Abstract
Purpose
To compare the efficacy and the safety of Tamsulosin 0.4 mg/day and 0.8 mg/day in patients suffering from lower urinary tract symptoms due to benign prostatic obstruction.
Patients and Methods
A prospective interventional, double-blinded, controlled study was carried out on 93 patients who met the criteria and divided randomly into two groups: group A for Tamsulosin 0.4 mg/day and group B for Tamsulosin 0.8 mg/day. International prostate symptom score, post void residual urine volume, and maximum flow rate of urine were assessed before and after 4 weeks of treatment.
Results
Both study groups showed a significant reduction in storage sub-score but only frequency was significantly reduced in group B (P < 0.001). On the other hand, Tamsulosin 0.8 mg was superior to Tamsulosin 0.4 mg regarding voiding sub-score except for straining (P = 0.325). Accordingly, the total international prostate symptom score was significantly improved in group B versus group A (P < 0.001). Furthermore, maximum flow rate and post-void residual urine volume were notably improved in Group B as compared to Group A (P < 0.001). Of all adverse events only dizziness was noted to be statistically significant in Group B versus Group A (P < 0.001).
Conclusion
Tamsulosin 0.8 mg has shown better outcomes in treating patients who suffer from lower urinary tract symptoms due to benign prostatic enlargement than Tamsulosin 0.4 mg, and besides that, it is well tolerated.
Trial registration number
M S 292/2020, SID: 373, date: 22/4/2020.
Keywords: Tamsulosin, Lower urinary tract symptoms, Benign prostatic enlargement, Maximum flow rate
Introduction
Benign prostatic enlargement (BPE) is the leading cause of lower urinary tract symptoms (LUTS) in elderly men. This condition is seen in 50% of men aged between 51 and 60 years, and more than 90% of men above 80 years old, increasing the need for efficient and enduring treatments. Management of BPE varies from watchful waiting to surgical intervention. The current medical therapies include α-adrenergic blockers (α-blockers), 5 α reductase inhibitors, Phosphodiesterase 5 enzyme inhibitors, and muscarinic receptor blockers (M3-blockers) [1].
Most physicians use α-blockers as the first line of treatment when treating patients with BPE-associated LUTS. The evidence that prostate smooth muscle contraction causes bladder outlet obstruction (BOO) justifies the use of α-blockers in treating BPE-associated LUTS. α1 adrenergic receptors have three subtypes: α1A, α1B, and α1D. 70% of human prostatic adrenoreceptors are made up of α1A which can reach 80% in BPE patients [2].
Tamsulosin, a highly selective α1-blocker, lowers the tone of the smooth muscle contraction in the prostate, urethra, and bladder neck, reducing urine flow resistance [3]. It has more affinity for α1A receptors than for α1B receptors. That is why it has fewer cardiovascular adverse effects, and no interactions with antihypertensive medications [4].
Uroflowmetry parameters like maximum flow rate (Qmax), average flow rate (Qavg), and post-void residual (PVR) urine volume, as well as International Prostate Symptom Score (IPSS), are used to evaluate the improvement in LUTS [5].
Compared to other α1-blockers, Tamsulosin causes fewer adverse effects such as dizziness, vertigo, first-dose syncope, and orthostatic hypotension. There was no statistically significant difference in blood pressure between Tamsulosin-treated, and Placebo-treated individuals according to the studies [6, 7]. On the other hand, Tamsulosin, frequently, causes delayed or retrograde ejaculation. This occurs by blocking the α1 adrenergic receptors in the vas deferens and the bladder neck, failing the internal sphincter to contract during ejaculation. Other less common adverse effects include headache, asthenia, and rhinitis-like symptoms which are likely to be brought on by suppression of serotonin’s release in the central nervous system [8].
In clinical experience, not all patients have reacted to Tamsulosin 0.4 mg once daily, necessitating the use of other therapies, or perhaps dose escalation. Tamsulosin 0.4 mg and 0.8 mg effects were compared in a small number of studies, mostly lacking blinded randomisation, or lack a control group. As a result, we aimed to evaluate the effectiveness of Tamsulosin 0.8 mg once daily compared to the traditional dose 0.4 mg, as well as the likelihood of any possible adverse events.
