Abstract
Poly(lactic acid) (PLA) stands as a compelling alternative to conventional plastic-based packaging, signifying a notable shift toward sustainable material utilization. This comprehensive analysis illuminates the manifold applications of PLA composites within the realm of the food industry, emphasizing its pivotal role in food packaging and preservation. Noteworthy attributes of PLA composites with phenolic active compounds (phenolic acid and aldehyde, terpenes, carotenoid, and so on) include robust antimicrobial and antioxidant properties, significantly enhancing its capability to bolster adherence to stringent food safety standards. The incorporation of microbial and synthetic biopolymers, polysaccharides, oligosaccharides, oils, proteins and peptides to PLA in packaging solutions arises from its inherent non-toxicity and outstanding mechanical as well as thermal resilience. Functioning as a proficient film producer, PLA constructs an ideal preservation environment by merging optical and permeability traits. Esteemed as a pioneer in environmentally mindful packaging, PLA diminishes ecological footprints owing to its innate biodegradability. Primarily, the adoption of PLA extends the shelf life of products and encourages an eco-centric approach, marking a significant stride toward the food industry’s embrace of sustainable packaging methodologies.
Graphical abstract
Keywords: Lactic acid, Polylactic acid, Biopolymer, Food packaging, Food preservation
Introduction
The mounting global concern for the environment is driven by anthropogenic activities leading to the proliferation of pollutants, the accelerated biodiversity loss, and the widespread degradation of ecosystems (Muller et al., 2017). Amongst, the pervasive use of plastics, exemplified by Bakelite’s impact since the early 1900s, marked a milestone in polymer industries, contributing significantly to environmental issues (Sreekumar, 2021). Plastic’s utilization surged since the 1950s due to its energy-saving benefits, becoming ubiquitous in our daily lives due to its adaptability and versatility in various applications (Algozeeb et al., 2022). By 2015, annual plastic production matched the total weight of the global human population, with an estimated 150 million metric tonnes dispersed in the oceans. Concerning projections indicate that by 2050, up to 10% of plastic waste could end up outnumbering marine life in oceanic environments. Forecasts also suggest that over half of the plastic waste generated by 2050 will likely infiltrate landfills, posing significant contamination risks to natural ecosystems such as lakes and seas (Gondal et al., 2023). The utilization of petroleum-based plastics is crucial in modern living and the reports show that plastic production increased from 2 million tonnes in 1950 to 381 million tonnes in 2015, signifying an almost 200-fold rise (Zainab-L and Sudesh, 2019). Many consumer plastics, designed for single use and with limited recyclability, have led to a surge in production and consumption, causing an unprecedented rise in plastic waste and widespread pollution.
Plastics are suitable for use in various consumer and industrial applications because they are affordable, strong, resistant to deterioration, and extremely adaptable. Despite the benefits of plastics made from petroleum, this has raised concerns about widespread pollution (Chia et al., 2020). Plastic packaging pollution poses environmental, animal, and human health risks globally due to poor collection and recycling rates. Managing plastic waste is urgent requirement to overcome these problem around the world (Tumu et al., 2023). The common plastics, primarily made from propylene and ethylene, are non-recyclable, leading to their accumulation in landfills and natural environment. The increasing demand for alternative materials, driven by environmental concerns and a strong emphasis on sustainability, addresses the persistence of non-degradable plastics like thermoplastics.
Sustainable development goal 14 achieves a reduction in plastic pollution and safeguards biodiversity and marine ecosystems through focused waste management, technological advancements, and international collaboration (Zainab-L and Sudesh, 2019). One such innovative solution is biobased thermoplastics which includes polylactic acid, low-density polyethylene, polyvinyl chloride, polypropylene, polyethylene terephthalate, high-density polystyrene, polyethylene, and polystyrene are indeed the thermoplastics commonly used (Shavandi and Ali, 2019). Transitioning thermoplastic materials to a renewable circular economy necessitates detaching from fossil fuels, optimizing recycling, and using biodegradable materials for littered articles. This shift faces challenges in meeting technical standards and managing diverse end-of-life scenarios. A proposed tool, biobased thermoplastic prioritizes material selection based on adaptability and performance, tested on 17 consumer goods and comparing the performance of 21 biobased plastics (Maaskant et al., 2023). They can be produced using biomass feedstocks sourced from renewable resources, including plant sugars, cellulose, vegetable oils, starches, and certain microorganisms. The production of biobased thermoplastics involves converting biomass feedstocks into monomers or polymers through various processes, such as fermentation, chemical synthesis, or polymerization (Lekrine et al., 2022). Unlike conventional plastics derived from fossil fuels, biobased thermoplastics offer a more sustainable and environmentally friendly alternative. These materials provide significant advantages which contributing towards resource conservation and carbon footprint reduction (Zhang et al., 2014).
Also, biobased thermoplastics reduce greenhouse gas emissions by minimizing the reliance on fossil fuels and have the potential to biodegrade under specific conditions, reducing their impact on ecosystems (Uitterhaegen et al., 2018). As self-degradable bioplastics break down into minuscule particles, their escalating release into the environment poses uncertain ecological impacts. Consequently, significant technological advancements are sought for the effective removal and degradation of plastics, including micro and nano-plastics (Kim et al., 2023). Thermoplastics, highly sought after across industries, offer unique advantages. They’re recyclable and reusable, enduring multiple melting cycles without quality loss. With notable strength, chemical resilience, and durability, they ensure reliability. Their adaptability caters to specific needs efficiently, adding significant value to manufacturing (Nguyen et al., 2023). Furthermore, these materials contribute to a circular economy by promoting the use of renewable resources and minimizing plastic waste (Grigore, 2017).
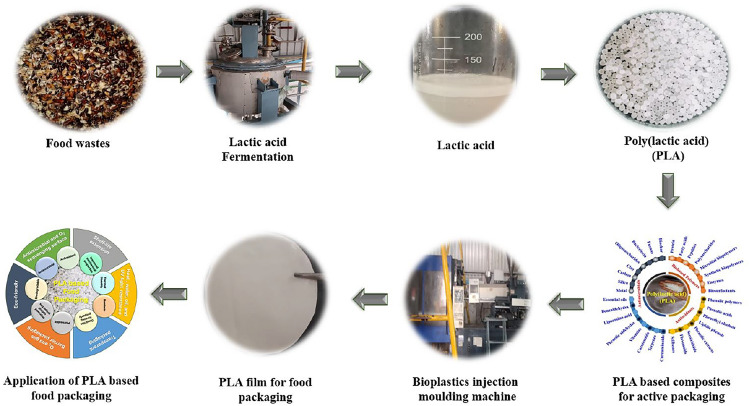
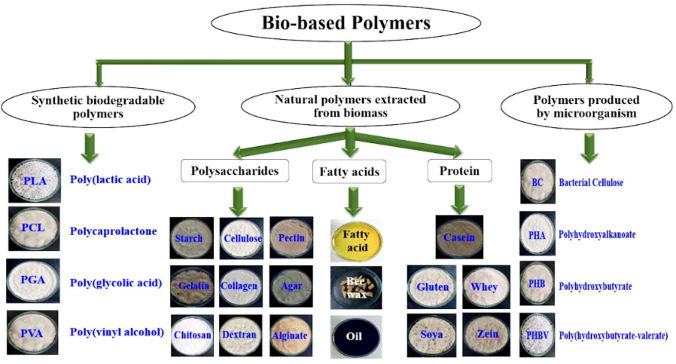
Traditional plastic production uses 65% more energy than bioplastic processing and emits 30–80% higher greenhouse gases. This unsustainable method faces urgent environmental concerns, requiring a rapid shift from conventional plastics to bio-based polymers (Taib et al., 2023). The classification of biobased polymers alternative to conventional polymers were elaborated in Fig. 1. Among bio-based thermoplastics, polylactic acid (PLA), an aliphatic polyester derived from renewable sources such as sugar, corn, and potatoes, garners considerable scientific attention due to its biocompatibility, biodegradability, and compostability within degradability categories (Gay et al., 2018).
Fig. 1.
Classification of various biobased polymers based on biodegradability
Nowadays, PLA finds widespread use in the food industry for packaging materials, disposable utensils, and cups. It is commonly employed in food containers, bakeware, and edible films due to its biodegradability and versatility. PLA’s eco-friendly properties make it a sustainable choice for reducing environmental impact in food applications (Jacob et al., 2020). PLA, is both biocompatible and non-toxic, ensuring its safety for applications involving contact with food (Milovanovic et al., 2022). Petroleum polymers, being affordable, have strong tensile qualities, and serve as an efficient shield against oxygen, CO2, and liquid–vapour, petrochemical polymers are used extensively in the packaging of foods. A wide range of these polymers, including both elastic and stiff forms, have been utilized in packaging, with the terms thermoset plastics or thermoplastics describing these materials. Heating can be used in the processing and recycling of thermoplastics. Since they can be readily molded into numerous forms and are recyclable, this class of polymers is more suited for use in food containers (Jacob et al., 2020).
This review comprehensively addresses the synthesis process of PLA, an environmentally friendly and biodegradable polymer derived from lactic acid. The detailed discourse not only outlines the synthesis methodology but also accentuates the ecological advantages intrinsic to PLA. Moreover, this review underscores the extensive commercial utilization of PLA based composites in food industry, particularly in the food packaging and preservation. As an eco-conscious choice for food packaging, PLA has garnered substantial acclaim owing to its remarkable biocompatibility and inherent ability to biodegrade.
Lactic acid—overview
Lactic acid (LA), derived from fermentable sugars, exhibits dual properties as an alcohol and an acid, characterized by minimal environmental impact. It manifests as the simplest lower hydroxyl carboxylic acid, featuring odorlessness, non-volatility, and solubility in water and compatible organic solvents, while insoluble in pure organic solvents (Castillo Martinez et al., 2013). The optical activity of LA, attributed to its chiral carbon, holds paramount importance in the synthesis of robust polymers such as poly-d-(−)-LA (PDLA) and poly-l-(+)-LA (PLLA) (Wang et al., 2015). The microbial process of lactic fermentation by specific bacterial strains predominantly generates lactic acid as a primary organic byproduct (Wang et al., 2023b).
Derived from fermentation processes involving crops like sugarcane and maize, lactic acid serves as the essential precursor for PLA. This versatile polymer, recognized by the FDA as safe, particularly finds utility in food packaging applications (Li et al., 2015). However, industrial-scale production often results in racemic mixtures, such as DL-LA, leading to the depletion of petroleum reserves and substantial economic costs (Daful et al., 2016). Consequently, current research focuses on bio-based lactic acid production to foster environmentally sustainable technologies (Henczka and Djas, 2016). Challenges persist in acquiring pure lactic acid through downstream processes, hindering the market expansion of PLA in various sectors (Kumar et al., 2020).
Emerging technologies, notably the emulsion liquid membrane separation method, gain traction due to their cost-effectiveness and limited environmental footprint (Liu et al., 2012). The increasing use of LA derivatives, replacing hazardous solvents in industrial applications, forecasts a rising global demand for LA, projected to reach 1970.1 kilo tonnes by 2020 (Abdel-Rahman and Sonomoto, 2016), and 1.8 million metric tons worldwide by 2023. Correspondingly, PLA’s demand is anticipated to surge, potentially displacing polyethylene terephthalate across multiple applications by 2025 (Abdel-Rahman et al., 2013) with a market size of 1.5 billon USD in 2023.
Polylactic acid—overview
Lactide polymerization, involving ring structure opening, produces d-lactide, l-lactide, and dl-lactide, distinguished solely by their optical isomers. Correspondingly, Poly [d-lactide] (PDLA), Poly [l-lactide] (PLLA), and Poly [dl-lactide] designate polymers from d-lactide, l-lactide, and dl-lactide respectively. Although l-lactide and d-lactide vary in steric structures, their chemical traits are comparable (Casalini et al., 2011). While PLLA and PDLA share similarities, PDLLA possesses distinct characteristics, finding use in bone engineering, while PLLA is prevalent in bioengineering. PLA compounds are widely employed in biodegradable packaging (Bose et al., 2013). Despite its inherent poor impact strength and brittleness, PLA can undergo traditional processing methods like blow molding, injection molding, sheet extrusion, and thermoforming, although some applications are limited. PLA’s properties, influenced by constituent isomers, processing factors, annealing duration, and molecular weight, directly affect its crystalline nature and overall characteristics (Madhavan Nampoothiri et al., 2010). PLA’s crystallinity significantly impacts various mechanical properties like toughness, modulus, tensile stress, and melting temperatures (Savioli Lopes et al., 2012).
PLA compounds display solubility in specific solvents like dioxane, chloroform, and acetonitrile, while showing partial solubility in chilled ethanol, benzene, acetone, toluene, and tetrahydrofuran, becoming easily soluble when heated. Notably, PLLA crystals remain insoluble in ethyl acetate, acetone, or tetrahydrofuran (Madhavan Nampoothiri et al., 2010). The breakdown pace of the polymer heavily relies on its interaction with moisture and catalysts. Factors altering responsiveness, including particle size and shape, temperature, humidity, crystalline nature, isomer content, residual lactic acid, molecular weight, water diffusion, and metallic catalyst contaminants, significantly impact the rate of polymer degradation (Auras et al., 2004).
Synthesis polylactic acid
Lactic acid is generated by fermentation or chemical synthesis which is the precussor for the PLA synthesis. Bacterial (heterofermentative and homofermentative) fermentation of carbohydrates produces its two optically active forms, the L ( +) and D (-) stereoisomers. The general procedure calls for the utilization of various species of the Lactobacillus genus, including L. delbrueckii, L. bulgaricus, L. amylophilus, and L. leichmanii, in addition to an ambient temperature of 38 to 42 ºC, a pH of 5.4 to 6.4, and a low oxygen concentration. In most cases, unadulterated l-lactic acid is often preferred during the synthesis of PLA (Mehta et al., 2006).
Polymerization methods include direct condensation, azeotropic solution polycondensation, and lactide-based polymerization. However, achieving high molecular weight via esterification poses challenges for mechanical properties (Sreekumar et al., 2021). Lactide formation, essential for high molecular weight PLA, involves mild conditions and solvent-free water removal, moreover, distillation simplifies lactide purification. Variations in molecular mass globally are achieved by adjusting obtained dimer quality (Payne et al., 2020). Industrially, stannous octoate serves as an effective accelerator in PLA polymerization. Its selection greatly influences polymer characteristics. Stannous octoate, non-toxic and FDA-approved, induces minimal racemization under extreme temperatures (Palak et al., 2022).
The advantages of PLA over petroleum polymers include reduced CO2 emissions. Some argue that PLA usage might result in fewer greenhouse emissions due to maize growth absorbing CO2, balancing the production’s carbon footprint (Jamshidian et al., 2010). Distinct synthesis methods include severe vacuum and high-temperature condensation for moderate molecular weight PLA. Industrial methods utilize transesterification catalysts like lead oxide to yield reasonably high molecular mass PLA (Balla et al., 2021). In summary, PLA’s production methods, which involves lactide formation and polymerization catalysts, significantly impact its molecular weight and properties, contributing to its environmental advantages over petroleum-based polymers.
Industrial applications of polylactic acid
The growing utilization of PLA stems from its cost-effectiveness with production costs at $1.41/kg and a 29.4% internal rate of return, which foster sustainable plastic production (Bressanin et al., 2022). PLA adopts a semicrystalline or amorphous state in its solid form. For amorphous variants, the glass transition temperature (Tg) serves as a pivotal parameter, delineating their upper limit of practical use across various industrial applications. This biodegradable polymer’s renewability and affordability have made it a preferred choice across diverse sectors like food packaging, electronics, transport, and medicine (Mehmood et al., 2023). Polylactic acid is a cornerstone material in the medical sector, particularly in the realm of bioresorbable implants (Lu et al., 2023). It finds extensive use in crafting essential medical implements such as sutures, drug delivery systems, and tissue scaffolds (Santoro et al., 2016). This widespread application owes to PLA’s remarkable biocompatibility and its unique capability to gradually degrade within the body, making it an invaluable material for a spectrum of medical applications (Baweja et al., 2016). Polylactic acid has emerged as a prominent material within the domain of 3D printing due to its inherent biodegradability and straightforward usability (Liu et al., 2019). Its commendable sustainability and adaptability render it an optimal choice for fabricating consumer products, prosthetic devices, prototypes, and an extensive spectrum of manufacturing applications (Liu et al., 2019). With its commendable environmental credentials, PLA stands as a pivotal element in reshaping food packaging. Derived from renewable sources and boasting inherent biodegradability, it’s becoming a prime substitute for traditional plastics.
Across various packaging applications, PLA seamlessly supplants conventional plastics, finding utility in fabricating films, cups, containers, and an array of food packaging materials. Its adaptability caters to the multifaceted packaging needs of the food industry, encompassing both rigid and flexible packaging solutions (Eissenberger et al., 2023). Notably, PLA exhibits the potential for compostability under specific conditions, presenting an environmentally favorable avenue for end-of-life disposal. This characteristic harmonizes with the global thrust toward sustainable packaging practices, offering an eco-conscious approach to managing packaging waste. Consequently, PLA embodies a significant stride toward fostering sustainability within the realm of food packaging (Chen et al., 2016). With a temperature range of 190 to 250 °C, PLA’s semicrystalline forms showcase critical thermal parameters such as Tg at 58 °C and a melting temperature (Tm) spanning from 130 to 230 °C based on its morphological aspects. The interplay of optical composition, fundamental structure, thermal properties, and molecular weight significantly influences the distinct phases of PLA, particularly impacting the Tg and Tm values. Above the Tg, amorphous PLAs exhibit viscous behaviour transitioning from a glassy to a rubbery state. Conversely, when cooled towards its transition point of approximately − 45 °C, PLA assumes a glassy state below the Tg, displaying a tendency to undergo creep. Only under temperatures below this threshold does PLA behave as a rigid polymer (Farah et al., 2016). The capacity of PLA to transition between flexible and rigid states within specific temperature ranges, particularly influenced by its glass transition temperature and subsequent state changes, renders it exceptionally suitable for food packaging. This polymer exhibits the ability to accommodate a diverse range of food types while maintaining structural integrity and minimizing deformation.
