Abstract
Premature death in diabetes is increasingly caused by cancer. The objectives were to estimate the excess mortality when individuals with type 2 diabetes(T2D) were diagnosed with cancer, and to examine the impact of modifiable diabetes-related risk factors. This longitudinal nationwide cohort study included individuals with T2D registered in the Swedish National Diabetes Register between 1998–2019. Poisson models were used to estimate mortality as a function of time-updated risk-factors, adjusted for sex, age, diabetes duration, marital status, country of birth, BMI, blood pressure, lipids, albuminuria, smoking, and physical activity. We included 690,539 individuals with T2D and during 4,787,326 person-years of follow-up 179,627 individuals died. Overall, the all-cause mortality rate ratio was 3.75 [95%confidence interval(CI):3.69–3.81] for individuals with T2D and cancer compared to those remaining free of cancer. The most marked risk factors associated to mortality among individuals with T2D and cancer were low physical activity, 1.59 (1.57–1.61) and smoking, 2.15 (2.08–2.22), whereas HbA1c, lipids, hypertension, and BMI had no/weak associations with survival. In a future with more patients with comorbid T2D and cancer diagnoses, these results suggest that smoking and physical activity might be the two most salient modifiable risk factors for mortality in people with type 2 diabetes and cancer.
Subject terms: Cancer, Endocrinology, Risk factors
Introduction
The incidence of type 2 diabetes (T2D) is increasing and affects 247 million people worldwide1. Also, the global cancer burden is expected to increase by 47%, rising from 19.3 million new cancer cases in 2020 to an estimated 28.4 million cases in 20402,3. Previously, the main cause of death in T2D was cardiovascular disease4. However, the leading cause of death among people with T2D has transitioned from vascular diseases to cancer during the past decade4.
It is evident, that T2D is associated with an increased risk of total cancer and many site-specific cancers5. The mechanisms behind the increased risk have not yet been clarified, but are probably a combination of different direct effects (hyperglycaemia, insulin resistance, and hyperinsulinemia)6 and indirect effects, through shared risk factors such as obesity, physical inactivity, diet, alcohol use and smoking7,8.
Today, focus on comorbidity among cancer patients is becoming more crucial as the long-term survival has improved for many types of cancer9. Therefore, the overall survival and morbidity not only depends on cancer specific treatment, but also to some extent depends on how other underlying lifestyle conditions are handled during and after cancer treatment8.
Several studies have showed that, compared with other patients with cancer, those with pre-existing diabetes have higher perioperative and long-term mortality10–12. Although the effects of a cancer diagnosis on diabetes management are mixed, a review by Pinheiro et al. concluded, that diabetes management appeared to generally decline after a cancer diagnosis primarily due to shifts in the priority of care from diabetes management to cancer treatment13.
A previous study utilizing the Swedish National Diabetes Register (NDR) revealed a heightened incidence of cancer development among individuals with T2D and reduced likelihood of survival compared to matched controls without diabetes. However, no nationwide healthcare studies have investigated the excess mortality among individuals with T2D who are diagnosed with cancer. Furthermore, it remains unclear whether certain modifiable diabetes-related risk factors exert a stronger influence on mortality following a cancer diagnosis in individuals with T2D, and whether this association differs depending on the type of cancer.
To address these gaps, we utilized data from the NDR linked to various nationwide registers in order to estimate the excess mortality among individuals with T2D upon receiving a diagnosis of one of the most prevalent cancers, including breast, lung, prostate, or colorectal cancer. Additionally, we aimed to examine the relationship between mortality risk and modifiable diabetes-related risk factors, such as HbA1c, total cholesterol, LDL cholesterol, hypertension, BMI, smoking, and physical activity.
Methods
Study population and design
The study was based on all individuals with T2D registered in NDR11,14 between 1998 until 2019 (Fig. 1). Both specialist clinics and primary health care clinics report to the NDR, and in 2017 the register included 90% of the diabetes population. We used an epidemiological definition of type 2 diabetes: patients of all ages receiving only dietary treatment, or oral glucose-lowering agents only, or persons diagnosed after the age of 40 years receiving insulin therapy or insulin and oral glucose-lowering agents.
Figure 1.
Flow chart for obtaining the study population.
The individual entry date (index date) was defined as the earliest date in the NDR. Excluded were those with cancer diagnosis other than breast, lung, prostate, colorectal before a diagnosis of T2D, except from and non-melanoma skin cancer, as it is usually not considered as a malignant disease in oncological epidemiological studies.
Data sources
The NDR is a clinical database containing time-updated clinical records on adult individuals with diabetes in Sweden. The NDR has detailed data on year of diabetes diagnosis, diabetic treatment (diet, tablets, insulin, and tablets and insulin), diabetes-related complications such as neuropathy, retinopathy and diabetic kidney disease. The NDR also holds information on both risk factors such as body mass index (BMI) and blood pressure, but also health behaviours and lifestyle habits such as physical activity level, and smoking. Smoking was defined as active smoking within 3 months prior inclusion (or the yearly update) and low physical activity as 30 min exercise less than 3 times per week. Laboratory measurements included urine samples and blood samples providing data on HbA1c and lipid levels.
