Abstract
Osteoarthritis (OA) is the most common joint disease, and good therapeutic results are often difficult to obtain due to its complex pathogenesis and diverse causative factors. After decades of research and exploration of OA, it has been progressively found that subchondral bone is essential for its pathogenesis, and pathological changes in subchondral bone can be observed even before cartilage lesions develop. Osteoclasts, the main cells regulating bone resorption, play a crucial role in the pathogenesis of subchondral bone. Subchondral osteoclasts regulate the homeostasis of subchondral bone through the secretion of degradative enzymes, immunomodulation, and cell signaling pathways. In OA, osteoclasts are overactivated by autophagy, ncRNAs, and Rankl/Rank/OPG signaling pathways. Excessive bone resorption disrupts the balance of bone remodeling, leading to increased subchondral bone loss, decreased bone mineral density and consequent structural damage to articular cartilage and joint pain. With increased understanding of bone biology and targeted therapies, researchers have found that the activity and function of subchondral osteoclasts are affected by multiple pathways. In this review, we summarize the roles and mechanisms of subchondral osteoclasts in OA, enumerate the latest advances in subchondral osteoclast-targeted therapy for OA, and look forward to the future trends of subchondral osteoclast-targeted therapies in clinical applications to fill the gaps in the current knowledge of OA treatment and to develop new therapeutic strategies.
Keywords: osteoarthritis, osteoclast, subchondral bone
Introduction
Osteoarthritis (OA) is one of the most common causes of disability among elderly individuals worldwide. It is essentially a degenerative disease involving lesions of the cartilage, subchondral bone, synovium, meniscus, and surrounding tissues [1]. Currently, the incidence of OA is increasing gradually, and most OA patients are elderly. It is estimated that one-third of people over the age of 65 suffer from OA, with a higher incidence among women than men [2]. The causes of OA are complex and varied and are influenced by a variety of factors, such as sex, age, genetics, diet and obesity; among them, population aging and obesity are the most important factors. Moreover, abnormal mechanical cues, such as joint instability, overuse of joints, and imbalance of muscle strength, are also important factors in the development of OA, and sports injuries can lead to structural and weight-bearing abnormalities in joints, increase the risk of cartilage damage, and contribute to OA [3]. Osteophytes are a prevalent anatomical manifestation of OA. They are believed to originate from cells in the periosteum. The process of osteophyte formation shares similarities with the process of bone repair observed during fracture healing, where the periosteum is deemed crucial. Stem cells derived from the periosteum, known as periosteum-derived cells (PDCs), reside in the cambium periosteum layer and exhibit pluripotent characteristics. PDCs play a pivotal role in bone repair and can be mobilized in response to inflammatory reactions and mechanical stimuli. Abnormal mechanical stress not only induces OA but also accelerates and promotes the production of bone encumbrances, which exacerbates the pain of OA patients [ 4, 5].
Joint pain and loss of function are the main reasons for treating OA. Common treatments include nonpharmacological treatments, such as weight control and physiotherapy. Pharmacological treatments include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and hyaluronic acid, whereas surgical treatments include joint replacement. Due to the numerous pathogenic factors and complex pathological processes involved, conventional treatments can alleviate only the symptoms of OA but cannot reverse the progression of OA or restore normal joint structure [ 6– 8]. Traditional therapies are frequently accompanied by serious effects. NSAIDs can relieve pain and inflammation, but long-term use may lead to gastrointestinal issues such as gastric ulcers and bleeding, and long-term use of anti-inflammatory drugs may also increase cardiovascular risk. Physiotherapy may initially cause some discomfort and pain but is generally safe; excessive exercise or improper management may aggravate joint damage. Surgical treatment may be accompanied by certain surgical risks, such as infection, bleeding, and anesthesia reactions; in addition, there are various problems, such as loosening of the artificial joint, joint durability, difficulty in recovery, and lack of function [ 9, 10]. Therefore, new treatment methods are urgently needed.
Osteoclasts are indispensable in many bone-related diseases, including osteoporosis, osteosclerosis, bone tumors, rheumatoid arthritis (RA), and Paget’s disease. These diseases usually involve abnormalities in bone structure, density, metabolism, or function [11]. In osteoporosis, overactivated osteoclasts absorb excess calcium and phosphorus from bone tissue, leading to decreased bone mineral density and fragility [ 12, 13]. Osteosclerosis, a genetic disorder characterized by increased bone mass, is caused by defects in osteoclast formation and function [ 14, 15]. In addition, Scr kinase deficiency has been reported to affect osteoclast activity, leading to osteoporosis [16]. Scr kinase deficiency leads to a lack of intact folds in osteoclasts, which impacts the adequate contact of osteoclasts with the bone surface and their ability to absorb minerals and proteins from bone tissue [ 17, 18]. In the case of bone tumors, surrounding malignant cells may activate osteoclasts, leading to destruction of the bone structure and irritation of nerve endings. Patients with bone tumors may experience bone-related pain [19]. Additionally, there are interactions between osteoclasts and malignant bone tumor cells. Tumor cells may produce chemokines that attract osteoclasts to migrate around tumors, thereby accelerating the destruction of bone tissue around tumors and enhancing the invasive ability of bone tumors [ 20, 21]. The primary function of osteoclasts in RA is related mainly to joint destruction and pain. During RA, inflammatory cells stimulate osteoclasts to release enzymes and cytokines related to bone resorption, destroy bone tissues, alter the joint microenvironment, and aggravate inflammation, resulting in severe joint damage and pain [ 22– 25]. The activity and number of osteoclasts in Paget’s disease are markedly increased, which leads to changes in bone mineral density and greatly increases the risk of fracture; concurrently, altered bone mineral density greatly increases the risk of fracture due to the significantly increased activity and number of osteoclasts. Moreover, excessive bone resorption increases the production rate of new bone tissue, but the new bone is usually arranged unevenly, resulting in an irregular bone shape and abnormal bone remodeling, which can cause bone deformity and pain [ 26– 28].
A growing body of evidence indicates that osteoclasts play an important role in the development of many diseases. However, the functions and mechanisms of action of subchondral osteoclasts in OA remain unclear. Therefore, this review summarizes the roles and mechanisms of subchondral osteoclasts in OA progression and regulation to provide new targets for treating OA.
Composition of Subchondral Bone
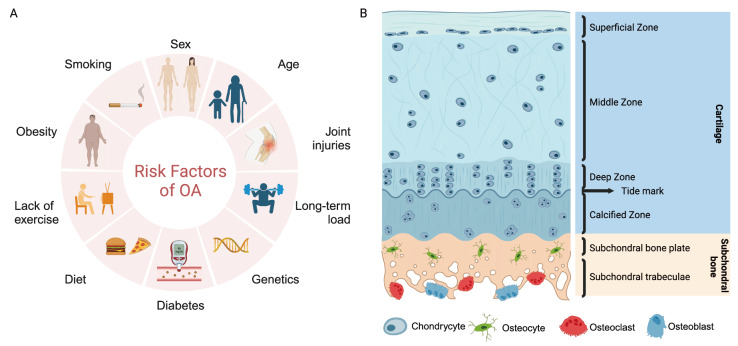
The subchondral bone is located distal to the calcified cartilage. It usually plays a role in maintaining joint elasticity, supporting articular cartilage, and influencing cartilage metabolism [29]. It can generally be divided into two anatomical entities: the subchondral bone plate and subchondral trabecular bone [30] ( Figure 1). The subchondral bone plate consists of a thin layer of cortical bone adjacent to calcified cartilage. The cortical bone plate has distinct pores and is a permeable structure that provides a direct connecting channel between the articular cartilage and subchondral trabeculae, through which many blood vessels and nerves pass. In contrast, the subchondral trabeculae are composed of cancellous bone close to the bone marrow cavity; this bone marrow has a sparser structure, is metabolically active, and is rich in blood vessels and nerves [ 31, 32]. Subchondral bone contains a variety of cells with different functions, including osteoclasts, osteocytes, osteoblasts, and endothelial cells. They collectively influence the microstructure and histopathological changes in subchondral bone through cell-mediated remodeling and modeling processes, and the four types of cells in subchondral bone interfere with each other [ 33, 34]. These cells are described below:
Figure 1 .
Many pathological factors, such as smoking, obesity, and aging, are involved in the progression of OA
These unfavorable factors mediate numerous pathological molecular signals within the knee joint, causing imbalances in multiple cellular homeostasis pathways (osteoclast, osteocyte, osteoblast, etc.) in the bone microenvironment and further exacerbating disease progression.
Osteoclast
Osteoclasts are specialized cells with multiple functions that are primarily responsible for bone resorption and remodeling to maintain the normal physiological state of the skeleton. They control bone growth and renewal by binding to the bone surface and releasing acids and enzymes from lysosomes to degrade and dissolve inorganic salts and organic matrices in the bone tissue [ 35, 36]. Osteoclasts are closely associated with the immune system and secrete cytokines and growth factors in response to proinflammatory stimuli [37]. Moreover, osteoclasts are essential for bone metabolism and angiogenesis. On the one hand, osteoclasts control the endocrine regulation of calcium and phosphate by regulating their response to parathyroid hormone and calcitonin; on the other hand, osteoclasts promote angiogenesis by facilitating endothelial cell migration and stimulating the paracrine secretion of endothelial cells to increase vascular endothelial growth factor (VEGF) [ 38, 39]. In addition, osteoclasts regulate osteoblast maturation and differentiation through RANK/RANKL/OPG, Ephrinb2-Ephb4 signaling, sphingolipid signaling, and other membrane-associated proteins [40]. Osteoclasts play an important role in many diseases. However, the function of subchondral osteoclasts in OA remains unclear.
Osteocyte
Osteocytes are the most abundant cells in bone and account for 90%–95% of bone cells. Osteocytes are responsible for maintaining bone homeostasis and mechanotransduction. They are the primary regulators of osteoblasts and osteoclasts. Upon stimulation, osteocytes maintain bone homeostasis by regulating the signals generated by mechanical loads and recruiting osteoclasts and osteoblasts to initiate the repair process [ 41, 42]. Osteocytes also modulate the extracellular matrix through specific molecular remodeling mechanisms. As endocrine cells, osteocytes can further influence bone metabolism by affecting phosphate uptake, insulin secretion, and skeletal muscle function, thereby regulating bone size and shape. Previous studies have demonstrated that osteocytes are crucial for bone aging [ 43, 44]. In addition to these functions, many other functions of osteocytes, such as interactions with the immune system [45], influencing hematopoiesis through the secretion of cytokines [46], and promoting the progression of bone cancer, are still being investigated [47].
