Abstract
BACKGROUND:
Lung adenocarcinoma (LUAD) is a major histological subtype of lung cancer with a high mortality rate worldwide. Heat shock protein family D member 1 (HSPD1, also known as HSP60) is reported to be increased in tumor tissues of lung cancer patients compared with healthy control tissues.
OBJECTIVE:
We aimed to investigate the roles of HSPD1 in prognosis, carcinogenesis, and immune infiltration in LUAD using an integrative bioinformatic analysis.
METHODS:
HSPD1 expression in LUAD was investigated in several transcriptome-based and protein databases. Survival analysis was performed using the KM plotter and OSluca databases, while prognostic significance was independently confirmed through univariate and multivariate analyses. Integrative gene interaction network and enrichment analyses of HSPD1-correlated genes were performed to investigate the roles of HSPD1 in LUAD carcinogenesis. TIMER and TISIDB were used to analyze correlation between HSPD1 expression and immune cell infiltration.
RESULTS:
The mRNA and protein expressions of HSPD1 were higher in LUAD compared with normal tissues. High HSPD1 expression was associated with male gender and LUAD with advanced stages. High HSPD1 expression was an independent prognostic factor associated with poor survival in LUAD patients. HSPD1-correlated genes with prognostic impact were mainly involved in aberrant ribosome biogenesis, while LUAD patients with high HSPD1 expression had low tumor infiltrations of activated and immature B cells and CD4+ T cells.
CONCLUSIONS:
HSPD1 may play a role in the regulation of ribosome biogenesis and B cell-mediated immunity in LUAD. It could serve as a predictive biomarker for prognosis and immunotherapy response in LUAD.
Keywords: Lung adenocarcinoma, HSPD1, ribosome biogenesis, immune infiltration, biomarker, prognosis
1. Introduction
Lung cancer is one of the most common cancers with a high mortality rate worldwide [1]. The majority of lung cancer is non-small cell lung cancer (NSCLC), in which lung adenocarcinoma (LUAD) is the most common subtype [2]. Although treatment options are currently available, LUAD patients still have a poor prognosis and low survival rate [2, 3]. Moreover, the effectiveness of chemotherapeutic and targeted drugs is often limited by the drug resistance [3, 4]. Therefore, identifying new prognostic markers and therapeutic targets for LUAD is necessary.
Tumor-infiltrating immune cells (TIICs) are one of the key components in the tumor microenvironment (TME) that influence tumor progression and aggressiveness [5]. Previous studies have shown that TIICs are related to clinical outcomes and prognosis of LUAD patients [6, 7, 8]. The composition of TIICs also affects patients’ response to immunotherapy [9, 10, 11]. Hence, TIICs are promising targets for predicting the prognosis and immune response in LUAD patients.
Heat shock protein family D member 1 (HSPD1), also known as HSP60, is a molecular chaperone that participates in various cellular functions. It is present in different cellular compartments and implicated in both intracellular and extracellular processes [12, 13, 14, 15]. Accumulating evidence has highlighted the critical role of HSPD1 in cancer development, progression, and chemoresistance across various types of cancer [16, 17]. HSPD1 has been shown to exert its oncogenic functions through several mechanisms, such as p53, Erk1/2, -catenin, and caspase 3 pathways [18, 19, 20, 21, 22]. Furthermore, apart from its intracellular roles, extracellular HSPD1 acts as a mediator in cell-cell communication, immune regulation, and TME modulation [14, 15]. Due to its diverse roles, HSPD1 has emerged as an interesting target for diagnosis and therapy in various cancers [16].
Previous studies have reported a correlation between HSPD1 overexpression and poor prognosis in NSCLC patients [25, 26]. HSPD1 has been shown to promote NSCLC development through modulation of mitochondrial metabolisms [27]. Additionally, extracellular HSPD1 can induce an inflammatory response in bronchial epithelial cells [28] and modulate the effector functions of immune cells, such as macrophages and neutrophils [23, 24]. The involvement of HSPD1 in regulating these critical aspects of tumor biology suggests its potential as a prognostic marker in LUAD. A deeper understanding of the precise mechanisms by which HSPD1 modulates these processes could provide valuable insights into LUAD pathogenesis and potentially reveal therapeutic targets.
