Abstract
Background:
Nirmatrelvir/ritonavir (N/R) reduces severe outcomes among patients with COVID-19; however, rebound after treatment has been reported. We compared symptom and viral dynamics in community-based individuals with COVID-19 who completed N/R and similar untreated individuals.
Methods:
We identified symptomatic participants who tested SARS-CoV-2 positive and were N/R eligible from a COVID-19 household transmission study: index cases from ambulatory settings and their households were enrolled, collecting daily symptoms, medication use, and respiratory specimens for quantitative PCR for 10 days, March 2022—May 2023. Participants who completed N/R (treated) were propensity score matched to untreated participants. We compared symptom rebound, viral load (VL) rebound, average daily symptoms, and average daily VL by treatment status measured after N/R completion or, if untreated, seven days after symptom onset.
Results:
Treated (n=130) and untreated participants (n=241) had similar baseline characteristics. After treatment completion, treated participants had greater occurrence of symptom rebound (32% vs 20%; p=0.009) and VL rebound (27% vs 7%; p<0.001). Average daily symptoms were lower among treated participants compared to untreated participants without symptom rebound (1.0 vs 1.6; p<0.01), but not statistically lower with symptom rebound (3.0 vs 3.4; p=0.5). Treated participants had lower average daily VLs without VL rebound (0.9 vs 2.6; p<0.01), but not statistically lower with VL rebound (4.8 vs 5.1; p=0.7).
Conclusions:
Individuals who completed N/R experienced fewer symptoms and lower VL but were more likely to have rebound compared to untreated individuals. Providers should still prescribe N/R, when indicated, and communicate possible increased rebound risk to patients.
Background
Since 2020, multiple treatments have been developed against SARS-CoV-2, the viral cause of COVID-19. Several outpatient treatments for people with COVID-19 and risk factors for severe outcomes are available —nirmatrelvir/ritonavir (N/R) being the most frequently used. N/R has been shown to reduce the risk of severe outcomes among patients with mild-to-moderate COVID-19. [1–5] Evidence is emerging that N/R can reduce the risk of post-COVID conditions.[6]
Soon after N/R became available clinically in January 2022, there were anecdotal reports, case series, and observational studies of symptom or viral rebound among individuals treated with N/R. [7–9] Understanding what happens both from a clinical standpoint (or the patient experience as assessed by symptoms) and epidemiologic standpoint (as assessed by viral loads and those associated with potential infectiousness[10]) is important for communicating treatment expectations and to understand potential changes in transmissibility. Few previous studies have assessed rebound among community-based individuals with COVID-19 or among comparable treated and untreated individuals, as people receiving N/R treatment tend to be older and have more medical comorbidities and risk factors than untreated people. The objective of this analysis was to compare COVID-19 symptom and viral dynamics among community-based individuals who completed N/R treatment compared to eligible individuals who were not treated.
Methods
Study Design and Population
Persons included in this analysis were drawn from a prospective COVID-19 case-ascertained household transmission study in the United States.[11] Briefly, individuals testing positive for SARS-CoV-2 by RT-PCR or antigen test between September 2021 and May 2023 were identified as eligible for enrollment as index cases. Upon enrollment, participants (index and household contacts) completed baseline questionnaires providing demographics, chronic conditions, vaccination, and prior infection history. Participants were followed prospectively for 10 days from enrollment, reporting daily COVID-19 symptoms and medications, and providing self-collected daily specimens for SARS-CoV-2 testing. Self-collected nasal swabs were tested for SARS-CoV-2 using real-time RT-PCR on the Hologic Panther platform. Viral loads of positive swabs were calculated via linear regression of cycle threshold values calibrated to WHO’s International Unit (IU)-based standard to estimate the number of viral RNA copies present in a mL of transport media (Supplemental Methods).[12]
Participants were eligible for this analysis if they met the following treatment eligibility criteria: tested positive for SARS-CoV-2, enrolled post-March, 2022 (the time of earliest report of N/R among participants), reported COVID-19 symptoms, ≥12 years old, and were not hospitalized. Participants who reported taking molnupiravir, remdesivir, bebtelovimab or >1 COVID-19 medication were excluded.
N/R Treated Group
Participants were considered “N/R treated” if N/R was started within five days of symptom onset and completed in 5–6 consecutive days both to align with FDA prescription guidelines and ensure similar timelines of symptom onset to N/R completion for post-treatment comparisons with untreated participants.[13] To allow inclusion of individuals whose first daily diary was after symptom onset (but still ≤5 days of onset), we considered 3–4 consecutive days of N/R as “N/R treated”. Treatment completion was defined as the last reported day of N/R. A summary of characteristics of all who initiated N/R is available (Table S1).
Propensity Score Matching for Untreated Group
We used propensity score (PS) matching to select similar untreated participants compared to N/R treated participants. PS matching was performed using logistic regression with the outcome of N/R treatment completion versus no COVID-19 treatment and baseline covariates (see Supplemental Methods for full PS matching methods). Among untreated participants, treatment completion proxy was defined as seven days since symptom onset — the median days from symptom onset to N/R treatment completion in the N/R treated group and used to align physiologic infection timelines between groups.
Definitions and Outcomes
For the main analysis, follow-up for study outcomes started at treatment completion for N/R treated participants or treatment completion proxy for untreated participants. Any comparisons performed before treatment completion or proxy were to understand comparability of course of illness between the N/R treated and untreated participants from symptom onset to the end of treatment. These comparisons include up to five pre-treatment days among some of the N/R treated participants. Follow-up from symptom onset continued through the earliest of last daily diary (symptom outcomes) or PCR result (viral outcomes), withdrawal date, or end of follow-up.
The primary outcomes were average daily number of symptoms, average daily VL, symptom rebound, and VL rebound after treatment completion or treatment completion proxy. Number of symptoms was calculated based on the reported presence of the 15 elicited COVID-19 symptoms in the daily diary (Supplemental Methods). A participant’s average daily number of symptoms was calculated by summing the number of symptoms reported each day after treatment completion then dividing by the number of days with symptom responses. Similarly, a participant’s average daily VL was calculated by summing the VL (as log10IU/mL) results each day after treatment completion then dividing by the number of days with VL results.
