Summary
Background
Patients with Barrett's oesophagus (BO) carry significant cancer worry, burden of symptoms, and lack disease-specific knowledge. Currently there is no validated BO patient reported outcome measure (PROM) to measure these factors for use in clinical practice and research, hence the aim of this study was to devise a novel, validated BO-specific tool, B-PROM.
Methods
Literature review, quantitative and qualitative research informed the initial item generation. The item bank was refined through a modified Delphi process between May and August 2021. The PROM was then tested through cognitive interviews and validated via multicentre testing between September 2021 and February 2023 with the aim to create a succinct tool which addresses the key important factors to BO patients and has strong psychometric properties.
Findings
B-PROM covers key themes of disease-specific knowledge, trust in clinicians, burden of symptoms, cancer worry and burden of surveillance. Validation results from 387 participants (response rate 40.8%) showed 93.3% of participants completed >95% of B-PROM. All individual items scored a completion rate of >95%. Mean completion time was 5 mins 34s for a sample group. Nineteen items showed a ceiling effect, 3 items showed a floor effect. Internal consistency overall demonstrated a Cronbach Alpha of 0.846, while predetermined subsections showed Cronbach alphas of 0.335, 0.718, 0.736, and 0.896. Inter-item analysis found 2 pairs of items with strong correlation, with only 6 items correlating weakly. Item-total correlation showed 19 items correlated well. Exploratory Factor analysis (EFA) with principal component analysis produced 5 components with Eigenvalues >1 of which 4/5 had satisfactory Cronbach alphas. Test-retest reliability showed no significant differences across single and average measures (p ≤ 0.001).
Interpretation
B-PROM is the first BO-specific PROM to be systematically evaluated. Validation findings show strong internal consistency, short completion time, low missingness and excellent test-retest reliability.
Funding
Medtronic Limited ISR-2016-1077.
Keywords: Barrett's oesophagus, Cancer, Endoscopy, Patient reported outcome measures, Health related quality of life
Research in context.
Evidence before this study
Barrett's oesophagus (BO) is a precursor lesion to oesophageal adenocarcinoma though progression rates are low at 0.33%/annum. Patients with the condition are advised to undergo routine regular invasive surveillance to detect early and treatable changes.
Studies have shown BO has an impact on patient reported health related quality of life particularly due to worry of cancer, impact of symptoms and impact of surveillance. Patients often lack disease specific knowledge.
We searched PubMed from database inception to May 2023 with no language restrictions using the search terms: “((“patient reported outcome measur∗”) OR (“PROM”) OR exp “PATIENT OUTCOME ASSESSMENT”/OR (“quality of life”) OR exp “QUALITY OF LIFE”/OR (“QOL”) OR (“HRQOL”)) AND ((dysplasia) AND ((“Barrett's Oesophagus”) OR (“Barrett∗ Oesophagus”) OR (Barrett∗ Oesophagus) OR (“Barrett's esophagus”) OR (“Barrett∗ esophagus”) OR (Barrett∗ esophagus) OR exp “BARRETT ESOPHAGUS”/))”. There was no validated BO specific patient reported outcome measure (PROM) that could be used to measure this impact in clinical work and research.
Added value of this study
At the time of writing this is the first rigorously validated BO specific patient reported outcome measure. B-PROM has been developed with very close involvement of patients from the background item generation phase, during a modified Delphi to item refine and during final multicentre validation.
Implications of all the available evidence
B-PROM's psychometric properties show strong internal consistency and low missingness, it covers the important priorities of BO patients found at systematic review of qualitative research. The development and validation process has been rigorous, and the psychometric properties of B-PROM are encouraging, it can now be taken forward to wider validation against other tools.
Introduction
Patient reported outcome measures (PROMs) are tools to assess how care impacts patients, going beyond simple measures of satisfaction, but rather systematically exploring both physical and psychological symptoms in addition to functional, social and economic aspects.1 PROMs and measures of health related quality of life (HRQOL) are extensively used in many aspects of healthcare from orthopaedic surgery2 to inflammatory bowel disease.3 HRQOL measures help assess the impact of conditions and disease states on a patient's quality of life, in areas such as physical functioning, mental health and social role and can be used to quantify quality adjusted life years in cost-utility analysis. PROMs offer a report of the patient's health state which comes directly from them without interpretation by a clinician, which can be used alongside clinical outcomes in measuring responses to interventions within healthcare and research. Critically, however, there remain a number of gaps in their use across the disease spectrum, impacting on delivering a high-quality health care service.
One area of gastroenterology where a validated disease-specific patient reported outcome measure (PROM) has not yet been developed is Barrett's oesophagus (BO). BO is a common condition thought to affect 2% of the population. It has been shown to be the key risk factor for adenocarcinoma of the oesophagus with estimated progression rates of <0.33% per annum.4,5 Patients with BO currently undergo regular surveillance endoscopies, as per national and international guidelines with mixed evidence around surveillance efficacy.6,7 Patients with BO lack disease specific knowledge and carry significant cancer worry above their actual risk, and have at best mixed quality follow up care and support.8, 9, 10 The British Society of Gastroenterology (BSG) guidelines do advise outpatient clinic follow up at index diagnosis, however there is no clear guidance provided for the follow up of BO patients who have already undergone invasive surveillance.6
There have been studies looking at the impact of BO on HRQOL using generic tools such as the Hospital anxiety and depression score (HADS),11 the generic measure SF-36,12 and measures such as gastroesophageal symptom rating scale (GSRS),13 and the gastroesophageal reflux disease health related quality of life (GERD-HRQOL).14 Moreover, while there have been some more oesophageal specific tools developed, such as the EORTC QLQ-C30 with the OES18 module15 and used in the BO population,16 these concentrate on cancer and specific symptoms such as dysphagia, with studies focused on those who are undergoing endotherapy. So far, only one study has used a specific BO tool but this was not validated.17 A systematic review of patient reported outcomes in BO was performed by van der Ende-van Loon et al. (2021),18 they showed most studies used generic tools and they reviewed qualitative studies in BO patients outlining the 18 key factors important to patients which, as yet, no single tool has explored fully.
Research in the field of BO has focused on clinical outcomes such as neoplasia detection or the use of advanced endoscopic methods.19 Studies exploring patient reported outcomes have often focused on those receiving endotherapy using PROMs not specifically designed with this patient group in mind. In a research priority setting exercise published in the Lancet Gastroenterology and Hepatology journal, 4th in the top ten research priorities was whether a dedicated service for BO care could improve detection of pre-cancerous lesions but also improve patient education and satisfaction.20 The British Society of Gastroenterology (BSG) BO guidelines also stated HRQOL and patient outcomes as a key future research consideration 10 years ago.6 This speaks to the desire of patients and clinicians to address patient reported outcomes. Generic tools or those focused on GORD do not cover the range of experience for this patient group—for example, though BO is associated with GORD many BO patients will have minimal physical symptoms of reflux or heartburn but may struggle with the psychological or physical burden of surveillance endoscopy or worry of cancer. Using a combination of pre-existing tools can create a questionnaire burden to the patient and may include less relevant items which might be confusing. Hence this work forms the design and validation of a BO-specific tool to help meet this need.
