Abstract
To investigate the screening and predicting functions of obesity- and lipid-related indices for type 2 diabetes (T2D) in middle-aged and elderly Chinese, as well as the ideal predicted cut-off value. This study's data comes from the 2011 China Health and Retirement Longitudinal Study (CHARLS). A cross-sectional study design was used to investigate the relationship of T2D and 13 obesity- and lipid-related indices, including body mass index (BMI), waist circumference (WC), waist–height ratio (WHtR), visceral adiposity index (VAI), a body shape index (ABSI), body roundness index (BRI), lipid accumulation product (LAP), conicity index (CI), Chinese visceral adiposity index (CVAI), triglyceride- glucose index (TyG index) and its correlation index (TyG-BMI, TyG-WC, TyG-WHtR). The unadjusted and adjusted correlations between 13 indices and T2D were assessed using binary logistic regression analysis. The receiver operating characteristic curve (ROC) was used to determine the usefulness of anthropometric indices for screening for T2D and determining their cut‑off value, sensitivity, specificity, and area under the curve (AUC). The study comprised 9488 people aged 45 years or above in total, of whom 4354 (45.89%) were males and 5134 (54.11%) were females. Among them were 716 male cases of T2D (16.44%) and 870 female cases of T2D (16.95%). A total of 13 obesity- and lipid-related indices were independently associated with T2D risk after adjusted for confounding factors (P < 0.05). According to ROC analysis, the TyG index was the best predictor of T2D among males (AUC = 0.780, 95% CI 0.761, 0.799) and females (AUC = 0.782, 95% CI 0.764, 0.799). The AUC values of the 13 indicators were higher than 0.5, indicating that they have predictive values for T2D in middle-aged and elderly Chinese. The 13 obesity- and lipid-related indices can predict the risk of T2D in middle‑aged and elderly Chinese. Among 13 indicators, the TyG index is the best predictor of T2D in both males and females. TyG-WC, TyG-BMI, TyG-WHtR, LAP, and CVAI all outperformed BMI, WC, and WHtR in predicting T2D.
Keywords: Type 2 diabetes, Obesity, Anthropometric indices, Cross-sectional study, Middle-aged and elderly Chinese
Subject terms: Diabetes, Dyslipidaemias, Obesity, Diabetes, Obesity, Risk factors, Predictive markers
Introduction
Diabetes is a chronic disease, which has become an observable global public health problem1. According to statistics, the global prevalence of diabetes among adults aged 20–79 will be 10.5% (536.6 million people) in 2021 and will rise to 12.2% (783.2 million people) in 20452. Type 2 diabetes (T2D) is the most common form of diabetes, accounting for more than 90% of diabetes cases3. Notably, macrovascular problems including coronary heart disease and stroke, as well as microvascular disorders like diabetic kidney disease, retinopathy, and peripheral neuropathy, are common complications of T2D patients4. The onset of T2D and the complications that it causes reduce people's quality of life and create significant economic and social burdens3,5. Particularly for China, which has the highest prevalence of T2D 6. According to the research data of He W et al7., the prevalence of T2D in Xiamen is significantly increasing, especially among young people. Similarly, Wang Z et al8. also found that the prevalence of T2D in Beijing is gradually increasing, and the prevalence of T2D in women is higher than that in men. Although the prevalence of T2D varies from place to place due to differences in economy, culture, lifestyle, eating habits, and other factors, it is generally on the rise.
Numerous studies have revealed that a wide range of lifestyle variables, such as sedentary behavior, psychological stress, smoking, and being overweight or obese, are extremely important in the development of T2D9. Obesity has been proven to increase the risk of T2D by leading to insulin resistance(IR), and lipid metabolism disorders. Obese individuals, due to functional disorders, release a large amount of free fatty acids, reactive oxygen species, and pro-inflammatory cytokines from their adipocytes, which can lead to IR early on and β cell dysfunction10,11. IR is a metabolic state in which insulin-dependent tissues become less sensitive to the action of insulin, resulting in ineffective conversion of blood sugar into energy, increasing blood sugar12. Long-term IR can lead to a decline in pancreatic islets β cell function and the reduction in insulin secretion may eventually lead to the occurrence of diabetes13,14. In addition, obesity can affect lipid metabolism, often accompanied by lipid abnormalities such as hypertriglyceridemia and high-density lipoprotein cholesterol, which further exacerbate IR15. When the lipid index increases, more lipid metabolites accumulate in the body, which increases the risk of diabetes, hyperlipidemia, and hyperuricemia16–18. As a result, the prevalence of T2D often increased simultaneously with the global rise in obesity prevalence19. Some studies20–22 have shown that about 90% of patients with diabetes develop T2D, which is mainly related to overweight. With the highest prevalence of overweight and obesity in the world, China may have 789.95 million overweight or obese people by 203023. Given the enormous number of obese people, it is crucial to use simple and efficient methods to identify high-risk populations for T2D, to prevent and treat it as early as possible.
Currently, many studies24–27 have shown that efficient and low-cost obesity- and lipid-related indices are associated with IR and T2D. In general, 13 obesity- and lipid-related indices can be used to describe the status of fat accumulation and the amount of lipid metabolites in the body: waist circumference (WC), body mass index (BMI), waist–height ratio (WHtR), visceral adiposity index (VAI), a body shape index (ABSI), body roundness index (BRI), lipid accumulation product (LAP), conicity index (CI), Chinese visceral adiposity index (CVAI), triglyceride-glucose (TyG) index and its correlation index (TyG-BMI, TyG-WC, TyG-WHtR). Mounting studies have suggested to use of these indices to identify high-risk populations for T2D28–30. However, there is still controversy over the usefulness of various indices in predicting T2D. This is because anthropometric indices are influenced by factors such as race, age, and gender, and body composition may vary by population and ethnicity31–33. Moreover, most studies33–35 did not comprehensively compare the predictive ability of obesity- and lipid-related indices for T2D.
Thus, the purpose of this study was to investigate the screening and prediction functions of obesity- and lipid-related indices for the risk of T2D in middle-aged and elderly Chinese, as well as the ideal predicted cut-off value for providing a foundation for T2D prevention and treatment.
Materials and methods
Study design and setting
This cross-sectional study followed the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ checklist, we confirm that all methods were performed in accordance with the relevant guidelines and regulations36. Data for the current study were obtained from The China Health and Retirement Longitudinal Study (CHARLS), a nationally representative cohort study of China's population of middle-aged and older adults aged from 45 to 10137. Without any direct interaction with people, all data are provided in the open as microdata at http://charls.pku.edu.cn/ index/zh-cn.html. Before data collection, all participants provided informed approval, and the study was approved by the Ethics Committee of Peking University's China Center for Economic Research.
Participants
The individuals for this study were drawn from the China Health and Retirement Longitudinal Study (CHARLS). This study used data from CHARLS Wave1, and participants recruited from May 2011 to March 2012 were included in our study. We excluded individuals who met any of the criteria at baseline (1) type 2 diabetes data missing, (2) one of the 13 indices missing, (3) age/sex/educational levels/marital status/current residence/current smoking/alcohol drinking/taking activities/having regular exercise/ chronic diseases missing. After missing data subjects were excluded, our study included 9,488 aged 45 years and above individuals after missing data subjects were eliminated. A total of 4354 (45.89%) were male and 5134 (54.11%) were female were included.
Definition of T2D
According to the latest definition of T2D by the International Diabetes Association. Fasting plasma glucose (FPG) levels of 7.0 mmol/L or higher, a 2-h plasma glucose level of 11.1 mmol/L or higher, and a prior self-reported diagnosis of T2D were used to describe the condition. The T2D diagnosis standards were the same as in earlier studies35,38.