Application of PLA in food packaging and preservation
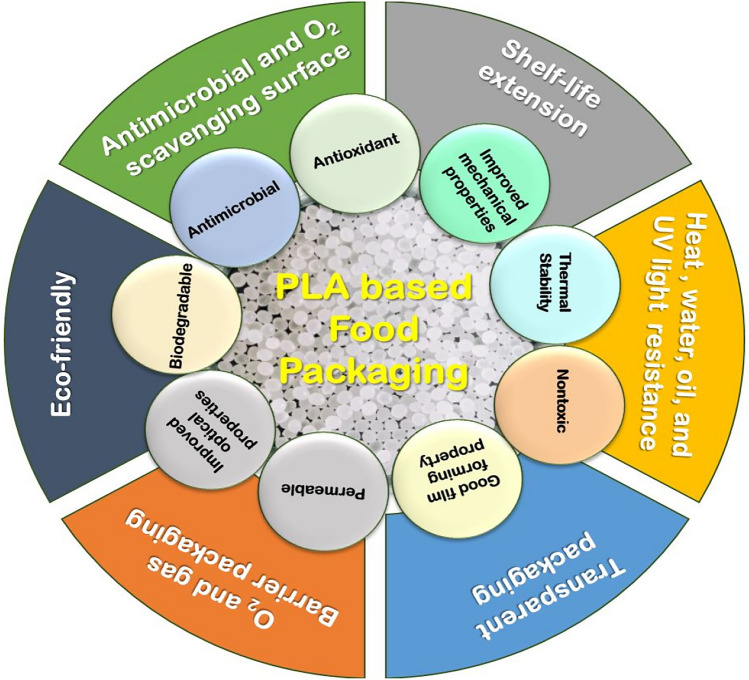
These biopolymers exhibit a significant characteristic potential for application in food packaging due to their ability to enhance the film’s structural integrity, strength, shelf-life extension, water, heat, and UV-light resistant, transparent packaging, O2 and gas barrier packaging and eco-friendly (Abou-alfitooh and El-hoshoudy, 2023) (Fig. 2). Packaging materials, encompassing films, coatings, and composites, are designed with three primary purposes namely, containment, communication, and protection (Farajinejad et al., 2023). Ensuring mechanical strength and thermal properties is crucial for containment, enabling the accommodation of a variety of food products, whether cold, hot, dense, or lightweight. Communication involves the conveyance of essential nutritional information and relevant details to consumers. Protection encompasses withstanding mechanical stresses during transportation and storage, and it also involves improving the water resistance and vapor barrier properties of packaging materials to extend the shelf life of the enclosed products.
Fig. 2.
Characteristics of the polylactic acid based polymeric composites utilized in the application of food packaging
Developing antimicrobial packaging is a compelling challenge in both academic research and industry, crucial for mitigating food spoilage and maintaining food safety. Contaminations during food production pose a significant risk, necessitating effective preventive measures. However, in the realm of PLA packaging, concerns arise regarding potential alterations in color and odor when active ingredients interact with food (Martins et al., 2018).
Assessing active materials for their antioxidant properties revealed dose-dependent benefits compared to inert matrices (Swetha et al., 2023). Although PLA generally lacks inherent antioxidant properties, some experiments suggested minimal activity, likely attributed to residual lactic acid (LA). The chemical structures of natural compounds, such as phenols and terpenes, exhibit a significant correlation with their antioxidant effects as well as antimicrobial nature (Iñiguez-Franco et al., 2012). Phenols, especially those with hydroxyl groups, function as radical scavengers, hindering oxidation by contributing hydrogen or electrons to disrupt the oxidation chain and counteract reactive oxygen species. The antimicrobial effectiveness of terpenes primarily stems from their lipophilic nature, which influences the structure and functionality of microbial cell membranes. This lipophilic character enables terpenes to interact with and affect the integrity of microbial cell membranes, contributing significantly to their antimicrobial properties (Stan et al., 2021). While the efficacy of certain metal oxide nanoparticles stems from their physical structures and metal ion release, many exert bactericidal effects by generating reactive oxygen species.
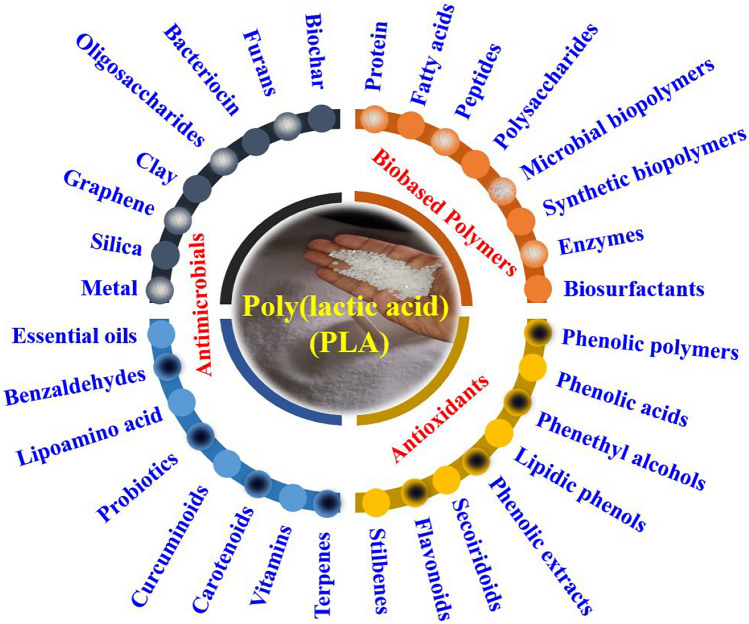
Numerous studies have investigated the use of organic and inorganic (metal) nanoparticles as antibacterial agents against both gram-positive and gram-negative bacteria (Munteanu et al., 2014). The biological traits of these materials hinge on factors like particle size, structure, and surface area, varying with the specific nanoparticles utilized. Plausible combinations are anticipated to exhibit enhanced antibacterial properties (Techawinyutham et al., 2019). Positively charged metal nanoparticles interact with negatively charged bacterial cell walls, disrupting and increasing their permeability. These nanoparticles also prompt the production of reactive oxygen species inside the cells, disrupting bacterial processes and contributing to their demise (Nisar et al., 2019; Sánchez-López et al., 2020). In addition to flavonoids (Martins et al., 2018), antioxidants (Freitas et al., 2024), and metallic nanoparticles (Akshaykranth et al., 2023), specific biopolymers can augment PLA to create films with robust mechanical and tensile strength. Bernini Lombardi and his coworker elaborated the synthesis of PLA composites and its application with natural plant extract in the field of food and biomedical (Lombardi et al., 2022). Polymeric materials activated by natural compounds and extracts, particularly active ingredients sourced from plants, are thoroughly examined in this study. The focus is on the chemical properties of these active compounds and the unconventional extraction technologies applied in activating PLA. The research delves into inventive techniques for seamlessly incorporating active ingredients into PLA, shedding light on characterization processes. Furthermore, the study explores the antioxidant and antimicrobial properties inherent in these PLA composites (Lombardi et al., 2022). In this review, the authors have elaborated the application of PLA with various molecules which includes polysaccharides, oligosaccharides, proteins, peptides, lipids, essential oils, microbial biopolymers, synthetic biopolymer, phenolic compounds, flavonoids, vitamins, carotenoids, terpenes, enzymes, biosurfactants, bacteriocins, lipoamino acids, and organic and inorganic nanomaterials towards the food packaging and preservation application (Table 1).
Table 1.
Preparative techniques and functional properties of polylactic acid based biodegradable polymeric composites for the food packaging applications
| Biodegradable polymers | Source | Additive compound and its concentration | Type of techniques | Methods for determination of specific activity | Application | Remarks | References |
|---|---|---|---|---|---|---|---|
| PLA—polysaccharides composite films | |||||||
| PLA/ethyl lauroyl arginate | Agricultural residue | Cellulose nanocrystals (1%) | Extrusion and electrospinning | Agar well diffusion method | Food packaging of diary and meat products | Zone of inhibition: 3 mm against L. innocua and 2.7 mm against S. enterica | Patiño Vidal et al. (2023) |
| PLA | Agricultural residue | Cellulose (0.3–1.2 g) | Solution casting method | DPPH and ABTS radical scavenging activity | Food packaging | Maximum scavenging rate of 71% by DPPH and 76% by ABTS method | Ren et al. (2023) |
| PLA | Commercial | Cellulose (1–5%) | Injection molding and solution casting method | Mechanical properties | Food packaging | Maximum tensile strength and elongation at break of 61.61 MPa and 9.52%, respectively | Guo et al. (2023) |
| PLA | Commercial | Starch (0.3 g) | Electrospinning | Mechanical properties and colony counting method | Food packaging | E. coli growth inhibition: 1.3 ± 0.1 CFU/mL; L. innocua: 2.5 ± 0.4 CFU/mL; Tensile strength: 30 MPa | Ordoñez et al. (2023) |
| PLA/poly(butylene adipate-co-terephthalate) | Cassava | Starch (40%) | Extrusion | Oxygen and water permeability | Food packaging | Tensile strength (17.5 MPa), elongation at break (295.9%), and water permeability of 8.3 × 103−9.7 × 103 g·mil/day·m2·atm | Katanyoota et al. (2023) |
| PLA/TiO2 | Commercial | Chitosan (3%) | Solution casting | Mechanical properties and disc diffusion method | Antimicrobial packaging | Tensile strength of 35.49 MPa; Zone of inhibition: 10.52 ± 0.63 mm against E. coli, 9.50 ± 0.15 mm against S.auerus | Zhang et al. (2023) |
| PLA | Commercial | Chitosan (3%) | Extrusion | Mechanical properties | Food packaging | Maximum tensile strength and elongation at break of 47 MPa and 11%, respectively | Elsawy et al. (2023) |
| PLA | Commercial | Chitosan (2 g/L) and cyclodextrin (2 g/L) | Solvent precipitation process | Stability and degrdation studies | Food packaging | Completely degraded within 17 days; Controlled release of carvacrol in 14 days | Andrade-Del Olmo et al. (2019) |
| PLA | Commercial | Pectin (4%) | Injection molding | Mechanical and thermal properties | Food packaging | Tensile strength: 59.9 MPa and 33.5% of crystallity | Satsum et al. (2022) |
| PLA | Commercial | Pectin | Electrospinning | Agar diffusion method | Citrus friut packaging | Inhibition rate: 95% against E. coli and S. auerus; Increase the shelf-life of citrus | Min et al. (2022) |
| PLA | Rosin gum | Pentaerythritol ester (1–5 phr) | Twin-screw extruder | Mechanical properties | Food additive and packaging | Tensile strength of 58.4 MPa and elongation break of 6.6%; Also utilized as lubricating agent | de la Rosa-Ramírez et al. (2023) |
| PLA | Commercial | Xanthum gum (10 g) | Solvent casting method | Thermal properties | Food coating and packaging of vegetables and fruits | Water vapour permeability of 10 to 15 g/m2; Withstand up to 120 °C | Abdenour et al. (2023) |
| PLA–protein composites | |||||||
| PLA | Commercial | Fish water-soluble protein (10%) | Twin screw extruder | Mechanical properties and permeability test | Food packaging | Tensile strength of 11.88 ± 1.82 MPa and elongation break of 462.96%; Water vapour permeability: 10.85 × 10–13 g m/m2.s.Pa | Saiwaew et al. (2014) |
| PLA | Commercial | Soy protein (50%) | Solvent casting method | Water vapour permeability and biodegradability | Soft cheese packaging | Water permeability rate: 2.3 × 10–11 g m Pa−1 s−1 m−2; Biodegradability rate: above 60% | González and Alvarez Igarzabal (2013) |
| PLA | Cotton industry | Cottonseed protein (1 g) | Solution casting method | Water vapour permeability and mechanical property | Food packaging | Tensile strength of 52 ± 8 MPa and elongation break of 2%; water vapour permeability: 0.00040 ± 0.00005 g-m/kPa-d-m2 | Biswas et al. (2023) |
| PLA/hydroxypropyl methylcellulose | Commercial | Zein (60%) | Electrospinning | DPPH method and agar diffusion method | Food packaging | Antioxidant activity: 68.83 ± 0.1%; zone of inhibition: 15.4 mm against S. aureus and 13 mm against E. coli | Aman Mohammadi et al. (2021) |
| PLA | Local market | Wheat gluten (10%) | Electrospinning | DPPH method | Edible packaging of solid food | Scavenging activity of 31.5%; Tensile strength of 4.9 MPa and elongation break of 140% | Hajikhani et al. (2020) |
| PLA | Commercial | Gelatin (2 g) | Solvent casting and electrospinning | DPPH and ABTS method | Packaging of sea food and fish oils | DPPH scavenging activity of 56.89 mmol TE/g sample and ABTS scavenging activity of 471.27 mmol TE/g sample | Ponnusamy et al. (2023) |
| PLA–lipid composites | |||||||
| PLA | Syzygium aromaticum | Essential oil (30%) | Solution casting method | DPPH method and Colony couting method | Cheese packaging | Antioxidant activity of 43%; reduce the food spoilage; Susceptibility order was: 98% inhibition of E. coli > 95% inhibition of S. typhimurium > 70% inhibition of L. monocytogenes | Stoleru et al. (2021) |
| PLA | Lippia citriodora/Laurus nobilis | Oil nanoemulsion (12%) | Solvent casting method | DPPH and agar diffusion method | Rainbow trout fillet packaging | Antioxidant activity of 92.66%; zone of inhibition: 19.46 ± 0.02 mm against E. coli and 20.79 ± 0.02 mm against S.aureus | Hojatoleslami et al. (2022) |
| PLA/CA | Commercial | oleic acid (5 g) | Solution casting method | Water vapour permeability | Food packaging |
Water permeability rate: 9.05 × 10–13 g m Pa−1 s−1 m−2; enhancement of fiber–matrix interface adhesion |
Almasi et al. (2015) |
| PLA/CA | Commercial | Cardanol Oil (5–15%) | Melt extrusion | DPPH method | Food packaging | Inhibition rate: 54%; IC50: 6.9 mg/mL | Mele et al. (2019) |
| PLA/PVOH | Commercial | Wax (20%) | Extrusion | Water permeability | Fresh cut vegetables to cheese | Water permeability rate: 120 g mm day−1 bar−1 m−2; | Barbato et al. (2023) |
| PLA–microbial biopolymer composites | |||||||
| PLA | Acetobacter pasterianus | Bacterial cellulose (5–30%) | Solvent casting method | Mechanical properties | Food packaging | Water–vapor barrier by 5 wt%; Tensile strength of 16.6 MPa, elongation at break of 81.5 5.0% | Patwa et al. (2019) |
| PLA | Commercial | Polyhydroxyalkanoates (5%) | Solution casting and electrospinning | Mechanical properties | Food packaging | Tensile strength of 66.4 MPa and elongation at break of 4.3% | Injorhor et al. (2023) |
| PLA | Commercial | Poly(hydroxybutyrate) (25%) | Compression molding | Oxygen transmission rate | Food packaging | Oxygen transmission rate: 11.0 cm3 mm m−2 day−1; Water contact angle: 70° | Arrieta et al. (2014) |
| PLA | Commercial | Polyhydroxybutyrate (75%) | Melt extrusion | Rheological properties | Food packaging | Tensile strength of 20 MPa and elasticity of 3.6 MPa | Aydemir and Gardner (2020) |
| PLA | Commercial | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (25%) | Melt extrusion | Minimum inhibitory concentration | Food packaging | MIC value: 750 mg/L against Escherichia coli and 700 mg/L against Listeria innocua | Hernández-García et al. (2022b) |
| PLA–synthetic biopolymer composites | |||||||
| PLA | Commercial | Polycaprolactone (30%) | Solvent casting method | Agar well diffusion and DPPH method | Biodegradable wrapping of sausage | Antioxidant activity IC50 of 0.251 mg/mL; Zone of inhibition: 12 mm against E. coli and 14 ± 0.02 mm against L. monocytogenes | Shahrampour et al. (2023) |
| PLA | Synthesize from L-lactide and glycolide | Polyglycolic acid (25%) | Melt extrusion | Degradation studies | Food packaging | Maximum degradation observed was 88%; Tensile strength of 52.3 MPa and elongation at break of 3.7% | Samadi et al. (2019) |
| PLA | Commercial | Polyvinyl alcohol (15%) | Electrospinning | FRAP and ABTS | Packaging for food-gatherers and artisanal fishers | Antimicrobial activity was also tested against E. coli; effectiveness with a 29.7% | Arrieta et al. (2018) |
| Phenolic active compounds | |||||||
| PLA–phenolic polymer composites | |||||||
| PLA | Commercial | Lignin (50 mg/mL) | Electrspraying | DPPH and mechanical properties | Food packaging | Antioxidant activity: 60%; Young’s modulus: 4.7 GPa; Elongation at break: 1.7% | Daassi et al. (2023) |
| PLA | Rice husk | Lignin (2.5%) | Solvent casting | DPPH and ABTS method | Food packaging | Inhibition rate by DPPH: 82.2%; Inhibition rate by ABTS: 83.6%; Minimize food oxidation | Fontes et al. (2021) |
| PLA | Commercial | Lignin (3%) | Solution casting method | DPPH | Food packaging | Antioxidant activity of 17.8% | Cavallo et al. (2020) |
| PLA/ethylene–vinyl acetate–glycidyl methacrylate | Commercial | Lignin (8 g) | Melt mixing | DPPH | Food packaging | Antioxidant activity: 81.2%; improvement of toughness and water vapor barrier properties | Yang et al. (2020) |
| PLA | Aesculus hippocastanum L. (seeds) | Procyanidins (0.8%) | Solvent casting | ABTS | Active food packaging | Shown the maximum inhibition rate; Incorporated photostabilizers | Havelt et al. (2021) |
| Phenolic acid composites | |||||||
| PLA | Commercial | 4-Hydrobenzoic acid (4%) | Melt extrusion | DPPH method | Food packaging | Antioxidant activity of 1.94 µmol TE/dm2 | Andrade et al. (2023) |
| PLA/hydroxypropyl-beta-cyclodextrin | Commercial | Gallic acid (5%) | Encapsulation/ Electrospinning | DPPH | Food processing | Antioxidant activity: 95%; increase the shelf life and quality of food | Aytac et al. (2016) |
| PLA | Cassava starch | Protocatechuic acid (2%) | Melt extrusion | Mechanical properties permeability property | Pork and meat packaging | Tensile strength: 8 MPa; elongation at break: 2%; water vapour permeability: 0.57 g mm kPa− 1 h−1 m−2 | Hernández-García et al. (2022a) |
| PLA/AgNPs | Commercial | Rosmarinic acid (8%) | Extrusion | DPPH | Food processing and packaging | Inhibition by antioxidant as 60%; determination of the migrated compounds based on their release at controlled rates | Ramos et al. (2020) |
| Phenethyl alcohols composites | |||||||
| PLA/poly(butylene adipate-co-terephthalate) | Olea europaea L. (by-products) | Hydroxytyrosol (20%) | Encapsulation | DPPH and Trolox | Preserving and packaging of fresh-cut avocado | Prevents oxidation/browning reactions of short shelf-life foods; Increased antioxidant activity from 28.21 to 64.86 mmol Trolox/dm2 | Apicella et al. (2021) |
| PLA | olive leaf extract | Hydroxytyrosol (1–5%) | Solvent evaporation method | DPPH method | Food packaging | Water vapour permeability: 11.01 ± 1.61 g m−2 h−1 | Grabska-Zielińska et al. (2021) |
| Lipidic phenols composites | |||||||
| PLA | Cannabis plant | Cannabidiol (3 phr) | Melt extrusion | Mechanical properties | Food packaging | Tensile strength: 39.4 MPa; elongation at break: 7.1% | Tutek and Masek (2023) |
| Phenolic aldehydes composites | |||||||
| PLA | Commercial | Cinnamaldeyde (5–8%) | Cast sheet extrusion | Colony forming method | Bakery product packaging | Cimmaldehyde reduced the mechanical strength and imorove the antibacterial activity against E. coli | Gonon et al. (2023) |
| PLA/bionanocomposite | Commercial | Cinnamaldeyde and thymol (17%) | Melt-extrution | Antimicrobial viability | Biodegradable food packaging | Antimicrobial activity against E. coli and S. aureus; Microbial community not detected after incorporation of thymol | Villegas et al. (2019) |
| PLA/nanocomposites | Commercial | Cinnamaldeyde and thymol (2.5%) | Impregnation | DPPH | Active food packaging | Exhibit 100% of antioxidant activity; Activation energies of the fast and slow diffusion process were found to be near 48 and 150 kJ/mol, respectively | Siddiqui et al. (2021) |
