Abstract
With the aim of deriving a definitive phylogenetic tree for as many mammalian and avian herpesvirus species as possible, alignments were made of amino acid sequences from eight conserved and ubiquitously present genes of herpesviruses, with 48 virus species each represented by at least one gene. Phylogenetic trees for both single-gene and concatenated alignments were evaluated thoroughly by maximum-likelihood methods, with each of the three herpesvirus subfamilies (the Alpha-, Beta-, and Gammaherpesvirinae) examined independently. Composite trees were constructed starting with the top-scoring tree based on the broadest set of genes and supplemented by addition of virus species from trees based on narrower gene sets, to give finally a 46-species tree; branching order for three regions within the tree remained unresolved. Sublineages of the Alpha- and Betaherpesvirinae showed extensive cospeciation with host lineages by criteria of congruence in branching patterns and consistency in extent of divergence. The Gammaherpesvirinae presented a more complex picture, with both higher and lower substitution rates in different sublineages. The final tree obtained represents the most detailed view to date of phylogenetic relationships in any family of large-genome viruses.
The Herpesviridae are a numerous family of large DNA viruses which have as their natural hosts humans, other mammals and vertebrates, and in one described case, an invertebrate (11, 16). The genomes of herpesviruses of mammals and birds clearly evince descent from a common ancestor, but with a great range of variation in terms of nucleotide substitution, gene content, and genomic arrangement (15). The Herpesviridae have been divided into three subfamilies, the Alpha-, Beta-, and Gammaherpesvirinae, initially from their distinct biological properties and latterly more precisely on the basis of their genomic attributes (16). Over the last two decades an extensive body of herpesvirus DNA sequence data has been built up, from single-gene analyses to studies of whole genomes (in the range 120 to 240 kbp). Phylogenetic studies using herpesvirus sequences have been undertaken, demonstrating clear division into the three subfamilies and, in some sublineages, patterns of divergence consistent with cospeciation of virus and host (7, 9, 13, 14). Herpesviruses of fish (2, 3), amphibians (4), and invertebrates (A. J. Davison, personal communication) are only remotely related to the mammalian and avian viruses, while certain turtle viruses (the only reptile herpesviruses for which some sequence is known) probably group with the mammalian and avian viruses (18).
We describe in this report a major update of herpesvirus phylogenetic analysis, using the greatly increased number of gene sequences now available from a wide range of mammalian and avian herpesviruses, and enabled by advances both in processing power of modern computers and in methods for analysis of relationships among gene sequences. We aimed to produce by good current practice a single phylogenetic tree that would be thoroughly justified, be accurate in terms of branch patterns and relative branch lengths, and contain as many mammalian and avian herpesviruses as possible.
MATERIALS AND METHODS
Amino acid sequences and alignments.
Coding sequences of herpesvirus genes were provided by colleagues and collected from the literature and the EMBL data library until December 1999. Table 1 lists abbreviations for viruses and sources for genes orthologous to HSV1 genes UL2, UL5, UL15, UL19, UL27, UL28, UL29, and UL30 (the set used in this study). Only complete gene sequences were used with the exception of the UL15 entry for PRV, for which only the second exon sequence (of two) was available. Sequence files were manipulated in Alpha (Open VMS and Unix) systems using Genetics Computer Group software (version 10). Amino acid sequences were aligned by each of the programs CLUSTAL W (20), Dialign2 (17), and PRRP (6), which use distinct algorithmic approaches and have been evaluated as good performers (21). Default values of program parameters were used. Combined alignments were produced by reextracting the individual sequences from these three alignment versions, with retention of the gapping characters introduced by each program, and then making a new alignment from this whole triple set of sequences using the program Pileup. All positions in the combined alignment that had a gap in any sequence were then excised, thus deleting both unanimously placed gaps and sections where the three primary alignments were in conflict. Regions regarded as unalignable were also removed (14).
TABLE 1.