Anthropometric indices
Certified medical personnel took anthropometric measures. Body weight and height were estimated to be the closest values of 0.1 kg and 0.5 cm, respectively, using standard medical equipment. After the expiratory breath, WC was measured on both sides between the iliac crest and the lower ribs. BMI was measured with weight (kg) / height2 (m2). WHtR is defined as WC (cm) / height (cm). The VAI, CVAI, LAP, and TyG-index require TG and high-density lipoprotein cholesterol (HDL-C) to be obtained by invasive examination for calculation. The TyG index is the result of a computation using TG and glucose39. Except for WC, the remaining anthropometric indices were calculated using the following Eqs40–44:
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
Males:
| 8 |
Females:
| 9 |
| 10 |
| 11 |
| 12 |
Covariates
Socio-demographic characteristics including age, sex (1 = male, 2 = female), education levels, marital status, current residence, current smoking, alcohol drinking, taking activities, having regular exercise, and chronic disease were collected by the self-reported questionnaire. These categories have been used extensively in our previous research45–48.
age was sorted into 45–54/55–64/65–74/above 75 years old.
education levels were classified into illiterate/less than elementary school/high school/and above vocational school.
marital status was classified into single/married;
current residence included urban/rural.
current smoking was categorized into no smoker/former smoker/current smoker.
alcohol drinking was divided into never drinking/ less than once a month/more than once a month.
taking activities were sorted into ever (at least once a month) /never.
having regular exercise included no exercise/less than exercises/regular exercises.
the counts of chronic disease were classified into 0/1–2/ 3–14.
Statistical analysis
All statistical analysis was performed using IBM SPSS version 25.0 (IBM Corp., Armonk, NY). Categorical variables were expressed as frequency and percent. Chi-square test and one-way ANOVA were performed for the comparison of dichotomous or multiple categorical variables. Means and standard deviations were used to express continuous variables. To evaluate changes in gender average distribution, an independent sample t-test was used. The unadjusted and adjusted correlations between obesity- and lipid-related indicators and T2D were assessed using binary logistic regression analysis. After adjusting for age, education level, marital status, current residence, current smoking, alcohol drinking, taking activities, having regular exercise, and chronic diseases, the odds ratio (OR) and 95% confidence intervals (95%CIs) of each obesity- and lipid-related indices with T2D were calculated. The receiver operating characteristic curve (ROC) was used to calculate the area under the curve (AUC) of each indicator as a predictive factor for T2D. The indicator with the highest AUC was regarded as the most accurate, and the closer the AUC was to 1, the more accurate the prediction would be. Calculations were made for the obesity- and lipid-related markers' sensitivity, specificity, cut-off points, and Youden index [maximum (sensitivity + specificity-1)]. Statistical significance was defined as a P < 0.05.
Ethics approval and consent to participate
All data are openly published as microdata at http://opendata.pku.edu.cn/dataverse/CHARLS with no direct contact with all participants. Approval for this study was given by the medical ethics committee of Wannan Medical College (approval number 2021–3). The patients/participants provided their written informed consent to participate in this study.
Results
Table 1 shows the baseline characteristics of participants with full samples. The study comprised 9488 people over the age of 45 in total, of whom 4354 (45.89%) were male and 5134 (54.11%) were female. The mean WC, BMI, WHtR, VAI, ABSI, BRI, LAP, CI, CVAI, TyG index, TyG-BMI, TyG-WC, TyG-WHtR, and HbA1c in females are higher than in males (P < 0.05). We also observed significant differences in age, education levels, marital status, current smoking, alcohol drinking, and chronic diseases between males and females (P < 0.05). However, there was no statistically significant difference in the current residence, taking activities, and having regular exercise between males and females (P > 0.05). Because of these significant differences between males and females (P < 0.05), we performed the main analyses separately by sex.
Table 1.
Characteristics of participants with full samples (N = 9488).
| Variables | Male | Female | Total | t/χ2 | P |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| N | 4354(45.89%) | 5134(54.11%) | 9488(100%) | ||
| Age(years) | 83.541 | 0.000 | |||
| 45–54 | 1274(29.26) | 1922(37.44) | 3196(33.68) | ||
| 55–64 | 1724(39.60) | 1930(37.59) | 3654(38.51) | ||
| 65–74 | 989(22.71) | 903(17.59) | 1892(19.94) | ||
| ≥ 75 | 367(8.43) | 379(7.38) | 746(7.86) | ||
| Education levels | 968.940 | 0.000 | |||
| Illiterate | 597(13.71) | 2185(42.56) | 2782(29.32) | ||
| Less than elementary school | 3195(73.38) | 2604(50.72) | 5799(61.12) | ||
| High school | 360(8.27) | 253(4.93) | 613(6.46) | ||
| Above vocational school | 202(4.64) | 92(1.79) | 294(3.10) | ||
| Marital status | 71.991 | 0.000 | |||
| Single | 406(9.32) | 775(15.10) | 1181(12.45) | ||
| Married | 3948(90.68) | 4359(84.90) | 8307(87.55) | ||
| Current residence | 0.750 | 0.387 | |||
| Rural | 4012(92.15) | 4755(92.62) | 8767(92.40) | ||
| Urban | 342(7.85) | 379(7.38) | 721(7.60) | ||
| Current smoking | 4521.237 | 0.000 | |||
| No | 1075(24.69) | 4733(92.19) | 5808(61.21) | ||
| Former smoke | 734(16.86) | 95(1.85) | 829(8.74) | ||
| Current smoke | 2545(58.45) | 306(5.96) | 2851(30.05) | ||
| Alcohol drinking | 2162.870 | 0.000 | |||
| No | 1920(44.10) | 4516(87.96) | 6436(67.83) | ||
| Less than once a month | 470(10.79) | 255(4.97) | 725(7.64) | ||
| More than once a month | 1964(45.11) | 363(7.07) | 2327(24.53) | ||
| Taking activities | 0.789 | 0.374 | |||
| No | 2138(49.10) | 2568(50.02) | 4706(49.60) | ||
| Yes | 2216(50.90) | 2566(49.98) | 4782(50.40) | ||
| Having regular exercises | 1.150 | 0.563 | |||
| No exercise | 2708(62.20) | 3145(61.26) | 5853(61.69) | ||
| Less than exercises | 814(18.70) | 1001(19.50) | 1815(19.13) | ||
| Regular exercises | 832(19.11) | 988(19.24) | 1820(19.18) | ||
| Chronic diseases(counts) | 20.702 | 0.000 | |||
| 0 | 1422(32.66) | 1474(28.71) | 2896(30.52) | ||
| 1–2 | 2161(49.63) | 2623(51.09) | 4784(50.42) | ||
| 3–14 | 771(17.71) | 1037(20.20) | 1808(19.06) | ||
| FPG(mg/dl) | 109.99 ± 35.44 | 109.77 ± 36.90 | 109.87 ± 36.24 | 0.297 | 0.766 |
| HbA1c | 5.24 ± 0.77 | 5.29 ± 0.84 | 5.27 ± 0.81 | − 2.888 | 0.004 |
| WC | 84.96 ± 9.81 | 85.64 ± 10.16 | 85.33 ± 10.01 | − 3.319 | 0.001 |
| BMI | 22.96 ± 3.64 | 23.99 ± 4.05 | 23.51 ± 3.90 | − 13.002 | 0.000 |
| WHtR | 0.52 ± 0.06 | 0.56 ± 0.07 | 0.54 ± 0.07 | − 33.311 | 0.000 |
| VAI | 3.96 ± 4.41 | 6.07 ± 5.72 | 5.10 ± 5.26 | − 20.256 | 0.000 |
| ABSI | 0.08 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | − 10.650 | 0.000 |
| BRI | 3.78 ± 1.14 | 4.66 ± 1.46 | 4.26 ± 1.39 | − 33.183 | 0.000 |
| LAP | 30.87 ± 33.31 | 43.74 ± 35.23 | 37.83 ± 34.95 | − 18.262 | 0.000 |
| CI | 1.27 ± 0.08 | 1.30 ± 0.10 | 1.29 ± 0.09 | − 15.487 | 0.000 |
| CVAI | 95.98 ± 47.50 | 107.11 ± 43.43 | 102.00 ± 45.68 | − 11.826 | 0.000 |
| TyG index | 8.62 ± 0.66 | 8.72 ± 0.63 | 8.68 ± 0.65 | − 7.594 | 0.000 |
| TyG-BMI | 198.67 ± 39.64 | 209.77 ± 41.67 | 204.68 ± 41.12 | − 13.285 | 0.000 |
| TyG-WC | 7.35 ± 1.18 | 7.49 ± 1.16 | 7.42 ± 1.17 | − 5.840 | 0.000 |
| TyG -WHtR | 4.48 ± 0.69 | 4.91 ± 0.76 | 4.71 ± 0.76 | − 28.240 | 0.000 |
FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; WC: waist circumference; BMI: body mass index; WHtR: waist to height ratio; VAI: visceral adiposity index; ABSI: A body shape index; BRI: body roundness index; LAP: lipid accumulation product; CVAI: Chinese visceral adiposity index; CI: conicity index; TyG index: triglyceride-glucose index; TyG-BMI: TyG related to BMI; TyG-WC: TyG related to WC; TyG-WHtR: TyG related to WHtR.