Patients and methods
Study design
This study was a double-blinded, randomized, prospective trial that was conducted from January 2020 to June 2021. A total of 211 patients from a single tertiary care facility were assessed for eligibility. Patients aged ≥ 50 and ≤ 90 years, who were diagnosed with BPE-associated LUTS and did not receive medical treatment for BPE in the last 2 weeks, were eligible. The exclusion criteria included a previous history of acute urinary retention (AUR) or prostate surgery, patients with chronic urinary retention, or prostate malignancy, and other causes of LUTS (urinary bladder stones, neurogenic bladder, or urethral stricture). 94 patients were excluded according to the exclusion criteria and 24 patients refused to participate. 93 patients were enrolled and consented to the study and its purpose. The study was approved by our institute’s ethical committee.
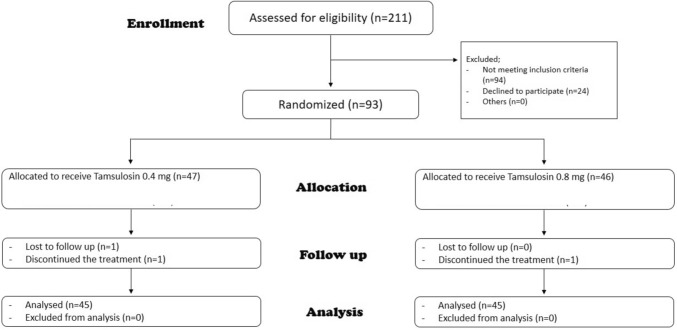
Before randomization, patients were evaluated by general history taking (smoking, lifestyle, past medical history, current medications, sexual life, and assessment of ejaculation activity), and physical examination (measurement of body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP)). IPSS questionnaires were administered, and PVR urine volume and Qmax were evaluated by an abdominopelvic ultrasound and uroflowmetry respectively. Participants were randomized into two groups in a manner of 1:1 ratio (Group A received Tamsulosin 0.4 mg and Group B received Tamsulosin 0.8 mg). All study subjects entered the double-blinded phase by giving the investigator coded pill boxes to deliver to the participants. Each box contained 28 compartments for the 28 days of the study. We chose to perform a preliminary study for a short period of time (4 weeks) for two reasons. The first was justified by the fact that most side effects of alpha blockers tend to express themselves in the initial doses. Secondly, we had concerns that subjects may not exhibit compliance with the drug under trial if the study duration was prolonged, especially Egyptian patients have a reputation of being non-compliant. So, to avoid a big segment of the patients aborting the trial, we chose to start with 4 weeks. In the event that the bigger dose proved its potency and safety, a second study would be designed on a longer scale. Each compartment had either 2 tablets of Tamsulosin 0.4 mg (for group B) or a tablet of Tamsulosin 0.4 mg and a placebo one with inactive ingredients (for group A). Both tablets were taken together as one dose. After 4 weeks of treatment, patients were re-evaluated by IPSS questionnaire, measurement of Qmax, PVR urine volume, SBP, and DBP, and asking about headache, dizziness, and ejaculation abnormality. There were 3 patients who dropped out of the study, 2 of them were due to adverse events (dizziness), and one failed to continue the study. Figure 1 demonstrates our consort flowchart.
Fig. 1.
Flow diagram of the number of patients enrolled, randomized, and completing the study per treatment group
Study assessment
Efficacy was determined by assessment of the primary endpoints, which were the changes in IPSS, PVR urine volume, and Qmax before and after the treatment. Regarding secondary endpoints, safety was assessed by summarizing the incidence of adverse effects and measurement of SBP and DBP.
Statistical analysis
Based on the postulated improvement of 50% of cases in Tamsulosin 0.4 mg compared to that of 80% of cases in Tamsulosin 0.8 mg, the alpha error is 5% and the power of the study is 80%. Therefore, the required sample size is 90 patients, 45 in each group. The program for sample size calculation is STATA 10.
