Abstract
Background
Chordomas are rare osseous neoplasms with a dismal prognosis when they recur. Here we identified cell surface proteins that could potentially serve as novel immunotherapeutic targets in patients with chordoma.
Methods
Fourteen chordoma samples from patients attending Xuanwu Hospital Capital Medical University were subjected to single-cell RNA sequencing. Target molecules were identified on chordoma cells and cancer metastasis-related signalling pathways characterised. VEGFR-targeting CAR-T cells and VEGFR CAR-T cells with an additional TGF-β scFv were synthesised and their in vitro antitumor activities were evaluated, including in a primary chordoma organoid model.
Results
Single-cell transcriptome sequencing identified the chordoma-specific antigen VEGFR and TGF-β as therapeutic targets. VRGFR CAR-T cells and VEGFR/TGF-β scFv CAR-T cells recognised antigen-positive cells and exhibited significant antitumor effects through CAR-T cell activation and cytokine secretion. Furthermore, VEGFR/TGF-β scFv CAR-T cells showed enhanced and sustained cytotoxicity of chordoma cell lines in vitro compared with VRGFR CAR-T cells.
Conclusions
This study provides a comprehensive single-cell landscape of human chordoma and highlights its heterogeneity and the role played by TGF-β in chordoma progression. Our findings substantiate the potential of VEGFR as a target for CAR-T cell therapies in chordoma which, together with modulated TGF-β signalling, may augment the efficacy of CAR-T cells.
Subject terms: Cancer immunotherapy, Immunotherapy, RNA sequencing
Background
Chordomas are rare primary bone tumours that originate from notochord remnants. There are only ~0.08 per 100,000 chordoma cases each year, with a male predominance and a peak incidence in the 5th decade [1]. There is no effective systemic treatment for chordoma [2], with current management relying on complete surgical resection and postoperative radiotherapy in patients with incomplete resections due to complicated surgery (e.g., due to adjacent nerves or vessels) [3–5]. Median overall survival (OS) for patients with chordoma is ~6 to 7 years, although the clinical course is variable [6–9]. Conventional chordomas are generally low to intermediate-grade, locally aggressive malignancies, but their behaviour can be quite variable, with some patients experiencing rapid progression. Poorly differentiated and dedifferentiated chordomas are clinically much more aggressive than conventional chordomas. About a third of chordoma patients will therefore develop local spread and metastases and, in the relapsed, refractory, or metastatic setting, chordoma is unlikely to be cured [6–9]. Therefore, there is a critical need for effective therapies that reduce recurrence and progression and that can treat recurrences when they do occur.
Single-cell RNA sequencing (scRNA-seq) technologies now provide the opportunity to comprehensively understand cellular heterogeneity, molecular regulation, and cancer evolution, including in chordoma. Indeed, a recent scRNA-seq analysis of human chordomas provided new insights into tumour composition and intrinsic mechanisms of tumour invasion [10]. While chimeric antigen receptor T cell (CAR-T) therapy has potential as a powerful treatment option for patients with chordoma, CAR-T cell therapy against solid cancers faces several challenges, including a scarcity of specific target antigens, restricted migration to tumour sites, antigen variability and loss, and the presence of an immunosuppressive tumour microenvironment (TME) [11]. To overcome this, numerous strategies have been developed to enhance the antitumor efficacy of CAR-T cells in diverse solid tumours, including the implementation of dual CAR designs capable of recognising multiple antigens simultaneously, the integration of oncolytic viruses, expression of cytokines or chemokines, and the elimination of other suppressive factors within the TME [12–15].
Like many tumours, chordomas have a complex heterotypic TME that includes cancer cells, vascular cells, fibroblasts, and immune cells. Intertumoral heterogeneity refers to the variability observed among patients sharing the same histological subtype, while intratumoral heterogeneity refers to the variability observed within an individual patient’s tumour [16]. The TGF-β signalling pathway is a central pathogenic mechanism in many tumours due to its diverse impacts on various cellular populations within the TME, both malignant and non-malignant [17]. Indeed, malignant cells in chordoma are TGF-β signalling dependent [18].
Here we explored the tumour landscape of chordomas using scRNA-seq. A primary objective of this study was to evaluate the extent of tumour heterogeneity and discover plausible therapeutic targets in chordomas. By considering the pathological attributes of the TME in chordoma, we devised a CAR-T agent, namely VEGFR/TGF-β scFv CAR-T cells, to effectively modulate the chordoma TME. This agent not only recognises the chordoma-specific antigen vascular endothelial growth factor receptor (VEGFR) but also targets soluble TGF-β molecules within solid tumours by secreting specific antibodies, thereby overcoming immunosuppression in the chordoma TME to deliver a robust therapeutic effect through a multimodal molecular targeting approach.
Methods
Data sources
Fourteen patients who underwent surgery at Xuanwu Hospital, Capital Medical University, Beijing, China between July 2019 to April 2023 were included in the analysis. Using the World Health Organisation classification of bone tumours, all patients were diagnosed with conventional chordomas [8]. Supplementary Table S1 details the characterisation of tumour samples from chordoma patients. Chordoma tissue samples were obtained from surgical specimens intraoperatively. Written informed consent was obtained from all patients, and the Ethics Committee of the Xuanwu Hospital, Capital Medical University, approved the study protocol.
Single-cell gene expression quantification and subclustering
Single-cell analysis was based on methods established in our previous study [19]. Utilising the Seurat R package (v4.3.0) [20], raw data were processed through a series of steps including quality control, normalisation, scaling, and subsequent data handling. The initial phase involved applying rigorous criteria to filter out low-quality cells. Specifically, cells with fewer than 501 expressed genes or with mitochondrial counts above 25% were excluded. The filtered, high-quality cells then underwent normalisation and scaling using the package’s default settings. To identify key features, we leveraged the ‘FindVariableFeatures’ function prior to principal component analysis (PCA), which was conducted on the scaled data with a focus on these variable features. Further analysis involved dimension reduction and clustering using the ‘FindNeighbors’ (using dimensions 1 to 10) and ‘FindClusters’ (with a resolution setting of 0.5) functions. To facilitate deeper exploration and effective visualisation of the data, we utilised t-SNE as a non-linear dimension reduction technique.
Cell type determination
In determining cell types, we integrated the ‘FindMarkers’ and ‘FindAllMarkers’ functions in our analytical toolkit to identify differentially expressed features unique to each cell cluster. To annotate these cell types, we used the CellMarker database (http://bio-bigdata.hrbmu.edu.cn/CellMarker/). This repository, rich in canonical marker genes, served as a reference, enabling us to accurately assign biological identities to our clusters. The process involved matching observed gene expression profiles with those catalogued in the database. Additionally, we used the SingleR package (v2.0.0) as a supplementary tool to further refine and verify our cell type identities [21].
Copy number variation analysis
To infer copy number variations (CNVs) from single-cell RNA sequencing (scRNA-seq) data, we used a previously published method [22]. The process was executed using the R code available at https://github.com/broadinstitute/inferCNV, applying the default parameters. Initially, we identified putative non-malignant cells, including manually annotated immune cells (T cells, B cells, myeloid cells, and neutrophils) as well as stromal cells (endothelial cells and fibroblasts). The CNV estimates derived from these cells were used to establish a baseline. Additionally, we classified cells expressing TBXT as putative malignant cells. The reference groups for the analysis were adjusted based on initial inferCNV results. These adjustments were then used to guide subsequent rounds of analysis, ensuring a refined and accurate CNV assessment.
