Abstract
Heptadecanoic acid (C17:0), an odd-chain saturated fatty acid (OCSFA) in ruminant lipid, has been demonstrated to be potential for treating cancers. Our results also showed that sheep tail fat (STF) with higher level of C17:0-containing saturated fatty acids (SFAs) whereas lower level of oleic acid (C18:1), performed remarkable inhibition against non-small-cell lung cancer (NSCLC) cells. To enrich the content of C17:0, a C17:0-rich SFA concentrate (HRSC) was prepared from STF by solvent crystallization and urea complexation methods (hexane/STF = 3.5/1, 4 °C for 8 h, and 80% ethanol/urea/free fatty acids = 8/1/1, 4 °C for 6 h). The content of C17:0 was up from 3.02 to 6.34% and the recovery was 4.17%. Biological experiments showed that HRSC exerted better antiproliferative effect against NSCLC cells. Moreover, HRSC performed enhanced inhibitory effect in A549 cell xenograft mouse model. Therefore, HRSC has the potential to be applied in adjuvant therapy for NSCLC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01504-w.
Keywords: Odd-chain saturated fatty acid, Heptadecanoic acid, Sheep tail fat, Solvent crystallization, Urea complexation
Introduction
Functional fatty acids and lipids are promising for adjuvant cancer therapy (Bian et al., 2021). Recently, accumulating evidences showed that saturated fatty acids (SFAs) exerted important functions in various diseases. For example, it was reported that intake of SFAs could reduce the severity of pancreatitis in humans (Khatua et al., 2021). Palmitic acid (C16:0) decreased the metastatic capacity of hepatocellular carcinoma cells through a series of detail mechanisms (Lin et al., 2017). Stearic acid (C18:0) was reported to blunt growth-factor signaling via protein modification, providing a mechanism for its anti-tumor effects (Nuskova et al., 2021). In addition, we recently reported that administration of heptadecanoic acid (C17:0), one odd-chain saturated fatty acid (OCSFA), could also dramatically suppress the proliferation of non-small-cell lung cancer (NSCLC) cells through downregulating the PI3K/Akt signaling pathway (Xu et al., 2019). C17:0 accounts for a small proportion in ruminant lipids. Our data also showed that sheep tail fat (STF), one dietary lipid rich in C17:0 (approximately 3 wt% in total fatty acids), could also perform similar antiproliferative effect against NSCLC cells in vitro and in vivo (Xu et al., 2022). Interestingly, our comparative results suggested that lipids with higher level of C17:0-containing SFAs whereas lower oleic acid (C18:1), could selectively inhibit NSCLC cell growth (Xu et al., 2022). C17:0 had the smallest IC50 value among the fatty acids in STF and fatty acid mimic experiment showed that C17:0 among SFAs performed the most counteracting effect against the pro-proliferative effect of C18:1 in NSCLC cells (Xu et al., 2022). These indicated that developing lipids with rational content of C17:0-containing SFAs is potential for optimal adjuvant NSCLC treatment.
Recently, there are more and more attractive methods applied to refine lipids due to their bioactive benefits, including solvent crystallization (Lei et al., 2016), urea complexation (Wang et al., 2020), transesterification (Aguedo et al., 2008), molecular distillation (Altuntas et al., 2018), and supercritical CO2 extraction (King et al., 2009). Transesterification, induced by lipases or relevant solvent (such as CH3ONa), could rearrange the location of fatty acids in triglyceride. However, transesterification is dependent on the utilization of expensive enzyme and the by-product is easily generated through the solvent transesterification; Fatty acids could separate from the total triglyceride in high vacuum condition, but the energy consumption and high vacuum conditions limits the application of molecular distillation (Altuntas et al., 2018); Also, a large investment in equipment and high extraction pressure dampened the use of supercritical fluid extraction of fatty acids (King et al., 2009). Comparatively, the solvent crystallization and urea complexation are more simple, easy to operation, and low-cost. There has been a series of reports referring to the enrichment of functional fatty acids from different lipids (Aguedo et al., 2008; Lei et al., 2016). However, these enriched fatty acids often belong to unsaturated fatty acids (UFAs). Only some reports focused on the enrichment of branched odd-chain saturated fatty acids (BOCSFAs) from lanolin (Wang et al., 2020) and butter oil (Mudgal et al., 2016). Little is known about the enrichment of OCSFAs, especially C17:0-rich SFAs.
In this study, we optimized the enrichment condition of C17:0-rich SFAs from STF via solvent crystallization and urea complexation methods. Meanwhile, its potential in suppressing NSCLC cells in vitro and in vivo was also investigated.
Materials and methods
Materials
STF was provided from Anhui Tianxiang grain and oil Food Co. Ltd. (Fuyang, China) and purchased from Xinjiang Uygur Autonomous Region, China. All the solvents and reagents with analytical grade were acquired from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). A standard mixture of fatty acid methyl esters was purchased from Sigma-Aldrich Chemical Co. Ltd. (Shanghai, China). Cisplatin was purchased from Meilun Biotech, China (Dalian, China). Matrigel was purchased from BD Biosciences (Franklin, NJ, USA).
