Abstract
Introduction
Acute kidney injury (AKI) is a common health problem that leads to high morbidity and potential mortality. The failure of conventional treatments to improve forms of this condition highlights the need for innovative and effective treatment approaches. Regenerative therapies with Renal Progenitor Cells (RPCs) have been proposed as a promising new strategy. A growing body of evidence suggests that progenitor cells differentiated from different sources, including human embryonic stem cells (hESCs), can effectively treat AKI.
Methods
Here, we describe a method for generating RPCs and directed human Embryoid Bodies (EBs) towards CD133+CD24+ renal progenitor cells and evaluate their functional activity in alleviating AKI.
Results
The obtained results show that hESCs-derived CD133+CD24+ RPCs can engraft into damaged renal tubules and restore renal function and structure in mice with gentamicin-induced kidney injury, and significantly decrease blood urea nitrogen levels, suppress oxidative stress and inflammation, and attenuate histopathological disturbances, including tubular necrosis, tubular dilation, urinary casts, and interstitial fibrosis.
Conclusion
The results suggest that RPCs have a promising regenerative potential in improving renal disease and can lay the foundation for future cell therapy and disease modeling.
Keywords: Embryonic stem cells, CD133+, CD24+, Renal progenitors, Kidney injury
1. Introduction
Acute kidney injury (AKI) is a decline in kidney function, leading to disruptions in metabolic, electrolyte, and fluid homeostasis [1,2]. Depending on the cause, AKI can precipitate chronic or end-stage renal disease (ESRD), which in turn, is a risk factor for various complications, including cardiovascular disease [2]. AKI affects millions of people, with an overall mortality rate of over 25% per year [3]. Volume loss, chemotherapeutic agents, sepsis, ischemia, diabetes, hypertension, and exposure to nephrotoxic drugs such as aminoglycosides are the most important causes of AKI [4,5]. Gentamicin, the most commonly used aminoglycoside, is one of the most important causes of kidney injury, affecting 10–15% of hospitalized patients [6].
Kidney transplantation and dialysis are the only effective options for treating End Stage Renal Disease (ESRD). However, despite being effective, kidney transplantation is not a readily accessible approach due to the lack of donors. Dialysis, however, reduces the patient's quality of life [7]. Thus, despite the limited success of conventional treatments in controlling the underlying cause and ameliorating AKI [8], developing new therapeutic strategies to improve kidney function seems essential. A notable approach involves renal progenitor-based regenerative medicine strategies. Various studies have shown the potent role of adult tissue-isolated progenitor cells in several models of kidney injury [[9], [10], [11]]. CD133+CD24+ cells are a group of renal progenitor cells in the human kidney that reside in different segments of the nephron and are known for multiple capabilities, such as self-renewal and differentiation into renal epithelial cells, in vitro and in vivo [[12], [13], [14]]. According to various studies, these cells are 2–4% of total kidney cells and regenerate kidney tissue during injury [12]. However, the limited number of these cells in the kidney, the lack of access to the cell source in patients, and their limited differentiation potentials have limited the use of these cells [15]. In recent years, stem cells, including embryonic stem cells (ESCs) and induced pluripotent stem cells (IPSCs), have received considerable attention in addressing this problem due to their potential to differentiate into different cell types. Many studies have shown the potent role of these cells in animal models of kidney injury [[16], [17], [18]]. However, their pluripotency has raised concerns about their use, such as the risk of tumorigenesis and ethical limitations. In more recent approaches, several groups have produced renal lineage cells, such as progenitor cells, by exposing embryonic cells and IPSC to various inducing factors such as fibroblast growth factor (FGF), retinoic acid (RA), activin (A), and bone morphogenetic proteins (BMPs) [19,20]. In addition, various findings show that pluripotent cell-derived renal progenitor cells can localize to and stably integrate into damaged kidney sites, such as the proximal tubule [10,21]. However, the effect of human embryonic cell-derived CD133+CD24+ progenitor cell transplantation on kidney regeneration and improvement of AKI has never been studied. Here, we enriched and isolated CD133+CD24+ renal progenitors and investigated their effects in a mouse model of gentamicin-induced kidney injury. The intraparenchymal administration of RPCs showed that they could engraft into injured tubules and attenuate oxidative stress and apoptosis. Moreover, they could ameliorate renal histological changes caused by gentamicin-induced injury and reduce fibrosis progression.
2. Materials and methods
2.1. Material
For this research, human ESCs (Royan H5) at passages 40–60 and CD133+CD24+ renal progenitor cells were purchased from Royan Institute. while Minimum Essential Medium were obtained from Thermo Fisher (Thermo Fisher Scientific, Massachusetts, USA). In this study we used fetal bovine serum (FBS, Gibco Life Technologies, USA), penicillin and streptomycin (Gibco Life Technologies, USA), osteogenic differentiation medium (Gibco Life Technologies, Germany) and alkaline phosphatase (Sigma-Aldrich, USA), alizarin red dye (Sigma-Aldrich, USA), oil red dye (Sigma-Aldrich, USA), knock-out serum replacement (Gibco), non-essential amino acids (Gibco Life Technologies, USA), glutamine (Gibco Life Technologies, USA), β-mercaptoethanol (Sigma-Aldrich, USA), basic fibroblast growth factor (bFGF, Sigma- Aldrich, USA), Accumax (Sigma-Aldrich, USA), bovine serum albumin (BSA, Sigma- Aldrich, USA), propidium iodide (PE) -conjugated mouse anti-human CD133 (Miltenyi Biotec, USA) and fluorescein isothiocyanate (FITC) -conjugated mouse anti-human CD24 (Abcam, USA) antibodies, primary anti-Human Nuclear Antigen antibodies (anti-HNA, Abcam, USA), anti-AQP1 (Abcam, USA), and anti-AQP2 (Abcam, USA). Additionally, TNF α, IL-1, IL-2, IL-6, IL-10, TGF-β, and IFN γ were obtained from Sigma Aldrich, USA.
2.2. Human ESCs culture
Human ESCs (Royan H5) obtained from the Royan Institute at passages 40–60 were expanded using on mitomycin C-treated mouse embryonic fibroblasts (MEFs) feeder cells in hESCs medium in a 5% (v/v) CO2 atmosphere with 95% humidity at 37 °C. Every other day, the medium was changed.
2.3. Embryoid body-based differentiation of human ESCs
Differentiation of human embryonic stem cells into CD133+CD24+ renal progenitor cells was carried out by forming embryoid bodies (EBs). For this purpose, hESCs were transferred to non-adherent bacterial dishes containing the same embryonic hESCs culture medium in the absence and presence of various concentrations of bFGF (100, 150, 200, and 250 ng/ml). The EBs were harvested for 5, 7, and 9 days after the inception of EB formation. The cell culture medium was refreshed every other day.
2.4. CD133+CD24+ renal progenitor cells sorting
To identify CD133+CD24+ renal progenitor cells derived from hESCs, the expression of CD133 and CD24 monoclonal antibodies against cell surface markers was screened, and two positive selection markers were isolated using FACS. On days 5, 7, and 9, the co-expression of CD133 and CD24 in EBs exposed to different concentrations of bFGF or absent bFGF was assessed. EBs were first treated with Accumax and disassociated into single cells. Following that, the cells were washed twice with PBS and incubated for 20 min at 4C in a blocking buffer (PBS with 5% bovine serum albumin (BSA). The cells were then washed with PBS again before being stained for 45 min at 4 °C with propidium iodide (PE) -conjugated mouse anti-human CD133 (1:100) and fluorescein isothiocyanate (FITC) -conjugated mouse anti-human CD24 (1:200) antibodies. FACS analysis was performed using BD FACSAria (BD Bioscience, San Jose, CA) according to the manufacturer's protocols, and sample analysis was performed using FlowJo software (version 10.6.2).
2.5. In vivo experiments and animal maintenance
This study was approved by the Research Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, under the code No. (IR.SBMU.LASER.REC.1402.012). All in-vivo experiments were carried out under ARRIVE guidelines (https://arriveguidelines.org). Male NMRI mice (6–8 weeks old, 30–35g weight) were purchased from Royan Institute and housed in a well-ventilated room with 12 h of light/12 h of darkness and free access to food and water. The Pasteur Institute in Iran provided male BALB/C nude mice (6–8 weeks old, 18–22 g body weight). Mice were kept in ventilated cages in sterile conditions, with free access to sterile water and food.