Table 2 shows the baseline characteristics of the study participants with and without T2D by sex. The cross-sectional analysis included 716 males with T2D (16.44%) and 870 females with T2D (16.95%). Males with T2D had significant differences in age, current smoking, chronic diseases, FPG, HbA1c, WC, BMI, WHtR, VAI, ABSI, BRI, LAP, CI, CVAI, TyG index, TyG-BMI, TyG-WC, and TyG-WHtR (P < 0.05). Females with T2D had significant differences in age, current residence, alcohol drinking, taking activities, chronic diseases, FPG, HbA1c, WC, BMI, WHtR, VAI, ABSI, BRI, LAP, CI, CVAI, TyG index, TyG-BMI, TyG-WC, and TyG-WHtR (P < 0.05).
Table 2.
Baseline characteristics of the study participants with and without T2D by sex.
| Variables | Male | χ2 | P | Female | χ2 | P | ||
|---|---|---|---|---|---|---|---|---|
| With T2D | Without T2D | With T2D | Without T2D | |||||
| N (%) | N (%) | N (%) | N (%) | |||||
| N | 716(16.44) | 3638(83.56) | 870(16.95) | 4264(83.05) | ||||
| Age(years) | 15.064 | 0.002 | 37.063 | 0.000 | ||||
| 45–54 | 167(23.32) | 1107(30.43) | 249(28.62) | 1673(39.24) | ||||
| 55–64 | 304(42.46) | 1420(39.03) | 368(42.30) | 1562(36.63) | ||||
| 65–74 | 175(24.44) | 814(22.37) | 187(21.49) | 716(16.79) | ||||
| ≥ 75 | 70(9.78) | 297(8.16) | 66(7.59) | 313(7.34) | ||||
| Education levels | 2.262 | 0.520 | 2.083 | 0.555 | ||||
| Illiterate | 96(13.41) | 501(13.77) | 378(43.45) | 1807(42.38) | ||||
| Less than elementary school | 526(73.46) | 2669(73.36) | 431(49.54) | 2173(50.96) | ||||
| High school | 54(7.54) | 306(8.41) | 41(4.71) | 212(4.97) | ||||
| Above vocational school | 40(5.59) | 162(4.45) | 20(2.30) | 72(1.69) | ||||
| Marital status | 0.099 | 0.753 | 0.019 | 0.890 | ||||
| Single | 69(9.64) | 337(9.26) | 130(14.94) | 645(15.13) | ||||
| Married | 647(90.36) | 3301(90.74) | 740(85.06) | 3619(84.87) | ||||
| Current residence | 2.673 | 0.102 | 7.135 | 0.008 | ||||
| Rural | 649(90.64) | 3363(92.44) | 787(90.46) | 3968(93.06) | ||||
| Urban | 67(9.36) | 275(7.56) | 83(9.54) | 296(6.94) | ||||
| Current smoking | 11.336 | 0.003 | 0.724 | 0.696 | ||||
| No | 182(25.42) | 893(24.55) | 796(91.49) | 3937(92.33) | ||||
| Former smoke | 149(20.81) | 585(16.08) | 18(2.07) | 77(1.81) | ||||
| Current smoke | 385(53.77) | 2160(59.37) | 56(6.44) | 250(5.86) | ||||
| Alcohol drinking | 2.224 | 0.329 | 6.967 | 0.031 | ||||
| No | 328(45.81) | 1592(43.76) | 786(90.34) | 3730(87.48) | ||||
| Less than once a month | 67(9.36) | 403(11.08) | 40(4.60) | 215(5.04) | ||||
| More than once a month | 321(44.83) | 1643(45.16) | 44(5.06) | 319(7.48) | ||||
| Taking activities | 1.423 | 0.233 | 4.393 | 0.036 | ||||
| No | 337(47.07) | 1801(49.51) | 407(46.78) | 2161(50.68) | ||||
| Yes | 379(52.93) | 1837(50.49) | 463(53.22) | 2103(49.32) | ||||
| Having regular exercises | 2.369 | 0.306 | 1.596 | 0.450 | ||||
| No exercise | 463(64.66) | 2245(61.71) | 549(63.10) | 2596(60.88) | ||||
| Less than exercises | 128(17.88) | 686(18.86) | 159(18.28) | 842(19.75) | ||||
| Regular exercises | 125(17.46) | 707(19.43) | 162(18.62) | 826(19.37) | ||||
| Chronic diseases(counts) | 114.524 | 0.000 | 196.794 | 0.000 | ||||
| 0 | 155(21.65) | 1267(34.83) | 152(17.47) | 1322(31.00) | ||||
| 1–2 | 341(47.63) | 1820(50.03) | 397(45.63) | 2226(52.20) | ||||
| 3–14 | 220(30.73) | 551(15.15) | 321(36.90) | 716(16.79) | ||||
| FPG(mg/dl) | 161.05 ± 61.26 | 99.94 ± 12.32 | − 26.586 | 0.000 | 157.79 ± 67.82 | 99.97 ± 11.62 | − 26.070 | 0.000 |
| HbA1c | 5.97 ± 1.44 | 5.10 ± 0.40 | − 16.192 | 0.000 | 6.20 ± 1.56 | 5.10 ± 0.39 | − 20.477 | 0.000 |
| WC | 88.72 ± 10.53 | 84.22 ± 9.49 | − 10.616 | 0.000 | 88.85 ± 10.10 | 84.99 ± 10.05 | − 10.325 | 0.000 |
| BMI | 24.17 ± 4.18 | 22.72 ± 3.48 | − 8.692 | 0.000 | 24.99 ± 4.48 | 23.78 ± 3.92 | − 7.378 | 0.000 |
| WHtR | 0.54 ± 0.06 | 0.51 ± 0.06 | − 11.286 | 0.000 | 0.58 ± 0.07 | 0.56 ± 0.07 | − 10.617 | 0.000 |
| VAI | 6.33 ± 7.38 | 3.49 ± 3.35 | − 10.087 | 0.000 | 8.99 ± 8.44 | 5.47 ± 4.77 | − 11.905 | 0.000 |
| ABSI | 0.08 ± 0.01 | 0.08 ± 0.01 | − 4.701 | 0.000 | 0.08 ± 0.01 | 0.08 ± 0.01 | − 4.655 | 0.000 |
| BRI | 4.24 ± 1.23 | 3.69 ± 1.10 | − 11.088 | 0.000 | 5.14 ± 1.57 | 4.57 ± 1.41 | − 10.584 | 0.000 |
| LAP | 50.25 ± 51.98 | 27.05 ± 26.61 | − 11.643 | 0.000 | 62.89 ± 47.06 | 39.83 ± 30.87 | − 13.859 | 0.000 |
| CI | 1.30 ± 0.08 | 1.27 ± 0.08 | − 8.380 | 0.000 | 1.32 ± 0.09 | 1.30 ± 0.10 | − 7.696 | 0.000 |
| CVAI | 118.90 ± 51.88 | 91.47 ± 45.25 | − 13.196 | 0.000 | 129.02 ± 43.40 | 102.64 ± 42.06 | − 16.770 | 0.000 |
| TyG index | 9.24 ± 0.78 | 8.50 ± 0.56 | − 24.176 | 0.000 | 9.30 ± 0.72 | 8.60 ± 0.54 | − 27.093 | 0.000 |
| TyG-BMI | 224.27 ± 48.84 | 193.63 ± 35.46 | − 15.976 | 0.000 | 232.67 ± 46.17 | 205.10 ± 39.08 | − 16.454 | 0.000 |
| TyG-WC | 821.92 ± 134.91 | 717.41 ± 106.25 | − 19.570 | 0.000 | 827.60 ± 121.56 | 732.57 ± 108.26 | − 21.392 | 0.000 |
| TyG-WHtR | 5.01 ± 0.78 | 4.38 ± 0.62 | − 20.603 | 0.000 | 5.43 ± 0.80 | 4.80 ± 0.71 | − 21.569 | 0.000 |
FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; WC: waist circumference; BMI: body mass index; WHtR: waist to height ratio; VAI: visceral adiposity index; ABSI: A body shape index; BRI: body roundness index; LAP: lipid accumulation product; CVAI: Chinese visceral adiposity index; CI: conicity index; TyG index: triglyceride-glucose index; TyG-BMI: TyG related to BMI; TyG-WC: TyG related to WC; TyG-WHtR: TyG related to WHtR.