| PLA | Ziziphora clinopodioides | Thymol (19.22%) | Solvent casting | Antimicrobial viability test | Meat packaging | Shown 7.88 log CFU/g of cell viability of Pseudomonas sp.; extended shelf life; final microbial population decreased approximately 1–3 log CFU/ | Shavisi et al. (2017) |
| PLA | Commercial | Vanillin (10%) | Melt extrusion | Mechanical properties | Food packaging | Tensile strength: 42 MPa; Elongation at break: 4% and impact strength of 17 kg/m2 | Zhao et al. (2018) |
| Secoiridoids composites | |||||||
| PLA | Olea europaea | Oleuropein (3 g) | Solvent casting | Agar diffusion method | Food packaging | Zone of inhibition of 16.20 mm against Staphylococcus aureus. Enhanced water absorption and degradability rates | Özge Erdohan et al. (2013) |
| Flavanoids composites | |||||||
| PLA | Commercial | Quercetin (0.97 g/L) | Solution casting method | ABTS method | Food packaging | Antioxidant activity: 98% | Deng and Zhou (2024) |
| PLA | Abies Nordmanniana | Catechin (0.05–1.0%) | Solvent casting | ABTS | Food packaging | Alternatives to common petrol-based antioxidants and photostabilisers; significant synergistic effects reaching as high as 726% | Havelt et al. (2021) |
| Poly(ester-urethane)/PLA/poly(ɛ-caprolactone) | Commercial | Catechin (5%) | Solvent evaporation | DPPH | Active heat-shrinkable advanced films | Improved water resistance; higheset antioxidant activity of 180 ± 20 mg/kg; decrease surface adhesiveness of 72.2 ± 1.5° of water contact angle | Arrieta et al. (2017) |
| Carboxymethyl cellulose/gelatin/PLA | Commercial | Quercetin (0.08 g) | Solvent casting | DPPH and ABTS | Food packaging | Blocked the UV transmission of the biopolymer films; PLA filmsʼ scavenging abilities increased considerably to 42%, in the ABTS method, and 96.9% in the DPPH method | Ezati and Rhim (2021) |
| Stilbenes composites | |||||||
| PLA | Vitis vinifera L. (cane) | trans-Resveratrol (10%) | Melt mixing | Water barrier property | Food packaging | Improved water vapor barrier property of 50%; Increase the self-life | Díaz-Galindo et al., 2020) |
| PLA | Vitis vinifera L. (cane) | trans-Viniferin (10%) | Melt mixing | Agar well diffusion | Food packaging | Inhibition of mycelium growth: 35.8%; helps in transport and storage of food | Díaz-Galindo et al. (2020) |
| Curcuminoids composites | |||||||
| PLA | Commercial | Curcumin (0–1.2%) | Melt extrusion | Thermal stability studies | Food packaging | Decomposition temperature: 334 °C; Maintaining 89% visible light transmittance | Redfearn and Goddard (2023) |
| PLA | Camelia sinensis | Curcumin (1 g) | Solvent casting | Agar well diffusion | Packaging of tea drink | Increasing of total microbial log was lower (2.2–4.39) than commercial plastic (3.24–5.82) | Hanafi et al. (2018) |
| PLA | Camelia sinensis | Curcumin | Solvent casting | Radical scavenging | Packaging of tea drink | Scavenging activity: IC50 value IC50 = 4.37–16.36% of produced membrane | Hanafi et al. (2017) |
| PLA | Curcuma longa L | Cucurmin (0.5–1.5%) | Solvent casting | DPPH and ABTS | Food packaging for food additives | UV-barrier property; antioxidant activity: 76.6% in DPPH method and 94.7% in ABTS method; Extend the shelflife of foods; antibacterial activity against foodborne pathogenic bacteria E. coli and L. monocytogenes | Roy and Rhim (2020) |
| Terpenes composites | |||||||
| PLA | Rosin gum | Abietic acid (5–15 phr) | Melt extrusion | Mechanical properties | Food packaging | Tensile strength: 65 MPa; elongation at break: 8% and impact strength of 17 kg/m2 | De La Rosa‐Ramírez et al., 2020) |
| PLA/cellulose | Tanacetum balsamita L | β- Bisabolene (2%) and Carvone | Solvent casting | Agar diffusion method | vacuum-packed foods.-cooked sausages | Extended shelf life period vacuum-packed foods; zone of inhibition: 26.85 mm for E. coli, 29.71 mm for B. cereus | Khodayari et al. (2019) |
| PLA/PHB | Commercial | Carvacrol (15 and 20%) | Extrusion | DPPH | Food packaging | Inhibition rate: 64%; ensure the safety in minimally processed foods | Burgos et al. (2017) |
| PLA/PBS | Alpinia officinarum | Eucalyptol (6%) | Cast extrusion | Mechanical and permeability properties | Food packaging | Decreasing tensile strength upto 22% and water and air permeability increased to 42% and 29%, respectively | Wongphan et al. (2023) |
| PLA | Commercial | Eugenol (5%) | Solvent casting method | Agar well diffusion | Food packaging | Zone of inhibition: 3.6 mm against E. coli and 6.5 mm against S.aureus | Yu et al. (2023) |
| PLA | Commercial | Geranial (20%) | Solvent casting method | Mechanical properties | Food packaging | Tensile strength: 16.6 MPa; Elongation at break: 230.7% | Gomez-Caturla et al. (2023) |
| PLA | Commercial | Limonene (5–20%) | Solution casting method | DPPH method | Fruit packaging | Maximum antioxidant activity reaching upto 70% | Bayer et al. (2023) |
| PLA | Commercial | Linalool (334 µL) | Solvent casting method | Agar diffusion method | Fresh food packaging | Zone of inhibition: 60 mm against E. coli and 32 mm against Salmonella | Silva et al. (2021) |
| PLA | Commercial | Myrcene (1.5%) | Screw extrusion | Mechanical properties | Food packaging | Tensile strength: 1.7 MPa; elongation at break: 30.2% | Brüster et al. (2019) |
| PLA | Cymbopogon citratus | Neral (1.21 m/m) | Encapsulation | Minimum inhibition concentration assay | Antimicrobial activity against Colletotrichum acutatum and Colletotrichum gloeosporioides | Reducing the development of bitter rot lesions in the longterm; MIC dosage of 0.1% (v/v) for Colletotrichum acutatum and Colletotrichum gloeosporioides | Antonioli et al. (2020) |
| PLA | Salvia sclarea | Terpineol (10%) | Electrospinning | Agar diffusion method | Smart food packaging | Bacteria inactivation efficiency of 76 and 100% against E. coli and S. epidermidis, respectively; antibacterial, anti-inflammatory or anti-oxidant properties | Wang and Mele (2018) |
| PLA | Commercial | Thymol (6% and 8%) | Extrusion | DPPH | Food packaging and food additives | Reduced the cell viability of E. coli and Staphylococcus aureus by around 40% after 24 h of storage; shelf-life extension; antioxidant activity: 60% inhibition | Ramos et al. (2020) |
| PLA/cellulose | Tanacetum balsamita | β-Thujone (2%) | Solvent casting | Agar diffusion method | Vacuum-packed foods-cooked sauages | Extended shelf life period vacuum-packed foods; zone of inhibition: 26.85 mm for E. coli, 29.71 mm for B. cereus | Khodayari et al. (2019) |
| Carotenoids composites | |||||||
| PLA | Tagetes erecta L. (flowers) | Astaxanthin (2%) | Extrusion | Water vapour permeability | Food packaging | 21% reduction of water vapor permeability; slower release of antioxidant | Samsudin et al. (2014) |
| PLA/TiO2 | Solanum lycopersicum L. (pulp) | Lycopene (5.5%) | Solvent casting | DPPH | Packaging of oxidation-sensitive food products | Maximum scavenging activity of 50%; enhanced biodegradable | Asadi and Pirsa (2020) |
| PLA/TiO2 | Commercial | Lycopene (3%) | Solvent casting method | DPPH | Packaging of margarine | Improved the mechanical properties; maximum antioxidant activity of 98.14% | Pirsa and Asadi (2021) |
| PLA/PHB | Commercial | Lutein (0.9%) | Extrusion | Mechanical properties | Food packaging | Tensile strength: 32.3 MPa; elongation at break: 3.3% | Latos-Brozio and Masek (2020) |
| Vitamins composites | |||||||
| PLA/PVA | Commercial | a-Tocopherol (3.5%) | Polymer extrusion technologies | Commercial | Food additive | Oxygen scavenging rate: with and without coated a-Tocopherol of 0.69 and 0.63 mL O2/gAT, respectively | Scarfato et al. (2017) |
| PLA | Commercial | Vitamin E (α-tocopherol) (5%) | Electrospinning | DPPH | Packaging of fruits and juices | Improved antioxidant activity of 94% was achieved by this membrane | Munteanu et al. (2014) |
| PLA | Commercial | Cholecalciferol (1–10%) | Solvent casting method | Agar well diffusion | Active food packaging | Showed effective antibacterial activity against food borne bacteria (S. aureus and E. coli) | Lawal et al. (2023) |
| Active compounds | |||||||
| Enzymes composites | |||||||
| PLA/chitosan | Commercial | Lysozyme (0.5 g) | Solution casting method | Minimum inhibitory concentration | Antibacterial food packaging | MIC value: 1.25 mg/mL against S. auerus and 2.5 mg/mL against E. coli | Hao et al. (2022) |
| PLA | Commercial | Proteinase k | Solution casting method | Water vapour transmission rate and biodegradable test | Food packaging | Water vapour transmission rate: 160 g/m2/d; utilized as plasticizing agents | Oliver-Ortega et al. (2021) |
| PLA/polyurethane | Bacillus ligniniphilus | Laccase (471.2 U/g) | Fused deposition method | Biodegradability rate | Food packaging | Increased the elastic modulus to 2.5 fold; 15% biodegradability rate after 6 month | Murillo-Morales et al. (2023) |
| Biosurfactants composites | |||||||
| PLA | Starmerella bombicola | Sophrolipid (5–20%) | Solvent casting method | Colony counting method | Food packaging |
Inhibition rate: more than 50%; water permeability rate: 4.99 × 10–11 g m Pa−1 s−1 m−2 |
Silveira et al. (2020) |
| Lipoaminoacids composites | |||||||
| PLA | Commercial | Lauric arginine (0.5–2%) | Electrospinning | Agar diffusion method | Fresh strawberry preservation | Extend the shelf life of strawberry | Li et al. (2021) |
| Oligosaccharides composites | |||||||
| PLA/CA/TiO2 | Commercial | β-cyclodextrin | Solution casting method | Colony counting method | Active food packaging | Inhibition rate: 25% against E. coli; Enhance thermo and photo degradation | Goñi-Ciaurriz and Vélaz (2022) |
| Bacteriocins composites | |||||||
| PLA/Silica nanoparticles | Commercial | Nisin (1–3%) | Solvent casting method | Permeability properties | Food packaging | Water vapour permeability decreased by 9.64% and oxygen permeability decreased by 35.19% | Jiang et al. (2023) |
| PLA/PEG | Lactococcus lactis | Nisin (2.5%) | Solvent casting method | Agar diffusion method | Food packaging | Limit of detection: 50 ng mL−1; Antibacterial reduced 25% after exposure 160 °C | Holcapkova et al. (2018a) |
| PLA/PEG | Commercial | Nisin (0.15%) | Solvent casting method | Agar diffusion method | Food packaging | Zone of inhibition: 25.9 mm against M. luteus | Holcapkova et al. (2018b) |
| Peptides composites | |||||||
| PLA/PBAT | Commercial | Natamycin (1%) | Melt extrusion | Colony counting method | Grape packaging | Inhibit the growth of fungi upto 0.42 log cfu g−1. tensile strength: 43.5 MPa; elongation at break: 258.2% | Zheng et al. (2023) |
| Furans composites | |||||||
| PLA/Poly(butylene 2,5-furan dicarboxylate) | Commercial | 2,5-Dimethyl Furandicarboxylate (0.75 mol) | Injection molding | Mechanical and thermal property | Food packaging | Thermal decomposition temperature: 368 °C; Young modulus: 412 ± 83 MPa | Long et al. (2017) |
| PLA/poly(pentamethylene 2,5-furanoate) | Commercial | 1,5- Pentanediol and furan-2,5-dicarboxylic acid (1%) | Solution casting method | Mechanical and thermal property | Sustainable Food Packaging | Gas-barrier performance improved upto 30%; Young modulus: 389 ± 44 MPa | Rigotti et al. (2021) |
| Organic/inorganic materials | |||||||
| Metallic nanoparticles | |||||||
| PLA | Commercial | Zinc oxide (1.11%) | Solven casting method | Mechanical properties and permeability test | Food packaging | Water permeability of 46.3%, young modulus of 52.6% and inhibition rate of 68% against E. coli | Liew et al. (2023) |
| PLA | Commercial | Zinc oxide (5%) | Melt extrusion | Colony counting method | Food packaging | Reduction (99.99%) of E. coli was observed; Improved tensile properties and surface free energy | Marra et al. (2016) |
| PLA/PCL | Clove essential oil | Zinc oxide (3%) | Solution casting method | Disc diffusion method | Scrambled egg packaging | Cell viability: 1 to 1.5 log reduction of S. aureus observed | Ahmed et al. (2019) |
| PLA | Commercial | Magnesium oxide (2%) | Solution casting method |
Fluorescence Activated Cell Sorting technique |
Food packaging | Inhibition rate: 46%; 20% increase in water vapour permeability | Swaroop and Shukla (2018) |
| PLA | Commercial | Silver nanoparticles (1 g) | Solvent casting method | Mechanical and permeability properties | Packaging of ground beef | Young modulus: 0.69 MPa; elongation at break: 41.35%; water permeability of 2.76 g mm kPa−1 h−1 m2 | Izadi et al. (2023) |
| PLA | Commercial | Copper nanocomposites (15%) | Melt extrusion | Agar diffusion method | Food packaging | Zone of inhibition: 22 mm against E. coli and 16 mm against P.aeruginsa | Popescu et al. (2023) |
| PLA | Commercial | Selenium dioxide (1.5%) | Extrusion method | – | Food packaging | Water vapour permeability of 77%; Increased antioxidant activity increased by incorporating Se nanoparticle | Zibaei et al. (2023) |
| Silica nanoparticles | |||||||
| PLA | Commercial | Halloysite nanotubes (14.3–25%) | Solvent casting method | – | Food packaging | Optimum performance by 30% of nanocomposites; loading capacity of cinnamaldehyde increased upto 25% | Li et al. (2023) |
| PLA/acetyl tri-n-butyl citrate | Commercial | Silica (0.5–5%) | Melt extrusion | Permeability measurements | Food packaging | Permeability of CO2: 3.56 barrier; permeability of O2: 0.76 barrier | Aragón-Gutierrez et al. (2020) |
| PLA | Commercial | Silica (1–3%) | Casting method | Cell viability test | Food packaging | Cell viability: approximately 5 log CFU/mL of E. coli and 3 log CFU/mL of S. aureus | Lu et al. (2021) |
| PLA/SiO2 | Commercial | Silica (1%) | Electrospinning | Rheological studies | Active packaging | Young modulus: 1.3 MPa; elongation at break: 45% | Cacciotti and Nanni (2016) |
| PLA | Commercial | Organoclay (5%) | supercritical impregnation | Cell viability test | Sustainable food packaging | MIC value: 250 μg/mL for thymol and 157 μg/mL for cinnamaldehyde; Increase the shelf life | Villegas et al. (2019) |
| PLA | Sodium montmorillonite | Nanoclay (40%) | Blown film co-extrusion method | Oxygen permeability and water vapor permeability | Food packaging | Water permeability: 0.192 (g cm)/(m2 day); oxygen permeability: 4.05 ± 0.7 (cm3 cm)/(m2 day atm−1) | Scarfato et al. (2017) |
| Carbon materials | |||||||
| PLA/PBAT | Commercial | Carbon nanotubes | Melt blending | Mechanical properties | Food packaging | Tensile strength: 33.7 MPa; Elongation at break: 341% | Wang et al. (2023a) |
| PLA | Commercial | Amorphous carbon | Solvent casting method | Oxygen and water permeability test | Sustainable food packaging | Oxygen transfer rate: 18.6 ± 3.3 cm3 mm m−2 day−1; water vapour permeability: 0.48 ± 0.12 kg m/s m2 Pa | Mattioli et al. (2013) |
| PLA/PCL | Commercial | Carbon nanotubes (7%) | Solution casting method | Colony counting method | Antibacterial food packaging | Water vapour permeability: 17.9 kg m/s m2 Pa; Colony reduction upto 5.65 log10CFU/mL against S. aureus | Cui et al. (2020) |
| PLA | Waste brewed coffee powder | Biochar (2.5%) | Solvent casting | Rheological studies | Food packaging | PLA/biochar withstand maximum temperature of 166 °C | Arrigo et al. (2020) |
| PLA | Beechwood | Biochar (5%) | Injection molding | Thermal and hygroscopic property studies | Food packaging | Water contact angle: 73°; biochar decreased the water repellence | Zouari et al. (2022) |
PLA polylactic acid, PCL polycaprolactone, CFU colony forming units, CA cellulose acetate, PEG polyethylene glycol, PVA polyvinyl alcohol, PBH polyhydroxybutyrate, DPPH 2,2-diphenyl-1-picrylhydrazyl, ABTS 2,2-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid, FRAP ferric ion reducing antioxidant power, phr parts per hundred
Polylactic acid with biopolymers
Biopolymers such as chitosan (Andrade-Del Olmo et al., 2019), starch (Muller et al., 2017), cellulose derivatives (Khodayari et al., 2019), alginate (Gu et al., 2013), pectin (Satsum et al., 2022), gum (Tripathi et al., 2017) can be combined with PLA to synthesize food packaging film for functional foods, meals, meats and other application. These biopolymers significantly enhance the mechanical strength (Muller et al., 2017), barrier function (Bang and Kim, 2012), and biodegradability of the films (González and Alvarez Igarzabal, 2013), meeting diverse packaging needs effectively. The incorporation of PLA with polysaccharides has been extensively studied and has received significant interest in scientific research. This combination of benefits has found practical use in the production of food packaging films, a subject that has been thoroughly discussed in scholarly literature (Aung et al., 2018; Grujić et al., 2017). Ren et al. (2023) developed the cellulose from agricultural residue incorporated with PLA by solution casting method which exhibited maximum scavenging rate of 71% by DPPH method for the application diary product packaging. Additionally, utilizing PLA with chitosan as polysaccharide instead of cellulose which maximize the tensile strength of 47 MPa and elongation at break of 11% can also be utilized in the food packaging application (Elsawy et al., 2023).