2.6. Gentamycin-induced kidney injury mouse model and experimental design
The kidney injury was induced by gentamicin (Exir Pharmaceutical Co.) 96 healthy male NMRI mice were randomly assigned to 12 experimental groups (n = 8 per group) to determine the effective dose for induction of injury. Mice were given gentamicin intraperitoneally every day for 7 days (80, 100, 150, and 200 mg/kg), and their renal function was tested 10, 14, and 21 days later. All animals died within three days of receiving the 200 mg/kg dose. The dose of 100 mg/kg was chosen as the effective dose of gentamicin-induced kidney injury based on renal function analysis, which was confirmed by the nude mice test. For evaluation of the nephroprotective effect of CD133 +/CD24+ ESCs-derived RPCs against gentamicin-induced kidney damage and their homing in the affected area, 32 BALB/C nude (n = 8 per group) mice were randomly divided into four groups. The first group was the control animals; the second was treated with gentamicin (100 mg/kg/i.p/7 days), and two other groups received an intraperitoneal injection of gentamicin (100 mg/kg/i.p/7 days), and after 24 h were received an intraparenchymal injection of saline or ESCs-derived of CD133 +/CD24 + RPCs (5 × 105 cells/mouse) into the right kidney.
2.7. Renal function analysis
Renal function was evaluated by serum urea, creatinine, sodium, and potassium levels. The animals were anesthetized with ketamine (100 mg/kg)/xylazine (10 mg/kg) on a predetermined day, and blood samples were collected. All blood samples were centrifuged for 10 min at 3000 rpm, and serum was stored at −80 °C. Bio diagnostics kits were used to assess the intended markers under the manufacturer's instructions.
2.8. Kidney index
The renal index was calculated by dividing the right kidney weight by body weight and then multiplied by 100. This was done after weighing each mouse at the end of the experiment period.
2.9. Renal histopathological analysis
The kidneys were immediately removed and fixed in 10% formalin after the mice were sacrificed on the predetermined days. After dehydrating and embedding in paraffin, all samples were sectioned into 5 μm thick sections and stained for histopathologic analysis with hematoxylin-eosin, periodic Acid-Schiff, and Masson's trichrome or Sirius red stain, and the severity of the injury was scored blindly. Light microscopy was used to assess the degree of renal tissue injury in a semi-quantitative way. Histological changes were graded on a scale of 0–4 based on the extent of tubular necrosis, tubular dilatation, urinary casts, and interstitial fibrosis: 0 = normal; 0.5 = minimal; 1 = mild; 1.5 = moderate; and 2 = marked.
2.10. Immunohistochemistry
Kidney tissue samples were fixed in 10% formalin for 24–48 h and embedded in paraffin after standard processing procedures for immunohistochemical analysis. After washing with PBS and cell permeabilization with 0.3% Triton, the slides were blocked with 10% goat serum and incubated overnight at 2–8 °C with the primary anti-Human Nuclear Antigen antibodies (anti-HNA, 1:100, Abcam, USA), anti-AQP1 (1:100, Abcam, USA), and anti-AQP2 (1:100, Abcam, USA). The slides were then incubated for 1 h with secondary antibodies. Finally, all slides were counterstained with DAPI and analyzed using fluorescent microscopy (Olympus) after adding glycerol and PBS solutions.
2.11. TUNEL assay for apoptosis analysis
Evaluating apoptosis in renal samples was analyzed by TUNEL (terminal-deoxynucleotidyl transferase-mediated nick end labeling) assay according to the manufacturer's protocol.
2.12. Enzyme-linked immunosorbent assay (ELISA) for inflammatory cytokine measurement
Tissue levels of TNF-α, IL-1, IL-2, IL-6, IL-10, TGF-β, and IFN-γ were measured using an ELISA kit under the manufacturer's recommendations. A few pieces of removed tissue pieces were homogenized with a homogenization medium, and the supernatant was then gathered after centrifugation. Following washing and incubation, an enzyme conjugate was added to each well along with 200 μl of the sample and standard. after more incubation and washing, a stop solution was then added to each well and the optical density was assessed at 405 nm using an ELISA reader. The concentration of each factor was calculated based on the slope of the line.
2.13. Statistical analyses
The results were expressed as the mean ± standard deviation (SD). Data analysis was performed by One-way analysis of variance (ANOVA) followed by the Tukey-Cicchetti test for group comparisons or t-test for unpaired data. P < 0.05 Values were considered to have statistical significance.
3. Result
3.1. Detection and isolation of ESC-derived renal progenitor cells using CD133/CD24 markers
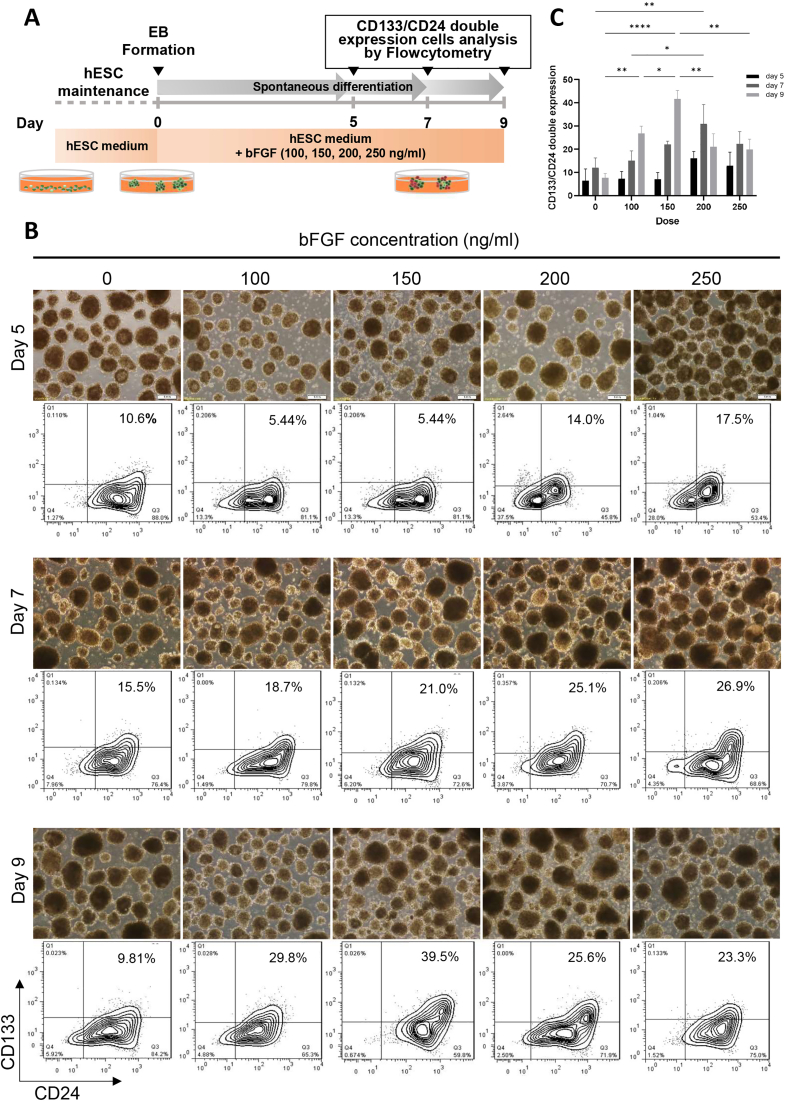
Fig. 1A depicts an overview of the spontaneous differentiation of hESCs into renal progenitor cells. CD24 and CD133 surface markers were used to distinguish RPCs from EBs, and cells were isolated using flow cytometry considering both markers. On day 9, the highest number of double-positive CD133+CD24+ cells (39.5%) were isolated from renal progenitor cells at a bFGF concentration of 150 ng/ml (Fig. 1B and C). FACS was used to isolate and purify differentiated cells under these conditions, which were then used in animal studies.
Fig. 1.
Differentiation and enrichment of human EBs into renal progenitor cells (RPCs). (a) Schematic description of human ESCs differentiation protocol to renal progenitor cells (RPCs). (b) Flow cytometric analysis of CD133 and CD24 co-expression in hESCs at days 5, 7, and 9 without or with different concentrations of bFGF (100, 150, 200, and 250 ng/ml). the EBs treated with 150 ng/ml bFGF on day 9 showed the highest double-expression of CD133 and CD24 and were selected for isolation of CD133+CD24+ RPCs by FACS. (c) The results of the antibodies screening are presented in the bar graph. Scale bars: 100 μm ∗∗∗∗P < 0.0001, ∗∗P < 0.01 and, ∗P < 0.05.