Symptom and VL rebound were determined based on the relative timing of and the magnitude difference of minimum and maximum daily number of symptoms or VL result after treatment completion (Figure S1). Symptom rebound was defined as an increase of at least two symptoms any time after treatment completion/proxy. VL rebound was defined as an increase of at least 1 log10IU/mL (increasing to or above 5 log10IU/mL) any time after treatment completion/proxy. For a sensitivity analysis, we evaluated alternative definitions of symptom and VL rebound including rebound from asymptomatic and rebound from negative PCR. We also evaluated rebound outcomes among all participants who started N/R within 5 days of symptom onset (and their PS selected untreated participants) compared to our analysis population.
Secondary outcomes included symptom resolution, PCR conversion to negative, average daily number of symptoms and VL before treatment completion or treatment completion proxy, peak number of symptoms reported on a single day, peak VL, and presence/absence of asymptomatic days and negative VL days. Symptom resolution was defined as the first day a participant was asymptomatic after which no symptoms were reported. Similarly, PCR conversion was defined as the first day a patient had a negative PCR result after which no additional positive results occurred. PCR conversion analysis used qualitative PCR results from both nasal swab and saliva specimens.
Statistical Analysis
N/R treated and untreated participant characteristics were compared before and after PS matching using Wilcoxon rank sum, Pearson’s Chi-squared, and Fisher’s exact tests, as appropriate. We compared proportions with any asymptomatic days or any negative PCR result using chi-squared tests. Average number of daily symptoms, peak number of symptoms, average daily VL, and peak VL were not normally distributed and thus group medians were compared using Wilcoxon rank sum tests. To understand the severity of rebound compared to the first week of illness, we compared average and peak daily symptoms and VL before and after treatment completion/proxy among individuals experiencing rebound using Wilcoxon signed-rank test. We used Kaplan-Meier survival analysis to compare cumulative probability of symptom resolution and PCR conversion by treatment status. Probability of resolution or conversion was reported as 1-survival probability. We used logistic regression to calculate odds of VL rebound by symptom rebound, overall and by treatment status. Because we attained covariate balance after PS matching, we did not account for matching status in our main analyses. [14] A 2-sided P<0.05 was defined as statistically significant. Analyses were performed in R Statistical Software (v4.2.3; R Core Team, 2023) and PS matching using MatchIt R package (v4.5.3, Ho, Imai, King, & Stuart, 2011).
Ethical Review
Study activities were reviewed and approved by the Institutional Review Boards (IRB) of Vanderbilt University and Westat; the Centers for Disease Control and Prevention IRB reviewed these activities and relied on these approvals (see 45 C.F.R. part 46; 21 C.F.R. part 56).
Results
Of 3,883 participants enrolled in the COVID-19 household transmission study, 2,661 tested positive for SARS-CoV-2 (Figure 1). Participants were excluded if they did not meet N/R eligibility criteria (n=1,286), took another COVID-19 medication (n=42), started N/R before symptom onset or after 5 days after symptom onset (n=10), or who did not complete N/R (n=89).
Figure 1. Participants eligible for analysis, nirmatrelvir/ritonavir treatment completion, and propensity score matched selection of untreated participants from a larger case-ascertained household transmission study of COVID-19 in the United States.
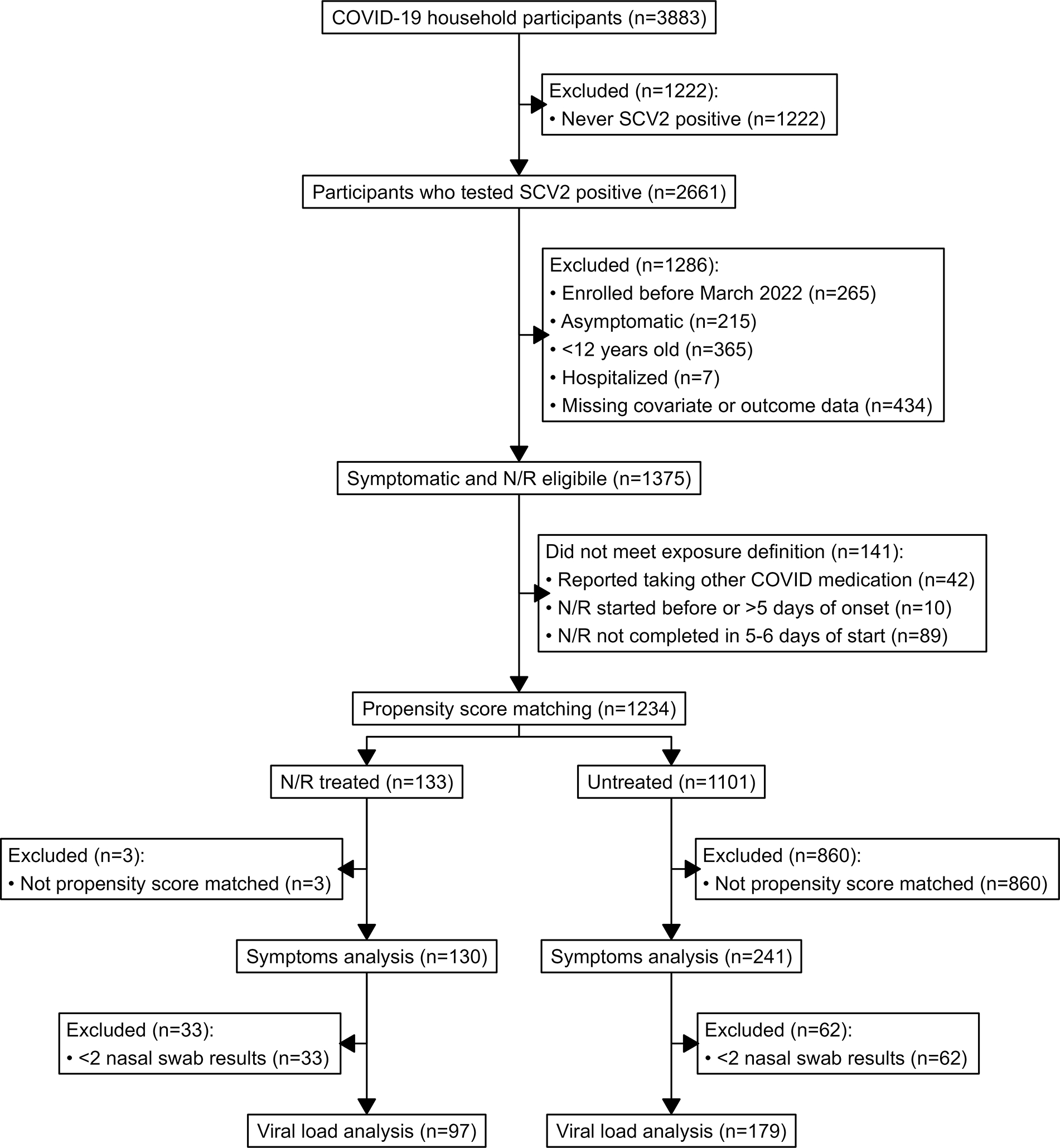
Footnote: Elicited symptoms included fever (including feeling feverish/chills), cough, sore throat, runny nose, nasal congestion, fatigue (including feeling run down), wheezing, trouble breathing (including shortness of breath), chest tightness (including chest pain), loss of smell or taste, headache, abdominal pain, diarrhea, vomiting, and body aches (including muscle aches). Elicited COVID-19 medications included molnupiravir, remdesivir, and nirmatrelvir/ritonavir; other medications were reported in a free-response section of the diary. PS matching was performed using logistic regression with the outcome of N/R treatment completion (N/R treated) versus no COVID-19 treatment (untreated) and covariates included age at enrollment, sex, race/ethnicity, Social Vulnerability Index, prior COVID-19, recruitment method, participant type (index vs. contact), accessed medical care after enrollment, received ≥3 verified COVID-19 vaccine doses, received a verified COVID-19 vaccine dose ≤6 months of index onset, SARS-CoV-2 variant circulating at the time of index onset, number of comorbidities, and whether the participant reported each of asthma or other lung disease, heart disease, diabetes, cancer, liver or kidney disease, immunocompromising condition or taking immunosuppressing medication, or any other chronic health condition. SCV2=SARS-CoV-2; N/R=nirmatrelvir/ritonavir