Cellular communication analysis
We used two independent approaches to analyse cellular communication. First, we used the CellChat package (v1.6.1) [23], an approach grounded in the analysis of ligand-receptor interactions, drawing on data from the KEGG signalling pathway database and recent experimental findings. The analysis involved several steps: first, we identified significantly differentially expressed signalling genes, which was complemented by calculating the ensemble average expression to obtain an inclusive view of gene expression across varied cell types. Finally, we assessed the likelihood of intercellular communication, a key factor in deciphering the signalling networks and interactions between the diverse cell populations identified in our study.
To complement this analysis, we used NicheNet (v2.0.4) to model intercellular communication, which linked ligands to their target genes and identified activated gene networks [24]. To select differentially expressed genes (DEGs), we set a threshold of p < 0.01 and an absolute log-fold change (FC) > 0.25. We designated myeloid cells as the receiver cells, while T and B cells were selected as sender cells. Prioritised ligands were estimated using NicheNet’s ‘predict_ligand_activities’ function.
Primary cell cultures
Chordoma tumour tissue was rinsed with 5 mL phosphate-buffered saline (PBS) in a 10 cm dish, followed by meticulous mincing using surgical scissors. Subsequently, tumours were cut into small fragments (1–3 mm3) using sterile scissors. Tissue was then digested with collagenase IV (200 U/mL) at 37 °C in a 5% CO2 environment, with vigorous shaking every 15 min, for 2 h. Following digestion, the sample was filtered through a 70 μm cell sieve into a fresh 15 mL centrifuge tube. The supernatant was extracted by centrifuging at 800 rpm for 3 min, resulting in the collection of the sample in the bottom of the tube. The supernatant was then completely aspirated and discarded. A mixture of MammoCult medium and Matrigel at a 3:4 ratio was introduced. Subsequently, 105 isolated primary chordoma cells were incorporated into a 70 μl suspension and placed in a single circular formation at the periphery of a 24-well plate. Following this, 1 ml of MammoCult medium was added and the plate was incubated.
Cell line cultures
Human chordoma cell lines UCH2 and JHC-7 were procured from the ATCC. The human embryonic kidney cell line HEK392T and the human colon cancer cell line HT-1080 were obtained from Wuhan Procell Biotech Company (Wuhan, China) for lentivirus preparation and titre assays. The cell lines used are identified and tested for mycoplasma.JHC-7 cells were cultured in DMEM/F12 medium supplemented with 10% FBS at 37 °C in a humidified atmosphere containing 5% CO2. The UCH2 cell line was cultured in a mixture of IMDM and RPMI 1640 (4:1) with the addition of 20% FBS. HEK293T and HT-1080 cell lines were maintained in DMEM supplemented with 10% FBS and 1 mM GlutaMax at 37 °C. Prior to experimentation, all cell lines were confirmed to be Mycoplasma free using the Rapid Mycoplasma Test kit (Cellapybio, Beijing, China) according to the manufacturer’s instructions. Mycoplasma was not detected in any cell line.
Plasmid construction
The construction of the anti-VEGFR involved following a previously established protocol with modifications [25]. The sequence encoding the anti-VEGFR scFv, which includes the VL and VH regions as laboratory work, was combined with a leading sequence derived from αCD8 and 4×G4S linkers and fused with the SD derived from human nuclear protein LaSS-B, as mentioned in the same publication [25]. The sequence was synthesised by Tsingke Biotechnology Co., Ltd. (Beijing, China). The sequence encoding the anti-TGFβ, which was designed by our laboratory, was also synthesised by Synbio Technologies Co. Ltd. Both sequences were subcloned into the pUC57 vector.
Lentivirus generation
The VEGFR CAR and VEGFR/TGF-β scFv CAR lentivirus constructs were produced in HEK293T cells using a third-generation lentivirus system. The viruses were collected 24, 48, and 72 hours after transfection, passed through 0.45-μm membrane filters (MilliporeSigma, Burlington, MA, USA), and subsequently subjected to ultracentrifugation in Ultratubes (MilliporeSigma) at 20,000 rpm for 2 hours at 4 °C. The resulting virus was resuspended in X-VIVO 15 (Lonza, Basel, Switzerland) and stored at −80 °C. Infectious titres of VEGFR CAR and VEGFR/TGF-β CAR lentivirus in HT-1080 cells were determined by flow cytometry.
CAR-T cell production
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque gradient centrifugation. CD3+ T cells were purified and stimulated using CD3/CD28 magnetic beads (Gibco, Thermo Fisher Scientific, Waltham, MA) at a ratio of 1:2. T cells were then sorted using magnetic beads placed in Ultra-Low Attachment Surface 6 wells at a density of 1.5 × 106/mL for activation in X-VIVO 15 medium (Lonza) supplemented with 500 IU/mL IL-2 (XinLuoEr, Shanghai, China), 80 ng/mL IL-7 (Jin’an, China), 20 ng/mL IL-15 (Jin’an, China), and 20 ng/mL IL-21 (Jin’an, China). Following a ten-day cell culture period, transduction efficiency was assessed.
Flow cytometry
The following anti-human antibodies were procured from BioLegend (San Diego, CA, USA): CD3, CD4, CD8, CD14, CD19, CD56, CD197 (CCR7), CD45RO, VEGFR-PE, PerCP, APC, and streptavidin-PE. Biotin-labelled Fab was acquired from Abcam (Abcam, Cambridge, UK) for the quantification of CAR-positive T cells, and the presence of biotin-Fab was detected through the addition of streptavidin-PE. Flow cytometry analysis was conducted using FACSCalibur (BD Biosciences, San Jose, CA, USA), and the obtained data were analysed using FlowJo v10.0.
In vitro cytotoxicity assay
CAR-T cells and chordoma cell lines were co-cultured at different effector-to-tumour (E/T) ratios [25]. In the transient cytotoxicity assay, the co-culture supernatant from various E/T ratios was collected after 20 h of cultivation. In the multi-round cytotoxic assay, effector cells were co-cultured with target cells at an E/T ratio of 4:1, with equal amounts of target cells added every other day. The supernatant samples were collected every 48 h, and the initial number of tumour cells was reintroduced into the co-culture system to re-challenge the CAR-T cells. Supernatant detection was conducted according to the Non-Radioactive Cytotoxicity kit (Promega, USA) instructions. The results were subsequently calculated following the manufacturer’s instructions.
Cytokine release assay
Cytotoxic cytokines IL-2, IFN-γ, IL-6, IL-15, and TNF-α were tested by ELISA in vitro. Supernatants were collected from samples and centrifuged at 800 x g for 15 min at 4 °C. Cytokines were quantified using ELISA kits (Neobioscience, Shenzhen, China) following the manufacturer’s instructions. Briefly, samples were collected and centrifuged at 800 x g for 15 min at 4 °C and the assay was performed following the manufacturer’s instructions. ELISA data were acquired using Varioskan Flash (Thermo Fisher Scientific), and LEGENDplex assay data were acquired by FACSCalibur flow cytometry (BD Biosciences).
