Abstract
Aedes aegypti is an important vector of arboviral diseases including dengue and yellow fever. Despite the wide distribution of this mosquito species, there are limited data on the ecology of Ae. aegypti in Ghana. In this study, we report on the oviposition preference and the larval life tables of Ae. aegypti mosquitoes in Accra, Ghana. The oviposition preference of the mosquitoes to three habitat types (car tyres, drums and bowls) was measured by setting up ovitraps. We recorded the presence and abundance of larvae every 3 days. Two-hour-old Ae. aegypti larvae were introduced and raised in three habitat types to undertake larval life tables. The number of surviving larvae at each developmental stage was recorded daily until they emerged as adults. Car tyres showed a higher abundance of Ae. aegypti larvae (52.3%) than drums (32.5%) and bowls (15.1%) (ANOVA, F(2,159) = 18.79, P < 0.001). The mean development time of Ae. aegypti larvae was significantly lower in car tyres (7 ± 1 days) compared to that of bowls (9 ± 0.0 days) and drums (12.6 ± 1.5 days) (P = 0.024). The differences in pupation rates and emergence rates were not significant across the habitat types; however, the highest pupation rate was observed in bowls (0.92 ± 0.17) and the emergence rate was highest in tyres (0.84 ± 0.10). The proportion of first-instar larvae that survived to emergence was significantly higher in car tyres (0.84 ± 0.10) compared to that of bowls (0.72 ± 0.20) and drums (0.62 ± 0.20) (P = 0.009). No mortalities were observed after 9 days in car tyres, 10 days in bowls and 15 days in drums. The results confirm that discarded car tyres were the preferred habitat choice for the oviposition of gravid female Ae. aegypti mosquitoes and provide the best habitat conditions for larval development and survival. These findings are necessary for understanding the ecology of Ae. aegypti to develop appropriate strategies for their control in Ghana.
Keywords: Aedes aegypti, Life tables, Oviposition, Ecology, Larval survivorship
Graphical abstract
Highlights
-
•
Higher Aedes aegypti larval abundance was observed in car tyres compared to bowls and drums.
-
•
This indicates that car tyres are the preferred choice for oviposition for gravid female Ae. aegypti mosquitoes.
-
•
Shorter larval development time and higher survival were observed in car tyres than in other habitat types (bowls and drums).
-
•
There was no significant difference in pupation and emergence rates of Ae. aegypti larvae in car tyres, bowls and drums.
1. Introduction
Yellow fever and dengue fever outbreaks have become more frequent in West Africa in the last five years (Suzuki et al., 2017; Amoako et al., 2018; Tarnagda et al., 2018; Weetman et al., 2018; Adogo and Ogoh, 2020; WHO, 2021). Aedes aegypti mosquitoes transmit arboviruses that cause diseases such as yellow fever, dengue fever, Zika, chikungunya and Rift Valley fever. Aedes aegypti is highly anthropophilic and mainly adapted to urban settings (Powell and Tabachnick, 2013). Domestic forms of African Ae. aegypti are more adapted to breeding in artificial containers close to human settlements (Brown et al., 2011; Crawford et al., 2017). However, the sylvatic forms of Ae. aegypti breed in natural breeding habitats such as rock pools, tree holes and fruit husks in forested areas (Powell and Tabachnick, 2013). The main vector control strategies for Aedes spp. are chemical interventions using insecticides and larval source management (Weeratunga et al., 2017).
The World Health Organization (WHO) recommends using larval source management (LSM) to control the immature stages of Aedes spp. mosquitoes (WHO, 2012). This will help in reducing the densities of these vectors within communities. Currently, evidence of insecticide resistance to the four main insecticides in adult Ae. aegypti has been detected in Ghana and across West Africa (Badolo et al., 2019; Konan et al., 2021; Kwame Amlalo et al., 2022; Owusu-Asenso et al., 2022; Toé et al., 2022). Controlling the immature stages of Aedes spp. using LSM helps reduce the dependence on the main insecticides by combining larviciding and habitat modifications and manipulation (WHO, 2013; Adetoro et al., 2022).
To control the immature stages of Aedes spp., it is critical to understand the behaviour of these vectors such as their preferred habitat for oviposition and their life history traits. Female Ae. aegypti in urban and suburban areas prefer to breed in artificial, man-made containers such as tyres, discarded containers, flower pots or drums (Maciel-de-Freitas et al., 2007). Aedes aegypti from different areas have been found to exhibit different oviposition preferences (Simard et al., 2005; Zahouli et al., 2016; Zuharah et al., 2016; Egid et al., 2022). Several studies in West Africa including Ghana have found Ae. aegypti breeding predominately in car tyres (Kamgang et al., 2013; Kudom, 2020; Tedjou et al., 2020; Wat'senga Tezzo et al., 2021; Owusu-Asenso et al., 2022). Tyres are especially useful for mosquito reproduction because they are mostly stored outdoors and can collect and maintain rainwater for a long period. Moreover, car tyres serve as an excellent breeding habitat for Aedes spp. mosquitoes because decaying leaves from neighbouring trees provide chemical conditions similar to tree holes (Kweka et al., 2018). However, other studies have also found Ae. aegypti larvae to breed predominately in other habitat types such as large water barrels, animal troughs, plastic containers and septic tanks (Kweka et al., 2018; Kampango et al., 2021; Badolo et al., 2022).
