | 0 (0.0) | 1 (16.7) | 1 (9.1) | 0 (0.0) | 2 (4.3) |
| Other | 1 (14.3) | 0 (0.0) | 2 (18.2) | 3 (13.0) | 6 (12.8) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (2.1) |
| Ethnicity (%) | |||||
| Hispanic or Latino | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (13.0) | 3 (6.4) |
| Not Hispanic or Latino | 7 (100.0) | 6 (100.0) | 10 (91.0) | 20 (87.0) | 41 (91.1) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (9.0) | 0 (0.0) | 1 (2.1) |
| Marital Status (%) | |||||
| Single | 3 (42.9) | 4 (66.7) | 4 (36.4) | 10 (43.5) | 21 (44.7) |
| Married | 4 (57.1) | 2 (33.3) | 6 (54.6) | 11 (47.8) | 23 (48.9) |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (9.0) | 2 (8.7) | 3 (6.4) |
| BMI | |||||
| Mean ± SD | 24.5 ± 3.7 | 25.2 ± 6.7 | 26.6 ± 5.9 | 25.4 ± 5.1 | 25.5 ± 5.2 |
| DMT (%) | |||||
| Infusion Therapy | 4 (57.1) | 5 (83.3) | 10 (90.9) | 17 (73.9) | 36 (76.6) |
| Oral Therapy | 1 (14.3) | 1 (16.7) | 0 (0.0) | 2 (8.7) | 4 (8.5) |
| First-Line Self-Injectables** | 1 (14.3) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 2 (4.3) |
| No DMT | 1 (14.3) | 0 (0.0) | 0 (0.0) | 4 (17.4) | 5 (10.6) |
| MS Type (%) | |||||
| Relapsing-Remitting | 7 (100.0) | 6 (100.0) | 10 (90.9) | 21 (91.3) | 44 (93.6) |
| Primary Progressive | 0 (0.0) | 0 (0.0) | 1 (9.1) | 2 (8.7) | 3 (6.4) |
| EDSS | |||||
| Median (IQR) | 1.5 (1.5–1.5) | 1.5 (1–1.5) | 1.5 (1–2) | 1.5 (1–2.5) | 1.5 (1–2) |
| Time On Study | |||||
| Mean N weeks ± SD | 17.9 ± 5.8 | 21.3 ± 4.2 | 20.8 ± 6.7 | 19.7 ± 6.1 | 19.9 ± 5.9 |
| Recruitment Site (%) | |||||
| UCSF | 3 (42.9) | 4 (66.7) | 10 (90.9) | 15 (65.2) | 32 (31.9) |
| Yale | 4 (57.1) | 2 (33.3) | 1 (9.1) | 8 (34.8) | 15 (68.1) |
Abbreviations: BMI: Body Mass Index; CIS: Clinically Isolated Syndrome; DMT: Disease Modifying Therapy; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; OC: oral contraceptive; IQR: Interquartile Range; SD: Standard deviation; IUD: intrauterine device.
The “no hormones” group included: Condom (n = 14), Copper IUD (n = 4), Tubal ligation (n = 3), FAM (n = 1) and Essure (n = 1).
First-line self-injectables = glatiramer, interferons.
4.1. Association between menstrual cycle phase and symptom severity in endogenously cycling women
Among the endogenously cycling participants (hormonal IUDs or non-hormonal contraception), when comparing scores across the four menstrual cycle phases, MFIS score was an average of 1 point lower in the perimenstrual period compared to the luteal period (p = 0.038), but this difference is unlikely to be clinically significant and there were no other differences in scores across the menstrual cycle (all p > 0.05). When menstrual cycle phases were collapsed into perimenstrual vs. non-perimenstrual (follicular, midcycle and luteal) phases, there were no significant differences in score severity for any symptom evaluated (SymptoMSScreen, MFIS, PHQ9, Perceived Stress or Hassles and Uplift).
4.2. Association between “ON hormone” and “OFF hormone” and symptom severity among OC users
In the females on exogenous OCs (cycling and continuous), there were no significant differences in symptom severity between the ON timepoints (11 weeks for continuous OCs and 3 weeks for cyclic OCs), and OFF timepoint.