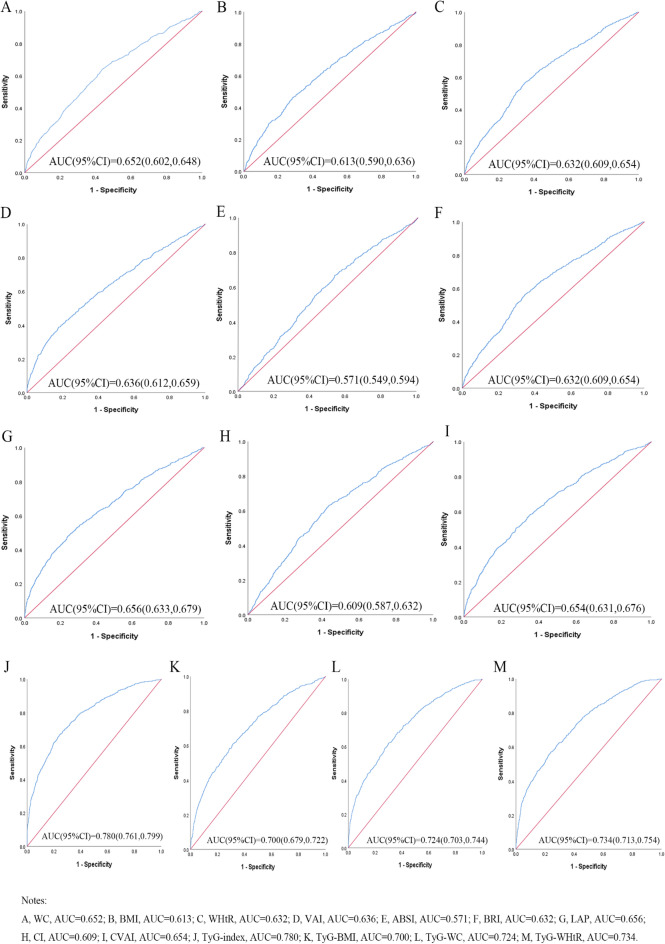
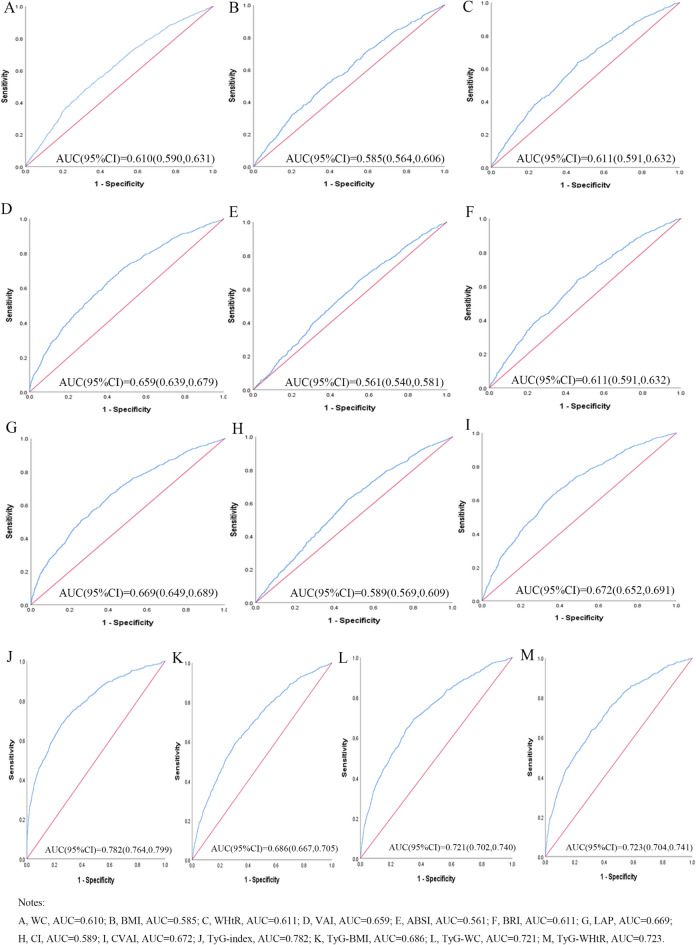
Table 3 shows the cut-off value between the area under the curve, sensitivity, and specificity for obesity- and lipid-related indices to detect T2D by sex. The ROC curves of each indicator in the prediction of T2D risk in males and females are shown in Figs. 1 and 2, respectively. As shown in the table and figures, among males, the TyG index was the best predictor of T2D (AUC = 0.780, 95% CI: 0.761, 0.799, and optimal cutoffs = 8.838). Meanwhile, TyG-WHtR (AUC = 0.734, 95%CI: 0.713, 0.754, and optimal cutoffs = 4.716) had similar predictive values. Moreover, among females, the TyG index was the most accurate predictor of T2D (AUC = 0.782, 95%CI: 0.764, 0.799, and optimal cutoffs = 8.940), followed by TyG-WHtR (AUC = 0.723, 95%CI: 0.704, 0.741, and optimal cutoffs = 5.131), and TyG-WC (AUC = 0.721, 95%CI: 0.702, 0.740, and optimal cutoffs = 769.129). All of the above indicators have statistical significance (P < 0.05). From the overall data, the AUC values of the above 13 indicators were higher than 0.5, indicating that they have predictive values for T2D in middle-aged and elderly Chinese.
Table 3.