The NDR data were linked with other nationwide registers at the individual level using unique personal identification numbers, which are assigned to all inhabitants of Sweden at birth or at the time of immigration. These data included information on demographic and socioeconomic status from Statistics Sweden15. Education was categorised as; 9 years or less, 10–12 years, and more than 12 years, and Country of birth as; Sweden, Europe except Sweden, and Rest of the world. Information on coexisting conditions were retrieved from the Swedish Inpatient Register16.
The main exposure was breast (only women), prostate (only men), colorectal or lung cancer. Date and type of cancer diagnosis was retrieved from The Swedish Cancer registry17, using ICD-7 codes (Breast: 170, Prostate: 177, Colorectal: 153, 154, and Lung: 162.0, 162.1, 162.8. Depending on the diagnosis date of T2D and a potential date of breast, prostate, colorectal, or lung cancer, the study population was divided into three groups: “No cancer” included those with T2D and no diagnosis of cancer, “Cancer before” included those with a diagnosis of breast, prostate, colorectal, or lung cancer before they were included in the NDR, and “Cancer after” included those with a diagnosis of breast, prostate, colorectal, or lung cancer after they were included in the NDR.
The primary outcome was all-cause mortality identified in the Causes of death register18.
Ethics
The study was approved by the Swedish Ethical Review Authority. Individual informed consent is not required to report patients to national quality registries of healthcare, or to be included in a study like this, according to Swedish law (Patient Data Act 2008:355, chapter 7). The methods were performed in accordance with the STROBE guideline for cohort studies (https://www.strobe-statement.org/checklists/). All methods were performed in accordance with the relevant guideline and the declaration of Helsinki.
Statistical analysis
Characteristics of included individuals are presented as means with standard deviations (SD) for continuous variables and as proportions (n, %) for categorical variables.
The individual entry date (index date) was defined as the earliest date in the NDR, and the participants were followed until death or end of follow up 31 December 2018, which ever came first. For patients with diabetes before the start of the NDR (1998), this date is not the same as the date of onset of diabetes. The study aimed to model mortality in those who had cancer before diabetes and those who developed cancer after inclusion in NDR. Therefore, patients could change state during follow-up i.e., those with “no cancer” transitioned from that state to “cancer after” when diagnosed with one of the four specific cancers under scope. To allow for time-updated analysis, follow-up time was split at each registration in the NDR and/or at cancer diagnosis. Each interval was assigned the time-updated values for age and duration of diabetes.
We plotted cumulative mortality proportion (CMP) curves both for those with “no cancer”, “cancer before” and “cancer after”. The CMP were both plotted for all cancers and stratified by cancer type (i.e., breast cancer, prostate cancer, colorectal cancer, and lung cancer). For those with “No cancer” and “Cancer before” the timescale in the CMP was time since inclusion in NDR, while the timescale for “Cancer after” was time since date of cancer to imitate the state transition and timescales in the Poisson models. Also, 5 year cumulative survival was extracted from the CMP.
We used Poisson models with log of the risk time (the length of each interval) as offset for mortality, while also including the indicators of diagnosis of cancer i.e., patients contributed with follow-up time in their specific risk state in each interval. In addition to the time scales, we included in Model 1: sex, age, country of birth, educational level, diabetes duration and the time-updated values for marital status, and Model 2 additionally the time-updated values for: smoking status, physical activity level, BMI, systolic blood pressure, HbA1c, albuminuria and low-density lipoprotein (LDL)-cholesterol.
We present both crude and adjusted mortality rate ratios (MRRs) between individuals with and without a diagnosis of breast, lung, prostate or colorectal cancer. All statistical analysis was conducted with Rstudio (version 4.0.5).
We imputed missing data to both maximize the sample size. Due to massive computational time required, we used a single imputation using changed equations (MICE) instead of multiple imputation.
Results
Table 1 displays the study population (N = 690 539) divided into 3 groups based on whether the participant had a diagnosis of cancer or not; “No cancer” (N = 588 095), “Cancer before” (N = 34 883), and “Cancer after” (N = 32 366).
Table 1.