Herpesvirus gene sequences
| Virus speciesa | Virus name abbreviation | Gene
|
Accession no. (reference) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UL2 | UL5 | UL15 | UL19 | UL27 | UL28 | UL29 | UL30 | |||
| Alphaherpesvirinae | ||||||||||
| Herpes simplex virus type 1 (human HV 1) | HSV1 | + | + | + | + | + | + | + | + | X14112 |
| Herpes simplex virus type 2 (human HV 1) | HSV2 | + | + | + | + | + | + | + | + | Z86099 |
| Simian agent 8 (cercopithecine HV 2) | SA8 | + | M57388 | |||||||
| HV papio 2 (cercopithecine HV 16) | HVP2 | + | U14662 | |||||||
| B virus (cercopithecine HV 1) | HVB | + | U14664 | |||||||
| Parma wallaby HV (macropodid HV 1) | WHV1 | + | AF061754 | |||||||
| Dorcopsis wallaby HV (macropodid HV 2) | WHV2 | + | AF061755 | |||||||
| Bovine HV 2 | BHV2 | + | + | + | M21628, P12639, AF181249 | |||||
| HV ateles 1 (ateline HV 1) | HVA1 | + | M95785 | |||||||
| HV saimiri 1 (saimiriine HV 1) | HVS1 | + | M95786 | |||||||
| Varicella-zoster virus (human HV 3) | VZV | + | + | + | + | + | + | + | + | X04370 |
| Simian varicella virus (cercopithecine HV 9) | SVV | + | U12388 | |||||||
| Bovine HV 1 | BHV1 | + | + | + | + | + | + | + | + | AJ004801 |
| Pseudorabies virus (suid HV 1) | PRV | + | + | Exon 2 | + | + | + | + | + | P52506, L20708, L00676, M17321, X14573, U80909, L24487 |
| Canid HV 1 | CHV1 | + | (12) | |||||||
| Seal HV 1 (phocid HV 1) | SHV1 | + | Z68147 | |||||||
| Felid HV 1 | FHV1 | + | + | + | AF022391, S49775, AJ224971 | |||||
| Equid HV 1 | EHV1 | + | + | + | + | + | + | + | + | M86664 |
| Equid HV 4 | EHV4 | + | + | + | + | + | + | + | + | AF030027 |
| Marek's disease virus 1 (gallid HV 2) | MDV1 | + | + | + | U04994, D00506, L40431 | |||||
| Marek's disease virus 2 (gallid HV 3) | MDV2 | + | + | U01886, AB024309 | ||||||
| HV of turkeys (meleagrid HV1) | HVT | + | + | Z54369, U01887 | ||||||
| Infectious laryngotracheitis virus (gallid HV 1) | ILTV | + | + | + | + | + | X97256, D00818, AF168792 | |||
| Betaherpesvirinae | ||||||||||
| Human cytomegalovirus (human HV 5) | HCMV | + | + | + | + | + | + | + | + | X17403, Y13735 |
| Chimpanzee cytomegalovirus | CCMV | + | + | + | + | + | + | + | + | (A. Dolan et al., unpublished data) |
| Rhesus cytomegalovirus (cercopithecine HV 8) | RHCM | + | + | U59238, AF033184 | ||||||
| Simian cytomegalovirus (cercopithecine HV 5) | SCMV | + | P13215 | |||||||
| Tupaiid HV 1 | THV1 | + | AF074327 | |||||||
| Murine cytomegalovirus (murid HV 1) | MCMV | + | + | + | + | + | + | + | + | U68299 |
| Rat cytomegalovirus (murid HV 2) | RCMV | + | + | + | + | U50550 | ||||
| Guinea pig cytomegalovirus (caviid HV 2) | GCMV | + | + | L25706 | ||||||
| Human HV 6, A variant | HHV6 | + | + | + | + | + | + | + | + | X83413 |
| Human HV 7 | HHV7 | + | + | + | + | + | + | + | + | U43400, AF037218 |
| Gammaherpesvirinae | ||||||||||
| Epstein-Barr virus (human HV 4) | EBV | + | + | + | + | + | + | + | + | V01555 |
| HV papio (cercopithecine HV 12) | HVP | + | U23857 | |||||||
| Human HV8 | HHV8 | + | + | + | + | + | + | + | + | U75698 |
| Retroperitoneal fibromatosis HV of macaques | RFHV | + | (19) | |||||||
| Rhesus rhadinovirus (cercopithecine HV 17) | RRV | + | + | + | + | + | + | + | + | AF083501, AF029302 |