A female mosquito’s choice of habitat type and oviposition may be influenced by several factors such as the season, habitat size and nutritional availability (Spencer et al., 2005; Maciel-de-Freitas et al., 2007). Female mosquitoes increase the survival and development of their offspring by selecting breeding habitats that reduce the risks of predators and competition (Blaustein et al., 2004; Wong et al., 2011). For instance, Ae. aegypti mostly rest and feed outdoors, so they can explore a wider range of habitat types that are far from human settlements (Wong et al., 2011). Several studies in Ghana found Aedes spp. abundance to be significantly higher outdoors than indoors (Captain-Esoah et al., 2020; Owusu-Asenso et al., 2022). Understanding the oviposition choices of female Aedes spp. mosquitoes will help in designing targeted LSM interventions for these vectors.
Mosquito larval development and survivorship are important determinants of vector densities within an area (Li et al., 2014). Several studies have found that there are variations in life history traits such as larval development and survival in Ae. aegypti in different areas (Bong et al., 2021; Cui et al., 2021). This is because different areas may have diverse environmental conditions which may affect larval development, survival and adult emergence (Afrane et al., 2008; Yang et al., 2020). Understanding the life history traits of Ae. aegypti in Ghana is very important for the development of appropriate vector control measures for the immature stages of these vectors. This study aims to investigate the oviposition preference and the larval life history traits of Ae. aegypti in Accra, Ghana. An experimental study was conducted to determine the oviposition preference and life history traits in three different habitat types (car tyres, bowls and drums). This information will greatly improve vector control strategies especially larval source management for arboviral disease vectors in Ghana.
2. Materials and methods
2.1. Study site
The study was conducted within Korle-bu (5°33′N, 0°12′W), Accra, Ghana, from November 2020 to March 2021. Korle-bu is a suburb located in the coastal savannah zone of the Greater Accra Region. This area has the largest teaching and referral hospital in Ghana and therefore it has an influx of people from different regions across Ghana. This is a populous cosmopolitan area in Accra with numerous vulcanizing shops (car tyre repair shops) within the area. The inhabitants also tend to store water in storage containers due to the irregular flow of pipe-borne water. There is an abundance of Aedes spp. breeding habitats ranging mostly from used car tyres, small containers and water storage drums.
2.2. Aedes aegypti oviposition in three habitat types
An oviposition experiment was set up to investigate the preferred habitat type for Ae. aegypti using three habitat types, i.e. car tyres, bowls and drums. Car tyre ovitraps were made by cutting a car tyre into three parts that can hold water. Each car tyre ovitrap was approximately 56 cm in length and 15 cm in height with an aperture of 5 cm. Bowls were plastic, 29 cm in diameter and 8 cm in height, and had a capacity of up to 5 l of water. Drums were plastic, 41 cm in diameter and 42 cm in height, and had a water capacity of 50 l. The bowls and drums were dark-coloured (black, grey and blue).
Each container type was filled with 2–5 l of rainwater. The containers were placed under trees with a canopy cover of about 50% to mimic where Ae. aegypti habitats were found in previous surveys (Owusu-Asenso et al., 2022; Abdulai et al., 2023). For the reproducibility of the experiment, 3 replicates of each habitat type were set up in 3 different spots (700–900 m apart). The spots where the replicates were set up were about 15–20 m from human dwellings. At each area, all three different habit types were set up 4–5 m apart to determine which type was preferred for oviposition by gravid female Ae. aegypti mosquitoes.
Larval abundance was used as a proxy for oviposition preference because of the difficulty in identifying and counting the eggs that were laid in the different habitats, especially tyres because of their dark surface. The replicates were checked for the presence of larvae every three days for 4 months (from December 2020 to March 2021). The number of larvae was recorded for each habitat type. The larvae were removed, and the water was replaced after every recording.
2.3. Larval life table in three habitat types
2.3.1. F1 eggs for Ae. aegypti larval life table
Aedes aegypti larvae were collected from breeding habitats in Korle-bu and transported to the insectary at the University of Ghana Medical School, Accra. In the insectary, the larvae were raised into adults. Adult female Ae. aegypti were blood-fed with a membrane feeder. After 48 h, egg-laying pads for oviposition by gravid females were prepared with damp cotton in a Petri dish lined with filter paper and placed in the cages. First filial generation (F1) eggs obtained were washed into larval bowls containing rainwater.
2.3.2. Aedes aegypti larval life-table experiments
Life-table experiments were performed to determine the life history traits of Ae. aegypti in different habitat types in November 2020. The life-table components investigated were larval development time, pupation rates, emergence rate and sex ratio. Experiments were set up in 3 replicates each, for 3 different habitat types, i.e. car tyres, drums and bowls. For each replicate, 30 2-h-old larvae were introduced into each habitat type, containing 2–5 l of rainwater and covered with a muslin net. Each habitat type was placed under trees in gardens and outdoors of human dwelling houses. The replicates were observed daily, and the volume of water was replenished daily to compensate for loss due to evaporation.
Developmental stages of surviving Ae. aegypti larvae were assessed daily; alive and dead larvae were recorded. Pupae collected from the habitat types were held in pre-labelled individual paper cups with water of a depth of 25 ml for adult emergence. The paper cups containing one pupa per cup were covered with muslin netting and observed daily for adult emergence. Pupal mortality, adult mosquitoes that emerged (emergence rate), and the sexes were recorded daily. When pupation started, habitats were visited twice a day, at 8:00 h and 17:00 h, for pupae collections.