3.2. Gentamicin induces kidney injury in mice
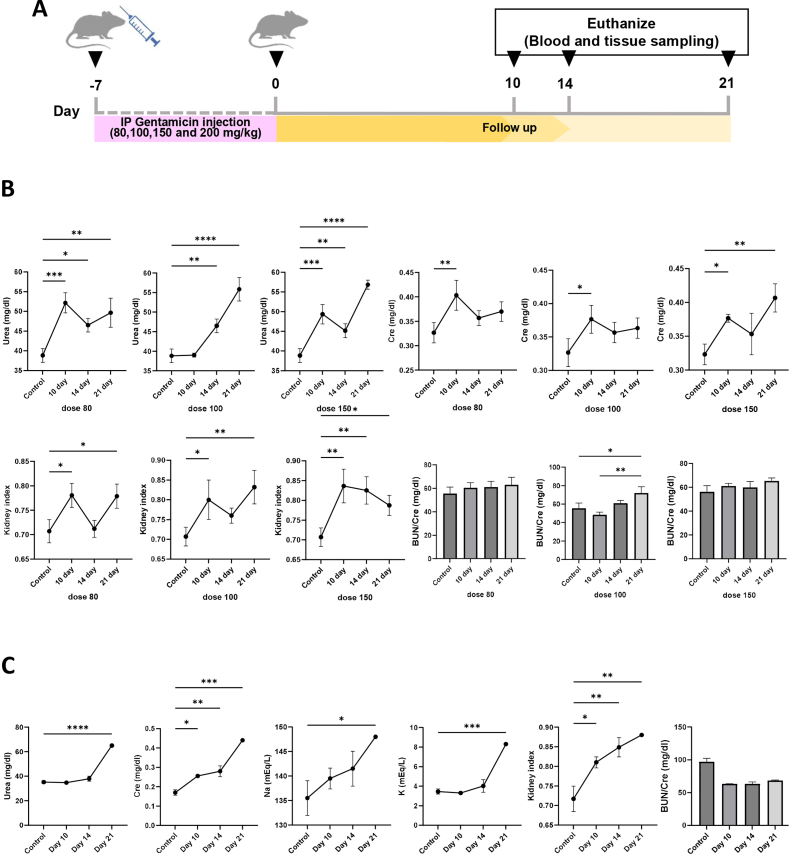
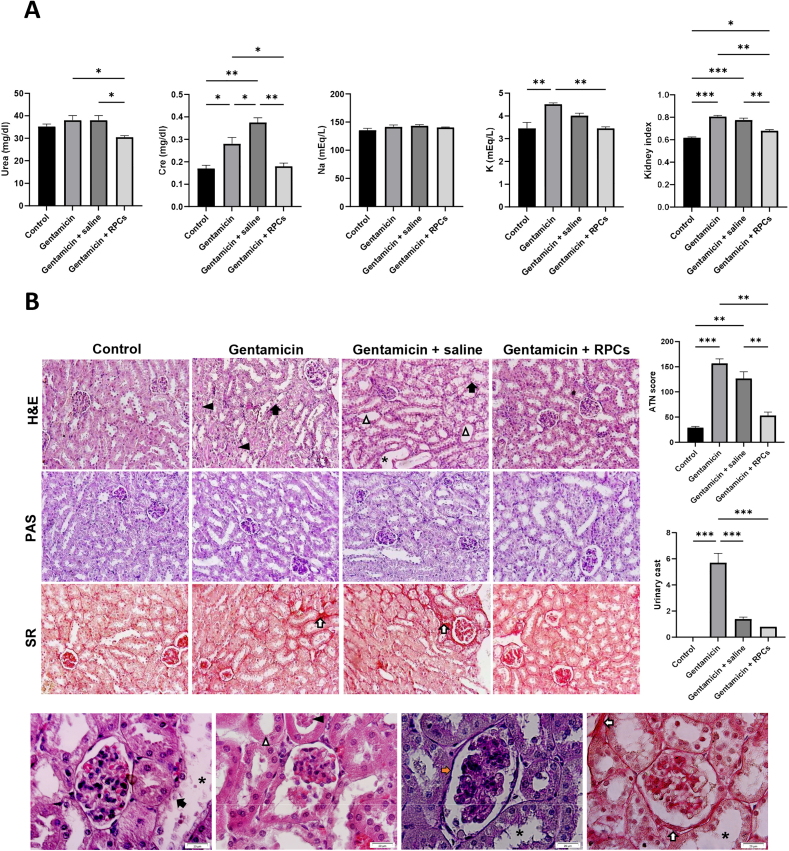
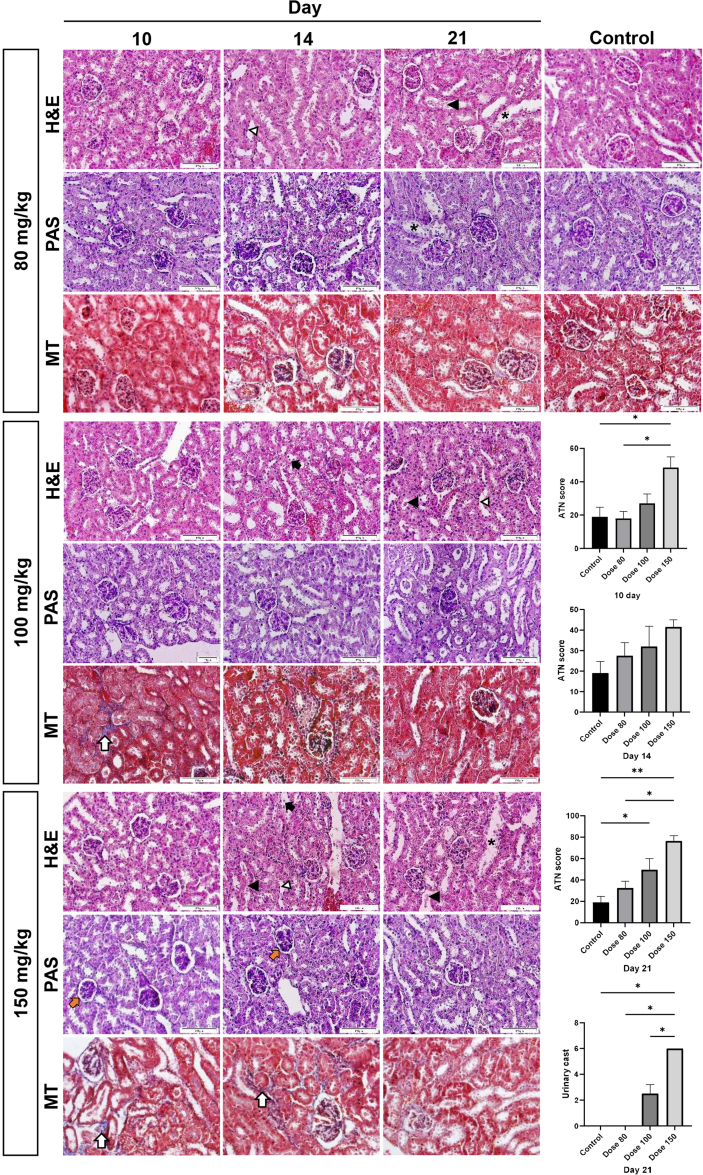
In a gentamicin dose-detection study, mice serum urea and creatinine levels were gradually increased until day 10 at drug doses of 80 and 150 mg/kg and then increased again after a temporary decrease on day 14. These levels were always significantly higher than the control values (Fig. 2). The serum creatinine level in mice given 100 mg/kg increased with a drop on day 14 and was significantly higher than in the control group. The urea level gradually increased from day 10 and was significantly higher than the control group on days 14 and 21. Gentamicin-induced renal lesions including epithelial cell necrosis, brush border loss, tubular dilation, urinary casts, and interstitial fibrosis were also seen in different doses (Supplementary Fig. 1). Kidney histopathology scores revealed that gentamicin (100 and 150 mg/kg) treatment caused more kidney damage than the control group; however, the damage was more severe at dose 150 than at other doses.
Fig. 2.