Cut‑off between area under the curve, sensitivity and specificity for obesity‑ and lipid‑related indices to detect T2D by sex.
| WC | BMI | WHtR | VAI | ABSI | BRI | LAP | CI | CVAI | TyG index | TyG-BMI | TyG-WC | TyG -WHtR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | |||||||||||||
| Area under curve | 0.625 | 0.613 | 0.632 | 0.636 | 0.571 | 0.632 | 0.656 | 0.609 | 0.654 | 0.780 | 0.700 | 0.724 | 0.734 |
| Std. Error | 0.012 | 0.012 | 0.011 | 0.012 | 0.012 | 0.011 | 0.012 | 0.012 | 0.011 | 0.010 | 0.011 | 0.011 | 0.010 |
| 95%CI | 0.602,0.648 | 0.590,0.636 | 0.609,0.654 | 0.612,0.659 | 0.549,0.594 | 0.609,0.654 | 0.633,0.679 | 0.587,0.632 | 0.631,0.676 | 0.761,0.799 | 0.679,0.722 | 0.703,0.744 | 0.713,0.754 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Optimal cutoffs | 84.650 | 24.238 | 0.532 | 4.881 | 0.082 | 3.971 | 33.755 | 1.278 | 106.900 | 8.838 | 206.505 | 767.931 | 4.716 |
| J-Youden | 0.202 | 0.179 | 0.219 | 0.211 | 0.130 | 0.219 | 0.240 | 0.195 | 0.231 | 0.426 | 0.296 | 0.323 | 0.331 |
| Sensitivity (%) | 64.2% | 46.5% | 56.4% | 40.1% | 67.0% | 56.4% | 49.6% | 63.1% | 58.1% | 66.8% | 61.0% | 62.2% | 61.5% |
| Specificity (%) | 56.0% | 71.4% | 65.5% | 81.0% | 46.0% | 65.5% | 74.4% | 56.4% | 65.0% | 75.8% | 68.6% | 70.1% | 71.6% |
| ( +) Likelihood ratio | 1.459 | 1.626 | 1.635 | 2.111 | 1.241 | 1.635 | 1.938 | 1.447 | 1.660 | 2.760 | 1.943 | 2.080 | 2.165 |
| ( −) Likelihood ratio | 0.639 | 0.749 | 0.666 | 0.740 | 0.717 | 0.666 | 0.677 | 0.654 | 0.645 | 0.438 | 0.569 | 0.539 | 0.538 |
| Female | |||||||||||||
| Area under curve | 0.610 | 0.585 | 0.611 | 0.659 | 0.561 | 0.611 | 0.669 | 0.589 | 0.672 | 0.782 | 0.686 | 0.721 | 0.723 |
| Std. Error | 0.010 | 0.011 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.009 | 0.010 | 0.010 | 0.010 |
| 95%CI | 0.590,0.631 | 0.564,0.606 | 0.591,0.632 | 0.639,0.679 | 0.540,0.581 | 0.591,0.632 | 0.649,0.689 | 0.569,0.609 | 0.652,0.691 | 0.764,0.799 | 0.667,0.705 | 0.702,0.740 | 0.704,0.741 |
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Optimal cutoffs | 89.350 | 24.599 | 0.561 | 4.810 | 0.082 | 4.571 | 36.918 | 1.301 | 115.018 | 8.940 | 221.078 | 769.129 | 5.131 |
| J-Youden | 0.165 | 0.127 | 0.178 | 0.235 | 0.106 | 0.178 | 0.253 | 0.152 | 0.266 | 0.432 | 0.285 | 0.343 | 0.323 |
| Sensitivity (%) | 48.5% | 50.8% | 63.7% | 63.7% | 65.2% | 63.7% | 66.3% | 62.4% | 63.2% | 68.5% | 59.0% | 69.2% | 63.4% |
| Specificity (%) | 68.0% | 61.9% | 54.1% | 59.8% | 45.4% | 54.1% | 59.0% | 52.8% | 63.4% | 74.7% | 69.5% | 65.1% | 68.9% |
| ( +) Likelihood ratio | 1.516 | 1.333 | 1.388 | 1.585 | 1.194 | 1.388 | 1.617 | 1.322 | 1.727 | 2.708 | 1.934 | 1.983 | 2.039 |
| ( −) Likelihood ratio | 0.757 | 0.795 | 0.671 | 0.607 | 0.767 | 0.671 | 0.571 | 0.712 | 0.580 | 0.422 | 0.590 | 0.473 | 0.531 |
WC: waist circumference; BMI: body mass index; WHtR: waist to height ratio; VAI: visceral adiposity index; ABSI: A body shape index; BRI: body roundness index; LAP: lipid accumulation product; CVAI: Chinese visceral adiposity index; CI: conicity index; TyG index: triglyceride-glucose index; TyG-BMI: TyG related to BMI; TyG-WC: TyG related to WC; TyG-WHtR: TyG related to WHtR.
Figure 1.
The ROC curves of each indicator in the prediction of T2D risk in males.
Figure 2.
The ROC curves of each indicator in the prediction of T2D risk in females.
Table 4 shows the odds ratios (ORs) and 95% confidence interval (CIs) for the associations of obesity- and lipid-related indices with T2D. According to the optimal cutoffs in Table 3, 13 obesity- and lipid-related indices were converted into two-category variables in this investigation. Table 4 is based on the transformed variables. A larger OR, in general, suggests a higher risk factor. Both before and after adjusting for age, educational levels, marital status, current residence, current smoking, alcohol drinking, taking activities, having regular exercise, and chronic disease counts, the odds of the risk of T2D increased progressively with increasing units of obesity- and lipid-related indices for both males and females. After adjusting for all covariates, each unit rises in TyG index, for example, was related to a 6.643-fold (95% CI: 5.547, 7.954) increase in the likelihood of developing T2D in males and 5.957-fold (95% CI: 5.068, 7.002) in females. Each unit increase in TyG-WHtR was linked to a 3.886-fold (95% CI: 3.270, 4.618) increase in the likelihood of developing T2D in males and 3.314-fold (95% CI: 2.833, 3.877) in females. Among the 13 indicators, the correlation between ABSI and T2D was weakest in males (OR = 1.516, 95% CI: 1.278, 1.797) and females (OR = 1.376, 95% CI: 1.163, 1.682). All indices had statistical significance after adjustment of confounding factors (P < 0.05).
Table 4.
Odds ratios (ORs) and 95% confidence interval (CIs) for the associations of obesity- and lipid-related indices with T2D.
| Variables | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| WC | 2.284(1.934,2.697) | 0.000 | 2.205(1.855,2.622) | 0.000 | 1.998(1.724,2.316) | 0.000 | 1.703(1.462,1.983) | 0.000 |
| BMI | 2.166(1.839,2.551) | 0.000 | 2.094(1.758,2.494) | 0.000 | 1.675(1.447,1.940) | 0.000 | 1.522(1.307,1.773) | 0.000 |
| WHtR | 2.446(2.079,2.878) | 0.000 | 2.251(1.903,2.662) | 0.000 | 2.058(1.771,2.393) | 0.000 | 1.760(1.506,2.057) | 0.000 |
| VAI | 2.843(2.397,3.373) | 0.000 | 2.868(2.403,3.423) | 0.000 | 2.603(2.238,3.028) | 0.000 | 2.337(2.002,2.728) | 0.000 |
| ABSI | 1.608(1.363,1.896) | 0.000 | 1.516(1.278,1.797) | 0.000 | 1.527(1.308,1.782) | 0.000 | 1.376(1.163,1.682) | 0.000 |
| BRI | 2.453(2.084,2.886) | 0.000 | 2.255(1.907,2.668) | 0.000 | 2.063(1.774,2.398) | 0.000 | 1.764(1.509,2.062) | 0.000 |
| LAP | 2.851(2.419,3.360) | 0.000 | 2.844(2.394,3.378) | 0.000 | 2.818(2.418,3.285) | 0.000 | 2.476(2.117,2.896) | 0.000 |
| CI | 2.203(1.867,2.599) | 0.000 | 2.024(1.709,2.397) | 0.000 | 1.848(1.591,2.146) | 0.000 | 1.619(1.379,1.900) | 0.000 |
| CVAI | 2.570(2.183,3.026) | 0.000 | 2.409(2.034,2.854) | 0.000 | 2.973(2.556,3.459) | 0.000 | 2.516(2.143,2.953) | 0.000 |
| TyG index | 6.266(5.271,7.450) | 0.000 | 6.643(5.547,7.954) | 0.000 | 6.421(5.478,7.526) | 0.000 | 5.957(5.068,7.002) | 0.000 |
| TyG-BMI | 3.415(2.893,4.030) | 0.000 | 3.508(2.943,4.182) | 0.000 | 3.269(2.814,3.798) | 0.000 | 2.978(2.549,3.479) | 0.000 |
| TyG-WC | 3.844(3.253,4.541) | 0.000 | 3.851(3.235,4.585) | 0.000 | 4.182(3.574,4.893) | 0.000 | 3.677(3.132,4.316) | 0.000 |
| TyG-WHtR | 4.009(3.393,4.737) | 0.000 | 3.886(3.270,4.618) | 0.000 | 3.829(3.288,4.460) | 0.000 | 3.314(2.833,3.877) | 0.000 |
WC: waist circumference; BMI: body mass index; WHtR: waist to height ratio; VAI: visceral adiposity index; ABSI: A body shape index; BRI: body roundness index; LAP: lipid accumulation product; CVAI: Chinese visceral adiposity index; CI: conicity index; TyG index: triglyceride-glucose index; TyG-BMI: TyG related to BMI; TyG-WC: TyG related to WC; TyG-WHtR: TyG related to WHtR.