| Mouse HV 68 (murid HV 4) | MHV4 | + | + | + | + | + | + | + | + | U97553, AF105037 |
| Bovine HV 4 | BHV4 | + | + | AF139096, Z15044 | ||||||
| HV ateles (ateline HV 3) | HVA | + | + | + | + | + | + | + | + | AF083424 |
| HV saimiri (saimiriine HV 2) | HVS | + | + | + | + | + | + | + | + | X64346 |
| Equid HV 2 | EHV2 | + | + | + | + | + | + | + | + | U20824 |
| Equid HV 5 | EHV5 | + | AF050671 | |||||||
| Wildebeest HV (alcelaphine HV 1) | AHV1 | + | + | + | + | + | + | + | + | AF005370 |
| Cottontail rabbit HV (leporid HV 1) | CRHV | + | L33971 | |||||||
| Porcine lymphotropic HV 1 | PLH1 | + | AF191042 | |||||||
| Porcine lymphotropic HV 2 | PLH2 | + | AF191043 | |||||||
For maximum intelligibility, both common and available systematic names are listed, the latter in italics. HV, herpesvirus.
Phylogenetic trees.
Trees were investigated in three stages. Preliminary examination used the neighbor-joining method with bootstrapping (programs Seqboot, Protdist, Neighbor, and Consense from the PHYLIP package, version 3.572 [5]). In the second stage of analysis, particular parts in the total tree were varied separately and examined with the maximum-likelihood program Protml from the MOLPHY package (1), which imposed a single rate of change on all sites in each sequence. We were able to examine up to 104 tree topologies for each subset of species by this approach. For each topology, Protml computed a log-likelihood (lnL) value, a bootstrap value, and a set of branch lengths. Final evaluation of high-scoring topologies and derivation of branch lengths were made with the maximum-likelihood program Codeml of the PAML package (version 2) (24), which allowed a distribution of rates of change across sites, in up to eight rate classes assigned by the program in a discrete gamma distribution (23); for each topology, Codeml computed lnL and branch lengths. Up to 20 trees for each data set were examined by this computer-intensive method. Substitution probabilities for Protml and Codeml were from reference 8. These maximum-likelihood trees are presented (see Fig. 2 and 3) in a rooted format with root locus as the midpoint of the distance from the mean position of branch tips in the Alphaherpesvirinae subfamily to the mean position of branch tips in the Beta- plus Gammaherpesvirinae subfamilies (i.e., the root is taken to lie on the branch from the Alphaherpesvirinae to the other subfamilies [14]). For purposes of presentation and for combining data from different trees, top-scoring tree topologies were input to Codeml to recompute branch lengths with overall rate constancy maintained among lineages; we refer to such trees as MC (molecular clock) trees. Minitab was used for analysis of numerical data. Details of alignments and trees are available by anonymous FTP from zippy.vir.gla.ac.uk/public/mcgeoch/.
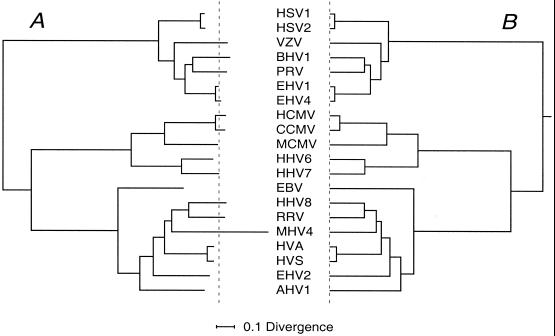
FIG. 2.
The 20-species phylogenetic trees. (A) Unconstrained maximum-likelihood tree obtained using Codeml and with alignment sites in eight rate classes. The tree is shown in a rooted format as specified in Materials and Methods, and the mean position of branch tips is indicated by the dashed line. (B) Equivalent rooted MC tree. Divergence scale (substitutions/site) for both is at the bottom.
