Summary
Background
The role of transarterial chemoembolization (TACE) in the treatment of advanced hepatocellular carcinoma (HCC) is unconfirmed. This study aimed to assess the efficacy and safety of immune checkpoint inhibitors (ICIs) plus anti-vascular endothelial growth factor (anti-VEGF) antibody/tyrosine kinase inhibitors (TKIs) with or without TACE as first-line treatment for advanced HCC.
Methods
This nationwide, multicenter, retrospective cohort study included advanced HCC patients receiving either TACE with ICIs plus anti-VEGF antibody/TKIs (TACE-ICI-VEGF) or only ICIs plus anti-VEGF antibody/TKIs (ICI-VEGF) from January 2018 to December 2022. The study design followed the target trial emulation framework with stabilized inverse probability of treatment weighting (sIPTW) to minimize biases. The primary outcome was overall survival (OS). Secondary outcomes included progression-free survival (PFS), objective response rate (ORR), and safety. The study is registered with ClinicalTrials.gov, NCT05332821.
Findings
Among 1244 patients included in the analysis, 802 (64.5%) patients received TACE-ICI-VEGF treatment, and 442 (35.5%) patients received ICI-VEGF treatment. The median follow-up time was 21.1 months and 20.6 months, respectively. Post-application of sIPTW, baseline characteristics were well-balanced between the two groups. TACE-ICI-VEGF group exhibited a significantly improved median OS (22.6 months [95% CI: 21.2–23.9] vs 15.9 months [14.9–17.8]; P < 0.0001; adjusted hazard ratio [aHR] 0.63 [95% CI: 0.53–0.75]). Median PFS was also longer in TACE-ICI-VEGF group (9.9 months [9.1–10.6] vs 7.4 months [6.7–8.5]; P < 0.0001; aHR 0.74 [0.65–0.85]) per Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1. A higher ORR was observed in TACE-ICI-VEGF group, by either RECIST v1.1 or modified RECIST (41.2% vs 22.9%, P < 0.0001; 47.3% vs 29.7%, P < 0.0001). Grade ≥3 adverse events occurred in 178 patients (22.2%) in TACE-ICI-VEGF group and 80 patients (18.1%) in ICI-VEGF group.
Interpretation
This multicenter study supports the use of TACE combined with ICIs and anti-VEGF antibody/TKIs as first-line treatment for advanced HCC, demonstrating an acceptable safety profile.
Funding
National Natural Science Foundation of China, National Key Research and Development Program of China, Jiangsu Provincial Medical Innovation Center, Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, and Nanjing Life Health Science and Technology Project.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Immune checkpoint inhibitors, Vascular endothelial growth factor, Tyrosine kinase inhibitors, Target trial emulation
Research in context.
Evidence before this study
Systemic therapy with immune checkpoint inhibitors (ICIs) in combination with anti-vascular endothelial growth factor (anti-VEGF) antibody/tyrosine kinase inhibitors (TKIs) or dual ICIs has been recommended as first-line treatments for advanced-stage hepatocellular carcinoma (HCC). We searched PubMed from database inception to November 20, 2023, for relevant articles using the following search terms: “hepatocellular carcinoma” AND “transarterial chemoembolization” AND “immune checkpoint inhibitor” AND (“tyrosine kinase inhibitor” OR “anti-VEGF”), without language restrictions. There were no randomized controlled trials or large-sample multicenter studies comparing the outcomes of TACE combined with ICIs and anti-VEGF antibody/TKIs against those of ICIs and anti-VEGF antibody/TKIs alone.
Added value of this study
To our knowledge, this is the largest, multicenter cohort study to date, comparing ICIs plus anti-VEGF antibody/TKIs with or without TACE as first-line treatment for advanced HCC. The results of this target trial emulation study demonstrated that those who received the TACE combination therapy experienced significantly longer overall survival, progression free survival and achieved a higher objective response rate than those who received ICIs and anti-VEGF antibody/TKIs alone. More AEs were observed for TACE plus ICIs and anti-VEGF antibody/TKIs therapy but well tolerated. The credibility of our findings was substantiated by the use of the target trial emulation framework that mimics prospective RCT and minimizes bias.
Implications of all the available evidence
Treatment with TACE plus ICIs and anti-VEGF antibody/TKIs therapy was associated with significantly better outcomes than with ICIs plus anti-VEGF antibody/TKIs alone in advanced HCC patients who had not received prior systemic therapy. This study supports the synergistic efficacy of combining TACE with ICIs plus anti-VEGF antibody/TKIs as first-line treatment for advanced HCC. Furthermore, these findings may offer research directions for future prospective studies in this area.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related mortality worldwide.1 Despite continued efforts to reduce HCC incidence, forecasts indicate a further escalation in these numbers over the ensuing two decades.2 Over 50% of HCC patients are diagnosed at an advanced stage (Barcelona Clinic Liver Cancer [BCLC] stage C), making them ineligible for curative treatments.3,4 These patients with advanced stage, as well as those with BCLC stage B HCC who are unsuitable for locoregional treatment, are candidates for systemic therapy.3
Systemic treatments with molecular and immune therapies have dramatically changed the management of advanced HCC.5 The success of the IMbrave150 trial using a combo of atezolizumab plus bevacizumab confirmed the efficacy of immune checkpoint inhibitors (ICIs) plus anti-vascular endothelial growth factor (anti-VEGF) antibody for HCC.6 Subsequent trials have further demonstrated the superiority of treatments combining anti-programmed death-(ligand)1 antibody with anti-VEGF antibody/tyrosine kinase inhibitors (TKIs) or cytotoxic T lymphocyte-associated antigen 4 inhibitors.7, 8, 9, 10, 11 Based on the strong evidence above, systemic therapy with ICIs plus anti-VEGF antibody/TKIs or dual ICIs has been recommended as first-line treatment for advanced HCC.3,12
Transarterial chemoembolization (TACE) is the primary treatment option for intermediate HCC.3,13 It is also widely used in advanced HCC in real-world practice, particularly in Asian countries, as recommended in several guidelines.4,13,14 Previous studies have explored the potential survival benefits of adding TACE to TKIs in advanced HCC.15,16 TACE plus lenvatinib as the first-line treatment for advanced HCC showed promising results, with significantly longer overall survival (OS, 17.8 vs 11.5 months) and progression-free survival (PFS, 10.6 vs 6.4 months) compared to lenvatinib alone.15 The rationale for combining TACE with ICIs plus anti-VEGF antibody/TKIs in the treatment of HCC is predicated on the potential for a synergistic anti-tumor effect through reprogramming tumor immune microenvironment by TACE as well as prohibiting tumor angiogenesis by anti-VEGF antibody/TKIs.5,17 However, several randomized controlled trials (RCTs) specifically investigating this combination in intermediate-stage HCC are in progress.18,19 Recently, the EMERALD-1 trial (durvalumab plus bevacizumab with TACE) was reported to meet its primary endpoint in patients with HCC who are eligible for TACE.20 A nationwide, multicenter, retrospective cohort study in China demonstrated that TACE combined with ICIs plus anti-VEGF antibody/TKIs significantly improved prognosis compared to TACE monotherapy for predominantly advanced HCC.21,22
Target trial emulation, a well-established statistical approach, is proposed to estimate treatment effectiveness across populations in an uncontrolled setting from a large observational dataset.23,24 This analysis framework applies the RCT's methodological and design principles to observational data.25,26 Thus, the emulation of a target trial can help avoid common methodologic pitfalls, such as selection bias due to imbalanced patient characteristics or immortal time bias. A directed acyclic graph (DAG) can be used to select covariates required to adjust for confounding factors. These approaches could offer generalizable information to clinical practice.