Enrichment of C17:0-rich SFA concentrate through solvent crystallization
STF was dissolved with the indicated organic solvent in a certain ratio, followed by an incubation at the setting temperature for a certain duration time. The solid and liquid phases were separated by centrifugation according to the protocol of solvent crystallization (Cheng et al., 2021).
Enrichment of C17:0 through urea complexation
STF (50 g) was dissolved with 120 mL of ethanol and 16 g of KOH, mixed and incubated at 65 °C for 2 h. The mixture was added with 200 mL of ultrapure water and extracted with 75 mL of n-hexane twice. The supernatant was collected and the pH was adjusted to 2, followed by extraction with 50 mL of n-hexane, washed with ultrapure water and dried with Cu2SO4. The free fatty acids (FFAs) were obtained after vacuuming at 50 °C and stored at − 20 °C.
Urea and ethanol–water with indicated ratio were added into triangular flask, heated at 80 °C and hold at 65 °C. Then certain amount of the FFAs of STF was added and mixed for 1 h, followed by cooling to room temperature and the liquid phase was acquired by centrifuging at 5000 rpm for 20 min. The solvent was recovered under a reduced pressure condition. The FFAs in both liquid and solid phases were obtained after water washing, 20 mL of n-hexane extraction for three times, and removing of n-hexane.
Fatty acid methylation and composition analysis through GC–MS
For the methylation of triglyceride, STF (0.04 g) was dissolved in two milliliters of KOH-CH3OH buffer, followed by addition of 6 ml of n-hexane, sufficiently mixing and incubating at 40 °C for 40 min. The supernatant was filtered with filter membrane (0.22 μm) and transferred into a sample tube and sealed; For the methylation of FFAs, 0.03 g of FFAs was dissolved in 2 mL of 5% HCl–CH3OH buffer, mixed and heated at 80 °C for 2 h. Then 3 mL of 10% Na2CO3 and 3 mL of n-hexane were added successively and mixed. The supernatant was filtered and transferred into a sample tube after centrifugation (2000 rpm, 5 min).
The fatty acid profile was analyzed through a GC system (QP2010, Shimadzu, Kyoto, Japan) with a flame ionization detector (GC–MS-QP2010 Ultra, Shimadzu, Kyoto, Japan) and a 100 m capillary column (0.25 mm internal diameter, SP-2560). The injector and detector temperatures were both 250 °C. Nitrogen carrier gas was supplied at 25 mL/min, and the split ratio was 20:1. Samples with an amount of 1 μL was injected (injector temperature, 240 °C). The column temperature was held at 100 °C for 5 min, increased to 190 °C at a rate of 4 °C/min, and increased from 190 to 220 °C at a rate of 2 °C/min, and maintained at 220 °C for 30 min, increased from 220 to 250 °C at a rate of 2 °C/min and maintained for 10 min. The fatty acid methyl esters were identified by comparing the retention times of sample peaks and MS.
Cell proliferation, colony formation and migration assays
A549 cells were stored in our laboratory and cultured as previously described (Xu et al., 2022). Cells were plated and subjected to proliferation, colony formation, and migration assays as previous described (Xu et al., 2019, 2022).
Immunoblotting assay
Cells were collected, lysed, and subjected to immunoblotting assay to detect the expression of relevant proteins as previous described (Xu et al., 2019, 2022).
Tumor xenograft experiments
The administration of tumor xenograft experiments, guided and approved by the Institutional Animal Care and Use Committee of the Hefei Institutes of Physical Science, Chinese Academy of Science, was described as previously (Xu et al., 2022). In brief, a 100 μL suspension of 2 × 106 A549 cells in an equal volume of Matrigel was injected subcutaneously in the right posterior limb of BALB/c-nude mice with six weeks of age. The mice were randomly divided into four groups once the tumor volumes were about 100–200 mm3. The vehicle group was treated with dimethyl sulfoxide (DMSO) (100 μL of saline containing 5% DMSO (v/v)). The STF/ HRSC group: 200 mg/kg of STF/ HRSC emulsified in DMSO. The mice in these groups were injected intraperitoneally once a day; The cisplatin group (5 mg/kg) were injected twice a week for 36 days. The weight of mice/tumors and the volume of tumors were measured. The Ki-67 positive cells were also counted based on the photograph of immunohistochemistry (IHC).
Statistical analysis
The statistical analyses of the data were performed as previously described (Xu et al., 2022).