Polylactic acid with proteins and lipids
Utilizing PLA as a biodegradable polymer could involve its application as a matrix or coating material for protein-based packaging which includes fish protein (Ma et al., 2015), wheat gluten (Hajikhani et al., 2020), soy protein (Dong et al., 2023), zein (Parlak et al., 2023) and gelatin (Ponnusamy et al., 2023), thereby contributing to the increased sustainability of the packaging material as a whole. Meanwhile, proteins like fish protein and soy protein incorporated with PLA improved the water vapour permeability of 10.85 × 10−13 g m m−2 s Pa. (Saiwaew et al., 2014) and 2.3 × 10–11 g m Pa−1 s−1 m−2, respectively (González and Alvarez Igarzabal, 2013) can be utilized in soft cheese packaging. These composites may involve combining lipids with polymers to create materials with specific properties like biocompatibility, biodegradability, flexibility and mechanical strength (Balla et al., 2021). In addition to the protein, essential oil also utilized in the food packaging application namely clove oil (Sharma et al., 2023), cardanol oil (Mele et al., 2019), and oleic acid (Almasi et al., 2015). In the study of Sharma et al. (2023), different concentrations (1 wt%, 5 wt%, and 10 wt%) of clove oil were incorporated into polylactic acid (PLA)-poly (butylene adipate)-terephthalate (PBAT) films to modify the antibacterial properties of the biodegradable films. The research assessed the inhibitory effects of these composite films on Escherichia coli and Staphylococcus aureus, revealing that clove oil exhibited superior antibacterial activity compared to the other essential oil tested. Specifically, for S. aureus, the addition of 10 wt% clove oil resulted in the most effective inhibition of growth (Sharma et al., 2023).
Polylactic acid with microbial and synthetic biopolymers
Biopolymers produced by microorganism are garnering attention for their potential use in eco-friendly food packaging applications. Nowadays, microbial biopolymers including bacterial cellulose (Patwa et al., 2019), polyhydroxyalkanotes (Injorhor et al., 2023), polyhydroxybutyrate (Aydemir and Gardner, 2020) and poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) (Hernández-García et al., 2022b) were incorporated with PLA and utilized in the food packaging application. The bacterial cellulose extracted from Acetobacter pasterianus blended with PLA which helps in the water vapour barrier by 5 wt% with tensile strength of 16.6 MPa (Patwa et al., 2019). Similarly, polyhydroxybutyrate with PLA exhibited the tensile strength of 20 MPa with elasticity of 3.6 MPa, which can be suitable for food packaging application (Aydemir and Gardner, 2020). Moreover, polylactic acid with synthetic polymer also utilized in food packaging application. One of the synthetic polymers such as polycaprolactone (PCL) with PLA films underwent supercritical CO2 impregnation to integrate thymol, resulting in enhanced antibacterial properties while maintaining a robust structure and excellent thermal stability. These films were assessed against E. coli and B. subtilis, demonstrating substantial inhibition. Remarkably, the film containing 35.8 wt% thymol exhibited exceptional antibacterial efficacy, completely eradicating bacterial cell viability (Milovanovic et al., 2018).
Polylactic acid with phenolic active compounds
Polylactic acid is devoid of toxicity and irritation, to enhance the antibacterial attributes of PLA matrix composites, the incorporation of phenolic active compounds becomes necessary. Active food packaging entails advanced packaging systems surpassing conventional passive containment and protective roles. Specifically engineered to actively engage with the food product or its surroundings, these packaging materials offer additional functionalities to boost food safety, prolong shelf life, and enhance the overall quality of the product. This approach not only preserves the degradability of PLA for environmental sustainability but also effectively impedes microbial growth in food, ensuring the safety of consumables. Phenolic compounds which include phenolic polymer (lignin (Daassi et al., 2023) and procyanidins (Havelt et al., 2021)), phenolic acids (hydrobenzoic acid (Andrade et al., 2023), gallic acid (Aytac et al., 2016), protocatechuic acid (Hernández-García et al., 2022a) and rosemarinic acid (Ramos et al., 2020)), phenethyl alcohol (Grabska-Zielińska et al., 2021), lipidic phenol (Gonon et al., 2023), phenolic aldehydes (Melesse et al., 2022), secoiridoids (Özge Erdohan et al., 2013), flavonoids (Ezati and Rhim, 2021), stilbenes (Díaz-Galindo et al., 2020), curcuminoids (Redfearn and Goddard, 2023), terpenes (Gomez-Caturla et al., 2023), carotenoids (Latos-Brozio and Masek, 2020) and vitamins (Aytac et al., 2017) are added into PLA to prepare the active packaging films for the enhancement of antioxidant and antibacterial activity for proper food preservation.
Lignin is one of phenolic polymers, complex organic material found in the cell walls of plants, providing structural support and rigidity (Shu et al., 2021). Lignin exhibits the capability to interact with numerous polymers, thereby altering their wettability, and mechanical properties which can be utilized in packaging application. Lignin from rice husk incorporated with PLA through the solvent casting method which improved the inhibition rate by DPPH and ABTS by 82.2% and 83.6%, respectively in food packaging (Fontes et al., 2021). Similarly, procyanidins with PLA showed the maximum inhibitory rate of ABTS and also utilized as photo stabilizers (Havelt et al., 2021).
Another phenolic active compounds of phenolic acid such as 4-hydrobenzoic acid, gallic acid, protocatechuic acid and rosmarinic acid are some of types of phenolic acid from the plant utilized with PLA for the active food packaging. For example, gallic acid incorporated into PLA with hydroxypropyl-beta-cyclodextrin increased the antioxidant activity of 95% and also improved the shelf life and quality of food (Aytac et al., 2017). While rosmarinic acid improved the antioxidant activity by 60% while blended with PLA and silver nanoparticles (Ramos et al., 2020). Likewise, phenethyl alcohol composites such as hydroxytyrosol increased the water vapour permeability of 11.01 ± 1.61 g m−2 h−1 for the packaging of food (Grabska-Zielińska et al., 2021).
In this context, lipidic phenols represents a category of phenolic active compound which harness the antioxidant capabilities of hydroxyl groups while incorporating conjugated double bonds along the aliphatic chain. This combination has the potential to actively scavenge free radicals. Cannabidiol is one of the lipidic phenols which improves the tensile strength by 39.4 MPa and 7.1% of elongation for the active food packaging (Tutek and Masek, 2023). Cinnamaldehyde, a phenolic aldehyde that gives cinnamon its distinct flavor and aroma (Ribeiro-Santos et al., 2017). Apart from its pleasant scent and taste, cinnamaldehyde has been studied for its potential benefits, including antioxidant and antimicrobial properties. Honorine Gonon and coworkers developed the bakery packaging material with cinnamaldehyde which reduced the mechanical strength of the material and enhanced the antibacterial activity against E. coli (Gonon et al., 2023). Another phenolic aldehyde of thymol with PLA utilized in the packaging of meat which improved the shelf life of the product (Shavisi et al., 2017). Secoridoids, such as oleuropein, in composites with PLA showed the zone of inhibition of 16.20 mm against S. aureus (Erdohan et al., 2013).
Flavonoids are a diverse group of polyphenolic compounds widely distributed in the plant kingdom, known for their antioxidant and antimicrobial properties (Mutha et al., 2021). They play a crucial role in various plant processes, such as pigmentation, UV filtration, and defence against pathogens. Flavonoids compounds such as catechin, quercetin blended with PLA which eventually improved the antioxidant activity in the active food packaging. Deng and Zhou (2024) demonstrated the quercetin incorporated with PLA through solution casting method improved the antioxidant activity of 98%. Flavonoids such as quercetin subjected to PLA with carboxymethyl cellulose for food packaging which increased 42% of antioxidant activity (Radusin et al., 2019). Another important phenolic active compounds is stilbenes, trans resveratrol and trans-viniferin with PLA utilized in the food packaging which improved the water vapour barrier property of 50% and also inhibit the mycelium growth of 35.8% (Díaz-Galindo et al., 2020).
Curcumin, commonly known as turmeric, is a type of polyphenol characterized by its chemical structure. The orange-yellow, water-insoluble crystal is derived from the rhizomes of plants belonging to the Zingiberaceae family, making it a natural pigment (Bakar et al., 2023). The study found that curcumin was incorporated into PLA to modify its properties for application in tea drink packaging (Hanafi et al., 2017). The customized PLA exhibited notable characteristics, including an antioxidant activity with an IC50 value of 4.37% and a reduced total microbial growth, as evidenced by a logarithmic value of 2.2. These attributes collectively suggest that the tailored PLA formulation surpasses its commercial counterparts in terms of performance and suitability for packaging tea drinks (Hanafi et al., 2017).
Furthermore, terpenes are a diverse class of organic compounds found in various plants and certain insects. They are characterized by their repeating units of isoprene, a five-carbon building block. Terpenes contribute to the distinct aromas and flavors of many plants, including fruits, flowers, and herbs (Tetali, 2019). PLA materials incorporating terpenes as antimicrobial agents find utility in the food packaging sectors. The antimicrobial effects of terpenes make them valuable in these applications. However, a critical consideration arises from the potential toxicity of certain terpenes and their ability to impact the organoleptic properties of food (Masyita et al., 2022). Eugenol is one of the terpenes which incorporated with PLA formed the food packaging film which exhibited the zone of inhibition of 3.6 mm against E. coli (Yu et al., 2023). Similarly, bisbolene and carvone exhibited the zone of inhibition of 26.85 mm against E. coli (Khodayari et al., 2019).
Similarly, the carotenoid composites of lycopene improved the antioxidant activity of 98.14% blended with PLA/TiO2 (Pirsa and Asadi, 2021). Vitamin E, including tocopherol, plays a crucial role in protecting cells from oxidative damage caused by free radicals. Its antioxidant properties and is often used in the food and cosmetic industries to extend the shelf life of products by preventing the oxidation of fats and oils. Scarfato et al. (2017) developed the membrane with PLA/PVA with additive tocopherol helps to improve the oxygen scavenging rate by 0.63 mL O2/gAT (Fig. 3).
Fig. 3.
Incorporating various compounds including antioxidant, antimicrobial and other biobased polymer into polylactic acid for the food packaging applications
Polylactic acid with active compounds
Various active compounds, including enzymes(Zhang et al., 2021), biosurfactants (Silveira et al., 2020), lipoamino acids (Li et al., 2021), oligosaccharides (Goñi-Ciaurriz and Vélaz, 2022), bacteriocins (Holcapkova et al., 2018a), peptides (Santos et al., 2018), and furans (Rigotti et al., 2021), have been extensively employed in conjunction with PLA for food packaging applications. Among these compounds in active packaging, the utilization of biosurfactants, specifically sophorolipids, has garnered significant attention for its widespread application in controlling foodborne pathogens (Cho et al., 2022; Hipólito et al., 2021; Silveira et al., 2020). In this context, PLA has been combined with sophorolipids to fabricate antimicrobial film packaging, which was prepared using the casting method. The observed films displayed robust antimicrobial efficacy against prevalent poultry pathogens such as Listeria monocytogenes, Staphylococcus aureus, and Salmonella spp. Incorporating sophorolipid into these films enhances thermomechanical properties and also acts as a natural antimicrobial agent. This multifunctional characteristic showcases potential of sophorolipid for effectively controlling foodborne pathogens in the food industry (Silveira et al., 2020). Besides sophorolipid, nisin has been integrated into biodegradable polymers for its role as a carrier of antimicrobial compounds in active food packaging applications to inhibit the growth of spoilage and pathogenic microorganisms, thus extending the shelf life of packaged foods. Its incorporation serves as an active barrier against microbial contamination, thereby ensuring food safety and preservation (Imran et al., 2014; Santos et al., 2023).
In a study, blending nisin with PLA facilitated the synthesis of active film packaging targeting foodborne pathogens, specifically aimed at preserving orange juice. This integration resulted in a substantial reduction in bacterial count, decreasing from an initial 6 log CFU/mL to 4.5 log CFU/mL (Jin and Zhang, 2008). In tandem with biosurfactants and bacteriocins, peptide composites namely, natamycin stands as a prominent constituent are integral to active food packaging. Renowned for its robust antifungal properties, natamycin effectively curbs mold proliferation, thereby significantly prolonging the shelf life of packaged food products (González and Alvarez Igarzabal, 2013; Santonicola et al., 2017).
Lantano et al. (2014) investigates the efficacy of a novel active packaging employing a hybrid organic–inorganic coating endowed with antimicrobial properties. The packaging system integrates PLA and natamycin as active agents. Through rigorous testing against mold growth on cheese surfaces, the coatings demonstrated substantial potential. The findings validate the viability of this film as an effective deterrent against microbial proliferation, specifically on cheese substrates (Lantano et al., 2014). In addition to the aforementioned active compounds, lipoamino acid—PLA composites have also been incorporated into the food packaging. These composites play a pivotal role in enhancing the overall efficacy and performance of the packaging, contributing to its antimicrobial properties and further augmenting its potential in preserving food quality and safety (Li et al., 2021; Ma et al., 2020). A novel environmentally friendly packaging with inherent antibacterial properties has been devised through the application of lauric arginate (LAE) coating on PLA films. The mechanical characteristics of the resulting film closely resemble those of neat PLA film, while exhibiting notable antibacterial efficacy against Listeria monocytogenes and Salmonella enterica. Particularly suitable for use as food-contact antimicrobial packaging for cooked cured ham, the film demonstrates heightened antibacterial activity corresponding to increased LAE coating levels (Theinsathid et al., 2012). Enzymes and furan compounds have been combined with PLA to craft advanced food packaging films. This innovative blend aims to enhance preservation capabilities, representing a progressive step in creating packaging that actively safeguards food quality (Bianchi et al., 2023; Fredi et al., 2022; Min et al., 2022).