The aim of this work was to develop and systematically evaluate a BO-specific PROM for clinical application in the NHS and healthcare settings more globally.
Desirable features of a BO PROM would include a short completion time, being suitable for use in routine practice, being relevant to all BO patients. It should measure key aspects relevant to patient issues or concerns and have a simple scoring system. It should identify BO patients in need of intervention e.g. via BO clinic and BO patients with unmet needs e.g. poor knowledge, and be used to support service improvement and quality assurance.
Methods
Study design
Best practice guidance on how to devise medical tools have been outlined in the COSMIN (COnsensus-based Standards for the selection of health status Measurement INstruments) criteria21 and strategies for creating a valid PROM have been outlined in the literature by Rothrock et al.22 These stages are: Input—derived from literature review, expert and patient opinion, output using the input to form a conceptual model and initial item generation. Thereafter is item refinement through readability analysis, patient interviews, and Rasch/factor/Cronbach Alpha analysis. Following this the tool can then go through initial population testing, validity and reliability testing before clinical validation studies.
Item generation: conceptual model and item generation
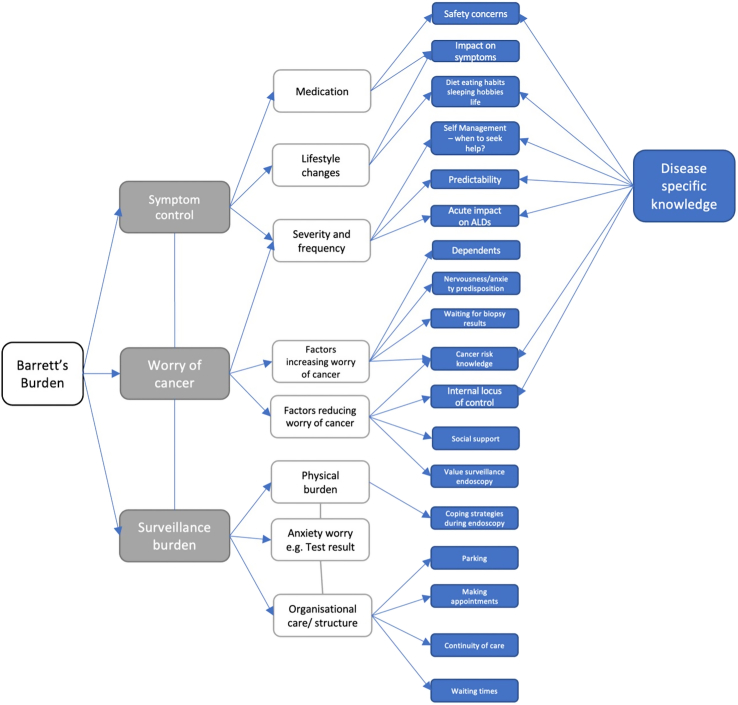
Our background work informing the item generation has been previously published and consisted of a literature review (a search of PubMed from database inception to May 2023), a qualitative interview study of BO patients and quantitative work using prior available non-BO specific tools.8,9,23 This work allowed the formation of a conceptual model (Fig. 1) which highlights the important disease-specific factors.
Fig. 1.
A conceptual model demonstrating the key areas of Barrett's oesophagus impact on patients from quantitative and qualitative work looking at health related quality of life in Barrett's oesophagus patients.
Using this as a framework, clinicians with experience in devising patient reported tools in other areas of gastroenterology were consulted for advice on methodology and a steering group was formed consisting of two patients, one with BO with dysplasia one with non-dysplastic BO, one gastroenterology clinical research fellow coordinating the PROM development and running a dedicated BO service, three clinical professors of gastroenterology—including one expert in BO endotherapy, a consultant gastroenterologist whose PhD work previously published forms the basis for the conceptual model and an advanced practitioner clinical endoscopist who performs BO surveillance as a core role.
The first iteration of B-PROM was devised by the steering group using the conceptual framework and the main subsections were: disease specific knowledge, symptom burden, cancer worry, burden of endoscopy and trust in physicians/medical team (this was additional to the conceptual model–Cooper et al.24 showed anxiety in BO patients correlates with lack of trust in the medical team).
This paper outlines the item refinement and population testing stages of the Barrett's PROM (B-PROM) development. These stages were undertaken in three phases:
-
-
Phase 1.–Item refinement: A patient and clinician modified Delphi process
-
-
Phase 2.–Item refinement: Patient cognitive interviews
-
-
Phase 3.–Population testing: Multicentre PROM testing for validity and reliability testing
The methods for each phase are outlined in turn below.
Phase 1–Item refinement: clinician and patient modified Delphi process–methods
The Delphi panel
The panel included clinicians from multidisciplinary backgrounds, patients, and patient advocates from a wide geographical area. Patients and patient advocates were invited via local and national patient support groups (Heartburn Cancer UK, Guts UK and Barrett's oesophagus campaign). Clinicians were invited via email from a known list of those involved in BO surveillance work and known experts in the field.
All involved received a participant information sheet (PIS) response to the online invitation and completion of the survey was deemed as consent.
Setting
The Delphi process was undertaken via an online survey platform (Qualrics) due to COVID-19 restrictions at the time. Participants were given a 14-day completion time with reminders.
Consensus
A consensus cut off of >80% remarking “agree” or “strongly agree” was used. The patient responses were analysed separately as well as the overall group as patient priorities may differ from those deemed important to clinicians. Hence, where patients favoured an item with >80% agreement but the overall group did not meet agreement, these items were retained and included in the second round Delphi.
Phase 2–Item refinement: cognitive interviews–methods
The participants, patients with BO, were asked to complete the PROM in real time whilst speaking about their experience completing it. This follows a technique outlined by Conrad and Blair.25 Participants involved in the interviews underwent face to face or virtual informed consent.
Setting
Participants were interviewed face to face (apart from one participant from London who was interviewed over Microsoft Teams) with only the interviewer also present.
Data
Audio recordings of the interviews were made and transcribed by the interviewer. Field notes were made on non-verbal information and were reviewed to contribute to the analysis.
Phase 3–Population testing: multicentre PROM testing for validity and reliability testing–methods
A questionnaire pack including the PROM was sent to patients with a diagnosis of BO across 4 UK hospital sites (Wrightington Wigan and Leigh NHS trust, Northern Care Alliance Salford, Bolton NHS Foundation Trust, Imperial College Hospitals NHS Trust) between April 2022 and February 2023.
Inclusion criteria
Participants were required to be over 18 years of age, with a known diagnosis of non-dysplastic BO or dysplastic BO, who were able to give informed consent.
Exclusion criteria
Potential participants were excluded if they did not have a confirmed diagnosis of BO, were under the age of 18 years, and if they were unable to provide informed consent to participate.
Consent
A cover letter and PIS were provided to all participants, return of the questionnaire by stamped addressed envelope was deemed as consent for the study.
Sample size
The purpose of the validation phase was to test the psychometric properties of the PROM. The sample size was determined by what would be adequate to perform exploratory factor analysis (EFA), hence we applied an assumed threshold of 10 participants/item.26 BO PROM version used during the validation phase had 31 items hence a sample size of 310–465 participants was desirable (n = 10–15/item).