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013. Quantitative normally distributed data was described as mean ± SD (standard deviation) after testing for normality using the Shapiro–Wilk test, then compared using independent t test (two independent groups) and paired t test (paired data). Qualitative data were described as numbers and percentages and compared using the Chi-square test. A P value < 0.050 was significant, otherwise was non-significant.
Results
Patients’ demographics and baseline characteristics
A total of 93 patients were randomized to Tamsulosin 0.4 mg (group A = 47), and Tamsulosin 0.8 mg (group B = 46). Regarding demographic characteristics, there was no significant difference between both groups as summarized in Table 1. Before starting the treatment, there were no significant differences between the 2 groups regarding IPSS, PVR urine volume, or Qmax.
Table 1.
Mean change ± SD of patients’ demographics
| Tamsulosin 0.4 mg | Tamsulosin 0.8 mg | P value* | |
|---|---|---|---|
| Age | 64.9 ± 6.6 | 63.8 ± 5.9 | 0.399 |
| BMI | 25.9 ± 1.9 | 26.2 ± 1.8 | 0.436 |
| SBP | 132.9 ± 12.0 | 135.2 ± 11.6 | 0.350 |
| DBP | 81.6 ± 8.7 | 81.4 ± 9.5 | 0.916 |
| Prostate Size | 50.4 ± 7.3 | 49.1 ± 7.3 | 0.372 |
*Independent t test (comparison between groups)
Efficacy
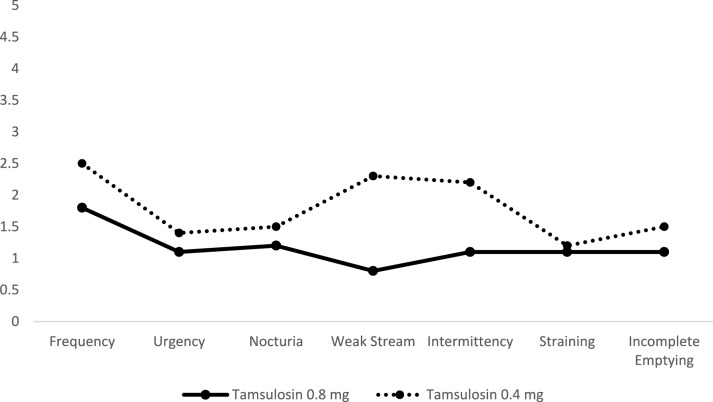
A statistically significant improvement in Total IPSS scores was observed from baseline (29.4 ± 2.6, severe) to the follow-up visit (8.5 ± 1.7, mild) in patients who received Tamsulosin 0.8 mg (P < 0.001). This improvement was seen in frequency, weak stream, intermittency, and incomplete emptying. On the other hand, no significant changes were noted between both groups for urgency, nocturia, or straining. Qmax was significantly greater in group B than in group A (P < 0.001). The mean change in Qmax was 6.1 ± 1.2 ml/s, and 1.9 ± 0.5 ml/s. for group B, and group A respectively. Furthermore, there was a significant reduction in PVR urine volume in group B. The mean change in group B was − 36 ± 6.5 ml. in comparison to that of group A (− 28 ± 6.8 ml., P < 0.001). These changes are summarized in Table 2 and Fig. 2.
Table 2.