Polylactic acid with organic and inorganic materials
The incorporation of metallic and inorganic composites into polymers represents a burgeoning trend aimed at augmenting polymer characteristics. This innovation has fostered the creation of polymer-metallic composites that exhibit heightened antimicrobial (Hao et al., 2022), UV-blocking, and antioxidant attributes (Cavallo et al., 2020; Hu et al., 2023). Nonetheless, apprehensions regarding toxicity and environmental repercussions have surfaced, prompting the need for a thorough assessment of their effectiveness, safety, and sustainability in the realm of food packaging (Mulla et al., 2021; Videira-Quintela et al., 2021).
Metallic nanoparticles such as titanium dioxide (Zheng et al., 2023), zinc oxide (Liew et al., 2023), magnesium oxide (Swaroop and Shukla, 2018), silver (Fortunati et al., 2013), copper (Popescu et al., 2023), and selenium (Zibaei et al., 2023) have been employed in food packaging for various purposes like UV protection, antimicrobial properties, and antioxidation. The research delineates the synthesis of PLA and magnesium oxide nanocomposite for food packaging films via an industrial melt-processing setup. Findings underscore notable enhancements in multiple facets such as heightened tensile strength, increased plasticity, augmented oxygen and water vapor barrier capabilities, and demonstrated antimicrobial efficacy against E. coli (Swaroop and Shukla, 2019). Fortunati et al., developed a high-performance nano-biocomposites, combining PLA with pristine cellulose nanocrystals (CNC) and surfactant-modified cellulose nanocrystals (s-CNC) containing silver (Ag) nanoparticles specifically crafted for superior film production. These films, designed for active food packaging applications, integrate PLA with cellulose nanostructures, offering enhanced performance and functionalities for food preservation (Fortunati et al., 2013).
In another study, composite films were engineered by combining PLA with zinc oxide nanoparticles, demonstrating UV-light barrier capabilities alongside improved thickness, tensile strength, and water vapor resistance upon nanoparticle integration. Furthermore, these films exhibited antibacterial efficacy against foodborne pathogens, specifically Escherichia coli and Listeria monocytogenes. Employed in the packaging of minced fish paste, these films effectively shielded against bacterial contamination, showcasing promising protective attributes for food preservation (Shankar et al., 2018).
Silica, specifically halloysite and clay, is crucial in modern food packaging due to its protective properties. These materials offer barrier qualities against moisture, oxygen, and gases, ensuring food quality. They also enhance the mechanical strength of packaging, ensuring product integrity during handling. Halloysite has antimicrobial properties, shelf life extension of perishable items. These nanomaterials enable intelligent packaging solutions, detecting temperature, gas composition, and freshness variations. They are also environmentally friendly, contributing to the search for biodegradable and eco-conscious packaging (Aragón-Gutierrez et al., 2020; Scarfato et al., 2017; Villegas et al., 2019).
In this context, halloysite nanotubes, a unique hollow tubular structure, significantly improve packaging performance in food packaging. They serve as ethylene scavengers, antimicrobial carriers, and fillers, enhancing shelf life, preventing microbial growth, and enhancing mechanical strength and durability (Boro et al., 2022; Deshmukh et al., 2023). In this regard, Risyon et al. (2020) demonstrates the potential of PLA/ halloysite nanotubes films in extending the shelf life of packaged cherry tomatoes, demonstrating a significant advancement in biopolymers and their potential applications in food packaging (Risyon et al., 2020). In addition to the carbon nanotubes, clay, bio-nanocomposite films can also be utilized in the food packaging. The study elaborated the PLA and nanoclay along with thymol and cinnamaldehyde have distinct properties, which exhibits a potent antibacterial property. All bio-nanocomposites completely disintegrated in compost, showcasing their potential application as compostable flexible active films (Villegas et al., 2019).
When integrated with PLA for food packaging, graphene and carbon compounds fortify the protective attributes of the packaging materials. Enhancing mechanical strength, moisture and oxygen barrier capabilities, and exhibiting antibacterial properties, thereby effectively extend the shelf life of packaged food items, ensuring heightened food safety standards (Arrigo et al., 2020; Goh et al., 2016; Zouari et al., 2022). Yakdoumi et al. (2022) explored the use of polylactic lactic acid blended with multiwalled carbon nanotubes as one of the nanofillers for food packaging with antimicrobial property. The PLA-based modified MWCNTs at 3 wt% loading showed better properties, with a 10% increase in crystallization rate and 7% increase in barrier. The Young modulus and hardness also improved, and the films showed strong antimicrobial and antifungal activity compared to pure PLA (Yakdoumi et al., 2022).
The sandwich-structured PLA–graphene composite film, which uses commercial PLA films as an external protective layer and impermeable reduced graphene oxide as an inner barrier, reduces water vapor permeability by 87.6%. Simulations show that this film can extend the shelf life of potato chips and edible oil by at least eightfold, making it a promising solution in food packaging (Goh et al., 2016). Arfat developed antimicrobial nano packaging films by incorporating clove essential oil and graphene oxide nanosheets into PLA for food packaging. The addition of GO enhanced the film’s optical and anti-UV properties. The composite film showed excellent antibacterial activity against Staphylococcus aureus and Escherichia coli, making it a potential food safety and preservation material (Arfat et al., 2018).
Future prospectives
Poly(lactic acid) (PLA) has been extensively utilized in active food packaging primarily due to its distinctive and commendable properties. However, PLA lauded for its ecological merits, confronts notable challenges such as suboptimal moisture and gas resistance, constrained heat resilience, and elevated production costs. Its inherent brittleness necessitates strategic treatment for enhanced mechanical performance, frequently achieved through amalgamation with supple biodegradable polymers. Moreover, PLA’s permeability to carbon dioxide (CO2), oxygen, and water vapor imposes constraints on its applicability in specific beverage bottle contexts. Despite its limitations, PLA’s significance is evident in its wide range of applications within biodegradable and bio-based polymer markets. Regarding future research endeavors, emphasis should be directed towards the development of antimicrobial packaging for PLA, thereby augmenting its utilization in various applications. In order to enhance the antimicrobial and antioxidant properties of the material, incorporation of microbial extracts is recommended. Particular attention should be given to siderophores, peptides, and enzymes, as these components play a pivotal role in augmenting the overall antimicrobial efficacy of the packaging material. To summarize, PLA represents a transition from petrochemical plastics to bioplastics, necessitating solutions to molecular synthesis limitations. Amidst an environmental crisis, substituting PLA for nondegradable polymers is a step in the right direction, addressing issues associated with the petroleum industry’s environmental impact. Overall, PLA’s versatility, affordability, recyclability, biocompatibility, and thermoplasticity make it a significant contender in various industrial sectors.
Acknowledgements
The authors would like to thank SRM Institute of Science and Technology for help in carrying out the research
Funding
No funding was obtained for this study.
Declarations
Competing interests
The authors declare that they have no know competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Rahman MA, Sonomoto K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. Journal of Biotechnology. 2016;236:176–192. doi: 10.1016/j.jbiotec.2016.08.008. [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances. 2013;31:877–902. doi: 10.1016/j.biotechadv.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Abdenour C, Eesaee M, Stuppa C, Chabot B, Barnabé S, Bley J, Tolnai B, Guy N, Nguyen-Tri P. Water vapor and air barrier performance of sustainable paper coatings based on PLA and xanthan gum. Materials Today Communications. 2023;36:106626. doi: 10.1016/j.mtcomm.2023.106626. [DOI] [Google Scholar]
- Abou-alfitooh SAM, El-hoshoudy AN. Eco-friendly modified biopolymers for enhancing oil production: a review. Journal of Polymers and the Environment. (2023). 10.1007/s10924-023-03132-1
- Ahmed J, Mulla M, Jacob H, Luciano G, Bini TB, Almusallam A. Polylactide/poly (ε-caprolactone)/zinc oxide/clove essential oil composite antimicrobial films for scrambled egg packaging. Food Packaging and Shelf Life. 2019;21:100355. doi: 10.1016/j.fpsl.2019.100355. [DOI] [Google Scholar]
- Akshaykranth A, Jayarambabu N, Venkatappa Rao T, Rakesh Kumar R, Srinivasa Rao L. Antibacterial activity study of ZnO incorporated biodegradable poly (lactic acid) films for food packaging applications. Polymer Bulletin. 2023;80:1369–1384. doi: 10.1007/s00289-022-04126-0. [DOI] [Google Scholar]
- Algozeeb WA, Savas PE, Yuan Z, Wang Z, Kittrell C, Hall JN, Chen W, Bollini P, Tour JM. Plastic waste product captures carbon dioxide in nanometer pores. ACS Nano. 2022;16:7284–7290. doi: 10.1021/acsnano.2c00955. [DOI] [PubMed] [Google Scholar]
- Almasi H, Ghanbarzadeh B, Dehghannya J, Entezami AA, Asl AK. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly(lactic acid): morphological and physical properties. Food Packaging and Shelf Life. 2015;5:21–31. doi: 10.1016/j.fpsl.2015.04.003. [DOI] [Google Scholar]
- Aman Mohammadi M, Ramezani S, Hosseini H, Mortazavian AM, Hosseini SM, Ghorbani M. Electrospun antibacterial and antioxidant zein/polylactic acid/hydroxypropyl methylcellulose nanofibers as an active food packaging system. Food and Bioprocess Technology. 2021;14:1529–1541. doi: 10.1007/s11947-021-02654-7. [DOI] [Google Scholar]
- Andrade-Del Olmo J, Pérez-Álvarez L, Hernáez E, Ruiz-Rubio L, Vilas-Vilela JL. Antibacterial multilayer of chitosan and (2-carboxyethyl)- β-cyclodextrin onto polylactic acid (PLLA) Food Hydrocolloids. 2019;88:228–236. doi: 10.1016/j.foodhyd.2018.10.014. [DOI] [Google Scholar]
- Andrade MA, Barbosa CH, Mariño-Cortegoso S, Barbosa-Pereira L, Sendón R, Buonocore GG, Stanzione M, Coelho A, Correia CB, Saraiva M. LDPE and PLA active food packaging incorporated with lemon by-products extract: preparation, characterization and effectiveness to delay lipid oxidation in almonds and beef meat. Foods. 2023;12:2450. doi: 10.3390/foods12132450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonioli G, Fontanella G, Echeverrigaray S, Delamare APL, Pauletti GF, Barcellos T. Poly (lactic acid) nanocapsules containing lemongrass essential oil for postharvest decay control: in vitro and in vivo evaluation against phytopathogenic fungi. Food Chemistry. 2020;326:126997. doi: 10.1016/j.foodchem.2020.126997. [DOI] [PubMed] [Google Scholar]
- Apicella A, Adiletta G, Albanese D, Di Matteo M, Incarnato L. Biodegradable films based on poly (lactic acid) coatings and natural olive-wastewater extracts for active food packaging. Chemical Engineering Transactions. 2021;87:85–90. [Google Scholar]
- Aragón-Gutierrez A, Arrieta MP, López-González M, Fernández-García M, López D. Hybrid biocomposites based on poly (lactic acid) and silica aerogel for food packaging applications. Materials. 2020;13:4910. doi: 10.3390/ma13214910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfat YA, Ahmed J, Ejaz M, Mullah M. Polylactide/graphene oxide nanosheets/clove essential oil composite films for potential food packaging applications. International Journal of Biological Macromolecules. 2018;107:194–203. doi: 10.1016/j.ijbiomac.2017.08.156. [DOI] [PubMed] [Google Scholar]
- Arrieta MP, Samper MD, López J, Jiménez A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. Journal of Polymers and the Environment. 2014;22:460–470. doi: 10.1007/s10924-014-0654-y. [DOI] [Google Scholar]
- Arrieta MP, Sessini V, Peponi L. Biodegradable poly (ester-urethane) incorporated with catechin with shape memory and antioxidant activity for food packaging. European Polymer Journal. 2017;94:111–124. doi: 10.1016/j.eurpolymj.2017.06.047. [DOI] [Google Scholar]
- Arrieta MP, de Dicastillo CL, Garrido L, Roa K, Galotto MJ. Electrospun PVA fibers loaded with antioxidant fillers extracted from Durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. European Polymer Journal. 2018;103:145–157. doi: 10.1016/j.eurpolymj.2018.04.012. [DOI] [Google Scholar]
- Arrigo R, Bartoli M, Malucelli G. Poly (lactic acid)–biochar biocomposites: effect of processing and filler content on rheological, thermal, and mechanical properties. Polymers. 2020;12:892. doi: 10.3390/polym12040892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi S, Pirsa S. Production of biodegradable film based on polylactic acid, modified with lycopene pigment and TiO2 and studying its physicochemical properties. Journal of Polymers and the Environment. 2020;28:433–444. doi: 10.1007/s10924-019-01618-5. [DOI] [Google Scholar]
- Aung SPS, Shein HHH, Aye KN, Nwe N. Environment-friendly biopolymers for food packaging: starch, protein, and poly-lactic acid (PLA). pp. 173-195. In: Bio-Based Materials for Food Packaging: Green and Sustainable Advanced Packaging Materials. Ahmed S (ed). Springer Singapore (2018)
- Auras R, Harte B, Selke S. An overview of polylactides as packaging materials. Macromolecular Bioscience. 2004;4:835–864. doi: 10.1002/mabi.200400043. [DOI] [PubMed] [Google Scholar]
- Aydemir D, Gardner DJ. Biopolymer blends of polyhydroxybutyrate and polylactic acid reinforced with cellulose nanofibrils. Carbohydrate Polymers. 2020;250:116867. doi: 10.1016/j.carbpol.2020.116867. [DOI] [PubMed] [Google Scholar]
- Aytac Z, Kusku SI, Durgun E, Uyar T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Materials Science and Engineering: c. 2016;63:231–239. doi: 10.1016/j.msec.2016.02.063. [DOI] [PubMed] [Google Scholar]
- Aytac Z, Keskin NOS, Tekinay T, Uyar T. Antioxidant α-tocopherol/γ-cyclodextrin-inclusion complex encapsulated poly(lactic acid) electrospun nanofibrous web for food packaging. Journal of Applied Polymer Science. 134: 48842 (2017)
- Bakar AEA, Othman R, Mohtar M, Latif NHM, Sulaiman WSHW, Hatta FAM, Ramya R. Antimicrobial activities of curcumin extracted from selected zingiberaceae species as potential halal active pharmaceutical ingredient. Journal of Halal Science and Technology. 2023;2:83–93. doi: 10.59202/jhst.v2i1.671. [DOI] [Google Scholar]
- Balla E, Daniilidis V, Karlioti G, Kalamas T, Stefanidou M, Bikiaris ND, Vlachopoulos A, Koumentakou I, Bikiaris DN. Poly (lactic acid): a versatile biobased polymer for the future with multifunctional properties—from monomer synthesis, polymerization techniques and molecular weight increase to PLA applications. Polymers. 2021;13:1822. doi: 10.3390/polym13111822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang G, Kim SW. Biodegradable poly (lactic acid)-based hybrid coating materials for food packaging films with gas barrier properties. Journal of Industrial and Engineering Chemistry. 2012;18:1063–1068. doi: 10.1016/j.jiec.2011.12.004. [DOI] [Google Scholar]
- Barbato A, Apicella A, Malvano F, Scarfato P, Incarnato L. High-barrier, biodegradable films with polyvinyl alcohol/polylactic acid + wax double coatings: influence of relative humidity on transport properties and suitability for modified atmosphere packaging applications. Polymers. 2023;15:4002. doi: 10.3390/polym15194002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baweja M, Nain L, Kawarabayasi Y, Shukla P. Current technological improvements in enzymes toward their biotechnological applications. Frontiers in Microbiology. 2016;7:965. doi: 10.3389/fmicb.2016.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer G, Shayganpour A, Bayer IS. Antioxidant activity of limonene modified cellulose pulp fiber-polylactic acid (PLA) composites. Cellulose. 2023;30:1599–1622. doi: 10.1007/s10570-022-05011-9. [DOI] [Google Scholar]