Data collection and handling
Data from the PROM results were obtained along with demographic information including age, sex, prior medical comorbidities, smoking history, family history and employment history. Clinical data were obtained on BO Prague C and M length at latest endoscopy/clinic, date of last endoscopy and/or clinic appointment, prior/current history of dysplasia/neoplasia and type. Study data were collected and managed by the research teams using REDCap electronic data capture tools hosted at the University of Manchester.27
Statistical analysis
Descriptive statistics were obtained on response rates, responder/non responder characteristics, rates of missingness, and responses based on demographic and clinical outcomes. Readability analysis was performed using an online Flesch-Kincaid Calculator.
Ceiling and floor effects were calculated based upon >40% of responders choosing the first or “top” response and >40% of responders choosing the lowest or bottom response respectively.
Item correlations were determined using Spearman's’ rank correlation. Cronbach Alpha calculations were used to look for internal consistency of the whole tool, of predetermined sections and of item groupings based upon the outcome of EFA. Test-retest reliability testing was performed with intraclass correlation coefficient. Statistical tests were performed using SPSS version 29 (Microsoft).
Missing data
Missing data for significant clinical/demographic variables when <10% of the cohort (e.g. age and length of BO) were assessed for likelihood of randomness with Expectation Maximisation analysis and then, where feasible, imputed values were used in the analysis.
Ethics statement
Prior ethical approval was obtained from the Yorkshire and Humber ethics committee (REC reference number 16/YH/0035) as part of a wider mixed methods study namely Quality of Life Measures in Barrett's oesophagus. For the interviews informed consent was obtained with consent forms in person, for the Delphi process and wider population testing, consent was implied by the completion and return of the Delphi or questionnaire pack as agreed by the ethics committee. All participants received a participant information sheet.
Role of the funding source
This study forms part of a larger research project which has received unrestricted external funding support from Medtronic Ltd (previously Covidien), grant/award Number: ISR-2016-10773. The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Phase 1–Item refinement: clinician and patient modified Delphi process-results
Two rounds of Delphi process were undertaken. Full details of participant backgrounds and demographics for each round can be found in Tables 1 and 2.
Table 1.
Table outlining the response rates for Delphi Round 1 and the characteristics of the respondents.
| ROUND 1 Delphi respondents N = 43 (Distributed to n = 58, 43 responses) | ||
|---|---|---|
| Patients N = 17 | ||
| Gender | Male | 7/17 (41%) |
| Female | 10/17 (59%) | |
| Duration of Barrett's Diagnosis | Recently Diagnosed | 3/17 (18%) |
| 0–5 years | 3/17 (18%) | |
| 6–10 years | 3/17 (18%) | |
| 10+ years | 8/17 (47%) | |
| Barrett's background | “I am a patient with Barrett's Oesophagus” | 12/17 (71%) |
| “I am a patient with Barrett's, and I have had dysplasia” | 3/17 (18%) | |
| “I am patient with Barrett's, and I have had cancer” | 1/17 (6%) | |
| Other– oesophagectomy | 1/17 (6%) | |
| Educational background | I hold a postgraduate degree (MD/PhD) | 3/17 (18%) |
| I have been to university and have a graduate degree | 5/17 (29%) | |
| A levels/AS levels | 3/17 (18%) | |
| O levels/GCSEs | 3/17 (18%) | |
| Vocation qualifications | 2/17 (12%) | |
| Prefer not to say | 1/17 (6%) | |
| Clinicians N = 23 | ||
| Sex | Male | 21/23 (91%) |
| Female | 2/23 (9%) | |
| Clinical discipline | Consultant gastroenterologist specialising in Barrett's oesophagus (main role/work) | 8/23 (35%) |
| Consultant gastroenterologist with an interest in Barrett's oesophagus | 9/23 (39%) | |
| Consultant gastroenterologist | 1/23 (4%) | |
| Consultant Upper GI surgeon | 1/23 (4%) | |
| Nurse Endoscopist specialising in Barrett's oesophagus (main work/role) | 2/23 (9%) | |
| Nurse endoscopist with an interest in Barrett's | 1/23 (4%) | |
| Other | 1/23 (4%) | |
| Patient advocates N = 3 | ||
| Sex | Male | 1/3 (33%) |
| Female | 2/3 (67%) | |
| Role | Chair of patient advocacy group/charity | 1 |
| Patient support group | 1 | |
| Nurse endoscopist | 1 | |
Table 2.
Responses rates and details about the participants involved in round 2 of the Delphi.
| Delphi round 2 response rates | |||
|---|---|---|---|
| Round 2 | Number invited N = 49 | Number responded N = 37 | Number completing the Delphi N = 37 |
| Round 2 Delphi respondents N = 37 | |||
| Patients N = 15 | |||
| Sex | Male | 6/15 (40%) | |
| Female | 9/15 (60%) | ||
| Duration of Barrett's Diagnosis | Recently Diagnosed | 3/15 (20%) | |
| 0–5 years | 3/15 (20%) | ||
| 6–10 years | 3/15 (20%) | ||
| 10+ years | 6/15 (40%) | ||
| Barrett's background | “I am a patient with Barrett's Oesophagus” | 10/15 (67%) | |
| “I am a patient with Barrett's, and I have had dysplasia” | 3/15 (20%) | ||
| “I am patient with Barrett's, and I have had cancer” | 1/15 (7%) | ||
| Other– oesophagectomy | 1/15 (7%) | ||
| Educational background | I hold a postgraduate degree (MD/PhD) | 3/15 (20%) | |
| I have been to university and have a graduate degree | 5/15 (33%) | ||
| A levels/AS levels | 2/15 (13%) | ||
| O levels/GCSEs | 3/15 (20%) | ||
| Vocation qualifications | 1/15 (7%) | ||
| Prefer not to say | 1/15 (7%) | ||
| Clinicians N = 19 | |||
| Sex | Male | 17/19 (89%) | |
| Female | 2/19 (11%) | ||
| Clinical discipline | Consultant gastroenterologist specialising in Barrett's oesophagus (main role/work) | 7/19 (37%) | |
| Consultant gastroenterologist with an interest in Barrett's oesophagus | 8/19 (42%) | ||
| Consultant gastroenterologist | 1/19 (5%) | ||
| Consultant Upper GI surgeon | 1/19 (5%) | ||
| Nurse Endoscopist specialising in Barrett's oesophagus (main work/role) | 1/19 (5%) | ||
| Nurse endoscopist with an interest in Barrett's | 1/19 (5%) | ||
| Other | 1/19 (5%) | ||
| Patient advocates N = 3 | |||
| Sex | Male | 1/3 (33%) | |
| Female | 2/3 (67%) | ||
| Role | Chair of patient advocacy group/charity | 1 | |
| Patient support group | 1 | ||
| Nurse endoscopist | 1 | ||
The results from Delphi round one and two are summarised in Tables 3 and 4 respectively. Out of the 49 items, 24 reached consensus and were kept unchanged, 14 were borderline or were favoured by patients and were revised. Nine items were merged with other items. Two items which were clearly not favoured by the group were reviewed and removed.