2.4. Data analysis
Larval abundance was calculated as the total number of larvae obtained per habitat type over the total number of larvae collected. This was used to measure the oviposition preference of Ae. aegypti female mosquitoes to the three habitat types. For the larval life-table experiment, the larval development time was recorded in days as the duration from the first-instar larval stage to the pupal stage. Mean larval development time was defined as the average duration, in days, of first-instar larvae to develop into pupae. The pupation rate was calculated as the sum of the total number of pupae per the sum of the total number of first-instar larvae. The emergence rate was calculated as the sum of the number of adults that emerged per the total number of pupae. Survivorship of Ae. aegypti larvae was calculated as the proportion of first-instar larvae that survived to adults. The ratio of females to males was determined by counting and recording the number of males and females that emerged per day over the total number of adults that emerged. Histograms with normal distribution curves and boxplots were used to assess the normality of the datasets. ANOVA and Kruskal-Wallis ranked sum test were used to test the statistical significance of the different habitat types on larval survivorship and larval development time wherever appropriate for normally distributed and skewed data respectively. Dunn’s test was used to compare the significance of the means. Pearson Chi-square test was used to test for significance in survivorship. All data analyses were performed using R version 3.6.3.
Air temperature and relative humidity data during the period of the experiments were obtained from the ECMWF Reanalysis v5. (ERA5 climate data) (ECMWF Reanalysis v5, 2018). ERA5 provides hourly estimates of a large number of atmospheric, land and oceanic climate variables. Generalized linear mixed model (GLMM) was used to determine the relationships and interactions between the environmental climatic conditions (air temperature and humidity), replication and type of breeding containers on larval abundance and larval life-table parameters of Ae. aegypti.
3. Results
3.1. Variations in the experimental conditions
Air temperature during the oviposition experiments (December to March) and the larval life-table experiments (November) is shown in Table 1. The mean temperatures during the oviposition experiments ranged between 27.7 °C and 28.7 °C, and the mean relative humidity ranged from 73.3% to 82.4%. For the larval life-table experiments, the mean temperature and relative humidity were 27.7 °C and 80%, respectively.
Table 1.
Air temperature and relative humidity during the oviposition and larval life-table experiments.
| Month | Temperature (°C) (Mean ± SD) |
Relative humidity (%) (Mean ± SD) |
|---|---|---|
| November | 27.72 ± 0.35 | 80.0 ± 0.10 |
| December | 27.74 ± 0.32 | 73.3 ± 0.05 |
| January | 27.71 ± 0.42 | 78.6 ± 0.20 |
| February | 28.42 ± 0.33 | 81.2 ± 0.15 |
| March | 28.70 ± 0.88 | 82.4 ± 0.31 |
Abbreviation: SD, standard deviation.
3.2. Abundance of immature Ae. aegypti in three different habitat types
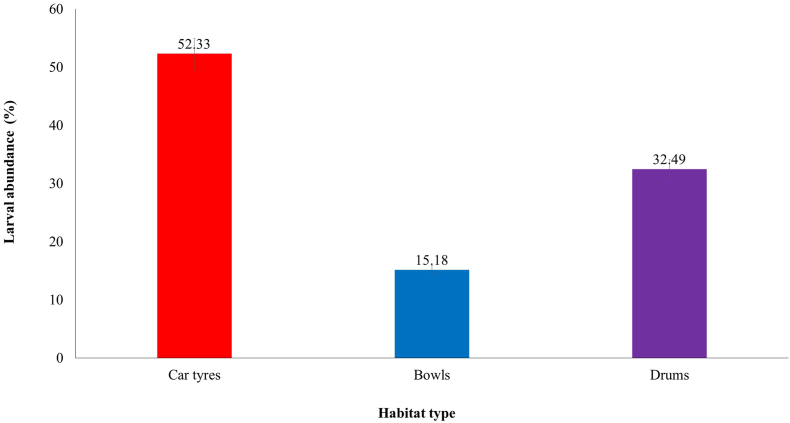
A total of 4059 immature Ae. aegypti mosquitoes were collected during the entire sampling period. The highest numbers of immatures were found in used tyres (2124/4059; 52.33%) as compared to that of drums (1319/4059; 32.49%). Bowls showed the lowest abundance of Aedes immatures (616/4059; 15.18%) (Fig. 1). There was a significant difference in the abundance of immatures in the three habitat types (ANOVA, F(2,159) = 18.79, P < 0.001). Pairwise post-hoc Tuckeyʼs tests conducted showed that all group comparisons were statistically significant (P < 0.05).
Fig. 1.
Abundance of immature Aedes aegypti collected from different habitat types (tyres, bowls and drums). Error bars represent the 95% confidence intervals.
3.3. Development time of immature stages of Ae. aegypti
The mean larval to pupal development time for immature Ae. aegypti mosquitoes was lower in tyres, 7 days as compared to bowls (9 days) and drums (12.7 days) (Kruskal-Wallis test, H = 7.448, df = 2, P = 0.024). A pairwise post-hoc Dunn test with Bonferroni adjustments showed that there was a significant difference in the mean larval to pupal development time between tyres and drums (P = 0.009) but no significant difference between tyres and bowls (P = 0.258). Furthermore, there was no significant difference in the mean larval to adult development time in males from the three habitat types (Kruskal-Wallis test, H = 5.728, df = 2, P = 0.06). There was a significant difference in the mean larval to adult development time of female Ae. aegypti mosquitoes (Kruskal-Wallis test, H = 6.054, df = 2, P = 0.048) (Table 2).
Table 2.
Larval development time of immature Aedes aegypti mosquitoes in different habitat types.
| Habitat type | Larval-pupal development time (days) | Development time of males (days) | Development time of females (days) |
|---|---|---|---|
| Tyres | 7.0 ± 1.0A | 8.0 ± 1.0A | 8.6 ± 0.5A |
| Bowls | 9.0 ± 0.0A | 8.3 ± 0.5A | 8.3 ± 0.5A |
| Drums | 12.6 ± 1.5B | 12.0 ± 1.0B | 14.3 ± 1.1B |
Notes: Values are means ± standard deviations. The superscript capital letters following the numerical values indicate the results of multiple comparison tests; values with the same letter were not statistically significant at P < 0.05 and those with different letters were statistically significant at P < 0.05.