Characteristics of patients at inclusion in the Swedish National Diabetes Registrer.
| No cancer | Cancer before | Cancer after | |
|---|---|---|---|
| N | 588,095 | 34,883 | 32,366 |
| Female sex | 255,147 (43.4) | 15,133 (43.4) | 10,780 (33.3) |
| Age (years) | 63 (13.1) | 73 (9.1) | 67 (9.1) |
| Age at diagnosis (years) | 59 (13.7) | 68 (10.8) | 62 (10.8) |
| Diabetes duration (years) | 4.4 (6.9) | 4.8 (7.4) | 5.4 (6.9) |
| Follow-up (years) | 7.3 (4.7) | 5.8 (4.1) | 9.2 (4.5) |
| Region of birth | |||
| Sweden | 462,893 (78.7) | 30,497 (87.4) | 27,906 (86.2) |
| Europe except Sweden | 30,052 (5.1) | 1711 (4.9) | 1774 (5.5) |
| Rest of the world | 95,150 (16.2) | 2675 (7.7) | 2686 (8.3) |
| Body mass index (kg/m2) | 30.2 (5.6) | 28.9 (5.0) | 29.4 (5.0) |
| Systolic blood pressure (mmHg) | 138 (17.9) | 139 (17.9) | 141 (17.9) |
| Diastolic blood pressure (mmHg) | 79 (10.1) | 77 (9.9) | 78.6 (9.7) |
| Diabetes treatment | |||
| Diet | 225,558 (38.4) | 14,625 (41.9) | 12,555 (38.8) |
| Tablets | 272,306 (46.3) | 14,632 (41.9) | 13,607 (42.0) |
| Insulin | 46,802 (8.0) | 3199 (9.2) | 3095 (9.6) |
| Tablets and insulin | 43,429 (7.4) | 2427 (7.0) | 3109 (9.6) |
| Antihypertensive medication | 368,419 (62.6) | 25,079 (71.9) | 21,947 (67.8) |
| Lipid-lowering medication | 233,899 (39.8) | 14,473 (41.5) | 13,905 (43.0) |
| HbA1c (mmol/mol) | 55 (16.8) | 53 (14.3) | 54 (14.3) |
| HbA1c (%) | 7.2 (1.6) | 7.0 (1.3) | 7.1 (1.3) |
| Total cholesterol (mmol/L) | 5.08 (1.18) | 5.04 (1.17) | 5.02 (1.13) |
| LDL cholesterol (mmol/L) | 2.92 (1.00) | 2.87 (1.00) | 2.87 (0.98) |
| HDL cholesterol (mmol/L) | 1.24 (0.41) | 1.28 (0.42) | 1.26 (0.41) |
| Triglycerol (mmol/L) | 2.19 (1.82) | 2.08 (1.65) | 2.10 (1.60) |
| NonHDL:HDL-ratio | 3.44 (1.72) | 3.27 (1.68) | 3.32 (1.59) |
| Albuminuria (> 20 ug/min) | 118,598 (20.2) | 7852 (22.5) | 6911 (21.4) |
| Creatinine (umol/L) | 79.4 (30.9) | 85.5 (35.5) | 83.4 (30.8) |
| Current smoker, n | 95,818 (16.3) | 3600 (10.3) | 5394 (16.7) |
| Physical active (< 3 times/week) | 175,449 (29.8) | 11,568 (33.2) | 9532 (29.5) |
| CVD at inclusion | 67,873 (11.5) | 5237 (15.0) | 3772 (11.7) |
| Unmarried | 280,787 (47.7) | 15,713 (45.0) | 13,367 (41.3) |
| Education (years) | |||
| 9 years or less | 235,042 (40.0) | 15,936 (45.7) | 14,952 (46.2) |
| 9–12 years | 247,165 (42.0) | 13,042 (37.4) | 12,435 (38.4) |
| College/University | 105,888 (18.0) | 5905 (16.9) | 4979 (15.4) |
Data are means (sd) or n (%).
The groups differed in mean age at diabetes onset and follow-up, the participants with “No Cancer” were younger at time of diabetes onset (59 years vs 62 years) but had a shorter mean follow-up (7.3 years vs 9.2 years) compared with the “Cancer after” group. The “Cancer before” group had the oldest mean age of diabetes onset (68 year) and the shortest follow-up (5.8 year). The proportion of women was lower in the “Cancer after” group (33%) when compared to the other two groups (43%).
Compared to the “No cancer” group, those with “Cancer after” had similar metabolic characteristics, level of physical activity and smoking status, whereas more patients received medication aimed for metabolic dysfunction. The “Cancer before” group was in general more similar to the “Cancer after” than the “No cancer group”.
Mortality risk
By the end of study, a total of 179,627 individuals had died during 4,787,326 person-years of follow-up (Table 2).
Table 2.