Model l: unadjusted.
Model 2: adjusting for age, educational levels, marital status, current residence, current smoking, alcohol drinking, taking activities, having regular exercises, and chronic diseases.
Discussion
In our study, we confirmed the correlation between 13 obesity- and lipid-related indicators and T2D, and compared the predictive ability of these indicators for the risk of T2D. Our study found that participants with T2D were higher than those without T2D in all indicator levels, and the higher the indicator level, the greater the risk of T2D. In addition, through ROC analysis, we found that among the 13 indicators, the TyG index performed the best, followed by TyG-WHtR, TyG-WC, and TyG-BMI.
Our research is consistent with the findings of Ahn N et al., who suggest using the TyG index as a reliable, convenient, and economical diagnostic measurement for the early identification of diabetes in the general population49. Previous research has shown that dyslipidemia, which is marked by elevated TG, and IR, is very common among individuals with T2D50,51. TyG index which is created through the multiplication of TG by glucose, has shown promise as substitute indices for IR because of their practical and widespread availability52,53. TyG-BMI, TyG-WC, and TyG-WHtR are obtained by multiplying TyG with BMI, WC, and WHtR. Several studies suggested that the indices had greater efficacy than the TyG index alone in predicting T2D31,54. In our study, the TyG index is the most effective indicator for identifying T2D (AUC = 0.780 in males and 0.782 in females) among all indicators, followed by TyG-WHtR (AUC = 0.734 in males and 0.723 in females), TyG-WC (AUC = 0.724 in males and 0.721 in females), and TyG-BMI (AUC = 0.700 in males and 0.686 in females).
It is worth noting that in addition to the TyG index and TyG-related factors, new visceral obesity indicators LAP and CVAI have also been reported as important predictors of T2D13,55. LAP is a lipid accumulation index calculated based on WC and TG, which performs best in predicting metabolic syndrome56 and it was superior to BMI in identifying T2D risk57. In this study, LAP is second only to TyG-related indices in males (AUC = 0.656, 95% CI: 0.633, 0.679, and optimal cutoff value = 33.755) and females (AUC = 0.669, 95% CI: 0.649, 0.689, and optimal cutoff value = 36.918). In addition, the CVAI calculated based on age, BMI, WC, TG, and HDL-C represents a novel indicator of visceral adipose tissue58,59. As described by Han et al., CVAI (AUC = 0.695 in men and 0.707 in women) performs better in predicting the risk of T2D compared to other visceral obesity indices (such as VAI, WC, and BMI)34. Similarly, Wei et al. also found that CVAI outperformed WC, BMI, and ABSI in diabetes screening among Chinese adults60. In this study, for males, the AUC of CVAI (AUC = 0.654, 95% CI: 0.631, 0.679, and optimal cutoff value = 106.900) was the largest except for the TyG index and its related indices, and LAP. For females, the AUC of CVAI (AUC = 0.672, 95% CI: 0.652, 0.691, and optimal cutoff value = 115.018) was also second only to the TyG index and its related indices. Compared with males, we found that CVAI has a better predictive ability for T2D in females. This interesting result was also found in Tsou MT et al.’s study, which may be related to physiological differences in visceral fat deposition and distribution between the genders61.
In this study, 13 obesity- and lipid-related indices were converted into two-category variables according to the optimal cutoff point in Table 3. Table 4 is based on the transformed variables. In general, a higher OR indicates a greater risk factor. In Table 4, the OR value of ABSI is much lower than that of the remaining 12 indices in both sexes (OR = 1.516 in males and 1.376 in females), after adjusting for all confounding factors. According to ROC analysis, the AUC value of ABSI is lower than that of the remaining 12 indices in both sexes (AUC = 0.571 in males and 0.561 in females). Consistent with our research results, Chang et al. also found that in terms of predicting the existence of diabetes among the rural population in Northeast China, compared with BRI, ABSI has the weakest predictive ability30.
Our research has several limitations. As this study is a cross-sectional study, it is not possible to make causal inferences about the relationship between the 13 indicators and the risk of T2D. Furthermore, the participants in this study are only Chinese aged 45 and above, therefore, this conclusion will limit the generalizability of the results to other countries and ethnic groups.
Additionally, our study also has several advantages. We examined the effects of 13 obesity- and lipid-related indicators on T2D and comprehensively compared their predictive ability for T2D, which was useful for early screening. Second, most of the indicators in this study were simple indicators with strong practicality for use in normal clinical practice to predict T2D. Finally, This study also included 9488 participants who were 45 years of age or older, the large sample size enhanced the generalizability and effectiveness of the research results.
Conclusion
In this study, 13 obesity- and lipid-related indices were able to significantly predict T2D in middle-aged and elderly Chinese. Among 13 indicators, the TyG index is the best indicator to predict T2D in males and females. At the same time, CVAI, LAP, TyG-BMI, TyG-WC, and TyG-WHtR performed better than traditional indicators (such as BMI, WC, and WHtR) in predicting T2D. It is worth noting that these indicators can serve as simple indicators for predicting T2D in clinical practice and epidemiological investigations, they can provide certain reference values for early identification and prevention of T2D.
Acknowledgements
We thank the members of the research as well as all participants for their contribution.
Abbreviations
- CHARLS
China Health and Retirement Longitudinal Study
- WC
Waist circumference
- BMI
Body mass index
- WHtR
Waist–height ratio
- VAI
Visceral adiposity index
- ABSI
A body shape index
- BRI
Body roundness index
- LAP
Lipid accumulation product
- CI
Conicity index
- CVAI
Chinese visceral adiposity index
- TyG index
Triglyceride-glucose index
- TyG-BMI
Triglyceride-glucose related to BMI
- TyG-WC
Triglyceride-glucose related to WC
- TyG-WHtR
Triglyceride-glucose related to WHtR
- T2D
Type 2 diabetes
- IR
Insulin resistance
- TG
Triglyceride
- HDL-C
High-density lipoprotein cholesterol
- FPG
Fasting plasma glucose
- HbA1c
Glycated hemoglobin
- SPSS
Statistical product service solutions
- ROC
Receiver operating characteristic curve
- AUC
Area under the curve
- ORs
Odds ratios
- 95% CIs
95% Confidence intervals
Author contributions
Conceived and designed the research: L.Z. Wrote the paper: X.Y.Z. Analyzed the data: X.Y.Z. and L.Z. Revised the paper: X.Y.Z, Y.W., Y.Q.L., J.F.G., Y.J.M., X.Y., L.Z., H.y.L., L.L.G., J.L.L., Y.X.L., X.P.L., L.S., L.Y., T.Y., C.Z.W., D.M.Z., J.L., M.M.L., and Y.H. All authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
CHARLS was supported by the NSFC (70910107022, 71130002), and National Institute on Aging (R03-TW008358-01; R01-AG037031-03S1), and World Bank (7159234) and the Support Program for Outstanding Young Talents from the Universities and Colleges of Anhui Province for Lin Zhang (gxyqZD2021118).