Herein, under the target trial emulation framework, we conducted this nationwide, multicenter, retrospective cohort study (CHANCE2201) to assess the efficacy and safety of ICIs plus anti-VEGF antibody/TKIs with or without TACE as first-line treatment for advanced HCC.
Methods
We emulated a hypothetical target trial in which patients with advanced HCC were treated with first-line ICIs plus anti-VEGF antibody/TKIs with or without TACE. The study used data from the nationwide, multicenter CHANCE registers in China, as detailed in prior publications.21,22 Only deidentified data were recorded in a central repository. The study followed the Declaration of Helsinki guidelines (ClinicalTrials.gov identifier: NCT05332821) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Ethics statement
Our study was approved by the ethics committee of Zhongda Hospital, Southeast University (2022ZDSYLL068-P01), and study protocol was approved by the institutional review boards of participating centers. The need for informed consent was waived by the institutional review board due to the retrospective nature of this study.
Patients
This study included advanced HCC patients treated at 63 participating centers in China from January 2018 to December 2022. Patients received either TACE combined with ICIs plus anti-VEGF antibody/TKIs (TACE-ICI-VEGF) or ICIs plus anti-VEGF antibody/TKIs (ICI-VEGF) as first-line therapy. The inclusion criteria were as follows: (1) age ≥18 years old; (2) diagnosis of HCC confirmed by histologic or cytologic analysis or clinical feature according to the American Association for the Study of Liver Diseases (AASLD) guideline27; (3) with at least one measurable intrahepatic lesion as per the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; (4) presence of vascular invasion or extrahepatic spread; (5) Eastern Cooperative Oncology Group (ECOG) performance status ≤2; (6) have received a first-line ICI-VEGF combination or TACE-ICI-VEGF combination within three months. More specifically, the administration of anti-VEGF antibody/TKIs was concomitant with ICIs, and TACE was performed either concurrently with, or up to three months before or after ICI-VEGF combination therapy (Figure S1& Table S1). Patients in TACE-ICI-VEGF group underwent at least one cycle of ICI-VEGF combination after the initial TACE session. The exclusion criteria included: (1) have received previous systemic treatment with ICIs or anti-VEGF antibody/TKIs for HCC; (2) Child-Pugh grade C liver function, uncontrollable ascites, or overt hepatic encephalopathy; (3) incomplete outcome data or missing key baseline adjustment factors for primary analysis. The baseline adjustment factors were defined as follows: sex, age, ECOG performance status, hepatitis B virus (HBV) infection, cirrhosis, Child-Pugh grade, tumor burden (up-to-seven criteria), macrovascular invasion, extrahepatic spread, and previous HCC-related treatment history.
Individual treatment choices regarding TACE-ICI-VEGF combination or ICI-VEGF alone were based on a multidisciplinary expert-led clinical decision-making process, involving a comprehensive consideration of the patient's profile, financial burden, and patient choice. Physicians would inform patients and their families about the potential benefits, complications, and costs of each treatment option before decision-making.
ICIs plus anti-VEGF antibody/TKIs administration
All patients received the first-line ICI-VEGF combinations. All agents were administrated in accordance with the prescription dose and frequency (detailed in Tables S2 and S3). Adverse events (AEs)-related dose reduction was allowed for TKIs, while it was not allowed for ICIs and bevacizumab or its biosimilar. AEs-related dose interruption was allowed for both ICIs and anti-VEGF antibody/TKIs. Systemic treatment continued until disease progression or unacceptable toxic effects.
TACE procedure
Either conventional TACE (cTACE) or drug-eluting beads TACE (DEB-TACE) was standardly performed in “on demand” mode in this study.28 All TACE procedures were performed with super-selective catheterization and embolization, prioritizing maximal liver function preservation. The choice and dosage of chemotherapeutic agents and embolic agents applied in the TACE session were based on the guidelines and their availability.28 Repeat TACE was considered in cases of vital viable tumors or intra-hepatic recurrence, as observed in the follow-up radiological examinations, in line with the guideline.28 Detailed TACE protocol is described in Supplement pp2 and 3.
Follow-up and assessments
Regular follow-up assessments included monitoring vital signs, clinical symptoms, treatment-related AEs (TRAEs), laboratory testing, and radiological examinations by contrast-enhanced computed tomography or magnetic resonance imaging. The follow-up interval was 6–9 weeks. The date of the last follow-up was July 30, 2023. Treatment response was then assessed by two independent radiologists with at least 10 years of experience at every participating center. Following a diagnosis of disease progression, subsequent anti-cancer treatments were determined by the local physician.
Outcomes
The primary outcome of this study was OS, defined as the interval from time zero (T0) to death from any cause, censoring, or the end of follow-up (July 30, 2023). T0 was the point when the eligibility criteria were met and the initial combination treatment commenced.
The secondary outcomes included PFS, objective response rate (ORR), and safety. PFS was defined as the period from T0 to first tumor progression as per RECIST v1.1 or modified RECIST (mRECIST), death from any cause, censoring, or the end of follow-up. ORR was defined as the proportion of patients exhibiting a confirmed complete or partial response as per RECIST v1.1 or mRECIST. Safety was monitored from T0 throughout the follow-up period. The severity of TRAEs was assessed by using the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 (NCI-CTCAE v 5.0).
Emulation of a target trial
For the primary analysis, stabilized inverse probability of treatment weighting (sIPTW) was used to adjust for observed confounding factors. The DAG was created to identify these confounders. The selection and classification of the confounders were informed by literature review and based on the availability of sufficient-quality data (Figure S2). A logistic regression model was then developed, with treatment receipt as the outcome and adjusting for the following covariates: sex, age, ECOG performance status, HBV infection, cirrhosis, Child-Pugh grade, tumor burden (up-to-seven criteria), macrovascular invasion, extrahepatic spread, and previous HCC-related treatment history. The sIPTW analyses, derived from the predictive probabilities of this model, were then applied to individual contributions in the survival curves and the Cox proportional hazards model.
The appropriate statistical methods were also used to compare the treatment strategies of interest. Firstly, clone and inverse probability of censoring weight (IPCW) methods were employed to eliminate the potential risk of immortal time bias, following a three-step analytical procedure accounting for cloning, censoring, and weighting (detailed in Supplement pp6). Secondly, the conditional landmark analysis with the landmark time set at three months after T0 was performed. Thirdly, an alternative time when eligible patients were diagnosed with advanced-stage HCC (Td) was also documented to recalculate OS. This is a sensitivity analysis for T0 which was defined in the primary study.