Of 1,234 participants who met analysis inclusion criteria, 133 completed N/R treatment (N/R treated) and 1,101 were untreated. Before PS matching selection of untreated participants, N/R treated participants were older, reported more comorbidities, were more likely to be White, non-Hispanic, and more likely to have received ≥3 vaccine doses (Table 1). After PS matching, 241 untreated participants were selected based on nearest PS match to 130 N/R treated participants (final ratio of 1.9:1) and included in our analysis. The selected untreated participants had similar baseline characteristics compared to N/R treated participants (Table 1, Figure S2). N/R treated participants started treatment a median of 2 days after symptom onset (Table S1). N/R treated and untreated participants were enrolled a median of 3 days after symptom onset and concluded follow-up a median of 13 days after onset.
Table 1.
Comparison of participant demographics and characteristics for nirmatrelvir/ritonavir (N/R) treated and untreated groups before and after propensity score matching
| All | Matched | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | N/R treated1 | Untreated1 | p-value2 | N/R treated1 | Untreated1 | p-value2 |
| N = 133 | N = 1,101 | N = 130 | N = 241 | |||
|
| ||||||
| Age at enrollment, years | 57 (42, 67) | 39 (29, 52) | <0.001 | 56 (41, 66) | 53 (43, 65) | 0.5 |
| Male sex | 55 (41%) | 423 (38%) | 0.5 | 54 (42%) | 99 (41%) | >0.9 |
| Race/ethnicity | <0.001 | >0.9 | ||||
| White, Non-Hispanic | 111 (83%) | 731 (66%) | 108 (83%) | 203 (84%) | ||
| Hispanic/Latino | 5 (3.8%) | 218 (20%) | 5 (3.8%) | 11 (4.6%) | ||
| Black, Non-Hispanic | 7 (5.3%) | 51 (4.6%) | 7 (5.4%) | 12 (5.0%) | ||
| Other | 8 (6.0%) | 88 (8.0%) | 8 (6.2%) | 12 (5.0%) | ||
| Unk/Refused | 2 (1.5%) | 13 (1.2%) | 2 (1.5%) | 3 (1.2%) | ||
| Social Vulnerability Index3 | 0.27 | 0.38 | <0.001 | 0.28 | 0.28 | 0.6 |
| (0.10, 0.47) | (0.15, 0.67) | (0.10, 0.50) | (0.11, 0.49) | |||
| Prior COVID-19 | 42 (32%) | 372 (34%) | 0.6 | 41 (32%) | 79 (33%) | 0.8 |
| Enrolled from a sentinel site | 100 (75%) | 663 (60%) | <0.001 | 97 (75%) | 181 (75%) | >0.9 |
| Index participant | 90 (68%) | 663 (60%) | 0.1 | 87 (67%) | 166 (69%) | 0.7 |
| Sought medical care after enrollment | 25 (19%) | 54 (4.9%) | <0.001 | 24 (18%) | 33 (14%) | 0.2 |
| Received 3+ COVID-19 vaccine doses | 120 (90%) | 792 (72%) | <0.001 | 117 (90%) | 217 (90%) | >0.9 |
| Received COVID-19 vaccine <6 months ago | 54 (41%) | 347 (32%) | 0.035 | 52 (40%) | 95 (39%) | >0.9 |
| Number of reported chronic medical conditions4 | 1 (1, 2) | 0 (0, 1) | <0.001 | 1 (1, 2) | 1 (0, 2) | 0.2 |
| Asthma or other chronic lung disease | 28 (21%) | 161 (15%) | 0.052 | 27 (21%) | 47 (20%) | 0.8 |
| Cardiovascular/heart disease | 41 (31%) | 180 (16%) | <0.001 | 40 (31%) | 66 (27%) | 0.5 |
| Diabetes | 18 (14%) | 55 (5.0%) | <0.001 | 17 (13%) | 27 (11%) | 0.6 |
| Cancer | 20 (15%) | 57 (5.2%) | <0.001 | 18 (14%) | 26 (11%) | 0.4 |
| Chronic kidney or liver disease | 2 (1.5%) | 18 (1.6%) | >0.9 | 2 (1.5%) | 4 (1.7%) | >0.9 |
| Immunocompromising condition or currently takes any immune suppressing medications | 12 (9.0%) | 52 (4.7%) | 0.035 | 12 (9.2%) | 23 (9.5%) | >0.9 |
| Any other chronic medical condition | 54 (41%) | 211 (19%) | <0.001 | 52 (40%) | 93 (39%) | 0.8 |
| Predominant variant at time of enrollment | 0.3 | >0.9 | ||||
| Omicron BA1/BA2: Dec 21 - Apr 22 | 5 (3.8%) | 62 (5.6%) | 5 (3.8%) | 9 (3.7%) | ||
| Omicron BA4/5: May 22 - mid Jan 23 | 109 (82%) | 922 (84%) | 108 (83%) | 202 (84%) | ||
| Omicron XBB: mid Jan 23 on | 19 (14%) | 117 (11%) | 17 (13%) | 30 (12%) | ||
Median (IQR); n (%)
Wilcoxon rank sum test; Pearson’s Chi-squared test; Fisher’s exact test
Social Vulnerability Index was determined based on the 2020 US decennial census tract location of the home. It uses 16 U.S. census variables to indicate the relative vulnerability of every U.S. census tract to a hazardous event with values closer to 1 representing highly vulnerable areas and values closer to 0 representing least vulnerable areas.