Data availability
Data can be accessed via http://opendata.pku.edu.cn/dataverse/CHARLS.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Harreiter J, Roden M. Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019) Wien Klin Wochenschr. 2019;131(Suppl 1):6–15. doi: 10.1007/s00508-019-1450-4. [DOI] [PubMed] [Google Scholar]
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad E, Lim S, Lamptey R, Webb DR, Davies MJ. Type 2 diabetes. Lancet. 2022;400(10365):1803–1820. doi: 10.1016/S0140-6736(22)01655-5. [DOI] [PubMed] [Google Scholar]
- 4.Thipsawat S. Early detection of diabetic nephropathy in patient with type 2 diabetes mellitus: A review of the literature. Diab. Vasc. Dis. Res. 2021;18(6):14791641211058856. doi: 10.1177/14791641211058856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022;18(9):525–539. doi: 10.1038/s41574-022-00690-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo Z, Fabre G, Rodwin VG. Meeting the challenge of diabetes in China. Int. J. Health Policy Manag. 2020;9(2):47–52. doi: 10.15171/ijhpm.2019.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Xu Q, Han L, Wu T, Shi X, Ye L, Yao G, Li X. Using real-world data to estimate the changing trends in the prevalence and incidence of type 2 diabetes mellitus in Xiamen of China from 2014 to 2019. BMC Endocr. Disord. 2021;21(1):92. doi: 10.1186/s12902-021-00759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Wu Y, Wu J, Wang M, Wang X, Wang J, et al. Trends in prevalence and incidence of type 2 diabetes among adults in Beijing, China, from 2008 to 2017. Diabet Med. 2021;38(9):e14487. doi: 10.1111/dme.14487. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 10.Kojta I, Chacińska M, Błachnio-Zabielska A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. 2020;12(5):1305. doi: 10.3390/nu12051305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ. Res. 2020;126(11):1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021;137:111315. doi: 10.1016/j.biopha.2021.111315. [DOI] [PubMed] [Google Scholar]
- 13.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metab. Res. Rev. 2018;34(7):e3048. doi: 10.1002/dmrr.3048. [DOI] [PubMed] [Google Scholar]
- 14.Yin Q, Yan X, Cao Y, Zheng J. Evaluation of novel obesity- and lipid-related indices as predictors of abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. BMC Endocr. Disord. 2022;22(1):272. doi: 10.1186/s12902-022-01179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locateli JC, Lopes WA, Simoes CF, de Oliveira GH, Oltramari K, Bim RH, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J. Pediatr. Endocrinol. Metab. 2019;32(10):1163–1170. doi: 10.1515/jpem-2019-0037. [DOI] [PubMed] [Google Scholar]
- 16.Ramdas Nayak VK, Nayak KR, Vidyasagar SPR. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabetes Metab. Syndr. 2020;14(5):1265–1272. doi: 10.1016/j.dsx.2020.06.045. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Zhu X, Wu J, Huang Z, Zhao Z, Zhang X, et al. Prevalence of hyperuricemia among chinese adults: Findings from two nationally representative cross-sectional surveys in 2015–16 and 2018–19. Front Immunol. 2021;12:791983. doi: 10.3389/fimmu.2021.791983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J. Diabetes Complic. 2013;27(5):436–442. doi: 10.1016/j.jdiacomp.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein S, Gastaldelli A, Yki-Jarvinen H, Scherer PE. Why does obesity cause diabetes? Cell Metab. 2022;34(1):11–20. doi: 10.1016/j.cmet.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issaka A, Cameron AJ, Paradies Y, Kiwallo JB, Bosu WK, Houehanou YCN, et al. Associations between obesity indices and both type 2 diabetes and impaired fasting glucose among West African adults: Results from WHO STEPS surveys. Nutr. Metab. Cardiovasc. Dis. 2021;31(9):2652–2660. doi: 10.1016/j.numecd.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014;11(11):1185–1200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toplak H, Leitner DR, Harreiter J, Hoppichler F, Wascher TC, Schindler K, et al. Diabesity-Obesity and type 2 diabetes (Update 2019) Wien. Klin. Wochenschr. 2019;131(Suppl 1):71–76. doi: 10.1007/s00508-018-1418-9. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet. Diabetes Endocrinol. 2021;9(7):446–461. doi: 10.1016/S2213-8587(21)00118-2. [DOI] [PubMed] [Google Scholar]
- 24.Jiang K, Luan H, Pu X, Wang M, Yin J, Gong R. Association between visceral adiposity index and insulin resistance: A cross-sectional study based on US adults. Front Endocrinol. Lausanne. 2022;13:921067. doi: 10.3389/fendo.2022.921067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Lin D, Feng Q, Li F, Qi Y, Feng W, et al. Assessment of adiposity distribution and its association with diabetes and insulin resistance: a population-based study. Diabetol. Metab. Syndr. 2019;11:51. doi: 10.1186/s13098-019-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DY, Lee ES, Kim JH, Park SE, Park CY, Oh KW, et al. Predictive value of triglyceride glucose index for the risk of incident diabetes: A 4-year retrospective longitudinal study. PLoS One. 2016;11(9):e0163465. doi: 10.1371/journal.pone.0163465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta R, Rebekah G, Jose A, Inbakumari MP, Finney G, Thomas N. Lipid accumulation product (LAP) as a potential index to predict risk of insulin resistance in young, non-obese Asian Indian males from Southern India: observations from hyperinsulinemic-euglycemic clamp studies. BMJ Open Diabetes Res. Care. 2021;9(1):e002414. doi: 10.1136/bmjdrc-2021-002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng X, Wang J, Wu S, Wang Z, Wei Y, Li L, et al. Correlation analysis of anthropometric indices and type 2 diabetes mellitus in residents aged 60 years and older. Front. Public Health. 2023;11:1122509. doi: 10.3389/fpubh.2023.1122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bawadi H, Abouwatfa M, Alsaeed S, Kerkadi A, Shi Z. Body shape index is a stronger predictor of diabetes. Nutrients. 2019;11(5):1018. doi: 10.3390/nu11051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y, Guo X, Chen Y, Guo L, Li Z, Yu S, et al. A body shape index and body roundness index: two new body indices to identify diabetes mellitus among rural populations in northeast China. BMC Public Health. 2015;15:794. doi: 10.1186/s12889-015-2150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng ZY, Liu SX, Xu H, Xu X, Liu XZ, Zhao XX. Association of triglyceride glucose index and its combination of obesity indices with prehypertension in lean individuals: A cross-sectional study of Chinese adults. J. Clin. Hypertens. Greenwich. 2020;22(6):1025–1032. doi: 10.1111/jch.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge Q, Li M, Xu Z, Qi Z, Zheng H, Cao Y, et al. Comparison of different obesity indices associated with type 2 diabetes mellitus among different sex and age groups in Nantong, China: a cross-section study. BMC Geriatr. 2022;22(1):20. doi: 10.1186/s12877-021-02713-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Peralta F, Abreu C, Cruz-Bravo M, Alcarria E, Gutierrez-Buey G, Krakauer NY, et al. Relationship between "a body shape index (ABSI)" and body composition in obese patients with type 2 diabetes. Diabetol. Metab. Syndr. 2018;10:21. doi: 10.1186/s13098-018-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han M, Qin P, Li Q, Qie R, Liu L, Zhao Y, et al. Chinese visceral adiposity index: A reliable indicator of visceral fat function associated with risk of type 2 diabetes. Diabetes Metab. Res. Rev. 2021;37(2):e3370. doi: 10.1002/dmrr.3370. [DOI] [PubMed] [Google Scholar]