Person years, numbers of events and mortality rates among individuals with type 2 by cancer overall and cancer type.
| Number of events | Person-years | Mortality rate (per 10,000 PY) | Mortality rate ratios (crude) | Mortality rate ratios (Adj 1) | Mortality rate ratios (Adj 2) | |
|---|---|---|---|---|---|---|
| Cancer overall | ||||||
| No cancer | 147,861 | 4,455,931 | 3.32 (3.30–3.34) | ref | ref | ref |
| Cancer before | 15,552 | 201,003 | 7.74 (7.62–7.86) | 2.33 (2.29–2.37) | 1.42 (.4–1.45) | 1.41 (1.38–1.43) |
| Cancer after | 16,214 | 130,392 | 12.43 (12.24–12.63) | 3.75 (3.69–3.81) | 2.42 (2.38–2.46) | 2.34 (2.31–2.38) |
| Breast | ||||||
| No cancer | 67,025 | 1,921,086 | 3.49 (3.46–3.52) | Ref | Ref | ref |
| Cancer before | 4590 | 73,158 | 6.27 (6.1–6.46) | 1.8 (1.75–1.85) | 1.32 (1.28–1.36) | 1.31 (1.27–1.35) |
| Cancer after | 1978 | 26,112 | 7.58 (7.25–7.92) | 2.17 (2.08–2.27) | 1.67 (1.60–1.74) | 1.61 (1.54–1.68) |
| Prostate | ||||||
| No cancer | 80,619 | 2,458,637 | 3.28 (3.26–3.30) | Ref | Ref | ref |
| Cancer before | 6342 | 77,127 | 8.22 (8.02–8.43) | 2.51 (2.44–2.57) | 1.38 (1.35–1.42) | 1.38 (1.34–1.42) |
| Cancer after | 4923 | 62,530 | 7.87 (7.66–8.1) | 2.40 (2.33–2.47) | 1.45 (1.41–1.49) | 1.43 (1.39–1.47) |
| Colorectal | ||||||
| No cancer | 147,666 | 4,337,358 | 3.40 (3.39–3.42) | ref | ref | ref |
| Cancer before | 3854 | 45,112 | 8.54 (8.28–8.82) | 2.51 (2.43–2.59) | 1.33 (1.29–1.38) | 1.30 (1.26–1.35) |
| Cancer after | 5023 | 33,991 | 14.78 (14.37–15.19) | 4.34 (4.22–4.46) | 2.53 (2.46–2.60) | 2.43 (2.36–2.50) |
| Lung | ||||||
| No cancer | 147,690 | 4,315,414 | 3.42 (3.4–3.44) | Ref | ref | ref |
| Cancer before | 732 | 5387 | 13.59 (12.64–14.61) | 3.97 (3.69–4.27) | 3.07 (2.85–3.30) | 2.80 (2.60–3.01) |
| Cancer after | 4261 | 7448 | 57.21 (55.52–58.95) | 16.72 (16.22–17.23) | 13.54 (13.14–13.96) | 11.75 (11.39–12.11) |
The diabetic population was categorized into three groups according to the presence/absence of cancer diagnosis in relation to the diagnosis of type 2 diabetes: “No cancer,” “Cancer before,” and “Cancer after.”
Model 1: sex, age, Country birth, educational level, diabetes duration and civil status.
Model 2: model 1, smoking, physical activity level, BMI, systolic blood pressure, HbA1c, albuminuria and LDL-cholesterol.
Figure 2 shows the cumulative mortality (CMP) and 95% CI for those with “No cancer”, “Cancer before”, and “Cancer after”. Compared to those with “No cancer” we saw a higher mortality in those with “Cancer after” that emerged very soon after inclusion, whereas the curves had almost similar slopes in the interval from 5 to 10 years follow-up. The cumulative mortality was highest among those with “Cancer after”. The cancer specific CMPs showed different patterns for the site-specific cancers. Lung cancer had the fastest increase in mortality, especially in the “cancer after” group while prostate cancer showed higher cumulative mortality in those with cancer, but no differences between those with “Cancer before” and “Cancer after”. 5 year cumulative survival was extracted from the CMP (Fig. 2). The cumulative survival after five years was 87.4% (95% CI: 87.4–87.5) in those with “No cancer”, 70.7% (70.2–71.3) in those with cancer before, and 54.7 (54.1–55.3) in those with “Cancer after”.
Figure 2.
