Abstract
Background
The early identification of responsive and resistant patients to androgen receptor-targeting agents (ARTA) in metastatic castration-resistant prostate cancer (mCRPC) is not completely possible with prostate-specific antigen (PSA) assessment and conventional imaging. Considering its ability to determine metabolic activity of lesions, positron emission tomography (PET) assessment might be a promising tool.
Patients and methods
We carried out a monocentric prospective study in patients with mCRPC treated with ARTA to evaluate the role of different PET radiotracers: 49 patients were randomized to receive 11C-Choline, Fluorine 18 fluciclovine (anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid - FACBC) (18F-FACBC), or Gallium-68-prostate-specific-membrane-antigen (68Ga-PSMA) PET, one scan before therapy and one 2 months later.
The primary aim was to investigate the performance of three novel PET radiotracers for the early evaluation of response to ARTA in metastatic CRPC patients; the outcome evaluated was biochemical response (PSA reduction ≥50%). The secondary aim was to investigate the prognostic role of several semiquantitative PET parameters and their variations with the different radiotracers in terms of biochemical progression-free survival (bPFS) and overall survival (OS).
The study was promoted by the Italian Department of Health (code RF-2016-02364809).
Results
Regarding the primary endpoint, at log-rank test a statistically significant correlation was found between metabolic tumor volume (MTV) (P = 0.018) and total lesion activity (TLA) (P = 0.025) percentage variation among the two scans with 68Ga-PSMA PET and biochemical response. As for the secondary endpoints, significant correlations with bPFS were found for 68Ga-PSMA total MTV and TLA at the first scan (P = 0.001 and P = 0.025, respectively), and MTV percentage variation (P = 0.031). For OS, statistically significant correlations were found for different 68Ga-PSMA and 18F-FACBC parameters and for major maximum standardized uptake value at the first 11C-Choline PET scan.
Conclusions
Our study highlighted that 11C-Choline, 68Ga-PSMA, and 18F-FACBC semiquantitative PET parameters and their variations present a prognostic value in terms of OS and bPFS, and MTV and TLA variations with 68Ga-PSMA PET a correlation with biochemical response, which could help to assess the response to ARTA.
Key words: ARTA, mCRPC, PET, PSMA, radiotracers, prostate cancer
Highlights
-
•
An unmet need in mCRPC is the early evaluation of response to ARTA.
-
•
We evaluated ARTA response with three different PET radiotracers, before therapy onset and after 2 months.
-
•
We analyzed PET-derived parameters to explore their prognostic value in terms of bPFS and OS.
-
•
68Ga-PSMA is the most reliable tracers to evaluate ARTA response, in view of the correlation with biochemical response.
-
•
Several PET-derived parameters could represent an effective prognostic factor.
Introduction
In the current year, prostate cancer (PCa) still represents the most common malignancy and the second cause of cancer-related death in men worldwide.1 Fortunately, the therapeutic landscape is constantly evolving in all settings of the disease, including metastatic castration-resistant prostate cancer (mCRPC).2 Abiraterone acetate and enzalutamide, two novel androgen receptor-targeting agents (ARTA), continue to play a crucial role in the treatment of mCRPC, regardless of the previous administration of docetaxel.3, 4, 5, 6 Of great impact on clinical practice could be the early identification of patients who develop resistance to these compounds or patients who are primary refractory. Nowadays, the monitoring of prostate-specific antigen (PSA) levels is commonly adopted to evaluate therapy response,7 but its determination could be impaired in case of non-producing tumors8 (for example, in de novo neuroendocrine PCa or induced by hormonal treatments for mCRPC)9 and in case of initial and transient increase in PSA levels due to the ‘flare’ phenomenon.10 Besides, the imaging evaluation with conventional imaging (CIM), consisting of computed tomography (CT), magnetic resonance imaging, or bone scintigraphy, is not completely able to identify responsive or resistant patients to ARTA. Otherwise, a promising assessment of response to therapy could be carried out with positron emission tomography (PET), as already demonstrated in other cancers,11, 12, 13 also in view of its ability to determine the extent of disease with respect to sites and number of metabolically active lesions.