Statistical analysis
The minimum sample size required for this target trial was 472 cases (236 cases in each group) estimated by PASS 15 (Supplement pp5). In the primary analysis, the log-rank test was utilized to compare OS between the two groups. The Kaplan–Meier curves were generated, while hazard ratios (HRs) along with 95% confidence intervals (CIs) were estimated using the Cox proportional hazards model. Details of secondary outcomes analyses and patients’ characteristics were displayed in Supplementary pp5 and 6.
Then, four sensitivity analyses and two post hoc analyses were conducted. First, rather than censoring patients during follow-up, we kept them in the risk set until the end of the follow-up. Second, without the use of sIPTW, we conducted a Kaplan–Meier curve analysis and a traditional Cox proportional hazards model. Third, propensity score matching was performed using a 1:1 nearest-neighbor method without replacement and a caliper width of 0.05. Fourth, we excluded patients in the ICI-VEGF group who received TACE after three months to account for the potential unidirectional crossover effect.
In the first post hoc analyses, we only included patients who received first-line atezolizumab-bevacizumab, sintilimab-bevacizumab biosimilar, or camrelizumab-apatinib with or without TACE and repeated the primary analysis. The second post hoc analysis only included patients with complete baseline data of serum alpha-fetoprotein (AFP) levels and albumin-bilirubin (ALBI) grade, including these two variables in the primary analysis to adjust for potential confounding factors.
A subgroup analysis was performed to assess the effect of TACE-ICI-VEGF therapy across the prespecified subgroups. Due to the multiple comparisons involved, findings from subgroup analyses should be interpreted as exploratory. All comparisons were 2-tailed, with P < 0.05 considered significant. All analyses were performed by using R software (version 4.3.1).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. GJT, ZGR, HDZ, ZCJ, and JJC had access to the dataset. GJT and ZGR had the final responsibility for the decision to submit for publication.
Results
Patient characteristics
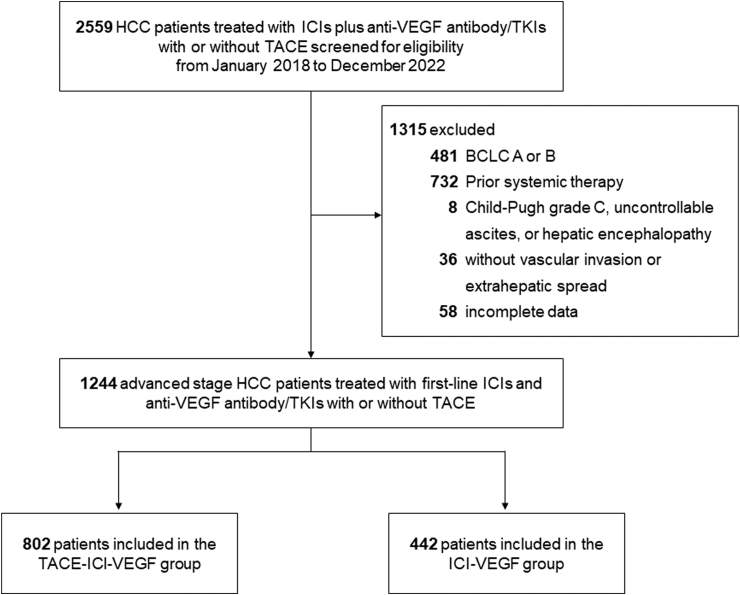
A total of 1244 patients were included in this analysis: 802 in TACE-ICI-VEGF group and 442 in ICI-VEGF group (Fig. 1). The median age was 55 (interquartile range [IQR], 48–62) years, 1067 patients (85.8%) were male, and 1047 patients (84.2%) had HBV-related infection. Compared with ICI-VEGF group, patients in TACE-ICI-VEGF group had higher tumor burden (beyond up-to-seven criteria, 664 [82.8%] vs 334 [75.6%]), accompanied by a higher proportion of vascular invasion (596 [74.3%] vs 286 [64.7%]) and a lower proportion of extrahepatic spread (437 [54.5%] vs 288 [65.2%]) before sIPTW (Table 1). After applying sIPTW, baseline characteristics were well-balanced between the two groups (Table 1). The standardized differences between the two groups for each baseline covariate before and after sIPTW are shown in Figure S3.
Fig. 1.
Study cohort. HCC, hepatocellular carcinoma; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer; TACE-ICI-VEGF, TACE with ICIs plus anti-VEGF antibody/TKIs; ICI-VEGF, ICIs plus anti-VEGF antibody/TKIs.
Table 1.
Patient baseline characteristics before and after applying sIPTW.a
| Characteristics | Before sIPTW |
After sIPTWb |
||||||
|---|---|---|---|---|---|---|---|---|
| TACE-ICI-VEGF (n = 802) | ICI-VEGF (n = 442) | P value | SMD | TACE-ICI-VEGF (n = 805) | ICI-VEGF (n = 437) | P value | SMD | |
| Median age (years) | 54 (48–62) | 56 (48–63) | 0.034 | 0.116 | 54 (48–63) | 56 (47–62) | 0.90 | 0.001 |
| Sex | 0.46 | 0.049 | 0.85 | 0.012 | ||||
| Male | 683 (85.2) | 384 (86.9) | 693 (86.1) | 378 (86.5) | ||||
| Female | 119 (14.8) | 58 (13.1) | 112 (13.9) | 59 (13.5) | ||||
| ECOG PS | 0.081 | 0.129 | 0.92 | 0.024 | ||||
| 0 | 455 (56.7) | 254 (57.5) | 464 (57.6) | 257 (58.8) | ||||
| 1 | 320 (39.9) | 162 (36.7) | 308 (38.3) | 162 (37.1) | ||||
| 2 | 27 (3.4) | 26 (5.9) | 33 (4.1) | 18 (4.1) | ||||
| Etiology | 0.089 | 0.107 | 0.64 | 0.029 | ||||
| Hepatitis B virus | 664 (82.8) | 383 (86.7) | 681 (84.6) | 374 (85.6) | ||||