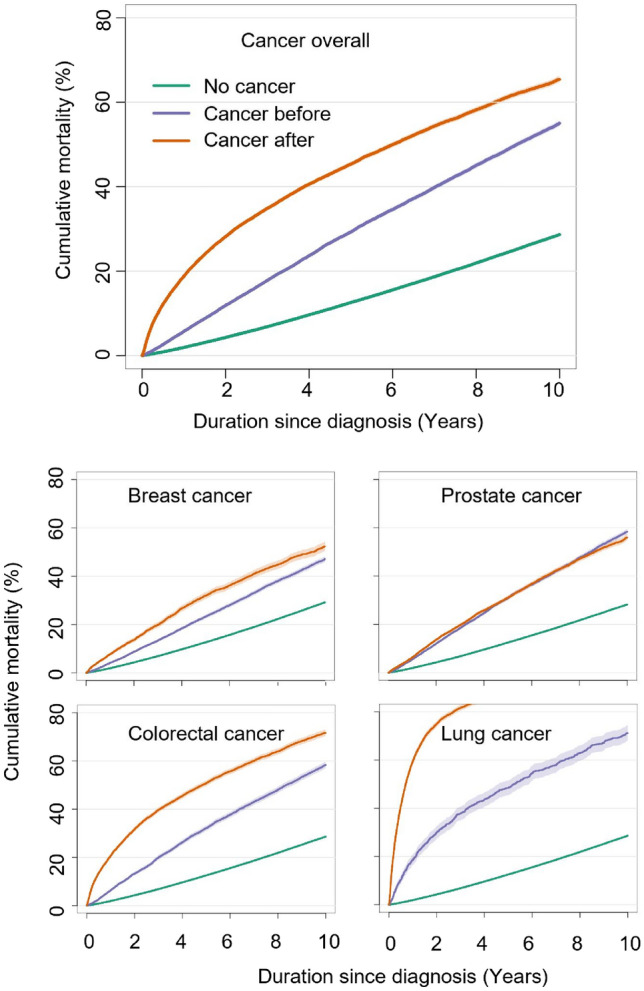
Cumulative mortality among individuals with Type 2 Diabetes, stratified by the presence or absence of cancer diagnosis in relation to the diagnosis of type 2 diabetes.
Table 2 shows the crude mortality rates and mortality rate ratios (MRR). We saw a higher all-cause MRR 3.75 [95% confidence interval (CI): 3.69–3.81] and a higher adjusted MRR: 2.42 (2.38—2.46) with similar results when taking metabolic measurements, smoking and activity into account (model 2). In the “Cancer after” group, the adjusted MRR was much higher for those diagnosed with lung cancer 13.5 (13.1–14.0), than those diagnosed with prostate cancer 1.44 (1.41–1.49), breast cancer 1.67 (1.60–1.74) or colorectal cancer 2.53 (2.46–2.60). Like the overall estimates, the cancer specific MRR were slightly lower in the adjusted analysis, with limited difference between model 1 and model 2.
Similar findings were observed among those diagnosed with “Cancer before”, but the associations were less pronounced than observed in the “Cancer after” group.
Risk factors associated to mortality
Table 3 demonstrates the excess mortality associated to each modifiable risk factor, reported as adjusted MRR within the 3 groups. Smoking turned out to be an important risk factor, smokers had a 1.65 (1.63–1.67) higher mortality rate compared to non-smokers. The association was strongest in those with “Cancer after”: 2.5 (2.40–2.61) when compared to “No cancer”: 1.57 (1.55–1.60) and “Cancer before”: 1.59 (1.50–1.68). The cancer specific adjusted MMR comparing smoker vs non-smokers (Supplementary table S1-4) were in general in the same range, regardless of type of cancer. The association was strongest in the “Lung cancer before” group: 2.32 (1.93–2.79) (Supplementary table S4) and weakest in the “Prostate cancer before” group:1.34 (1.22–1.46) (Supplementary table S2).
Table 3.
Excess mortality (adjusted Mortality Rate Ratio) associated with various modifiable risk factors among individuals with Type 2 Diabetes: overall and stratified by the presence/absence of cancer diagnosis in relation to the diagnosis of type 2 diabetes.
| Overall | No cancer | Cancer before | Cancer after | |
|---|---|---|---|---|
| HbA1c (per 10 mmol/mol) | 1.08 (1.07–1.08) | 1.09 (1.08–1.09) | 1.07 (1.05–1.08) | 0.99 (0.98–1.00) |
| LDL (per 1 mmol/L) | 1.02 (1.02–1.03) | 1.02 (1.01–1.02) | 1.01 (1.00–1.03) | 1.07 (1.05–1.09) |
| Non-HDL ratio | 1.06 (1.06–1.06) | 1.06 (1.06–1.06) | 1.06 (1.05–1.06) | 1.05 (1.04–1.06) |
| Systolic blood pressure (per 10 mmHg) | 0.97 (0.96–0.97) | 0.97 (0.96–0.97) | 0.96 (0.95–0.96) | 0.97 (0.97–0.97) |
| BMI (per 5 kg/m2) | 0.93 (0.93–0.94) | 0.93 (0.92–0.93) | 0.91 (0.89–0.93) | 0.99 (0.98–1.01) |
| Smoking (any smoking vs. never smoking) | 1.65 (1.63–1.67) | 1.57 (1.55–1.6) | 1.59 (1.5–1.68) | 2.5 (2.4–2.61) |
| Physical activity (less than 3 times/week vs more) | 1.95 (1.93.1.97) | 2.03 (2.01–2.05) | 1.74 (1.68–1.79) | 1.48 (1.43–1.52) |
All estimates are adjusted for sex, age, country birth, educational level, diabetes duration and civil status.