Currently, 11C-Choline and Gallium-68-prostate-specific-membrane-antigen (68Ga-PSMA) PET radiotracers are approved for biochemical recurrence and initial staging of intermediate/high-risk localized or locally advanced disease.14 Fluorine 18 fluciclovine (anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid - FACBC) (18F-FACBC) radiotracer is only adopted for experimental purposes.14 Nonetheless, there is no unanimous consensus for PET use for monitoring response to therapy in the metastatic setting.15 According to that, it is still unclear how to evaluate mCRPC patients undergoing treatment, whether with CIM or with next-generation imaging (i.e. PET), along with PSA monitoring.15 Of note, a systematic review and meta-analysis pointed out the quite relevant discordance (about 25% of cases) between PSA and 68Ga-PSMA PET response assessments in mCRPC patients undergoing systemic therapies.16
A single-arm study that enrolled 16 mCRPC patients treated with abiraterone acetate or enzalutamide showed that the decrease in the uptake at the PSMA PET carried out 2-4 months after the start of ARTA was strongly correlated with treatment response.17
Regarding the potential prognostic role of PET in PCa, several studies have begun to investigate this topic. In patients undergoing radiotherapy (RT) for localized disease or as salvage therapy, 11C-Choline and 68Ga-PSMA PET/CT were found to play an important prognostic role, as emerged in a recent systematic review of the literature, while their utility in course of systemic therapies was controversial.18 A European, multicenter, retrospective analysis highlighted that patients with a positive 68Ga-PSMA PET, who already underwent salvage treatments after radical prostatectomy and PSA relapse, presented worse outcomes if compared to men with no uptake at the PET scan, while the result of the PET scan in patients who have never received salvage therapies did not affect their oncologic outcomes.19 Another retrospective, observational trial showed that 68Ga-PSMA PET seems to be a more reliable prognostic factor for progression-free survival (PFS) than PSA levels in mCRPC.20
In addition to 11C-Choline, 68Ga-PSMA, and 18F-FACBC, several other PET radiotracers are under evaluation in PCa,21 but limited data are available on which the radiotracer is more effective in predicting a patient’s outcome and early response to therapy. The most reliable PET-derived parameter in terms of prognostic and predictive value is also yet to be defined. The possibility of discriminating responder patients from resistant ones could help clinicians in mCRPC management, leading to a more tailored therapeutic approach. According to that, several trials have already revealed that carrying out 68Ga-PSMA PET in metastatic castration-sensitive PCa could lead to management changes.22, 23, 24
In this prospective monocentric interventional study, we tried to shed light on these unveiled and controversial topics.
Patients and methods
Study design
We carried out a prospective, interventional, monocentric, explorative study that enrolled patients with mCRPC assigned to treatment with abiraterone acetate or enzalutamide (before or after docetaxel chemotherapy). Patients were randomly assigned to receive 11C-Choline, 18F-FACBC, or 68Ga-PSMA PET, one scan before therapy onset (PET1) and one 2 months later (PET2). PET scans have been evaluated by three experienced nuclear medicine physicians visually and semi-quantitatively and the maximum standardized uptake value (SUVmax) and SUVmean have been measured in all hot lesions outside the normal tracer distribution. PET scans were achieved in conformity with the Joint European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI) procedure guidelines for PCa imaging. The study has been conducted according to Good Clinical Practices, after local ethical committee and AIFA (Associazione Italiana del Farmaco) approval. This study was approved by the local institutional review board (Comitato Etico Indipendente, IRCCS Azienda Ospedaliero-Universitaria di Bologna, protocol code: 133/2018/Farm/AOUBo) and was conducted in accordance with the principles of the Declaration of Helsinki. The response at PET2 has been evaluated according to the European Organisation for Research and Treatment of Cancer (EORTC) PET response criteria. In Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103448, a brief representation of the study design is reported.
The study is part of the research project with code RF-2016-02364809 promoted by the Italian Department of Health. This is the first report of the study results.
Study population
The study enrolled patients from January 2019 to August 2022.
The inclusion criteria were as follows: (i) diagnosis of mCRPC as defined by the European Association of Urology (EAU); (ii) radiological evidence of metastatic disease at either CT or bone scintigraphy; (iii) eligible for ARTA (abiraterone acetate or enzalutamide), before or after docetaxel treatment (could have received docetaxel for metastatic hormone-sensitive or castration-resistant setting); (iv) Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1; (v) abiraterone acetate or enzalutamide naïve; (vi) age ≥ 18 years old; (vii) signed informed consent.