Evaluation of different doses of gentamicin on renal function. (a) An overview of the protocol used to determine gentamicin doses in NMRI mice. (b) The effect of different doses of gentamicin on serum biochemical factors in NMRI mice. (c) verification of gentamicin-determined dose (100 mg/kg) in Nude mice. Cr, serum creatinine. ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
3.3. Transplantation of ESCs-derived CD133+CD24+ renal progenitor cells ameliorate kidney injury in mice
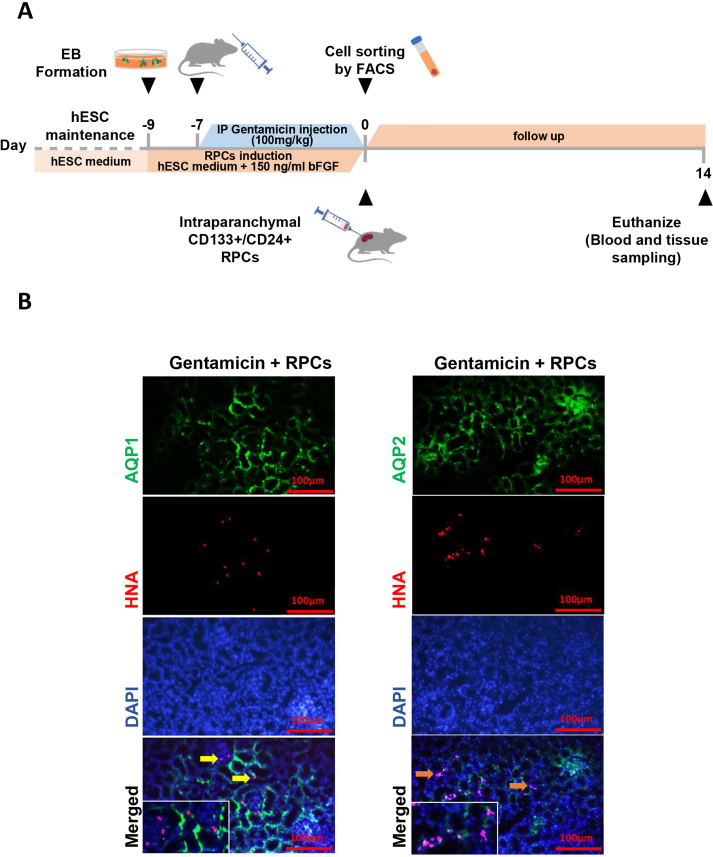
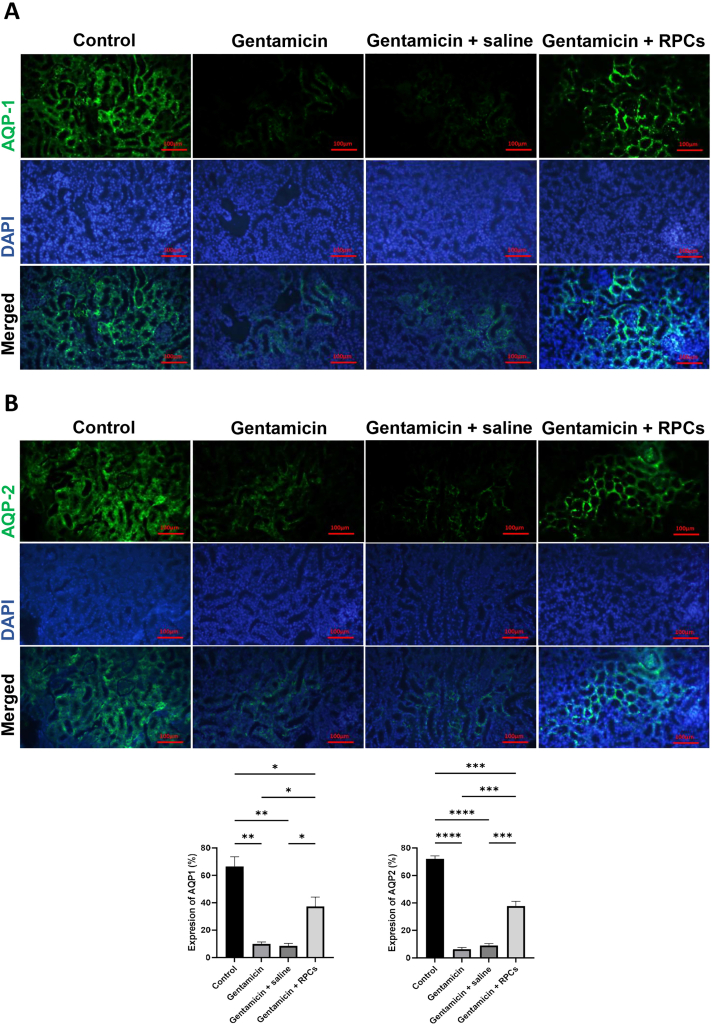
As shown in schematic Fig. 3A, we tested the renoprotective potential of ESC-derived CD133+CD24+ renal progenitor cells obtained on day 9 in a murine model of gentamicin-induced kidney injury. Following 14 days, intraparenchymal injected ESC-derived RPCs were found in various parts of the kidneys of mice, as shown by human nuclear antigen (HNA) (Fig. 3B); stained areas show engraftment of about 11% of HNA-positive cells per high power field (HPF). Furthermore, immunofluorescence staining with AQP1 and AQP2 shows that CD133+CD24+ cells were transplanted into mouse proximal convoluted tubules and collecting ducts (Fig. 4A and B). Serum Urea, creatinine and potassium levels were significantly lower in the group transplanted with CD133+CD24+ progenitor cells than in the gentamicin group and gentamicin-treated mice given saline 14 days after transplantation. The levels of serum sodium were measured and compared, and no significant differences was found (Fig. 5A). Renal histology in mice treated with ESC-derived RPCs was greatly improved, as shown by a considerable reduction in renal histological score in terms of tubule dilatation, urinary cast, loss of brush boundary, and epithelial cell necrosis (Fig. 5B).
Fig. 3.
CD133+CD24+RPCs regenerative potential in gentamicin-induced AKI. (a) A schematic description of cell therapy and sample collection protocol. (b) CD133+CD24+ RPCs identified in gentamicin-treated mice by HNA staining (red), expressed AQP1 (yellow arrow), and AQP2 (orange arrow). Scale bars: 100 μm.
Fig. 4.
The effects of CD133+CD24+RPCs transplantation on expression of proximal convoluted tubule and collecting duct markers. Immunostaining of different groups' renal sections with AQP1 (A) and AQP2 (B) antibodies revealed that the expression level of these markers was significantly higher in the cell administration group than in the gentamicin- or saline-treated groups. Scale bars: 100 μm. ∗∗∗∗P < 0.0001, ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
Fig. 5.