Elicited chronic medical conditions included asthma, non-asthma chronic lung disease, cancer, diabetes, cardiovascular/heart disease, immunocompromising conditions, immune suppressing medications, kidney disease, liver disease, and other chronic medical conditions.
Symptom Outcomes
Before treatment completion/proxy, participants had similar average number of daily symptoms (3.9 vs. 4.0; p=0.9) and peak number of symptoms (6.5 vs. 6; p=0.8) by treatment status. The cumulative probability of symptom resolution from symptom onset to the end of follow-up was 34%. N/R treated and untreated participants had similar cumulative probability of symptom resolution (31% vs. 35%; p=0.8, Figure 2A).
Figure 2. Symptom dynamics during the first two weeks after symptom onset by nirmatrelvir/ritonavir (N/R) treatment and symptom rebound1 visualized as (A) cumulative probability of symptom resolution2, (B) median number of symptoms each day since symptom onset and proportion of patients experiencing symptom rebound, and (C) average daily symptoms after N/R completion (or seven days since symptom onset for untreated group).
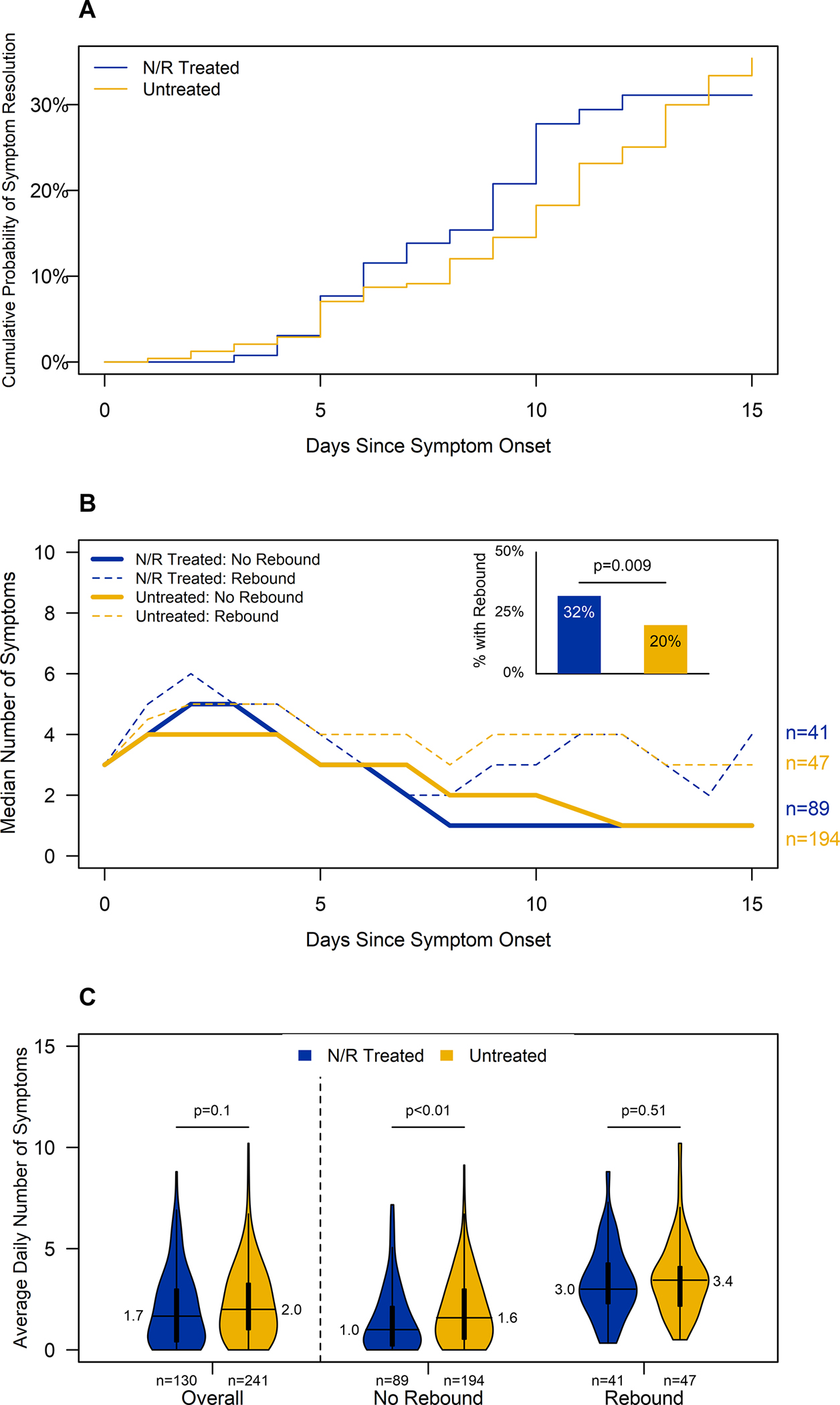
Footnote: Participants reported symptoms daily from a list of 15 symptoms including fever (including feeling feverish/chills), cough, sore throat, runny nose, nasal congestion, fatigue (including feeling run down), wheezing, trouble breathing (including shortness of breath), chest tightness (including chest pain), loss of smell or taste, headache, abdominal pain, diarrhea, vomiting, and body aches (including muscle aches).
1Symptom rebound was defined as an increase of at least two reported symptoms after treatment completion or treatment completion proxy
2Symptom resolution was defined as the first day in which a participant was asymptomatic after which no later symptoms were reported
After treatment completion/proxy, participants still had similar average number of daily symptoms (1.7 vs. 2.0; p=0.1, Figure 2B) and proportion with ≥1 asymptomatic day (48% vs. 36%; p=0.05) by treatment status. One-quarter of participants (88/371, 23.7%) experienced symptom rebound. Compared to participants who did not experience symptom rebound, participants who did had higher average daily symptoms (4.6 vs. 3.7; p<0.001) and peak symptoms (7 vs. 6; p<0.001) before completion/proxy irrespective of treatment. Among participants who experienced symptom rebound (n=88), average daily symptoms and peak symptoms were higher before completion/proxy than after (4.6 vs. 3.3; p<0.001; 7 vs. 5; p<0.001; respectively).