Results and discussion
Preparation of the C17:0-rich triglyceride through solvent crystallization
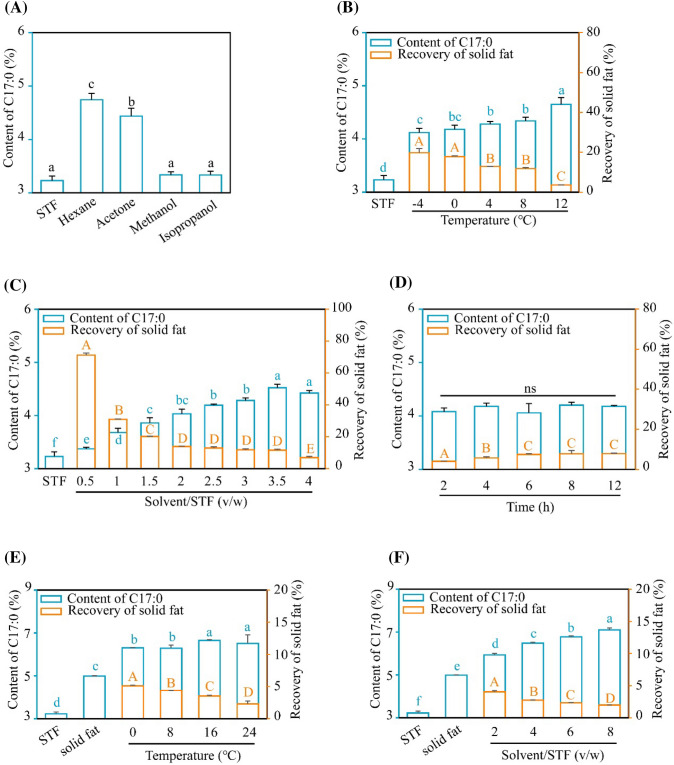
To enrich C17:0, we firstly seek to refine STF through the solvent crystallization method. The type of solvent, the ratio of solvent/STF, temperature, and duration time are key factors affecting the content of C17:0 and the recovery of the concentrate. Five common solvents (hexane, acetone, methanol, isopropanol, and ethanol) were employed here. STF could not be dissolved in ethanol in our experiment. As shown in Fig. 1A, the content of C17:0 was found to be the richest in hexane among the solvents tested. Next, temperatures were screened based on the melting points of STF and hexane. We found that the content of C17:0 is highest at 12 °C, while the recovery is the lowest among the tested temperatures (Fig. 1B). Comprehensively, 4 °C was selected based on the C17:0 content and the recovery. Further, the ratio of solvent/STF was screened from 0.5/1 to 4/1 and our results showed that 3.5/1 was the optimal condition (Fig. 1C). Based on the above conditions, the content of C17:0 was not changed significantly along with the duration time of crystallization from 2 to 12 h and the recovery of solid fat was not altered from 6 h onwards (Fig. 1D). To ensure the sufficiency of the reaction, 8 h was selected here. Collectively, the optimal conditions of the first round of solvent crystallization were set (hexane/STF = 3.5/1, at 4 °C for 8 h). The content of C17:0 and recovery were (4.29 ± 0.01)% and (11.51 ± 0.62)%, respectively.
Fig. 1.
Optimization of enrichment of C17:0 from STF through crystallization solvent. Based on the indicated condition, the impact of solvent (A), temperature (B), the ratio of solvent/STF (C) and duration time (D) was screened. Similarly, the temperature (E) and the ratio of solvent/STF (F) were screened to enrich C17:0 in the secondary round of solvent crystallization. Values in groups with different alphabet were significantly different at p < 0.05. ns: no significance. Uppercase letters were used to present the difference between the values of “Recovery of solid fat”; lowercase letters were harnessed to analysis the difference between the values of “Content of C17:0”
Next, the second round of solvent crystallization was performed. According to the above conditions, the impact of temperature and the ratio of hexane/STF were further investigated. As shown in Fig. 1E, the content of C17:0 was higher when the temperature was set beyond 16℃, while the recovery declined with the going up of the temperature. Meanwhile, we found that the content of C17:0 was the richest (up to 7.10%) under the ratio of hexane/STF = 8/1 at 16 °C (Fig. 1F). However, the recovery of solid fat was only (1.98 ± 0.02)%.
Optimization of urea complexation conditions for the C17:0-rich SFAs
We directly investigated the effect of urea complexation for C17:0 enrichment. The solvent was screened when STF was saponified to FFAs under the setting condition (solvent/urea/FFA = 8/1/1(v/w/w), at 4 °C for 6 h). As shown in Fig. 2A, the content of C17:0 in 80% ethanol is highest among our tested groups. Next, the ratio of 80% ethanol/urea/FFAs was identified to be more optimal at 8/1/1 (v/w/w) (Fig. 2B and C). The optimal temperature and duration time were found to be still 4 °C and 6 h, respectively (Fig. 2D and E). Together, our results showed that the content of C17:0 could reach to (5.92 ± 0.15)% and the recovery could remain at (20.42 ± 0.93)% through the urea complexation method.
Fig. 2.