3.4. Pupation rate, emergence and survivorship of immature Ae. aegypti in the different habitat types
A higher proportion of larvae pupated in bowls (0.92), compared to that of tyres (0.88) and drums (0.75) but the observed differences were not significant (Kruskal-Wallis test, H = 2.667, df = 2, P = 0.263). Out of the proportion of larvae that pupated, those that emerged as adults were 0.84 in tyres, 0.8 in drums and 0.77 in bowls (ANOVA, F(2,6) = 0.38, P = 0.697). The proportion of first-instar larvae that survived to adult stage was greater in tyres (0.84 ± 0.10) than in bowls (0.72 ± 0.20) and drums (0.62 ± 0.2). However, the differences in survivorship were not significant across the three habitat types (Kruskal-Wallis test, H = 2.822, df = 2, P = 0.238) (Table 3).
Table 3.
Pupation rate and emergence rate of immature Aedes aegypti mosquitoes in different habitat types.
| Habitat type | Total no. of larvae | Pupation (Mean ± SD) | Emergence (Mean ± SD) | Survivorship (Mean ± SD) |
|---|---|---|---|---|
| Tyres | 90 | 0.88 ± 0.02 | 0.84 ± 0.10 | 0.84 ± 0.1A |
| Bowls | 90 | 0.92 ± 0.17 | 0.77 ± 0.70 | 0.72 ± 0.2A |
| Drums | 90 | 0.75 ± 0.22 | 0.80 ± 0.12 | 0.62 ± 0.2B |
Notes: Values are means ± standard deviations (SD). The differences in the proportion of larvae that pupated and emerged between the three habitat types, tyres, bowls and drums were not statistically significant (P > 0.05). The superscript capital letters following the numerical values indicate the results of multiple comparison tests; values with the same letter were not statistically significant at P < 0.05 and those with different letters were statistically significant at P < 0.05.
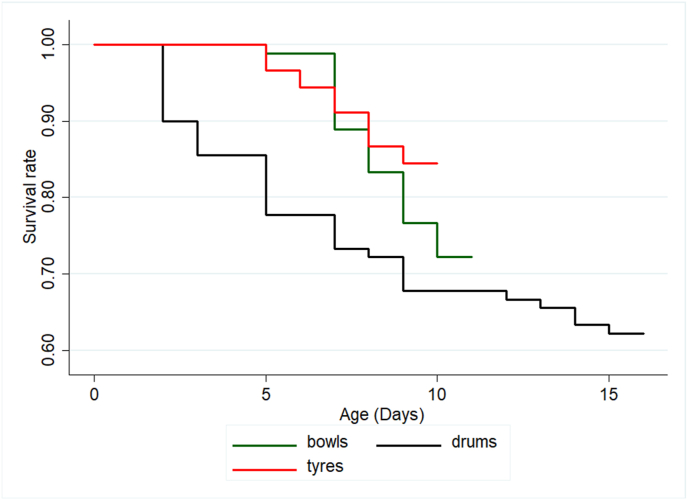
Fig. 2 shows the Kaplan-Meier survivorship dynamics of Ae. aegypti in the three habitat types. The survival rate of immatures was higher in immature Ae. aegypti larvae in tyres compared to those in bowls and drums. No mortalities were observed after 9 days in car tyres, 10 days in bowls, and 15 days in drums. The log-rank test showed that all the survival curves were significantly different (P < 0.05 for all comparisons).
Fig. 2.
Kaplan-Meier survival curves for Aedes aegypti larvae in the different habitat types (tyres, bowls and drums).
3.5. Sex ratio of immature Ae. aegypti mosquitoes in different habitat types
The proportion of males that emerged was higher in tyres (41.67%) and bowls (40.83%) compared to that of drums (17.50%). The proportion of emerged females was higher in drums (42.17%) compared to that of car tyres (31.33%) and bowls (26.51%) (Table 4). There was a significant difference between habitat type and the sex of emerged adults (χ2 = 15.1045, df = 2, P = 0.001). Sex ratio was male-biased in tyres (males: 65.79%; females: 34.21%) and bowls (males: 69%; females: 31%). However, in drums, more females emerged (62.5%) compared to males (37.5%).
Table 4.
The number of males and females that emerged from the different habitat types.
| Habitat type | Sample size | Males n (%) | Females n (%) | Sex ratio (Females: Males) |
|---|---|---|---|---|
| Tyres | 76 | 50 (41.67) | 26 (31.33) | 1:1.8A |
| Bowls | 71 | 49 (40.83) | 22 (26.51) | 1:1.9A |
| Drums | 56 | 21 (17.50) | 35 (42.17) | 1:0.6B |
| Total | 203 | 120 (100) | 83 (100) | 1:1.3 |
Notes: Frequencies are indicated by “n”. The superscript capital letters following the numerical values indicate the results of multiple comparison tests; values with the same letter in the sex ratio column were not statistically significant at P < 0.05 and those with different letters in the same column were statistically significant at P < 0.05.
3.6. Relationship between larval abundance, larval life-table parameters and environmental variables
Generalized Linear Mixed Model (GLMM) analysis showed that temperature and humidity had a significantly negative relationship with larval abundance in the oviposition experiments (beta-values: temperature = −56.30, humidity = −15.96, P < 0.05). In tyres and drums, temperature had a significantly negative influence on larval abundance (P < 0.05) (Supplementary Table S1). There was no significant relationship between temperature, humidity and replication on larval life-table parameters of Ae. aegypti mosquitoes (P > 0.05) (Supplementary Table S2).