In this study, we analyzed HSPD1 expression and its correlation with clinicopathologic features in LUAD patients using various databases, including TIMER, GEPIA2, HPA, and UALCAN. Prognostic value of HSPD1 expression in LUAD was investigated by KM plotter and OSluca. Survival prognosis, interaction network, and enrichment analyses of HSPD1-correlated genes in LUAD were performed using GEPIA2, GeneMANIA, and DAVID, respectively. In addition, association between HSPD1 expression and immune cell infiltration was also explored with TIMER and TISIDB. Our findings provide new insights into the role of HSPD1 and its relationship to tumor immunity in LUAD.
2. Materials and methods
2.1. Differential expression analysis of HSPD1 in LUAD and normal tissues – mRNA level
We investigated the mRNA expression of HSPD1 in tumor and normal samples across various human cancers using Tumor Immune Estimation Resource (TIMER) database (https://cistrome.shinyapps.io/timer/) [29, 30]. The Gene Expression Profiling Interactive Analysis 2 (GEPIA2) database (http://gepia2.cancer-pku.cn/) [31] was used to analyze the mRNA expression of HSPD1 in normal ( 59) and LUAD ( 483) samples from The Cancer Genome Atlas (TCGA) dataset. The differential mRNA expression of HSPD1 was further confirmed in LUAD-TCGA dataset ( 574) through the University of ALabama at Birmingham CANcer data analysis portal (UALCAN) database (http://ualcan.path.uab.edu) [32]. A statistical difference was analyzed using one-way ANOVA and Student’s -test with unequal variance in GEPIA2 and UALCAN, respectively.
2.2. Differential expression analysis of HSPD1 in LUAD and normal tissues – protein level
Differential protein expression of HSPD1 was analyzed from normal ( 111) and LUAD ( 111) samples in the Clinical Proteomic Tumor Analysis Consortium (CPTAC) dataset through UALCAN database. Statistical comparison was made using Student’s -test with unequal variance on the database. The Human Protein Atlas (HPA) database (http://www.proteinatlas. org) [33, 34] was used to explore HSPD1 protein expression in normal lung and LUAD tissues.
2.3. Association analysis of HSPD1 expression and clinicopathologic features of LUAD patients
Relationships between HSPD1 mRNA expression and clinicopathologic features, including age, race, gender, and stages were analyzed in normal ( 59) and LUAD ( 515) samples in TCGA dataset using UALCAN database, with statistical analysis by Student’s -test assuming unequal variance.
2.4. Survival analysis
To evaluate the prognostic value of HSPD1 in LUAD, we used two different tools: Kaplan-Meier (KM) plotter database (www.kmplot.com/lung) [35] and Online consensus Survival for Lung Cancer (OSluca) database (http://bioinfo.henu.edu.cn/LUCA/LUCAList.jsp) [36] In KM plotter, the Affymetrix probe ID: 200806_s_at was used to analyze the overall survival ( 719) and first progression survival ( 461) in LUAD patients, comparing low- and high-expression groups based on the median of HSPD1 expression values. We employed OSluca to assess the association of HSPD1 expression with overall survival ( 597) and disease-specific survival ( 551) in LUAD, using the TCGA dataset and splitting patients by “upper 50%”. KM survival curves of the patients were plotted, and the log-rank -value and hazard ratio (HR) were calculated by the web tools. Additionally, we performed univariate and multivariate Cox hazard regression analyses of HSPD1 and other variables for survival in LUAD patients using TIMER.
2.5. Integrative gene interaction network and enrichment analyses of HSPD1-correlated genes
To comprehensively analyze the potential role of HSPD1 in LUAD, we retrieved the top 25 genes that are positively and negatively correlated with HSPD1 in the LUAD-TCGA dataset based on the Pearson correlation coefficient value from the UALCAN database. Survival impact of HSPD1-correlated genes on overall survival in LUAD patients was explored using GEPIA2. The GeneMANIA (https://genemania.org/) [37] was used to construct an interaction network of HSPD1-correlated genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/summary.jsp) [38, 39]. The dot plots of enriched pathways were depicted by using GraphBio online tool (http://www.graphbio1.com/en/) [40].