FIG. 3.
The 28-species phylogenetic tree. The unconstrained maximum-likelihood tree from the UL27-plus-UL30 alignment is shown as for Fig. 1A, with species contributed to the 28-species composite tree marked with circles.
RESULTS AND DISCUSSION
Aligning amino acid sequences.
The gene complements of mammalian and avian herpesviruses contain a subset of some 30 genes that have homologues with clearly conserved encoded amino sequences in all of the genomes sequenced. In previous work on herpesvirus phylogeny, we used collections of eight genes from this set to build phylogenetic trees (14). As a preliminary to the present analysis, and with the greater number of herpesvirus gene sequences now available, we examined alignments of additional gene collections whose amino acid sequences were relatively highly conserved; however, we concluded that in all of these cases the alignment process introduced too many gapping characters for them to be useful for tree inference across the whole herpesvirus family. Table 1 summarizes sequences for the eight-gene set used for the present study. Forty-eight viruses were represented with at least one gene sequence, and 19 had data for all eight genes. HHV6 variants A and B were very close by applicable criteria, and only variant A was included in the analysis. Our goal was to infer from these various sequences a single integrated, well-founded phylogenetic tree.
The wide divergences among herpesvirus gene sequences require that the appropriate level for examining phylogeny based on molecular sequence comparison is that of the amino acid, rather than nucleotide, sequences (13). Alignments for amino acid sequences from each of the eight gene sets were constructed with each of three programs that used distinct computational approaches. Alignments from the three programs were identical in regions that were of uniform length in all sequences, but placements of gaps by the programs in regions that exhibited length inequalities among sequences often differed. Inappropriate gap placement represents a potential source of noise or bias for subsequent phylogenetic analysis; therefore, these contentious regions were removed via a procedure involving a further alignment of alignments.
Inference of phylogenetic trees.
We aimed to carry through the phylogenetic analysis as thoroughly as practicable with the best currently available approach, which we judged to comprise maximum-likelihood evaluation of sets of candidate trees with provision for different rates of evolutionary change at different sites in the alignment. Limitations are that this method is relatively computationally intensive and that the number of possible trees to be evaluated rapidly becomes gigantic as number of species increases. We therefore proceeded by establishing a small subset of candidate best trees using more rapid methods and then subjecting only this subset to the most time-consuming computation. Preliminary analysis by the neighbor-joining method with bootstrapping demonstrated that, as expected, for all sets of sequences individual viruses were assigned unambiguously to one of the three subfamilies of the Alpha-, Beta-, and Gammaherpesvirinae; that is, it was reasonable to pursue maximum-likelihood evaluation independently within each subfamily, much reducing the search space. Tree topologies for each of the eight sets of genes were found to be closely similar in most respects; a notable exception was the variable locus for MHV4 within the Gammaherpesvirinae. We next chose a set of alignments, including concatenated alignments (containing sequences from all eight genes down to two-gene sets) and UL2, UL27, and UL30 single-gene alignments, to give the maximum representation for the greatest number of species (Table 2). For each data set, tree topologies within each subfamily were evaluated as exhaustively as possible with Protml while holding the pattern in the two other subfamilies constant (initially in the topology derived by neighbor joining). We were able to handle up to seven operational taxonomic units in a subfamily, requiring analysis of 10,395 trees. For those cases where the subfamily contained more than seven species, we proceeded either by treating closely related species together in uncontentious cases (e.g., putting HSV1 and HSV2 into one operational taxonomic unit) or by analyzing separately parts of the subfamily that we were confident were distinct. The trees examined by Protml were ranked by their lnL scores and then evaluated by lnL differences and the bootstrap value. The top trees (typically 10 to 20) were then taken for examination by Codeml, with relative rates of change at individual alignment sites assigned to one of eight values by the program. Evidently this procedure relies on Protml and Codeml yielding similar rankings of trees, and in practice this was observed to be so with no major discordances. Top topologies derived for subsets of virus species were then brought together, and branch lengths for the overall best topology were obtained with Codeml. The final outcome for a given set of aligned sequences was (i) identification of the top-scoring topology; (ii) evaluation of the robustness of each branch point obtained from comparison with scores of runner-up trees; and (iii) a set of branch lengths for the top-scoring tree.