Immunohistochemical staining
For immunohistochemical (IHC) staining, sections were immersed in citrate buffer (11 mmol, pH 6.0; Zhongshan Biotechnology Co., Zhongshan City, China) and subjected to antigen heat retrieval in a microwave oven at 100 °C for 10 min. Subsequently, sections were subjected to three washes with 1× PBS and then incubated in 1× PBS containing 3% (v/v) H2O2 (Zhongshan Biotechnology Co.) for 15 min to suppress endogenous peroxidase activity. Following a triple washing procedure, sections were blocked using 5% (v/v) normal goat serum (Zhongshan Biotechnology Co.) in PBS for 1 h at room temperature. Subsequently, sections were incubated overnight with the primary antibodies (TGF-β,1:200; VEGFR, 1:100, Servicebio, Hubei, China) at 4 °C. After three additional PBS washes, sections were exposed to appropriate HRP-conjugated secondary antibodies (1:200, Servicebio) for 30 min at room temperature. HRP was visualised using the DAB method. To serve as negative controls, sections were incubated with pre-immune rabbit sera (Zhongshan Biotechnology Co.) instead of primary antibodies. Following this, sections were counterstained with hematoxylin and mounted with neutral balsam.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analyses were performed using Prism (GraphPad Software, La Jolla, CA, USA) and R software (version 4.2.3). Student’s t-test was employed as a two-sided paired test with 95% confidence intervals for comparisons between two groups. For comparisons involving three or more groups, two-way analysis of variance (ANOVA) was conducted with Dunnett’s multiple comparisons test. Results with a p-value less than 0.05 were deemed statistically significant.
Results
Single-cell heterogeneity landscape of human chordoma
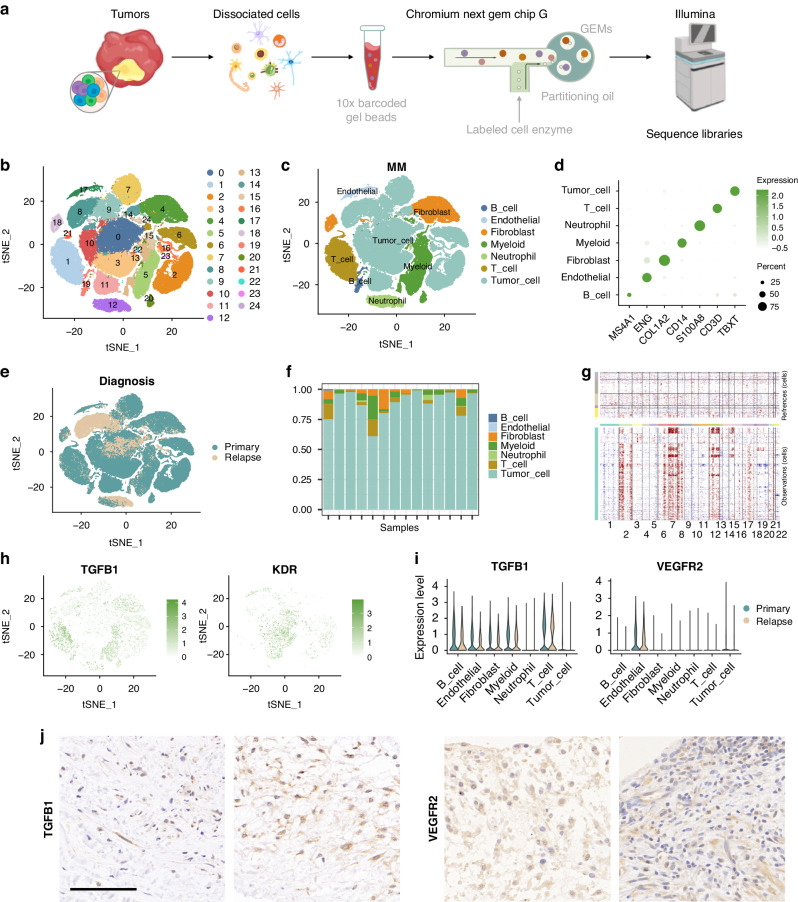
We conducted scRNA-seq on samples obtained from fourteen chordoma patients, with the diagnoses confirmed by histopathological assessment (Table S1). This analysis yielded 105,840 high-quality cells, distributed across 25 distinct clusters (Fig.1a, b and Fig. S1). Detailed annotation, employing canonical markers, was performed to map the intricate heterogeneity landscape of human chordoma, which included various cell types: tumour cells (TBXT-positive, 66,960 cells, 63.27%), T cells (CD3D-positive, 10,012 cells, 9.46%), B cells (MS4A1-positive, 1392 cells, 1.32%), myeloid cells (CD14-positive, 11,947 cells, 11.29%), neutrophils (S100A8-positive, 3616 cells, 3.42%), endothelial cells (ENG-positive, 1,609 cells, 1.52%), and fibroblasts (COL1A2-positive, 10,304 cells, 9.74%) (Fig. 1c, d).
Fig. 1. Single-cell heterogeneity landscape of human chordoma.
a Schematic of the single-cell RNA sequencing process for human chordoma samples. b t-SNE plot displaying clustering of 105,840 high-quality cells. c Detailed cell type annotation within the human chordoma samples. d Dot plots illustrating the expression of canonical marker genes across different cell types. e t-SNE plot highlighting the spatial distribution of cells, differentiating between primary and relapse samples. f Bar plots representing the proportion of each cell type across the fourteen chordoma samples. g Visualisation of the chromosomal landscape showing inferred large-scale copy number variations (CNVs) in both non-malignant (top) and malignant chordoma cells. The rows indicate individual cells, while the columns correspond to chromosomal positions. Amplifications and deletions are denoted in red and blue, respectively, calculated by averaging expression over 100-gene segments on each chromosome. h t-SNE plots depicting the expression levels of TGF-β1 and VEGFR2. i Violin plots comparing the expression of TGF-β1 and VEGFR2 across various cell types in both primary and relapse samples. j Immunohistochemistry images showing the presence of TGF-β1 and VEGFR2 in chordoma tissues. Scale bars represent 100 μm for inset images.
An intriguing aspect was the marked difference between primary and relapse samples, particularly with respect to tumour cells (Fig. 1e, f). Tumour cells were identified through inference, using large-scale copy number variations (CNVs) to differentiate them from other cells (Fig. 1g). In the tumour microenvironment (TME), notable findings included the increased expression of TGF-β1 in immune cells, predominantly in T and B cells, and VEGFR2 in endothelial and tumour cells, which was consistent across both primary and relapse samples (Fig. 1h, i). These expression patterns were further corroborated by immunohistochemistry (IHC) of chordoma tumour samples (Fig. 1j).
Cell communication network analysis in the chordoma TME
We next conducted cellular communication analysis to identify potential interaction pathways within the chordoma TME, using various complementary methodologies (Fig. S2). The analysis, particularly when using the CellChat technique, highlighted prominent TGF-β signalling within the TME (Table S2). This pathway predominantly featured T and B cells, along with endothelial cells, as the primary senders of TGF-β signals, while myeloid cells and fibroblasts acted as receivers. The most notable ligand-receptor interaction within this pathway was TGFβ1-TGFβR1/TGFβR2 (Fig. 2a–c).
Fig. 2. Cell communication network analysis of the chordoma tumour microenvironment.
a, b The distribution and contribution of the TGF-β signalling pathway network. c, d The distribution and contribution of the VEGF signalling pathway network. e Dot plots showed the expression of signal-related genes between T and B cells. f Dot plots showed the expression of the receiver network for myeloid cells between primary and relapse samples. g NicheNet’s ligand–target matrix denoting the regulatory potential from the chordoma programme.
In addition, the VEGF signalling pathway emerged as a significant network involving tumour cells, myeloid cells, fibroblasts, and endothelial cells. This network was characterised by key ligand-receptor pairs, notably VEGFα/VEGFβ-VEGFR1 and VEGFα-VEGFR2 (Fig. 2c, d).