N/R treated participants were more likely to experience symptom rebound compared to untreated participants (32%; 95%CI=24–40% vs. 20%; 95%CI=15–25%; p=0.009). Sensitivity analyses of symptom rebound definitions showed similar trends (Table S2). N/R treated and untreated participants who experienced symptom rebound reported similar average daily symptoms (3.0 vs 3.4; p=0.5), peak symptoms (5 vs. 5; p=0.9), and proportion with ≥1 asymptomatic day (29% vs. 15%; p=0.1) after treatment completion/proxy. Among those who did not experience symptom rebound, N/R treated had lower average daily symptoms (1.0 vs 1.6; p=0.003; Figure 2C) and peak symptoms (2 vs 3; p=0.004), and higher proportion with ≥1 asymptomatic day (56% vs 42%; p=0.03) after treatment completion/proxy compared to untreated.
Viral Outcomes
All 371 participants included in the symptoms analysis had ≧2 qualitative PCR results from nasal swab or saliva specimens and were included in the PCR conversion analysis. The cumulative probability of PCR conversion to negative before the end of follow-up was 63% and was not statistically different by treatment status (N/R treated=56% vs untreated=65%; p=0.7, Figure 3A).
Figure 3. Viral dynamics during the first two weeks after symptom onset by nirmatrelvir/ritonavir treatment completion and viral load rebound1 visualized by (A) cumulative probability of RT-PCR conversion to negative2, (B) median viral load each day since symptom onset and proportion with viral load rebound, and (C) median of each participant’s average daily viral load after nirmatrelvir/ritonavir completion or seven days since symptom onset.
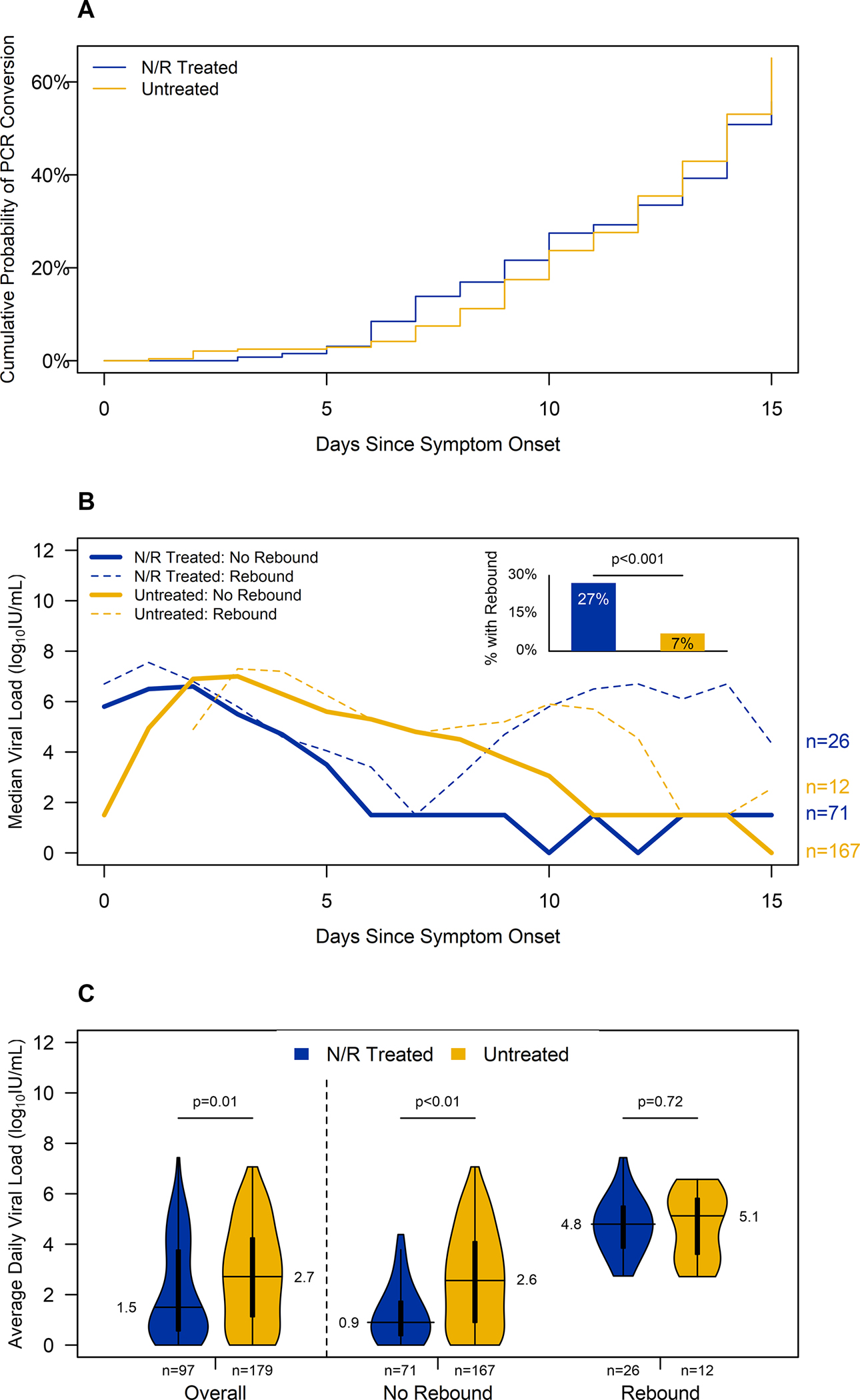
Footnote: N/R=nirmatrelvir/ritonavir;
1VL rebound was defined as an increase of at least 1 log10IU/mL for which the maximum VL exceeded 5 log10IU/mL after treatment completion/proxy;
2PCR conversion was defined as the first day in which a patient had a negative PCR result after which no additional positive results occurred.