Low physical activity defined as 30 min exercise less than 3 times per week doubled the mortality rate (Table 3) when compared to more active people, 1.95 (1.93–1.97). The associations were more pronounced in those with “No cancer” 2.03 (2.01–2.05) compared to “Cancer before”: 1.74 (1.68–1.79) and “Cancer after”: 1.48 (1.43–1.52). Looking at each cancer type separately (Supplementary table S1-4), low physical activity had the strongest prognostic impact among those with prostate cancer 1.86 (1.77–1.96) in the “Cancer before” and 1.78 (1.68–1.88) in the “Cancer after”(Supplementary table S2) and the weakest among those with “Lung cancer after”: 1.09 (1.02–1.16) (Supplementary table S4).
In contrast, the other modifiable risk factors such as HbA1c, total cholesterol, LDL cholesterol, hypertension, and BMI only had little or no prognostic impact on mortality, both overall and by each specific cancer type.
Discussion
Our study revealed a significant elevation of more than three times in all-cause mortality among individuals with T2D who received a diagnosis of breast, prostate, colorectal, or lung cancer. The magnitude of excess mortality varied depending on the type of cancer—highest for those diagnosed with lung cancer. Smoking and lack of exercise emerged as the most influential modifiable risk factors associated with mortality in the Swedish diabetic population.
Previous research has predominantly focused on comparing individuals with and without T2D, establishing a well-established link between T2D and an elevated risk of developing cancer5, as well as higher post-cancer mortality11. Furthermore, the leading cause of death in T2D has transitioned from cardiovascular disease to cancer4 and consequently, a significant proportion of individuals with T2D also experience the burden of cancer. However, despite the clinical importance of this comorbidity as part of the diabetes care, there is a notable paucity of nationwide studies elucidating the precise influence of cancer on mortality in people with T2D.
Post-cancer mortality
In this study we examined how mortality in diabetes is affected by the concurrent presence of cancer, occurring either before or after the T2D diagnosis. The excess mortality was highest in those with prevalent diabetes and subsequent cancer development (the “Cancer after” group). Although the group of patients with “Cancer before” did have higher mortality than those with “No cancer”, the excess in mortality was more modest than in the “Cancer after” group. These results are expected, as it is well-known that the excess mortality in cancer is highest in the period early after diagnoses and declines with time. The “cancer before” group is most likely biased as a survivor group, as they have already survived for some time before inclusion in the NDR, while those with more aggressive cancer and/or poorer general health, died before the potential inclusion in the NDR. The excess mortality in those with “Cancer after” is mainly driven by higher mortality rates in the first few years after diagnosis i.e. the slopes of the cumulative mortality curves are highly comparable after few years.
As expected, the excess mortality differed with cancer type. Patients with prevalent diabetes and subsequent lung cancer (“Cancer after”) had a 13 times higher mortality rate compared to those without cancer. The excess in mortality is reflecting the aggressive nature of lung cancer. While the 5- survival in our study was 87,4% in those with “No cancer” the 5 year survival for those with lung cancer after T2D was only 13.1%. For comparison, patients with lung cancer, but no diabetes, are reported to have a 5 year survival rate of < 30% in the general Swedish population19.In contrast, the 5 year survival rate in the general Swedish population after prostate cancer is 95% among men, 92% after breast cancer in women, and 70% after colorectal cancer19. Mortality was higher in the “Cancer after” group, compared with the “Cancer before” group for all specific cancer sites, except, prostate cancer. This may be explained by the overall high survival in those with prostate cancer.
A previous study has examined cancer deaths in the Swedish diabetes population compared to the background population (matched controls), and the same pattern of associations across cancer types was observed11. That study showed that diabetes has no impact on post-cancer mortality after lung cancer (reflecting poor prognosis for everyone, regardless of diabetes status), whereas diabetes is associated to a higher post-cancer mortality after prostate cancer (HR: 1.29 (1.25–1.35)) and breast cancer (HR:1.25 (1.18–1.33)), and to a lesser degree colorectal cancer (HR: 1.09 (1.05–1.13). These findings highlight that in cancer types with long-term survival (good prognosis), the overall survival becomes dependent on how comorbidity is handled during and after cancer treatment.
Diabetes related risk factors
In our study smoking and lack of exercise were the two most salient modifiable risk factors associated with mortality in those with diabetes and cancer.
In general it is well-established, that smoking is strongly associated to incident smoking-related as well as non-smoking related cancer and cancer mortality20, and there is dose–response association between physical activity and all cancer mortality21. Recently, a study based on 5 different cohorts of individuals with diabetes reported that those with the healthiest lifestyle (current nonsmoking, low-to-moderate alcohol drinking, adequate physical activity, healthy diet and optimal bodyweight) have a 45% lower cancer mortality than those with a less healthy lifestyle8. When looking at each individual lifestyle factor, smoking had the most profound impact on cancer mortality—in line with our results. In contrast, the authors did not find a significant association between physical activity and cancer mortality. There are several reasons for this discrepancy. First of all, the difference in outcome. They used cancer specific mortality, whereas in our study, the outcome was all cause mortality. Furthermore, our study was based on nation-wide registers linked to the NDR (including almost all Swedish residents with diabetes with time-updated assessments), whereas the study by Zhang et al. was based on survey data from 5 different cohorts, and diabetes was defined based on single-measure biomarkers or self-reports. Finally, unhealthy physical activity was defined very differently within the 5 studies (≤ 20 min less than 3 times/week, < 80 or 150 min/week exercise, bottom 2/3 of total activity or frequency of leisure-time physical activity) and different from our definition of 30 min exercise less than 3 times per week.