To corroborate these findings, we used NicheNet as an independent technique. This analysis revealed that T cells expressed more signalling pathways than B cells, particularly those involving TGF-β1 (Fig. 2e). Furthermore, we observed that TME signalling was higher in relapsed samples than in primary samples (Fig. 2f). Lastly, our analysis identified a broader range of predicted target genes for TGF-β1 (Fig. 2g).
Generation and characterisation of CAR-T cells
Having established dominant TGFβ and VEGF signalling in chordomas, two CARs incorporating 4-1BB costimulatory domains and targeting VEGFR were generated: VEGFR CAR and VEGFR CAR combined with TGF-β scFv (Fig. 3a). VEGFR and VEGFR/TGF-β scFv CARs were expressed on the surface of transduced T cells (Fig. 3b). VEGFR/TGF-β scFv CAR T cells were significantly more frequent than VEGFR CAR T cells (p < 0.01) (Fig. 3c). Furthermore, flow cytometry analysis revealed that CD4+ VEGFR and VEGFR/TGF-β scFv CAR-T cells were more frequent than CD8+ VEGFR and VEGFR/TGF-β scFv CAR-T cells (Fig. 3d). After expansion, VEGFR/TGF-β scFv CAR-T cells were enriched for effector memory cells (Tem) compared with VEGFR CAR-T cells (Fig. 3e).
Fig. 3. Generation and characterisation of VEGFR CAR and VEGFR/TGF-β CAR-T cells.
a Schematic of the construction of VEGFR-specific CARs. b Representative flow cytometry analysis showing CAR expression on T cells transduced with VEGFR or VEGFR/TGF-β scFv on day 7. c CAR expression on T cells transduced with VEGFR or VEGFR/TGF-β scFv on day 10. d Population assay of the CD8+ and CD4+ subpopulations in VEGFR or VEGFR/TGF-β scFv CAR-T cells after incubation for 12 days. e Subpopulations of memory T cells in the final VEGFR or VEGFR/TGF-β scFv CAR-T cells were measured by flow cytometry on days 7–10. Subpopulations of different T cells were identified as follows: Tem: CD45RO+/CD197-; naïve T, CD45RO-/CD197+; effector T, CD45RO-/CD197-; Tcm, CD45RO+/CD197+. f Expansion of T cells transduced with VEGFR or VEGFR TGF-β CAR. g Competitive binding of CAR-T cell supernatants to TGF-β antibodies. Data shown are mean ± SD (n = 3). **p < 0.01 and ***p < 0.001 (two-way ANOVA).
To assess and contrast the in vitro expansion efficiencies of various VEGFR CAR-T cell structures, cells were cultured and quantified at multiple time points. VEGFR CAR-T cells demonstrated notably greater proliferation than VEGFR/TGF-β scFv CAR-T cells following a 12-day expansion period (Fig. 3f). ELISA assays were performed to assess the binding capacity of VEGFR/TGF-β scFv CAR-T cells to TGF-β, where the cell supernatants of VEGFR CAR-T and VEGFR/TGF-β scFv CAR-T cells were mixed with a TGF-β protein standard (10 ng/mL) at varying ratios (as shown in Fig. 3g). At various ratios of standard and supernatant, the VEGFR CAR-T group exhibited higher expression of TGF-β than the VEGFR/TGF-β scFv CAR-T group, demonstrating the ability of VEGFR/TGF-β scFv CAR-T cell supernatant to effectively bind TGF-β.
Enhanced anti-tumour cytotoxicity of VEGFR/TGF-β scFv CAR-T cells in vitro
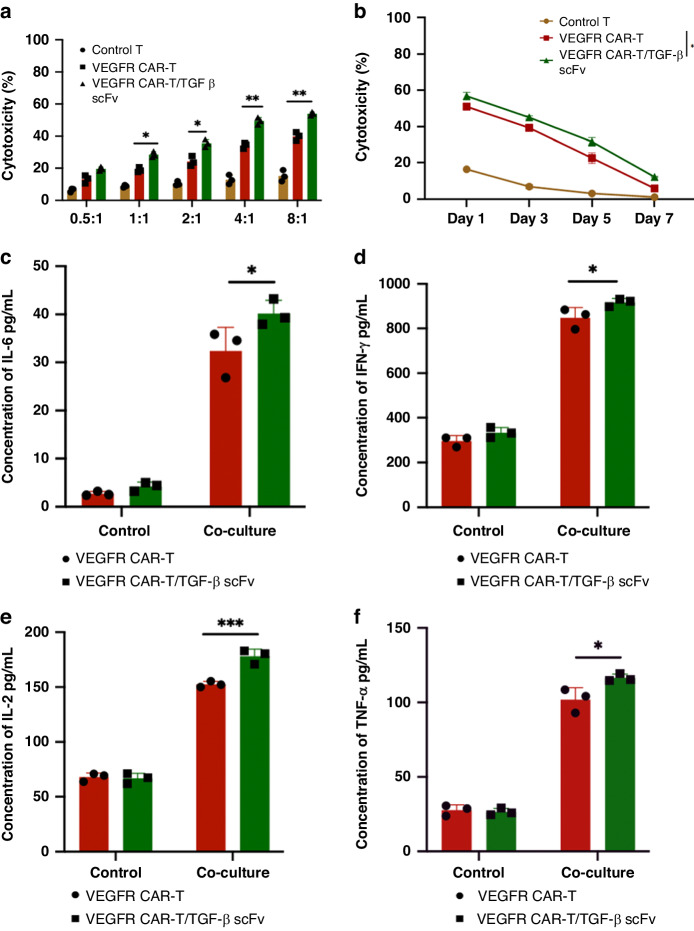
We next assessed the activation and killing effects of our CAR-T cells on chordoma cells expressing VEGFR using JHC-7 and UCH2 chordoma cell lines, which showed malignant proliferative potential (Fig. S3). Control, VEGFR, and VEGFR/TGF-β scFv CAR-T cells were incubated with JHC-7 and UCH2 cells at different effector-to-target (E/T) ratios. VEGFR CAR-T and VEGFR/TGF-β scFv CAR-T cells both showed cytotoxicity against JHC-7 cells that was positively correlated with E/T ratios. Cells were quantified based on the number of positive CAR-T cells. Remarkably, VEGFR/TGF-β scFv CAR-T cells exhibited greater cytotoxicity than VEGFR CAR-T cells (Fig. 4a). Cytotoxic T cell therapy requires effector cells to systematically eliminate target cells so, to assess the persistent effects of CAR-T cell variants on tumour cells, JHC-7 tumour cells were introduced into the culture system on days 0, 2, 4, and 6. The subsequent killing effect of re-challenged VEGFR and VEGFR/TGF-β scFv CAR-T cells on tumour cells was observed at different time intervals (Fig. 4b). Control T cells showed little cytotoxicity towards tumour cells and became largely ineffective by day 5. Conversely, VEGFR and VEGFR/TGF-β scFv CAR-T cells demonstrated cytotoxicity until day 7, with VEGFR/TGF-β scFv CAR-T cells displaying superior cytotoxicity. Incubation of the two CAR-T cells with JHC-7 cells at an 8:1 ratio resulted in the release of proinflammatory cytokines including interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-α), interleukin-2 (IL-2), and IL-6 (Fig. 4c–f). VEGFR/TGF-β scFv CAR-T cells exhibited greater release of all four cytokines compared with VEGFR CAR-T cells. Similarly, when incubated with UCH2 cells, VEGFR/TGF-β scFv CAR-T cells significantly enhanced tumour-killing activity and promoted the release of proinflammatory cytokines (Fig. S4).