Table 3.
Outcome of the first round Delphi process including steering group review.
| Item round 1 Delphi | Overall agreement | Patients only | Outcome |
|---|---|---|---|
| What is Barrett's oesophagus? | 92% | 100% | Strong consensus–retained |
| What causes Barrett's oesophagus? | 86% | 100% | Strong consensus–retained |
| What are the outcomes for Barrett's Oesophagus? | 72% | 100% | Revised to “What are the possible outcomes for Barrett's oesophagus” and merged with next item |
| What happens when Barrett's progresses (e.g. Dysplasia)? | 81% | 88% | Merged with item above |
| What is my risk of oesophageal cancer? | 93% | 94% | Strong consensus–retained |
| What are you looking for during surveillance? | 93% | 94% | This achieved consensus but reworded |
| Are there other options to surveillance? | 79% | 82% | Favoured by patients and borderline for consensus hence reworded |
| When can I stop surveillance? | 72% | 71% | Reworded to “When might regular Barrett's checkups be stopped or no longer required?” |
| Why do I need to take anti-acid medication (e.g. PPI) long term?? | 93% | 100% | Strong consensus–retained |
| Are anti-acid medications safe to take long term? | 84% | 94% | Strong consensus–retained |
| Can Barrett's oesophagus be reversed? | 72% | 94% | This was not favoured overall but favoured by patients hence revised for round 2 |
| If things change what treatment options are there? | 88% | 100% | Reached strong consensus but review of comments revised for round 2 |
| What can I do to improve my symptoms? | 93% | 100% | Strong consensus–retained |
| What can I do to reduce my risk of cancer? | 93% | 94% | Strong consensus–retained |
| How can I manage symptom flare-ups? | 81% | 94% | Strong consensus—retained |
| When should I seek medical advice? | 100% | 100% | Strong consensus–retained |
| My clinician (doctors/nurse/person who looks after my Barrett's) is knowledgeable about my condition: | 79% | 76% | These were borderline but repetition hence merged for round 2 to “I trust my medical team to prioritise my needs when making decisions about my Barrett's” |
| I trust my clinician's (doctor/nurse/person who looks after my Barrett's) judgement about my Barrett's: | 79% | 77% | See above |
| I trust my clinician (doctor/nurse/person who looks after my Barrett's) to prioritise my needs when making decisions about my Barrett's: | 79% | 82% | See above |
| In general, would you say your health is: Excellent/Good/Fair/Not good/Poor | 81% | 82% | Strong consensus–retained |
| In general, would you say your outlook on life is: | 71% | 88% | Patients favoured inclusion hence reworded as “In general would you say your outlook on life is positive or negative?” |
| How would you rate the control of your reflux symptoms (visual analogue scale) | 86% | 82% | Changed from a visual analogue scale to options. |
| In terms of my swallow, I am not able to swallow, able to swallow liquids, able to swallow solids with some problems, able to swallow a normal diet | 83% | 94% | Strong consensus–retained |
| How often have you had any heartburn (burning discomfort behind breastbone) during the last 2 weeks? | 84% | 94% | Strong consensus–retained |
| How severe has your heartburn been during the past 2 weeks? | 77% | 88% | This was favoured by patients for inclusion hence it was revised |
| Have you had any reflux during the past 2 weeks? (Fluid coming up or stomach contents coming up) | 81% | 94% | Consensus achieved particularly for patients hence kept in. |
| How severe has your reflux been during the past 2 weeks? (Fluid coming up or stomach contents coming up) | 65% | 82% | This did not reach overall consensus, but it was felt to be important to have separate questions about frequency and severity hence these were kept in |
| Has your stomach felt bloated during the past 2 weeks? | 65% | 94% | This did not reach consensus decision was made to streamline two items into one question for the second round Delphi. |
| How severe has your bloating been during the past 2 weeks? | 53% | 77% | See above |
| Have you been bothered by stomachache or pain during the past 2 weeks? | 65% | 88% | See above |
| Overall–my symptoms have stopped me from sleeping properly? | 88% | 94% | Strong consensus–retained |
| Overall–My symptoms have made me worry about cancer? | 88% | 76% | The group felt this should be covered in the worry of cancer section hence removed. |
| Overall–My symptoms have prevented me doing the things I want to do (e.g., Going for a meal/socialising/exercising) | 91% | 94% | Strong consensus–retained |
| Overall–My symptoms have prevented me from going to work? | 77% | 77% | Deemed repetitive hence removed. |
| Overall–My symptoms have made me feel anxious or depressed? | 88% | 88% | Strong consensus–retained |
| How often have you thought about your chances of getting oesophageal (gullet) cancer? | 81% | 77% | Repetition hence cancer worry items were streamlined to two items |
| Have these thoughts affected your mood? | 86% | 82% | See above |
| Have these thoughts interfered with your ability to do daily activities? | 72% | 77% | See above |
| How concerned are you about getting oesophageal (gullet) cancer one day? | 84% | 77% | See above |
| How often do you worry about getting oesophageal (gullet) cancer? | 77% | 71% | See above |
| I worry or get anxious knowing my Barrett's procedure is coming up | 81% | 71% | It was agreed to keep an item which covered discomfort during the procedure and one about anxiety of the test approaching for round 2. |
| I struggle with the preparation for the procedure | 72% | 71% | See above |
| I struggle with the time required for my Barrett's procedure e.g. coming to hospital, time out of work or normal activities | 67% | 53% | See above |
| I worry or get anxious about the findings on the day of my procedure | 74% | 59% | See above |
| I worry more about gullet cancer near the time of my Barrett's procedure | 63% | 47% | See above |
| I worry or get anxious whilst I wait for my results | 77% | 65% | See above |
| Overall, how would you rate your Barrett's oesophagus care | 91% | 88% | Strong consensus–retained |
| Overall, how would you describe your Barrett's condition | 51% | 65% | Removed |
Table 4.
Outcome of the round 2 Delphi process including the steering group review.
| Item round 2 Delphi | Overall agreement | Patients only | Outcome |
|---|---|---|---|
| What are the possible outcomes for Barrett's Oesophagus | 92% | 100% | Strong consensus–retained |
| Are there other options to endoscopy (camera test) check-ups? | 81% | 93% | Consensus achieved hence kept in |
| When might regular check-ups be no longer indicated | 76% | 67% | Did not achieve consensus, the steering group agreed it was an important part of clinical discussions but perhaps too complicated for the PROM. |
| Can Barrett's oesophagus get better on its own? | 75% | 93% | As with the item above, this was deemed important by patients, but clinicians were concerned this is a complicated issue which may cause confusion in the context of the PROM. |
| I trust my medical team in their ability to care for my Barrett's | 81% | 67% | Though this reached consensus. The steering group agreed to combine all the trust questions into “I trust my medical team to prioritise my preferences and needs when making decisions about my Barrett's” which did reach consensus. |
| I trust my medical team to prioritise my preferences and needs when making decisions about my Barrett's | 81% | 87% | See above |
| In general would you say your outlook on life is positive or negative? | 54% | 53% | This was felt to be too ambiguous by the Delphi group hence it was removed. |
| How severe has your heart burn been over the past 4 weeks? | 89% | 87% | Strong consensus–retained |
| How severe has your reflux been during the past 4 weeks? (Fluid coming up or stomach contents coming up) | 89% | 100% | Strong consensus–retained |
| Has your stomach felt bloated over the past 4 weeks? | 65% | 80% | Complicated by functional gut disorders hence removed |
| How concerned are you about getting gullet cancer one day? | 94% | 87% | Strong consensus–retained |
| Have these thoughts affected your mood? | 71% | 60% | Did not achieve consensus–removed |
| I know where to go to get support for if these thoughts or feelings arise? | 75% | 93% | This was favoured by the patients but not overall—however steering group discussion felt inclusion was useful in terms of sign posting patients |
| I get anxious knowing my Barrett's check-up is coming up | 73% | 60% | Consensus not achieved on two rounds hence removed. |
| I find the Barrett's checkup difficult to complete due to discomfort | 83% | 80% | Strong consensus–retained |
In round two, revised and merged items were reviewed by the panel and a further 8 items achieved consensus, the other items did not achieve consensus and final agreed items were reviewed by the steering group.