| Others | 138 (17.2) | 59 (13.3) | 124 (15.4) | 63 (14.4) | ||||
| Cirrhosis | 0.066 | 0.114 | 0.87 | 0.010 | ||||
| Absent | 225 (28.1) | 102 (23.1) | 211 (26.2) | 112 (25.6) | ||||
| Present | 577 (71.9) | 340 (76.9) | 594 (73.8) | 325 (74.4) | ||||
| Child-Pugh class | 0.81 | 0.019 | 0.93 | 0.005 | ||||
| A | 659 (82.2) | 360 (81.4) | 659 (81.8) | 357 (81.7) | ||||
| B | 143 (17.8) | 82 (18.6) | 146 (18.2) | 80 (18.3) | ||||
| ALBI gradec | 0.13 | 0.122 | 0.37 | 0.087 | ||||
| 1 | 293 (38.7) | 182 (43.3) | 291 (38.2) | 173 (41.7) | ||||
| 2 | 445 (58.8) | 223 (53.1) | 451 (59.2) | 227 (55.0) | ||||
| 3 | 19 (2.5) | 15 (3.6) | 20 (2.6) | 13 (3.2) | ||||
| Up-to-seven criteria | 0.0028 | 0.179 | 0.96 | 0.003 | ||||
| Within | 138 (17.2) | 108 (24.4) | 163 (20.2) | 89 (20.4) | ||||
| Beyond | 664 (82.8) | 334 (75.6) | 642 (79.8) | 348 (79.6) | ||||
| Macrovascular invasion | 0.00046 | 0.210 | 0.92 | 0.006 | ||||
| Absent | 206 (25.7) | 156 (35.3) | 235 (29.2) | 129 (29.5) | ||||
| Present | 596 (74.3) | 286 (64.7) | 570 (70.8) | 308 (70.5) | ||||
| Extrahepatic spread | 0.00033 | 0.219 | 0.85 | 0.012 | ||||
| Absent | 365 (45.5) | 154 (34.8) | 334 (41.5) | 179 (41.0) | ||||
| Present | 437 (54.5) | 288 (65.2) | 471 (58.5) | 258 (59.0) | ||||
| Serum AFP leveld | 0.79 | 0.020 | 0.67 | 0.027 | ||||
| ≤400 ng/mL | 384 (47.9) | 215 (48.6) | 394 (48.9) | 208 (47.6) | ||||
| >400 ng/mL | 361 (45.0) | 194 (43.9) | 354 (44.0) | 197 (45.1) | ||||
| HCC-related treatment history | <0.0001 | 0.423 | 0.86 | 0.011 | ||||
| Absent | 626 (78.1) | 260 (58.8) | 570 (70.8) | 307 (70.3) | ||||
| Present | 176 (21.9) | 182 (41.2) | 235 (29.2) | 130 (29.7) | ||||
| Surgery | 50 (6.2) | 49 (11.1) | 0.0035 | 0.173 | 69 (8.6) | 34 (7.8) | 0.60 | 0.030 |
| Ablation | 31 (3.9) | 25 (5.7) | 0.19 | 0.084 | 42 (5.2) | 17 (4.0) | 0.33 | 0.056 |
| TACEe | 121 (15.1) | 142 (32.1) | <0.0001 | 0.410 | 161 (20.0) | 102 (23.3) | 0.15 | 0.085 |
| Other LRTsf | 15 (1.9) | 26 (5.9) | 0.00029 | 0.209 | 19 (2.3) | 18 (4.1) | 0.088 | 0.097 |
Data are median (interquartile range) or n (%).
Abbreviations: sIPTW, stabilized inverse probability of treatment weighting; TACE-ICI-VEGF, TACE with ICIs plus anti-VEGF antibody/TKIs; ICI-VEGF, ICIs plus anti-VEGF antibody/TKIs; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors; SMD, standardized mean difference; ECOG PS, Eastern Cooperative Oncology Group performance status; ALBI, albumin-bilirubin; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; LRT, locoregional therapy.
Unless otherwise indicated, data are expressed as number (percentage) of patients. Percentages have been rounded and may not total 100.
The total number of patients in two groups is slightly different in the post-sIPTW pseudo-data set as a result of the weighting.
ALBI grade for 45 and 22 patients are missing in the respective group.
Serum AFP data for 57 and 33 patients are missing in the respective group.
History of TACE treatment more than 3 months prior to systemic therapy.
Includes any history of radiotherapy, percutaneous ethanol injection, and hepatic arterial infusion chemotherapy.
During the period of triple (TACE-ICI-VEGF) or dual (ICI-VEGF) treatment regimens, patients in TACE-ICI-VEGF group underwent a median of 5 cycles of ICIs (IQR, 3–8), 5.8 months of anti-VEGF antibody/TKIs (IQR, 2.9–10.0), and 2 sessions of TACE procedures (IQR, 1–4), whereas patients in ICI-VEGF group had a median of 5 cycles of ICIs (IQR, 3–9) and 5.1 months of anti-VEGF antibody/TKIs (IQR, 3.0–9.0). Subsequent post-line therapies are summarized in Table S4.
Efficacy
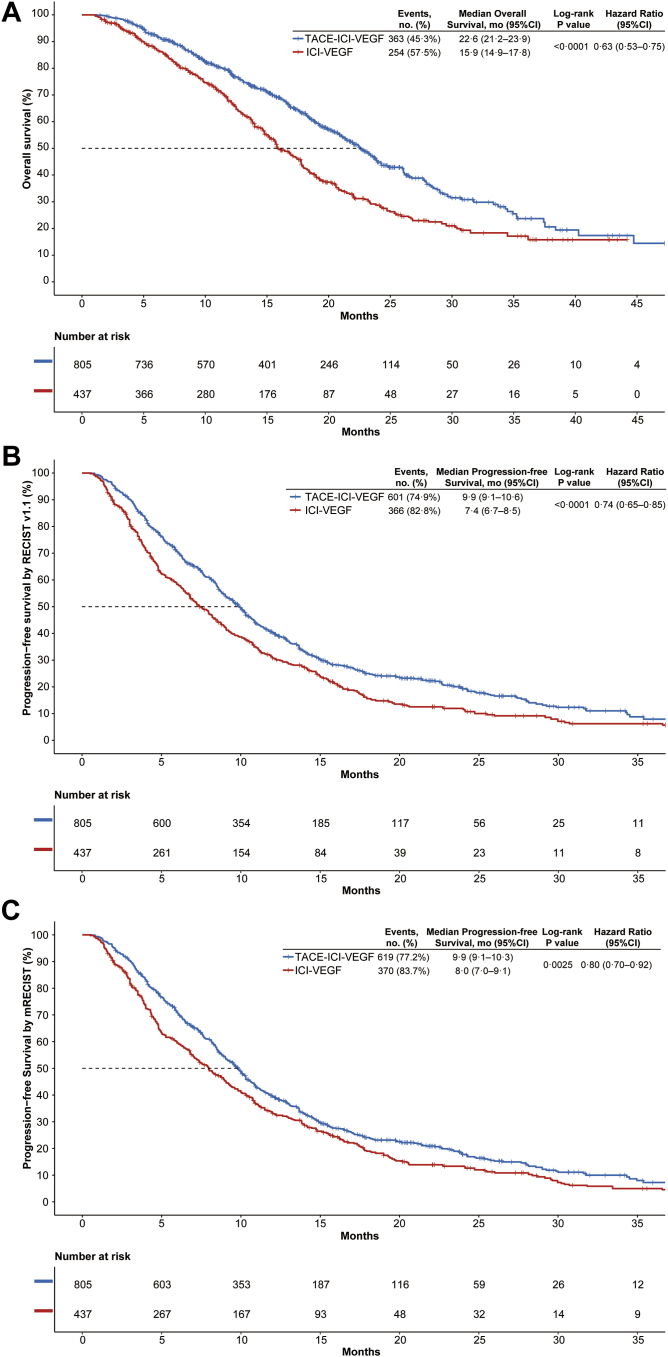
The median follow-up time was 21.1 (95% confidence interval [CI]: 19.9–22.2) months in TACE-ICI-VEGF group, vs 20.6 (95% CI: 19.6–24.1) months in ICI-VEGF group (P = 0.50). At the time of data cutoff, 363 patients (45.3%) in TACE-ICI-VEGF group and 254 patients (57.5%) in ICI-VEGF group died. After sIPTW, the median OS of the TACE-ICI-VEGF group was significantly longer than that of the ICI-VEGF group (22.6 months [95% CI: 21.2–23.9] vs 15.9 months [95% CI: 14.9–17.8]; log-rank P < 0.0001; adjusted HR for death, 0.63; 95% CI: 0.53–0.75; Fig. 2).