Exclusion criteria: (i) patients not eligible for ARTA; (ii) life expectancy ≤ 6 months.
Statistical analysis
The primary aim was to investigate the performance of PET scan with different novel radiotracers for early therapy assessment in mCRPC patients treated with an ARTA. With regard to this aim, the primary endpoint was biochemical response (PSA response ≥50%), which was correlated with PET parameters difference and percentage variation. Biochemical response was defined as a ≥50% reduction of PSA at the time of PET2 from baseline. PSA values taken into account were PSA at baseline (≤4 weeks before ARTA start) and PSA at the time of PET2 (+4 weeks).
The secondary aim was to investigate the prognostic role of PET with different radiotracers. Secondary endpoints were biochemical PFS (bPFS) and overall survival (OS).
Data resulting from pre-treatment PET parameters and their variations among the two PET scans have been analyzed in relation to bPFS and OS.
bPFS was defined as the time of ARTA start to the time of PSA increase >50% from baseline value. OS was defined from therapy start to death from any cause.
Evaluated semiquantitative PET parameters and variations were: SUVmax; metabolic tumor volume (MTV: the volume of the metabolically active areas of the disease); total lesion activity (TLA: MTV × SUVmean); major value of SUVmax reported in each PET/CT scan (majSUVmax); difference in the parameter at PET2 compared to PET1 (DIFF_majSUVmax, DIFF_MTV, DIFF_TLA); percentage of changes among the two PET scans (majSUVmax_VARIATION%, MTV_VARIATION%, TLA_VARIATION%). The total MTV and TLA values at the two scans were calculated and reported as: MTV_TOT_PET1, MTV_TOT_PET2, TLA_TOT_PET1, TLA_TOT_PET2.
For the statistical analysis, we used the Wilcoxon signed rank test (for continuous variables with non-normal distribution), log-rank test, receiver operating characteristic (ROC) curves, Kaplan–Meier curve, and univariate analysis. Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) program version 26.0 (IBM, Armonk, NY).
Results
From January 2019 to August 2022, we enrolled 49 mCRPC patients treated with abiraterone acetate or enzalutamide, randomized 1 : 1 : 1 to receive PET scan with 11C-Choline (n = 16), 68Ga-PSMA (n = 18), or 18F-FACBC (n = 15). The median follow-up was 16 months (range 2-39 months).
Five patients were excluded from the analysis because they did not receive PET2 for death or worsening of ECOG PS. Previous docetaxel was administered in five patients for metastatic hormone-sensitive prostate cancer (mHSPC).
All the main patients’ characteristics are reported in Table 1.
Table 1.
Patients’ characteristics for each randomized group
| 11C-CHOLINE PET (n = 16 patients) n (%) | 68Ga-PSMA PET (n = 18 patients) n (%) | 18F-FACBC PET (n = 15 patients) n (%) | |
|---|---|---|---|
| General characteristics | |||
| Median age at PET1, years | 76 | 76 | 78 |
| Median PSA at PET1, ng/ml | 11.3 | 19.4 | 17.8 |
| Median first PSA on ARTA, ng/ml | 26.1 | 7.8 | 8.0 |
| Previous docetaxel | 3 (18.8%) | 2 (11.1%) | 0 |
| Bisphosphonates use | 5 (31.3%) | 4 (22.2%) | 2 (13.3%) |
| Biochemical response (reduction of PSA >50% from baseline) | 6 (33.3%) | 4 (22.2%) | 5 (33.3%) |
| Biochemical progression (increase of PSA >50% from baseline) | 6 (33.3%) | 2 (11.1%) | 7 (46.7%) |
| EORTC response at PET2 | 0 CR, 2 PR, 4 SD | 1 CR, 3 PR, 4 SD | 0 CR, 3 PR, 1 SD |
| EORTC progression at PET2 | 9 PD | 7 PD | 7 PD |
| Median bPFS, months | 5 | 2 | 6 |
| PET-derived parameters | |||
| Median major SUVmax at PET1 | 9.20 | 30.65 | 11.2 |
| Median major SUVmax at PET2 | 11.50 | 30.40 | 11 |
| Median total MTV at PET1 | 162.10 | 56 | 278.2 |
| Median total MTV at PET2 | 216.55 | 75.5 | 109.7 |
| Median total TLA at PET1 | 467.45 | 515.8 | 969.1 |
| Median total TLA at PET2 | 789.80 | 1403.4 | 470.1 |
| Patients not undergoing PET2 | 1 (6.3%) | 3 (16.7%) | 4 (26.7%) |
| Sites with PET uptake at PET1 | |||
| Prostate | 9 (56.3%) | 5 (27.8%) | 6 (40%) |
| Pelvic lymph nodes | 7 (43.8%) | 7 (38.9%) | 2 (13.3%) |
| Extra-pelvic lymph nodes | 11 (68.8%) | 10 (55.6%) | 3 (20%) |
| Bone | 12 (75%) | 12 (66.7%) | 10 (66.7%) |
| Lung | 1 (6.3%) | 4 (22.0%) | 2 (13.3%) |
| Liver | 0 | 0 | 0 |
| Abdominal nodules | 0 | 0 | 0 |
ARTA, androgen receptor-targeting agents; bPFS, biochemical progression-free survival; CR, complete response; EORTC, European Organisation for Research and Treatment of Cancer; MTV, metabolic tumor volume; PD, progression disease; PET, positron emission tomography; PR, partial response; PSA, prostate-specific antigen; SD, stable disease; SUVmax, maximum standard uptake value; TLA, total lesion activity.