Fig. 4. VEGFR/TGF-β scFv CAR-T cells enhance anti-tumour cytotoxicity of cells in vitro.
a VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with JHC-7, a chordoma cell line, at different E/T ratios (from 0.5:1 to 8:1). Cytotoxicity was measured by the LDH release assay after a 20 h incubation. b VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with multiple rounds of JHC-7 (E/T ratios 4:1). Long-term cytotoxicity was measured by the LDH release assay after 1–7 days of incubation. c–f ELISA data showing the quantification of cytokines (IL-6, IFN-γ, IL-2, and TNF-α) in the supernatants after VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with JHC-7 at an E:T ratio of 8:1 for 20 h. Data shown are mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (two-way ANOVA). Not significant (ns).
VEGFR/TGF-β scFv CAR-T cells can modulate the TME to enhance anti-tumour effects
To assess the impact of CAR-T cells on the TME, we conducted in vitro experiments to investigate the eradication of chordoma cells by CAR-T cells under varying conditions. It is well-established that TGF-β plays a crucial role in immunosuppression in solid cancers [26, 27]. We therefore co-cultured VEGFR CAR-T and VEGFR/TGF-β CAR-T cells with JHC-7 cells at an E:T ratio of 4:1 for 20 h. TGF-β was introduced to the culture system at a final concentration of 10 ng/mL. VEGFR/TGF-β scFv CAR-T cells exhibited superior cytotoxicity compared with the experimental group without the addition of TGF-β (Fig. 5a). When cultured with TGF-β, there was a reduction in cytotoxicity by both types of CAR-T cells. However, the introduction of VEGFR/TGF-β scFv CAR-T reduced TGF-β within the system, improving the killing effect. Additionally, VEGFR/TGF-β scFv CAR-T cells showed consistently enhanced killing capacity (Fig. 5b). By day 7, VEGFR CAR-T cells in the presence of TGF-β failed to eradicate JHC tumour cells, while VEGFR/TGF-β scFv CAR-T cells demonstrated sustained killing capacity against JHC tumour cells.
Fig. 5. VEGFR/TGF-β scFv CAR-T cells can manipulate the TME to enhance anti-tumour efficacy.
a VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with JHC-7 cells at an E:T of 4:1 for 20 h. NC: normal control, TGF-β + : TGFβ was introduced into the culture system at a final concentration of 10 ng/mL. b The impact of 10 ng/mL TGF-β within the biological system on the persistence of JHC-7 eradication by CAR-T cells. E:T ratio 4:1, equal numbers of target cells added every other day. c VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with JHC-7 cells at an E:T of 4:1 for 20 h. NC: normal control, M2 Co-culture: the inclusion of M2 macrophages into the culture system. d The impact of M2 macrophages within the biological system on the persistence of JHC-7 cell eradication by CAR-T cells. E:T is 4:1, equal numbers of target cells added every other day. Data shown are mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (two-way ANOVA). Not significant (ns).
“M2” phenotype macrophages have been reported to exert an immunosuppressive function within the TME [28] and secrete TGF-β [29, 30]. In an experimental setup where M2 macrophages were cultured on a transwell filter, CAR-T cells and JHC-7 tumour cells were cultured in the lower compartment. The presence of M2 macrophages reduced the cytotoxicity of CAR-T cells. However, the VEGFR/TGF-β scFv CAR-T cells alleviated immunosuppression caused by M2 macrophages; when co-cultured with M2 macrophages, VEGFR/TGF-β scFv CAR-T cells demonstrated superior cytotoxicity (Fig. 5c). Furthermore, VEGFR/TGF-β scFv CAR-T cells exhibited sustained tumour cytotoxicity in the presence of M2 macrophages (Fig. 5d). These findings highlight the enhanced adaptability of VEGFR/TGF-β scFv CAR-T cells in complex tumour microenvironments. Moreover, VEGFR/TGF-β scFv CAR-T therapy effectively mitigated immunosuppression while concurrently preserving the cytotoxicity of CAR-T cells against chordoma cells.
VEGFR/TGF-β scFv CAR-T cells show enhanced therapeutic efficacy against primary chordoma cells
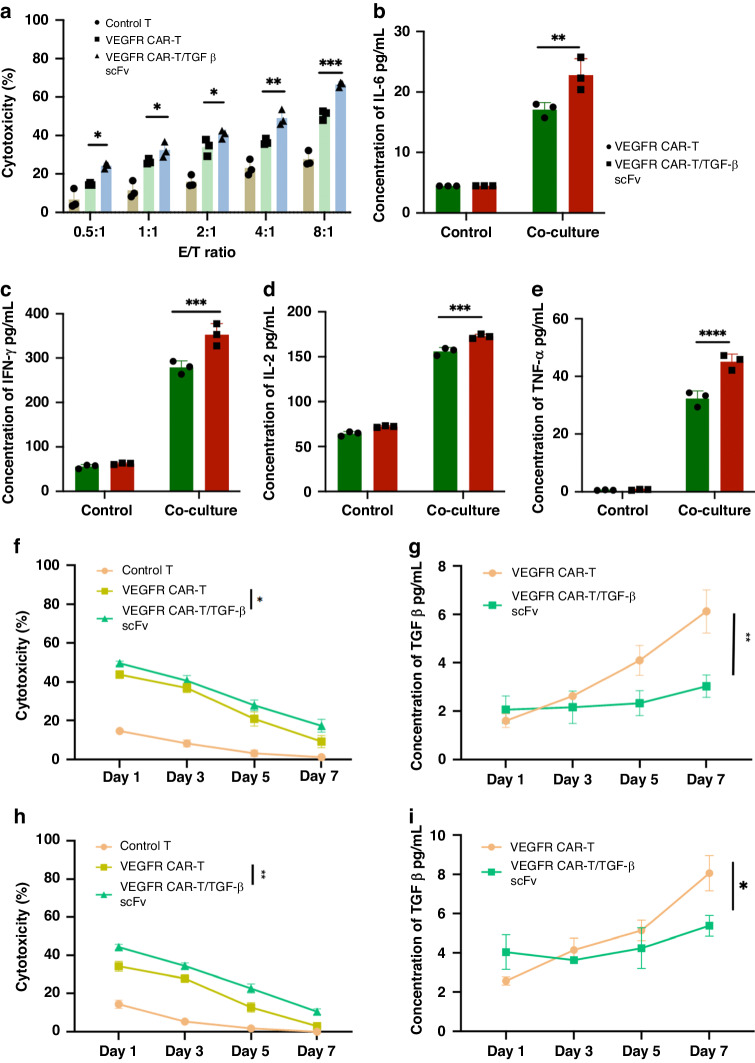
To evaluate the effectiveness of our CAR-T cells in a clinical setting, we tested them in a patient-derived chordoma organoid model expressing VEGFR. VEGFR was confirmed as highly expressed in chordoma organoid cells (Fig. S5). Organoids were incubated with control, VEGFR, and VEGFR/TGF-β scFv CAR-T cells at different E/T ratios. VEGFR CAR-T and VEGFR/TGF-β scFv CAR-T cells exhibited distinct cytotoxicity against organoids, with cytotoxicity positively associated with E/T ratios. The cytotoxicity of VEGFR/TGF-β scFv CAR-T cells was superior to that of VEGFR CAR-T cells (Fig. 6a). Furthermore when organoids were incubated with both types of CAR-T cells at a 4:1 ratio, there was the secretion of proinflammatory cytokines IFN-γ, TNF-α, IL-2, and IL-6. Notably, VEGFR/TGF-β scFv CAR-T cells exhibited greater release of all four cytokines compared with VEGFR CAR-T cells (Fig. 6b–e). To assess the persistent cytotoxicity of CAR-T cells against primary chordoma cells, we developed a CAR-T cell/primary chordoma cell co-culture system, with primary tumour cells introduced at intervals to evaluate the sustained cytotoxicity of CAR-T cells. Notably, VEGFR/TGF-β scFv CAR-T cells exhibited superior sustained cytotoxicity towards chordoma organoids (Fig. 6f). VEGFR/TGF-β scFv CAR-T cells demonstrated enhanced affinity for TGF-β within the culture system, thereby preserving the cytotoxicity of CAR-T cells (Fig. 6g). Co-cultivation of chordoma organoids and CAR-T cells with M2 macrophages revealed that VEGFR/TGF-β scFv CAR-T cells modulate TGF-β secretion by M2 cells and extend the duration of CAR-T cell-mediated tumour cell eradication (Fig. 6h, i). Moreover, VEGFR/TGF-β scFv CAR-T cells effectively bound TGF-β within the system, mitigating immunosuppression and enhancing the cytotoxicity of CAR-T cells against chordoma cells [31].