Osteoblast
Osteoblasts are derived from bone marrow mesenchymal stem cells (BMSCs), and their differentiation is a key step in osteogenesis. There are three stages of differentiation from BMSCs to osteoblasts: osteogenitor cells, preosteoblasts, and osteoclasts [ 48, 49]. This differentiation process is regulated by various transcription factors, signaling pathways, and genes, such as bone morphogenetic proteins (Bmp), Runx2 transcription factors, and the Wnt signaling pathway [ 50, 51]. Osteoblasts play an important role in bone development and the maintenance of homeostasis. VEGF-derived proteins affect bone repair and regeneration and contribute to bone defect healing by stimulating vascular and osteoclast recruitment [52]. Osteoblasts, which have abundant basophilic cytoplasm, a large number of mitochondria, and high Golgi capacity, produce a unique extracellular protein assemblage consisting of large amounts of collagen type I, osteocalcin, alkaline phosphatase, and the extracellular matrix [53] and simultaneously affect the development and differentiation of osteoclasts. Previous studies have demonstrated that osteoblasts can influence the cellular behavior, survival, and differentiation of osteoclasts through direct contact between osteoblasts and osteoclasts through the bidirectional transactivation of activation signals such as EFNB2-EPHB4 and FASL-FAS or secreted proteins such as PANKL/OPG, M-CSF, Wnt5a, and Wnt16 [ 54, 55].
Endothelial cell
Subchondral bone endothelial cells are smooth monolayers of cells tightly arranged in the lining of the vascular lumen that serve various important physiological functions. Endothelial cells can regulate angiogenesis and blood flow by secreting vascular and secretory factors or engaging in molecular crosstalk with osteoblasts, playing a crucial role in fracture healing and the maintenance of bone homeostasis [ 56, 57]. Endothelial cells can participate in the inflammatory response in the subchondral bone region in various ways, such as through the calcium signaling pathway, which produces several immune factors and chemokines that direct immune cells to the damaged site and promote tissue repair and regeneration [58]. Simultaneously, endothelial cells produce a variety of cytokines, such as basic fibroblast growth factor (bFGF) and ADAMTS, thereby promoting the proliferation and differentiation of BMSCs and maintaining the regenerative capacity of bone tissue [ 59, 60]. Additionally, endothelial cells partially regulate the maturation and differentiation of osteoblasts. Endothelial cells can produce a variety of cytokines and growth factors, such as VEGF, or through cell crosstalk to influence osteoblasts [61].
Origin of Osteoclasts
Since the end of the 19th century, when osteoclasts were first discovered and observed under a microscope, the multinucleated morphology of osteoclasts has given rise to a great deal of discussion about their origin and function. Since then, various experiments have been conducted, and many theories have been proposed to explore and explain the origin of osteoclasts. As common osteocytes and osteoblasts are involved in the regulation of bone remodeling, it was initially thought that there was a commonality between the two in the early 20th century. However, a growing body of evidence is beginning to support a ‘biphyletic origin’ theory between the two types of osteocytes, and morphological similarities have been observed between mature osteoclasts and macrophage-derived cells [62].
The hematopoietic origin of osteoclasts was confirmed by Walker’s pioneering experiments in the 1970s, in which cells from the spleen and bone marrow of normal mice were transplanted into mice suffering from hereditary osteosclerosis and osteoclast deficiency. As a result, bone resorption was restored in the mice, suggesting that the hematopoietic organs could produce certain cells to resorb hardened bone tissue [63]. Subsequently, an increasing number of scholars have shown that osteoclast production is inextricably linked to monocyte/macrophage production. Scheven et al. [64] demonstrated that populations of hematopoietic stem cells (HSCs) can produce osteoclasts and that the ability to produce osteoclasts increases with the purity of stem cells. Previous studies reported that macrophage colony-stimulating factor (M-CSF) activated the differentiation of HSCs into monocytes/macrophages; furthermore, mature cells of the monocyte/macrophage lineage could form osteoclasts, and immature monocytes and macrophages could also form osteoclasts when bone marrow stromal cells provided the appropriate microenvironment [ 65, 66].
In addition, bone and bone marrow contain three distinct macrophage populations, namely, osteoclasts and bone marrow macrophages, haematopoietic stem cell macrophages and osteal macrophages, which also suggests that osteoclasts and macrophages may have similar origins. It has been shown that cells from the monocyte/macrophage system, such as hematopoietic marrow cells, blood monocytes and peritoneal macrophages, can develop into bone-resorbing osteoclasts; therefore, the osteoclast population can be classified within these series of cells. In fact, osteoclasts and macrophages are two differentiation products of myeloid precursors that compete with each other [ 67, 68]. Osteoclasts are generated through a series of processes. The transformation of hematopoietic stem cells (HSCs) to monocytes/phagocytes initiates the differentiation of osteoclasts, followed by the proliferation and differentiation of osteoclast precursors and finally maturation into osteoblasts with bone resorption capacity.
The Role of Subchondral Osteoclasts in OA
Subchondral bone is crucial for OA onset. Under normal physiological conditions, the osteochondral unit comprises subchondral bone and articular cartilage. Articular cartilage provides a smooth surface for movement, whereas subchondral bone provides stability and support. Together, they ensure the proper function and health of joints. A growing body of evidence suggests that abnormal remodeling of the subchondral bone in OA patients occurs before and, to some extent, accelerates articular cartilage degeneration. Additionally, the subchondral bone may be the primary source of pain in OA patients, making it essential to the pathogenesis of this disease [ 69, 70]. In the early stages of OA, hyperactivation of subchondral bone remodeling due to excessive bone resorption has been proposed as a major pathological hallmark of OA [71]. Bone remodeling is a highly coordinated process, and under normal conditions, osteoclast-mediated bone resorption and osteoblast-mediated bone formation are balanced to ensure the maintenance of bone homeostasis [72]. However, when OA occurs, the number of osteoclasts in the subchondral bone significantly increases, leading to enhanced bone resorption and alterations in the microstructure and microenvironment of the subchondral bone. Multiple signaling pathways and molecules are involved in the recruitment of subchondral osteoclasts. First, RANKL binds to the RANK receptor on the surface of osteoclasts and activates osteoclasts, which is a critical step that drives the migration of osteoclasts to bone tissue. Several chemokines and chemotactic proteins, such as CCL2 and CX3CL1, can be produced in subchondral bone and attract osteoclasts to these regions [ 73, 74]. During osteoclast migration, proteases such as collagenase are involved in the degradation of the bone matrix, providing a pathway for the movement of osteoclasts. Adhesion molecules on the cell surface, such as integrins, are also involved in the migration of osteoclasts through bone [75]. The periosteal microenvironment in the bone marrow also plays an important role in the recruitment of osteoclasts, providing a suitable environment for survival and differentiation. It affects angiogenesis and the innervation of subchondral bone, thereby accelerating articular cartilage damage and causing joint pain [ 76, 77]. Hence, subchondral osteoclasts have extraordinary significance in OA.
The role of subchondral osteoclasts in OA is mainly reflected in the structural destruction of subchondral bone and articular cartilage, angiogenesis, and joint pain. In the early stage of OA, the number and activity of subchondral osteoclasts increase abnormally, and the rate of bone resorption increases significantly. This disrupts the balance of bone remodeling, resulting in increased loss of subchondral bone, enlarged bone marrow cavities, and decreased bone mineral density. Excessive bone resorption leads to irregularities in the subchondral bone, which can cause the formation of bone cysts. In the subchondral bone, these cysts are liquid or semisolid cysts that can cause bone pain and discomfort. Moreover, overactive osteoclasts secrete proteases and degrading enzymes, such as MMPs, and capture enzymes, which degrade the cartilage matrix and lead to structural destruction of articular cartilage [ 78– 80].
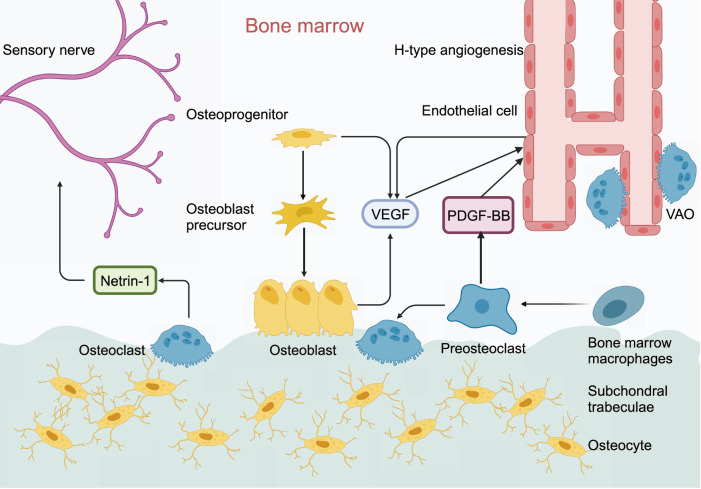
CD31 hiEmcn hi, a specific vascular subtype, is an important feature of OA and was recently found to be closely associated with angiogenesis and osteogenesis [ 81, 82]. It is characterized by strong positive expression of platelet endothelial cell adhesion molecule (PECAM-1/CD31) and endothelial mucin (EMCN), which can exacerbate cartilage erosion in OA. Excessive secretion of PDGF-BB by osteoclast precursors induces the formation of the CD31 hiEmcn hi vascular isoform ( Figure 2), and the number of CD31 hiEmcn hi vessels increases significantly in OA. Moreover, vascular-associated osteoclasts (VAOs), a subtype of osteoclasts, can assist H-type blood vessels in eliminating cartilage [ 83– 86]. Moreover, excessive angiogenesis promotes osteogenesis. Despite increasing bone mass, it does not enhance bone strength. Instead, this leads to insufficient bone mineralization and destruction of the mechanical properties of the subchondral bone, which accelerates articular cartilage damage and exacerbates the vicious cycle of OA [87]. Sensory nerve fibers and nerve trunks are distributed within the vascular channels of articular cartilage and around the blood vessels of subchondral bone. Osteoclasts secrete Netrin-1, a protein that plays a key role in neural development and the function of the nervous system. This helps to establish neural connections by guiding the growth and migration of neuronal axons; thus, sensory innervation of the subchondral bone is related to the activity and number of osteoclasts during OA. Osteoclasts in subchondral bone introduce abnormal sensory innervation during OA, causing joint pain in OA patients [ 88, 89].
Figure 2 .
PDGF-BB secreted by subchondral osteoclast precursor cells promotes H-type angiogenesis in OA
In the bone microenvironment, osteoblasts and endothelial cells secrete VEGF to promote H-type angiogenesis; at the same time, osteoclasts secrete Netrin-1 to act on sensory nerves, leading to joint pain in patients with OA.