- Bianchi E, Guidotti G, Soccio M, Siracusa V, Gazzano M, Salatelli E, Lotti N. Biobased and compostable multiblock copolymer of poly(l-lactic acid) containing 2,5-furandicarboxylic acid for sustainable food packaging: the role of parent homopolymers in the composting kinetics and mechanism. Biomacromolecules. 2023;24:2356–2368. doi: 10.1021/acs.biomac.3c00216. [DOI] [PubMed] [Google Scholar]
- Biswas A, Cheng HN, Kuzniar G, He Z, Kim S, Furtado RF, Alves CR, Sharma BK. Bilayer films of poly (lactic acid) and cottonseed protein for packaging applications. Polymers. 2023;15:1425. doi: 10.3390/polym15061425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boro U, Priyadarsini A, Moholkar VS. Synthesis and characterization of poly(lactic acid)/clove essential oil/alkali-treated halloysite nanotubes composite films for food packaging applications. International Journal of Biological Macromolecules. 2022;216:927–939. doi: 10.1016/j.ijbiomac.2022.07.209. [DOI] [PubMed] [Google Scholar]
- Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Materials Today. 2013;16:496–504. doi: 10.1016/j.mattod.2013.11.017. [DOI] [Google Scholar]
- Bressanin JM, de Mesquita Sampaio IL, Geraldo VC, Klein BC, Chagas MF, Bonomi A, Maciel Filho R, Cavalett O. Techno-economic and environmental assessment of polylactic acid production integrated with the sugarcane value chain. Sustainable Production and Consumption. 2022;34:244–256. doi: 10.1016/j.spc.2022.09.009. [DOI] [Google Scholar]
- Brüster B, Adjoua Y-O, Dieden R, Grysan P, Federico CE, Berthé V, Addiego F. Plasticization of polylactide with myrcene and limonene as bio-based plasticizers: conventional vs. reactive extrusion. Polymers. 2019;11:1363. doi: 10.3390/polym11081363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos N, Armentano I, Fortunati E, Dominici F, Luzi F, Fiori S, Cristofaro F, Visai L, Jiménez A, Kenny JM. Functional properties of plasticized bio-based poly (lactic acid)_poly (hydroxybutyrate)(PLA_PHB) films for active food packaging. Food and Bioprocess Technology. 2017;10:770–780. doi: 10.1007/s11947-016-1846-3. [DOI] [Google Scholar]
- Cacciotti I, Nanni F. Poly (lactic) acid fibers loaded with mesoporous silica for potential applications in the active food packaging. In: AIP Conference Proceedings. Vol. 1738. AIP Publishing, St. Louis (2016)
- Casalini T, Rossi F, Santoro M, Perale G. Structural characterization of poly-l-lactic Acid (PLLA) and poly(glycolic acid) (PGA) oligomers. International Journal of Molecular Sciences. 2011;12:3857–3870. doi: 10.3390/ijms12063857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo Martinez FA, Balciunas EM, Salgado JM, Domínguez González JM, Converti A, Oliveira RPS. Lactic acid properties, applications and production: a review. Trends in Food Science & Technology. 2013;30:70–83. doi: 10.1016/j.tifs.2012.11.007. [DOI] [Google Scholar]
- Cavallo E, He X, Luzi F, Dominici F, Cerrutti P, Bernal C, Foresti ML, Torre L, Puglia D. UV protective, antioxidant, antibacterial and compostable polylactic acid composites containing pristine and chemically modified lignin nanoparticles. Molecules. 2020;26:126. doi: 10.3390/molecules26010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Geever LM, Killion JA, Lyons JG, Higginbotham CL, Devine DM. Review of multifarious applications of poly (lactic acid) Polymer—Plastics Technology and Engineering. 2016;55:1057–1075. doi: 10.1080/03602559.2015.1132465. [DOI] [Google Scholar]
- Chia WY, Ying Tang DY, Khoo KS, Kay Lup AN, Chew KW. Nature’s fight against plastic pollution: algae for plastic biodegradation and bioplastics production. Environmental Science and Ecotechnology. 2020;4:100065. doi: 10.1016/j.ese.2020.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WY, Ng JF, Yap WH, Goh BH. Sophorolipids—bio-based antimicrobial formulating agents for applications in food and health. Molecules. 2022;27:5556. doi: 10.3390/molecules27175556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Jiang K, Yuan M, Cao J, Li L, Tang Z, Qin Y. Antimicrobial film based on polylactic acid and carbon nanotube for controlled cinnamaldehyde release. Journal of Materials Research and Technology. 2020;9:10130–10138. doi: 10.1016/j.jmrt.2020.07.016. [DOI] [Google Scholar]
- Daassi R, Durand K, Rodrigue D, Stevanovic T. Optimization of the electrospray process to produce lignin nanoparticles for PLA-based food packaging. Polymers. 2023;15:2973. doi: 10.3390/polym15132973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daful AG, Haigh K, Vaskan P, Görgens JF. Environmental impact assessment of lignocellulosic lactic acid production: integrated with existing sugar mills. Food and Bioproducts Processing. 2016;99:58–70. doi: 10.1016/j.fbp.2016.04.005. [DOI] [Google Scholar]
- de la Rosa-Ramírez H, Dominici F, Ferri JM, Luzi F, Puglia D, Torre L, López-Martínez J, Samper MD. Pentaerythritol and glycerol esters derived from gum rosin as bio-based additives for the improvement of processability and thermal stability of polylactic acid. Journal of Polymers and the Environment. 2023;31:5446–5461. doi: 10.1007/s10924-023-02949-0. [DOI] [Google Scholar]
- Deng P, Zhou Y. A sustainable strategy for promoting the colour strength and ultraviolet/oxidation resistance of polylactic acid fabric integrating quercetin and food-grade ester. Industrial Crops and Products. 2024;208:117875. doi: 10.1016/j.indcrop.2023.117875. [DOI] [Google Scholar]
- Deshmukh RK, Kumar L, Gaikwad KK. Halloysite nanotubes for food packaging application: a review. Applied Clay Science. 2023;234:106856. doi: 10.1016/j.clay.2023.106856. [DOI] [Google Scholar]
- Díaz-Galindo EP, Nesic A, Cabrera-Barjas G, Dublan-García O, Ventura-Aguilar RI, Vázquez-Armenta FJ, Aguilar-Montes-de-Oca S, Mardones C, Ayala-Zavala JF. Physico-chemical and antiadhesive properties of poly (lactic acid)/grapevine cane extract films against food pathogenic microorganisms. Polymers. 2020;12:2967. doi: 10.3390/polym12122967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Xue Y, Huang H, Shen D, Gao W, Xu F, Weng Y, Zhang Y. Facile synthesis of soybean protein-based phosphorus-nitrogen flame retardant for poly (lactic acid) Polymer Degradation and Stability. 2023;214:110412. doi: 10.1016/j.polymdegradstab.2023.110412. [DOI] [Google Scholar]
- Eissenberger K, Ballesteros A, De Bisschop R, Bugnicourt E, Cinelli P, Defoin M, Demeyer E, Fürtauer S, Gioia C, Gómez L. Approaches in sustainable biobased multilayer packaging solutions. Polymers. 2023;15:1184. doi: 10.3390/polym15051184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsawy MA, Fekry M, Sayed AM, Maziad NA, Saad GR. Physico-chemical characteristics of biodegradable poly(lactic acid) and poly(lactic acid)/chitosan nano-composites under the influence of gamma irradiation. Journal of Polymers and the Environment. 2023;31:2705–2714. doi: 10.1007/s10924-022-02693-x. [DOI] [Google Scholar]
- Erdohan ZÖ, Çam B, Turhan KN. Characterization of antimicrobial polylactic acid based films. Journal of Food Engineering. 2013;119:308–315. doi: 10.1016/j.jfoodeng.2013.05.043. [DOI] [Google Scholar]
- Ezati P, Rhim J-W. Fabrication of quercetin-loaded biopolymer films as functional packaging materials. ACS Applied Polymer Materials. 2021;3:2131–2137. doi: 10.1021/acsapm.1c00177. [DOI] [Google Scholar]
- Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications—a comprehensive review. Advanced Drug Delivery Reviews. 2016;107:367–392. doi: 10.1016/j.addr.2016.06.012. [DOI] [PubMed] [Google Scholar]
- Farajinejad Z, Sani IK, Alizadeh M, Amiri S. A review of recent advances in the photocatalytic activity of protein and polysaccharide-based nanocomposite packaging films: antimicrobial, antioxidant, mechanical, and strength properties. Journal of Polymers and the Environment. 2023 doi: 10.1007/s10924-023-03159-4. [DOI] [Google Scholar]
- Fontes MRV, da Rosa MP, Fonseca LM, Beck PH, da Rosa Zavareze E, Dias ARG. Thermal stability, hydrophobicity and antioxidant potential of ultrafine poly (lactic acid)/rice husk lignin fibers. Brazilian Journal of Chemical Engineering. 2021;38:133–144. doi: 10.1007/s43153-020-00083-1. [DOI] [Google Scholar]
- Fortunati E, Peltzer M, Armentano I, Jiménez A, Kenny JM. Combined effects of cellulose nanocrystals and silver nanoparticles on the barrier and migration properties of PLA nano-biocomposites. Journal of Food Engineering. 2013;118:117–124. doi: 10.1016/j.jfoodeng.2013.03.025. [DOI] [Google Scholar]
- Fredi G, Dorigato A, Dussin A, Xanthopoulou E, Bikiaris DN, Botta L, Fiore V, Pegoretti A. Compatibilization of polylactide/poly (ethylene 2, 5-furanoate)(PLA/PEF) blends for sustainable and bioderived packaging. Molecules. 2022;27:6371. doi: 10.3390/molecules27196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas EES, Dias ÊR, Albuquerque MMS, Biondi IB, Branco CRC, Cruz RS, Branco A, Camilloto GP. Antioxidant films based on poly (lactic acid) incorporated with crude extract from Malpighia emarginata DC pomace for use in food packaging. Packaging Technology and Science. 2024;37:39–50. doi: 10.1002/pts.2778. [DOI] [Google Scholar]
- Gay S, Lefebvre G, Bonnin M, Nottelet B, Boury F, Gibaud A, Calvignac B. PLA scaffolds production from thermally induced phase separation: effect of process parameters and development of an environmentally improved route assisted by supercritical carbon dioxide. The Journal of Supercritical Fluids. 2018;136:123–135. doi: 10.1016/j.supflu.2018.02.015. [DOI] [Google Scholar]
- Goh K, Heising JK, Yuan Y, Karahan HE, Wei L, Zhai S, Koh J-X, Htin NM, Zhang F, Wang R, Fane AG, Dekker M, Dehghani F, Chen Y. Sandwich-architectured poly(lactic acid)–graphene composite food packaging films. ACS Applied Materials & Interfaces. 2016;8:9994–10004. doi: 10.1021/acsami.6b02498. [DOI] [PubMed] [Google Scholar]
- Gomez-Caturla J, Tejada-Oliveros R, Ivorra-Martinez J, Garcia-Sanoguera D, Balart R, Garcia-Garcia D. Development and characterization of new environmentally friendly polylactide formulations with terpenoid-based plasticizers with improved ductility. Journal of Polymers and the Environment. 2023;32:749–762. doi: 10.1007/s10924-023-03000-y. [DOI] [Google Scholar]
- Gondal AH, Bhat RA, Gómez RL, Areche FO, Huaman JT. Advances in plastic pollution prevention and their fragile effects on soil, water, and air continuums. International Journal of Environmental Science and Technology. 2023;20:6897–6912. doi: 10.1007/s13762-022-04607-9. [DOI] [Google Scholar]
- Goñi-Ciaurriz L, Vélaz I. Antibacterial and degradable properties of β-cyclodextrin-TiO2 cellulose acetate and polylactic acid bionanocomposites for food packaging. International Journal of Biological Macromolecules. 2022;216:347–360. doi: 10.1016/j.ijbiomac.2022.06.202. [DOI] [PubMed] [Google Scholar]
- Gonon H, Srisa A, Promhuad K, Chonhenchob V, Bumbudsanpharoke N, Jarupan L, Harnkarnsujarit N. PLA thermoformed trays incorporated with cinnamaldehyde and carvacrol as active biodegradable bakery packaging. Food Packaging and Shelf Life. 2023;38:101123. doi: 10.1016/j.fpsl.2023.101123. [DOI] [Google Scholar]
- González A, Alvarez Igarzabal CI. Soy protein–poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocolloids. 2013;33:289–296. doi: 10.1016/j.foodhyd.2013.03.010. [DOI] [Google Scholar]
- Grabska-Zielińska S, Gierszewska M, Olewnik-Kruszkowska E, Bouaziz M. Polylactide films with the addition of olive leaf extract—physico-chemical characterization. Materials. 2021;14:7623. doi: 10.3390/ma14247623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigore ME. Methods of recycling, properties and applications of recycled thermoplastic polymers. Recycling. 2017;2:24. doi: 10.3390/recycling2040024. [DOI] [Google Scholar]
- Grujić R, Vujadinović D, Savanović D. Biopolymers as food packaging materials. In: Pellicer E, Nikolic D, Sort J, Baró M, Zivic F, Grujovic N, Grujic R, Pelemis S, editors. Advances in Applications of Industrial Biomaterials. Cham: Springer; 2017. pp. 139–160. [Google Scholar]
- Gu C-H, Wang J-J, Yu Y, Sun H, Shuai N, Wei B. Biodegradable multilayer barrier films based on alginate/polyethyleneimine and biaxially oriented poly(lactic acid) Carbohydrate Polymers. 2013;92:1579–1585. doi: 10.1016/j.carbpol.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhang Q, Yang X, Fang Y, Qian X, Sheng K. Microcrystalline cellulose reinforced polylactic acid composites: comparison of fabrication method and cellulose content. Journal of Polymer Research. 2023;30:402. doi: 10.1007/s10965-023-03790-8. [DOI] [Google Scholar]
- Hajikhani M, Emam-Djomeh Z, Askari G. Fabrication and characterization of gluten film reinforced by lycopene-loaded electrospun polylactic acid nano-fibers. Food and Bioprocess Technology. 2020;13:2217–2227. doi: 10.1007/s11947-020-02561-3. [DOI] [Google Scholar]
- Hanafi S, Sirait SM, Irawan C, Rochaeni H. Poly(lactic acid) packaging modified curcumin as bioactive substance in tea drink (Camelia sinensis) Asian Journal of Chemistry. 2017;30:145–147. doi: 10.14233/ajchem.2018.20953. [DOI] [Google Scholar]
- Hao Y, Zhang M, Wang L, Tao N, Li L, Zhu W, Xu C, Deng S, Wang Y. Mechanism of antimicrobials immobilized on packaging film inhabiting foodborne pathogens. LWT. 2022;169:114037. doi: 10.1016/j.lwt.2022.114037. [DOI] [Google Scholar]
- Havelt T, Brettschneider S, Schmitz M. Evaluation of practical applicability and synergistic effects of bio-based food packaging materials combined with plant-based stabilisers. Processes. 2021;9:1838. doi: 10.3390/pr9101838. [DOI] [Google Scholar]
- Henczka M, Djas M. Reactive extraction of acetic acid and propionic acid using supercritical carbon dioxide. The Journal of Supercritical Fluids. 2016;110:154–160. doi: 10.1016/j.supflu.2015.11.018. [DOI] [Google Scholar]
- Hernández-García E, Vargas M, Chiralt A. Starch-polyester bilayer films with phenolic acids for pork meat preservation. Food Chemistry. 385: 132650 (2022a) [DOI] [PubMed]
- Hernández-García E, Vargas M, Chiralt A. Effect of active phenolic acids on properties of PLA-PHBV blend films. Food Packaging and Shelf Life 33: 100894 (2022b)
- Hipólito A, Caretta TO, Silveira VAI, Bersaneti GT, Mali S, Celligoi MAPC. Active biodegradable cassava starch films containing sophorolipids produced by Starmerella bombicola ATCC® 22214TM. Journal of Polymers and the Environment. 2021;29:3199–3209. doi: 10.1007/s10924-021-02103-8. [DOI] [Google Scholar]
- Hojatoleslami M, Ahari H, Larijani K, Sharifan A. Preservation effect of Lippia citriodora and Laurus nobilis nanoemulsions incorporated with polylactic acid composite film for rainbow trout fillet packaging. Food Science and Technology. 2022;42:e83921. doi: 10.1590/fst.83921. [DOI] [Google Scholar]
- Holcapkova P, Hurajova A, Bazant P, Pummerova M, Sedlarik V. Thermal stability of bacteriocin nisin in polylactide-based films. Polymer Degradation and Stability 158: 31-39 (2018a)
- Holcapkova P, Hurajova A, Kucharczyk P, Bazant P, Plachy T, Miskolczi N, Sedlarik V. Effect of polyethylene glycol plasticizer on long-term antibacterial activity and the release profile of bacteriocin nisin from polylactide blends. Polymers for Advanced Technologies 29: 2253-2263 (2018b)
- Hu B, Li L, Guan D, Yang B, Li G, Wang Z, Han R. Enhanced UV-shielding performance of poly (lactic acid) composite with POSS-modified bamboo powder. Industrial Crops and Products. 2023;192:116133. doi: 10.1016/j.indcrop.2022.116133. [DOI] [Google Scholar]
- Imran M, Klouj A, Revol-Junelles A-M, Desobry S. Controlled release of nisin from HPMC, sodium caseinate, poly-lactic acid and chitosan for active packaging applications. Journal of Food Engineering. 2014;143:178–185. doi: 10.1016/j.jfoodeng.2014.06.040. [DOI] [Google Scholar]
- Iñiguez-Franco F, Soto-Valdez H, Peralta E, Ayala-Zavala JF, Auras R, Gámez-Meza N. Antioxidant activity and diffusion of catechin and epicatechin from antioxidant active films made of poly(l-lactic acid) Journal of Agricultural and Food Chemistry. 2012;60:6515–6523. doi: 10.1021/jf300668u. [DOI] [PubMed] [Google Scholar]