Optimization of enrichment of C17:0 from STF through urea complexation. Under the setting condition, the solvent (A), the ratio of solvent/urea (B), urea/fatty acid (C), temperature (D), and duration time (E) were examined to enrich C17:0. Values in groups with different alphabet were significantly different at p < 0.05. Upper case letters were used to present the difference between the value of “Recovery of solid fat”; lower case letters were harnessed to analysis the difference between the values of “Content of C17:0”
Enrichment of C17:0-rich SFAs through solvent crystallization and urea complexation
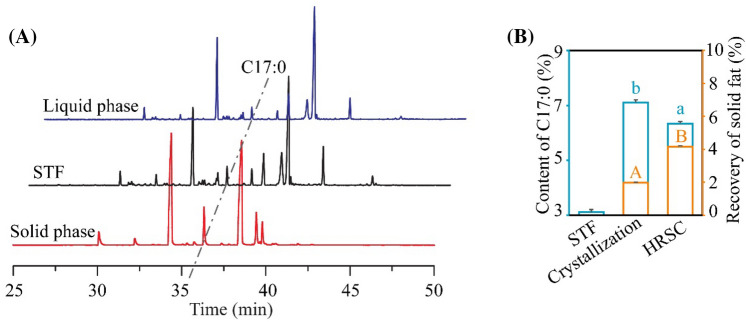
To acquire more HRSC, STF was processed successively by solvent crystallization and urea complexation. Combining the above optimal conditions (hexane/STF = 3.5/1, 4 °C for 8 h, and 80% ethanol/urea/free fatty acids = 8/1/1, 4 °C for 6 h), HRSC from STF was obtained. As shown in Fig. 3A and B, the content of C17:0 reached (6.34 ± 0.07)% and the recovery was about (4.17 ± 0.04)%. Although the content of C17:0 is slightly lower when compared to that in two rounds of solvent crystallization method alone, the recovery of solid fat in the combination method is higher. In Table 1, all the content of SFAs increased, including C16:0, C17:0, and C18:0, while the content of C18:1 and linoleic acid (C18:2) dropped significantly, consistent with our purpose to obtain a concentrate with higher C17:0-rich SFAs, and lower level of C18:1. C17:0 is hard to further separated from C16:0 and C18:0, due to the similar melting points and length of carbon chain. Therefore, sophisticated methods based on the characteristic of C17:0 should be investigated in the future study. For example, the detailed conditions and multiple rounds of solvent crystallization and urea complexation should be further investigated. Meanwhile, the melting point of the C17:0 concentrate in solvent crystallization method is too high to perform antitumor experiments. Therefore, HRSC with above combined methods was selected to perform further antitumor experiments due to its solubility in culture medium when dissolved in DMSO (Li et al., 2018).
Fig. 3.
The C17:0 content and recovery in STF after solvent crystallization and urea complexation. Under the combination of two rounds of solvent crystallization and urea complexation, the C17:0 content was examined with GC–MS (A) and compared statistically (B). Solid phase and liquid phase represent the two phases of lipid after centrifugation in the methods. Crystallization: the C17:0 concentrate after two rounds of solvent crystallization. HRSC: the C17:0 concentrate after solvent crystallization and urea complexation. Values in groups with different alphabet were significantly different at p < 0.05. Upper case letters were used to present the difference between the value of “Recovery of solid fat”; lower case letters were harnessed to analysis the difference between the values of “Content of C17:0”
Table 1.
The fatty acid profile of STF and HRSC
| Fatty acids | STF | HRSC |
|---|---|---|
| C14:0 | 1.96 ± 0.02 | – |
| C14:1 | 0.16 ± 0.01 | – |
| C15:0 | 1.38 ± 0.02 | 0.27 ± 0.47 |
| C16:0 | 18.69 ± 0.39 | 41.69 ± 0.38 |
| C16:1 | 2.86 ± 0.22 | 0.33 ± 0.31 |
| C17:0 | 3.12 ± 0.09 | 6.34 ± 0.07 |
| C17:1 | 2.23 ± 0.05 | 0.25 ± 0.21 |
| C18:0 | 9.86 ± 0.04 | 37.31 ± 0.11 |
| C18:1 | 48.86 ± 0.05 | 13.45 ± 0.21 |
| C18:2 | 5.54 ± 0.22 | – |
| C18:3 | 0.05 ± 0.04 | – |
| C19:0 | – | – |
| C20:1 | 0.07 ± 0.06 | – |
| others | 5.25 ± 0.40 | 0.36 ± 0.32 |
| SFA | 35.0 ± 0.29 | 85.62 ± 0.18 |
| MUFA | 54.18 ± 0.27 | 14.02 ± 0.50 |
| PUFA | 5.59 ± 0.26 | – |
“–” not detected; SFA saturated fatty acid; MUFA monounsaturated fatty acid; PUFA polyunsaturated fatty acid
Response surface methodology (RSM) was also applied in our study. According to the rules of Box Behnken experimental design principle, RSM was used to optimize the condition in urea complexation (Tables S1–S3 and Fig. S1). The theoretical predicted content of C17:0 in the product is 6.36%. Relatively, the content in our practical verification was 6.08%, consistent with the predicted content, indicating that the model has a certain degree of reliability. However, more optimal condition with other methods should be explored in the future study.