Mean change ± SD from baseline to follow-up in primary efficacy parameters
| Tamsulosin 0.8 mg | Tamsulosin 0.4 mg | P value** | |
|---|---|---|---|
| Total IPSS | |||
| Baseline | 29.4 ± 2.6 | 29.1 ± 1.3 | 0.577 |
| Follow up | 8.5 ± 1.7 | 12.4 ± 1.0 | < 0.001* |
| Change | − 20.8 ± 1.7 | − 16.8 ± 1.7 | < 0.001* |
| Qmax | |||
| Baseline | 8.8 ± 1.5 | 8.4 ± 1.1 | 0.154 |
| Follow up | 14.9 ± 2.0 | 10.4 ± 1.3 | < 0.001* |
| Change | 6.1 ± 1.2 | 1.9 ± 0.5 | < 0.001* |
| PVR | |||
| Baseline | 59.7 ± 7.1 | 61.5 ± 8.2 | 0.281 |
| Follow up | 23.6 ± 4.3 | 32.9 ± 7.0 | < 0.001* |
| Change | − 36.1 ± 6.5 | − 28.6 ± 6.8 | < 0.001* |
*Significant
**Independent t test (comparison between groups)
Fig. 2.
The difference between both groups at follow-up in IPSS sub-scores
Safety
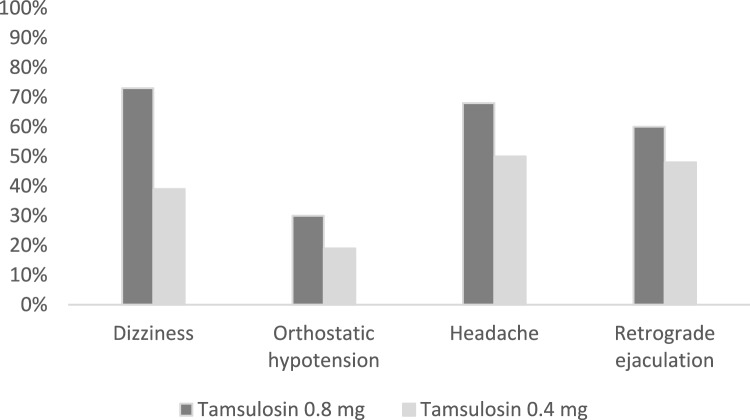
Dizziness was statistically more frequent in group B (73%) than in group A (39%) (P < 0.001). Retrograde ejaculation was also a frequent adverse event in both groups, especially in group B (60%). Despite that both groups had no significant differences (P = 0.290). Orthostatic hypotension occurred by 30% in group B and 19% in group A without significant difference (P = 0.227). Also, both groups reported drug-related headaches but without a statistically significant difference (P = 0.085). Adverse events are summarized in Fig. 3.
Fig. 3.
Percentage of patients in both groups who experienced adverse events
Discussion
This was a prospective, randomized, double-blind study, conducted on 90 patients who had BPE-associated LUTS.
In this study, the total IPSS score improved significantly with group B who received Tamsulosin 0.8 mg (P < 0.001), without noted changes between the 2 groups in straining, nocturia, or urgency. In a phase 3 multicenter placebo-controlled study [9], patients with BPH were randomized to receive Tamsulosin 0.8 mg, Tamsulosin 0.4 mg, and placebo. The mean change in IPSS was significantly greater in both Tamsulosin groups than that of placebo (P < 0.001) with the superiority of Tamsulosin 0.8 mg over Tamsulosin 0.4 mg in voiding sub-scores (P = 0.007). Another study done on 81 Taiwanese patients who were dissatisfied with the usual dose of Tamsulosin (0.2 mg, due to the lower BMI in Asian people) and asked to escalate the dose to 0.4 mg, found a significant improvement in total IPSS from baseline (14.94 ± 7.41) to the end of 12-week period (7.36 ± 5.77, P < 0.001) [10]. The results of these 2 studies were in agreement with ours. In contrast, no statistically significant difference was noted in the mean change in IPSS from the baseline to the endpoint between Tamsulosin 0.4 mg and Tamsulosin 0.8 mg (− 5.09 ± 0.41 and − 5.76 ± 0.41, respectively) in another study [11]. In a multicenter double-blind study for 12 weeks of treatment with a placebo, Tamsulosin modified release (MR) 0.4 mg, Tamsulosin oral-controlled absorption system (OCAS) 0.4 mg and Tamsulosin OCAS 0.8 mg, no statistically significant change was found in IPSS between Tamsulosin MR 0.4 mg and Tamsulosin OCAS 0.8 mg (P = 0.999) [12].