- Injorhor P, Trongsatitkul T, Wittayakun J, Ruksakulpiwat C, Ruksakulpiwat Y. Biodegradable polylactic acid-polyhydroxyalkanoate-based nanocomposites with bio-hydroxyapatite: preparation and characterization. Polymers. 2023;15:1261. doi: 10.3390/polym15051261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izadi F, Pajohi-Alamoti M, Emamifar A, Nourian A. Fabrication and characterization of active poly(lactic acid) films containing Thymus daenensis essential oil/beta-cyclodextrin inclusion complex and silver nanoparticles to extend the shelf life of ground beef. Food and Bioprocess Technology. 2023 doi: 10.1007/s11947-023-03200-3. [DOI] [Google Scholar]
- Jacob J, Lawal U, Thomas S, Valapa RB. Biobased polymer composite from poly(lactic acid): processing, fabrication, and characterization for food packaging. pp. 97-115. In: Processing and Development of Polysaccharide-Based Biopolymers for Packaging Applications. Elsevier, Amsterdam (2020)
- Jamshidian M, Tehrany EA, Imran M, Jacquot M, Desobry S. Poly-lactic acid: production, applications, nanocomposites, and release studies. Comprehensive Reviews in Food Science and Food Safety. 2010;9:552–571. doi: 10.1111/j.1541-4337.2010.00126.x. [DOI] [PubMed] [Google Scholar]
- Jiang K, Lu W, Zhu B, Chen H, Li L, Qin Y. Study on the property of mesoporous silica nanoparticles loaded with nisin for poly (lactic acid)-based packaging film. Polymer Composites. 2023;44:6676–6690. doi: 10.1002/pc.27588. [DOI] [Google Scholar]
- Jin T, Zhang H. Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. Journal of Food Science. 2008;73:M127–M134. doi: 10.1111/j.1750-3841.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Katanyoota P, Jariyasakoolroj P, Sane A. Mechanical and barrier properties of simultaneous biaxially stretched polylactic acid/thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Polymer Bulletin. 2023;80:5219–5237. doi: 10.1007/s00289-022-04312-0. [DOI] [Google Scholar]
- Khodayari M, Basti AA, Khanjari A, Misaghi A, Kamkar A, Shotorbani PM, Hamedi H. Effect of poly (lactic acid) films incorporated with different concentrations of Tanacetum balsamita essential oil, propolis ethanolic extract and cellulose nanocrystals on shelf life extension of vacuum-packed cooked sausages. Food Packaging and Shelf Life. 2019;19:200–209. doi: 10.1016/j.fpsl.2018.11.009. [DOI] [Google Scholar]
- Kim MS, Chang H, Zheng L, Yan Q, Pfleger BF, Klier J, Nelson K, Majumder EL-W, Huber GW. A review of biodegradable plastics: chemistry, applications, properties, and future research needs. Chemical Reviews. 2023;123:9915–9939. doi: 10.1021/acs.chemrev.2c00876. [DOI] [PubMed] [Google Scholar]
- Kumar S, Yadav N, Nain L, Khare SK. A simple downstream processing protocol for the recovery of lactic acid from the fermentation broth. Bioresource Technology. 2020;318:124260. doi: 10.1016/j.biortech.2020.124260. [DOI] [PubMed] [Google Scholar]
- Lantano C, Alfieri I, Cavazza A, Corradini C, Lorenzi A, Zucchetto N, Montenero A. Natamycin based sol–gel antimicrobial coatings on polylactic acid films for food packaging. Food Chemistry. 2014;165:342–347. doi: 10.1016/j.foodchem.2014.05.066. [DOI] [PubMed] [Google Scholar]
- Latos-Brozio M, Masek A. The application of natural food colorants as indicator substances in intelligent biodegradable packaging materials. Food and Chemical Toxicology. 2020;135:110975. doi: 10.1016/j.fct.2019.110975. [DOI] [PubMed] [Google Scholar]
- Lawal U, Samyuktha R, Robert V, Sreelakshmi K, Gopi A, Poochi M, Loganathan S, Thomas S, Valapa RB. Poly(lactic acid)/cholecalciferol based composites for active food packaging application. International Journal of Biological Macromolecules. 2023;246:125637. doi: 10.1016/j.ijbiomac.2023.125637. [DOI] [PubMed] [Google Scholar]
- Lekrine A, Belaadi A, Makhlouf A, Amroune S, Bourchak M, Satha H, Jawaid M. Structural, thermal, mechanical and physical properties of Washingtonia filifera fibres reinforced thermoplastic biocomposites. Materials Today Communications. 2022;31:103574. doi: 10.1016/j.mtcomm.2022.103574. [DOI] [Google Scholar]
- Li X, Chen Y, Zhao S, Chen H, Zheng X, Luo J, Liu Y. Efficient production of optically pure l-lactic acid from food waste at ambient temperature by regulating key enzyme activity. Water Research. 2015;70:148–157. doi: 10.1016/j.watres.2014.11.049. [DOI] [PubMed] [Google Scholar]
- Li T, Liu Y, Qin Q, Zhao L, Wang Y, Wu X, Liao X. Development of electrospun films enriched with ethyl lauroyl arginate as novel antimicrobial food packaging materials for fresh strawberry preservation. Food Control. 2021;130:108371. doi: 10.1016/j.foodcont.2021.108371. [DOI] [Google Scholar]
- Li Q, Hu X, Perkins P, Ren T. Antimicrobial film based on poly(lactic acid) and natural halloysite nanotubes for controlled cinnamaldehyde release. International Journal of Biological Macromolecules. 2023;224:848–857. doi: 10.1016/j.ijbiomac.2022.10.171. [DOI] [PubMed] [Google Scholar]
- Liew WC, Muhamad II, Chew JW, Karim KJA. Synergistic effect of graphene oxide/zinc oxide nanocomposites on polylactic acid-based active packaging film: properties, release kinetics and antimicrobial efficiency. International Journal of Biological Macromolecules. 2023;253:127288. doi: 10.1016/j.ijbiomac.2023.127288. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhu Y, Li J, Wang M, Lee P, Du G, Chen J. Microbial production of propionic acid from Propionibacteria: current state, challenges and perspectives. Critical Reviews in Biotechnology. 2012;32:374–381. doi: 10.3109/07388551.2011.651428. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wang Y, Wu B, Cui C, Guo Y, Yan C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. The International Journal of Advanced Manufacturing Technology. 2019;102:2877–2889. doi: 10.1007/s00170-019-03332-x. [DOI] [Google Scholar]
- Lombardi A, Fochetti A, Vignolini P, Campo M, Durazzo A, Lucarini M, Puglia D, Luzi F, Papalini M, Renzi M. Natural active ingredients for poly (lactic acid)-based materials: state of the art and perspectives. Antioxidants. 2022;11:2074. doi: 10.3390/antiox11102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y, Zhang R, Huang J, Wang J, Jiang Y, Hu G, Yang J, Zhu J. Tensile property balanced and gas barrier improved poly (lactic acid) by blending with biobased poly (butylene 2, 5-furan dicarboxylate) ACS Sustainable Chemistry & Engineering. 2017;5:9244–9253. doi: 10.1021/acssuschemeng.7b02196. [DOI] [Google Scholar]
- Lu W, Cui R, Zhu B, Qin Y, Cheng G, Li L, Yuan M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly (lactic acid) biocomposite food packaging film. Journal of Materials Research and Technology. 2021;11:1152–1161. doi: 10.1016/j.jmrt.2021.01.098. [DOI] [Google Scholar]
- Lu Y, Cheng D, Niu B, Wang X, Wu X, Wang A. Properties of poly (lactic-co-glycolic acid) and progress of poly (lactic-co-glycolic acid)-based biodegradable materials in biomedical research. Pharmaceuticals. 2023;16:454. doi: 10.3390/ph16030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Shen J, Yang Q, Zhou J, Xia S, Cao J. Effect of the introduction of fish collagen on the thermal and mechanical properties of poly (lactic acid) Industrial & Engineering Chemistry Research. 2015;54:10945–10951. doi: 10.1021/acs.iecr.5b02969. [DOI] [Google Scholar]
- Ma Q, Davidson PM, Zhong Q. Properties and potential food applications of lauric arginate as a cationic antimicrobial. International Journal of Food Microbiology. 2020;315:108417. doi: 10.1016/j.ijfoodmicro.2019.108417. [DOI] [PubMed] [Google Scholar]
- Maaskant E, Post W, Brouwer MT, van Es DS, van Velzen EUT. Strategic selection tool for thermoplastic materials in a renewable circular economy: identifying future circular polymers. Sustainable Production and Consumption. 2023;38:174–185. doi: 10.1016/j.spc.2023.04.005. [DOI] [Google Scholar]
- Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresource Technology. 2010;101:8493–8501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- Marra A, Silvestre C, Duraccio D, Cimmino S. Polylactic acid/zinc oxide biocomposite films for food packaging application. International Journal of Biological Macromolecules. 2016;88:254–262. doi: 10.1016/j.ijbiomac.2016.03.039. [DOI] [PubMed] [Google Scholar]
- Martins C, Vilarinho F, Silva AS, Andrade M, Machado AV, Castilho MC, Sá A, Cunha A, Vaz MF, Ramos F. Active polylactic acid film incorporated with green tea extract: development, characterization and effectiveness. Industrial Crops and Products. 2018;123:100–110. doi: 10.1016/j.indcrop.2018.06.056. [DOI] [Google Scholar]
- Masyita A, Sari RM, Astuti AD, Yasir B, Rumata NR, Emran TB, Nainu F, Simal-Gandara J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chemistry X. 2022;13:100217. doi: 10.1016/j.fochx.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli S, Peltzer M, Fortunati E, Armentano I, Jiménez A, Kenny JM. Structure, gas-barrier properties and overall migration of poly(lactic acid) films coated with hydrogenated amorphous carbon layers. Carbon. 2013;63:274–282. doi: 10.1016/j.carbon.2013.06.080. [DOI] [Google Scholar]
- Mehmood A, Raina N, Phakeenuya V, Wonganu B, Cheenkachorn K. The current status and market trend of polylactic acid as biopolymer: awareness and needs for sustainable development. Materials Today: Proceedings. 2023;72:3049–3055. [Google Scholar]
- Mehta R, Kumar V, Bhunia H, Upadhyay SN. Synthesis of poly(lactic acid): a review. Journal of Macromolecular Science, Part C. 45: 325-349 (2006). 10.1080/15321790500304148
- Mele G, Bloise E, Cosentino F, Lomonaco D, Avelino F, Marcianò T, Massaro C, Mazzetto SE, Tammaro L, Scalone AG. Influence of cardanol oil on the properties of poly (lactic acid) films produced by melt extrusion. ACS Omega. 2019;4:718–726. doi: 10.1021/acsomega.8b02880. [DOI] [Google Scholar]
- Melesse GT, Hone FG, Mekonnen MA. Extraction of cellulose from sugarcane bagasse optimization and characterization. Advances in Materials Science and Engineering. 2022 doi: 10.1155/2022/1712207. [DOI] [Google Scholar]
- Milovanovic S, Hollermann G, Errenst C, Pajnik J, Frerich S, Kroll S, Rezwan K, Ivanovic J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Research International. 2018;107:486–495. doi: 10.1016/j.foodres.2018.02.065. [DOI] [PubMed] [Google Scholar]
- Milovanovic S, Pajnik J, Lukic I. Tailoring of advanced poly(lactic acid)-based materials: a review. Journal of Applied Polymer Science. 2022;139:51839. doi: 10.1002/app.51839. [DOI] [Google Scholar]
- Min T, Zhou L, Sun X, Du H, Bian X, Zhu Z, Wen Y. Enzyme-responsive food packaging system based on pectin-coated poly (lactic acid) nanofiber films for controlled release of thymol. Food Research International. 2022;157:111256. doi: 10.1016/j.foodres.2022.111256. [DOI] [PubMed] [Google Scholar]
- Mulla MZ, Rahman MRT, Marcos B, Tiwari B, Pathania S. Poly lactic acid (PLA) nanocomposites: effect of inorganic nanoparticles reinforcement on its performance and food packaging applications. Molecules. 2021;26:1967. doi: 10.3390/molecules26071967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, González-Martínez C, Chiralt A. Combination of poly (lactic) acid and starch for biodegradable food packaging. Materials. 2017;10:952. doi: 10.3390/ma10080952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munteanu BS, Aytac Z, Pricope GM, Uyar T, Vasile C. Polylactic acid (PLA)/silver-NP/Vitamin E bionanocomposite electrospun nanofibers with antibacterial and antioxidant activity. Journal of Nanoparticle Research. 2014;16:1–12. doi: 10.1007/s11051-014-2643-4. [DOI] [Google Scholar]
- Murillo-Morales G, Sethupathy S, Zhang M, Xu L, Ghaznavi A, Xu J, Yang B, Sun J, Zhu D. Characterization and 3D printing of a biodegradable polylactic acid/thermoplastic polyurethane blend with laccase-modified lignin as a nucleating agent. International Journal of Biological Macromolecules. 2023;236:123881. doi: 10.1016/j.ijbiomac.2023.123881. [DOI] [PubMed] [Google Scholar]
- Mutha RE, Tatiya AU, Surana SJ. Flavonoids as natural phenolic compounds and their role in therapeutics: an overview. Future Journal of Pharmaceutical Sciences. 2021;7:1–13. doi: 10.1186/s43094-020-00161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Nguyen B-S, Le D-T, Alomar TS, AlMasoud N, Ghotekar S, Oza R, Raizada P, Singh P, Nguyen V-H. A concept for the biotechnological minimizing of emerging plastics, micro- and nano-plastics pollutants from the environment: a review. Environmental Research. 2023;216:114342. doi: 10.1016/j.envres.2022.114342. [DOI] [PubMed] [Google Scholar]
- Nisar P, Ali N, Rahman L, Ali M, Shinwari ZK. Antimicrobial activities of biologically synthesized metal nanoparticles: an insight into the mechanism of action. JBIC Journal of Biological Inorganic Chemistry. 2019;24:929–941. doi: 10.1007/s00775-019-01717-7. [DOI] [PubMed] [Google Scholar]
- Oliver-Ortega H, Vandemoortele V, Bala A, Julian F, Méndez JA, Espinach FX. Nanoclay effect into the biodegradation and processability of poly (lactic acid) nanocomposites for food packaging. Polymers. 2021;13:2741. doi: 10.3390/polym13162741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordoñez R, Atarés L, Chiralt A. Multilayer antimicrobial films based on starch and PLA with superficially incorporated ferulic or cinnamic acids for active food packaging purposes. Food Chemistry Advances. 2023;2:100250. doi: 10.1016/j.focha.2023.100250. [DOI] [Google Scholar]
- Özge Erdohan Z, Çam B, Turhan KN. Characterization of antimicrobial polylactic acid based films. Journal of Food Engineering. 2013;119:308–315. doi: 10.1016/j.jfoodeng.2013.05.043. [DOI] [Google Scholar]
- Palak H, Güler E, Nofar M, Karagüzel Kayaoğlu B. Effects of D-lactide content and molecular weight on the morphological, thermal, and mechanical properties of electrospun nanofiber polylactide mats. Journal of Industrial Textiles. 2022;51:3030S–3056S. doi: 10.1177/15280837221090260. [DOI] [Google Scholar]
- Parlak ME, Uzuner K, Kirac FT, Ozdemir S, Dundar AN, Sahin OI, Dagdelen AF, Saricaoglu FT. Production and characterization of biodegradable bi-layer films from poly (lactic) acid and zein. International Journal of Biological Macromolecules. 2023;227:1027–1037. doi: 10.1016/j.ijbiomac.2022.11.278. [DOI] [PubMed] [Google Scholar]
- Patiño-Vidal C, Luzi F, Puglia D, López-Carballo G, Rojas A, Galotto MJ, López de Dicastillo C. Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl arginate. Food Packaging and Shelf Life. 2023;36:101050. doi: 10.1016/j.fpsl.2023.101050. [DOI] [Google Scholar]
- Patwa R, Saha N, Sáha P, Katiyar V. Biocomposites of poly (lactic acid) and lactic acid oligomer-grafted bacterial cellulose: it’s preparation and characterization. Journal of Applied Polymer Science. 2019;136:47903. doi: 10.1002/app.47903. [DOI] [Google Scholar]
- Payne J, McKeown P, Mahon MF, Emanuelsson EAC, Jones MD. Mono-and dimeric zinc (ii) complexes for PLA production and degradation into methyl lactate—a chemical recycling method. Polymer Chemistry. 2020;11:2381–2389. doi: 10.1039/D0PY00192A. [DOI] [Google Scholar]
- Pirsa S, Asadi S. Innovative smart and biodegradable packaging for margarine based on a nano composite polylactic acid/lycopene film. Food Additives & Contaminants: Part A. 2021;38:856–869. doi: 10.1080/19440049.2021.1891299. [DOI] [PubMed] [Google Scholar]
- Ponnusamy A, Rajasekaran B, Tagrida M, Prodpran T, Kim JT, Benjakul S. Bilayer polylactic acid and chitosan/gelatin film containing epigallocatechin gallate prepared through solvent casting and electrospinning: properties, bioactivities and release kinetics. Journal of Polymers and the Environment. 2023;32(1):1–17. [Google Scholar]
- Popescu V, Prodan D, Cuc S, Saroşi C, Furtos G, Moldovan A, Carpa R, Bomboş D. Antimicrobial poly (lactic acid)/copper nanocomposites for food packaging materials. Materials. 2023;16:1415. doi: 10.3390/ma16041415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radusin T, Tomšik A, Šarić L, Ristić I, GiacintiBaschetti M, Minelli M, Novaković A. Hybrid PLA/wild garlic antimicrobial composite films for food packaging application. Polymer Composites. 2019;40:893–900. doi: 10.1002/pc.24755. [DOI] [Google Scholar]
- Ramos M, Beltran A, Fortunati E, Peltzer M, Cristofaro F, Visai L, Valente AJM, Jiménez A, Kenny JM, Garrigós MC. Controlled release of thymol from poly (lactic acid)-based silver nanocomposite films with antibacterial and antioxidant activity. Antioxidants. 2020;9:395. doi: 10.3390/antiox9050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfearn HN, Goddard JM. Reactive extrusion of poly(lactic acid)-graft-curcumin antioxidant and intelligent packaging. ACS Applied Polymer Materials. 2023 doi: 10.1021/acsapm.3c1820. [DOI] [PubMed] [Google Scholar]