HRSC inhibits NSCLC cell proliferation through PI3K/Akt signaling pathway
Similar to our previous study, cell growth, colony formation, migration and underlying mechanism experiments were conducted directly. As shown in Fig. 4A, HRSC performed enhanced inhibitory effect against cell growth compared to that of initial STF. Results in colony formation assay also confirmed this comparison (Fig. 4B and C). Further, HRSC exerted enhanced inhibitory effect on cell migration (Fig. 4D and E). The impact of HRSC on PI3K/Akt signaling pathway and the expression of GLUT1 was also investigated. As shown in Fig. 4F, the administration of HRSC could also inhibit the activation of p-Akt, p-S6K and GLUT1. Further, the levels of these proteins were all decreased in response to the addition of fatty acid mixtures containing increased ratios of C17:0 (Fig. 4F and Table S4). These data suggested that HRSC also downregulate PI3K/Akt signaling and the expression of GLUT1. One report indicated that OCSFAs could serve as the inhibitors of HDAC6 to counteract tumor cell growth (Ediriweera et al., 2021). However, the potential of C17:0 was not explored. C17:0 may also function as HDAC inhibitor to suppress NSCLC cell proliferation. Therefore, more potential mechanisms for C17:0 need to be investigated.
Fig. 4.
HRSC inhibits NSCLC cell proliferation through PI3K/Akt signaling pathway. A The effect of HRSC on the proliferation of A549 cells. B The effect of HRSC on the formation of A549 cell clones. 1 g/L of STF, or HRSC was added. C The comparison of colony numbers. D The impact of the HRSC on A549 cell migration. Equal amount of A549 cells were plated overnight. 1 g/L of STF, or HRSC was added. E The comparison of healing ratios. F The effect of HRSC and fatty acid mixture A–C containing increased ratios of C17:0 on the PI3K/Akt signaling pathway and GLUT1 expression. **p < 0.01, ***p < 0.001
As shown in Table 1, the contents of C17:0, C16:0, and C18:0 were both increased in the HRSC. To assess each contribution of the three SFAs in HRSC, fatty acid mixtures containing different ratios were generated. As shown in Fig. S2A, fatty acids mixture did not inhibit A549 cell growth. As described in our previous study, C18:1 performed pro-proliferative effect, counteracting the impact of SFAs (Xu et al., 2022). Therefore, fatty acid mixtures without C18:1 were obtained and showed similar effect with those of STF or HRSC (Figure S2B). To assess the contribution of C17:0, different mixtures with varying ratio of C17:0 were added into cells. As shown in Fig. S2C, the inhibition was increased companied with the growing of C17:0. The change of C16:0 or C18:0 did not alter the trends. Combined with our previous study, these experiments suggested that the observed beneficial biological effects are specifically attributed to the increased C17:0.
HRSC inhibits A549 cell growth in xenograft model
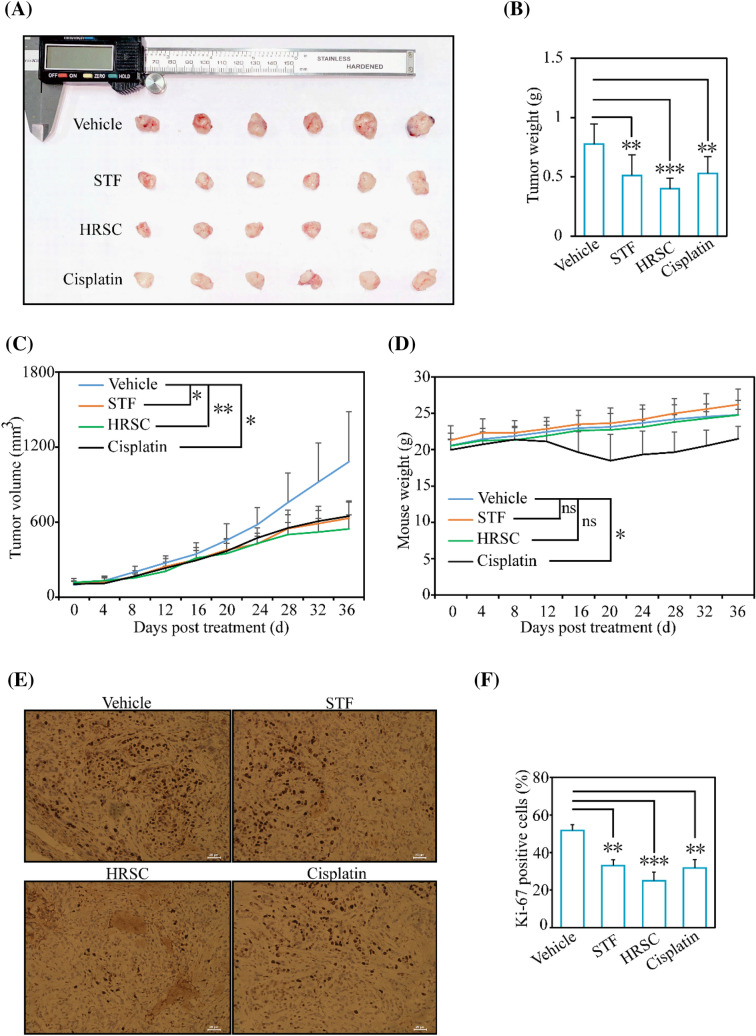