TABLE 2.
Characteristics of alignment sets
| Alignment | No. of species | Lengtha |
|---|---|---|
| 8 genes | 19 | 4,792 |
| 7 genes + UL15 exon 2 | 20 | 4,580 |
| 5 genes | 21 | 2,648 |
| Genes UL27 and UL30 | 28 | 1,223 |
| Genes UL15 and UL27 | 20 | 1,087 |
| Gene UL27 | 41 | 554 |
| Gene UL30b | 32 | 669 |
| 30 | 754 | |
| Gene UL2 | 25 | 183 |
Number of amino acids after trimming and degapping.
Two UL30 alignments were used, the longer omitting ILTV and MDV2 (which had unique deletions).
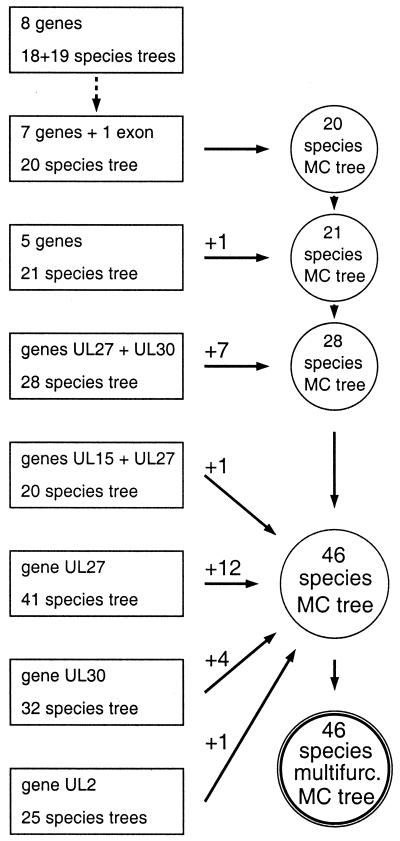
To combine the various top-scoring trees (which contained distinct subsets of the 48 virus species) into one composite tree, we first used Codeml to compute for each top-scoring topology a rooted maximum-likelihood tree with molecular clock imposed. This gave a tree with branch tips in register, in contrast to the unconstrained rate tree that was the direct output of the topology evaluation process (see Fig. 2). These MC trees could then be used straightforwardly to add locations for additional virus species successively to the MC tree based on the largest gene set, by interpolation and scaling into the appropriate locus. We emphasize that the use of MC trees is primarily a simplifying and presentational device, justified pragmatically in the present work but readily alterable. Figure 1 presents a flow diagram for the composite-tree derivation process. The eight-gene set gave a 19-species tree that was unambiguous except for the location of MHV4 (a single, robust, equivalent tree was obtained when MHV4 was excluded) (Fig. 1, top left). With a gene set that omitted only UL15 exon 1, a 20-species tree (including PRV) was obtained that was topologically equivalent and very close in branch lengths to the eight-gene trees, and this 20-species tree was therefore used as the starting point for building composite trees. Reducing the number of genes to five (UL2, UL5, UL27, UL29, and UL30) allowed addition of ILTV. An alignment containing UL27 plus UL30 sequences increased the number of species in the MC tree to 28. Four alignments (UL15 plus UL27, and single-gene alignments for UL27, UL30, and UL2) then independently added species to the 28-species MC tree to give a 46-species MC tree. These later additions were facilitated by the distribution of species in different trees; for instance, all but one of the species represented by UL27 alone were alphaherpesviruses, while no UL30-only species were from this subfamily. Finally, the 46-species MC tree was reduced to a multifurcated form that eliminated branching uncertainties at three loci (Fig. 1, bottom right).
FIG. 1.
Flow diagram for creation of composite trees. The box at top left indicates the starting maximum-likelihood tree based on the eight-gene alignment; the other boxes represent trees that contributed to building composite trees. Successive composite MC trees are shown as circles with numbers of species added from different data sets shown above arrows; the final 46-species multifurcated tree is shown at bottom right.