4. Discussion
Understanding the ecology and biology of Aedes spp. mosquitoes is crucial for the control of Aedes-borne diseases. Development times and survivorship of various stages of mosquitoes under different environments are of particular importance, as they affect the vectorial capacity, which is tightly linked to mosquito-borne disease transmission (David et al., 2018). This study provides evidence of the oviposition preferences and larval life history traits of Ae. aegypti in different habitat types in Accra, Ghana. The findings from our study showed high larval abundance in tyres compared to that of bowls and drums. Aedes aegypti larvae from tyres showed a significantly shorter development time and a high survivorship compared to the other habitat types.
The immature abundance of Ae. aegypti was used as a proxy for oviposition preference in the three different habitat types studied. The highest larval abundance was observed in tyres, suggesting that Ghanaian Ae. aegypti mosquitoes prefer to breed in tyres compared to other habitat types. This finding is in line with that of Owusu-Asenso et al. (2022) where the highest densities of Ae. aegypti larvae were registered in car tyres (Owusu-Asenso et al., 2022). Car tyres are among the most productive aquatic habitats across West Africa (Ouattara et al., 2019; Tedjou et al., 2020; Egid et al., 2022). This may be because discarded car tyres are less prone to disturbance as compared to other habitat types such as containers, tin cans or coconut shells. Also, the internal conditions in car tyres such as reduced light and low humidity attract gravid female Aedes spp. mosquitoes (Dom et al., 2013). Car tyres have a narrow opening thus providing some level of shade to the immature larvae and reducing the amount of light entering the habitat (Egid et al., 2022). High mosquito densities have been associated with habitats that have some level of shading (Yee et al., 2012).
Thus, car tyres may be targeted for larval source management in the control of Aedes spp. Although car tyres are the most productive habitat type, discarded containers are also a common source of aquatic habitats. Drums were the second most abundant habitat in our study. A risk factor for the presence of Aedes spp. is the storage of water in drums for drinking or domestic use. A study from Cape Coast, Ghana found more storage containers to be infested with Ae. aegypti larvae in areas with water storage compared to areas with adequate access to piped water (Kudom, 2020).
This study observed that the mean development time was significantly longer in the larvae of Ae. aegypti from drums compared to that from tyres. The mean development time was similar between tyres and bowls and shorter as compared to that of drums. The short mean development time of Aedes spp. mosquito larvae has important epidemiological implications. Rapid larval development favours higher vector densities, which may increase disease transmission. This is because the longer development time will expose the larvae to predation and loss of habitat through desiccation (Egid et al., 2022). This negatively impacts the vectorial capacity of the vectors (Rivero et al., 2010).
There were no significant differences in the emergence of Ae. aegypti mosquitoes across the three habitat types. However, the proportion of emerging adults was higher in car tyres and drums compared to bowls. Larval survivorship was higher in car tyres coupled with high pupation and emergence rates. These findings suggest that car tyres may be responsible for the high densities of adult Ae. aegypti mosquitoes, which may facilitate arboviral transmission in Accra, Ghana. Car tyres are known to contain soluble chemicals and metals such as barium, cadmium and zinc, that gradually leach into the larval habitat water and have negative effects on larval survival of Aedes spp. (Wik and Dave, 2009; Villena et al., 2017). However, high densities of Aedes spp. larvae were associated with car tyres in our study and other studies (Ouattara et al., 2019; Tedjou et al., 2020; Owusu-Asenso et al., 2022). This suggests that local Ae. aegypti populations may have developed tolerance mechanisms to the compounds that leach from the tyres and hence further studies are needed.
Significant male bias was observed in the emerged adults from car tyres and bowls. Sex-specific responses such as responses to temperature and humidity have been associated with sex ratio imbalances (Lounibos and Escher, 2008). Male bias in Aedes spp. mosquitoes was found to be likely associated with year-to-year variations in temperatures (Lounibos and Escher, 2008). Another factor that has been associated with sex ratio differences is larval diet. Larvae of Ae. aegypti used for our larval life-table experiments were not provided any diet. Some studies have reported that Ae. aegypti larvae reared with less food produce more males whereas the sex ratio was female-skewed when the larvae were fed with more food (Puggioli et al., 2017; Gunathilaka et al., 2018). This may explain the high male bias observed in the tyres and bowls. However, in drums, more females emerged as adults than males, which may suggest that other factors may also be involved in the sex ratio differences observed.
Environmental conditions such as temperature and relative humidity affect the larval ecology of mosquitoes. The environmental temperature alters mosquito population dynamics by affecting the development of immature stages (Couret and Benedict, 2014). GLMM results from this study showed that temperature and relative humidity had a negative association with larval abundance. This suggests that higher environmental temperatures (above 32 °C) may decrease the survival and development of mosquitoes. Similar findings have been observed in other studies (Christiansen-Jucht et al., 2014; Yang et al., 2020). Aedes aegypti can develop at temperatures as low as 16 °C, with an upper limit of 34 °C. However, development time has been found to be shorter at higher temperatures (30 °C vs 21 °C) (Couret and Benedict, 2014).
Our study had several limitations. The experiments were conducted in a controlled environment thus this study did not take into account potential biological factors such as predation and competition, which may affect survivorship. The larvae were not provided any diet hence its effect on larval survival and productivity cannot be ascertained. Previous studies reported that the size of Ae. aegypti is dependent on variations in food and population density of the immature stages (Jirakanjanakit et al., 2007). Larvae of Ae. aegypti are recognized for their high plasticity, demonstrating the ability to undergo development under different microorganism-based diets (Souza et al., 2019). Therefore, bacteria that are found in the rainwater used may have provided a source of feed to the larvae. Lastly, the number of replicates used was small, hence the results may not be generalized. Therefore, further studies can be conducted with a higher number of replicates to provide stronger conclusions on Ae. aegypti larval ecology.