4.3. Association between symptom variability and contraceptive type: comparisons between continuous OC users and other groups
Continuous OC users had less symptom variability between menstrual cycle phases than cyclic OC users for fatigue (MFIS) (one-way ANOVA: F(1, 235)=[45.7], p < 0.001)(Fig. 3); there were trends for decreased variability in scores for the other domains (SymptoMScreen, PHQ9, Hassles and Uplift and Perceived Stress Scale) among continuous OC users, but these were not statistically significant (all p > 0.05) (Table 2).
Fig. 3. Comparison of symptom score variability between Continuous, Cyclic and No OC groups. Continuous OC users appear to have less variability in fatigue and hassles scores over their menstrual cycles.
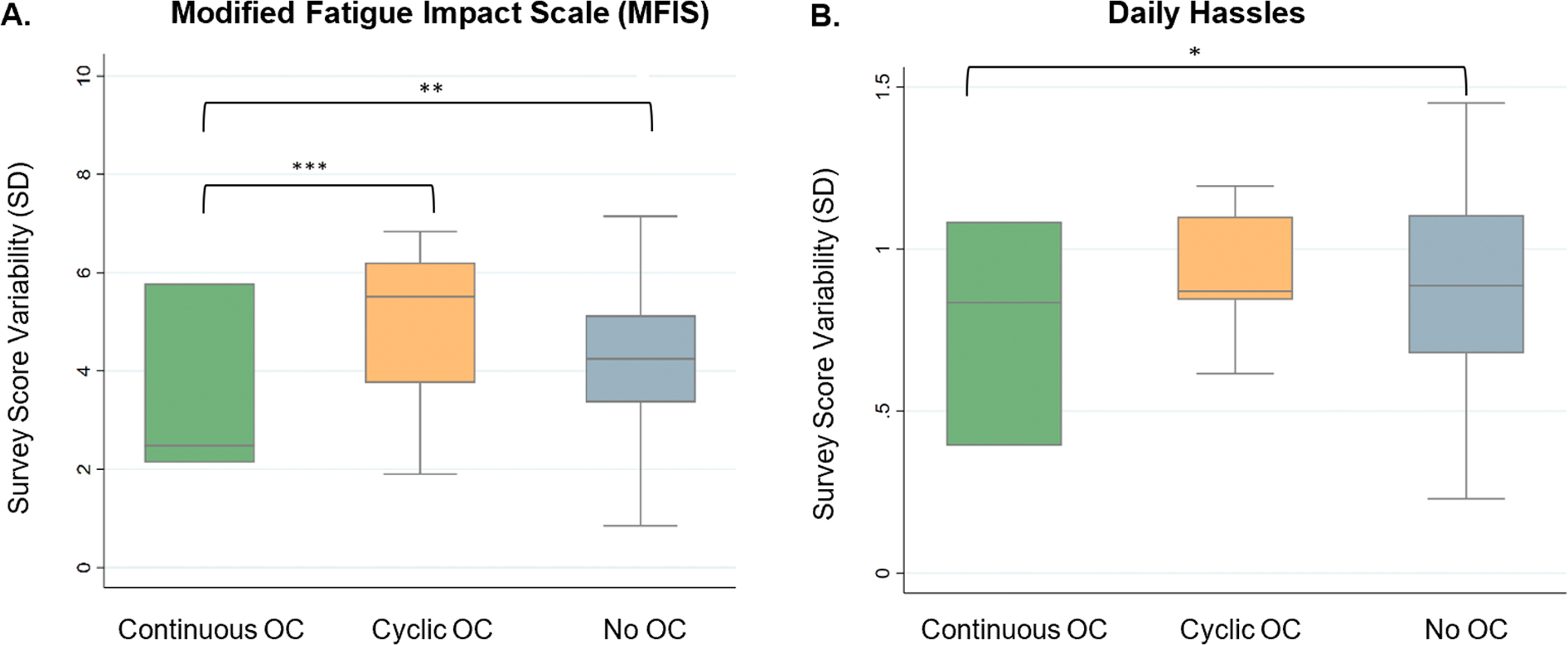
Comparison of survey score variability in Modified Fatigue Impact Scale (MFIS) (A) and Daily Hassles (B) between Continuous, Cyclic, and No OC groups. Comparisons performed using ANOVA. * p < 0.05, ** p < 0.01, *** p < 0.001.
Table 2.