Phase 2–Item refinement: cognitive interviews–results
Following the Delphi phase the refined PROM tool was then taken to patients to undergo cognitive interviews.
Recruitment and study size
Participants were recruited purposively to aim for a range of clinical and demographic backgrounds (Table 5).
Table 5.
Outline of participant demographics and date of interview.
| Date of interview | Sex | Age |
|---|---|---|
| 11/11/2021 | Male | 66 |
| 15/11/2021 | Male | 83 |
| 15/11/2021 | Male | 74 |
| 30/11/2021 | Female | 60 |
| 08/12/2021 | Male | 78 |
| 16/12/2021 | Female | 85 |
| 16/12/2021 | Male | 73 |
| 02/02/2022 | Female | 75 |
| 09/02/2022 | Female | 74 |
| 24/02/2022 | Male | 61 |
| 25/02/2022 | Male | 77 |
| 15/03/2022 | Male | 39 |
| 10/03/2022 | Male | 70 |
| 16/03/2022 | Male | 62 |
To check for grounding of the PROM in patient experience, some of the participants who had contributed to the initial qualitative interviews that informed the conceptual model were invited to participate. Recruitment continued until no further changes were needed, 13 cognitive interviews were performed between November 2021 and March 2022 with 9 male and 4 female patients (mean age 69.8 years range 39–85).
Analysis
Transcripts were analysed using a framework outlined by Conrad and Blair using the criteria to define issues arising with completion of any items during the interview.25 The results are summarised in Table 6. After each round of interviews the PROM was refined, and a new version shared with the next participant.
Table 6.
Summary of cognitive interview results and analysis using Conrad and Blair25 terminology of response stage and problem class.
| Item | Problem stage | Problem type | Change |
|---|---|---|---|
| Year of diagnosis | Producing a response | Temporal | Added in “approximate” |
Type of diagnosis (choose one of the following options:
|
Understanding | Lexical | Type of diagnosis (choose one of the following options): I am a patient with Barrett's’ oesophagus without biopsy changes (dysplasia) I am a patient with Barrett's, and I have had changes in my biopsies (dysplasia) or gullet (oesophagus) cancer |
| Education background | Understanding | Omission/inclusion -query as to why included | Removed question re: education |
| Knowledge section: | |||
| All items | Mapping a response | Computational | Changed options from a scale of confidence to a Yes/No/Not sure response with appropriate rewording of the items to correspond with a yes/no response. |
| 1.3 What are the possible consequences of Barrett's oesophagus 1.5 What are you looking for during regular Barrett's check ups? |
Understanding/Mapping response | Lexical | I understand the possible consequences of Barrett's oesophagus I understand what the medical team are looking for during regular Barrett's check ups |
| 1.4 What is my risk of oesophageal cancer? | Understanding | Lexical | I understand my own risk of gullet (oesophageal) cancer |
| 1.7 Why do I need to take antacid medication long term? | Understanding | Lexical | I understand why I need to take antacid medication (e.g. omeprazole, lansoprazole famotidine or similar) Are antacid medications (e.g. omeprazole, lansoprazole famotidine or similar) safe to take long term? |
| 1.9 Can Barrett's oesophagus get better on its own? | Mapping a response | Logical | Change responses to yes/no/not sure |
| 1.10 If my Barrett's progresses/worsens what treatment options are there? | Understanding | Lexical | I know what treatments are available if my Barrett's progresses/worsens (biopsies or samples show abnormalities) |
| 1.12: What can I do to reduce my risk of cancer? | Understanding | Omission/inclusion | I know what I can do to reduce my risk of gullet (oesophagus) cancer |
| No option for people without symptoms for 2 questions regarding symptoms 1.13: How can I manage symptom flair ups? |
Mapping response and understanding of the term “flare ups” when no symptoms | Omission/inclusion and lexical | Added no Symptom options for question 1.11 and 1.13 |
| Trust and symptoms section | |||
| I trust my clinical team to prioritise my needs and preferences when making decisions about my Barrett's | Understanding | Lexical | I trust my clinical (hospital) team to prioritise my needs and preferences when making decisions about my Barrett's |
| 4: how would you rate your overall control of your reflux symptoms? | Mapping response | Omission/inclusion | Added in an option for no symptoms and separated it from the other options and reworded: “How would you rate your overall reflux symptoms?” |
| All items | Mapping response | Computational | Adjusted the order of responses to make it more logical throughout the PROM |
| Overall my symptoms have made me feel anxious or depressed | Mapping response | Omission/inclusion—people with no symptoms excluded | My symptoms or my Barrett's make me feel anxious or depressed. |
| Worry of cancer section | |||
| 13: how concerned have you been getting oesophageal (gullet) cancer in the future over the past four weeks? | Producing a response Mapping response |
Temporal Lexical |
How concerned have you been about getting oesophageal (gullet) cancer in the future? Changed the responses to not at all, rarely, occasionally, frequently and constantly |
| 14: I know how to get support for my worry of gullet cancer if I need it? | Mapping response | Computational | Kept the scale responses to tie in with rest of PROM, most able to use agreement scale and allowed for nuance |
| Procedure section | |||
| I find my Barrett's check up (procedure) difficult due to discomfort | Understanding | Lexical | Kept open ended to cover minimally invasive testing in the future and dysplasia treatment. |
| Barrett's procedure section | Mapping a response | Omission | Add in two items I get anxious or worried knowing my Barrett's check up or endoscopy is coming up I get anxious or worried whilst waiting for the results of my samples |
Phase 3–Population testing: multicentre PROM testing for validity and reliability testing–results
In total, 387/949 participants completed and returned the pack giving a 40.8% response rate overall. A breakdown of responder and non-responder characteristics can be found in Table 7.
Table 7.