5. Conclusions
This study showed that car tyres are the preferred choice for oviposition for gravid female Ae. aegypti mosquitoes. Larvae of Ae. aegypti from car tyres showed a shorter larval development time and higher survivorship compared to other habitat types. This suggests that car tyres may be playing a significant role in the ecology of Ae. aegypti mosquitoes, thus facilitating arboviral transmission. This study provides baseline information that is essential for wider studies towards a better understanding of the ecology of Ae. aegypti to develop appropriate strategies for their control in Ghana.
Funding
This study was supported by grants from the National Institute of Health (D43 TW 011513).
Ethical approval
Not applicable.
CRediT authorship contribution statement
Anisa Abdulai: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Christopher Mfum Owusu-Asenso: Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. Christodea Haizel: Investigation, Writing – review & editing. Sebastian Kow Egyin Mensah: Investigation, Writing – review & editing. Isaac Kwame Sraku: Investigation, Writing – review & editing. Daniel Halou: Investigation, Writing – review & editing. Richard Tettey Doe: Investigation, Writing – review & editing. Abdul Rahim Mohammed: Investigation, Writing – review & editing. Yaw Akuamoah-Boateng: Investigation, Writing – review & editing. Akua Obeng Forson: Conceptualization, Data curation, Supervision, Writing – review & editing. Yaw Asare Afrane: Conceptualization, Data curation, Methodology, Funding acquisition, Supervision, Project administration, Resources, Writing – review & editing. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the residents of the study sites for their support during our study. Our sincere gratitude goes to all the staff of the animal house unit of the Department of Medical Microbiology, University of Ghana for their field assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crpvbd.2024.100176.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
The data supporting the conclusions of this article are included within the article and its supplementary files. All datasets generated and/or analysed during this study are available on request.
References
- Abdulai A., Owusu-Asenso C.M., Akosah-Brempong G., Mohammed A.R., Sraku K.I., Attah S., et al. Insecticide resistance status of Aedes aegypti in southern and northern Ghana. Parasites Vectors. 2023;16:135. doi: 10.1186/s13071-023-05752-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetoro F.A., Anikwe J.C., Makanjuola W.A., Omotayo A.I., Awolola S.T. Comparative evaluation of larvicides for larval source management of mosquitoes in Lagos, Nigeria. Egypt. Acad. J. Biol. Sci. A Entomol. 2022;15:33–46. [Google Scholar]
- Adogo L.Y., Ogoh M.O. Yellow fever in Nigeria: A review of the current situation. Afr. J. Clin. Exp. Microbiol. 2020;21:1–13. doi: 10.4314/ajcem.v21i1.1. [DOI] [Google Scholar]
- Afrane Y.A., Little T.J., Lawson B.W., Githeko A.K., Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerg. Infect. Dis. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoako N., Duodu S., Dennis F.E., Bonney J.H.K., Asante K.P., Ameh J., et al. Detection of dengue virus among children with suspected malaria, Accra, Ghana. Emerg. Infect. Dis. 2018;24:1544–1547. doi: 10.3201/eid2408.180341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolo A., Sombié A., Pignatelli P.M., Sanon A., Yaméogo F., Wangrawa D.W., et al. Insecticide resistance levels and mechanisms in Aedes aegypti populations in and around Ouagadougou, Burkina Faso. PLoS Negl. Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badolo A., Sombié A., Yaméogo F., Wangrawa D.W., Sanon A., Pignatelli P.M., et al. First comprehensive analysis of Aedes aegypti bionomics during an arbovirus outbreak in west Africa: Dengue in Ouagadougou, Burkina Faso, 2016–2017. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein L., Kiflawi M., Eitam A., Mangel M., Cohen J.E. Oviposition habitat selection in response to risk of predation in temporary pools: Mode of detection and consistency across experimental venue. Oecologia. 2004;138:300–305. doi: 10.1007/s00442-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Bong L.J., Tu W.C., Neoh K.B. Interpopulation variations in life history traits and reproductive tactics in Aedes aegypti: A test on populations 50 km apart. Acta Trop. 2021;213 doi: 10.1016/j.actatropica.2020.105750. [DOI] [PubMed] [Google Scholar]
- Brown J.E., McBride C.S., Johnson P., Ritchie S., Paupy C., Bossin H., et al. Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proc. R. Soc. B Biol. Sci. 2011;278:2446–2454. doi: 10.1098/rspb.2010.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Captain-Esoah M., Kweku Baidoo P., Frempong K.K., Adabie-Gomez D., Chabi J., Obuobi D., et al. Biting behavior and molecular identification of Aedes aegypti (Diptera: Culicidae) subspecies in some selected recent yellow fever outbreak communities in Northern Ghana. J. Med. Entomol. 2020;57:1239–1245. doi: 10.1093/jme/tjaa024. [DOI] [PubMed] [Google Scholar]