Mechanism by Which Subchondral Osteoclasts Regulate OA
Autophagy-related signaling pathways
Increasing evidence suggests that autophagy may play a pivotal role in regulating the proliferation, differentiation, and function of osteoclasts. Multiple signaling pathways, including the Beclin-1/Becn1, p62/sqstm1, mTOR, and HIF-1α pathways, play key roles in this process. Molecules such as CD147, G protein-coupled receptor kinase-interacting protein 1 (GIT1), IL-17A, and TRAF6 can regulate osteoclast autophagy through Beclin-1 [ 90, 91]. After CD147-mediated autophagy is activated, the levels of Beclin-1 and soluble RANKL increase, promoting osteoclastogenesis [92]. GIT1 promotes autophagy in osteoclasts by promoting the phosphorylation of the Beclin1 Thr119 site and disrupting the binding of Beclin1 to BCL2 [93]. IL-17A regulates RANKL-induced osteoclast formation by modulating Beclin-1-mediated autophagy [94]. TRAF6 mediates the ubiquitination of Beclin1 at Lys117 and promotes RANKL-stimulated osteoclast differentiation [95]. As a characteristic autophagy adaptor protein, p62/sqstm1 activates autophagy and is affected by LC3 accumulation and F-actin loop formation, which are involved in RANKL-induced osteoclast differentiation [96]. Additionally, mTOR regulates autophagy through the AMPK/mTOR/70-kDa ribosomal protein S6 kinase (P70S6K) signaling pathway, affecting osteoclast differentiation [97]. Among the protein signaling pathways through which HIF-1α regulates autophagy, the upregulation of BNIP3 is involved in hypoxia-induced autophagy activation [98]. Furthermore, HIF-1α mediates the involvement of miRNAs in autophagy regulation in osteoclasts [99].
Noncoding RNAs (ncRNAs)
NcRNAs are important epigenetic regulators of osteoclast biological behavior. MiRNAs, circRNAs, and lncRNAs form a complex network that profoundly affects the biological activity of osteoclasts [100]. Among the miRNAs studied, miR-31 is one of the most upregulated miRNAs during osteoclastogenesis and regulates osteoclasts by affecting RhoA activity [101]. Moreover, miR-21, a new player in bone disease, promotes osteoclast formation and bone resorption through the PI3K/Akt signaling pathway [102]. Moreover, miR-34c promotes osteoclast survival by targeting leucine-rich repeat G-protein-coupled receptor 4 (lgr4) [103]. Mir-29b promotes osteoclast survival by inhibiting osteoclast apoptosis through the targeting of the proapoptotic factor Bcl-2 modifier (BMF) [104]. Mir-146-5p and mir-539 have been implicated in promoting osteoclast survival, bone resorption, and secretion, but their targets remain to be explored [ 105, 106]. A large number of circRNAs are upregulated during the early and late stages of osteoclastogenesis, suggesting that the expression profile of circRNAs is highly regulated during osteoclastogenesis. However, studies on the regulation of circRNAs by osteoclasts are rare. The available data indicate that circRNAs may function as miRNAs in the regulation of osteoclasts [107]. Exosomes are also involved in ncRNA communication. However, further studies are needed on the targeted regulation of subchondral osteoclasts by exosome-based ncRNAs.
RANK/RANKL/OPG axis
The RANKL/RANK/OPG axis plays an essential regulatory role in osteoclast formation. This biological process begins with osteoblasts and stromal cells secreting RANKL, which interacts with osteoclast precursors and binds to RANK receptors. This interaction triggers the activation of a series of transcription factors, including NF-κB, activator protein 1, AKT, nuclear factor of activated T-cell cytoplasm 1 (NFATc1), and MAPK-related macromolecules such as ERK, JNK, and p38. Activation of these downstream factors initiates the transcription of genes related to osteoclast differentiation and bone resorption, including genes encoding anti-tartrate acid phosphatase (TRAP), cathepsin K (CTSK), the calcitonin receptor (CTR) and MMP-9. This process ultimately results in the formation of mature multinucleated osteoclasts [ 108– 113]. TNF receptor-associated factor 6 (TRAF6) is also an essential component of the RANKL-RANK signaling pathway, and recruitment of TRAF6 activates signaling pathways such as the NF-κB, MAPK, and PI3K/AKT pathways, thereby promoting osteoclast differentiation and maturation [ 114, 115]. In this process, the activation of NF-κB and the subsequent upregulation of transcription factors such as NFATc1 and c-Fos are critical steps in osteoclast differentiation and maturation [ 116, 117].
Moreover, OPG, a secreted protein and cytokine receptor protein produced by osteoblasts, plays a pivotal role in this process. It plays an important negative regulatory role in bone tissue. As a receptor antagonist, it can act as a decoy receptor to replace RANK and bind to RANKL, thereby inhibiting the formation of mature osteoclasts and consequently downregulating bone resorption. This mechanism is responsible for maintaining the homeostasis of bone tissue and ensuring that an appropriate ratio of bone resorption to osteogenesis is maintained. The expression of OPG is usually low but may be reduced in OA, leading to an increase in the combined effect of RANKL and RANK, which in turn increases the number and activity of osteoclasts, significantly increasing bone resorption and disrupting the balance of bone remodeling, thereby causing joint injury [ 118, 119]. Additionally, OPG can cause the breakdown of osteoclast pseudopods and protect the bone cortex through MAPK signaling and other pathways [120]. Overall, the RANKL/RANK/OPG axis is crucial for the development and function of osteoclasts. The ratio of OPG/RANKL determines the degree of bone resorption and the process of bone metabolism and is a crucial factor in bone and tissue metabolism.
Oxidative stress
Oxidative stress-induced reactive oxygen species (ROS) play a large role in regulating the balance of the bone remodeling process. ROS include a variety of reactive molecules and free radicals, such as superoxide anions, hydrogen peroxide and hydroxyl radicals. These molecules are produced through the electron transport chain during aerobic respiration and can affect biological functions such as cell signaling and homeostasis [121]. Several recent studies have shown that under normal conditions, ROS are indispensable intracellular secondary messengers that perform numerous functions, including apoptosis, gene expression and activation of cellular signaling cascades, and play important roles in regulating cell proliferation, survival, metabolism, apoptosis, differentiation and migration [122]. However, ROS play dual roles. They are harmful when their levels increase due to aging or the onset of diseases such as OA. Excessive amounts of ROS can lead to bone destruction and even death of bone cells.
Recent studies have shown that oxidative stress and the consequent generation of ROS promote osteoclast differentiation. It has been demonstrated that RANKL stimulation increases ROS production in BMMs via the TRAF6/Rac1/nicotinamide adenine dinucleotide phosphate oxidase 1 (Nox1) signaling cascade, leading to enhanced osteoclast differentiation. In contrast, the antioxidant N-acetylcysteine (NAC) inhibited the response of BMMs to RANKL, which included ROS generation, MAPK pathway activation, and osteoclastogenesis. Similarly, in a glucose-induced diabetic rat model of osteoporosis, an increase in ROS production in osteoclasts was observed, followed by enhanced expression of MAPK, the NF- κB signaling pathway and NLRP3-related proteins, which promoted osteoclast differentiation and bone resorption [ 123, 124]. The production of ROS not only directly promotes osteoclast differentiation but also interacts with osteoblasts to regulate osteoclast formation and differentiation. The OPG/RANK/RANKL axis causes osteoblasts and osteoclasts to be inseparable [125]. High levels of H 2O 2-induced ROS in osteoblasts and BMSCs can stimulate RANKL mRNA and protein expression via the ERK and PKA-CREB pathways. The cocultivation of osteoblasts and osteoclast precursor cells demonstrated that the upregulation of RANKL expression induced by ethanol relied on the activation of intracellular ROS through NADPH oxidase activity in osteoblasts. Furthermore, the generated ROS actively facilitate the differentiation of osteoclasts [ 126, 127]. These findings imply that ROS play a pivotal role in enhancing RANKL secretion from osteoblasts, thereby modulating the differentiation of osteoclasts. In addition, the ROS/endoplasmic reticulum and ROS/TFEB pathways regulate osteoclast production and differentiation to a certain extent by affecting autophagy [ 128, 129].
Inflammatory signaling pathway
Inflammatory cells are formed by immune cells, such as macrophages, T lymphocytes, B lymphocytes, and other leukocytes that infiltrate bone tissues and produce a variety of cytokines and chemokines, such as IL-1β, IL-6, TNF-α, nerve growth factor (NGF), and the anamnestic toxin C5a, all of which can regulate osteoclast activity to a certain degree, thus affecting OA. Accordingly, IL-1β can stimulate osteoclastogenesis by upregulating the expression of RANKL in osteoblasts or stromal cells, thereby significantly increasing the rate of bone resorption and disrupting the balance of bone metabolism, leading to the destruction of bone structure and accelerating the progression of OA [ 130, 131]. Simultaneously, IL-1β can affect osteoclasts through the NF-κB signaling pathway. When IL-1β binds to its cell surface receptor IL-1 receptor type I (IL-1RI), it causes receptor activation and the formation of a receptor complex consisting of IL-1RI, IL-1 receptor accessory protein (IL-1RAcP), and myeloid mediator protein 88 (MyD88). MyD88 activates IL-1RI and TRAFs through the IL-1 receptor-associated kinase (IRAK) to activate TRAF6. Activated TRAF6 stimulates TGF-β-activated kinase 1 (TAK1), which induces the expression of the kinase kappa B (IKK), leading to IκB protein degradation and NF-κB nuclear translocation, thereby regulating osteoclasts [132]. In addition, IL-1β activates the JAK-STAT and MAPK pathways to stimulate osteoclastogenesis [133]. Moreover, compared with IL-1β, IL-6 activates the JAK-STAT pathway and preferentially stimulates osteoclastogenesis. When osteoclasts are stimulated by IL-6 family cytokines, these factors bind to the gp130 receptor and activate gp130, activating JAK kinases, especially JAK1 and JAK2, which phosphorylate IL-6Rα. Activated STAT3 enters the nucleus and affects gene expression, thereby regulating osteoclast differentiation and activity [134]. In addition, IL-6 activates the NF-κB and MAPK signaling pathways, thereby regulating osteoclast activity and function. Like the RANKL/RANK system, TNF-α induces osteoclast differentiation but independently of this process. TNF-α recruits TRAFs to activate the transcription factors NF-κB, c-Fos, and NFATc1, which in turn induces osteoclast differentiation. However, TNF-α alone has a very limited role in inducing osteoclast formation, and several inhibitory proteins, including TRAF3, IRF8, and RBP-j, regulate this process [135]. Furthermore, NGF and the anamorphic toxin C5a can influence osteoclasts by activating the RANKL and MAPK signaling cascades, respectively [ 136– 138].