Characteristics of responders and non-responders for patient reported outcome measure (PROM) validation phase.
| Variable | Responders (n = 387) | Non-responders (n = 562) |
|---|---|---|
| Sex | ||
| Male | 290/387 (75% | 402 (72%) (58% of males were non-responders) |
| Female | 93/387 (24%) (4 missing) | 156 (28%) (63% of females were non-responders) (4 missing) |
| Age | ||
| Mean | 70.3 years (37–90 years) | Mean 66.2 years (30–94 years) |
| Hospital site | ||
| NCA | 100 (26%) | 145 (26%) |
| WWL | 221 (57%) | 358 (64%) |
| ICH | 38 (10%) | 20 (4%) |
| BHT | 28 (7%) | 39 (7%) |
| Length of Barrett's (Prague M in cm) | ||
| <3 cms | 169/377 (45%) | |
| >3 cm | 208 (55%) | |
| Range | 0–16 cms | |
| Mean | 3.8 cms (11 missing) | |
| Prior dysplasia | ||
| Yes | 91 (23.5%) | |
| No | 286 (73.7%) 11 missing (2.8%) | |
| Type of Dysplasia if present/known | ||
| IDD | 16 | |
| LGD | 32 | |
| HGD | 27 | |
| OAC | 15 (1 missing) |
BHT, Bolton Hospitals trust; cm/s, centimetres; HGD, high grade dysplasia; ICH, Imperial College Hospitals; IDD, Indefinite for dysplasia; LGD, low grade dysplasia; NCA, Northern Care Alliance; OAC, oesophageal adenocarcinoma; WWL, Wrightington Wigan and Leigh NHS trust.
Response distributions
There were 31 items in the main PROM divided into subsections relating to different themes.
On the first page there were some introductory questions to establish basic clinical and demographic details, the results of these are summarised in Supplementary Material 1.
Participants completed the year of diagnosis in varying ways and only 47/91 of patients with prior dysplasia accurately reported dysplasia, 37 marked they had non dysplastic Barrett's and 7 did not complete. The steering group felt this was an important knowledge deficit and should be moved to the knowledge section.
Feasibility analysis
Readability testing was performed of the content of the PROM using an online version of the Flesch–Kincaid test. This showed a grade level of 5.2 and reading ease score of 66.6 (8th and 9th grade level).
Completion time
Five participants were timed whilst completing the PROM. The mean completion time was 5 min 34 s (Range 03:51–07:00 min).
Missingness
Overall missingness was low, with no individual items in the main PROM <95% completion. There were 361/387 participants who completed >95% of the main PROM items (93%).
Response distributions
Different sections of the PROM had different response options (summarised in Supplementary Material 2) and the responses for each item are summarised in Supplementary Material 3.
Floor and ceiling effects
Within the items of the PROM, 19 items showed a ceiling effect, 3 items showed a floor effect. Most items showing a ceiling/floor effect were in the knowledge section, where there were fewer options making either floor or ceiling more likely. Symptom questions were also often affected by ceiling effects, this may relate to BO patients being established on antisecretory drugs, so most could be expected to have well controlled symptoms. Clinically it would be very relevant to find those with uncontrolled symptoms even if uncommon, especially swallowing difficulties, hence these items were retained.
Correlations
Items were compared to look for correlations in responses. Of the correlation pairs two strongly correlated together (rs >0.8). All other items had some correlation with at least one other item with rs 0.34–0.8, six items correlated weakly with any other item (rs <0.34), and 19 items correlated with the total (rs >0.3).
Two changes were suggested firstly that the term “antacid” was not strictly correct for PPI and H2A as they are antisecretory medications, so it was advised to change to “medications that reduce stomach acid” as a patient friendly term. Also “Can Barrett's get better on its own?” correlated poorly with the total and other items and it was more of a yes no question. The steering group advised to remove this item from the PROM for clarity.
Exploratory factor analysis
EFA was used to explore the relationship between items in the PROM and look for subsections that could inform sub-scores in future research. Items were excluded from the EFA if they had weak ITC or inter-item correlations, 18 items were included.
Adequacy of sampling
The Kaiser-Meyer-Olkin (KMO) measure of sampling adequacy was 0.827 which is very good. The Bartlett's test of sphericity was significant (p ≤ 0.001) hence it was possible to proceed with the further analysis.
Principle components analysis
A Scree plot (Supplementary Material 4) produced showed a sharp inflection corresponding with 2 components. However 5 components had Eigenvalues greater than 1 and accounted for 66% of the variance hence 5 components were used. The average communality was 0.653 which allowed for inclusion of all 5 components.
A rotated component matrix was produced from the outcomes (Supplementary Material 5). The subthemes found with EFA were correlated in transformation matrix which suggested correlation between components 3 and 4, and weakly between 2 and 3, and 2 and 4.
Reliability of scale
These EFA components and predetermined PROM themes were tested for internal consistency with Cronbach Alpha (Table 8).
Table 8.
A summary of the components derived from the exploratory factor analysis (EFA) and the pre-determined patient reported outcome measure (PROM) subthemes and their Cronbach Alpha internal consistency values.
| EFA component | Theme | Items included | Cronbach alpha |
|---|---|---|---|
| 1 | Symptom burden and impact on lifestyle | reflux_symptoms swallow_issues heartburn_frequency heartburn_severity recent_reflux severity_of_reflux symptoms_and_sleep symptoms_and_socialising |
0.896 |
| 2 | Worry and anxiety | anxiety_about_results worry_of_cancer symptoms_and_anxiety_depression |
0.775 |
| 3 | Medical team and overall care | trust_my_team overall_bo_care help_with_worry_of_cancer |
0.705 (0.815 if help with worry of cancer deleted) |
| 4 | Patient's own management of symptoms | symptoms symptom_flares |
0.696 |
| 5 | Understanding risk and available help | my_own_risk_oac help_with_worry_of_cancer |
0.379 |
| Pre-determined subthemes | |||
| Disease specific knowledge | what_is_barretts_no_1 cause_of_barretts consequences_of_bo my_own_risk_oac bo_check_ups other_options use_of_ppi ppi_safety better_on_its_own treatments_for_bo symptoms reduce_risk_of_oac symptom_flares when_to_seek_help |
0.736 | |
| Symptom burden | reflux_symptoms swallow_issues heartburn_frequency heartburn_severity recent_reflux severity_of_reflux symptoms_and_sleep symptoms_and_socialising symptoms_and_anxiety_depression |
0.896 | |
| Worry of cancer | worry_of_cancer help_with_worry_of_cancer |
0.335 | |
| Burden of Endoscopy | anxiety_re_endoscopy discomfort_from_endoscopy anxiety_about_results |
0.718 | |
| Overall B-PROM | 0.846 | ||
The full items corresponding with the item codes in the third column are in Supplementary Material 2.
There were two EFA components and one predetermined subthemes which showed lower Cronbach Alphas, each of these had only two items included and the Cronbach alphas reflect the low number of items tested within that subtheme. Components and subthemes with >2 items tests all had good internal consistency.
Steering group review of EFA
Reviewing the findings of the EFA, one component in EFA and one predetermined subsection had low Cronbach alpha scores, despite this, the items contained within were felt to be very useful clinically. It was suggested however that “I know where to get help for my worry of cancer” was perhaps a yes/no question and could be amalgamated with item 1.14 which asked about when to get medical help.