Regarding Qmax, we found a statistically significant improvement in both groups relative to the baseline. The mean change in Qmax was significantly higher in group B (6.1 ± 1.2 ml/s and 1.9 ± 0.5 ml/s for group B and A respectively, P < 0.001). Two studies [10, 13] compared the effect of Tamsulosin 0.4 mg versus Tamsulosin 0.2 mg, and found a significant improvement in Qmax for Tamsulosin 0.4 mg. In one study [10], Qmax increased significantly from baseline (11.37 ± 6.04 ml/s) to Week 12 (13.06 ± 6.18 ml/s) (P = 0.0037). In the other study [13], the mean change in Qmax from the baseline to Week 12 for Tamsulosin 0.2 was − 0.25 ± 0.3 ml/s and 3.0 ± 0.48 ml/s for Tamsulosin 0.4 mg (P < 0.001). The results from the fore mentioned studies were in agreement with ours that doubling the dose of Tamsulosin had better outcomes. On the other hand, another study [9], found no significant difference in the mean change between Tamsulosin 0.4 mg and 0.8 mg (1.75 ± 3.5 and 1.78 ± 3.3 ml/s, respectively). And in yet another study [11], although the results in patients treated with Tamsulosin were significant in comparison to placebo (P < 0.05), there was no significant difference between Tamsulosin 0.4 mg and 0.8 mg.
Our study found that the reduction in PVR urine volume was more significant in group B (− 36.1 ± 6.5 ml) than in group A (− 28.6 ± 6.8 ml) (P < 0.001). Unlike our study, others found no significant change in PVR urine volume when the dose of Tamsulosin was upscaled from 0.2 mg to 0.4 mg (P = 0.5486) [10]. Another study also found no significant change between Tamsulosin 0.2 mg and 0.4 mg [13].
Overall, Tamsulosin was well tolerated at doses of 0.4 mg and 0.8 mg. The incidence of adverse events like headache, abnormal ejaculation, and orthostatic hypotension was more frequent with Tamsulosin 0.8 mg but not significant. Only dizziness was significantly more frequent in group B (73%, n = 33) than in group A (39%, n = 18, P < 0.001). On the other hand, abnormal ejaculation was significantly frequent with Tamsulosin 0.8 mg in some studies [9, 11, 12].
Conclusion
Treating patients who have symptomatic BPE and complain of severe LUTS with Tamsulosin 0.8 mg once daily is more effective than Tamsulosin 0.4 mg with significant improvement in IPSS, Qmax, and PVR urine volume. Tamsulosin 0.8 mg is well tolerated showing no significant difference from Tamsulosin 0.4 mg. Consequently, we do believe it is safe to increase the dose to 0.8 mg according to the severity of the symptoms without increasing the incidence of adverse events.
Limitations of the study
Absence of Tamsulosin 0.8 mg as one tablet in our country was one of the obstacles that was solved by giving the patients 2 tablets of Tamsulosin 0.4 mg. In addition, other adverse events might not have been detected due to the short-term period of the study like impacts on sexual function. Furthermore, will the drug effect decline with time, is a question yet to be answered. We therefore recommend a second trial studying more subjects for a longer duration before any solid recommendations on the role of a double dose tamsulosin could be made.
Acknowledgements
We acknowledge the effort and time spent by our residents and medical staff to help us gather the necessary data, their generous contribution is much appreciated.
Abbreviations
- LUTS
Lower urinary tract symptoms
- BPE
Benign prostatic enlargement
- BOO
Bladder outlet obstruction
- Qmax
Maximum urinary flow rate
- Qavg
Average urinary flow rate
- PVR
Post-void residual
- IPSS
International prostate symptom score
- QoL
Quality of life
- AUR
Acute urinary retention
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- MR
Modified release
- OCAS
Oral-controlled absorption system
Author contributions
TO: project development, manuscript editing. HE: data analysis, manuscript writing. KF: manuscript writing. MS: data collection, data analysis. MDE: protocol development, data collection, manuscript writing. DO: manuscript writing. KOE: project development, data management, data analysis, manuscript editing.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
Data availability applicable upon reasonable request.