Fig. 6. The therapeutic efficacy of VEGFR/TGF-β scFv CAR-T cells is superior to VEGFR CAR-T cells when targeting primary chordoma cells.
a VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with chordoma organoids derived from patients at different E/T ratios (from 0.5:1 to 8:1). Cytotoxicity was measured by the LDH release assay after a 20 h incubation. b–e ELISA data showing the quantification of cytokines (IL-6, IFN-γ, IL-2, and TNF-α) in the supernatants after VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with chordoma organoids at an E:T of 8:1 for 20 h. f VEGFR CAR-T and VEGFR/TGF-β CAR-T cells were co-cultured with multiple rounds of chordoma organoids. Long-term cytotoxicity was measured by the LDH release assay after 1–7 days of incubation. g The alterations in TGF-β expression within the cell supernatants were observed at various time intervals during the eradication of tumour cells by CAR-T cells. h The impact of M2 macrophages within the biological system on the durability of chordoma organoid eradication by CAR-T cells. Equal amounts of target cells were added every other day. i Alterations in TGF-β expression within the cell supernatants was examined at various time intervals during the eradication of tumour cells by CAR-T cells in a co-culture setting with M2 macrophages. Data shown are mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 (two-way ANOVA). Not significant (ns).
Discussion
Angiogenesis, the process of neovascularization from pre-existing vessels, represents one of the six recognised mechanisms used by solid tumours to acquire the blood vessels needed for tumour initiation, proliferation, and metastasis. Nearly four decades ago, the VEGF-VEGFR axis was identified as the most potent pro-angiogenic stimulus in tumour angiogenesis and metastasis [32]. Since then, over twelve pharmaceuticals targeting the VEGF/VEGFR pathway have been approved for use in approximately twenty solid tumour types, typically in conjunction with other treatments [33, 34]. Originally intended to deprive tumours of nutrients, these agents have been shown to temporarily “normalise” tumour blood vessels in both preclinical and clinical investigations. Moreover, in clinical settings, enhanced tumour blood flow or oxygenation resulting from these agents is associated with enhanced outcomes [35–37]. VEGFR has been shown to be expressed in chordoma cells, indicating its potential as a therapeutic target in patients with chordoma [38–40].
TGF-β exhibits dual effects in oncogenesis and cancer progression. Early in the process, it can trigger apoptosis in precancerous cells and inhibit cancer cell proliferation [41]. Nevertheless, as the tumour progresses, tumour cells acquire tolerance to TGF-β by inhibiting the TGF-β pathway or rendering themselves TGF-β tolerant. In chordoma, the secretion of CCL5 by tumour cells plays a significant role in promoting lesion progression, recruiting macrophages, and polarising them towards an M2 phenotype that promotes proliferation, invasion, and migration [42]. M2 macrophages also highly express TGF-β which, when accumulating in the TME, further facilitates tumorigenesis and augments immunosuppression [43–46]. Our results also show that both TGF-β and M2 macrophages inhibit CAR-T cell function. Consequently, TGF-β transitions from tumour suppressive to facilitating cancer evolution, such as by promoting pro-metastatic epithelial-mesenchymal transition (EMT) [10, 47]. Simultaneously, TGF-β assumes a crucial regulatory function in the progression, stimulation, and differentiation of lymphocytes in cancer. TGF-β prevents activation of primary CD4+ T cells, Tregs, primary CD8+ T cells, and memory CD8+ T cells by dendritic cells (DCs). This effect is mediated by its impact on tissue-level angiogenesis, tumour tissue healing, M2-like macrophage polarisation, and eosinophil responses, which collectively exert an influence on tumour progression by targeting cancer cells and the TME [17, 48, 49].
The relatively recent introduction of scRNA-seq has significantly contributed to the elucidation of intratumoral heterogeneity, drug resistance mechanisms, and immune infiltration patterns with translational relevance for diagnostic and therapeutic purposes. Preliminary results from our lab revealed high expression of VEGFR in chordoma cells, and here we expanded our analysis to detect VEGFR expression in chordomas from patients. We found that VEGFR was expressed by chordoma cells in all patients and was highly expressed in some patients, while TGF-β was highly expressed in tumour-infiltrating immune cells such as T/NK cells and macrophages. In cell communication network analysis, TGF-β exerted a regulatory effect on tumour cells, stromal cells, and immune cells, potentially modulating their proliferation, differentiation, migration, invasion, and immune evasion [50]. High VEGFR and TGF-β expression in chordoma was also confirmed at the protein level by IHC. Currently, several drugs are in clinical development that target the TGF-β pathway [17, 51], but they lack persistent efficacy. Our VEGFR/TGF-β scFv CAR-T cells demonstrated the capacity to persistently produce antibodies that effectively bind TGF-β upon interaction with tumour cells, thereby ameliorating the TME, mitigating immunosuppression, and augmenting of the anti-tumour efficacy of CAR-T cells.
The complex TME of solid tumours has been a significant barrier to immunotherapy. Compared to other attempts to treat chordoma with CAR structures alone [52], we selected VEGFR as a therapeutic target informed by our scRNA-seq analysis of clinical samples, further incorporating the TGF-β scFv structure into the CAR structure and hypothesising that the VEGFR/TGF-β scFv CAR-T cell construct would have enhanced therapeutic effects by modulating the immune microenvironment while directly killing tumour cells. The proliferation and positivity of CAR-T cells were not diminished following the coupling of VEGFR/TGF-β scFv CAR-T cells with the TGF-β scFv sequence. VEGFR/TGF-β scFv CAR-T cells exhibited significantly increased CAR-T positivity. Furthermore, structural modifications in CARs did not impact the proportions of the final CAR-T cell subsets. Therefore, the incorporation of the TGF-β scFv structure with the CAR structure does not have any discernible impact on T cells. The TGF-β scFv was engineered with the purpose of augmenting CAR-T cell cytotoxicity towards tumour cells through (i) its binding affinity for TGF-β present in the surrounding milieu, and (ii) its ability to ameliorate the TME. Chordomas, especially recurrent chordomas, can be highly aggressive, and TGF-β is widely acknowledged as the primary catalyst for the initiation of EMT in various contexts, including in development, cancer progression, and other diseases. Notably, in certain in vitro models employing cultured epithelial cell lines, EMT can be induced solely through stimulation with TGF-β [10, 53]. There exists a distinct prognostic correlation between various forms of solid cancers and their infiltration with M1 or M2 macrophage subtypes, where M2 macrophages release TGF-β [54, 55]. Based on our co-culture results with M2 macrophages, VEGFR/TGF-β scFv CAR-T effectively reduced the expression of TGF-β within the surrounding milieu, thereby augmenting the cytotoxic effect of CAR-T cells. Moreover, due to its ability to bind to TGF-β present in the environment, this therapeutic intervention holds promise in reducing TGF-β levels in the TME and regulating EMT, thereby potentially mitigating chordoma invasion in clinical settings.