Figures 2 to 4 illustrate selected stages in the above process. Figure 2A depicts the unconstrained maximum-likelihood tree with 20 species. The atypical excess length of the terminal branch for MHV4 is striking, while EBV has a short terminal branch. Figure 2B shows the corresponding maximum-likelihood MC tree. In view of the unusual behavior of MHV4, we excluded this species when computing MC trees and placed it in the 20-species MC tree by interpolation from the unconstrained tree's data. Figure 3 shows the 28-species tree as obtained from the analytical process and used to compute an MC tree to add seven species to the 21-species MC tree. We emphasize that in this and the other trees from narrower gene sets not illustrated, congruence of branching pattern for virus subfamilies that added new species with the equivalent parts of the 20-species tree emerged from the analysis as distinct from being imposed via the topologies chosen for evaluation. Figure 4A shows the final 46-species MC tree constructed, and Fig. 4B shows the subfamily portions of the same tree with three regions of uncertain branching order drawn as unresolved multifurcations; major sublineages in each subfamily, taxonomically equivalent to genera (16), are labeled for the following discussion. Two of the starting 48 virus species, SCMV and CRHV, were withheld from the final tree. SCMV, for which only gene UL29 was available, locates to a position in the β1 lineage equivalent to that of RHCM in Fig. 4 but not resolved from RHCM because that species was represented by only UL27 and UL30. The only CRHV sequence was that for the short UL2 gene. Our analysis placed CRHV near the clade occupied by HHV8 through to HVS in the γ2 sublineage (Fig. 4) but lacked resolution in what is also in other respects a problematic locality (see below).
FIG. 4.
The 46-species composite trees. (A) The 46-species composite MC tree. (B) Subfamily portions of the same tree with regions of uncertain branching drawn as multifurcations (heavy lines). Subfamily and sublineage designations are at the right.
Characteristics of the herpesvirus phylogenetic tree.
The final tree obtained in this work is consistent with less populated trees derived previously by less rigorous methods (13, 14). We consider that the present tree is likely to be accurate in almost all of its topology, while estimates of branch lengths specific to the many viruses that are represented by one- or two-gene data sets must be relatively imprecise. We believe that this analysis sets a presently unequaled standard in phylogenetic analysis of large-genome virus families.
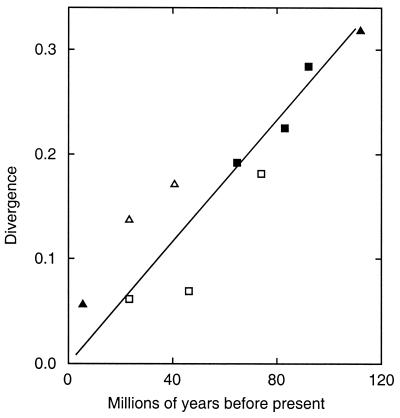
We previously discussed for a smaller data set the high level of congruence between the herpesvirus phylogenetic tree and that of the virus hosts' lineages, indicating that cospeciation has been a prominent feature in herpesvirus evolution (13, 14), and the 46-species tree now provides fuller evidence of this trend. The most cogent examples of congruence are in the α2 sublineage, involving primate, perissodactyl, carnivore, and artiodactyl viruses, and in the β1 sublineage, with primate, rodent, and tree shrew viruses. The Old and New World primate viruses in the α1 sublineage provide another example. Other features in the Alphaherpesvirinae, i.e., the locations of BHV2, WHV1, and WHV2 and (from consideration of branch lengths) the α3 and α4 avian viruses, clearly do not represent cospeciation. We postpone discussion of the Gammaherpesvirinae. For the α2 and β1 examples noted above, we plotted selected pairs of divergence values for virus lineages present in the 20-species tree (i.e., those with the best quantitative support) against host lineage divergence times as shown in Fig. 5. The host datings are from a recent analysis using DNA sequences of vertebrate genes (10). The straight line indicates an overall consistency of divergences with cospeciation and a global substitution rate of 3 × 10−9 substitutions per site per year in each lineage for the sets of amino acid sequences used. Further α1, α2, and β1 divergence values based on shorter alignments are consistent with an equivalent overall rate but greater variability.