Cell therapy using CD133+CD24+RPCs for acute gentamicin injured kidney in Nude mice. (a) Analysis of serum levels of biochemical factors in different groups, including control, gentamicin-treated group, and mice receiving RPC transplant or saline injection. (b) Representative photographs of mice's kidney sections from different groups on day 14 after RPC transplantation. loss of the brush border (white triangle arrow), tubular necrosis (black arrow), tubular dilatation (black asterisks), urinary casts (black triangle arrow), fibrosis (white arrows), and glomerular basement membrane thickness (orange arrow) were shown in 20x and 100x. H&E staining shows a marked improvement in renal tissue construct in the cell-receiving group compared to the untreated control group. As seen in the PAS and SR stained slides, the preservation of renal tubule integrity and interstitial fibrosis in the cell recipient group were lower than in the untreated group, respectively. The renal injury score was calculated using a semi-quantitative approach. Cre, creatinine; Na, sodium; K, potassium H&E, Hematoxylin, and eosin; PAS, periodic Acid-Schiff; SR, Sirius Red; Scale bars: 100 μm, 20 μm ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
3.4. Treatment with ESC-derived CD133+CD24+ RPCs protecting against the gentamicin-induced renal oxidative state
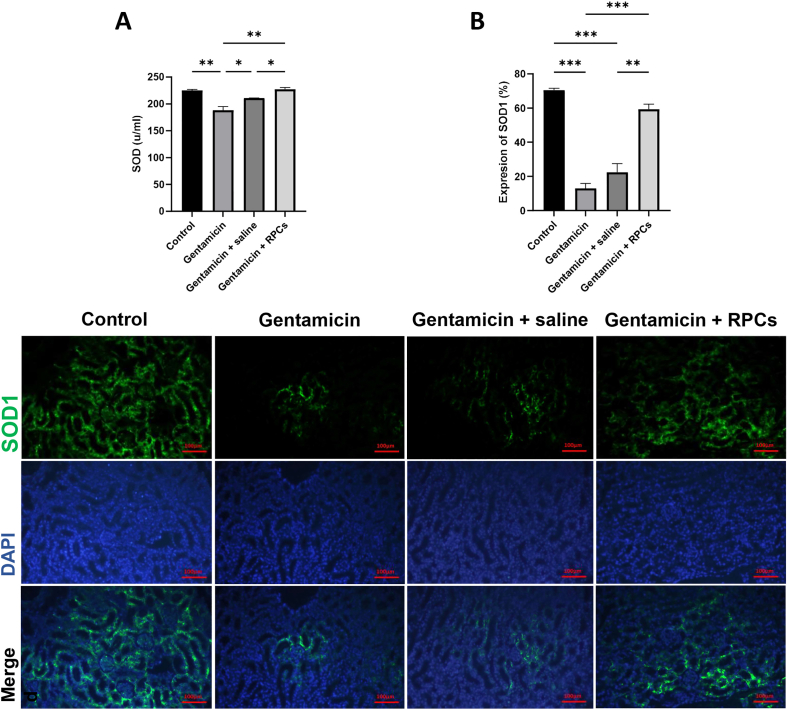
Superoxide dismutase (SOD) concentration in serum was used to assess gentamicin-induced renal tissue oxidative stress, which was confirmed by immunofluorescence staining with a SOD1 antibody. Serum SOD concentrations in the RPC-treated group increased significantly more than in the gentamicin-treated and gentamicin receiving groups (Fig. 6A). Furthermore, SOD1 IFC staining confirmed this issue, revealing that the protein expression level of SOD1 in the RPCs-treated group is significantly higher than in the gentamicin and gentamicin/saline groups (Fig. 6B).
Fig. 6.
Effect of CD133+cd24+ RPCs administration on oxidative damage in gentamicin-treated AKI mice. (a) The SOD level in gentamicin-treated nude mice kidney samples was measured by serum biochemical analysis (a) and immunostaining (b). Scale bars: 100 μm. ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
3.5. CD133+CD24+ RPCs relieve inflammation in gentamicin-induced kidney injury
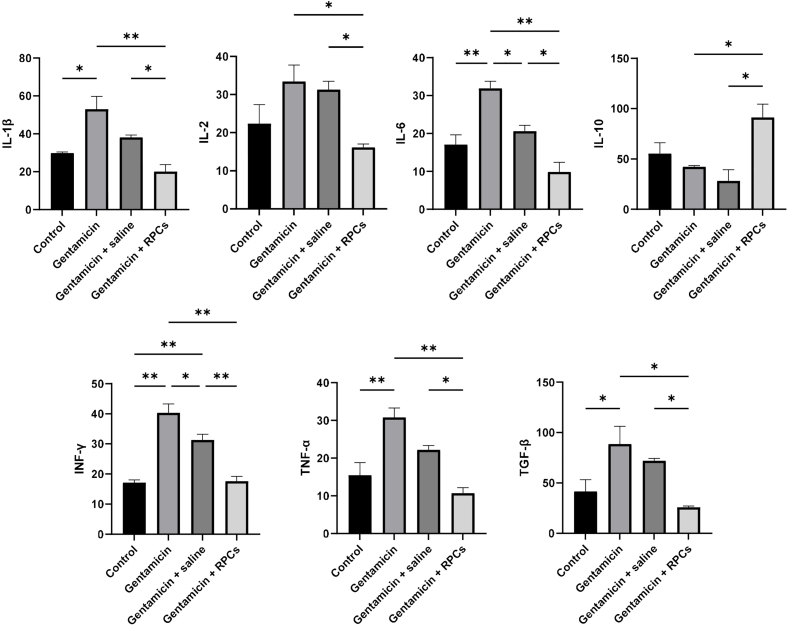
To assess the potential effects of CD133+CD24+ RPC transplantation on tissue inflammation, the levels of cytokines such as IL1, IL2, IL6, IL10, TGF-β, TNF-α, and IFN-γ were measured using an ELISA assay after following 14 days. The renal value of pro-inflammatory cytokines including IL-1β, IL2, IL-6, TNF-α, TGF-β, and IFN-γ was significantly higher in the gentamicin group than in the control group, but significantly lower after cell transplantation in the gentamicin group (Fig. 7). In addition, the level of these cytokines was significantly higher compared to the cell-receiving group, showing the transplantation of RPCs had a beneficial impact on the reduction of inflammation. The level of these cytokines increased with a similar pattern in the gentamicin/saline group but not significantly compared to the control group. In contrast, the anti-inflammatory cytokine IL-10 levels were significantly higher in the RPCs receiving group compared to the gentamicin and gentamicin/saline groups, and slightly lower in these groups compared to the control group (Fig. 7). Overall, transplantation of CD133+CD24+ RPCs reduced pro-inflammatory cytokine levels while increasing anti-inflammatory cytokine IL-10 levels.
Fig. 7.
Effect of CD133+CD24+RPCs administration on the expression of proinflammatory, anti-inflammatory, and fibrotic factors following acute renal gentamicin injury 14 days post-treatment. CD133+CD24+ RPC treatment contributed to lower expression of IL-1β, IL2, IL-6, TNF-α, and IFN-γ as the proinflammatory cytokines and TGF-β as a proinflammatory and fibrotic marker in mice compared with gentamicin- or saline-treated groups. The expression level of the anti-inflammatory factor IL-10 was significantly higher in the cell-treated group than in other groups. ∗∗P < 0.01, and ∗P < 0.05.
3.6. Effects of CD133+CD24+ RPCs on apoptosis in the injured kidney
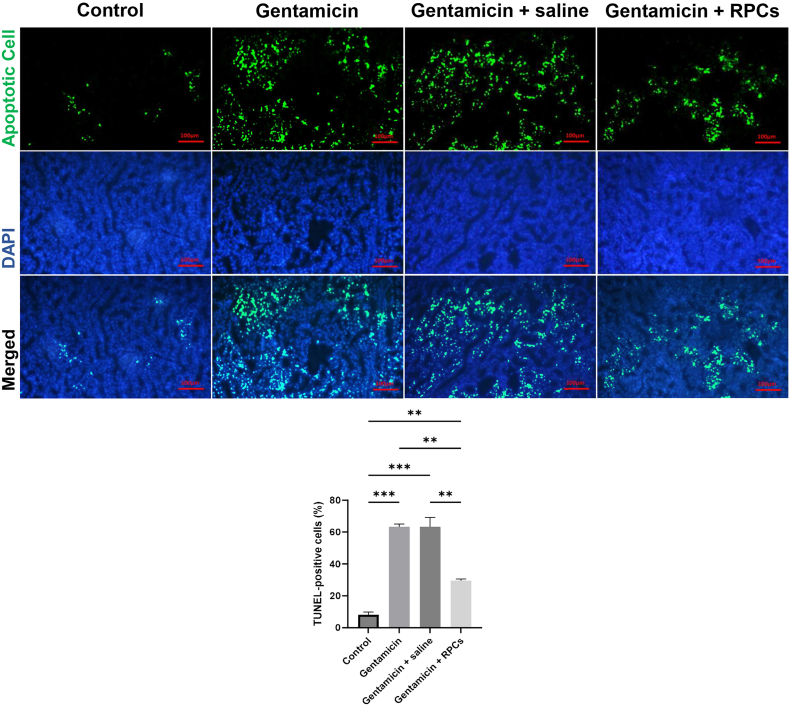
TUNEL assay was used to assess apoptosis in renal tissue. The number and proportion of TUNEL-positive cells were significantly higher in animals with kidney damage and mice given gentamicin/saline, but lower in the RPCs-treated group (Fig. 8). ImageJ 1.8.0 software analysis revealed that the color intensity of the cell-receiving group was lower than that of the gentamicin and gentamicin/saline groups.
Fig. 8.
TUNEL staining of the nude mice kidney sections after gentamicin-induced AKI. Administration of CD133+CD24+ RPCs contributed to reduced apoptotic cells in damaged kidneys compared with gentamicin- and saline-treated groups. Scale bars: 100 μm. ∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05.