Of 371 participants included in symptoms analysis, ≥2 nasal VL results were available for 276 (74%) participants, who were included in VL analysis; similar proportions of N/R treated and untreated participants were excluded, and participant characteristics remained similar (Figure 1, Table S3). N/R treated and untreated participants had similar days from onset to first VL result (3 vs 4; p=0.06) and similar first VL quantity (6.0 vs 6.0 log10IU/mL; p=0.6). Before treatment completion/proxy, N/R treated participants had lower average daily VLs (4.2 vs 5.6; p<0.001) compared to untreated participants.
After treatment completion/proxy, N/R treated participants had lower average daily VLs (1.5 vs 2.7, p=0.007). VL rebound occurred among 14% of participants (38/276). Before treatment completion/proxy, average daily VLs were similar for participants who would later have VL rebound and those who did not (4.8 vs. 5.2; p=0.3). Among participants who had VL rebound (n=38), pre- and post-treatment completion/proxy average daily VLs (4.8 vs. 4.8; p=0.7) were similar, but peak VL (6.3 vs. 6.7; p=0.01) was slightly higher after treatment.
N/R treated participants were more likely to have VL rebound compared to untreated participants (27%, 95%CI=19–37% vs. 7%, 95%CI=4–12%; p<0.001). Sensitivity analysis of various VL rebound definitions illustrated similar results (Table S4). Of note, VL rebound from a negative PCR result to at least 5 log10IU/mL after treatment occurred among 7% (95% CI: 3%, 15%) of N/R treated participants compared to 0% (95% CI: 0%, 3%) of the untreated participants (p<0.001). Among those with VL rebound (n=38), N/R treated participants had a larger VL increase after treatment completion than untreated participants: median VL increase was 5.4 log10IU/mL (IQR: 4.1, 6.5) among N/R treated compared to 1.6 log10IU/mL (IQR: 1.2, 2.4) among untreated (p<0.001; Figure 3B). N/R treated participants with VL rebound had similar average daily VL after treatment completion (4.8 vs. 5.1; p=0. 7) and similarly likely to have at least one negative result (27% vs. 25%; p>0.9) compared to untreated participants with VL rebound. However, N/R treated participants with rebound had significantly higher peak VL (7.3 vs. 6.2; p=0.03) than untreated participants. There were no significant differences in VL before treatment completion among participants who experienced rebound or did not, overall and stratified by treatment status.
Overlap of symptom and viral rebound
Over two-thirds (69%; n=190) of participants experienced neither symptom nor VL rebound after treatment completion/proxy and only 7% (n=18) experienced both (Figure 4A). Participants who experienced symptom rebound had 3.56 increased odds of VL rebound compared to those who did not experience symptom rebound (95% CI: 1.74, 7.28; Figure 4B; VL rebound event rate: 27% with symptom rebound vs 10% without symptom rebound). Among N/R treated, odds of VL rebound were 5.55 greater among participants who experienced symptom rebound compared to those who did not experience symptom rebound (95% CI: 2.13, 15.1; VL rebound event rate: 52% with vs 16% without symptom rebound). Among untreated, there was no association between symptom rebound and VL rebound (OR: 1.30, 95%CI: 0.28, 4.65; VL rebound event rate: 8% with vs 6% without symptom rebound).
Figure 4. Proportion of participants experiencing symptom and viral load rebound outcomes (A) and odds of viral load rebound when symptom rebound experienced (B), overall and stratified by nirmatrelvir/ritonavir treatment completion status.
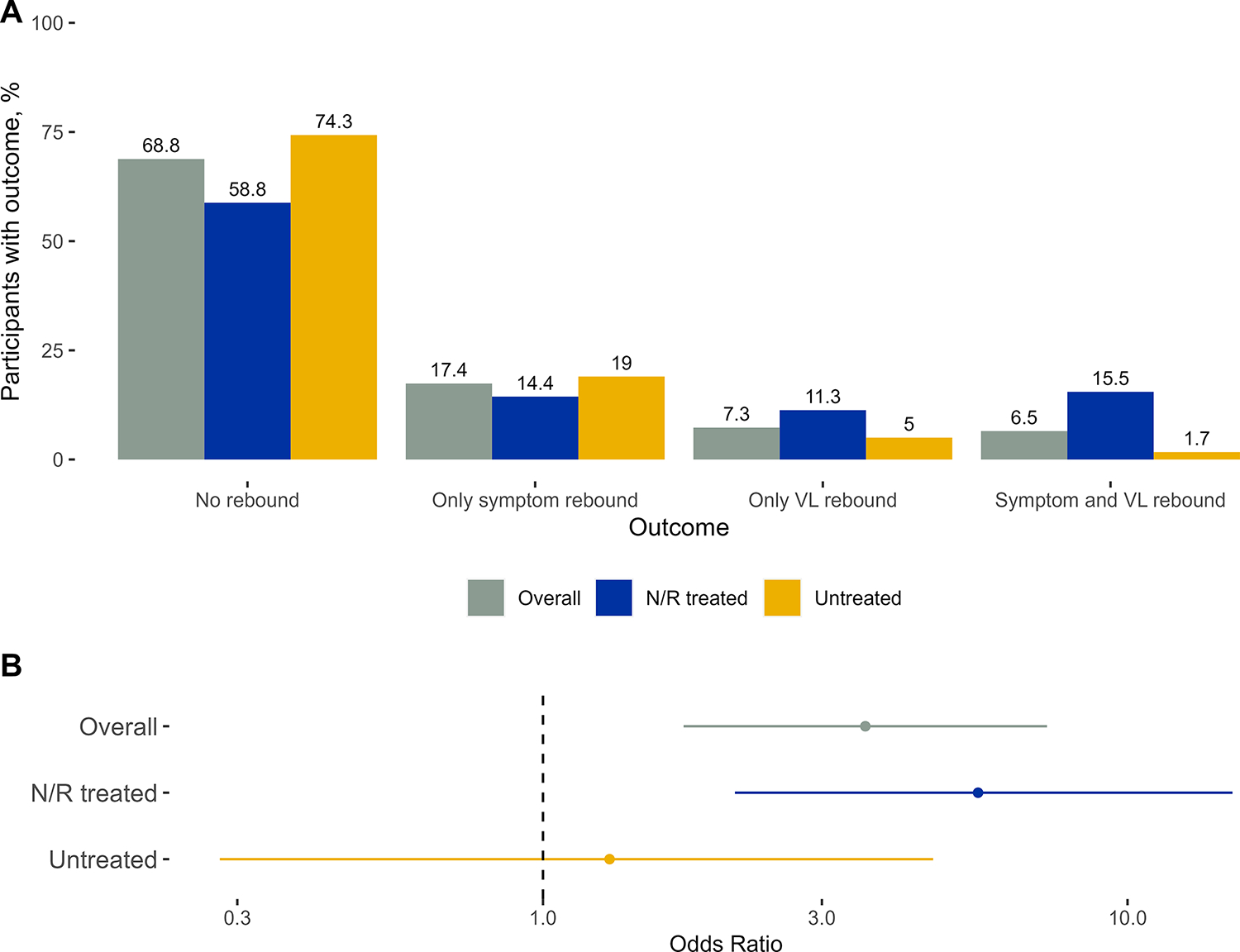
Footnote: VL=viral load; N/R=nirmatrelvir/ritonavir
Discussion
This analysis is among the first to describe symptom and VL dynamics and rebound among community-based individuals in a nation-wide sample who completed N/R treatment compared to similar untreated individuals. Like other studies, we found that individuals who completed N/R had fewer symptoms and lower VL than individuals who did not take treatment. However, after completing treatment, N/R recipients were more likely to experience symptom and VL rebound compared to untreated participants. Independent of treatment status, participants who experienced symptom rebound reported more daily symptoms in the first week of illness than those who did not experience symptom rebound, and participants experiencing rebound reported fewer daily symptoms during rebound compared to before treatment completion. Individuals who completed N/R and experienced symptom rebound were more likely to have VL rebound than those without symptom rebound.