To further assess the function of HRSC in antitumor use, the in vivo experiment was performed. In A549 xenograft mice model, we found that the antiproliferative effect of HRSC group was much more significant than those in STF or cisplatin groups, evidenced by the statistical comparison of tumor volume (p < 0.05 vs p < 0.01) and tumor weight (p < 0.01 vs p < 0.001) (Fig. 5A–C). During the administration, the mouse weight only dropped in cisplatin group (Fig. 5D). Further, the Ki-67 positive cells in HRSC group were much less than those in STF or cisplatin group (p < 0.01 vs p < 0.001) (Fig. 5E and F). Collectively, our in vivo results implied that HRSC exerted enhanced antitumor effect against NSCLC cells. OCSFAs account for less than 0.5% total plasma fatty acids (0.5% total plasma fatty acids is equal to 1.5 μmol/L) (Abdullah et al., 2015; Qian et al., 2022). However, its level could be elevated through the intake of C17:0-rich food. According to the concentration in xenograft model (200 mg/kg) (Xu et al., 2022), 10–15 g of HRSC may be required in normal human body with 50–70 kg weight. This demand seems reasonable. However, the exact quantity needs to be further studied if it could be consumed in reality. Here, 200 mg/kg of STF or HRSC were selected. This concentration is a commonly used dosage for potential lipids in antitumor experiments according to the normalization method of body surface weight area (BSA) (Reagan-Shaw et al., 2008). However, other concentration was not performed and this is indeed a limitation here. HRSC with more concentrations needs to be performed in the future study.
Fig. 5.
Effects of HRSC in NSCLC tumor xenograft model. A The antitumor potential of HRSC was performed in A549 cell xenograft nude mice. The final tumors were isolated and photographed. B–D The final tumor weight (B), tumor volume (C), and mouse weight (D) were determined. E and F The IHC staining in each group was obtained (E) and the Ki-67 positive cells ratio was compared statistically (F). ns, no significance. *p < 0.05, **p < 0.01, ***p < 0.001
In the landscape of NSCLC management, there is a great progress by innovative therapeutic agents and strategies, including immunotherapy and targeted approaches. However, the overall survival outcomes for NSCLC patients remain below expectations, due to the challenges of drug resistance, recurrence and metastasis. Lung cancer cells exhibit a profound metabolic adaptation, optimizing their metabolic and energetic circuits for proliferation in microenvironments marked by nutrient scarcity and hypoxia. These oncogenic cells tailor their carbohydrate, amino acid, and lipid metabolic pathways, ensuring growth and resilience even under a lack or shortage of nutrients. Such metabolic shifts serve dual purposes: facilitating the demands of accelerated cell division and circumventing pro-apoptotic cues. Notably, recent scholarly attention underscores alterations in lipid metabolism as pivotal in the context of lung cancer pathophysiology. C16:0 was reported to be negatively correlated with the development of hepatocellular carcinoma (Lin et al., 2017). Interestingly, one study showed that the intake of ruminant lipid might be involved in the occurrence of lung cancer in China (Ma et al., 2014). In addition, as one of the top five different metabolites differing lung cancer patients from normal group, C17:0 level is much lower in lung cancer patients, implying that the addition of C17:0 could be benefit for NSCLC treatment (Qi et al., 2021).