Our study suggests, that both smoking and physical activity not only contribute to the development of diabetes and cancer22,23, but they may be linked with increased mortality in individuals with diabetes regardless of cancer status. It is also conceivable that the physical activity levels recorded in the NDR are already affected by ill-health, possibly connected to pre-clinical stages of cancer. As such, there is some potential for reverse causality. In reality the association between physical inactivity and cancer is likely to be bi-directional, as it is for diabetes. Based on our observational data it is impossible to elucidate the most likely sequence.
Pinheiro et al. described the impact of cancer on diabetes management in a review including 36 studies (22 of them conducted in US and 7 in Korea), and the authors concluded that the effects are mixed13. In our study, metabolic risk factors such as HbA1c, LDL-cholesterol, systolic and blood pressure and BMI had almost no association with mortality in both the diabetic population without cancer and with cancer, corresponding with results from the Zhang study22. In line with these findings a study based on UK Clinical Practice Research Datalink data (N > 11.3 million patients) comparing three cohorts of individuals with diabetes and a subsequent diagnosis of breast, colorectal or prostate cancer matched to non-cancer controls with diabetes found no difference in micro- or macrovascular complication between the groups24.
Overall, the BMI in the three groups was similar, however we observed a slightly higher BMI in the “no cancer” group compared to those with cancer. This may partly be explained by the catabolic state caused by the concomitant cancer disease. The Swedish diabetic population has almost achieved the cardio-metabolic treatment goals for LDL cholesterol (2.92 mmol/l), and hypertension (systolic blood pressure 138 mmHg)—and consequently there is little room for improvement in cardiovascular protection, which may explain the small association with mortality for these risk factors. HbA1c was fairly well-regulated, but still approximately 50% of the population had a HbA1c above 7.2% (mean HbA1c 55 mmol/mol (7.2%)), and may benefit from more strict glycaemic control—especially those with cancer before inclusion in the NDR.
Strengths and limitations
One of the strengths of this study was that the NDR has high coverage, with > 95% of adults with type 2 diabetes in Sweden and almost 100% of outpatient diabetes clinics represented in the register. The data we used consist of detailed time-updated information with repeated measures of clinical, socioeconomic and outcome data, making it possible to isolate the excess mortality associated to being diagnosed with cancer while accounting for the effects carried by age, duration of diabetes and other covariates. Our findings should be interpreted in the light of the observational nature of the data, which renders definitive causal inference impossible. Both smoking, physical activity, and the other self-reported lifestyle outcome may be prone to recall bias. Nevertheless, the study design mitigated this potential limitation through repeated measurement, which fragmented the recall period into minor time spans. An important limitation in this study is the lack of data on cancer state (regional or progressive disease), cancer status (active or cured cancer), and cancer treatment. Cancer and/or cancer treatment may induce diabetes (e.g. corticosteroid treatment), however, as we do not have access to the cancer state or the cancer specific treatment we are not able to assess any potential bias caused by diabetes induced by cancer and/or cancer treatment. Nor can we assess the potential effect of cancer status (active versus cured cancer). A potential limitation is misclassification. The slowly progressive nature and the hidden symptoms early in diabetes mean that up to 50% of individuals with type 2 diabetes are unaware—and undiagnosed—early in the disease25. As such patients may be misclassified in the “cancer before” group although they had diabetes first. Also, most cancers (especially prostate) are slowly developing and have some months/years of lead time which could also lead to misclassification if a diabetes diagnoses lead to more clinical attention—and a subsequent cancer diagnosis. In either case, any potential bias would reduce the differences between the groups, however, we would not expect the excess mortality risk compared to those without cancer to be largely affected.
We did include patients with cancer before inclusion in the NDR. This group represents individuals with diverse characteristics, and as such, the results must be interpreted with caution. While this group may be considered the “health survivors”—i.e., those who survived long enough to be included in the registry—their mortality rates are still higher than patients with “No cancer.” Additionally, in this group, there is a risk of reverse causality, where cancer may have led to a diabetes diagnosis rather than the other way around.
Although our results are relevant to other countries with similar health care systems, we acknowledge that the findings are mainly generalizable to countries with similar demographic and socio-economic characteristics.
We show that smoking and physical inactivity continue to affect mortality risk in patients with diabetes, even after an additional cancer diagnosis. These results might inform the design of new strategies to manage modifiable lifestyle risk factors in the growing group of patients with this specific comorbidity.