Fig. 2.
Kaplan–Meier curves of (A) overall survival, (B) progression-free survival assessed by RECIST v1.1, and (C) progression-free survival assessed by mRECIST after sIPTW. TACE-ICI-VEGF, TACE with ICIs plus anti-VEGF antibody/TKIs; ICI-VEGF, ICIs plus anti-VEGF antibody/TKIs; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors; CI, confidence interval; mRECIST, modified RECIST; sIPTW, stabilized inverse probability of treatment weighting.
The median PFS was also significantly improved in TACE-ICI-VEGF group vs ICI-VEGF group (9.9 months [95% CI: 9.1–10.6] vs. 7.4 months [95% CI: 6.7–8.5]; log-rank P < 0.0001; adjusted HR, 0.74; 95% CI: 0.65–0.85; Fig. 2) according to RECIST v1.1. This finding was consistent with the median PFS following mRECIST (9.9 months [95% CI: 9.1–10.3] vs. 8.0 months [95% CI: 7.0–9.1]; log-rank P = 0.0025; adjusted HR, 0.80 [95% CI: 0.70–0.92]; Fig. 3). The confirmed ORRs were 41.2% (95% CI: 37.7–44.6) in TACE-ICI-VEGF group and 22.9% (95% CI: 19.0–27.1) in ICI-VEGF group, as per RECIST 1.1 (P < 0.0001), and 47.3% (95% CI: 43.7–50.7) and 29.7% (95% CI: 25.5–34.3), respectively, according to mRECIST (P < 0.0001).
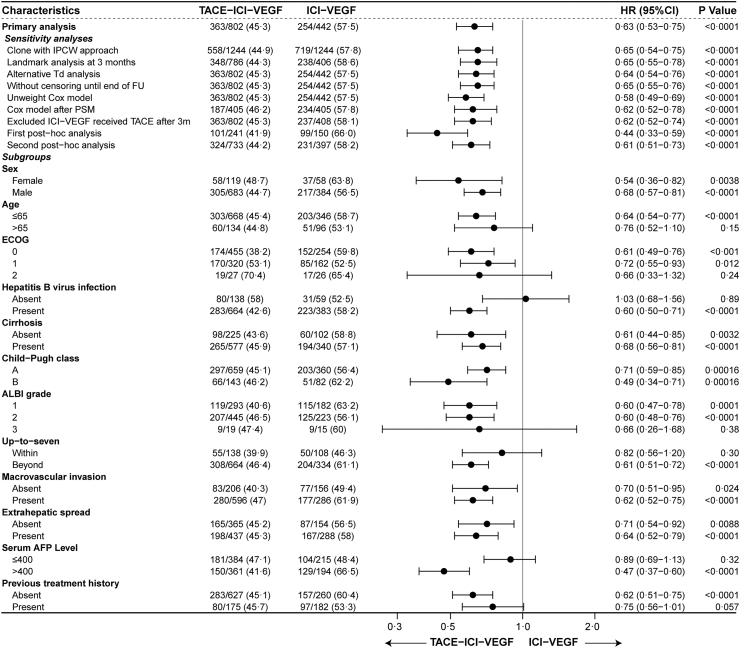
Fig. 3.
Subgroup analysis of overall survival. TACE-ICI-VEGF, TACE with ICIs plus anti-VEGF antibody/TKIs; ICI-VEGF, ICIs plus anti-VEGF antibody/TKIs; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors; HR, hazard ratio; CI, confidence interval; IPCW, inverse probability of censoring weight; FU, follow-up; PSM, propensity score matching; ECOG, Eastern Cooperative Oncology Group; ALBI, albumin-bilirubin; AFP, alpha-fetoprotein.
After applying IPCW, the median OS in TACE-ICI-VEGF was 22.5 months (95% CI: 21.1–23.9) vs 15.9 months (95% CI: 14.8–17.8) in ICI-VEGF group, with a HR = 0.65 (95% CI: 0.54–0.75) by 1000 resamples bootstrap. Similar results were observed in both the landmark analysis and alternative Td analysis (Fig. 3 & Figure S4). The sensitivity analyses and post hoc analyses also supported these results (Fig. 3 & Figure S5). For patients receiving standard first-line combinations (including atezolizumab plus bevacizumab, sintilimab plus bevacizumab biosimilar, or camrelizumab plus apatinib), the median OS in TACE-ICI-VEGF group was 26.0 months (95% CI: 22.4–33.4) compared to 14.7 months (95% CI: 12.9–17.8) in ICI-VEGF group. This yielded a log-rank P value <0.0001 with an adjusted HR of 0.44 (95% CI: 0.33–0.59; Figure S5). There are 34 patients in ICI-VEGF group treated with TACE after three months. After excluding these patients, the OS in TACE-ICI-VEGF group was 22.5 (21.1–23.8) months compared with 15.9 (15.1–17.8) months (log-rank P < 0.0001) in ICI-VEGF group (adjusted HR = 0.62 [95% CI: 0.52–0.74]; Figure S5). Detailed results were described in Supplement pp9–11.
The OS benefits of TACE-ICI-VEGF treatment were generally consistent across various clinical subgroups (Fig. 3 & Figure S6). A similar trend of improved PFS was observed in TACE-ICI-VEGF group (Figure S7). An exception was for levels of serum AFP, where the 95% confidence intervals for patients with serum AFP above or below 400 ng/mL did not overlap, with higher treatment differences at higher levels of AFP.
Safety
AEs of any grade were reported in 573 patients (71.4%) in TACE-ICI-VEGF group and 247 patients (55.9%) in ICI-VEGF group (Table S5). Grade 3–4 AEs occurred in 176 patients (21.9%) in TACE-ICI-VEGF group and 79 (17.9%) patients in ICI-VEGF group, while grade 5 AEs occurred in 2 patients (0.2%) and 1 patient (0.2%), respectively. The most common AEs of any grade in each group are shown in Table 2.
Table 2.