OCSFAs play a key role and function in preventing a plethora of diseases (Vlaeminck et al., 2006). OCSFAs were reported to be inversely associated with cardiovascular diseases, type 2 diabetes, and cancers (Marcia et al., 2013; Jenkins et al., 2015). Triheptanoin, a triglyceride of heptanoate (C7:0), was also reported to function as a positive auxiliary regulator in treating epilepsy (Borges et al., 2012). We also reported that C17:0 and C17:0-rich STF could dramatically suppress the growth of NSCLC cells in vitro and in vivo (Xu et al., 2019, 2022). Although some reports suggested that C17:0 could be synthesized endogenously (Jenkins et al., 2017; Kornsteiner et al., 2008), the concentration of C17:0 in human body is maintained at relatively low level. Dairy is one type of effective dietary resource containing C17:0, however, a positive correlation was reported between dairy consumption and risks of total and certain cancers, especially hepatocellular carcinoma cells and breast cancers in Chinese adults (Kakkoura et al., 2022) and prostate cancers in north Americans (Orlich et al., 2022). Alternatively, STF is frequently used in food industry and traditional medicine in China to treat various diseases (Kulxax et al., 2011). Therefore, enrichment of C17:0 and other functional SFAs may be a potential approach for optimal adjuvant cancer therapy. Our data shed light on the potential of C17:0-rich HRSC in adjuvant NSCLC treatment through solvent crystallization and urea complexation methods. We believe that the inhibitory effect might be further enhanced by rational increasement of the content of C17:0.
In conclusion, a C17:0-rich SFAs concentrate, termed HRSC, was obtained through solvent crystallization and urea complexation methods from STF. Further, our data revealed that HRSC performed better anti-proliferative effect in vitro and antiproliferative effect against NSCLC cells in vivo, shedding light on its potential application in adjuvant therapy for NSCLC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was funded by the Science and Technology Major Project of Anhui Province, China (No. 202003a06020026), the Innovation and Entrepreneurship Project of High-level Scientific and Technological Talent Teams in Anhui Province, the Natural Science Research Project of Colleges and Universities in Anhui Province, China (KJ2021A0077), and the College Students’ Innovation and Entrepreneurship Training Project in Anhui University, Anhui, China (S205011002), and Applied Medicine Research Project of Hefei Health Commission, Anhui Province, China (Hwk2022zd001).
Declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could appeared to influence the work reported in this paper.
Research involving human and animal rights
This work followed all institutional and national guidelines regarding the care and use of experimental animals.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiaoqi Yu, Xiaoyi Liu and Yuanli Li have contributed equally to this work and share first authorship.
Contributor Information
Changzhi Xu, Email: changzhixu007@163.com.
Buchang Zhang, Email: zhbc@ahu.edu.cn.
References
- Abdullah MM, Cyr A, Lepine MC, Labonte ME, Couture P, Jones PJ. Recommended dairy product intake modulates circulating fatty acid profile in healthy adults: a multi-centre cross-over study. British Journal of Nutrition. 2015;113:435–444. doi: 10.1017/S0007114514003894. [DOI] [PubMed] [Google Scholar]
- Aguedo M, Hanon E, Danthine S, Paquot M, Lognay G, Thomas A. Enrichment of anhydrous milk fat in polyunsaturated fatty acid residues from linseed and rapeseed oils through enzymatic interesterification. Journal of Agricultural and Food Chemistry. 2008;56:1757–1765. doi: 10.1021/jf0722203. [DOI] [PubMed] [Google Scholar]
- Altuntas AH, Ketenoglu O, Cetinbas S, Erdogdu F, Tekin A. Deacidification of crude hazelnut oil using molecular distillation - Multiobjective optimization for free fatty acids and tocopherol. European Journal of Lipid Science and Technology. 2018;120:1700369. doi: 10.1002/ejlt.201700369. [DOI] [Google Scholar]
- Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. Journal of Experimental Medicine. 2021;1:e20201606. doi: 10.1084/jem.20201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Sonnewald U. Triheptanoin-a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment? Epilepsy Research. 2012;3:239–244. doi: 10.1016/j.eplepsyres.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Huang Y, Yang Z, Wang T, Wang X. Enrichment of palmitoleic acid by a combination of two-step solvent crystallization and molecular distillation. Journal of Oleo Science. 2021;70:599–606. doi: 10.5650/jos.ess20273. [DOI] [PubMed] [Google Scholar]
- Ediriweera MK, To NB, Lim Y, Cho SK. Odd-chain fatty acids as novel histone deacetylase 6 (HDAC6) inhibitors. Biochimie. 2021;186:147–156. doi: 10.1016/j.biochi.2021.04.011. [DOI] [PubMed] [Google Scholar]