Symptom variability between Continuous OC compared to cyclic and non-OC groups
For each outcome evaluated, the first row presents data on the Continuous OC users. The second row presents comparisons between Continuous and Cyclic OC users, and the third row presents comparisons between Continuous OC users and non-OC users. Rows with significant differences relative to Continuous OCs are shaded in gray.
| Groups | Mean | Std. Deviation | P value* | Barlett’s test** | |
|---|---|---|---|---|---|
|
| |||||
| MFIS | Cont. | 3.44 | 1.68 | ||
| OC | |||||
| Cyclic | 5.05 | 1.63 | 0.000 | 0.818 | |
| OC | |||||
| No OC | 4.24 | 1.80 | 0.001 | 0.435 | |
| Hassles | Cont. | 0.79 | 0.30 | ||
| OC | |||||
| Cyclic | 0.93 | 0.18 | 0.000 | 0.000 | |
| OC | |||||
| No OC | 0.88 | 0.29 | 0.011 | 0.680 | |
| PHQ9 | Cont. | 1.20 | 011 | ||
| OC | |||||
| Cyclic | 2.30 | 1.42 | 0.000 | 0.000 | |
| OC | |||||
| No OC | 1.89 | 1.05 | 0.000 | 0.000 | |
| SymptoMScreen | Cont. | 1.22 | 0.47 | ||
| OC | |||||
| Cyclic | 2.49 | 0.72 | 0.000 | 0.000 | |
| OC | |||||
| No OC | 2.14 | 1.15 | 0.000 | 0.000 | |
| PSS10 | Cont. | 1.85 | 0.66 | ||
| OC | |||||
| Cyclic | 1.96 | 0.39 | 0.111 | 0.000 | |
| OC | |||||
| No OC | 2.47 | 1.30 | 0.000 | 0.000 | |
| Uplift | Cont. | 0.87 | 0.08 | ||
| OC | |||||
| Cyclic | 0.84 | 0.25 | 0.395 | 0.000 | |
| OC | |||||
| No OC | 0.82 | 0.32 | 0.199 | 0.000 | |
p-value in comparison with Cont. OC users, derived from ANOVA where p ≤ 0.05 represents that the score means are significantly different between the groups.
p-value in comparison with Cont. OC users, derived from Bartlett’s test for homogeneity of variances where p > 0.05 represents that the score variance is significantly different between the groups.
Continuous OC users were also compared with endogenously cycling females (i.e., those not on OCs). There was less variability in the scores for fatigue (MFIS) (F(1, 815)=[12.21], p < 0.01) and Daily Hassles (F(1, 815)=[6.46], p < 0.05) for females in the continuous OC group (Fig. 3). Again, there were trends for decreased variability in scores for the other domains (SymptoMScreen, PHQ9, PSS10, Uplift) but these were not statistically significant (p > 0.05 for each) (Table 2).
4.4. Overall symptom severity across all contraceptive groups
There was no statistical evidence that the symptom score means in the menstrual cycle phases were significantly different across the four contraceptive groups studied.
5. Discussion
A prior retrospective observational study of females with MS found continuous OC use to be associated with fewer inflammatory lesions on MRI (Chen et al., 2020), but prospective data on symptom severity as it relates to menstrual phases are limited (Zorgdrager and De Keyser, 2002; Holmqvist et al., 2009). This pilot study evaluated the feasibility of answering two important clinical questions, namely: whether day-to-day MS symptoms demonstratably fluctuate across the menstrual cycle and whether certain forms of contraception may reduce these fluctuations. We observed less week-to-week variability in symptom scores among continuous OC users compared to those using other types of contraception.
For each outcome evaluated, the first row presents data on the Continuous OC users. The second row presents comparisons between Continuous and Cyclic OC users, and the third row presents comparisons between Continuous OC users and non-OC users. Rows with significant differences relative to Continuous OCs are shaded in gray.
Day-to-day symptoms of MS vary considerably from person to person and may include pain, spasticity, “brain fog” and fatigue. Many of these symptoms are nonspecific and could overlap with the menstrual associated symptoms frequently observed in healthy females. As such, selecting appropriate patient reported outcomes is challenging. We selected a diverse spectrum of reporting tools, including scales targeting MS symptoms, overall health, fatigue, and stress. We used the Fitbit platform to collect information about systemic symptoms often associated with menstruation like mood swings or bloating. Using such a broad spectrum of outcomes, while informative, may have contributed to survey fatigue and participant attrition. The degree to which symptoms fluctuate in MS patients compared to healthy menstruating females also remains to be quantified.