These cytokines and chemokines influence chondrocytes by interacting with osteoblasts and osteoclasts to regulate each other and maintain normal bone structure, thus indirectly affecting subchondral osteoclasts. The effects of the inflammatory factors IL-1β, IL-6, and TNF-α on chondrocytes can generally be summarized as catabolism, which induces further induction of inflammatory mediators as well as degradation of the cartilage extracellular matrix through the upregulation of a series of hydrolytic enzymes and moreover contributes to chondrocyte apoptosis [ 133, 139]. Chondrocytes can promote subchondral bone loss by regulating osteoclasts. Abnormal mechanical stress induces primary chondrocytes to produce IL-1b, which indirectly induces the differentiation and maturation of osteoclasts by increasing RANKL expression via osteoblasts. In the medial meniscus (DMM) instability-induced OA model, chondrocytes produce large amounts of TNF-α and IL-6. Moreover, TNF-α activated NF-κB and JNK in a Rankl-independent manner, which directly induced osteoclast differentiation and indirectly induced their production. In addition, senescent chondrocytes and hypertrophic chondrocytes produce proinflammatory mediators, catabolic enzymes, and chemokines, collectively referred to as senescence-associated secretory phenotypes (SASPs), which affect subchondral osteoclast lineage cells. In addition, osteoclast precursors invade the hypertrophic cartilage region and interact with chondrocytes to remodel the cartilage matrix and form ossification centers. Moreover, mature osteoclasts can regulate nearby chondrocytes, disrupting bone-cartilage connections and aggravating cartilage damage. TGF-β1 expression in osteoblasts was upregulated in a time-dependent and dose-dependent manner under mechanical stimulation. Chondrocyte apoptosis was aggravated when the cells were cocultured with osteoclasts. Intraperitoneal injection of a TGF-β1R inhibitor in OA rats effectively reduced chondrocyte apoptosis and cartilage degradation. TGF-β1 is transported from the subchondral bone to the cartilage layer by diffusion or blood circulation, which adversely affects chondrocytes. Chondrocytes affected by inflammatory factors can also indirectly affect osteoblast differentiation and maturation through ERK, NF-κB and other signaling pathways [ 33, 140].
Other factors
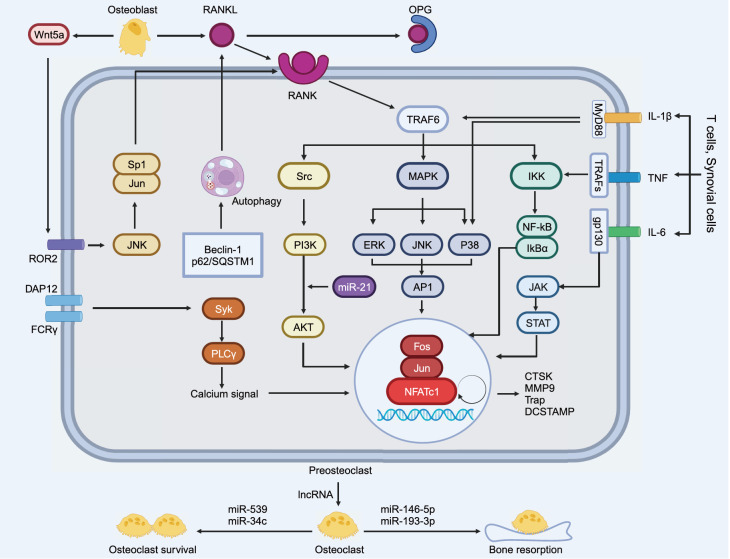
The activity and function of osteoclasts in osteoarthritic subchondral bone are also affected by a variety of other factors, such as apoptosis [141], calmodulin [142], estrogen, thyroid proteins, Nrf2 [ 143, 144], RUNX2 [145], and other genes. These signaling pathways and factors work together to regulate osteoclast activity in OA ( Figure 3), leading to joint destruction and pain. An in-depth analysis of these regulatory mechanisms is important for understanding the pathophysiological processes of OA and developing relevant therapeutic approaches. By interfering with these signaling pathways, it is possible to alleviate symptoms and slow disease progression in patients with OA.
Figure 3 .
Mechanistic crosstalk of subchondral osteoclasts in OA
The TRAF6 gene in the RANK/RANKL/OPG axis is the central factor that triggers the activation of a series of transcription factors, including NF-κB, AKT, MAPK, and NFATc1. Moreover, inflammatory factors and autophagy pathways are related to RANKL/RANK and interactively affect osteoclast differentiation; in addition, ncRNAs regulate osteoclast survival and bone resorption capacity.
Conclusions and Perspectives
Bone remodeling, which is essential for maintaining the mechanical capacity of the skeleton and coordinating the replacement of old and new bone, is a complex and subtle process mediated by all osteoblasts. Osteoclasts are the predominant cells involved in bone resorption and play an important role in maintaining bone remodeling. It has an indispensable function. OA is a chronic, degenerative joint disease that progresses irreversibly. Its main features are cartilage degeneration and abnormal remodeling of the subchondral bone. These lesions are inextricably linked to inflammatory processes. Different types of cells are involved in the inflammatory process. With the increasing understanding of the bone remodeling process and cellular activities in OA, subchondral osteoclasts appear to be the key to early pathological changes in OA and are expected to be a new target for the treatment of OA. In contrast to traditional NSAIDs or analgesics, treatments targeting subchondral osteoclasts focus on the underlying causes and pathophysiological mechanisms of OA, as opposed to pain and symptom relief, which is the main focus of traditional treatments. Targeted therapy can intervene in the pathophysiological processes that lead to subchondral osteolysis, such as inflammation, osteoporosis, and cartilage damage, thereby slowing disease progression and protecting joint structures, whereas traditional treatments can relieve only pain. Moreover, targeted therapies usually formulate treatment plans based on the disease characteristics and genotype of the patient and thus better meet the patient’s requirements. Moreover, targeted therapies can provide longer-lasting efficacy, whereas conventional therapies may require constant maintenance and lose effectiveness over time. Additionally, traditional therapies may cause gastrointestinal problems, liver and kidney damage, and other adverse effects. In contrast, targeted therapies typically have fewer systemic side effects due to their capacity to target arthritis-associated biomolecules with greater precision [ 146, 147].
Consequently, how can subchondral osteoclasts be targeted and regulated to achieve therapeutic effects? Currently, a large number of studies have shown that by inhibiting RANKL and activating AMPK, NF-κB. In addition, the generation of subchondral osteoclasts can be inhibited, bone resorption can be attenuated, and osteoclast-mediated abnormal remodeling of the subchondral bone can be reduced to alleviate OA. Drugs such as metformin, paroxetine, irisin, and some phenolactones significantly inhibit subchondral osteoclast differentiation and maturation ( Table 1). Additionally, from a mechanobiological point of view, mechanical cues and biochemical factors can modulate OA by affecting subchondral osteoclasts. There is growing evidence that appropriate mechanical loading reduces cartilage destruction, subchondral bone changes and secondary inflammation in OA joints. Some experiments have shown that early tibial axial mechanical loading may reduce the abnormal remodeling of subchondral bone and protect the cartilage from damage [148]; appropriate mechanical loading significantly reduces the level of IL-1β, as well as cox-2 and iNOS, and reduces the inflammatory state of OA joints through the NLRP3/caspase-1/IL-1β axis [149]; knee loading increases the expression of Wnt3a, and decreases the expression of NFATc1, RANKL, TNF-α, and Cathepsin K [150].
Table 1 List of drugs inhibiting subchondral osteoclast differentiation and maturation
|
Agent |
Target |
Signaling pathway |
Function |
Ref. |
|
Metformin |
BMMs |
AMPK/NF-κB/ERK signaling |
Inhibition osteoclast formation |
|
|
Diterbutyl phthalate |
BMMs/RAW264.7 |
ERK/c-fos/NFATc1 signaling |
Inhibition of subchondral osteoclast formation and related angiogenesis and neurogenesis |
|
|
Dihydroartemisinin |
Osteoclast precursors |
NF-κB/MAPK/NFATc1 signaling |
Inhibition of osteoclast formation and bone resorption in the early stage of OA |
|
|
Halofuginone |
BMMs |
Smad2/3-dependent TGF-β signaling |
Inhibition of osteoclast bone resorption |
|
|
Neratinib |
ATDC5 cells/BMMs |
MAPK/NF-κB signaling |
Protect cartilage and inhibit osteoclast formation |
|
|
Paroxetine |
ATDC5 cells/BMMs |
NF-κB signaling |
Inhibition of pyrosis and osteoclast formation |
|
|
Total lignans |
BMMs |
ERK/NFATc1 signaling |
Inhibition of osteoclast differentiation |
|
|
PP121 |
BMMs |
RANKL/Src/MAPK/Akt signaling |
Inhibition of osteoclast formation and bone resorption |
|
|
Isorhamnetin |
ATDC5 cells/BMMs |
RANKL/ MAPK/ NF-κB signaling |
Inhibit osteoclast formation and protect chondrocytes by regulating ROS homeostasis |
|
|
Nirogacestat |
BMMs |
RANKL/NFATc1 signaling |
Inhibition of osteoclast formation and bone resorption |
|
|
USP13 |
BMMs |
AKT/ NF-κB signaling |
Inhibition of osteoclastogenesis and osteoclast related gene expression |
|
|
Ruboxistaurin |
BMMs |
PKCδ/MAPKs signaling |
Inhibition of osteoclast formation and absorption activity |
|
|
Curcuminoid |
RAW264.7 |
RANKL/OPG signaling |
Reduce osteoclast activity and maintain osteoblast function |
|
|
Irisin |
ATDC5 cells/BMMs |
RANKL/OPG/NF-κB signaling |
Inhibition of osteoclast formation |
|
|
FICZ |
BMMs |
RANKL signaling |
Inhibit osteoclast differentiation and activity |
|
|
Velutin |
BMMs |
RANKL/p38 signaling |
Inhibits osteoclast formation and bone resorption |
|
|
Parthenolide |
NF-κB signaling |
Inhibits osteoclast formation and survival |
||
|
Lenalidomide |
BMMs |
RANKL/NF-κB signaling |
Inhibits osteoclast formation and function |
|
|
C-176 |
Osteoclast precursors |
NF-κB/NFATc1 signaling |
Inhibits osteoclast formation and activation |
|
|
AZ-628 |
ATDC5 cells/BMMs |
NF-κB/MAPK signaling |
Inhibition of chondrocyte decomposition, osteoclast formation and bone resorption |
|
|
Urolithin A |
RAW264.7 |
RANKL/NF-κB signaling |
Inhibition of osteoclast differentiation |
|
|
HIF-2α inhibitor |
BMMs |
Akt/MAPK/NF-κB signaling |
Inhibition of osteoclast differentiation |
|
|
Diallyl disulfide |
RAW264.7 |
RANKL/NF-κB/ NFATc1 signaling |
Inhibition of osteoclast formation, fusion and bone resorption |
|
|
Tyrosine kinase inhibitor |
BMMs |
RANKL/ NF-κB/ STAT3 signaling |
Inhibition of osteoclast differentiation |
|
|
Gypenoside |
BMMs |
RANKL/NF-κB/AKT/MAPK signaling |
Inhibition of osteoclast formation |
Additionally, biochemical factor levels are likewise not negligible in the regulation of subchondral osteoclasts. It has been shown that human OA articular cartilage stem cells suppress osteoclasts and improve subchondral bone remodeling in experimental knee OA partially by releasing TNFAIP3 [175]. It also inhibits overactive osteoclastogenesis and maintains the microarchitecture of subchondral bone by suppressing ROS production [176] and the expression of inflammatory mediators [177]. Additionally, lentiviral small hairpin RNA can knock down macrophage inflammatory protein 1 γ, thereby inhibiting osteoclast formation [178]. It can also inhibit osteoclast activity by inhibiting osteoclast-associated receptors (Oscar) [ 179, 180]. Moreover, exosomes derived from dental pulp stem cells (DPSCs) have been revealed to inhibit osteoclast activation in vivo by inhibiting TRPV4 activation and reducing cartilage degradation and synovial inflammation in vivo [181]. Targeted gene therapy can also regulate the activity and function of osteoclasts to achieve therapeutic effects. According to a previous article, ncRNAs, such as mir-21-5p, which targets Skp2 and can decrease osteoclast production, have great potential for treating OA [182]. Moreover, it has been shown that targeting and upregulating HMOX1 signaling can inhibit BMM-induced osteoclast activation, whereas selective knockdown of PDGF-BB in osteoclasts reduces subchondral bone angiogenesis and attenuates joint damage [183]. Moreover, numerous studies have shown that autophagy plays an indispensable role in regulating bone homeostasis and is expected to be a new target for regulating subchondral osteoclasts [ 184, 185]. Treatment of OA is a long-term process that focuses on improving disease management and quality of life. Future studies will continue to explore the molecular regulatory mechanisms of osteoclasts to identify new drug targets and pave the way for more effective treatment of osteoarthritis, and the development of individualized treatment strategies will provide more precise and effective treatments for OA patients, which will be a key focus of future research. In addition, the collaboration of multidisciplinary research teams will facilitate the integration of various studies on inflammation, bone resorption, and osteoclasts. In addition, the collaboration of interdisciplinary research teams will help to integrate multiple fields, such as inflammation, bone resorption, and osteoclast research, thereby providing new perspectives on the comprehensive treatment of OA.