Test-retest reliability
Test-retest reliability testing was undertaken with 25 participants who completed the PROM again approximately 4 weeks from their initial completion. When analysed for absolute consistency using intraclass correlation coefficient using a two-way mixed effects model there were no statistical differences between the total scores (0.670, 95% CI−0.4 to 0.878 p ≤ 0.001), knowledge section score (0.706, 95% CI −0.130 to 0.902 p ≤ 0.001) and rest of PROM scores (0.752, 95% CI 0.432–0.901 p ≤ 0.001).
Final PROM version
A final version of B-PROM is found in Supplementary Material 6.
Discussion
B-PROM is the first BO-specific PROM to be designed and systematically evaluated and has been developed with patient involvement throughout. There is an increasing recognition for the need for measures that capture the patient experience beyond clinical factors, with BO issues such as lack of disease specific knowledge and worry of cancer are significant. What the process of developing B-PROM has shown is a strong indication that patient education and support is needed in BO, and that the day-to-day burden of the disease is only starting to be recognised.
Yacavone et al.28 described 3 main uses of HRQOL assessment; I) “Descriptive” to measure and compare aspects of health-related quality of life in multiple disease states. II) “Discriminative” to differentiate subgroups within a disease. III) “Evaluative” to help assess treatment efficacy or the quality of health care delivery. Ultimately patients are at the centre of our care, and though clinicians may value time consuming technological interventions which improve neoplasia detection, long procedures may be unacceptable to patients so it is important to measure clinical and patient outcomes. BO patients are not routinely offered outpatient appointments after diagnosis, but many feel uninformed and carry significant cancer worry and anxiety. B-PROM may help measure these factors and help to guide interventions. Evaluating B-PROM against the 18 factors reported to be important to BO patients by van der Ende-van Loon et al.,18 14 factors are clearly covered, 2 factors were debated at the Delphi stage and felt to be too non-specific, and 2 factors are covered partially (detailed in Supplementary Table S7).
The modified Delphi process gave additional weight to the PROM refinement given the breadth of experience of both the clinicians and patients involved. Comparing our Delphi process with a previous systematic review,29 key strengths of our process were that we had clarity around how the items were devised, a heterogenous panel who were willing to engage as evidenced by our response rates. Though we did not use two methods of sending the questionnaire, our response rates were very good.
A strength of the cognitive interview phase was the use of rounds of interviews creating an iterative approach to refine the tool. The use of the interviewees who had been involved in the prior qualitative study helped provide content validity. Participants came from a range of clinical and educational backgrounds, with one participant recruited from another region.
B-PROM offers a streamlined tool that is short, straightforward to participants to complete and has strong internal consistency. Key strengths are the short completion time, strong internal consistency, that it was devised with dysplastic BO patients and non-dysplastic BO patients from multiple centres with low rates of missingness and strong test-retest reliability. It is also generic and future-proofed for use if non-endoscopic modalities of surveillance become part of routine care.
The research which ground B-PROM showed there is a range of factors important to BO patients which can be independent from one another, but also interact. Our group's work showed poor disease specific knowledge made BO patients more anxious about their disease and we also showed a link between poor symptom control and anxiety. Cooper et al.24 showed a link between poor trust in physicians was associated with increased worry around BO. Scoring B-PROM may need to be a panel of results as disease knowledge is a different entity to symptoms. However, these factors are all important to capture, having a panel of scores may allow for a future clinical contact e.g. a clinic appointment, to address poor scoring areas of need for that patient. Future multicentre study will involve developing the scoring for B-PROM and look at responsiveness to changes in the patient's state. Future testing will also include comparison to prior validated tools for symptoms and worry of cancer.
There were some limitations to this work, due to the COVID-19 pandemic we used an online questionnaire to provide the Delphi. This may have prevented some discussion but however it allowed individuals time to consider their own responses and no individuals could dominate the discussion.30 The time frame for response was quite short compared with other Delphi processes, however there were good response rates. The online platform restricted involvement to persons with access to computers. The inclusion of clinicians in the Delphi, though felt to be important to help ground the tool in clinical application, may be criticised for their influence on the item refinement in the sense of prioritising clinician views alongside patients. Care was taken however to review the patient responses separately and take forward items which were valued by patients when there was disparity with the overall group.
Most of the participants interviewed were from a single centre in the Northwest of England, a predominantly white ethnic area, though a range of male and female participants of differing ages were used purposively to try to gain a breadth of responses. Given the location of the participants for the cognitive interviews, some may have had contact with the interviewer previously. It is vital for future validation and testing that a broad range of centres are used and data on racial background will be obtained. The tool will be tested in different cultural settings with care taken to account for nuances in language when translating into different languages.
There was a 40.8% response rate to the B-PROM validation, though this is similar to prior studies with postal questionnaires, there is a potential for missed data in the non-responders. Ways to improve response could be explored, namely face to face delivery in clinics or at points of patient contact or use of an electronic version. B-PROM relied on paper and postal services, a validated electronic version would be beneficial for ease of data collection and analysis, but care must be taken not to exclude those without access to computers or electronic devices. This response rate also could represent feedback on the tool as a whole, suggesting an issue with engagement which could be a response to the content as well as the delivery. With future work it is key to continue revising the tool to meet the needs of this population.
In conclusion, at the time of writing this is the first systematically evaluated PROM specifically designed for use in BO. Further work will be undertaken of B-PROM to test responsiveness to changes in participant's clinical situation and discriminant validity which can be performed in multicentre testing over a longer time. Comparison studies will also be undertaken to compare B-PROM with prior existing tools that cover some aspects relevant to Barrett's patients. Further B-PROM work will also include using participants from a wider cultural and ethnic background and B-PROM should be adapted into other languages and cultural settings.
The strength of B-PROM is the involvement of patients throughout the development process, making it relevant to patient priorities and not only those felt to be important by clinicians. The development and validation process has been rigorous, and the psychometric properties of B-PROM are encouraging, it can now be taken forward to wider clinical testing in multicentre trials.
Contributors
The project was devised by ER, JB, JM, SH and YA. A project steering group consisted of ER, JB, SB, JM, SH and YA along with two patient representatives. ER organised and coordinated the phases of the PROM development, carrying out the modified Delphi, cognitive interviews and design and validation of the PROM. The steering group were involved in the initial item bank and revision of the after each phase. YA, NP, RK, MM, MMont and EV were principal investigators at their respective sites and responsible for the study coordination and data collection. ER and YA accessed and verified the data. ER produced the first draft of the manuscript. All authors contributed to and reviewed the final manuscript and with oversight of the final data.
Data sharing statement
Anonymised data are available to researchers who provide a methodologically sound proposal to the corresponding author beginning 3 months and ending 5 years after publication. Requesters will be required to sign a data access agreement.
Declaration of interests
YA has received funding from cancer research UK and Medtronic Ltd.
ER has received research funding from Medtronic UK and speaker honoraria from Janssen and Takeda.
SH has NIHR funding for other research studies, is on the board of ESSD and holds shares in the company Phagenesis Ltd.
Acknowledgements
The authors would like to extend specific thanks to the patient representatives on our steering group Malcolm Childs and Tom Vose for their time and input.