Symptom and VL rebound are multidimensional phenomena. As a result, the definition of rebound impacts its frequency. We used a sensitive symptom rebound definition to answer the clinical patient-centered question “after treatment completion, will I feel worse again (even if my symptoms haven’t resolved during treatment)?” Consequently, symptom rebound was more common in untreated participants in our study than in other studies where rebound required resolution of symptoms before a subsequent increase in symptoms, [15, 16] but comparable to one with a similar definition. [17] We defined VL rebound in a similarly sensitive way to answer the epidemiologic-centered question: “Will I be infectious after treatment completion?” VL rebound was more common among untreated participants in our study than in studies defining rebound only on days 10 or 14 post-treatment, [16, 18] but comparable to a trial with similar sampling and definition to ours.[17] Across the diverse definitions for rebound, most studies evaluating rebound by treatment with SARS-CoV-2 antivirals have found higher proportions among treated participants though few were statistically significant. [15, 18–21]
Our analysis demonstrated that VL and symptom rebound are distinct clinical and epidemiologic phenomena that can occur in patients with mild-to-moderate COVID-19. However, individuals who completed N/R were over five times more likely to have VL rebound when they experienced symptom rebound compared to those without symptom rebound. Given that inhibiting viral replication is the intended mechanism of action of nirmatrelvir, VL rebound after treatment completion is not surprising. [22] Though VL rebound was more frequent among N/R treated participants, they were more likely to have ≥1 negative result compared to untreated participants, further illustrating how N/R quickly reduces viral load.[1, 23]
Our analysis has several limitations. First, medications and symptoms were self-reported; there could have been unreported COVID-19 medication use causing misclassification, and bias in symptom reporting. Second, daily symptoms and VL were available for 10 days following enrollment. We may have insufficient follow-up time to detect all outcomes. Third, there could be unmeasured differences between N/R treated and untreated participants. By including many pre-treatment patient characteristics in the PS model, much of the unmeasured confounding may be mitigated. Fourth, this study population may not be representative of all community-based COVID-19 patients in the US. Fifth, included participants had mild disease not requiring hospitalization, most household contacts did not seek care, and symptom severity was not captured. It is unclear how these results extrapolate to severe disease. Finally, participants are from a prospective cohort study with varying days from symptom onset to enrollment. To mitigate potential bias from our choice of comparison timeline among untreated participants, we used median observed days from symptom onset to N/R completion our proxy for treatment completion in the untreated group and limited N/R treated participants to those who started ≤5 days of symptom onset and completed in 5–6 days.
Among community-based individuals with mild-to-moderate COVID-19, individuals who completed N/R had fewer symptoms and lower VL, but greater risk of symptom and viral rebound after treatment completion compared to similar untreated individuals. Our results, in combination with other clinical trial data showing reduction in severe outcomes following nirmatrelvir/ritonavir, support that nirmatrelvir/ritonavir be prescribed for all high-risk individuals. [1–5] Future studies are needed to understand rebound predictors and the association with COVID-19 treatments. Healthcare providers should initiate timely N/R treatment for patients when indicated and inform patients about the benefits of N/R as well as the potential increased risk for rebound.
Supplementary Material
Key Points:
Among community-based individuals with mild-to-moderate COVID-19, participants who completed nirmatrelvir/ritonavir had fewer symptoms and lower viral load but rebound occurred more often compared to untreated participants. Providers should prescribe N/R, when indicated, and communicate possible increased rebound risk to patients.
Acknowledgements
The authors would like to acknowledge all members of the Respiratory Virus Transmission Network study teams. Vanderbilt University Medical Center: Chris Lindsell, Judy King, John Meghreblian, Samuel Massion, Brittany Creasman, Lauren Milner, Andrea Stafford Hintz, Jorge Celedonio, Ryan Dalforno, Maria Catalina Padilla-Azain, Daniel Chandler, Paige Yates, Brianna Schibley-Laird, Alexis Perry, Ruby Swaimn, Mason Speirs, Erica Anderson, Suryakala Sarilla, Amelia Dodds, Dayton Marchlewski, Timothy Williams, Afan Swan, Onika Abrams, Jackson Resser, Ine Sohn, Cara Lwin, Hsi-nien (Jubilee) Tan, Stephen Yeargin, James Grindstaff, Heather Prigmore, Jessica Lai, Zhouwen Liu, James D. Chappell, Marcia Blair, Rendie E. McHenry, Bryan P. M. Peterson, Lauren J. Ezzell. Columbia University: Lisa Saiman, Raul A. Silverio Francisco, Anny L. Diaz Perez, Ana M. Valdez de Romero. Stanford University: Rosita Thiessen, Marcela Lopez, Alondra A. Aguilar, Emma Stainton, Grace K-Y. Tam, Jonathan Altamirano, Leanne X. Chun, Rasika Behl, Samantha A. Ferguson, Yuan J. Carrington, Frank S. Zhou. Marshfield Clinic Research Institute: Hannah Berger, Vicki Moon, Gina Burbey, Brianna Breu, Leila Deering, Garrett Heuer, Sarah Kopitzke, Carrie Marcis, Jennifer Meece, Jennifer Moran, DeeAnn Polacek, Miriah Rotar, Carla Rottscheit, Elisha Stefanski, Sandy Strey, Melissa Strupp. University of Arizona: Ferris Alaa Ramadan, Flavia Maria Nakayima Miiro, Josue Ortiz, Mokenge Ndiva Mongoh. University of North Carolina: Jessica Lin, Julienne Reynolds, Katherine “Katie” Murray, Miriana Moreno Zivanovich, Michelle Floris-Moore. University of Colorado: Kathleen Grice, Cameron Bendalin, Sonia Chavez, Jolie Granger. Finally, we would like to acknowledge the households for their participation.