Primary aim: correlation of PET parameters at baseline and percentage variation with biochemical response
For each radiotracer, we correlated PET parameters at baseline and their variations with biochemical response using the Wilcoxon signed rank test. The only statistically significant correlation was found for MTV_VARIATION% with 68Ga-PSMA (P = 0.043, Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103448).
Cut-off values of PET parameters at baseline and their percentage variation (reported in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103448) were assessed through ROC curve and dichotomized into lower or higher of cut-offs.
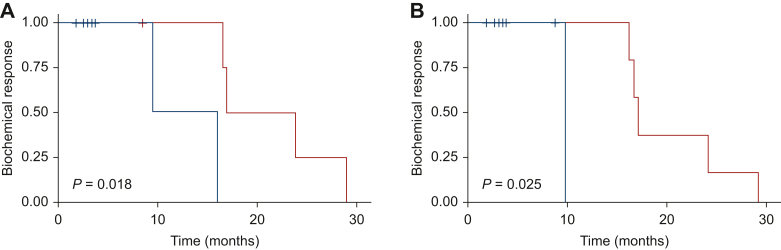
The cut-offs for each variable were correlated with biochemical response through the log-rank test. A statistically significant correlation was found between MTV_VARIATION% (P = 0.018, Figure 1) and TLA_VARIATION% (P = 0.025, Figure 1) with 68Ga-PSMA PET and biochemical response (Table 2).
Figure 1.
Biochemical response. (A) Kaplan–Meier estimates of biochemical response according to 68Ga-PSMA PET MTV_VARIATION%. (B) Kaplan–Meier estimates of biochemical response according to 68Ga-PSMA PET TLA_VARIATION%. Blue curve: higher than cut-off. Red curve: lower than cut-off.
Table 2.
Log-rank test for the correlation of PET parameters and percentage variation and biochemical response for each radiotracer
| 11C-CHOLINE PET | 68Ga-PSMA PET | 18F-FACBC PET | |
|---|---|---|---|
| MTV_TOT_PET1 | 0.22 | 0.84 | 0.5 |
| TLA_TOT_PET1 | 0.58 | 0.47 | 0.8 |
| MAJ_SUV_MAX_PET1 | 0.33 | 0.26 | 0.78 |
| SUVMAX_VARIATION% | 0.11 | 0.62 | 0.84 |
| MTV_VARIATION% | 0.63 | 0.018 | 0.96 |
| TLA_VARIATION% | 0.055 | 0.025 | 0.71 |
In bold, statistically significant variables.
Maj, Major; MTV, metabolic tumor volume; PET, positron emission tomography; SUVmax, maximum standard uptake value; TLA, total lesion activity.
Secondary aim: correlation of pre-treatment PET parameters and their variations with biochemical PFS
PET parameters at baseline and their variations were correlated with bPFS using the Wilcoxon signed rank test for each radiotracer and no statistically significant correlation was found. Using ROC curve, cut-off values of PET parameters at baseline and their percentage variation were found and subsequently dichotomized according to lower or higher of cut-offs.