FIG. 5.
Comparison between divergences for branch points in the herpesvirus tree and dates of corresponding events in mammalian evolution. The graph compares divergence values (substitutions/site in one lineage) for features in the composite tree (Fig. 4) with dates of possible equivalents in host evolution (10) in millions of years before the present (My). Filled symbols, data from 20-species MC tree; open symbols, data from 46-species tree; squares, α2 sublineage; triangles, β1 sublineage. The line was drawn through the origin and the four highest-value filled symbols. Divergence events and times for filled symbols: humans/chimpanzees, 5.5 My; suidae/ruminants, 64.7 My; artiodactyls/perissodactyls, 83.4 My; primates/ungulates, 92.0 My; primates/rodents, (sciurognathi), 112 My. Similarly for open symbols: human/cercopithecidae, 23.3 My; mice/rats, 40.7 My; feliformia/caniformia, 46.2 My; carnivores/perissodactyls, 74.0 My. Dates in reference 10 included error estimates.
The phylogeny of the Gammaherpesvirinae presents the most complex and least interpretationally satisfactory region in the herpesvirus tree and thus is also of particular interest. The exact phylogenetic loci for MHV4 and the associated BHV4 remain unresolved, but a cospeciational history is not indicated. From Fig. 2 and 3, the MHV4 lineage has been evolving at a higher rate than other species (a phenomenon not visible with MCMV, the other rodent virus studied with the full data set), while the γ1 EBV lineage may have a lower evolutionary rate. The branching pattern for separation of Old from New World primate γ2 viruses is consistent with a cospeciational process, but the magnitude of divergence between these groups would then imply a rate of change about twice that seen for the Alpha- and Betaherpesvirinae in Fig. 5, possibly another indication of more rapid evolution in the γ2 group. Separately, the perissodactyl and artiodactyl γ2 viruses do not form a clade (as seen in the α2 viruses and there taken to indicate a cospeciational history); this finding could be genuine or possibly an artifact of tree inference associated with aberrant substitution rates. It may be possible to improve resolution in γ-only or γ2-only phylogenetic analyses with an enlarged gene set.
The final tree gives an integrated view of many features of herpesvirus phylogeny. It will be of utility for illuminating evolutionary and functional relationships among herpesvirus species and in developing taxonomy. The procedures we have described should represent a route for future analysis of the Herpesviridae with additional sequences. There are other herpesviruses, not included in this study, for which the only sequences available are of gene fragments, often obtained via PCR (18, 22). We consider that inferring phylogenetic trees de novo from such limited data is an uncertain undertaking, but that the extensive, high-quality tree now available should allow an alternative approach, namely, varying the locus of the test species on the standard topology and comparing likelihoods.
ACKNOWLEDGMENTS
We thank A. J. Davison for a critical review, and we thank B. Ehlers, T. M. Rose, and the CCMV Genome Sequence Group for early sight of data.
This work was supported by the UK Medical Research Council.