To the experts, patients and representatives who participated in our modified Delphi process we are extremely grateful for your time and enthusiasm. We are grateful for help the upper GI nurses at Wrightington Wigan and Leigh, Guts UK, Heartburn cancer UK and the Barrett's oesophagus campaign for their help publicising the project.
Specific thanks also to Dr Clare Ormerod and Dr Laura Neilson for their methodological advice regarding development of patient reported measures.
This study forms part of a larger research project which has received unrestricted external funding support from Medtronic Ltd (previously Covidien). Medtronic have not influenced the design of this study, writing of the manuscript or decision to submit for publication. (Medtronic, Grant/Award Number: ISR-2016-10773).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102606.
Contributor Information
Elizabeth Ratcliffe, Email: Elizabeth.ratcliffe@nca.nhs.uk, Elizabeth.ratcliffe-2@postgrad.manchester.ac.uk.
B-PROM Delphi contributors:
Anuj Abraham, Abhay Bagewadi, Ian Beales, Iosif Beintaris, Philip Boger, Sara Brogden, Rosie Bray, Jeffrey Butterworth, John De Caestecker, Anjan Dhar, Massimillano Di Petro, Guy Finch, Stephen Foley, David Gorard, Hasan Haboubi, Rehan Haidry, Chris Haigh, Jo Harvey, Neil Hawkes, Jamal Hayat, Kar Lau, Pradeep Mundre, Neeraj Prasad, Alix Rankin, and Nigel Trudgill
Methodological advice collaborators:
Appendix A. Supplementary data
References
- 1.Fitzpatrick R., Davey C., Buxton M.J., Jones D.R. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess. 1998;2(i–iv):1–74. [PubMed] [Google Scholar]
- 2.Griffin D., Wall P., Realpe A., et al. UK FASHIoN: feasibility study of a randomised controlled trial of arthroscopic surgery for hip impingement compared with best conservative care. Health Technol Assess. 2016;20:1–172. doi: 10.3310/hta20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodger K., Ormerod C., Shackcloth D., Harrison M. Development and validation of a rapid, generic measure of disease control from the patient's perspective: the IBD-control questionnaire. Gut. 2014;63:1092–1102. doi: 10.1136/gutjnl-2013-305600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hvid-Jensen F., Pedersen L., Drewes A.M., Sφrensen H.T., Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 5.Bhat S., Coleman H.G., Yousef F., et al. Risk of malignant progression in Barrett's Esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103(13):1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald R.C., Di Pietro M., Ragunath K., et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut. 2014;63(1):7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen N.J., Falk G.W., Iyer P.G., Gerson L.B. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111(1):30–50. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton J., Hamdy S., McLaughlin J., Horne M., Ang Y. Barrett's oesophagus: a qualitative study of patient burden, care delivery experience and follow-up needs. Health Expect. 2019;22:21–33. doi: 10.1111/hex.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britton J., Taxiarchi P., Martin G., et al. Comparative quantitative survey of patient experience in Barrett's oesophagus and other gastrointestinal disorders. BMJ Open Gastroenterol. 2020;7 doi: 10.1136/bmjgast-2019-000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Ende-van Loon M., Brouwers M., de Munnik S., Nieuwkerk P., Curvers W., Schoon E. Factors influencing health-related quality of life in patients with Barrett's esophagus: a qualitative focus group study. Eur J Gastroenterol Hepatol. 2021;34:161–167. doi: 10.1097/MEG.0000000000002070. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Ware J.E., Sherbourne C.D. The MOS 36-ltem Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 13.Svedlund J., Sjödin I., Dotevall G. GSRS-A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129–134. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 14.Velanovich V., Vallance S.R., Gusz J.R., Tapia F.V., Harkabus M.A. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217–224. [PubMed] [Google Scholar]
- 15.Blazeby J.M., Conroy T., Hammerlid E., et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer. 2003;39:1384–1394. doi: 10.1016/s0959-8049(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 16.Peerally M.F., Bhandari P., Ragunath K., et al. Radiofrequency ablation compared with argon plasma coagulation after endoscopic resection of high-grade dysplasia or stage T1 adenocarcinoma in Barrett's esophagus: a randomized pilot study (BRIDE) Gastrointest Endosc. 2019;89:680–689. doi: 10.1016/j.gie.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 17.Shaheen N.J., Sharma P., Overholt B.F., et al. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 18.van der Ende-van Loon M.C.M., Stoker A., Nieuwkerk P.T., Curvers W.L., Schoon E.J. How are we measuring health-related quality of life in patients with a Barrett Esophagus? A systematic review on patient-reported outcome measurements. Qual Life Res. 2021;31:1639–1656. doi: 10.1007/s11136-021-03009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canto M.I., Anandasabapathy S., Brugge W., et al. In vivo endomicroscopy improves detection of Barrett's esophagus-related neoplasia: a multicenter international randomized controlled trial (with video) Gastrointest Endosc. 2014;79:211–221. doi: 10.1016/j.gie.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britton J., Gadeke L., Lovat L., et al. Research priority setting in Barrett's oesophagus and gastro-oesophageal reflux disease. Lancet Gastroenterol Hepatol. 2017;2:824–831. doi: 10.1016/S2468-1253(17)30250-9. [DOI] [PubMed] [Google Scholar]
- 21.Mokkink L.B., Terwee C.B., Patrick D.L., et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539–549. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothrock N.E., Kaiser K.A., Cella D. Developing a valid patient-reported outcome measure. Clin Pharmacol Ther. 2011;90:737–742. doi: 10.1038/clpt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britton J., Keld R., Prasad N., Hamdy S., McLaughlin J., Ang Y. Effect of diagnosis, surveillance, and treatment of Barrett's oesophagus on health-related quality of life. The Lancet Gastroenterol Hepatol. 2018;3:57–65. doi: 10.1016/S2468-1253(17)30213-3. [DOI] [PubMed] [Google Scholar]
- 24.Cooper S.C., El-Agib A., Dar S., et al. Endoscopic surveillance for Barrett's oesophagus: the patients' perspective. Eur J Gastroenterol Hepatol. 2009;21(8):850–854. doi: 10.1097/MEG.0b013e328318ed2d. [DOI] [PubMed] [Google Scholar]
- 25.Conrad F. From impressions to data increasing the objectivity of cognitive interviews. 1996. [Google Scholar]
- 26.Hogarty K.Y., Hines C.V., Kromrey J.D., Ferron J.M., Mumford K.R. The quality of factor solutions in exploratory factor analysis: the influence of sample size, communality, and overdetermination. Educ Psychol Meas. 2005;65:202–226. [Google Scholar]
- 27.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yacavone R.F., Locke G.R., 3rd, Provenzale D.T., Eisen G.M. Quality of life measurement in gastroenterology: what is available? Am J Gastroenterol. 2001;96:285–297. doi: 10.1111/j.1572-0241.2001.03509.x. [DOI] [PubMed] [Google Scholar]
- 29.Boulkedid R., Abdoul H., Loustau M., Sibony O., Alberti C. Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jairath N., Weinstein J. The Delphi methodology (Part one): a useful administrative approach. Can J Nurs Adm. 1994;7:29–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.