Adverse events after treatment in whole population.
| Adverse events | Any grade |
Grade ≥3 |
||
|---|---|---|---|---|
| TACE-ICI-VEGF (n = 802) | ICI-VEGF (n = 442) | TACE-ICI-VEGF (n = 802) | ICI-VEGF (n = 442) | |
| Abdominal pain | 200 (24.9) | 8 (1.8) | 44 (5.5) | 1 (0.2) |
| Pyrexia | 193 (24.1) | 22 (5.0) | 8 (1.0) | 3 (0.7) |
| Increased ALT | 158 (19.7) | 61 (13.8) | 23 (2.9) | 9 (2.0) |
| Increased AST | 151 (18.8) | 60 (13.6) | 22 (2.7) | 10 (2.3) |
| Hypertension | 135 (16.8) | 87 (19.7) | 26 (3.2) | 19 (4.3) |
| Pain | 115 (14.3) | 15 (3.4) | 3 (0.4) | 0 |
| HFSR | 96 (12.0) | 41 (9.3) | 15 (1.9) | 4 (0.9) |
| Fatigue | 106 (13.2) | 64 (14.5) | 13 (1.6) | 5 (1.1) |
| Nausea | 97 (12.1) | 22 (5.0) | 2 (0.2) | 0 |
| Vomiting | 92 (11.5) | 10 (2.3) | 2 (0.2) | 1 (0.2) |
| Elevated bilirubin | 76 (9.5) | 42 (9.5) | 14 (1.7) | 11 (2.5) |
| Diarrhea | 75 (9.4) | 50 (11.3) | 10 (1.2) | 8 (1.8) |
| Rash | 73 (9.1) | 52 (11.8) | 8 (1.0) | 4 (0.9) |
| Proteinuria | 52 (6.5) | 39 (8.8) | 6 (0.7) | 3 (0.7) |
| Anorexia | 62 (7.7) | 41 (9.3) | 4 (0.5) | 2 (0.5) |
| Hypothyroidism | 45 (5.6) | 28 (6.3) | 2 (0.2) | 0 |
| RCCEP | 41 (5.1) | 12 (2.7) | 3 (0.4) | 0 |
| Thrombocytopenia | 36 (4.5) | 41 (9.3) | 8 (1.0) | 8 (1.8) |
| Gastrointestinal hemorrhage | 23 (2.9) | 18 (4.1) | 8 (1.0) | 9 (2.0) |
| Decreased WBC count | 21 (2.6) | 13 (2.9) | 3 (0.4) | 1 (0.2) |
| Abdominal distension | 18 (2.2) | 5 (1.1) | 1 (0.1) | 0 |
| Ascites | 17 (2.1) | 17 (3.8) | 1 (0.1) | 1 (0.2) |
| Pruritus | 11 (1.4) | 7 (1.6) | 2 (0.2) | 0 |
| Pneumonitis | 11 (1.4) | 7 (1.6) | 4 (0.5) | 2 (0.5) |
| Infusion-related reaction | 5 (0.6) | 0 | 0 | 0 |
| Weight decrease | 4 (0.5) | 2 (0.5) | 0 | 0 |
| Constipation | 3 (0.4) | 3 (0.7) | 0 | 0 |
TACE-ICI-VEGF, TACE with ICIs plus anti-VEGF antibody/TKIs; ICI-VEGF, ICIs plus anti-VEGF antibody/TKIs; TACE, transarterial chemoembolization; ICIs, immune checkpoint inhibitors; VEGF, vascular endothelial growth factor; TKIs, tyrosine kinase inhibitors; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HFSR, hand-foot skin reaction; RCCEP, reactive cutaneous capillary endothelial proliferation; WBC, white blood cell.
ICIs were discontinued due to AEs in 41 patients (5.1%) in TACE-ICI-VEGF group, compared to 19 patients (4.3%) in ICI-VEGF group, while discontinuation of anti-VEGF antibody/TKIs occurred in 64 patients (8.0%) vs 37 patients (8.4%), respectively. Thirty-four patients (4.2%) in TACE-ICI-VEGF group and 26 patients (5.9%) in ICI-VEGF group experienced dose interruptions of ICIs due to AEs. Moreover, dose reductions or interruptions of anti-VEGF antibody/TKIs due to AEs were reported in 154 patients (19.2%) in TACE-ICI-VEGF group vs 92 patients (20.8%) in ICI-VEGF group (Table S5).
Discussion
To the best of our knowledge, this is the most extensive multicenter study conducted to evaluate the efficacy and safety of combining TACE with ICIs and anti-VEGF antibody/TKIs as a first-line treatment for advanced HCC in the real-world setting. Our results support the combination of TACE with ICIs and anti-VEGF antibody/TKIs, showing significantly better OS, PFS, and ORR compared to the use of ICIs and anti-VEGF antibody/TKIs alone. An increase in AEs was observed but was acceptable. These survival benefits were consistent in multiple sensitivity analyses and across clinical subgroups. The credibility of our results was further reinforced by the use of a target trial emulation framework, which approximates prospective RCTs to minimize bias from confounding factors.
Compared to those in reported trials evaluating first-line ICIs combined with anti-VEGF antibody/TKIs for advanced HCC (Table S6), patients in our study had a higher prevalence of poor liver function, with approximately 18% of patients in the Child-Pugh B class. Additionally, a higher proportion of patients had vascular invasion (∼70%) and HBV infection (∼80%). 27 (3.4%) and 26 (5.9%) patients with ECOG PS of 2 from the two groups were included in this study. This demographic more accurately reflected the real-world clinical practice than the highly selected populations typically involved in clinical trials. Notwithstanding these differences, patients in the TACE-ICI-VEGF group achieved a median OS of 22.6 months, a median PFS of 9.9 months, and an ORR of 41.2%. These results are comparable to those of previous clinical trials,6, 7, 8, 9 which indicate that adding TACE to the regimen of ICIs plus anti-VEGF antibody/TKIs is a feasible approach for patients with advanced HCC in a real-world setting.
There are sound reasons to integrate TACE with ICIs and anti-VEGF antibody/TKIs in treatment regimens. TACE can induce a hypoxia microenvironment, leading to upregulated VEGF expression and tumor angiogenesis.5 Elevated VEGF levels foster an immunosuppressive tumor environment, hindering dendritic cell maturation and function, and increasing T regulatory cells and myeloid-derived suppressor cell recruitment.29 By targeting VEGF, there is potential to restore anti-tumor activity and enhance the effectiveness of ICIs.30 Furthermore, TACE prompts the release of tumor antigens and proinflammatory cytokines, fostering immunogenic cell death and converting non-immunogenic tumors into immunogenic ones.17 These potential mechanisms might explain why, compared to the PFS benefits primarily attributed to locoregional treatment, the OS outcomes in our study showed more significant improvements, likely enhanced by the combination therapy.
In alignment with the combination rationales, the EMERALD-1 trial, the first global phase 3 study, demonstrated a significant improvement in PFS—its primary endpoint, in unresectable HCC patients receiving the combination of durvalumab plus bevacizumab with TACE.20 The median PFS was 15.0 months (95% CI: 11.1–18.9) with combination therapy and 8.2 months (95% CI: 6.9–11.1) with TACE alone (HR = 0.77 [95% CI: 0.61–0.98]; stratified log-rank P = 0.032). This benefit was generally consistent across subgroups. Furthermore, time to progression (22.0 months [95% CI: 16.6–24.9] vs. 10.0 months [95% CI: 7.1–13.6]) and ORR (43.6% vs. 29.6%) were also significantly improved with combination therapy. The safety profile was manageable and in line with known profiles of the treatments involved. However, it should be noted that the study population and treatment approaches in the EMERALD-1 trial, which involves HCC patients eligible for embolization across different stages and compares durvalumab plus bevacizumab with TACE to TACE alone, are different from our study.