4. Discussion
Acute kidney injury, a potentially fatal clinical condition, is a common problem characterized by decreased renal function and affects 10–15% of hospitalized patients [22]. Gentamicin, a common treatment for gram-negative bacterial infections, can lead to renal damage, limiting its use [23,24]. Our findings show that transplanting embryonic stem cell-derived enriched CD133+CD24+ RPCs into nude mice can reverse gentamicin-induced kidney injury and improve renal function and construction. Several studies have shown that renal progenitors derived from hESCs/iPSCs administration can improve renal injury in a variety of experimental models. However, because all cells, including pluripotent stem cells, RPCs, and other differentiated cell types, were transplanted in these studies, it is unclear whether the administration of pluripotent stem cell-derived renal progenitors contributes to the attenuative effects on kidney injury and ameliorates the injury or not [[19], [20], [21],[25], [26], [27]]. In this study, we showed the therapeutic potential of embryonic stem cell-derived renal progenitor cells by transplanting the purified population and using double expression of CD 133 and CD 24 markers and flowcytometric sorting.
Researchers have used a variety of methods for the administration of stem/progenitor cells into damaged tissue, including tail vein and intrarenal artery injection, as well as intraparenchymal injection [21,[28], [29], [30], [31]]. Cellular uptake, kidney preservation, and systemic safety are the most important therapeutic considerations for cell-based therapy, regardless of approach [29]. However, intravenous cell delivery is often associated with the accumulation of injected cells in non-target organs such as lungs, reducing treatment effectiveness [32,33]; Although cell delivery through the intrarenal artery is associated with an increase in the number of transplanted cells, microvasculature occlusion by the injected cells remains a challenge for this method [16,34]. As a result, the intraparenchymal method seems more effective. In our study, we used the intraparenchymal injection method to transfer the cells. The exact process of gentamicin-induced kidney damage is not completely known, but it is primarily linked to oxidative stress, inflammation, and tubular necrosis [35]. After filtering by the kidneys, a small fraction of gentamicin (about 5–10%) is absorbed and accumulated in certain kidney cells' organelles [36], leading to their disruption and the activation of pathways that result in cell death [37]. This process by affecting mitochondria [38] involves oxidative stress and an increase in apoptotic cells [39,40]. like other studies, we found that gentamicin increased oxidative stress and the number of apoptotic cells and showed the administration of progenitor cells effectively reduced these items, however, their exact role and mechanism of action were not investigated in this study.
Gentamicin-induced oxidative stress triggers an inflammatory response [41]. This process involves NF-κB, a factor responsible for regulating inflammatory pathways and cytokines. Gentamicin has been found to increase the expression of NF-κB [41,42], which, when released in response to increased ROS production, leads to the transcription of several inflammatory factors like TNF-α and IL-6. These cytokines promote leukocyte/macrophage infiltration and ROS production, which exacerbates renal injury [43]. The accumulation of mononuclear cells in the renal interstitium and the activation of macrophages and lymphocytes, is linked to the release of more inflammatory, pro-inflammatory, and pro-fibrotic cytokines, which intensify the phenotypic response in epithelial and endothelial cells and cause apoptosis and EMT [44,45]. TGF- is a key regulator of fibrosis and an important stimulator of EMT, and it plays a role in the development of fibrosis by inducing the generation of myofibroblasts [46,47]. In our study, as in earlier studies, the expression of pro-inflammatory and fibrotic factors such as IL1, IL2, IL6, TGF--β, TNF-α, and IFN-γ was elevated in the gentamicin group [36,48].
The administration of CD133+CD24+ RPCs reduced pro-inflammatory factors while increasing anti-inflammatory IL-10 production. Given the decrease in TGF-β factor expression in the cell-treated group, RPCs are likely to contribute to the improvement of fibrosis by participating in the pathways involved in TGF-β inhibition. However, a more detailed analysis of their signaling mediators is required to determine exactly how these cells function in alleviating inflammation and fibrosis. The role of renal progenitor cells in improving the renal injury process is hotly debated. There are two main mechanisms by which these cells participate in reducing damage and developing regeneration: The first mechanism, known as the “cell pathway,” is the homing and differentiation of progenitor cells into functional cells and the replacement of damaged cells. Another mechanism is the “paracrine pathway,” which is the secretion of humoral and soluble factors by these cells [49,50]. Several studies have found that exogenous progenitor cells can be integrated into damaged tubes. Progenitor cells' ability to differentiate into different cell types is thought to explain their protective effects [10,11,21,51]. Even when paracrine mechanisms are involved, the deployment of injected cells is thought to be more effective in achieving the desired therapeutic properties. Indeed, cells in damaged tissue are exposed to a beneficial inflammatory environment, which can influence their behavior in a bidirectional manner [52]. CD133, a surface molecule expressed on progenitor cells, is involved in the Wnt/-catenin signaling pathway and plays a role in inducing and regulating cellular proliferation after damage by stabilizing β-catenin as the primary target of Wnt [53].
Several studies have shown that paracrine mechanisms are primarily responsible for the healing and preservation of the damaged organ during cell injection [33,54,55]; As the lack of kidney transplantation of injected cells in some studies and the improvement of kidney structure and function in these studies clearly confirm this matter [33,54]. According to research, renal progenitor cells secrete a variety of trophic factors that promote kidney regeneration by increasing cell proliferation and decreasing apoptosis [54,55]. Several growth factors with renoprotective properties, such as HGF, VEGF, and NAGF, have been identified in these cells [[56], [57], [58], [59]]. HGF is a potential anti-fibrotic cytokine that inhibits TGF/Smad signaling and prevents EMT [59]; therefore, renal progenitor cells can be said to play a role in upregulating HGF and downregulating TGF. According to the findings of one study, CD 133+ progenitor cells secrete FGF2 and inhibin-A in addition to HGF [39,60]. FGF2 acts as a growth factor, accelerating the process of post-injury regeneration and preventing apoptosis [61]. Inhibin-A functions as a TGF antagonist and is involved in the processes of proliferation, differentiation, and apoptosis [60]. By secreting erythropoietin, CD 133+ progenitor cells can limit renal fibrosis after injury [6]. Other therapeutic factors effective in kidney injury have been reported to be secreted by renal progenitor cells as vesicles and microvesicles [39,55,[62], [63], [64]]. Functional analysis of EV proteins secreted from CD 133 + cells in a study identified a set of proteins related to cell maintenance and cell cycle, negative regulation of apoptosis, specific proteins related to protection against oxidative stress such as SOD, and proteins related to angiogenesis as Von Willebrand [56,65]. Decorin and cyclin D1 are two other factors secreted by CD 133+ progenitor cells as microvesicles [39]. Decorin is a potent TGF-β signaling antagonist, and its expression reduces fibrosis and inflammation [[66], [67]]; This factor is also involved in apoptosis and the cell cycle, and it can be activated via WNT signaling [68,69]. Although these evidences support the efficacy of CD 133+ progenitors, we did not specifically investigate their paracrine function and effectiveness in our study, and more detailed evaluations are required. It is recommended to evaluate the impact of these cells in more advanced models and within the clinical setting.
5. Conclusion
Our findings show hESCs- CD133+CD24+ RPC intraparenchymal administration significantly attenuates renal injury and helps with the restoration of renal function following gentamicin-induced injury. Cell therapy using CD133+CD24+ RPCs may represent a cutting-edge therapeutic strategy to treat renal failure.
Ethical statement
This study was approved by the Research Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, under the code No. (IR.SBMU.LASER.REC.1402.012). All in-vivo experiments were carried out under ARRIVE guidelines (https://arriveguidelines.org).
Author contributions
MB: Conceptualization - Methodology - formal analysis and writing. HA: Conceptualization – review and editing. MN: review and editing. MAA: review and editing. NAR: review and editing. MS: Methodology. MA: Methodology. EE: review and editing. MB: review and editing. AL. S: review and editing. RM: Conceptualization – review and editing.
Funding sources
This study is based on a PhD dissertation by Maryam Bahrami at the Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran. This work was financially supported by Laser application in medical sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran and Royan Institute for Stem Cell Biology and Technology, Tehran, Iran.
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgment
The authors wish to thank the Laser Applications in Medical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran and Royan Institute for their collaboration.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2024.04.015.
Contributor Information
Hojjat Allah Abbaszadeh, Email: Dr.Abbaszadeh@sbmu.ac.ir.
Reza Moghadasali, Email: Reza.Moghadasali@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Supplementary 1.