Cut-off of PET parameters and their variation for each radiotracer are reported in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103448.
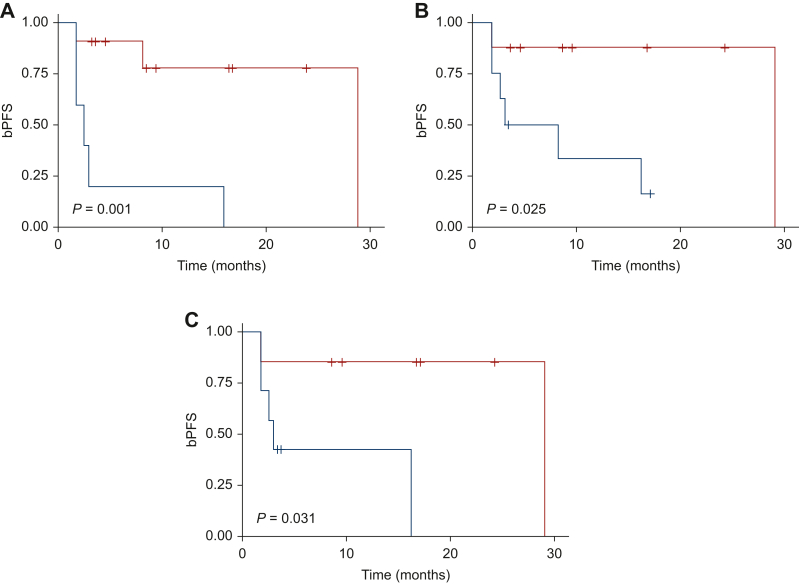
Subsequently, these variables were correlated with bPFS through the log-rank test and a statistically significant correlation was found only for 68Ga-PSMA PET MTV_TOT_PET1 (P = 0.001, Figure 2), TLA_TOT_PET1 (P = 0.025, Figure 2), and MTV_VARIATION% (P = 0.031, Figure 2). The log-rank test for the correlation of PET parameters and percentage variation and bPFS for each radiotracer are reported in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103448.
Figure 2.
Biochemical progression-free survival. (A) Kaplan–Meier estimates of bPFS according to 68Ga-PSMA PET MTV_TOT_PET1. (B) Kaplan–Meier estimates of bPFS according to 68Ga-PSMA PET TLA_TOT_PET1. (C) Kaplan–Meier estimates of bPFS according to 68Ga-PSMA PET MTV_VARIATION%. Blue curve: higher than cut-off. Red curve: lower than cut-off. bPFS, biochemical progression-free survival; PET, positron emission tomography.
Secondary aim: correlation of pre-treatment PET parameters and their variations with OS
PET parameter at baseline and their percentage variation were correlated with OS using the Wilcoxon signed rank test for each radiotracer. Significant correlations with OS were found for MTV_TOT_PET1 of 68Ga-PSMA (P = 0.044) and 18F-FACBC PET (P = 0.025, Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103448), MTV_VARIATION% of 68Ga-PSMA PET (P = 0.04, Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103448), and TLA_VARIATION% of 18F-FACBC PET (P = 0.044, Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103448).
Cut-off values of each PET parameter were calculated using ROC curves and were subsequently dichotomized into lower or higher than cut-off (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103448). Then we evaluated the correlation of each PET parameter variable, calculated with ROC curve, and OS with the log-rank test.
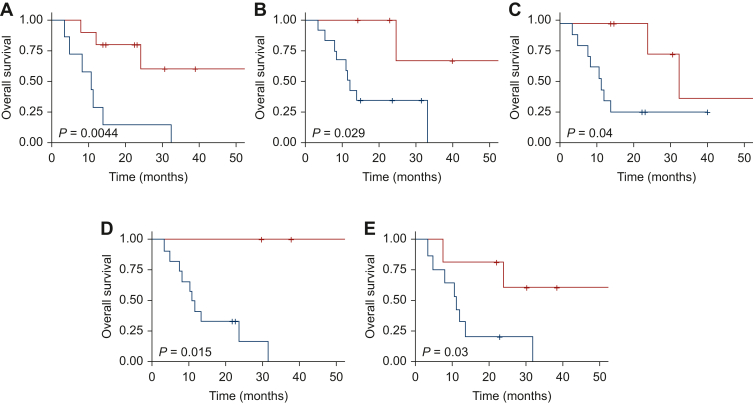
Then we evaluated the correlation of each PET parameter variable, calculated with ROC curve, and OS with the log-rank test (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103448). With regard to 11C-Choline PET, a significant correlation was found for MAJ_SUV_MAX_PET1 (P = 0.007, Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2024.103448). For 68Ga-PSMA PET, parameters statistically correlated with OS were MTV_TOT_PET1 (P = 0.004, Figure 3), MAJ_SUV_MAX_PET1 (P = 0.029, Figure 3), SUVMAX_VARIATION% (P = 0.04, Figure 3), MTV_VARIATION% (P = 0.015, Figure 3), and TLA_VARIATION% (P = 0.03, Figure 3). With 18F-FACBC radiotracer, a statistically significant correlation was found for MTV_TOT_PET1 (P = 0.011, Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103448), TLA_TOT_PET1 (P = 0.009, Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103448), MAJ_SUV_MAX_PET1 (P = 0.027, Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103448), and MTV_VARIATION% (P = 0.048, Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103448).