The survival benefits of adding TACE to systemic therapy were also examined across several clinical subgroups. While the results from subgroup analyses are considered exploratory and should be interpreted with caution, there are several noteworthy observations that merit discussion. A significant concern regarding the addition of TACE to systemic therapy is the potential deterioration of liver function, which could increase the risk of mortality resulting from liver decompensation. Previous studies found that both TACE and ICI-VEGF treatment are safe and potentially effective for patients with Child-Pugh B class.31,32 Our study did not observe any additional risk associated with combination therapy in this population. For patients with high intrahepatic or extrahepatic tumor burden, TACE could effectively reduce intrahepatic tumor burden. Consequently, the efficacy of ICI-VEGF may be improved as the tumor burden diminishes.33 In line with this, patients with serum AFP levels>400 ng/mL, reflecting a higher tumor burden, may benefit more from combination therapy. Considering the confidence intervals for high and low AFP level do not even overlap in subgroup analysis (Fig. 3 & Figure S7), the relationship between therapeutic outcomes and serum AFP levels was then specifically examined. A significant interaction was found both for overall survival (P = 0.00028) and progression-free survival (P = 0.0028). These results suggest that incorporating patient-specific AFP levels into future treatment strategies could potentially tailor treatment plans, possibly optimizing therapeutic outcomes while minimizing adverse effects. Future research is warranted to explore these observations further.
It is crucial to consider whether the AEs might have a cumulative effect in combination therapy. In this study, the overall AE rate was higher in the TACE-ICI-VEGF group compared to the ICI-VEGF group (71.4% vs 55.9%), though the rates of grade 3–4 AE were similar (21.9% vs 17.9%), and grade 5 AEs occurred in 0.2% of patients in both groups. Patients in the TACE-ICI-VEGF group experienced increased incidences of abdominal pain, nausea, fever, and temporary rises in liver enzyme elevations and hyperbilirubinemia, probably attributed to TACE. It would be interesting to explore the impact of AEs on treatment efficacy in future studies.34 Comparable rates of drug discontinuation, dose reduction, or interruption were exhibited in the two groups. Hence, combining TACE with ICIs and anti-VEGF antibody/TKIs in advanced HCC was generally well-tolerated.
We acknowledge that the use of multiple drugs and their various combinations is a major limitation in our study. Inspired by the concept of “umbrella trials”, we pooled various combination protocols. Several ICIs and TKIs, approved by the National Medical Products Administration and available in China, have demonstrated efficacy in HCC in RCTs.7,8,35,36 Drug combination choices were based on a multidisciplinary expert-led clinical decision-making process, considering the patient's medical profile, financial considerations, and personal preferences. To mitigate the influence of different drugs, we performed a post-hoc analysis including only atezolizumab-bevacizumab, sintilimab-bevacizumab biosimilar, or camrelizumab-apatinib. The results were consistent with our primary analysis. Moreover, there was heterogeneity in the TACE procedures in different centers. The standardization of TACE procedures across participating centers minimized such heterogeneity.14 Besides, this study did not collect data on liver-specific responses or patterns of progression, which are important for evaluating intrahepatic tumor shrinkage resulting from the addition of TACE to systemic treatment. Our study included only Chinese patients, mostly with HBV-related HCC, therefore our findings cannot be easily generalized to Western populations, where other etiologies of liver disease are prevalent and baseline patient characteristics are different.
In conclusion, first-line treatment combining TACE with ICIs plus anti-VEGF antibody/TKIs was associated with significantly improved OS, PFS, and ORR compared to ICIs plus anti-VEGF antibody/TKIs alone in patients with advanced HCC. AEs were generally well-tolerated, although slightly more commonly observed in the TACE combination group. The results of this study support the use of TACE combined with ICIs and anti-VEGF antibody/TKIs as first-line treatment for advanced HCC.
Contributors
GJT and ZGR conceptualised and designed the study, conducted the investigation. ZCJ, JJC, XLZ, XHD, YJX, BYZ, JZC, JT, KSZ, ZL, MH, MJP, HBS, RBL, ABX, FPJ, JBW, GLS, HLL, MSH, ZYP, JSJ, CWY, XFL, ZCH, WZY, GWY, JHH, NJG, XL, XLQ, GHX, QT, HLL, YJZ, HJ, HBS, YJS, TSC, BQS, WGX, SZG, WDW, SW, SWW, WFL, XZ, WM, WXR, ZML, YF, JPL, WZ, CSZ, XYZ, HZ, JX, WHH, YML, QHW, HZN, JRL, JJH, DPF, ZC, QDL, ZYD, RSS, YC, WJW, LNY, XZ, HTZ, HDZ, and ZGR collected the data. YZ, JWZ, ZCJ, and JJC analysed the data. ZCJ, JJC, BYZ, and HDZ wrote the original draft. GJT, ZGR, HDZ, ZCJ, and JJC have accessed and verified the data. GJT and ZGR provided supervision. All authors reviewed and approved the final version of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Data are not available for sharing due to privacy and ethical or legal issues. Summary statistical data will be available from the corresponding author on reasonable request with the permission of the Chinese multicenter registry.
Declaration of interests
We declare no competing interests.
Acknowledgements
The study was supported by National Natural Science Foundation of China (82130060, 61821002), National Key Research and Development Program (2018YFA0704100, 2018YFA0704104), Jiangsu Provincial Medical Innovation Center (CXZX202219), Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, and Nanjing Life Health Science and Technology Project (202205045). The funding sources had no role in the writing of the report, or decision to submit the paper for publication. We thank all the investigators at each site, as well as the staff involved in the CHANCE study.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102622.
Contributor Information
Zheng-Gang Ren, Email: ren.zhenggang@zs-hospital.sh.cn.
Gao-Jun Teng, Email: gjteng@seu.edu.cn.