- Jenkins B, de Schryver E, Van Veldhoven PP, Koulman A. Peroxisomal 2-hydroxyacyl-coa lyase is involved in endogenous biosynthesis of heptadecanoic acid. Molecules. 2017;10:1718. doi: 10.3390/molecules22101718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (C15:0) and heptadecanoic Acid (C17:0) in health and disease. Molecules. 2015;20:2425–2444. doi: 10.3390/molecules20022425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkoura MG, Du H, Guo Y, Yu C, Yang L, Pei P. Dairy consumption and risks of total and site-specific cancers in Chinese adults: An 11-year prospective study of 0.5 million people. BMC Medicine. 2022;1:134. doi: 10.1186/s12916-022-02330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua B, El-Kurdi B, Patel K, Rood C, Noel P, Crowell M. Adipose saturation reduces lipotoxic systemic inflammation and explains the obesity paradox. Science Advances. 2021;7:eabd6449. doi: 10.1126/sciadv.abd6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulxax R. The application of sheep tail fat in Kazakh medicine. Journal of Medicine & Pharmacy of Chinese Minorities. 2011;9:91–92. [Google Scholar]
- Kornsteiner M, Singer I, Elmadfa I. Very low N-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Annals of Nutrition and Metabolism. 2008;52:37–47. doi: 10.1159/000118629. [DOI] [PubMed] [Google Scholar]
- King JW, Srinivas K. Multiple unit processing using sub- and supercritical fluids. Journal of Supercritical Fluids. 2009;47:598–610. doi: 10.1016/j.supflu.2008.08.010. [DOI] [Google Scholar]
- Lei Q, Ba S, Zhang H, Wei Y, Lee JY, Li T. Enrichment of omega-3 fatty acids in cod liver oil via alternate solvent winterization and enzymatic interesterification. Food Chemistry. 2016;199:364–371. doi: 10.1016/j.foodchem.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Li X, Shen Y, Wu G, Qi X, Zhang H, Wang L. Determination of key active components in different edible oils affecting lipid accumulation and reactive oxygen species production in hepg2 cells. Journal of Agricultural and Food Chemistry. 2018;66:11943–11956. doi: 10.1021/acs.jafc.8b04563. [DOI] [PubMed] [Google Scholar]
- Lin L, Ding Y, Wang Y, Wang Z, Yin X, Yan G. Functional lipidomics: palmitic acid impairs hepatocellular carcinoma development by modulating membrane fluidity and glucose metabolism. Hepatology. 2017;66:432–448. doi: 10.1002/hep.29033. [DOI] [PubMed] [Google Scholar]
- Ma X, Ji W, Wang C, Fang X, Zhao F. The clinical and pathological characteristics of primary lung cancer in xinjiang uygur and han patients. Journal of Xinjiang Medical University. 2014;37(3):325–327. [Google Scholar]
- Marcia CO, Nettleton JA, Lemaitre RN, Steffen LM, Kromhout D, Rich SS. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the multi-ethnic study of atherosclerosis. Journal of the American Heart Association. 2013;2:e000092. doi: 10.1161/JAHA.113.000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgal S, Ran-Ressler RR, Liu L, Brenna JT, Rizvi SSH. Branched chain fatty acids concentrate prepared from butter oil via urea adduction. European Journal of Lipid Science and Technology. 2016;118:669–674. doi: 10.1002/ejlt.201500110. [DOI] [Google Scholar]
- Nuskova H, Serebryakova MV, Ferrer-Caelles A, Sachsenheimer T, Luchtenborg C, Miller AK. Stearic acid blunts growth-factor signaling via Oleoylation of Gnai proteins. Nature Communications. 2021;12:4590. doi: 10.1038/s41467-021-24844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlich MJ, Mashchak AD, Jaceldo-Siegl K, Utt JT, Knutsen SF, Sveen LE. Dairy foods, calcium intakes, and risk of incident prostate cancer in adventist health study-2. American Journal of Clinical Nutrition. 2022;2:314–324. doi: 10.1093/ajcn/nqac093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Wu Q, Chen Z, Zhang W, Zhou Y, Mao K. High resolution metabolomic biomarkers for lung cancer diagnosis and prognosis. Scientific Reports. 2021;11(1):11805. doi: 10.1038/s41598-021-91276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Li Y, Feng Y, Wang S, Zhong D. Optimization of the gas chromatography and mass spectrometry method for the determination of 37 fatty acids in human plasma. Physical Testing and Chemical Analysis. 2022;58:5. [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb journal. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Vlaeminck B, Fievez V, Cabrita ARJ, Fonseca AJM, Dewhurst RJ. Factors affecting odd- and branched-chain fatty acids in milk: a review. Animal Feed Science and Technology. 2006;131:389–417. doi: 10.1016/j.anifeedsci.2006.06.017. [DOI] [Google Scholar]
- Wang X, Wang X, Chen Y, Jin W, Jin Q, Wang X. Enrichment of branched chain fatty acids from lanolin via urea complexation for infant formula use. Lwt-Food Science and Technology. 2020;117:108627. doi: 10.1016/j.lwt.2019.108627. [DOI] [Google Scholar]
- Xu C, Wu P, Gao J, Zhang L, Ma T, Ma B. Heptadecanoic acid inhibits cell proliferation in pc9 non-small-cell lung cancer cells with acquired Gefitinib resistance. Oncology Reports. 2019;41:3499–3507. doi: 10.3892/or.2019.7130. [DOI] [PubMed] [Google Scholar]
- Xu C, Zhang L, He H, Liu X, Pei X, Ma T. Sheep tail fat inhibits the proliferation of non-small-cell lung cancer cells in vitro and in vivo. Frontiers in Pharmacology. 2022;13:917513. doi: 10.3389/fphar.2022.917513. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.