The effects of different doses of gentamicin on renal histology in NMRI mice. Representative section photographs of NMRI mice's kidneys treated with different doses of gentamicin. A semi-quantitative score was used to assess renal damage, as explained in the Methods section. H&E, hematoxylin and eosin; PAS, Periodic Acid-Schiff; MT, Masson's trichrome. ATN: acute tubular necrosis. Scale bars: 100μm. ∗∗P < 0.01 and, ∗P < 0.05.
References
- 1.Kellum J.A., Romagnani P., Ashuntantang G., Ronco C., Zarbock A., Anders H.-J. Acute kidney injury. Nat Rev Dis Prim. 2021;7:52. doi: 10.1038/s41572-021-00284-z. [DOI] [PubMed] [Google Scholar]
- 2.Mamillapalli R., Cho S., Mutlu L., Taylor H.S. Therapeutic role of uterine-derived stem cells in acute kidney injury. Stem Cell Res Ther. 2022;13:107. doi: 10.1186/s13287-022-02789-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohaney R., Yin H., Shahinian V., Saran R., Burrows N.R., Pavkov M.E., et al. In-hospital and 1-year mortality trends in a national cohort of US veterans with acute kidney injury. Clin J Am Soc Nephrol. 2022;17:184–193. doi: 10.2215/CJN.01730221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havasi A., Borkan S.C. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchan J.R., Jhaveri K., Berns J.S., Lam A.Q. UpToDate; Waltham, MA: 2021. Chemotherapy nephrotoxicity and dose modification in patients with kidney impairment: conventional cytotoxic agents. UpToDate. [Google Scholar]
- 6.Aggarwal S., Grange C., Iampietro C., Camussi G., Bussolati B. Human CD133+ renal progenitor cells induce erythropoietin production and limit fibrosis after acute tubular injury. Sci Rep. 2016;6:1–13. doi: 10.1038/srep37270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshina A., Kawamoto T., Sueta S.-I., Mae S.-I., Araoka T., Tanaka H., et al. Development of new method to enrich human iPSC-derived renal progenitors using cell surface markers. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-24714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertow G.M., Burdick E., Honour M., Bonventre J.V., Bates D.W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 9.Bussolati B., Bruno S., Grange C., Buttiglieri S., Deregibus M.C., Cantino D., et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ronconi E., Sagrinati C., Angelotti M.L., Lazzeri E., Mazzinghi B., Ballerini L., et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol. 2009;20:322–332. doi: 10.1681/ASN.2008070709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angelotti M.L., Ronconi E., Ballerini L., Peired A., Mazzinghi B., Sagrinati C., et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem cells. 2012;30:1714–1725. doi: 10.1002/stem.1130. [DOI] [PubMed] [Google Scholar]
- 12.Sagrinati C., Netti G.S., Mazzinghi B., Lazzeri E., Liotta F., Frosali F., et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 13.Smeets B., Boor P., Dijkman H., Sharma S.V., Jirak P., Mooren F., et al. Proximal tubular cells contain a phenotypically distinct, scattered cell population involved in tubular regeneration. J Pathol Clin Res. 2013;229:645–659. doi: 10.1002/path.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward H.H., Romero E., Welford A., Pickett G., Bacallao R., Gattone I.I.V.H., et al. Adult human CD133/1+ kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta, Mol Basis Dis. 2011;1812(10):1344–1357. doi: 10.1016/j.bbadis.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussolati B., Camussi G. Therapeutic use of human renal progenitor cells for kidney regeneration. Nat Rev Nephrol. 2015;11:695–706. doi: 10.1038/nrneph.2015.126. [DOI] [PubMed] [Google Scholar]
- 16.Lee P.-Y., Chien Y., Chiou G.-Y., Lin C.-H., Chiou C.-H., Tarng D.-C. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia–reperfusion rats. Cell Transplant. 2012;21:2569–2585. doi: 10.3727/096368912X636902. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y., Zhang M., Li M., Zhang H., Zhang F., Zhang H., et al. Generation of urine-derived induced pluripotent stem cell line from patients with acute kidney injury. Cell Reprogr. 2021;23:290–303. doi: 10.1089/cell.2021.0051. [DOI] [PubMed] [Google Scholar]
- 18.de Carvalho Ribeiro P., Oliveira L.F., Caldas H.C. Differentiating induced pluripotent stem cells into renal cells: a new approach to treat kidney diseases. Stem Cell Int. 2020;2020 doi: 10.1155/2020/8894590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang M., Han Y.-M. Differentiation of human pluripotent stem cells into nephron progenitor cells in a serum and feeder free system. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam A.Q., Freedman B.S., Morizane R., Lerou P.H., Valerius M.T., Bonventre J.V. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imberti B., Tomasoni S., Ciampi O., Pezzotta A., Derosas M., Xinaris C., et al. Renal progenitors derived from human iPSCs engraft and restore function in a mouse model of acute kidney injury. Sci Rep. 2015;5:1–7. doi: 10.1038/srep08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ronco C., Bellomo R., Kellum J.A. Acute kidney injury. J Lancet. 2019;394(10212):1949–1964. doi: 10.1016/S0140-6736(19)32563-2. [DOI] [PubMed] [Google Scholar]
- 23.Dandachi I., Sokhn E.S., Dahdouh E.A., Azar E., El-Bazzal B., Rolain J.-M., et al. Prevalence and characterization of multi-drug-resistant gram-negative bacilli isolated from lebanese poultry: a nationwide study. Front Microbiol. 2018;9:550. doi: 10.3389/fmicb.2018.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui J., Bai X.-Y., Sun X., Cai G., Hong Q., Ding R., et al. Rapamycin protects against gentamicin-induced acute kidney injury via autophagy in mini-pig models. Sci Rep. 2015;5:1–17. doi: 10.1038/srep11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Tian S-f, Guo Y., Niu X., Hu B., Guo S-c, et al. Transplantation of induced pluripotent stem cell-derived renal stem cells improved acute kidney injury. Cell Biosci. 2015;5:1–9. doi: 10.1186/s13578-015-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takasato M., Er P., Becroft M., Vanslambrouck J.M., Stanley E., Elefanty A.G., et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1186/s13578-015-0040-z. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi A., Kaku Y., Ohmori T., Sharmin S., Ogawa M., Sasaki H., et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Alfarano C., Roubeix C., Chaaya R., Ceccaldi C., Calise D., Mias C., et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012;21:2009–2019. doi: 10.3727/096368912X640448. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L., Xu L., Shen J., Song Q., Wu R., Ge Y., et al. Preischemic administration of nonexpanded adipose stromal vascular fraction attenuates acute renal ischemia/reperfusion injury and fibrosis. Stem Cells Transl Med. 2016;5:1277–1288. doi: 10.5966/sctm.2015-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghadasali R., Azarnia M., Hajinasrollah M., Arghani H., Nassiri S.M., Molazem M., et al. Intra-renal arterial injection of autologous bone marrow mesenchymal stromal cells ameliorates cisplatin-induced acute kidney injury in a rhesus Macaque mulatta monkey model. Cytotherapy. 2014;16:734–749. doi: 10.1016/j.jcyt.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L., Song Q., Shen J., Xu L., Xu Z., Wu R., et al. Comparison of human adipose stromal vascular fraction and adipose-derived mesenchymal stem cells for the attenuation of acute renal ischemia/reperfusion injury. Sci Rep. 2017;7:1–9. doi: 10.1038/srep44058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freyman T., Polin G., Osman H., Crary J., Lu M., Cheng L., et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 33.Santeramo I., Herrera Perez Z., Illera A., Taylor A., Kenny S., Murray P., et al. Human kidney-derived cells ameliorate acute kidney injury without engrafting into renal tissue. Stem Cells Transl Med. 2017;6:1373–1384. doi: 10.1002/sctm.16-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih Y.-C., Lee P.-Y., Cheng H., Tsai C.-H., Ma H., Tarng D.-C. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plast Reconstr Surg. 2013;132:940e. doi: 10.1097/PRS.0b013e3182a806ce. 51e. [DOI] [PubMed] [Google Scholar]