CHANCE2201 Investigators:
Zhi-Cheng Jin, Jian-Jian Chen, Xiao-Li Zhu, Xu-Hua Duan, Yu-Jing Xin, Bin-Yan Zhong, Jin-Zhang Chen, Jun Tie, Kang-Shun Zhu, Lan Zhang, Ming Huang, Ming-Jian Piao, Xiao Li, Hai-Bin Shi, Rui-Bao Liu, Ai-Bing Xu, Fan-Pu Ji, Jian-Bing Wu, Guo-Liang Shao, Hai-Liang Li, Ming-Sheng Huang, Zhi-Yi Peng, Jian-Song Ji, Chun-Wang Yuan, Xiu-Feng Liu, Zhou-Chao Hu, Wei-Zhu Yang, Guo-Wen Yin, Jin-Hua Huang, Nai-Jian Ge, Xiao-Long Qi, Yang Zhao, Jia-Wei Zhou, Guo-Hui Xu, Qiang Tu, Hai-Lan Lin, Yao-Jun Zhang, Hua Jiang, Hai-Bo Shao, Yong-Jie Su, Ting-Song Chen, Bao-Qi Shi, Wen-Ge Xing, Shan-Zhi Gu, Wei-Dong Wang, Song Wang, Shu-Wei Wen, Wei-Fu Lv, Xu Zhu, Wei Mu, Wei-Xin Ren, Zai-Ming Lu, Yong Fan, Jia-Ping Li, Wei Zhao, Chuan-Sheng Zheng, Xu-Ya Zhao, Hui Zhao, Jian Xu, Wen-Hao Hu, Yan-Ming Lei, Qing-Hua Wu, Huan-Zhang Niu, Jia-Rui Li, Jian-Jun Han, Dui-Ping Feng, Zheng Cai, Qing-Dong Li, Zhen-Yu Dai, Rong-Shu Shi, Yong Chen, Wen-Jun Wang, Li-Nan Yin, Xiang Zhou, Hai-Tao Zhao, Hai-Dong Zhu, Zheng-Gang Ren, and Gao-Jun Teng
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rumgay H., Arnold M., Ferlay J., et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77(6):1598–1606. doi: 10.1016/j.jhep.2022.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reig M., Forner A., Rimola J., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park J.W., Chen M., Colombo M., et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet J.M., De Baere T., Kulik L., et al. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18(5):293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 6.Finn R.S., Qin S., Ikeda M., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 7.Ren Z., Xu J., Bai Y., et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22(7):977–990. doi: 10.1016/S1470-2045(21)00252-7. [DOI] [PubMed] [Google Scholar]
- 8.Qin S., Chan S.L., Gu S., et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): a randomised, open-label, international phase 3 study. Lancet. 2023;402(10408):1133–1146. doi: 10.1016/S0140-6736(23)00961-3. [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa G.K., Lau G., Kudo M., et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence. 2022;1(8) doi: 10.1056/EVIDoa2100070. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A.L., Qin S., Ikeda M., et al. Updated efficacy and safety data from IMbrave150: atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862–873. doi: 10.1016/j.jhep.2021.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Sangro B., Chan S., Kelley R., et al. SO-15 Four-year overall survival update from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. 2023;34:S168. doi: 10.1016/j.annonc.2024.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Chen J.J., Jin Z.C., Luo B., et al. New first-line immunotherapy-based therapies for unresectable hepatocellular carcinoma: a living network meta-analysis. J Clin Transl Hepatol. 2024;12(1):15–24. doi: 10.14218/JCTH.2023.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J., Sun H., Wang Z., et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition) Liver Cancer. 2020;9(6):682–720. doi: 10.1159/000509424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu J., Zhao M., Arai Y., et al. Clinical practice of transarterial chemoembolization for hepatocellular carcinoma: consensus statement from an international expert panel of International Society of Multidisciplinary Interventional Oncology (ISMIO) Hepatobiliary Surg Nutr. 2021;10(5):661–671. doi: 10.21037/hbsn-21-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Z., Fan W., Zhu B., et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (launch) J Clin Oncol. 2023;41(1):117–127. doi: 10.1200/JCO.22.00392. [DOI] [PubMed] [Google Scholar]
- 16.Park J.W., Kim Y.J., Kim D.Y., et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 17.Chang X., Lu X., Guo J., Teng G.J. Interventional therapy combined with immune checkpoint inhibitors: emerging opportunities for cancer treatment in the era of immunotherapy. Cancer Treat Rev. 2019;74:49–60. doi: 10.1016/j.ctrv.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Llovet J.M., Vogel A., Madoff D.C., et al. Randomized phase 3 LEAP-012 study: transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc Intervent Radiol. 2022;45(4):405–412. doi: 10.1007/s00270-021-03031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben Khaled N., Seidensticker M., Ricke J., et al. Atezolizumab and bevacizumab with transarterial chemoembolization in hepatocellular carcinoma: the DEMAND trial protocol. Future Oncol. 2022;18(12):1423–1435. doi: 10.2217/fon-2021-1261. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R., Kudo M., Erinjeri J., et al. EMERALD-1: a phase 3, randomized, placebo-controlled study of transarterial chemoembolization combined with durvalumab with or without bevacizumab in participants with unresectable hepatocellular carcinoma eligible for embolization. J Clin Oncol. 2024;42(3_suppl):LBA432. [Google Scholar]
- 21.Zhu H.D., Li H.L., Huang M.S., et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001) Signal Transduct Target Ther. 2023;8(1):58. doi: 10.1038/s41392-022-01235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Z.C., Zhong B.Y., Chen J.J., et al. Real-world efficacy and safety of TACE plus camrelizumab and apatinib in patients with HCC (CHANCE2211): a propensity score matching study. Eur Radiol. 2023;33(12):8669–8681. doi: 10.1007/s00330-023-09754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernán M.A., Robins J.M. Using big data to emulate a target trial when a randomized trial is not available. Am J Epidemiol. 2016;183(8):758–764. doi: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou J.W., Huang L.H., You D.F., Chen F., Zhao Y. The emulation of clinical trials with real-world data: development and application of target trial. Chin J Epidemiol. 2024;45(2):279–285. doi: 10.3760/cma.j.cn112338-20230821-00081. [DOI] [PubMed] [Google Scholar]
- 25.Matthews A.A., Danaei G., Islam N., Kurth T. Target trial emulation: applying principles of randomised trials to observational studies. BMJ. 2022;378 doi: 10.1136/bmj-2022-071108. [DOI] [PubMed] [Google Scholar]
- 26.Hernán M.A., Wang W., Leaf D.E. Target trial emulation: a framework for causal inference from observational data. JAMA. 2022;328(24):2446–2447. doi: 10.1001/jama.2022.21383. [DOI] [PubMed] [Google Scholar]
- 27.Heimbach J.K., Kulik L.M., Finn R.S., et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 28.Chinese College of Interventionalists CMD, Association Chinese clinical practice guidelines for transarterial chemoembolization of hepatocellular carcinoma. J Interv Radiol. 2018;27(12):1117–1126. [Google Scholar]
- 29.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Llovet J.M., Castet F., Heikenwalder M., et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi: 10.1038/s41571-021-00573-2. [DOI] [PubMed] [Google Scholar]
- 31.Takayasu K., Arii S., Kudo M., et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol. 2012;56(4):886–892. doi: 10.1016/j.jhep.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Xie E., Yeo Y.H., Scheiner B., et al. Immune checkpoint inhibitors for child-pugh class B advanced hepatocellular carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2023;9(10):1423–1431. doi: 10.1001/jamaoncol.2023.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S.I., Cassella C.R., Byrne K.T. Tumor burden and immunotherapy: impact on immune infiltration and therapeutic outcomes. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.629722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granito A., Facciorusso A., Sacco R., et al. TRANS-TACE: prognostic role of the transient hypertransaminasemia after conventional chemoembolization for hepatocellular carcinoma. J Pers Med. 2021;11(10):1041. doi: 10.3390/jpm11101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin S., Kudo M., Meyer T., et al. Tislelizumab vs sorafenib as first-line treatment for unresectable hepatocellular carcinoma: a phase 3 randomized clinical trial. JAMA Oncol. 2023;9(12):1651–1659. doi: 10.1001/jamaoncol.2023.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qin S., Bi F., Gu S., et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol. 2021;39(27):3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.