We categorized menstrual cycle phases relative to self-reported menses, assuming that ovulation would take place 14 days later. Given the physiologically variable nature of the menstrual cycle (Mihm et al., 2011), precise determination of the menstrual cycle phase might require hormonal measurements, and this could be considered in future studies.
We found recruitment for this study to be difficult and were limited by low participant numbers and attrition: our initial goal was to collect data for up to 6 months, but only 67% of participants provided more than 4 weeks of data. Additionally, the inclusion criteria of having stable MS and normal menstrual cycles led to many potential participants being excluded. These challenges will need to be considered in future work examining the impact of hormonal fluctuations on day-to-day symptoms of MS, as it is likely that adjustments to the study design may impact the success of future work in this space.
Conclusions:
Females with MS reported a variety of symptoms across their menstrual cycles. In this pilot cohort, we observed decreased variability in patient-reported fatigue among the subset of females taking continuous oral contraceptives. Larger studies are needed to ascertain whether menstruation definitively impacts MS-related symptoms and whether suppressing endogenous hormone cycling using contraceptives could be a viable strategy for minimizing symptom fluctuations.
Supplementary Material
Acknowledgements
The authors thank the study participants for their contributions to the study.
Role of the funding source
No specific funding was provided for this project.
Study Funding
This work did not receive specific support. Dr. Bove is supported by the National Multiple Sclerosis Harry Weaver Award.
Footnotes
Declaration of Competing Interest
Helga Taylor, Saleh Alhasan, Maha Saleem, Shane Poole, Fei Jiang report no disclosures.
Erin E. Longbrake has received research support from Genetech, NIH. She has received consulting or advisory board fees from Genentech, Janssen, TG Therapeutics, NGM Bio, Bristol Myers Squibb, EMD Serono and Genzyme.
Riley Bove is supported by a National Multiple Sclerosis Society Harry Weaver Award. She receives research support from NIH, NSF, Department of Defense, National Multiple Sclerosis Society, as well as from Biogen, Novartis and Roche Genentech. She also reports scientific advisory board and consulting fees from Alexion, EMD Serono, Horizon, Genzyme Sanofi, Janssen, and TG Therapeutics.
CRediT authorship contribution statement
Helga Taylor: Conceptualization, Formal analysis, Investigation, Writing – original draft. Saleh Alhasan: Conceptualization, Formal analysis, Investigation, Writing – original draft. Maha Saleem: Data curation, Investigation. Shane Poole: Data curation, Writing – review & editing. Fei Jiang: Conceptualization, Methodology. Erin E Longbrake: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing. Riley Bove: Conceptualization, Investigation, Project administration, Supervision, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2023.104864.
References
- Bove R, Chitnis T, 2014. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult. Scler. 20 (5), 520–526. Apr. [DOI] [PubMed] [Google Scholar]
- Chen CS, Krishnakumar T, Rowles W, Anderson A, Zhao C, Do L, et al. , 2020. Comparison of MS inflammatory activity in women using continuous versus cyclic combined oral contraceptives. Mult. Scler. Relat. Disord. 41, 101970. Jun. [DOI] [PubMed] [Google Scholar]
- Cooper DB, Patel P, Mahdy H, 2022. Oral contraceptive pills. StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL) [cited 2022 Sep 8]. Available from. http://www.ncbi.nlm.nih.gov/books/NBK430882/. [Google Scholar]
- De Leo V, Scolaro V, Musacchio MC, Di Sabatino A, Morgante G, Cianci A, 2011. Combined oral contraceptives in women with menstrual migraine without aura. Fertil. Steril. 96 (4), 917–920. Oct. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Coyne JC, Dakof G, Folkman S, Lazarus RS, 1982. Relationship of daily hassles, uplifts, and major life events to health status. Health Psychol. 1, 119–136. [Google Scholar]