Declarations
Conflict of interest
None declared.
Human and animal participants
This research involves human participants.
Informed consent
All participants signed an informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xiong Y, Zhang Y, Li X, et al. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male. 2020;23(5):1432–1439. doi: 10.1080/13685538.2020.1781806. [DOI] [PubMed] [Google Scholar]
- 2.Sugianto R, Tirtayasa PMW, Duarsa GWK. A comprehensive review of medical therapy on benign prostatic hyperplasia. Sexologies. 2022;31(1):52–60. doi: 10.1016/j.sexol.2021.07.002. [DOI] [Google Scholar]
- 3.Laxman Prabhu GG, Prajapati H, Chaturvedi A, et al. Tamsulosin in urology: beyond benign prostatic hyperplasia. Drugs Therap Perspect. 2019;35(4):181–184. doi: 10.1007/s40267-019-00611-1. [DOI] [Google Scholar]
- 4.Kapoor A. Benign prostatic hyperplasia (BPH) management in the primary care setting. Can J Urol. 2012;19(1):10–17. [PubMed] [Google Scholar]
- 5.Chen Y, Zhang X, Hu X, et al. The potential role of a self-management intervention for benign prostate hyperplasia. Urology. 2012;79(6):1385–1389. doi: 10.1016/j.urology.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 6.Abrams P, Schulman CC, Vaage S. Tamsulosin, a selective α1c-adrenoceptor antagonist: a randomized, controlled trial in patients with benign prostatic ‘obstruction’ (symptomatic BPH) Br J Urol. 1995;76(3):325–336. doi: 10.1111/j.1464-410x.1995.tb07709.x. [DOI] [PubMed] [Google Scholar]
- 7.Schulman CC, Cortvriend J, Jonas U et al (1996) Tamsulosin, the first prostate-selective alpha 1A-adrenoceptor antagonist. Analysis of a multinational, multicentre, open-label study assessing the long-term efficacy and safety in patients with benign prostatic obstruction (symptomatic BPH). European Tamsulosin Study Group. Eur Urol 29(2):145–154 [PubMed]
- 8.Lee M. Tamsulosin for the treatment of benign prostatic hypertrophy. Ann Pharmacother. 2000;34(2):188–199. doi: 10.1345/aph.18263. [DOI] [PubMed] [Google Scholar]
- 9.Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Urology. 1998;5(6):892–900. doi: 10.1016/s0090-4295(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang PS, Chen CL, Hou CP, et al (2018) An open-label, prospective interventional study of the tolerability and efficacy of 0.4 mg oral tamsulosin oral controlled absorption system in men with lower urinary tract symptoms associated with benign prostatic hyperplasia who are unsatisfied with treatment with 0.2 mg tamsulosin. Clin Interv Aging 13: 235–242. 10.2147/CIA.S152701 [DOI] [PMC free article] [PubMed]
- 11.Narayan P, Tewari A. A second phase III multicenter placebo-controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. J Urol. 1998;160(5):1701–1706. doi: 10.1016/S0022-5347(01)62386-3. [DOI] [PubMed] [Google Scholar]
- 12.Chapple CR, Al-Shukri SH, Gattegno B, et al. Tamsulosin oral controlled absorption system (OCAS) in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH): efficacy and tolerability in a placebo and active comparator-controlled phase 3a study. Eur Urol Suppl. 2005;4(2):33–44. doi: 10.1016/j.eursup.2004.10.010. [DOI] [Google Scholar]
- 13.Kim JJ, Han DH, Sung HH, et al (2014) Efficacy and tolerability of tamsulosin 0.4 mg in Asian patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia refractory to tamsulosin 0.2 mg: a randomized placebo-controlled trial. Int J Urol 21(7): 677–682. 10.1111/iju.12412 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability applicable upon reasonable request.