Supplementary Information
Author contributions
T.L drafted the manuscript. L.B. analyzed data and confirms full access to all the data, and final responsibility for accuracy of data analysis. T.L., L.B., S.G., B.E, and D.R.W. conceived. the idea, designed the study, interpreted data, reviewed and edited the manuscript. All authors. accepted the final version of the manuscript for publication.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to the Swedish law, but are available from the corresponding author on reasonable request.
Competing interests
D.R.W. own shares in Novo Nordisk A/S. BE reports personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp and Dohme, Mundipharma, NovoNordisk, RLS Global and Sanofi, all outside the submitted work. T.L., L.B., S.G. have no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-61563-9.
References
- 1.Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020;10(1):14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Maddams J, Utley M, Møller H. Projections of cancer prevalence in the United Kingdom, 2010–2040. Br. J. Cancer. 2012;107(7):1195–1202. doi: 10.1038/bjc.2012.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson-Stuttard J, Bennett J, Cheng YJ, et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: An epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 2021;9(3):165–173. doi: 10.1016/s2213-8587(20)30431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 6.González N, Prieto I, Del Puerto-Nevado L, et al. 2017 update on the relationship between diabetes and colorectal cancer: Epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget. 2017;8(11):18456–18485. doi: 10.18632/oncotarget.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanders MM, Vissers PA, Haak HR, van de Poll-Franse LV. Colorectal cancer, diabetes and survival: Epidemiological insights. Diabetes Metab. 2014;40(2):120–127. doi: 10.1016/j.diabet.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YB, Pan XF, Lu Q, et al. Associations of combined healthy lifestyles with cancer morbidity and mortality among individuals with diabetes: Results from five cohort studies in the USA, the UK and China. Diabetologia. 2022;65(12):2044–2055. doi: 10.1007/s00125-022-05754-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: A population-based study. Lancet. 2015;385(9974):1206–1218. doi: 10.1016/s0140-6736(14)61396-9. [DOI] [PubMed] [Google Scholar]
- 10.Barone BB, Yeh HC, Snyder CF, et al. Postoperative mortality in cancer patients with preexisting diabetes: Systematic review and meta-analysis. Diabetes Care. 2010;33(4):931–939. doi: 10.2337/dc09-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjornsdottir HH, Rawshani A, Rawshani A, et al. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Sci. Rep. 2020;10(1):17376. doi: 10.1038/s41598-020-73668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barone BB, Yeh HC, Snyder CF, et al. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA. 2008;300(23):2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinheiro LC, Kaur H, Nilo D, Safford MM, DeRosa AP, Kern LM. Determining the impact of a cancer diagnosis on diabetes management: A systematic literature review. Am. J. Clin. Oncol. 2019;42(11):870–883. doi: 10.1097/coc.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 14.Bjerg L, Gudbjörnsdottir S, Franzén S, et al. Duration of diabetes-related complications and mortality in type 1 diabetes: A national cohort study. Int. J. Epidemiol. 2021;50(4):1250–1259. doi: 10.1093/ije/dyaa290. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson JF, Svedberg P, Olén O, Bruze G, Neovius M. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur. J. Epidemiol. 2019;34(4):423–437. doi: 10.1007/s10654-019-00511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish cancer register: A sample survey for year 1998. Acta Oncol. 2009;48(1):27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 18.Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish cause of death register. Eur. J. Epidemiol. 2017;32(9):765–773. doi: 10.1007/s10654-017-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Registries N-AotNC. Available from https://nordcan.iarc.fr/en/dataviz/survival?populations=752
- 20.Kenfield SA, Stampfer MJ, Rosner BA, Colditz GA. Smoking and smoking cessation in relation to mortality in women. JAMA. 2008;299(17):2037–2047. doi: 10.1001/jama.299.17.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Gu M, Jing F, et al. Association between physical activity and all cancer mortality: Dose-response meta-analysis of cohort studies. Int. J. Cancer. 2016;138(4):818–832. doi: 10.1002/ijc.29828. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Pan XF, Chen J, et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia. 2020;63(1):21–33. doi: 10.1007/s00125-019-04985-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YB, Pan XF, Chen J, et al. Combined lifestyle factors, incident cancer, and cancer mortality: A systematic review and meta-analysis of prospective cohort studies. Br. J. Cancer. 2020;122(7):1085–1093. doi: 10.1038/s41416-020-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths RI, Valderas JM, McFadden EC, et al. Outcomes of preexisting diabetes mellitus in breast, colorectal, and prostate cancer. J. Cancer Surviv. 2017;11(5):604–613. doi: 10.1007/s11764-017-0631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogurtsova K, Guariguata L, Barengo NC, et al. IDF diabetes Atlas: Global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res. Clin. Pract. 2022;183:109118. doi: 10.1016/j.diabres.2021.109118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to the Swedish law, but are available from the corresponding author on reasonable request.