- Christiansen-Jucht C., Parham P.E., Saddler A., Koella J.C., Basáñez M.-G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasites Vectors. 2014;7:489. doi: 10.1186/s13071-014-0489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couret J., Benedict M.Q. A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae) BMC Ecol. 2014;14:3. doi: 10.1186/1472-6785-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J.E., Alves J.M., Palmer W.J., Day J.P., Sylla M., Ramasamy R., et al. Population genomics reveals that an anthropophilic population of Aedes aegypti mosquitoes in West Africa recently gave rise to American and Asian populations of this major disease vector. BMC Biol. 2017;15:16. doi: 10.1186/s12915-017-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G., Zhong S., Zheng T., Li Z., Zhang X., Li C., et al. Aedes albopictus life table: Environment, food, and age dependence survivorship and reproduction in a tropical area. Parasites Vectors. 2021;14:568. doi: 10.1186/s13071-021-05081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David M.R., Garcia G.A., Valle D., Maciel-de-Freitas R. Insecticide resistance and fitness: the case of four Aedes aegypti populations from different Brazilian regions. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/6257860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dom N.C., Ahmad A.H., Ismail R. Habitat characterization of Aedes sp. breeding in urban hotspot area. Procedia Social Behav. Sci. 2013;85:100–109. [Google Scholar]
- ECMWF Reanalysis v5 ERA5 hourly data on single levels from 1940 to present. 2018. https://cds.climate.copernicus.eu/cdsapp#!/dataset/reanalysis-era5-single-levels?tab=overview
- Egid B.R., Coulibaly M., Dadzie S.K., Kamgang B., McCall P.J., Sedda L., et al. Review of the ecology and behaviour of Aedes aegypti and Aedes albopictus in Western Africa and implications for vector control. Curr. Res. Parasitol. Vector Borne Dis. 2022;2 doi: 10.1016/j.crpvbd.2021.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunathilaka P.A., Uduwawala U.M., Udayanga N.W., Ranathunge R.M., Amarasinghe L.D., Abeyewickreme W. Determination of the efficiency of diets for larval development in mass rearing Aedes aegypti (Diptera: Culicidae) Bull. Entomol. Res. 2018;108:583–592. doi: 10.1017/S0007485317001092. [DOI] [PubMed] [Google Scholar]
- Jirakanjanakit N., Leemingsawat S., Thongrungkiat S., Apiwathnasorn C., Singhaniyom S., Bellec C., Dujardin J.P. Influence of larval density or food variation on the geometry of the wing of Aedes (Stegomyia) aegypti. Trop. Med. Int. Health. 2007;12:1354–1360. doi: 10.1111/j.1365-3156.2007.01919.x. [DOI] [PubMed] [Google Scholar]
- Kamgang B., Ngoagouni C., Manirakiza A., Nakouné E., Paupy C., Kazanji M. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl. Trop. Dis. 2013;7 doi: 10.1371/journal.pntd.0002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampango A., Furu P., Sarath D.L., Haji K.A., Konradsen F., Schiøler K.L., et al. Risk factors for occurrence and abundance of Aedes aegypti and Aedes bromeliae at hotel compounds in Zanzibar. Parasites Vectors. 2021;14:544. doi: 10.1186/s13071-021-05005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konan L.Y., Oumbouke W.A., Silué U.G., Coulibaly I.Z., Ziogba J.-C.T., N'Guessan R.K., et al. Insecticide resistance patterns and mechanisms in Aedes aegypti (Diptera: Culicidae) populations across Abidjan, Côte d'Ivoire, reveal emergent pyrethroid resistance. J. Med. Entomol. 2021;58:1808–1816. doi: 10.1093/jme/tjab045. [DOI] [PubMed] [Google Scholar]
- Kudom A.A. Entomological surveillance to assess potential outbreak of Aedes-borne arboviruses and insecticide resistance status of Aedes aegypti from Cape Coast, Ghana. Acta Trop. 2020;202 doi: 10.1016/j.actatropica.2019.105257. [DOI] [PubMed] [Google Scholar]
- Kwame Amlalo G., Akorli J., Etornam Akyea-Bobi N., Sowa Akporh S., Aqua-Baidoo D., Opoku M., et al. Evidence of high frequencies of insecticide resistance mutations in Aedes aegypti (Culicidae) mosquitoes in Urban Accra, Ghana: Implications for insecticide-based vector control of Aedes-borne arboviral diseases. J. Med. Entomol. 2022;59:2090–2101. doi: 10.1093/jme/tjac120. [DOI] [PubMed] [Google Scholar]
- Kweka E., Baraka V., Mathias L., Mwang’onde B., Baraka G., Lyaruu L., Mahande A.M. In: Dengue fever - a resilient threat in the face of innovation. Falcón-Lezama J.A., Betancourt-Cravioto M., Tapia-Conyer R., editors. IntechOpen; London, UK: 2018. Ecology of Aedes mosquitoes, the major vectors of arboviruses in human population. [DOI] [Google Scholar]
- Li Y., Kamara F., Zhou G., Puthiyakunnon S., Li C., Liu Y., et al. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L.P., Escher R.L. Sex ratios of mosquitoes from long-term censuses of Florida tree holes. J. Am. Mosq. Control Assoc. 2008;24:11–15. doi: 10.2987/5656.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R., Codeço C.T., Lourenço-De-Oliveira R. Body size-associated survival and dispersal rates of Aedes aegypti in Rio de Janeiro. Med. Vet. Entomol. 2007;21:284–292. doi: 10.1111/j.1365-2915.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Ouattara L.P.E., Sangaré I., Namountougou M., Hien A., Ouari A., Soma D.D., et al. Surveys of arboviruses vectors in four cities stretching along a railway transect of Burkina Faso: Risk transmission and insecticide susceptibility status of potential vectors. Front. Vet. Sci. 2019;6:140. doi: 10.3389/fvets.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Asenso C.M., Mingle J.A.A., Weetman D., Afrane Y.A. Spatiotemporal distribution and insecticide resistance status of Aedes aegypti in Ghana. Parasites Vectors. 2022;15:61. doi: 10.1186/s13071-022-05179-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell J.R., Tabachnick W.J. History of domestication and spread of Aedes aegypti - a review. Mem. Inst. Oswaldo Cruz. 2013;108(Suppl. l.):11–17. doi: 10.1590/0074-0276130395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puggioli A., Carrieri M., Dindo M.L., Medici A., Lees R.S., Gilles J.R., Bellini R. Development of Aedes albopictus (Diptera: Culicidae) larvae under different laboratory conditions. J. Med. Entomol. 2017;54:142–149. doi: 10.1093/jme/tjw127. [DOI] [PubMed] [Google Scholar]