- 35.El Gamal A.A., AlSaid M.S., Raish M., Al-Sohaibani M., Al-Massarani S.M., Ahmad A., et al. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/983952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H., Jin W.W., Huang M., Ji H., Capen D.E., Xia Y., et al. Gentamicin-induced acute kidney injury in an animal model involves programmed necrosis of the collecting duct. J Am Soc Nephrol. 2020;31:2097–2115. doi: 10.1681/ASN.2019020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randjelovic P., Veljkovic S., Stojiljkovic N., Sokolovic D., Ilic I. Gentamicin nephrotoxicity in animals: current knowledge and future perspectives. EXCLI J. 2017;16:388. doi: 10.17179/excli2017-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morales A.I., Detaille D., Prieto M., Puente A., Briones E., Arévalo M., et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–869. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 39.Sallustio F., Costantino V., Cox S.N., Loverre A., Divella C., Rizzi M., et al. Human renal stem/progenitor cells repair tubular epithelial cell injury through TLR2-driven inhibin-A and microvesicle-shuttled decorin. Kidney Int. 2013;83:392–403. doi: 10.1038/ki.2012.413. [DOI] [PubMed] [Google Scholar]
- 40.Cuzzocrea S., Mazzon E., Dugo L., Serraino I., Di Paola R., Britti D., et al. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur J Pharmacol. 2002;450:67–76. doi: 10.1016/s0014-2999(02)01749-1. [DOI] [PubMed] [Google Scholar]
- 41.Mahmoud Y.I. Kiwi fruit (Actinidia deliciosa) ameliorates gentamicin-induced nephrotoxicity in albino mice via the activation of Nrf2 and the inhibition of NF-κB (Kiwi & gentamicin-induced nephrotoxicity) Biomed Pharmacother. 2017;94:206–218. doi: 10.1016/j.biopha.2017.07.079. [DOI] [PubMed] [Google Scholar]
- 42.Tak P.P., Firestein G.S. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quiros Y., Vicente-Vicente L., Morales A.I., López-Novoa J.M., López-Hernández F.J. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicol Sci. 2011;119:245–256. doi: 10.1093/toxsci/kfq267. [DOI] [PubMed] [Google Scholar]
- 44.Puthumana J., Thiessen-Philbrook H., Xu L., Coca S.G., Garg A.X., Himmelfarb J., et al. Biomarkers of inflammation and repair in kidney disease progression. J Clin Invest. 2021;131 doi: 10.1172/JCI139927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng X.-M., Nikolic-Paterson D.J., Lan H.Y. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10:493–503. doi: 10.1038/nrneph.2014.114. [DOI] [PubMed] [Google Scholar]
- 46.Dees C., Chakraborty D., Distler J.H. Cellular and molecular mechanisms in fibrosis. Exp Dermatol. 2021;30:121–131. doi: 10.1111/exd.14193. [DOI] [PubMed] [Google Scholar]
- 47.Zavadil J., Böttinger E.P. TGF-β and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 48.Jaikumkao K., Pongchaidecha A., Thongnak L-o, Wanchai K., Arjinajarn P., Chatsudthipong V., et al. Amelioration of renal inflammation, endoplasmic reticulum stress and apoptosis underlies the protective effect of low dosage of atorvastatin in gentamicin-induced nephrotoxicity. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossol-Allison J., Ward C.J. Exosomes to the rescue. Am Soc Nephrol. 2015:2303–2304. doi: 10.1681/ASN.2015030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun X., Meng H., Wan W., Xie M., Wen C. Application potential of stem/progenitor cell-derived extracellular vesicles in renal diseases. Stem Cell Res Ther. 2019;10:1–9. doi: 10.1186/s13287-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranghino A., Bruno S., Bussolati B., Moggio A., Dimuccio V., Tapparo M., et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther. 2017;8:1–15. doi: 10.1186/s13287-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grange C., Moggio A., Tapparo M., Porta S., Camussi G., Bussolati B. Protective effect and localization by optical imaging of human renal CD 133+ progenitor cells in an acute kidney injury model. Phys Rep. 2014;2 doi: 10.14814/phy2.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brossa A., Papadimitriou E., Collino F., Incarnato D., Oliviero S., Camussi G., et al. Role of CD133 molecule in Wnt response and renal repair. Stem Cells Transl Med. 2018;7:283–294. doi: 10.1002/sctm.17-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyohara T., Mae S.-I., Sueta S.-I., Inoue T., Yamagishi Y., Kawamoto T., et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. 2015;4:980–992. doi: 10.1002/sctm.17-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuji K., Kitamura S., Sang Y., Fukushima K., Wada J. Adult kidney stem/progenitor cells contribute to regeneration through the secretion of trophic factors. Stem Cell Res. 2020;46 doi: 10.1016/j.scr.2020.101865. [DOI] [PubMed] [Google Scholar]
- 56.Miyasaki D.M., Senegaglia A.C., de Moura S.A.B., Leitolis A., Capriglione L.G.A., Fracaro L., et al. Treatment of chronic kidney disease with extracellular vesicles from mesenchymal stem cells and CD133+ expanded cells: a comparative preclinical analysis. Int J Mol Sci. 2022;23:2521. doi: 10.3390/ijms23052521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barasch J., Qiao J., McWilliams G., Chen D., Oliver J.A., Herzlinger D. Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol Ren Physiol. 1997;273:F757–F767. doi: 10.1152/ajprenal.1997.273.5.F757. [DOI] [PubMed] [Google Scholar]
- 58.Brown A.C., Muthukrishnan S.D., Guay J.A., Adams D.C., Schafer D.A., Fetting J.L., et al. Role for compartmentalization in nephron progenitor differentiation. Proc Natl Acad Sci India B Biol Sci. 2013;110:4640–4645. doi: 10.1073/pnas.1213971110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang J., Dai C., Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 60.Sallustio F., Curci C., Aloisi A., Toma C.C., Marulli E., Serino G., et al. Inhibin-A and decorin secreted by human adult renal stem/progenitor cells through the TLR2 engagement induce renal tubular cell regeneration. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-08474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villanueva S., Cespedes C., Gonzalez A.A., Roessler E., Vio C.P. Inhibition of bFGF-receptor type 2 increases kidney damage and suppresses nephrogenic protein expression after ischemic acute renal failure. Am J Physiol, Integrative Comparative Physiology. 2008;294:R819–R828. doi: 10.1152/ajpregu.00273.2007. [DOI] [PubMed] [Google Scholar]
- 62.Camussi G., Deregibus M.C., Bruno S., Cantaluppi V., Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 63.Cantaluppi V., Gatti S., Medica D., Figliolini F., Bruno S., Deregibus M.C., et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia–reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012;82:412–427. doi: 10.1038/ki.2012.105. [DOI] [PubMed] [Google Scholar]
- 64.Bahrami M., Darabi S., Rozbahany N.A., Abbaszadeh H.A., Moghadasali R. Great potential of renal progenitor cells in kidney: from the development to clinic. Exp Cell Res. 2023;11 doi: 10.1016/j.yexcr.2023.113875. [DOI] [PubMed] [Google Scholar]
- 65.Angulski A.B., Capriglione L.G., Batista M., Marcon B.H., Senegaglia A.C., Stimamiglio M.A., et al. The protein content of extracellular vesicles derived from expanded human umbilical cord blood-derived CD133+ and human bone marrow-derived mesenchymal stem cells partially explains why both sources are advantageous for regenerative medicine. Stem Cell Rev Rep. 2017;13:244–257. doi: 10.1007/s12015-016-9715-z. [DOI] [PubMed] [Google Scholar]
- 66.Fetting J.L., Guay J.A., Karolak M.J., Iozzo R.V., Adams D.C., Maridas D.E., et al. FOXD1 promotes nephron progenitor differentiation by repressing decorin in the embryonic kidney. Development. 2014;141:17–27. doi: 10.1242/dev.089078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaefer L., Macakova K., Raslik I., Micegova M., Gröne H.-J., Schönherr E., et al. Absence of decorin adversely influences tubulointerstitial fibrosis of the obstructed kidney by enhanced apoptosis and increased inflammatory reaction. Am J Pathol. 2002;160:1181–1191. doi: 10.1016/S0002-9440(10)64937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schönherr E., Sunderkötter C., Iozzo R.V., Schaefer L. Decorin, a novel player in the insulin-like growth factor system. J Biol Chem. 2005;280:15767–15772. doi: 10.1074/jbc.M500451200. [DOI] [PubMed] [Google Scholar]
- 69.Long K., Moss L., Laursen L., Boulter L. Integrin signalling regulates the expansion of neuroepithelial progenitors and neurogenesis via Wnt7a and Decorin. Nat Commun. 2016;7:1–14. doi: 10.1038/ncomms10354. [DOI] [PMC free article] [PubMed] [Google Scholar]