- Gollenberg AL, Hediger ML, Mumford SL, Whitcomb BW, Hovey KM, Wactawski-Wende J, et al. , 2010. Perceived stress and severity of perimenstrual symptoms: the BioCycle Study. J. Womens Health (Larchmt.) 19 (5), 959–967. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Kalina J, Ford R, Pandey K, Kister I, 2017. SymptoMScreen: a tool for rapid assessment of symptom severity in MS across multiple domains. Appl. Neuropsychol. Adult 24 (2), 183–189. Apr. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Massaro JM, 2015. Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia 56 (5), e58–e62. May. [DOI] [PubMed] [Google Scholar]
- Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J, 2009. Symptoms of multiple sclerosis in women in relation to cyclical hormone changes. Eur. J. Contracept. Reprod. Health Care 14 (5), 365–370. Oct. [DOI] [PubMed] [Google Scholar]
- Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J, 2006. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas 54 (2), 149–153. May 20. [DOI] [PubMed] [Google Scholar]
- Huck LC, Truhn D, Wilpert C, Zanderigo E, Raaff V, Dethlefsen E, et al. , 2022. Background parenchymal enhancement in contrast-enhanced MR imaging suggests systemic effects of intrauterine contraceptive devices. Eur. Radiol. 32 (11), 7430–7438. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson RD, 2013. Psychometric Properties of the Modified Fatigue Impact Scale. Int. J. MS Care 15 (1), 15–20. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulu S, Graves J, Waubant E, 2016. Menarche increases relapse risk in pediatric multiple sclerosis. Mult. Scler. 22 (2), 193–200. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihm M, Gangooly S, Muttukrishna S, 2011. The normal menstrual cycle in women. Anim. Reprod. Sci. 124 (3–4), 229–236. Apr. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Terreno E, Sances G, Martini E, Tonani S, Santamaria V, et al. , 2013. Effect of a contraceptive pill containing estradiol valerate and dienogest (E2V/DNG) in women with menstrually-related migraine (MRM). Contraception 88 (3), 369–375. Sep. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Tiranini L, Sacco S, De Matteis E, De Icco R, Tassorelli C, 2022. Role of estrogens in menstrual migraine. Cells 11 (8), 1355. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick S, Connick P, 2019. Psychometric properties of the PHQ-9 depression scale in people with multiple sclerosis: a systematic review. PLoS ONE 14 (2), e0197943. Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penovich PE, Helmers S, 2008. Catamenial epilepsy. Int. Rev. Neurobiol. 83, 79–90. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Falaschi P, Mainero C, Martocchia A, D’Urso R, Proietti A, et al. , 1999. MRI in multiple sclerosis during the menstrual cycle: relationship with sex hormone patterns. Neurology 53 (3), 622–624. Aug 11. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Giglio LD, Barletta VT, Marinelli F, Angelis FD, Gallo V, et al. , 2015. Oral contraceptives combined with interferon β in multiple sclerosis. Neurol. - Neuroimmunol. Neuroinflamm. [Internet] 2 (4). Aug 1 [cited 2022 Dec 28]Available from: https://nn.neurology.org/content/2/4/e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubøll E, Isojärvi JIT, Herzog AG, 2021. The interactions between reproductive hormones and epilepsy. Handb. Clin. Neurol. 182, 155–174. [DOI] [PubMed] [Google Scholar]
- Vickrey BG, Hays RD, Harooni R, Myers LW, Ellison GW, 1995. A health-related quality of life measure for multiple sclerosis. Qual. Life Res. 4 (3), 187–206. Jun. [DOI] [PubMed] [Google Scholar]
- Voskuhl RR, Wang H, Wu TCJ, Sicotte NL, Nakamura K, Kurth F, et al. , 2016. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 15 (1), 35–46. Jan. [DOI] [PubMed] [Google Scholar]
- Wu SM, Amtmann D, 2013. Psychometric evaluation of the perceived stress scale in multiple sclerosis. ISRN Rehabil. 2013, 1–9. Dec 22. [Google Scholar]
- Xiao B, Zeng T, Wu S, Sun H, Xiao N, 1995. Effect of levonorgestrel-releasing intrauterine device on hormonal profile and menstrual pattern after long-term use. Contraception 51 (6), 359–365. Jun 1. [DOI] [PubMed] [Google Scholar]
- Zorgdrager A, De Keyser J, 2002. The premenstrual period and exacerbations in multiple sclerosis. Eur. Neurol. 48 (4), 204–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