- Rivero A., Vezilier J., Weill M., Read A.F., Gandon S. Insecticide control of vector-borne diseases: When is insecticide resistance a problem? PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard F., Nchoutpouen E., Toto J.C., Fontenille D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005;42:726–731. doi: 10.1093/jmedent/42.5.726. [DOI] [PubMed] [Google Scholar]
- Souza R.S., Virginio F., Riback T.I.S., Suesdek L., Barufi J.B., Genta F.A. Microorganism-based larval diets affect mosquito development, size and nutritional reserves in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae) Front. Physiol. 2019;10:152. doi: 10.3389/fphys.2019.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer C.Y., Pendergast T.H., Harrington L.C. Fructose variation in the dengue vector, Aedes aegypti, during high and low transmission seasons in the Mae Sot region of Thailand. J. Am. Mosq. Control Assoc. 2005;21:177–181. doi: 10.2987/8756-971X(2005)21[177:FVITDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Kutsuna S., Taniguchi S., Tajima S., Maeki T., Kato F., et al. Dengue virus exported from Côte d'Ivoire to Japan, June 2017. Emerg. Infect. Dis. 2017;23:1758. doi: 10.3201/eid2310.171132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnagda Z., Cissé A., Bicaba B.W., Diagbouga S., Sagna T., Ilboudo A.K., et al. Dengue fever in Burkina Faso, 2016. Emerg. Infect. Dis. 2018;24:170. doi: 10.3201/eid2401.170973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedjou A.N., Kamgang B., Yougang A.P., Wilson-Bahun T.A., Njiokou F., Wondji C.S. Patterns of ecological adaptation of Aedes aegypti and Aedes albopictus and Stegomyia indices highlight the potential risk of arbovirus transmission in Yaoundé, the Capital City of Cameroon. Pathogens. 2020;9:491. doi: 10.3390/pathogens9060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toé H.K., Zongo S., Guelbeogo M.W., Kamgang B., Viana M., Tapsoba M., et al. Multiple insecticide resistance and first evidence of V410L kdr mutation in Aedes (Stegomyia) aegypti (Linnaeus) from Burkina Faso. Med. Vet. Entomol. 2022;36:309–319. doi: 10.1111/mve.12602. [DOI] [PubMed] [Google Scholar]
- Villena O.C., Terry I., Iwata K., Landa E.R., LaDeau S.L., Leisnham P.T. Effects of tire leachate on the invasive mosquito Aedes albopictus and the native congener Aedes triseriatus. PeerJ. 2017;5 doi: 10.7717/peerj.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wat’senga Tezzo F., Fasine S., Manzambi Zola E., Marquetti M.D.C., Binene Mbuka G., Ilombe G., et al. High Aedes spp. larval indices in Kinshasa, Democratic Republic of Congo. Parasites Vectors. 2021;14:92. doi: 10.1186/s13071-021-04588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeratunga P., Rodrigo C., Fernando S.D., Rajapakse S. Control methods for Aedes albopictus and Aedes aegypti. Cochrane Database Syst. Rev. 2017;2017 [Google Scholar]
- Weetman D., Kamgang B., Badolo A., Moyes C.L., Shearer F.M., Coulibaly M., et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Publ. Health. 2018;15:220. doi: 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2013. Larval source management: A supplementary measure for malaria vector control - an operational manual.https://www.who.int/publications/i/item/9789241505604 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2012. Global strategy for dengue prevention and control, 2012–2020.https://www.who.int/publications/i/item/9789241504034 [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2021. Yellow fever - West and Central Africa.https://www.who.int/emergencies/disease-outbreak-news/item/yellow-fever---west-and-central-africa [Google Scholar]
- Wik A., Dave G. Occurrence and effects of tire wear particles in the environment - a critical review and an initial risk assessment. Environ. Pollut. 2009;157:1–11. doi: 10.1016/j.envpol.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Wong J., Stoddard S.T., Astete H., Morrison A.C., Scott T.W. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl. Trop. Dis. 2011;5 doi: 10.1371/journal.pntd.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., He Y., Ni W., Lai Q., Yang Y., Xie J., et al. Semi-field life-table studies of Aedes albopictus (Diptera: Culicidae) in Guangzhou, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0229829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee D.A., Allgood D., Kneitel J.M., Kuehn K.A. Constitutive differences between natural and artificial container mosquito habitats: Vector communities, resources, microorganisms, and habitat parameters. J. Med. Entomol. 2012;49:482–491. doi: 10.1603/me11227. [DOI] [PubMed] [Google Scholar]
- Zahouli J.B.Z., Utzinger J., Adja M.A., Müller P., Malone D., Tano Y., Koudou B.G. Oviposition ecology and species composition of Aedes spp. and Aedes aegypti dynamics in variously urbanized settings in arbovirus foci in southeastern Côte d'Ivoire. Parasites Vectors. 2016;9:523. doi: 10.1186/s13071-016-1778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuharah W.F., Fadzly N., Wei W.O.K., Hashim Z.H. Oviposition habitat selection of dengue vectors, Aedes aegypti and Aedes albopictus in response to fish predator. Trop. Life Sci. Res. 2016;27:117. doi: 10.21315/tlsr2016.27.3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the conclusions of this article are included within the article and its supplementary files. All datasets generated and/or analysed during this study are available on request.