- Ren H, Li S, Gao M, Xing X, Tian Y, Ling Z, Yang W, Pan L, Fan W, Zheng Y. Preparation and characterization of microcrystalline cellulose/polylactic acid biocomposite films and its application in Lanzhou Lily (Lilium davidii var. unicolor) bulbs preservation. Sustainability. 2023;15:13770. doi: 10.3390/su151813770. [DOI] [Google Scholar]
- Ribeiro-Santos R, Andrade M, Madella D, Martinazzo AP, de Moura LAG, de Melo NR, Sanches-Silva A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends in Food Science & Technology. 2017;62:154–169. doi: 10.1016/j.tifs.2017.02.011. [DOI] [Google Scholar]
- Rigotti D, Soccio M, Dorigato A, Gazzano M, Siracusa V, Fredi G, Lotti N. Novel biobased polylactic acid/poly(pentamethylene 2,5-furanoate) blends for sustainable food packaging. ACS Sustainable Chemistry & Engineering. 2021;9:13742–13750. doi: 10.1021/acssuschemeng.1c04092. [DOI] [Google Scholar]
- Risyon NP, Othman SH, Basha RK, Talib RA. Characterization of polylactic acid/halloysite nanotubes bionanocomposite films for food packaging. Food Packaging and Shelf Life. 2020;23:100450. doi: 10.1016/j.fpsl.2019.100450. [DOI] [Google Scholar]
- Roy S, Rhim J-W. Preparation of bioactive functional poly (lactic acid)/curcumin composite film for food packaging application. International Journal of Biological Macromolecules. 2020;162:1780–1789. doi: 10.1016/j.ijbiomac.2020.08.094. [DOI] [PubMed] [Google Scholar]
- Saiwaew R, Suppakul P, Boonsupthip W, Pechyen C. Development and characterization of poly (lactic acid)/fish water soluble protein composite sheets: a potential approach for biodegradable packaging. Energy Procedia. 2014;56:280–288. doi: 10.1016/j.egypro.2014.07.159. [DOI] [Google Scholar]
- Samadi K, Francisco M, Hegde S, Diaz CA, Trabold TA, Dell EM, Lewis CL. Mechanical, rheological and anaerobic biodegradation behavior of a poly (lactic acid) blend containing a poly (lactic acid)-co-poly (glycolic acid) copolymer. Polymer Degradation and Stability. 2019;170:109018. doi: 10.1016/j.polymdegradstab.2019.109018. [DOI] [Google Scholar]
- Samsudin H, Soto-Valdez H, Auras R. Poly (lactic acid) film incorporated with marigold flower extract (Tagetes erecta) intended for fatty-food application. Food Control. 2014;46:55–66. doi: 10.1016/j.foodcont.2014.04.045. [DOI] [Google Scholar]
- Sánchez-López E, Gomes D, Esteruelas G, Bonilla L, Lopez-Machado AL, Galindo R, Cano A, Espina M, Ettcheto M, Camins A. Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials. 2020;10:292. doi: 10.3390/nano10020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonicola S, García Ibarra V, Sendón R, Mercogliano R, Rodríguez-Bernaldo de Quirós A. Antimicrobial films based on chitosan and methylcellulose containing natamycin for active packaging applications. Coatings. 2017;7:177. doi: 10.3390/coatings7100177. [DOI] [Google Scholar]
- Santoro M, Shah SR, Walker JL, Mikos AG. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Advanced Drug Delivery Reviews. 2016;107:206–212. doi: 10.1016/j.addr.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JCP, Sousa RCS, Otoni CG, Moraes ARF, Souza VGL, Medeiros EAA, Espitia PJP, Pires ACS, Coimbra JSR, Soares NFF. Nisin and other antimicrobial peptides: production, mechanisms of action, and application in active food packaging. Innovative Food Science & Emerging Technologies. 2018;48:179–194. doi: 10.1016/j.ifset.2018.06.008. [DOI] [Google Scholar]
- Santos CVM, Vieira IMM, Santos BLP, de Souza RR, Ruzene DS, Silva DP. Biosurfactant production from pineapple waste and application of experimental design and statistical analysis. Applied Biochemistry and Biotechnology. 2023;195:386–400. doi: 10.1007/s12010-022-04159-1. [DOI] [PubMed] [Google Scholar]
- Satsum A, Busayaporn W, Rungswang W, Soontaranon S, Thumanu K, Wanapu C. Structural and mechanical properties of biodegradable poly (lactic acid) and pectin composites: using bionucleating agent to improve crystallization behavior. Polymer Journal. 2022;54:921–930. doi: 10.1038/s41428-022-00637-9. [DOI] [Google Scholar]
- Savioli Lopes M, Jardini AL, Maciel Filho R. Poly (lactic acid) production for tissue engineering applications. Procedia Engineering. 2012;42:1402–1413. doi: 10.1016/j.proeng.2012.07.534. [DOI] [Google Scholar]
- Scarfato P, Avallone E, Galdi MR, Di Maio L, Incarnato L. Preparation, characterization, and oxygen scavenging capacity of biodegradable α-tocopherol/PLA microparticles for active food packaging applications. Polymer Composites. 2017;38:981–986. doi: 10.1002/pc.23661. [DOI] [Google Scholar]
- Shahrampour D, Razavi SMA, Sadeghi A. Evaluation of green tea extract incorporated antimicrobial/antioxidant/biodegradable films based on polycaprolactone/polylactic acid and its application in cocktail sausage preservation. Journal of Food Measurement and Characterization. 2023;17:1058–1067. doi: 10.1007/s11694-022-01670-1. [DOI] [Google Scholar]
- Shankar S, Wang L-F, Rhim J-W. Incorporation of zinc oxide nanoparticles improved the mechanical, water vapor barrier, UV-light barrier, and antibacterial properties of PLA-based nanocomposite films. Materials Science and Engineering: C. 2018;93:289–298. doi: 10.1016/j.msec.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Sharma S, Byrne M, Perera KY, Duffy B, Jaiswal AK, Jaiswal S. Active film packaging based on bio-nanocomposite TiO2 and cinnamon essential oil for enhanced preservation of cheese quality. Food Chemistry. 2023;405:134798. doi: 10.1016/j.foodchem.2022.134798. [DOI] [PubMed] [Google Scholar]
- Shavandi A, Ali MA. Keratin based thermoplastic biocomposites: a review. Reviews in Environmental Science and Bio/technology. 2019;18:299–316. doi: 10.1007/s11157-019-09497-x. [DOI] [Google Scholar]
- Shavisi N, Khanjari A, Basti AA, Misaghi A, Shahbazi Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Science. 2017;124:95–104. doi: 10.1016/j.meatsci.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Shu F, Jiang B, Yuan Y, Li M, Wu W, Jin Y, Xiao H. Biological activities and emerging roles of lignin and lignin-based products—a review. Biomacromolecules. 2021;22:4905–4918. doi: 10.1021/acs.biomac.1c00805. [DOI] [PubMed] [Google Scholar]
- Siddiqui MN, Redhwi HH, Tsagkalias I, Vouvoudi EC, Achilias DS. Development of bio-composites with enhanced antioxidant activity based on poly (lactic acid) with thymol, carvacrol, limonene, or cinnamaldehyde for active food packaging. Polymers. 2021;13:3652. doi: 10.3390/polym13213652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CG, Yudice EDC, Campini PAL, Rosa DS. The performance evaluation of Eugenol and Linalool microencapsulated by PLA on their activities against pathogenic bacteria. Materials Today Chemistry. 2021;21:100493. doi: 10.1016/j.mtchem.2021.100493. [DOI] [Google Scholar]
- Silveira VAI, Marim BM, Hipólito A, Gonçalves MC, Mali S, Kobayashi RKT, Celligoi MAPC. Characterization and antimicrobial properties of bioactive packaging films based on polylactic acid-sophorolipid for the control of foodborne pathogens. Food Packaging and Shelf Life. 2020;26:100591. doi: 10.1016/j.fpsl.2020.100591. [DOI] [Google Scholar]
- Sreekumar MG. Feasibility of plastic waste as reinforcement in the mechanical properties of stabilized lateritic soil blocks. In: PREPARE@ u®| IEI Conferences (2021)
- Sreekumar K, Bindhu B, Veluraja K. Perspectives of polylactic acid from structure to applications. Polymers from Renewable Resources. 2021;12:60–74. doi: 10.1177/20412479211008773. [DOI] [Google Scholar]
- Stan D, Enciu A-M, Mateescu AL, Ion AC, Brezeanu AC, Stan D, Tanase C. Natural compounds with antimicrobial and antiviral effect and nanocarriers used for their transportation. Frontiers in Pharmacology. 2021;12:723233. doi: 10.3389/fphar.2021.723233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru E, Vasile C, Irimia A, Brebu M. Towards a bioactive food packaging: poly (lactic acid) surface functionalized by chitosan coating embedding clove and argan oils. Molecules. 2021;26:4500. doi: 10.3390/molecules26154500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop C, Shukla M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. International Journal of Biological Macromolecules. 2018;113:729–736. doi: 10.1016/j.ijbiomac.2018.02.156. [DOI] [PubMed] [Google Scholar]
- Swaroop C, Shukla M. Development of blown polylactic acid-MgO nanocomposite films for food packaging. Composites Part A: Applied Science and Manufacturing. 2019;124:105482. doi: 10.1016/j.compositesa.2019.105482. [DOI] [Google Scholar]
- Swetha TA, Bora A, Mohanrasu K, Balaji P, Raja R, Ponnuchamy K, Muthusamy G, Arun A. A comprehensive review on polylactic acid (PLA)—synthesis, processing and application in food packaging. International Journal of Biological Macromolecules. 2023;234:123715. doi: 10.1016/j.ijbiomac.2023.123715. [DOI] [PubMed] [Google Scholar]
- Taib N-AAB, Rahman MR, Huda D, Kuok KK, Hamdan S, Bakri MKB, Julaihi MRMB, Khan A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polymer Bulletin. 2023;80:1179–1213. doi: 10.1007/s00289-022-04160-y. [DOI] [Google Scholar]
- Techawinyutham L, Siengchin S, Parameswaranpillai J, Dangtungee R. Antibacterial and thermomechanical properties of composites of polylactic acid modified with capsicum oleoresin-impregnated nanoporous silica. Journal of Applied Polymer Science. 2019;136:47825. doi: 10.1002/app.47825. [DOI] [Google Scholar]
- Tetali SD. Terpenes and isoprenoids: a wealth of compounds for global use. Planta. 2019;249:1–8. doi: 10.1007/s00425-018-3056-x. [DOI] [PubMed] [Google Scholar]
- Theinsathid P, Visessanguan W, Kruenate J, Kingcha Y, Keeratipibul S. Antimicrobial activity of lauric arginate-coated polylactic acid films against listeria monocytogenes and Salmonella typhimurium on cooked sliced ham. Journal of Food Science. 2012;77:M142–M149. doi: 10.1111/j.1750-3841.2011.02526.x. [DOI] [PubMed] [Google Scholar]
- Tripathi N, Monika, Katiyar V. Poly (lactic acid)/modified gum Arabic based bionanocomposite films: thermal degradation kinetics. Polymer Engineering & Science. 57: 1193-1206 (2017)
- Tumu K, Vorst K, Curtzwiler G. Global plastic waste recycling and extended producer responsibility laws. Journal of Environmental Management. 2023;348:119242. doi: 10.1016/j.jenvman.2023.119242. [DOI] [PubMed] [Google Scholar]
- Tutek K, Masek AM. Biodegradable PLA-based materials modified by hemp extract. Research Square. 2023 doi: 10.21203/rs.3.rs-2775247/v1rs. [DOI] [Google Scholar]
- Uitterhaegen E, Parinet J, Labonne L, Mérian T, Ballas S, Véronèse T, Merah O, Talou T, Stevens CV, Chabert F. Performance, durability and recycling of thermoplastic biocomposites reinforced with coriander straw. Composites Part A: Applied Science and Manufacturing. 2018;113:254–263. doi: 10.1016/j.compositesa.2018.07.038. [DOI] [Google Scholar]
- Videira-Quintela D, Martin O, Montalvo G. Recent advances in polymer–metallic composites for food packaging applications. Trends in Food Science & Technology. 2021;109:230–244. doi: 10.1016/j.tifs.2021.01.020. [DOI] [Google Scholar]
- Villegas C, Arrieta MP, Rojas A, Torres A, Faba S, Toledo MJ, Gutierrez MA, Zavalla E, Romero J, Galotto MJ. PLA/organoclay bionanocomposites impregnated with thymol and cinnamaldehyde by supercritical impregnation for active and sustainable food packaging. Composites Part B: Engineering. 2019;176:107336. doi: 10.1016/j.compositesb.2019.107336. [DOI] [Google Scholar]
- Wang P, Mele E. Effect of antibacterial plant extracts on the morphology of electrospun poly (lactic acid) fibres. Materials. 2018;11:923. doi: 10.3390/ma11060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang Q, Xu Z, Zhang W, Xiang J. Effect of fermentation conditions on l-lactic acid production from soybean straw hydrolysate. Journal of Microbiology and Biotechnology. 2015;25:26–32. doi: 10.4014/jmb.1405.05025. [DOI] [PubMed] [Google Scholar]
- Wang Z, Tu J, Gao Y, Xu P, Ding Y. Fabricating super tough polylactic acid based composites by interfacial compatibilization of imidazolium polyurethane modified carbon nanotubes. International Journal of Biological Macromolecules 242: 125079 (2023a) [DOI] [PubMed]
- Wang Y, Zhang C, Liu F, Jin Z, Xia X. Ecological succession and functional characteristics of lactic acid bacteria in traditional fermented foods. Critical Reviews in Food Science and Nutrition 63: 5841-5855 (2023b) [DOI] [PubMed]
- Wongphan P, Nampanya P, Chakpha W, Promhuad K, Laorenza Y, Leelaphiwat P, Bumbudsanpharoke N, Sodsai J, Lorenzo JM, Harnkarnsujarit N. Lesser galangal (Alpinia officinarum Hance) essential oil incorporated biodegradable PLA/PBS films as shelf-life extension packaging of cooked rice. Food Packaging and Shelf Life. 2023;37:101077. doi: 10.1016/j.fpsl.2023.101077. [DOI] [Google Scholar]
- Yakdoumi FZ, Hadj-Hamou AS, Rahoui N, Rahman MM, Abetz V. Polylactic acid nanocomposites containing functionalized multiwalled carbon nanotubes as antimicrobial packaging materials. International Journal of Biological Macromolecules. 2022;213:55–69. doi: 10.1016/j.ijbiomac.2022.05.142. [DOI] [PubMed] [Google Scholar]
- Yang W, Weng Y, Puglia D, Qi G, Dong W, Kenny JM, Ma P. Poly (lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. International Journal of Biological Macromolecules. 2020;144:102–110. doi: 10.1016/j.ijbiomac.2019.12.085. [DOI] [PubMed] [Google Scholar]
- Yu H, Huang X, Zhou L, Wang Y. Incorporation of cinnamaldehyde, carvacrol, and eugenol into zein films for active food packaging: enhanced mechanical properties, antimicrobial activity, and controlled release. Journal of Food Science and Technology. 2023;60:2846–2857. doi: 10.1007/s13197-023-05802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zainab-L I, Sudesh K. High cell density culture of Cupriavidus necator H16 and improved biological recovery of polyhydroxyalkanoates using mealworms. Journal of Biotechnology. 2019;305:35–42. doi: 10.1016/j.jbiotec.2019.09.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rempel C, Liu Q. Thermoplastic starch processing and characteristics—a review. Critical Reviews in Food Science and Nutrition. 2014;54:1353–1370. doi: 10.1080/10408398.2011.636156. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhou X, Wang D, Fang C, Zhang W, Wang C, Huang Z. Lysozyme-based composite membranes and their potential application for active packaging. Food Bioscience. 2021;43:101078. doi: 10.1016/j.fbio.2021.101078. [DOI] [Google Scholar]
- Zhang Q, Zhai W, Cui L, Liu Y, Xie W, Yu Q, Luo H. Physicochemical properties and antibacterial activity of polylactic acid/starch acetate films incorporated with chitosan and tea polyphenols. Polymer Bulletin. 2023;80:13319–13341. doi: 10.1007/s00289-023-04691-y. [DOI] [Google Scholar]
- Zhao P, Liu Z, Wang X, Pan Y-T, Kuehnert I, Gehde M, Wang D-Y, Leuteritz A. Renewable vanillin based flame retardant for poly (lactic acid): a way to enhance flame retardancy and toughness simultaneously. RSC Advances. 2018;8:42189–42199. doi: 10.1039/C8RA08531E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Jia X, Zhao Z, Ran Y, Du M, Ji H, Pan Y, Li Z, Ma X, Liu Y, Duan L, Li X. Innovative natural antimicrobial natamycin incorporated titanium dioxide (nano-TiO2)/poly (butylene adipate-co-terephthalate) (PBAT) /poly (lactic acid) (PLA) biodegradable active film (NTP@PLA) and application in grape preservation. Food Chemistry. 2023;400:134100. doi: 10.1016/j.foodchem.2022.134100. [DOI] [PubMed] [Google Scholar]
- Zibaei R, Ebrahimi B, Rouhi M, Hashami Z, Roshandel Z, Hasanvand S, de Toledo Guimarães J, Goharifar M, Mohammadi R. Development of packaging based on PLA/POE/SeNPs nanocomposites by blown film extrusion method: physicochemical, structural, morphological and antioxidant properties. Food Packaging and Shelf Life. 2023;38:101104. doi: 10.1016/j.fpsl.2023.101104. [DOI] [Google Scholar]
- Zouari M, Devallance DB, Marrot L. Effect of biochar addition on mechanical properties, thermal stability, and water resistance of hemp-polylactic acid (PLA) composites. Materials. 2022;15:2271. doi: 10.3390/ma15062271. [DOI] [PMC free article] [PubMed] [Google Scholar]