- 35.Zhang FL, Ren JX, Zhang P, Jin H, Qu Y, Yu Y, et al. Strong association of waist circumference (WC), body mass index (BMI), waist-to-height ratio (WHtR), and waist-to-hip ratio (WHR) with diabetes: A population-based cross-sectional study in jilin province China. J. Diabetes Res. 2021;2021:8812431. doi: 10.1155/2021/8812431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandenbroucke JP, Elm EV, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strobe initiative strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Ann. Int. Med. 2007;147(8):163. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: The China health and retirement longitudinal study (CHARLS) Int. J. Epidemiol. 2014;43(1):61–68. doi: 10.1093/ije/dys203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Zhang X, Fang L, Guan Q, Guan L, Li Q. Prevalence, awareness, treatment and control of diabetes mellitus among middle-aged and elderly people in a rural Chinese population: A cross-sectional study. PLoS One. 2018;13(6):e0198343. doi: 10.1371/journal.pone.0198343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Liu J, Cheng Z, Zhong Y, Chen X, Song W. Triglyceride glucose-body mass index and the risk of diabetes: A general population-based cohort study. Lipids. Health Dis. 2021;20(1):99. doi: 10.1186/s12944-021-01532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji M, Zhang S, An R. Effectiveness of a body shape index (ABSI) in predicting chronic diseases and mortality: A systematic review and meta-analysis. Obes. Rev. 2018;19(5):737–759. doi: 10.1111/obr.12666. [DOI] [PubMed] [Google Scholar]
- 41.Amiri P, Javid AZ, Moradi L, Haghighat N, Moradi R, Behbahani HB, et al. Associations between new and old anthropometric indices with type 2 diabetes mellitus and risk of metabolic complications: A cross-sectional analytical study. J. Vasc. Bras. 2021;20:e20200236. doi: 10.1590/1677-5449.200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefanescu A, Revilla L, Lopez T, Sanchez SE, Williams MA, Gelaye B. Using a body shape index (ABSI) and body roundness index (BRI) to predict risk of metabolic syndrome in Peruvian adults. J. Int. Med. Res. 2020;48(1):300060519848854. doi: 10.1177/0300060519848854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozorgmanesh M, Hadaegh F, Azizi F. Diabetes prediction, lipid accumulation product, and adiposity measures; 6-year follow-up: Tehran lipid and glucose study. Lipids. Health Dis. 2010;9:45. doi: 10.1186/1476-511X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao T, Luo T, Pei H, Yimingniyazi B, Aili D, Aimudula A, et al. Association between abdominal obesity indices and risk of cardiovascular events in Chinese populations with type 2 diabetes: A prospective cohort study. Cardiovasc. Diabetol. 2022;21(1):225. doi: 10.1186/s12933-022-01670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L, Li JL, Zhang LL, Guo LL, Li H, Yan W, et al. Relationship between adiposity parameters and cognition: The fat and jolly hypothesis in middle-aged and elderly people in China. Med. Baltim. 2019;98(10):e14747. doi: 10.1097/MD.0000000000014747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Combined effect of famine exposure and obesity parameters on hypertension in the midaged and older adult: A population-based cross-sectional study. Biomed. Res. Int. 2021;2021:5594718. doi: 10.1155/2021/5594718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Individual and combined association analysis of famine exposure and serum uric acid with hypertension in the mid-aged and older adult: a population-based cross-sectional study. BMC Cardiovasc. Disord. 2021;21(1):420. doi: 10.1186/s12872-021-02230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Yang L, Wang C, Yuan T, Zhang D, Wei H, et al. Mediator or moderator? The role of obesity in the association between age at menarche and blood pressure in middle-aged and elderly Chinese: A population-based cross-sectional study. BMJ Open. 2022;12(5):e051486. doi: 10.1136/bmjopen-2021-051486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci. Rep. 2019;9(1):9693. doi: 10.1038/s41598-019-46187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Athyros VG, Doumas M, Imprialos KP, Stavropoulos K, Georgianou E, Katsimardou A, et al. Diabetes and lipid metabolism. Horm. Athens. 2018;17(1):61–67. doi: 10.1007/s42000-018-0014-8. [DOI] [PubMed] [Google Scholar]
- 51.Kane JP, Pullinger CR, Goldfine ID, Malloy MJ. Dyslipidemia and diabetes mellitus: Role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. Curr. Opin. Pharmacol. 2021;61:21–27. doi: 10.1016/j.coph.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 52.Gui J, Li Y, Liu H, Guo LL, Li J, Lei Y, et al. Obesity- and lipid-related indices as a predictor of obesity metabolic syndrome in a national cohort study. Front Public Health. 2023;11:1073824. doi: 10.3389/fpubh.2023.1073824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007–2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2019;14(3):e0212963. doi: 10.1371/journal.pone.0212963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng S, Shi S, Ren X, Han T, Li Y, Chen Y, et al. Triglyceride glucose-waist circumference, a novel and effective predictor of diabetes in first-degree relatives of type 2 diabetes patients: Cross-sectional and prospective cohort study. J. Trans. Med. 2016;14:260. doi: 10.1186/s12967-016-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li X, Li HY, Yu ZW, Zhang YT, Tong XW, Gao XY. Association among lipid accumulation product, chinese visceral obesity index and diabetic retinopathy in patients with type 2 diabetes: A cross-sectional study. Diabetes Metab. Syndr. Obes. 2021;14:4971–4979. doi: 10.2147/DMSO.S348195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duan Y, Zhang W, Li Z, Niu Y, Chen Y, Liu X, et al. Predictive ability of obesity- and lipid-related indicators for metabolic syndrome in relatively healthy Chinese adults. Front. Endocrinol. Lausanne. 2022;13:1016581. doi: 10.3389/fendo.2022.1016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayundini G, Astrella C, Tahapary D, Soewondo P. A systematic review on the association between lipid accumulation product index and type 2 diabetes mellitus. J. Asean. Fed. Endocr. Soc. 2019;34(1):16–20. doi: 10.15605/jafes.034.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie X, Li Q, Zhang L, Ren W. Lipid accumulation product, visceral adiposity index, and chinese visceral adiposity index as markers of cardiometabolic risk in adult growth hormone deficiency patients: A cross-sectional study. Endocr. Pract. 2018;24(1):33–39. doi: 10.4158/EP-2017-0007. [DOI] [PubMed] [Google Scholar]
- 59.Wu J, Gong L, Li Q, Hu J, Zhang S, Wang Y, et al. A novel visceral adiposity index for prediction of type 2 diabetes and pre-diabetes in chinese adults: a 5-year prospective study. Sci. Rep. 2017;7(1):13784. doi: 10.1038/s41598-017-14251-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J, Liu X, Xue H, Wang Y, Shi Z. Comparisons of visceral adiposity index, body shape index, body mass index and waist circumference and their associations with diabetes mellitus in adults. Nutrients. 2019;11(7):1580. doi: 10.3390/nu11071580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsou MT, Chang YC, Hsu CP, Kuo YC, Yun CH, Huang WH, et al. Visceral adiposity index outperforms conventional anthropometric assessments as predictor of diabetes mellitus in elderly Chinese: A population-based study. Nutr. Metab. Lond. 2021;18(1):87. doi: 10.1186/s12986-021-00608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be accessed via http://opendata.pku.edu.cn/dataverse/CHARLS.