In conclusion, this article reviews the latest progress on subchondral osteoclast differentiation in OA and targeted interventions for osteoclasts. Subchondral osteoclasts may play a central role in OA pathogenesis. We hope that this work will help us understand and develop new strategies for the targeted treatment of OA.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Program of Jiangsu Science and Technology Department (Nos. BK20211083 and BE2022737), the Program of Suzhou Health Commission (Nos. GSWS2020078 and SZXK202111), the Program of Suzhou Science and Technology Department (No. SKY2023062) and the Jiangsu Graduate Student Cultivation Innovative Engineering Graduate Research and Practice Innovation Program (No. SJCX23_0683).
References
- 1.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. . 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol. 2019, 37 Suppl 120: 3–6 . [PubMed]
- 3.Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatology. . 2014;28:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Venne G, Tse MY, Pang SC, Ellis RE. Mechanically-induced osteophyte in the rat knee. Osteoarthritis Cartilage. . 2020;28:853–864. doi: 10.1016/j.joca.2020.02.834. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Duan M, Zhang D, Xie J. The role of mechano growth factor in chondrocytes and cartilage defects: a concise review. Acta Biochim Biophys Sin. . 2023;55:701–712. doi: 10.3724/abbs.2023086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Fan X, Xing L, Tian F. Wnt signaling: a promising target for osteoarthritis therapy. Cell Commun Signal. . 2019;17:97. doi: 10.1186/s12964-019-0411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. . 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 8.Roman-Blas JA, Bizzi E, Largo R, Migliore A, Herrero-Beaumont G. An update on the up and coming therapies to treat osteoarthritis, a multifaceted disease. Expert Opin Pharmacother. . 2016;17:1745–1756. doi: 10.1080/14656566.2016.1201070. [DOI] [PubMed] [Google Scholar]
- 9.Quicke JG, Conaghan PG, Corp N, Peat G. Osteoarthritis year in review 2021: epidemiology & therapy. Osteoarthritis Cartilage. . 2022;30:196–206. doi: 10.1016/j.joca.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology & therapy. Osteoarthritis Cartilage. . 2021;29:180–189. doi: 10.1016/j.joca.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Yamauchi K, Mitsunaga T. A review on osteoclast diseases and osteoclastogenesis inhibitors recently developed from natural resources. Fitoterapia. . 2020;142:104482. doi: 10.1016/j.fitote.2020.104482. [DOI] [PubMed] [Google Scholar]
- 12.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. . 2001;285:785–795. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein RS, Manolagas SC. Apoptosis and osteoporosis. Am J Med. . 2000;108:153–164. doi: 10.1016/S0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 14.Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. . 2013;9:522–536. doi: 10.1038/nrendo.2013.137. [DOI] [PubMed] [Google Scholar]
- 15.Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. . 2009;4:5. doi: 10.1186/1750-1172-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, Faraggiana T, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. . 2000;151:311–320. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-Src proto-oncogene leads to osteopetrosis in mice. Cell. . 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy JB, Baron R. c-Cbl is downstream of c-Src in a signalling pathway necessary for bone resorption. Nature. . 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 19.Kawatani M, Osada H. Osteoclast‐targeting small molecules for the treatment of neoplastic bone metastases. Cancer Sci. . 2009;100:1999–2005. doi: 10.1111/j.1349-7006.2009.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. . 2005;328:679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 21.Kan C, Vargas G, Pape F, Clézardin P. Cancer cell colonisation in the bone microenvironment. Int J Mol Sci. . 2016;17:1674. doi: 10.3390/ijms17101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda K, Yoshida K, Nishizawa T, Otani K, Yamashita Y, Okabe H, Hadano Y, et al. Inflammation and bone metabolism in rheumatoid arthritis: molecular mechanisms of joint destruction and pharmacological treatments. Int J Mol Sci. . 2022;23:2871. doi: 10.3390/ijms23052871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Q, Zhou C, Nandakumar KS. Molecular and cellular pathways contributing to joint damage in rheumatoid arthritis. Mediators Inflamm. . 2020;2020:1–20. doi: 10.1155/2020/3830212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikuta J, Ishii M. Osteoclast migration, differentiation and function: novel therapeutic targets for rheumatic diseases. Rheumatology. . 2013;52:226–234. doi: 10.1093/rheumatology/kes259. [DOI] [PubMed] [Google Scholar]
- 25.Niu Q, Gao J, Wang L, Liu J, Zhang L. Regulation of differentiation and generation of osteoclasts in rheumatoid arthritis. Front Immunol. . 2022;13:1034050. doi: 10.3389/fimmu.2022.1034050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabjohns EM, Hurst K, Ghosh A, Cuellar MC, Rampersad RR, Tarrant TK. Paget’s disease of bone: osteoimmunology and osteoclast pathology. Curr Allergy Asthma Rep. . 2021;21:23. doi: 10.1007/s11882-021-01001-2. [DOI] [PubMed] [Google Scholar]
- 27.Reddy SV. Etiology of Paget’s disease and osteoclast abnormalities. J Cell Biochem. . 2004;93:688–696. doi: 10.1002/jcb.20256. [DOI] [PubMed] [Google Scholar]
- 28.Gennari L, Rendina D, Falchetti A, Merlotti D. Paget’s disease of bone. Calcif Tissue Int. . 2019;104:483–500. doi: 10.1007/s00223-019-00522-3. [DOI] [PubMed] [Google Scholar]
- 29.Imhof H, Sulzbacher I, Grampp S, Czerny C, Youssefzadeh S, Kainberger F. Subchondral bone and cartilage disease. Investig Radiol. . 2000;35:581–588. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. . 2010;18:419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 31.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. . 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmdahl DE, Ingelmark BE. The contact between the articular cartilage and the medullary cavities of the bone. Acta Orthop Scand. . 1950;20:156–165. doi: 10.3109/17453675009043414. [DOI] [PubMed] [Google Scholar]
- 33.Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. . 2021;80:413–422. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann New York Acad Sci. . 2010;1192:230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 35.Udagawa N, Koide M, Nakamura M, Nakamichi Y, Yamashita T, Uehara S, Kobayashi Y, et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. . 2021;39:19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 36.Bar-Shavit Z. The osteoclast: a multinucleated, hematopoietic‐origin, bone‐resorbing osteoimmune cell. J Cell Biochem. . 2007;102:1130–1139. doi: 10.1002/jcb.21553. [DOI] [PubMed] [Google Scholar]
- 37.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. . 2013;92:860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cappariello A, Maurizi A, Veeriah V, Teti A. The great beauty of the osteoclast. Arch Biochem Biophys. . 2014;558:70–78. doi: 10.1016/j.abb.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 39.Holtrop ME, King GJ. The ultrastructure of the osteoclast and its functional implications. Clin Orthop Relat Res. 1977: 177–196 . [PubMed]
- 40.Zaidi M, Kim SM, Mathew M, Korkmaz F, Sultana F, Miyashita S, Gumerova AA, et al. Bone circuitry and interorgan skeletal crosstalk. Elife. . 2023;12:e83142. doi: 10.7554/eLife.83142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. . 2000;159:7–13. doi: 10.1016/S0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Wen C. Osteocyte dysfunction in joint homeostasis and osteoarthritis. Int J Mol Sci. . 2021;22:6522. doi: 10.3390/ijms22126522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robling AG, Bonewald LF. The osteocyte: new insights. Annu Rev Physiol. . 2020;82:485–506. doi: 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui J, Shibata Y, Zhu T, Zhou J, Zhang J. Osteocytes in bone aging: advances, challenges, and future perspectives. Ageing Res Rev. . 2022;77:101608. doi: 10.1016/j.arr.2022.101608. [DOI] [PubMed] [Google Scholar]
- 45.Zhou M, Li S, Pathak JL. Pro-inflammatory cytokines and osteocytes. Curr Osteoporos Rep. . 2019;17:97–104. doi: 10.1007/s11914-019-00507-z. [DOI] [PubMed] [Google Scholar]
- 46.Divieti Pajevic P, Krause DS. Osteocyte regulation of bone and blood. Bone. . 2019;119:13–18. doi: 10.1016/j.bone.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atkinson EG, Delgado‐Calle J. The emerging role of osteocytes in cancer in bone. JBMR Plus. . 2019;3:e10186. doi: 10.1002/jbm4.10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Titorencu I, Pruna V, Jinga VV, Simionescu M. Osteoblast ontogeny and implications for bone pathology: an overview. Cell Tissue Res. . 2014;355:23–33. doi: 10.1007/s00441-013-1750-3. [DOI] [PubMed] [Google Scholar]