The clinical implementation of CAR-T therapy is often accompanied by notable adverse events, especially cytokine release syndrome (CRS). Furthermore, CAR-T cell efficacy directly correlates with CRS severity, and an excessively robust inflammatory response can even result in patient death [56]. Therefore, the expression of inflammatory factors is also an important parameter to consider when evaluating CAR-T cells. We found that the TGF-β scFv structure did not have a direct effect on the differentiation of T cell subsets in CAR-T cells. Interestingly, the co-cultivation of chordoma cells with CAR-T cells resulted in a greater release of inflammatory factors from VEGFR/TGF-β scFv CAR-T cells than from VEGFR CAR-T cells. TGF-β is a potent immunosuppressant that helps tumour cells evade host immune surveillance and inhibits inflammation [17, 57]. VEGFR/TGF-β scFv CAR-T cells can reduce TGF-β, enhance immune responses, and enable CAR-T cells to release more inflammatory factors.
In summary, we analysed single-cell sequencing data from 14 chordoma patients and in doing so provided important insights into chordoma biology and an atlas of malignant, stromal, and immune cells in chordoma. Our computational analyses revealed VEGFR as a promising target for CAR-T treatment of chordoma together with TGF-β for chordoma immunotherapy. Our experiments demonstrated that VEGFR/TGF-β scFv CAR-T cells showed high and persistent cytotoxicity towards chordoma cells, including in primary chordoma organoids. While further preclinical validation of our CAR-T cells in patient-derived xenograft models of chordoma or other valid in vivo models is needed, this study paves the way for a new treatment option for patients with chordoma.
Supplementary information
Author contributions
Zan Chen, Wanru Duan, Huantong Wu, and Xinqiang Li contributed to study’s conception and design. Huantong Wu, Xinqiang Li, Boyan Zhang, Penghao Liu, Maoyang Qi, Yueqi Du, Can Zhang performed the experiments and analysed the data. Zan Chen, Wanru Duan, Huantong Wu, Xinqiang Li, and Boyan Zhang wrote and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Beijing Natural Science Foundation Grant (L212039), National High Level Hospital Clinical Research Funding (2022-PUMCH-D-004), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202138), The “Young Talents” Programme, supported by Beijing Municipal Hospital Administration (QML20210801), the Research and Application of Clinical Characteristic Diagnosis and Treatment Programme, Supported by Beijing Municipal Science & Technology Commission (Z221100007422019), and the CAMS Innovation Fund for Medical Sciences (CIFMS #2021-1-I2M-025).
Data availability
The datasets generated and analysed during this study are publicly accessible from the NGDC Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa-human/), accession number HRA006471.
Code availability
Additionally, the code utilised in our study is available from a dedicated GitHub repository, accessible at https://github.com/restore1997/chordoma2024.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Medical Ethics Committee of Xuanwu Hospital, Capital Medical University, Beijing, China (Ethics Committee Approval No: [2021]021). Informed consent was obtained from all individual participants included in the study. The study was performed in accordance with the Declaration of Helsinki.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huantong Wu, Xinqiang Li, Boyan Zhang.
Contributor Information
Wanru Duan, Email: duanwanru@xwhosp.org.
Zan Chen, Email: chenzan@ccmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-024-02635-5.
References
- 1.Walcott BP, Nached BV, Mohyeldin A, Coumans J-V, Kahle KT, Ferreira MJ. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13:e69–76. doi: 10.1016/S1470-2045(11)70337-0. [DOI] [PubMed] [Google Scholar]
- 2.Shihabi AAI, Davarifar A, Nguyen HTL, Tavanaie N, Nelson SD, Yanagawa J, et al. Personalized chordoma organoids for drug discovery studies. Sci Adv. 2022;8:eabl3674. doi: 10.1126/sciadv.abl3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denaro L, Berton A, Ciuffreda M, Loppini M, Candela V, Brandi ML, et al. Surgical management of chordoma: a systematic review. J Spinal Cord Med. 2020;43:797–812. doi: 10.1080/10790268.2018.1483593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongers MER, Dea N, Ames CP, Schwawb JH. Surgical Strategies for Chordoma. Neurosurg Clin N. Am. 2020;31:251–61. doi: 10.1016/j.nec.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Kuang L, Lv G, Wang B, Li L, Dai Y, Li Y. Overexpression of adenosine deaminase acting on RNA 1 in chordoma tissues is associated with chordoma pathogenesis by reducing miR‑125a and miR‑10a expression. Mol Med Rep. 2015;12:93–8. doi: 10.3892/mmr.2015.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung YP, Diaz-Perez JA, Cote GM, Wejde J, Schwab,V Nardi JH, et al. Dedifferentiated chordoma: clinicopathologic and molecular characteristics with integrative analysis. Am J Surg Pathol. 2020;44:1213–23. doi: 10.1097/PAS.0000000000001501. [DOI] [PubMed] [Google Scholar]
- 7.Colia V, Stacchiotti S. Medical treatment of advanced chordomas. Eur J Cancer. 2017;83:220–8. doi: 10.1016/j.ejca.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CBJ, Anonescu C, Merens F. WHO classification of tumours: soft tissue and bone tumours. Vol. 3 (WHO Classification of Tumours Editorial Board; 2020).