REFERENCES
- 1.Adachi J, Hasegawa M. The MOLPHY 2.2 package. Tokyo, Japan: Institute of Statistical Mathematics; 1994. [Google Scholar]
- 2.Davison A J. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- 3.Davison A J. The genome of salmonid herpesvirus 1. J Virol. 1998;72:1974–1982. doi: 10.1128/jvi.72.3.1974-1982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davison A J, Sauerbier E, Dolan A, Addison C, McKinnell R G. Genomic studies of the Lucké tumor herpesvirus (RaHV-1) J Cancer Res Clin Oncol. 1999;125:232–238. doi: 10.1007/s004320050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenstein J. PHYLIP—phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 6.Gotoh O. Significant improvement in accuracy of multiple protein sequence alignments by iterative refinement as assessed by reference to structural alignments. J Mol Biol. 1996;264:823–838. doi: 10.1006/jmbi.1996.0679. [DOI] [PubMed] [Google Scholar]
- 7.Hannenhalli S, Chappey C, Koonin E V, Pevzner P A. Genome sequence comparison and scenarios for gene rearrangements: a test case. Genomics. 1995;30:299–311. doi: 10.1006/geno.1995.9873. [DOI] [PubMed] [Google Scholar]
- 8.Jones D T, Taylor W R, Thornton J M. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 9.Karlin S, Mocarski E S, Schachtel G A. Molecular evolution of herpesviruses: genomic and protein sequence comparisons. J Virol. 1994;68:1886–1902. doi: 10.1128/jvi.68.3.1886-1902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Hedges S B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 11.Le Deuff R M, Nicolas J L, Renault T, Cochennec N. Experimental transmission of a herpes-like virus to axenic larvae of Pacific oyster Crassostrea gigas. Bull Eur Assoc Fish Pathol. 1994;14:69–72. [Google Scholar]
- 12.Limbach K J, Limbach M P, Conte D, Paoletti E. Nucleotide sequence of the genes encoding the canine herpesvirus gB, gC, and gD homologues. J Gen Virol. 1994;75:2029–2039. doi: 10.1099/0022-1317-75-8-2029. [DOI] [PubMed] [Google Scholar]
- 13.McGeoch D J, Cook S. Molecular phylogeny of the Alphaherpesvirinae subfamily and a proposed evolutionary timescale. J Mol Biol. 1994;238:9–22. doi: 10.1006/jmbi.1994.1264. [DOI] [PubMed] [Google Scholar]
- 14.McGeoch D J, Cook S, Dolan A, Jamieson F E, Telford E A R. Molecular phylogeny and evolutionary timescale for the family of mammalian herpesviruses. J Mol Biol. 1995;247:443–458. doi: 10.1006/jmbi.1995.0152. [DOI] [PubMed] [Google Scholar]
- 15.McGeoch D J, Davison A J. The molecular evolutionary history of the herpesviruses. In: Domingo E, Webster R, Holland J, editors. Origin and evolution of viruses. London, England: Academic Press; 1999. pp. 441–446. [Google Scholar]
- 16.Minson A C, Davison A J, Desrosiers R C, Fleckenstein B, McGeoch D J, Pellett P E, Roizman B, Studdert D M J. Herpesviridae. In: Van Regenmortel M H V, Fauquet C M, Bishop D H L, Carstens E B, Estes M K, Lemon S M, Maniloff J, Mayo M A, McGeoch D J, Pringle C R, Wickner R B, editors. Virus taxonomy. New York, N.Y: Academic Press; 2000. pp. 203–225. [Google Scholar]
- 17.Morgenstern B, Dress A, Werner M S. Multiple DNA and protein sequence alignment based on segment-to-segment comparison. Proc Natl Acad Sci USA. 1996;93:12098–12103. doi: 10.1073/pnas.93.22.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quackenbush S L, Work T M, Balazs G H, Casey R N, Rovnak J, Chaves A, duToit L, Baines J D, Parrish C R, Bowser P R, Casey J W. Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virology. 1998;246:392–399. doi: 10.1006/viro.1998.9207. [DOI] [PubMed] [Google Scholar]
- 19.Schultz E R, Rankin G W, Jr, Blanc M P, Raden B W, Tsai C C, Rose T M. Characterization of two divergent lineages of macaque rhadinoviruses related to Kaposi's sarcoma-associated herpesvirus. J Virol. 2000;74:4919–4928. doi: 10.1128/jvi.74.10.4919-4928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson J D, Higgins D J, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson J D, Plewniak F, Poch O. A comprehensive comparison of multiple sequence alignment programs. Nucleic Acids Res. 1999;27:2682–2690. doi: 10.1093/nar/27.13.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanDevanter D R, Warrener P, Bennett L, Schultz E R, Coulter S, Garber R L, Rose T M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J Clin Microbiol. 1996;34:1666–1671. doi: 10.1128/jcm.34.7.1666-1671.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z. Among-site rate variation and its impact on phylogenetic analyses. Trends Ecol Evol. 1996;11:367–372. doi: 10.1016/0169-5347(96)10041-0. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]