- 49.Ambrosi TH, Longaker MT, Chan CKF. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. . 2019;7:189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kito H, Ohya S. Role of K + and Ca 2+-permeable channels in osteoblast functions . Int J Mol Sci. . 2021;22:10459. doi: 10.3390/ijms221910459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salhotra A, Shah HN, Levi B, Longaker MT. Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. . 2020;21:696–711. doi: 10.1038/s41580-020-00279-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu K, Olsen BR. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest. . 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long F. Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol. . 2011;13:27–38. doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 54.Kim JM, Lin C, Stavre Z, Greenblatt MB, Shim JH. Osteoblast-osteoclast communication and bone homeostasis. Cells. . 2020;9:2073. doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrine Rev. . 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 56.Ribatti D, d′Amati A. Bone angiocrine factors. Front Cell Dev Biol. . 2023;11:1244372. doi: 10.3389/fcell.2023.1244372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramasamy SK, Kusumbe AP, Wang L, Adams RH. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. . 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dalal PJ, Muller WA, Sullivan DP. Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol. . 2020;190:535–542. doi: 10.1016/j.ajpath.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grellier M, Bordenave L, Amédée J. Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol. . 2009;27:562–571. doi: 10.1016/j.tibtech.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Xing Z, Jiang X, Si Q, Finne-Wistrand A, Liu B, Xue Y, Mustafa K. Endochondral ossification induced by cell transplantation of endothelial cells and bone marrow stromal cells with copolymer scaffold using a rat calvarial defect model. Polymers. . 2021;13:1521. doi: 10.3390/polym13091521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu G, Corn PG, Shen P, Song JH, Lee YC, Lin SC, Pan J, et al. Retinoic acid receptor activation reduces metastatic prostate cancer bone lesions by blocking the endothelial-to-osteoblast transition. Cancer Res. . 2022;82:3158–3171. doi: 10.1158/0008-5472.CAN-22-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walker DG. Spleen cells transmit osteopetrosis in mice. Science. . 1975;190:785–787. doi: 10.1126/science.1198094. [DOI] [PubMed] [Google Scholar]
- 63.Walker DG. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science. . 1975;190:784–785. doi: 10.1126/science.1105786. [DOI] [PubMed] [Google Scholar]
- 64.Scheven BAA, Visser JWM, Nijweide PJ. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population . Nature. . 1986;321:79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- 65.Veis DJ, O′Brien CA. Osteoclasts, master sculptors of bone. Annu Rev Pathol Mech Dis. . 2023;18:257–281. doi: 10.1146/annurev-pathmechdis-031521-040919. [DOI] [PubMed] [Google Scholar]
- 66.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. . 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theill LE, Boyle WJ, Penninger JM. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. . 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 68.Roodman GD. Regulation of osteoclast differentiation. Ann New York Acad Sci. . 2006;1068:100–109. doi: 10.1196/annals.1346.013. [DOI] [PubMed] [Google Scholar]
- 69.Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G, Rosen V, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. . 2013;24:297–310. doi: 10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. . 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Mu W, Xu B, Ren J, Wahafu T, Wuermanbieke S, Ma H, et al. Artesunate, an anti-malaria agent, attenuates experimental osteoarthritis by inhibiting bone resorption and CD31 hiEmcn hi vessel formation in subchondral bone . Front Pharmacol. . 2019;10:685. doi: 10.3389/fphar.2019.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka Y, Okada Y, Nakamura T. Inter- and intracellular signaling in secondary osteoporosis. J Bone Mineral Metab. . 2003;21:61–66. doi: 10.1007/s007740300010. [DOI] [PubMed] [Google Scholar]
- 73.Siddiqui JA, Partridge NC. CCL2/Monocyte chemoattractant protein 1 and parathyroid hormone action on bone. Front Endocrinol. . 2017;8:49. doi: 10.3389/fendo.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wojdasiewicz P, Turczyn P, Dobies-Krzesniak B, Frasunska J, Tarnacka B. Role of CX3CL1/CX3CR1 signaling axis activity in osteoporosis. Mediators Inflamm. . 2019;2019:1–9. doi: 10.1155/2019/7570452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duong LT, Lakkakorpi P, Nakamura I, Rodan GA. Integrins and signaling in osteoclast function. Matrix Biol. . 2000;19:97–105. doi: 10.1016/S0945-053X(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 76.Zhu S, Zhu J, Zhen G, Hu Y, An S, Li Y, Zheng Q, et al. Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest. . 2019;129:1076–1093. doi: 10.1172/JCI121561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, Li C, et al. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med. . 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tateiwa D, Yoshikawa, Kaito Cartilage and bone destruction in arthritis: pathogenesis and treatment strategy: a literature review. Cells. . 2019;8:818. doi: 10.3390/cells8080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bultink IEM, Lems WF. Osteoarthritis and osteoporosis: what is the overlap? Curr Rheumatol Rep. . 2013;15:328. doi: 10.1007/s11926-013-0328-0. [DOI] [PubMed] [Google Scholar]
- 80.Strassle BW, Mark L, Leventhal L, Piesla MJ, Jian Li X, Kennedy JD, Glasson SS, et al. Inhibition of osteoclasts prevents cartilage loss and pain in a rat model of degenerative joint disease. Osteoarthritis Cartilage. . 2010;18:1319–1328. doi: 10.1016/j.joca.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 81.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. . 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. . 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kubota K, Sakikawa C, Katsumata M, Nakamura T, Wakabayashi K. Platelet-derived growth factor BB secreted from osteoclasts acts as an osteoblastogenesis inhibitory factor. J Bone Mineral Res. . 2002;17:257–265. doi: 10.1359/jbmr.2002.17.2.257. [DOI] [PubMed] [Google Scholar]
- 84.Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. . 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 85.Romeo SG, Alawi KM, Rodrigues J, Singh A, Kusumbe AP, Ramasamy SK. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat Cell Biol. . 2019;21:430–441. doi: 10.1038/s41556-019-0304-7. [DOI] [PubMed] [Google Scholar]
- 86.Quan H, Ren C, He Y, Wang F, Dong S, Jiang H. Application of biomaterials in treating early osteonecrosis of the femoral head: research progress and future perspectives. Acta Biomater. . 2023;164:15–73. doi: 10.1016/j.actbio.2023.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y, Xu J, Zhang X, Wang C, Huang Y, Dai K, Zhang X. TNF-α-induced LRG1 promotes angiogenesis and mesenchymal stem cell migration in the subchondral bone during osteoarthritis. Cell Death Dis. . 2017;8:e2715. doi: 10.1038/cddis.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suri S, Gill SE, Massena de Camin S, McWilliams DF, Wilson D, Walsh DA. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann Rheumatic Dis. . 2007;66:1423–1428. doi: 10.1136/ard.2006.063354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. . 2012;51:204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Deng Z, Ma Y, Jin J, Qi F, Li S, Liu C, et al. The role of autophagy and mitophagy in bone metabolic disorders. Int J Biol Sci. . 2020;16:2675–2691. doi: 10.7150/ijbs.46627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo YF, Su T, Yang M, Li CJ, Guo Q, Xiao Y, Huang Y, et al. The role of autophagy in bone homeostasis. J Cell Physiol. . 2021;236:4152–4173. doi: 10.1002/jcp.30111. [DOI] [PubMed] [Google Scholar]
- 92.Su B, Li D, Xu J, Zhang Y, Cai Z, Kauther MD, Ma R. Wear particles enhance autophagy through up-regulation of CD147 to promote osteoclastogenesis. Iran J Basic Med Sci. 2018, 21: 806–812 . [DOI] [PMC free article] [PubMed]
- 93.Zhao SJ, Kong FQ, Cai W, Xu T, Zhou ZM, Wang ZB, Xu AD, et al. GIT1 contributes to autophagy in osteoclast through disruption of the binding of Beclin1 and Bcl2 under starvation condition. Cell Death Dis. . 2018;9:1195. doi: 10.1038/s41419-018-1256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xue Y, Liang Z, Fu X, Wang T, Xie Q, Ke D. IL-17A modulates osteoclast precursors’ apoptosis through autophagy-TRAF3 signaling during osteoclastogenesis. Biochem Biophys Res Commun. . 2019;508:1088–1092. doi: 10.1016/j.bbrc.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 95.Arai A, Kim S, Goldshteyn V, Kim T, Park NH, Wang CY, Kim RH. Beclin1 modulates bone homeostasis by regulating osteoclast and chondrocyte differentiation. J Bone Mineral Res. . 2019;34:1753–1766. doi: 10.1002/jbmr.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li RF, Chen G, Ren JG, Zhang W, Wu ZX, Liu B, Zhao Y, et al. The adaptor protein p62 is involved in RANKL-induced autophagy and osteoclastogenesis. J Histochem Cytochem. . 2014;62:879–888. doi: 10.1369/0022155414551367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tong X, Gu J, Song R, Wang D, Sun Z, Sui C, Zhang C, et al. Osteoprotegerin inhibit osteoclast differentiation and bone resorption by enhancing autophagy via AMPK/mTOR/p70S6K signaling pathway in vitro . J Cell Biochem. . 2019;120:1630–1642. doi: 10.1002/jcb.27468. [DOI] [PubMed] [Google Scholar]
- 98.Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J, Sun ZJ, et al. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J Cell Physiol. . 2012;227:639–648. doi: 10.1002/jcp.22768. [DOI] [PubMed] [Google Scholar]
- 99.Sun KT, Chen MYC, Tu MG, Wang IK, Chang SS, Li CY. MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone. . 2015;73:145–153. doi: 10.1016/j.bone.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 100.Duan L, Liang Y, Xu X, Wang J, Li X, Sun D, Deng Z, et al. Noncoding RNAs in subchondral bone osteoclast function and their therapeutic potential for osteoarthritis. Arthritis Res Ther. . 2020;22:279. doi: 10.1186/s13075-020-02374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizoguchi F, Murakami Y, Saito T, Miyasaka N, Kohsaka H. miR-31 controls osteoclast formation and bone resorption by targeting RhoA. Arthritis Res Ther. . 2013;15:R102. doi: 10.1186/ar4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang S, Liu Z, Wang J, Ji X, Yao Z, Wang X. miR-21 promotes osteoclastogenesis through activation of PI3K/Akt signaling by targeting Pten in RAW264.7 cells. Mol Med Report. . 2020;21:1125–1132. doi: 10.3892/mmr.2020.10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cong F, Wu N, Tian X, Fan J, Liu J, Song T, Fu H. MicroRNA-34c promotes osteoclast differentiation through targeting LGR4. Gene. . 2017;610:1–8. doi: 10.1016/j.gene.2017.01.028. [DOI] [PubMed] [Google Scholar]