- 9.Wang L, Wu Z, Tian K, Wang K, Li D, Ma J, et al. Clinical features and surgical outcomes of patients with skull base chordoma: a retrospective analysis of 238 patients. J Neurosurg. 2017;127:1257–67. doi: 10.3171/2016.9.JNS16559. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Fei L, Han R, Huang R, Wang Y, Chen H, et al. Single-cell transcriptome reveals cellular hierarchies and guides p-EMT-targeted trial in skull base chordoma. Cell Discov. 2022;8:94. doi: 10.1038/s41421-022-00459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer JW, Bhattarai N. CAR-T cell therapy: mechanism, management, and mitigation of inflammatory toxicities. Front Immunol. 2021;12:693016. doi: 10.3389/fimmu.2021.693016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Tang X, Zhang Z, Gu L, Wei H, Zhao S, et al. Tandem CAR-T cells targeting CD70 and B7-H3 exhibit potent preclinical activity against multiple solid tumors. Theranostics. 2020;10:7622–34. doi: 10.7150/thno.43991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian Y, Xie D, Yang L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct Target Ther. 2022;7:117. doi: 10.1038/s41392-022-00951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanitis E, Kosti P, Ronet C, Cribioli E, Rota G, Spill A, et al. VEGFR-2 redirected CAR-T cells are functionally impaired by soluble VEGF-A competition for receptor binding. J Immunother Cancer. 2021;9:e002151. doi: 10.1136/jitc-2020-002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, et al. Dominant-negative TGF-beta receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26:1855–66. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derynck R, Turley SJ, Akhurst RJ. TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9–34. doi: 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan W, Zhang B, Li X, Chen W, Jia S, Xin Z, et al. Single-cell transcriptome profiling reveals intra-tumoral heterogeneity in human chordomas. Cancer Immunol Immunother. 2022;71:2185–95. doi: 10.1007/s00262-022-03152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Li S, Wu B, Xu Q, Teng D, Yang T, et al. Landscape of immune cells heterogeneity in liver transplantation by single-cell RNA sequencing analysis. Front Immunol. 2022;13:890019. doi: 10.3389/fimmu.2022.890019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell. 2021;184:3573–87.e3529. doi: 10.1016/j.cell.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. 2019;20:163–72. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venteicher AS, Tirosh I, Hebert C, Yizhak K, Neftel C, Filbin MG, et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science. 2017;355:eaai8478. doi: 10.1126/science.aai8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuang C-H, et al. Inference and analysis of cell-cell communication using CellChat. Nat Commun. 2021;12:1088. doi: 10.1038/s41467-021-21246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Browaeys R, Saelens W, Saeys Y. NicheNet: modeling intercellular communication by linking ligands to target genes. Nat Methods. 2020;17:159–62. doi: 10.1038/s41592-019-0667-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Liu Z, Wang X, Wu H, Zhang J, Yang J, et al. Treatment with humanized selective CD19CAR-T cells shows efficacy in highly treated B-ALL patients who have relapsed after receiving murine-based CD19CAR-T therapies. Clin Cancer Res. 2019;25:5595–607. doi: 10.1158/1078-0432.CCR-19-0916. [DOI] [PubMed] [Google Scholar]
- 26.Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–8. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Xu J, Zhang B, Liu J, Liang C, Meng Q, et al. The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications. Mol Cancer. 2019;18:184. doi: 10.1186/s12943-019-1117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu Y, Chen T, Hu R, Zhu R, Li C, Ruan Y, et al. Next frontier in tumor immunotherapy: macrophage-mediated immune evasion. Biomark Res. 2021;9:72. doi: 10.1186/s40364-021-00327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han S, Wang W, Wang S, Yang T, Zhang G, Wang D, et al. Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics. 2021;11:2892–916. doi: 10.7150/thno.50928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Cao HH, Chu JH, Kwan HY, Su T, Yu H, Cheng C-Y, et al. Inhibition of the STAT3 signaling pathway contributes to apigenin-mediated anti-metastatic effect in melanoma. Sci Rep. 2016;6:21731. doi: 10.1038/srep21731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elaimy AL, Guru S, Chang C, Ou J, Amante JJ, Zhu LJ, et al. VEGF-neuropilin-2 signaling promotes stem-like traits in breast cancer cells by TAZ-mediated repression of the Rac GAP beta2-chimaerin. Sci Signal. 2018;11:eaao6897. doi: 10.1126/scisignal.aao6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palazon A, Tyrakis PA, Macias D, Velica P, Rundqvist H, Fitzpatrick S, et al. An HIF-1alpha/VEGF-A axis in cytotoxic T cells regulates tumor progression. Cancer Cell. 2017;32:669–83.e665. doi: 10.1016/j.ccell.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le X, Nilsson M, Goldman J, Reck M, Nakagawa K, Kato T, et al. Dual EGFR-VEGF pathway inhibition: a promising strategy for patients with EGFR-mutant NSCLC. J Thorac Oncol. 2021;16:205–15. doi: 10.1016/j.jtho.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci USA. 2016;113:4470–5. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laura Joszt MA. FDA approves third bevacizumab biosimilar. https://www.ajmc.com/view/fda-approves-third-bevacizumab-biosimilar (2022).
- 38.Morimoto Y, Ramura R, Ohara K, Kosugi K, Oishi Y, Kuranari Y, et al. Prognostic significance of VEGF receptors expression on the tumor cells in skull base chordoma. J Neurooncol. 2019;144:65–77. doi: 10.1007/s11060-019-03221-z. [DOI] [PubMed] [Google Scholar]
- 39.Akhavan-Sigari R, Gaab MR, Rohde V, Abili M, Ostertag H. Prognostic significance of immunohistochemical expression of VEGFR2 and iNOS in spinal chordoma. Eur Spine J. 2014;23:2416–22. doi: 10.1007/s00586-014-3417-5. [DOI] [PubMed] [Google Scholar]
- 40.Akhavan-Sigari R, Gaab MR, Rohde V, Brandis A, Tezval H, Abili M, et al. Expression of vascular endothelial growth factor receptor 2 (VEGFR-2), inducible nitric oxide synthase (iNOS), and Ki-M1P in skull base chordoma: a series of 145 tumors. Neurosurg Rev. 2014;37:79–88. doi: 10.1007/s10143-013-0495-5. [DOI] [PubMed] [Google Scholar]
- 41.Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. doi: 10.1186/s12943-022-01569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Shi Q, Lou J, Wang B, Wang W, Niu J, et al. Chordoma recruits and polarizes tumor-associated macrophages via secreting CCL5 to promote malignant progression. J Immunother Cancer. 2023;11:e006808. doi: 10.1136/jitc-2023-006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tzavlaki K, Moustakas A. TGF-beta signaling. Biomolecules. 2020;10:487. doi: 10.3390/biom10030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity. 2019;50:924–40. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foley M. No convincing evidence. Br Dent J. 2020;229:72. doi: 10.1038/s41415-020-1939-2. [DOI] [PubMed] [Google Scholar]
- 47.Ma J, Tian K, Wang L, Wang K, Du J, Li D, et al. High expression of TGF-beta1 predicting tumor progression in skull base chordomas. World Neurosurg. 2019;131:e265–e270. doi: 10.1016/j.wneu.2019.07.128. [DOI] [PubMed] [Google Scholar]
- 48.Nixon BG, Gao S, Wang X, Li MO. TGFbeta control of immune responses in cancer: a holistic immuno-oncology perspective. Nat Rev Immunol. 2023;23:346–62. doi: 10.1038/s41577-022-00796-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ciardiello D, Elez E, Tabernero J, Seoane J. Clinical development of therapies targeting TGFbeta: current knowledge and future perspectives. Ann Oncol. 2020;31:1336–49. doi: 10.1016/j.annonc.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman SE, AI Abdulmohsen SA, Gupta S, Hauser BM, Meredith DM, Dunn LF, et al. Translational windows in chordoma: a target appraisal. Front Neurol. 2020;11:657. doi: 10.3389/fneur.2020.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim B-G, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J Hematol Oncol. 2021;14:55. doi: 10.1186/s13045-021-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long C, Li G, Zhang C, Jiang T, Li Y, Duan X, et al. B7-H3 as a target for CAR-T cell therapy in skull base chordoma. Front Oncol. 2021;11:659662. doi: 10.3389/fonc.2021.659662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cendrowicz E, Sas Z, Bremer E, Rygiel TP. The role of macrophages in cancer development and therapy. Cancers (Basel) 2021;13:1946. doi: 10.3390/cancers13081946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5:eaax7969. doi: 10.1126/sciimmunol.aax7969. [DOI] [PubMed] [Google Scholar]
- 57.Chen W, Dijke PT. Immunoregulation by members of the TGFbeta superfamily. Nat Rev Immunol. 2016;16:723–40. doi: 10.1038/nri.2016.112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during this study are publicly accessible from the NGDC Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa-human/), accession number HRA006471.
Additionally, the code utilised in our study is available from a dedicated GitHub repository, accessible at https://github.com/restore1997/chordoma2024.