Figure 3.
Overall survival. (A) Kaplan–Meier estimates of OS according to 68Ga-PSMA PET MTV_TOT_PET1. (B) Kaplan–Meier estimates of OS according to 68Ga-PSMA PET MAJ_SUV_MAX_PET1. (C) Kaplan–Meier estimates of OS according to 68Ga-PSMA PET SUVMAX_VARIATION%. (D) Kaplan–Meier estimates of OS according to 68Ga-PSMA PET MTV_VARIATION%. (E) Kaplan–Meier estimates of OS according to 68Ga-PSMA PET TLA_VARIATION%. Blue curve: higher than cut-off. Red curve: lower than cut-off. OS, overall survival; PET, positron emission tomography.
Discussion
A crucial aspect about the management of cancer patients is represented by the early identification of responders and resistant subjects, thus enabling replacement of the ongoing oncologic treatment if indicated, and by the identification of patients with a more aggressive disease and a worse expected outcome, for whom an intensified therapy program may be considered. CIM or PSA assessment are not completely able to fulfill this task, while PET assessment could represent a promising option. Our study was designed to explore the role and the utility of PET scan with three different radiotracers in the early therapy response assessment of mCRPC patients and to determine its prognostic value.
In this study, MTV and TLA variations with 68Ga-PSMA PET resulted to be associated with biochemical response, thus appearing to be valuable parameters to include in the assessment of response. Moreover, several PET parameters presented a correlation with bPFS and OS, underlining their prognostic role. The results of our study suggest that, along with SUVmax, other semiquantitative parameters, such as TLA and MTV, should be included routinely in the PET/CT reports, helping the clinicians in identifying responder patients.
Regarding the prognostic role, 11C-Choline or 68Ga-PSMA PET are known to be associated with prognosis in patients undergoing RT, but no sufficient data are available about their role during systemic therapy.18,25 Currently, a minor role is played by 11C-Choline and 18F-FACBC PET and their use in routine clinical practice in CRPC is not strongly supported, especially in the early therapy assessment. It must be stressed that the limited sample size of these two groups of patients in our study may have influenced the results of the analysis regarding these tracers. Nonetheless, our analysis adds to previous preliminary data in the literature supporting the use of 68Ga-PSMA PET for the precocious assessment of therapy with ARTA.18,20,26,27 However, even if carried out on a larger sample size, three of these above-mentioned studies were characterized by a retrospective design,20,26,27 of which one enrolled both mHSPC and mCRPC patients,26 and the other one was a systematic review of the literature.18 In detail, while the analysis conducted by Esen et al. showed considerable concordance between 68Ga-PSMA PET data and PSA response,26 Calderoni and colleagues suggested that 68Ga-PSMA PET could possibly represent a better prognostic factor than PSA in terms of PFS,20 suggesting that this novel imaging technique may play a crucial role in treatment response evaluation in mCRPC.
A strength of our study is clearly its prospective and randomized design, along with the accrual of only a specific population of patients with PCa (consisting of mCRPC). Moreover, the adherence to international protocols for PET imaging evaluation guarantees high reliability in terms of diagnostic results. Thirdly, another merit of the study was the investigation of three different novel radiotracers. It also has to be underlined that patients’ characteristics of each randomized group of the study are quite homogenous, also regarding the burden of the disease, baseline PSA levels, and previous therapies received.