Funding
The parent household transmission study (the Respiratory Virus Transmission Network) was funded by the CDC (contract numbers 75D30121C11656 and 75D30121C11571).
Footnotes
Conflict of Interest Disclosures
Dr. Rao received grant support from BioFire. Dr. McLean received grant/research support from CSL Sequiris and CSK. Dr. Petrie was a former consultant for CSL Seqirus and received grants from NIH and CSL Seqirus. Dr. Asturias was a former consultant for Hillevax and Moderna, Merck supported lecture at the Latin American Vaccine Summit, and received grant/research support from Pfizer. Dr. Grijalva was a former advisor to Merck and has received grant/research support from AHRQ, CDC, FDA, NIH, and Syneos Health. Dr. Bowman received grant/contracts from NIH, Doris Duke Charitable Foundation, and North Carolina Collaboratory. Dr. McLaren received grants/contracts from NIH, American Academy of Pediatrics, and Doris Duke Charitable Foundation. Dr. Schmitz has received grants from NIH. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention.
Data Availability
Data described in this report can be made available from the corresponding author upon reasonable request and upon completion of required approvals.
References
- 1.Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. New England Journal of Medicine 2022; 386(15): 1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah MM, Joyce B, Plumb ID, et al. Paxlovid Associated with Decreased Hospitalization Rate Among Adults with COVID-19 - United States, April-September 2022. MMWR Morbidity and mortality weekly report 2022; 71(48): 1531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal NR, Molina KC, Beaty LE, et al. Real-world use of nirmatrelvir–ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: a retrospective cohort study. The Lancet Infectious Diseases 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. The Lancet 2022; 400(10359): 1213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis 2022; 22(12): 1681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Choi T, Al-Aly Z. Association of Treatment With Nirmatrelvir and the Risk of Post–COVID-19 Condition. JAMA Internal Medicine 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charness ME, Gupta K, Stack G, et al. Rebound of SARS-CoV-2 Infection after Nirmatrelvir–Ritonavir Treatment. New England Journal of Medicine 2022; 387(11): 1045–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonelli G, Focosi D, Turriziani O, et al. Virological and clinical rebounds of COVID-19 soon after nirmatrelvir/ritonavir discontinuation. Clinical Microbiology and Infection 2022; 28(12): 1657–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boucau J, Uddin R, Marino C, et al. Characterization of Virologic Rebound Following Nirmatrelvir-Ritonavir Treatment for Coronavirus Disease 2019 (COVID-19). Clin Infect Dis 2023; 76(3): e526–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boucau J, Marino C, Regan J, et al. Duration of Shedding of Culturable Virus in SARS-CoV-2 Omicron (BA.1) Infection. New England Journal of Medicine 2022; 387(3): 275–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellis AM, Lauring AS, Talbot HK, et al. Changes in Transmission and Symptoms of SARS-CoV-2 in United States Households, April 2020-September 2022. medRxiv 2023; 2023/290185. [Google Scholar]
- 12.Bentley E, Mee ET, Routley S, et al. Collaborative Study for the Establishment of a WHO International Standard for SARS-CoV-2 RNA. Geneva, 2020. [Google Scholar]
- 13.U.S. Food and Drug Administration. Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid: Health and Human Services FDA, 2023. 02/2023.
- 14.Ho DE, Imai K, King G, Stuart EA. Matching as Nonparametric Preprocessing for Reducing Model Dependence in Parametric Causal Inference. Political Analysis 2007; 15(3): 199–236. [Google Scholar]
- 15.Pandit JA, Radin JM, Chiang DC, et al. The Coronavirus Disease 2019 (COVID-19) Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated With Nirmatrelvir Plus Ritonavir Versus Untreated Controls. Clinical Infectious Diseases 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Food and Drug Administration. UPDATED INFORMATION: March 16, 2023: Antimicrobial Drugs Advisory Committee Meeting Announcement. Available at: https://www.fda.gov/advisory-committees/advisory-committee-calendar/updated-information-march-16-2023-antimicrobial-drugs-advisory-committee-meeting-announcement#event-information. Accessed May 10.
- 17.Deo R, Choudhary MC, Moser C, et al. Symptom and Viral Rebound in Untreated SARS-CoV-2 Infection. Annals of Internal Medicine 2023; 176(3): 348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson AS, Caubel P, Rusnak JM. Nirmatrelvir–Ritonavir and Viral Load Rebound in Covid-19. New England Journal of Medicine 2022; 387(11): 1047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong CKH, Lau KTK, Au ICH, et al. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. The Lancet Infectious Diseases 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong GL-H, Yip TC-F, Lai MS-M, Wong VW-S, Hui DS-C, Lui GC-Y. Incidence of Viral Rebound After Treatment With Nirmatrelvir-Ritonavir and Molnupiravir. JAMA Netw Open 2022; 5(12): e2245086–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilling WHK, Jittamala P, Watson JA, et al. Antiviral efficacy of molnupiravir versus ritonavir-boosted nirmatrelvir in patients with early symptomatic COVID-19 (PLATCOV): an open-label, phase 2, randomised, controlled, adaptive trial. The Lancet Infectious Diseases 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lam C, Patel P. Nirmatrelvir-Ritonavir. StatPearls [Internet]: StatPearls Publishing, 2023. [PubMed] [Google Scholar]
- 23.Wang Y, Zhao D, Chen X, Liu X, Xiao W, Feng L. The effect of nirmatrelvir-ritonavir on viral clearance and length of hospital stay in patients infected with SARS-CoV-2 omicron variants. Influenza and Other Respiratory Viruses 2023; 17(2): e13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this report can be made available from the corresponding author upon reasonable request and upon completion of required approvals.