- 104.Sul OJ, Rajasekaran M, Park HJ, Suh JH, Choi HS. MicroRNA-29b enhances osteoclast survival by targeting BCL-2-modifying factor after lipopolysaccharide stimulation. Oxid Med Cell Longev. . 2019;2019:1–11. doi: 10.1155/2019/6018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin SH, Ho JC, Li SC, Chen JF, Hsiao CC, Lee CH. MiR-146a-5p expression in peripheral CD14 + monocytes from patients with psoriatic arthritis induces osteoclast activation, bone resorption, and correlates with clinical response . J Clin Med. . 2019;8:110. doi: 10.3390/jcm8010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corney DC, Flesken-Nikitin A, Godwin AK, Wang W, Nikitin AY. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth . Cancer Res. . 2007;67:8433–8438. doi: 10.1158/0008-5472.CAN-07-1585. [DOI] [PubMed] [Google Scholar]
- 107.Chen X, Ouyang Z, Shen Y, Liu B, Zhang Q, Wan L, Yin Z, et al. CircRNA_28313/miR-195a/CSF1 axis modulates osteoclast differentiation to affect OVX-induced bone absorption in mice. RNA Biol. . 2019;16:1249–1262. doi: 10.1080/15476286.2019.1624470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kitaura H, Marahleh A, Ohori F, Noguchi T, Shen WR, Qi J, Nara Y, et al. Osteocyte-related cytokines regulate osteoclast formation and bone resorption. Int J Mol Sci. . 2020;21:5169. doi: 10.3390/ijms21145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ding D, Yan J, Feng G, Zhou Y, Ma L, Jin Q. Dihydroartemisinin attenuates osteoclast formation and bone resorption via inhibiting the NF‑κB, MAPK and NFATc1 signaling pathways and alleviates osteoarthritis. Int J Mol Med. . 2021;49:4. doi: 10.3892/ijmm.2021.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang X, Liang J, Wang Z, Su Y, Zhan Y, Wu Z, Li J, et al. Sesamolin protects mice from ovariectomized bone loss by inhibiting osteoclastogenesis and RANKL-Mediated NF-κB and MAPK signaling pathways. Front Pharmacol. . 2021;12:664697. doi: 10.3389/fphar.2021.664697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. . 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yasuda H. Discovery of the RANKL/RANK/OPG system. J Bone Miner Metab. . 2021;39:2–11. doi: 10.1007/s00774-020-01175-1. [DOI] [PubMed] [Google Scholar]
- 113.Takayanagi H. RANKL as the master regulator of osteoclast differentiation. J Bone Miner Metab. . 2021;39:13–18. doi: 10.1007/s00774-020-01191-1. [DOI] [PubMed] [Google Scholar]
- 114.Armstrong AP, Tometsko ME, Glaccum M, Sutherland CL, Cosman D, Dougall WC. A RANK/TRAF6-dependent signal transduction pathway is essential for osteoclast cytoskeletal organization and resorptive function. J Biol Chem. . 2002;277:44347–44356. doi: 10.1074/jbc.M202009200. [DOI] [PubMed] [Google Scholar]
- 115.Martin TJ, Sims NA. RANKL/OPG; Critical role in bone physiology. Rev Endocr Metab Disord. . 2015;16:131–139. doi: 10.1007/s11154-014-9308-6. [DOI] [PubMed] [Google Scholar]
- 116.Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. . 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- 117.Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. . 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 118.Eriksen EF. Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord. . 2010;11:219–227. doi: 10.1007/s11154-010-9153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Y, Liang J, Liu P, Wang Q, Liu L, Zhao H. The RANK/RANKL/OPG system and tumor bone metastasis: potential mechanisms and therapeutic strategies. Front Endocrinol. . 2022;13:1063815. doi: 10.3389/fendo.2022.1063815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amin N, Boccardi V, Taghizadeh M, Jafarnejad S. Probiotics and bone disorders: the role of RANKL/RANK/OPG pathway. Aging Clin Exp Res. . 2020;32:363–371. doi: 10.1007/s40520-019-01223-5. [DOI] [PubMed] [Google Scholar]
- 121.Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y. Oxidative stress in bone remodelling and disease. Trends Mol Med. . 2009;15:468–477. doi: 10.1016/j.molmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 122.Agidigbi TS, Kim C. Reactive oxygen species in osteoclast differentiation and possible pharmaceutical targets of ros-mediated osteoclast diseases. Int J Mol Sci. . 2019;20:3576. doi: 10.3390/ijms20143576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee NK, Choi YG, Baik JY, Han SY, Jeong D, Bae YS, Kim N, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood. . 2005;106:852–859. doi: 10.1182/blood-2004-09-3662. [DOI] [PubMed] [Google Scholar]
- 124.An Y, Zhang H, Wang C, Jiao F, Xu H, Wang X, Luan W, et al. Activation of ROS/MAPKs /NF‐κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J. . 2019;33:12515–12527. doi: 10.1096/fj.201802805RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang B, Li S, Chen Z, Feng F, He L, Liu B, He T, et al. Amyloid β peptide promotes bone formation by regulating Wnt/β-catenin signaling and the OPG/RANKL/RANK system. FASEB J. . 2020;34:3583–3593. doi: 10.1096/fj.201901550R. [DOI] [PubMed] [Google Scholar]
- 126.Bai X, Lu D, Liu A, Zhang Z, Li X, Zou Z, Zeng W, et al. Reactive oxygen species stimulates receptor activator of NF-κB ligand expression in osteoblast. J Biol Chem. . 2005;280:17497–17506. doi: 10.1074/jbc.M409332200. [DOI] [PubMed] [Google Scholar]
- 127.Chen JR, Shankar K, Nagarajan S, Badger TM, Ronis MJJ. Protective effects of estradiol on ethanol-induced bone loss involve inhibition of reactive oxygen species generation in osteoblasts and downstream activation of the extracellular signal-regulated kinase/signal transducer and activator of transcription 3/Receptor activator of nuclear Factor-κB ligand signaling cascade. J Pharmacol Exp Ther. . 2008;324:50–59. doi: 10.1124/jpet.107.130351. [DOI] [PubMed] [Google Scholar]
- 128.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. . 2007;9:2277–2294. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 129.Martini-Stoica H, Xu Y, Ballabio A, Zheng H. The autophagy-lysosomal pathway in neurodegeneration: a TFEB perspective. Trends Neuroscis. . 2016;39:221–234. doi: 10.1016/j.tins.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu T, Zhang Z, Deng C, Ma X, Liu X. Effects of β2 integrins on osteoclasts, macrophages, chondrocytes, and synovial fibroblasts in osteoarthritis. Biomolecules. . 2022;12:1653. doi: 10.3390/biom12111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bergdolt S, Kovtun A, Hägele Y, Liedert A, Schinke T, Amling M, Huber-Lang M, et al. Osteoblast-specific overexpression of complement receptor C5aR1 impairs fracture healing. PLoS One. 2017, 12: e0179512 . [DOI] [PMC free article] [PubMed]
- 132.Mercurio F, Manning AM. Multiple signals converging on NF-κB. Curr Opin Cell Biol. . 1999;11:226–232. doi: 10.1016/S0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- 133.Jenei-Lanzl Z, Meurer A, Zaucke F. Interleukin-1β signaling in osteoarthritis - chondrocytes in focus. Cell Signal. . 2019;53:212–223. doi: 10.1016/j.cellsig.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 134.Hu L, Liu R, Zhang L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int Immunopharmacol. . 2022;111:109095. doi: 10.1016/j.intimp.2022.109095. [DOI] [PubMed] [Google Scholar]
- 135.Yao Z, Getting SJ, Locke IC. Regulation of TNF-Induced osteoclast differentiation. Cells. . 2022;11:132. doi: 10.3390/cells11010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu L, Nwosu LN, Burston JJ, Millns PJ, Sagar DR, Mapp PI, Meesawatsom P, et al. The anti-NGF antibody muMab 911 both prevents and reverses pain behaviour and subchondral osteoclast numbers in a rat model of osteoarthritis pain. Osteoarthritis Cartilage. . 2016;24:1587–1595. doi: 10.1016/j.joca.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mödinger Y, Rapp A, Pazmandi J, Vikman A, Holzmann K, Haffner-Luntzer M, Huber-Lang M, et al. C5aR1 interacts with TLRL2 in osteoblasts and stimulates the osteoclast-inducing chemokine CXCL10. J Cell Mol Medi. . 2018;22:6002–6014. doi: 10.1111/jcmm.13873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shi Y, Tao H, Li X, Zhang L, Li C, Sun W, Chu M, et al. κ-Opioid receptor activation attenuates osteoarthritis synovitis by regulating macrophage polarization through the NF-κB pathway. Acta Biochim Biophys Sin. . 2024;56:82–95. doi: 10.3724/abbs.2023223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu B, Wang C, Weng Z, Yang Y, Zhao H, Zhang Y, Fei Q, et al. Glycolytic enzyme PKM2 regulates cell senescence but not inflammation in the process of osteoarthritis. Acta Biochim Biophys Sin. . 2023;55:1425–1433. doi: 10.3724/abbs.2023062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z, Huang Z, Bai L. Cell interplay in osteoarthritis. Front Cell Dev Biol. . 2021;9:720477. doi: 10.3389/fcell.2021.720477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. . 2013;54:264–271. doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williams JP, Micoli K, McDonald JM. Calmodulin-an often-ignored signal in osteoclasts. Ann New York Acad Sci. . 2010;1192:358–364. doi: 10.1111/j.1749-6632.2009.05242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han J, Yang K, An J, Jiang N, Fu S, Tang X. The role of NRF2 in bone metabolism—friend or foe? Front Endocrinol. . 2022;13:813057. doi: 10.3389/fendo.2022.813057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li W, Tao C, Mao M, Zhu K. The Nrf2/HMGB1/NF-κB axis modulates chondrocyte apoptosis and extracellular matrix degradation in osteoarthritis. Acta Biochim Biophys Sin. . 2023;55:818–830. doi: 10.3724/abbs.2023078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zeng L, He H, Sun M, Gong X, Zhou M, Hong Y, Wu Y, et al. Runx2 and Nell-1 in dental follicle progenitor cells regulate bone remodeling and tooth eruption. Stem Cell Res Ther. . 2022;13:486. doi: 10.1186/s13287-022-03140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kodama J, Kaito T. Osteoclast multinucleation: review of current literature. Int J Mol Sci. . 2020;21:5685. doi: 10.3390/ijms21165685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Boyce BF. Advances in osteoclast biology reveal potential new drug targets and new roles for osteoclasts. J Bone Mineral Res. . 2013;28:711–722. doi: 10.1002/jbmr.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu J, Pan Y, Yu Y, Yang Q, Liu Q, Liu Y, Zhong J, et al. Axial compressive loading attenuates early osteoarthritis by reducing subchondral bone remodeling. Am J Sports Med. . 2023;51:1752–1764. doi: 10.1177/03635465231164644. [DOI] [PubMed] [Google Scholar]