Conversely, several limitations have to be pointed out. The first limitation is the relatively restricted number of patients enrolled in each randomized group of the study and the percentage of patients lost to follow-up. These characteristics suggest that the results emerged in this study should be interpreted with caution and need further investigations to confirm them. Secondly, the short follow-up time did not allow the evaluation of long-term outcomes. Thirdly, this study did not include an imaging re-assessment of disease under treatment with CIM and, consequently, a direct comparison with PET in terms of prognostic value and early response evaluation could not be carried out. Moreover, the lack of CIM did not allow to assess the radiographic PFS as endpoint.
The importance of a valid imaging to evaluate treatment response can be reflected in the potential savings from unnecessary collateral effects of ARTA if progressive disease could be detected early. Additionally, an early evaluation of disease progression can eventually lead to a benefit in oncologic outcome and in the effectiveness of subsequent treatment, in view of the possibility to replace ARTA with other active therapies when the burden of the disease is still restricted and to address locoregional strategy (such as RT or surgery) to the oligoprogressive sites.
The PET parameters evaluated in our study could be helpful in identifying less respondent patients who need to be strictly observed. These parameters may not be enough to justify a change in the therapeutic approach, but could be integrated into a multidimensional assessment of response that should possibly include a clinical, biochemical, and imaging evaluation in order to tailor the treatment strategy on the single patient.
Conclusion
Our prospective study highlighted that several semiquantitative PET-derived parameters and their variations present a prognostic value in terms of OS and bPFS, which could help to identify responsive or resistant patients to ARTA. Furthermore, MTV and TLA variations with 68Ga-PSMA PET appeared to be correlated with biochemical response. The ability of these radiotracers to give information on prognostically worse disease at baseline or throughout novel antiandrogen therapy is extremely relevant in order to define the best therapeutic strategy, considering the wide plethora of treatment options currently at disposal for PCa patients.
Acknowledgements
We thank the patients, their families, and all of the investigators involved in this study.
Funding
The work reported in this publication was financed by the Italian Ministry of Health with the code RF-2016-02364809 and RC-2022-2773335.
Disclosure
FM has received research support and/or honoraria from Astellas, BMS, Janssen, Ipsen, MSD, and Pfizer outside the submitted work. All other authors have declared no conflicts of interest.
Data sharing
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti A., Tassinari E., Rosellini M., Rizzo A., Massari F., Mollica V. Prostate cancer and novel pharmacological treatment options-what’s new for 2022? Expert Rev Clin Pharmacol. 2023;16(3):231–244. doi: 10.1080/17512433.2023.2181783. [DOI] [PubMed] [Google Scholar]
- 3.Fizazi K., Scher H.I., Molina A., et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012;13(10):983–992. doi: 10.1016/S1470-2045(12)70379-0. [Erratum in: Lancet Oncol. 2012;13(11):e464; Lancet Oncol. 2014;15(9):e365] [DOI] [PubMed] [Google Scholar]
- 4.Ryan C.J., Smith M.R., Fizazi K., et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 5.Scher H.I., Fizazi K., Saad F., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 6.Nigro M.C., Mollica V., Marchetti A., et al. Current androgen receptor antagonists under investigation for resistant prostate cancer. Expert Rev Anticancer Ther. 2022;22(2):191–202. doi: 10.1080/14737140.2022.2020651. [DOI] [PubMed] [Google Scholar]
- 7.Scher H.I., Morris M.J., Stadler W.M., et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pezaro C., Omlin A., Lorente D., et al. Visceral disease in castration-resistant prostate cancer. Eur Urol. 2014;65(2):270–273. doi: 10.1016/j.eururo.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Wang Y., Ci X., et al. Molecular events in neuroendocrine prostate cancer development. Nat Rev Urol. 2021;18(10):581–596. doi: 10.1038/s41585-021-00490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgio S.L., Conteduca V., Rudnas B., et al. PSA flare with abiraterone in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2015;13(1):39–43. doi: 10.1016/j.clgc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Farag S., Geus-Oei L.F., van der Graaf W.T., et al. Early evaluation of response using 18F-FDG PET influences management in gastrointestinal stromal tumor patients treated with neoadjuvant imatinib. J Nucl Med. 2018;59(2):194–196. doi: 10.2967/jnumed.117.196642. [DOI] [PubMed] [Google Scholar]
- 12.Foley K.G., Jeffries J., Hannon C., Coles B., Bradley K.M., Smyth E. Response rate and diagnostic accuracy of early PET-CT during neo-adjuvant therapies in oesophageal adenocarcinoma: a systematic review and meta-analysis. Int J Clin Pract. 2021;75(6) doi: 10.1111/ijcp.13906. [DOI] [PubMed] [Google Scholar]
- 13.Vos J.L., Zuur C.L., Smit L.A., et al. [18F]FDG-PET accurately identifies pathological response early upon neoadjuvant immune checkpoint blockade in head and neck squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2022;49(6):2010–2022. doi: 10.1007/s00259-021-05610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker C., Castro E., Fizazi K., et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi: 10.1016/j.annonc.2020.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Gillessen S., Bossi A., Davis I.D., et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185:178–215. doi: 10.1016/j.ejca.2023.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Han S., Woo S., Kim Y.I., et al. Concordance between response assessment using prostate-specific membrane antigen PET and serum prostate-specific antigen levels after systemic treatment in patients with metastatic castration resistant prostate cancer: a systematic review and meta-analysis. Diagnostics (Basel) 2021;11(4):663. doi: 10.3390/diagnostics11040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zukotynski K.A., Emmenegger U., Hotte S., et al. Prospective, single-arm trial evaluating changes in uptake patterns on prostate-specific membrane antigen-targeted 18F-DCFPyL PET/CT in patients with castration-resistant prostate cancer starting abiraterone or enzalutamide. J Nucl Med. 2021;62(10):1430–1437. doi: 10.2967/jnumed.120.259069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alongi P., Laudicella R., Lanzafame H., et al. PSMA and choline PET for the assessment of response to therapy and survival outcomes in prostate cancer patients: a systematic review from the literature. Cancers (Basel) 2022;14(7):1770. doi: 10.3390/cancers14071770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi L., Ceci F., Costa F., et al. The impact of PSMA-PET on oncologic control in prostate cancer patients who experienced PSA persistence or recurrence. Cancers (Basel) 2022;15(1):247. doi: 10.3390/cancers15010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calderoni L., Maietti E., Farolfi A., et al. Prostate-specific membrane antigen expression on positron emission tomography/computed tomography in patients with metastatic castration-resistant prostate cancer: a retrospective observational study. J Nucl Med. 2023;64(6):910–917. doi: 10.2967/jnumed.122.264964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niaz M.J., Sun M., Skafida M., et al. Review of commonly used prostate specific PET tracers used in prostate cancer imaging in current clinical practice. Clin Imaging. 2021;79:278–288. doi: 10.1016/j.clinimag.2021.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Hope T.A., Aggarwal R., Chee B., et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58(12):1956–1961. doi: 10.2967/jnumed.117.192476. [DOI] [PubMed] [Google Scholar]
- 23.Donswijk M.L., van Leeuwen P.J., Vegt E., et al. Clinical impact of PSMA PET/CT in primary prostate cancer compared to conventional nodal and distant staging: a retrospective single center study. BMC Cancer. 2020;20(1):723. doi: 10.1186/s12885-020-07192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain M., Carducci M.A., Clarke N., et al. Evolving role of prostate-specific membrane antigen-positron emission tomography in metastatic hormone-sensitive prostate cancer: more questions than answers? J Clin Oncol. 2022;40(26):3011–3014. doi: 10.1200/JCO.22.00208. [DOI] [PubMed] [Google Scholar]
- 25.Maurer T., Eiber M., Schwaiger M., Gschwend J.E. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13(4):226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 26.Esen B., Seymen H., Tarim K., et al. Diagnostic performance of 68Ga-PSMA-11 positron emission tomography/computed tomography to monitor treatment response in patients with metastatic prostate cancer: the concordance between biochemical response and prostate-specific membrane antigen results. Eur Urol Focus. 2023;9(5):832–837. doi: 10.1016/j.euf.2023.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Plouznikoff N., Artigas C., Sideris S., et al. Evaluation of PSMA expression changes on PET/CT before and after initiation of novel antiandrogen drugs (enzalutamide or abiraterone) in metastatic castration-resistant prostate cancer patients. Ann Nucl Med. 2019;33(12):945–954. doi: 10.1007/s12149-019-01404-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.