- 149.He Z, Nie P, Lu J, Ling Y, Guo J, Zhang B, Hu J, et al. Less mechanical loading attenuates osteoarthritis by reducing cartilage degeneration, subchondral bone remodelling, secondary inflammation, and activation of NLRP3 inflammasome. Bone Joint Res. . 2020;9:731–741. doi: 10.1302/2046-3758.910.BJR-2019-0368.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li X, Yang J, Liu D, Li J, Niu K, Feng S, Yokota H, et al. Knee loading inhibits osteoclast lineage in a mouse model of osteoarthritis. Sci Rep. . 2016;6:24668. doi: 10.1038/srep24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guo H, Ding D, Wang L, Yan J, Ma L, Jin Q. Metformin attenuates osteoclast-mediated abnormal subchondral bone remodeling and alleviates osteoarthritis via AMPK/NF-κB/ERK signaling pathway. PLos One. 2021, 16: e0261127 . [DOI] [PMC free article] [PubMed]
- 152.Fang C, Guo J, Wang Y, Li X, Zhang H, Cui J, Hu Y, et al. Diterbutyl phthalate attenuates osteoarthritis in ACLT mice via suppressing ERK/c-fos/NFATc1 pathway, and subsequently inhibiting subchondral osteoclast fusion. Acta Pharmacol Sin. . 2022;43:1299–1310. doi: 10.1038/s41401-021-00747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cui Z, Crane J, Xie H, Jin X, Zhen G, Li C, Xie L, et al. Halofuginone attenuates osteoarthritis by inhibition of TGF-β activity and H-type vessel formation in subchondral bone. Ann Rheum Dis. . 2016;75:1714–1721. doi: 10.1136/annrheumdis-2015-207923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qiu J, Jiang T, Yang G, Gong Y, Zhang W, Zheng X, Hong Z, et al. Neratinib exerts dual effects on cartilage degradation and osteoclast production in osteoarthritis by inhibiting the activation of the MAPK/NF-κB signaling pathways. Biochem Pharmacol. . 2022;205:115155. doi: 10.1016/j.bcp.2022.115155. [DOI] [PubMed] [Google Scholar]
- 155.Zheng X, Qiu J, Gao N, Jiang T, Li Z, Zhang W, Gong Y, et al. Paroxetine attenuates chondrocyte pyroptosis and inhibits osteoclast formation by inhibiting NF-κB pathway activation to delay osteoarthritis progression. Drug Des Devel Ther. . 2023;17:2383–2399. doi: 10.2147/DDDT.S417598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ban Y, Wang Y, Qiao L, Zhang C, Wang H, He X, Jia D, et al. Total lignans from Vitex negundo seeds attenuate osteoarthritis and their main component vitedoin A alleviates osteoclast differentiation by suppressing ERK/NFATc1 signaling . Phytother Res. . 2023;37:1422–1434. doi: 10.1002/ptr.7750. [DOI] [PubMed] [Google Scholar]
- 157.Zhou Z, Chen X, Chen X, Qin A, Mao Y, Pang Y, Yu S, et al. PP121 suppresses RANKL-induced osteoclast formation in vitro and LPS-Induced bone resorption in vivo . Exp Cell Res. . 2020;388:111857. doi: 10.1016/j.yexcr.2020.111857. [DOI] [PubMed] [Google Scholar]
- 158.Zhou F, Mei J, Yuan K, Han X, Qiao H, Tang T. Isorhamnetin attenuates osteoarthritis by inhibiting osteoclastogenesis and protecting chondrocytes through modulating reactive oxygen species homeostasis. J Cell Mol Medi. . 2019;23:4395–4407. doi: 10.1111/jcmm.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen X, Chen X, Zhou Z, Mao Y, Wang Y, Ma Z, Xu W, et al. Nirogacestat suppresses RANKL-Induced osteoclast formation in vitro and attenuates LPS-induced bone resorption in vivo . Exp Cell Res. . 2019;382:111470. doi: 10.1016/j.yexcr.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 160.Huang J, Ye Z, Wang J, Chen Q, Huang D, Liu H. USP13 mediates PTEN to ameliorate osteoarthritis by restraining oxidative stress, apoptosis and inflammation via AKT-dependent manner. Biomed Pharmacother. . 2021;133:111089. doi: 10.1016/j.biopha.2020.111089. [DOI] [PubMed] [Google Scholar]
- 161.Pang C, Wen L, Lu X, Luo S, Qin H, Zhang X, Zhu B, et al. Ruboxistaurin maintains the bone mass of subchondral bone for blunting osteoarthritis progression by inhibition of osteoclastogenesis and bone resorption activity. Biomed Pharmacother. . 2020;131:110650. doi: 10.1016/j.biopha.2020.110650. [DOI] [PubMed] [Google Scholar]
- 162.Yeh CC, Su YH, Lin YJ, Chen PJ, Shi CS, Chen CN, Yeh CC. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des Devel Ther. . 2015;9:2285. doi: 10.2147/DDDT.S78277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.He Z, Li H, Han X, Zhou F, Du J, Yang Y, Xu Q, et al. Irisin inhibits osteocyte apoptosis by activating the Erk signaling pathway in vitro and attenuates ALCT-induced osteoarthritis in mice . Bone. . 2020;141:115573. doi: 10.1016/j.bone.2020.115573. [DOI] [PubMed] [Google Scholar]
- 164.Yoshikawa Y, Izawa T, Hamada Y, Takenaga H, Wang Z, Ishimaru N, Kamioka H. Roles for B[a]P and FICZ in subchondral bone metabolism and experimental temporomandibular joint osteoarthritis via the AhR/Cyp1a1 signaling axis. Sci Rep. . 2021;11:14927. doi: 10.1038/s41598-021-94470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang K, Lu X, Li X, Zhang Y, Xu R, Lou Y, Wang Y, et al. Dual protective role of velutin against articular cartilage degeneration and subchondral bone loss via the p38 signaling pathway in murine osteoarthritis. Front Endocrinol. . 2022;13:926934. doi: 10.3389/fendo.2022.926934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu J, Wu HF, Ang ESM, Yip K, Woloszyn M, Zheng MH, Tan RX. NF-κB modulators in osteolytic bone diseases. Cytokine Growth Factor Rev. . 2009;20:7–17. doi: 10.1016/j.cytogfr.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 167.Qu X, Mei J, Yu Z, Zhai Z, Qiao H, Dai K. Lenalidomide regulates osteocytes fate and related osteoclastogenesis via IL-1β/NF-κB/RANKL signaling. Biochem Biophys Res Commun. . 2018;501:547–555. doi: 10.1016/j.bbrc.2018.05.035. [DOI] [PubMed] [Google Scholar]
- 168.Yu ZC, Fu R, Li Y, Zhao DY, Jiang H, Han D. The STING inhibitor C-176 attenuates osteoclast-related osteolytic diseases by inhibiting osteoclast differentiation. FASEB J. . 2023;37:e22867. doi: 10.1096/fj.202201600R. [DOI] [PubMed] [Google Scholar]
- 169.Gong Y, Qiu J, Ye J, Jiang T, Zhang W, Zheng X, Zhu Z, et al. AZ-628 delays osteoarthritis progression via inhibiting the TNF-α-induced chondrocyte necroptosis and regulating osteoclast formation. Int Immunopharmacol. . 2022;111:109085. doi: 10.1016/j.intimp.2022.109085. [DOI] [PubMed] [Google Scholar]
- 170.Wang Z, Qi G, Li Z, Cui X, Guo S, Zhang Y, Cai P, et al. Effects of urolithin A on osteoclast differentiation induced by receptor activator of nuclear factor-κB ligand via bone morphogenic protein 2. Bioengineered. . 2022;13:5064–5078. doi: 10.1080/21655979.2022.2036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bae WJ, Shin MR, Kang SK, Zhang J, Kim JY, Lee SC, Kim EC. HIF-2 inhibition supresses inflammatory responses and osteoclastic differentiation in human periodontal ligament cells. J Cell Biochem. . 2015;116:1241–1255. doi: 10.1002/jcb.25078. [DOI] [PubMed] [Google Scholar]
- 172.Yang J, Tang R, Yi J, Chen Y, Li X, Yu T, Fei J. Diallyl disulfide alleviates inflammatory osteolysis by suppressing osteoclastogenesis via NF-κB–NFATc1 signal pathway . FASEB J. . 2019;33:7261–7273. doi: 10.1096/fj.201802172R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gyurkovska V, Stefanova T, Dimitrova P, Danova S, Tropcheva R, Ivanovska N. Tyrosine kinase inhibitor tyrphostin AG490 retards chronic joint inflammation in mice. Inflammation. . 2014;37:995–1005. doi: 10.1007/s10753-014-9820-6. [DOI] [PubMed] [Google Scholar]
- 174.Han J, Gao W, Su D, Liu Y. Gypenoside inhibits RANKL-induced osteoclastogenesis by regulating NF‐κB, AKT, and MAPK signaling pathways. J Cell Biochem. . 2018;119:7310–7318. doi: 10.1002/jcb.27028. [DOI] [PubMed] [Google Scholar]
- 175.Li ZL, Li XT, Hao RC, Wang FY, Wang YX, Zhao ZD, Li PL, et al. Human osteoarthritic articular cartilage stem cells suppress osteoclasts and improve subchondral bone remodeling in experimental knee osteoarthritis partially by releasing TNFAIP3. Stem Cell Res Ther. . 2023;14:253. doi: 10.1186/s13287-023-03411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yajun W, Jin C, Zhengrong G, Chao F, Yan H, Weizong W, Xiaoqun L, et al. Betaine attenuates osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. Front Pharmacol. . 2021;12:723988. doi: 10.3389/fphar.2021.723988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang ZM, Chen YC, Wang DP. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomed Pharmacother. . 2016;83:763–770. doi: 10.1016/j.biopha.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 178.Shen PC, Lu CS, Shiau AL, Lee CH, Jou IM, Hsieh JL. Lentiviral small hairpin RNA knockdown of macrophage inflammatory protein-1γ ameliorates experimentally induced osteoarthritis in mice. Hum Gene Ther. . 2013;24:871–882. doi: 10.1089/hum.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim GM, Park H, Lee SY. Roles of osteoclast-associated receptor in rheumatoid arthritis and osteoarthritis. Joint Bone Spine. . 2022;89:105400. doi: 10.1016/j.jbspin.2022.105400. [DOI] [PubMed] [Google Scholar]
- 180.Park DR, Kim J, Kim GM, Lee H, Kim M, Hwang D, Lee H, et al. Osteoclast-associated receptor blockade prevents articular cartilage destruction via chondrocyte apoptosis regulation. Nat Commun. . 2020;11:4343. doi: 10.1038/s41467-020-18208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fu Y, Cui S, Zhou Y, Qiu L. Dental pulp stem cell-derived exosomes alleviate mice knee osteoarthritis by inhibiting TRPV4-mediated osteoclast activation. Int J Mol Sci. . 2023;24:4926. doi: 10.3390/ijms24054926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Huang Y, Yang Y, Wang J, Yao S, Yao T, Xu Y, Chen Z, et al. miR-21-5p targets SKP2 to reduce osteoclastogenesis in a mouse model of osteoporosis. J Biol Chem. . 2021;296:100617. doi: 10.1016/j.jbc.2021.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Su W, Liu G, Liu X, Zhou Y, Sun Q, Zhen G, Wang X, et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. . 2020;5:e135446. doi: 10.1172/jci.insight.135446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang J, Zhang Y, Cao J, Wang Y, Anwar N, Zhang Z, Zhang D, et al. The role of autophagy in bone metabolism and clinical significance. Autophagy. . 2023;19:2409–2427. doi: 10.1080/15548627.2023.2186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, et al. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. . 2011;21:966–974. doi: 10.1016/j.devcel.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]