2.6. Analysis of correlation between HSPD1 and ribosome biogenesis marker gene expression
Correlation between HSPD1 expression and ribosome biogenesis marker genes (i.e. NHP2, H/ACA ribonucleoprotein complex subunit 2; NMD3, 60S ribosomal export protein NMD3; NOP56, Nucleolar protein 56; RCL1, RNA 3’-terminal phosphate cyclase-like protein; and TCOF1, Treacher Collins syndrome protein) expression was analyzed in LUAD-TCGA dataset using GEPIA2 with Pearson’s correlation analysis.
2.7. Analysis of correlation between HSPD1 expression and immune cell infiltration in LUAD
TIMER was used to analyze the correlation between HSPD1 expression with tumor infiltrating immune cells in LUAD, including B cells, CD8+ T cells, CD4+ T cells, neutrophils, macrophages, and dendritic cells. KM survival curves of each immune cell infiltration were plotted with the log-rank -value using the TIMER. Additionally, we confirmed the correlation between HSPD1 expression and abundance of activated B cells, immature B cells, and memory B cells using Tumor and Immune System Interaction Database (TISIDB) (http:// cis.hku.hk/TISIDB) [41] with Spearman’s correlation test.
3. Results
3.1. Expression of HSPD1 in human cancers and LUAD tumor tissues
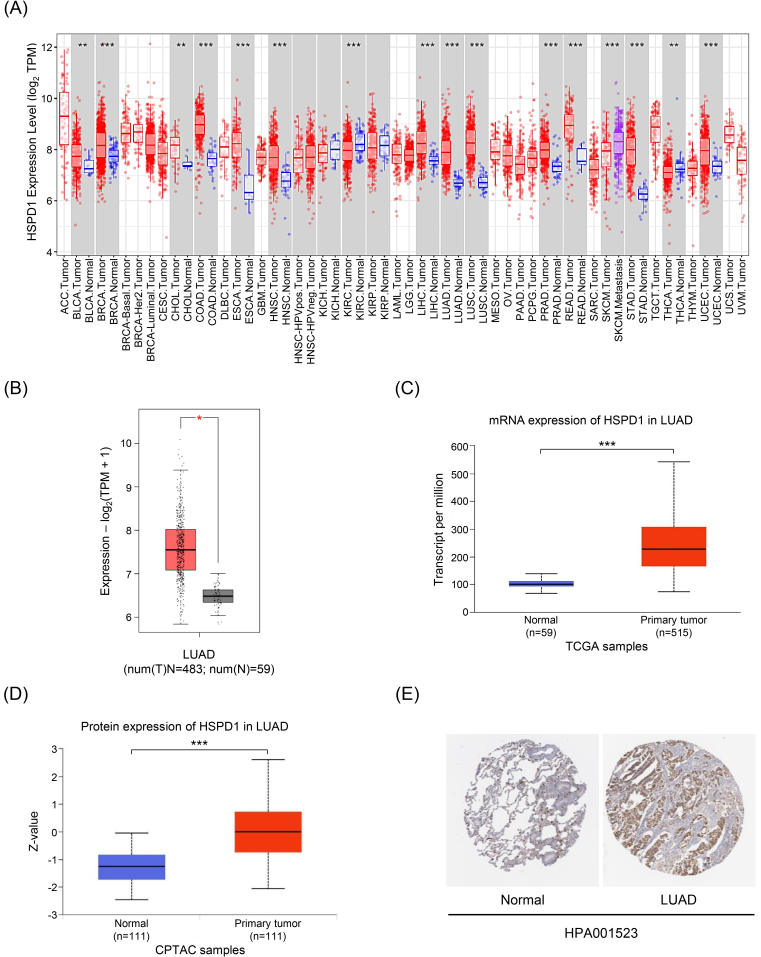
The workflow chart of this study is showed in Fig. 1. The expression level of HSPD1 in human cancers was initially investigated using TIMER database. As shown in Fig. 2A, HSPD1 mRNA expression was markedly increased in LUAD and various other types of cancers. Similarly, an elevated HSPD1 mRNA expression in LUAD tissues was found in GEPIA2 database (Fig. 2B). Furthermore, UALCAN analysis demonstrated a significant increase in HSPD1 expression in LUAD at both mRNA and protein levels (Fig. 2C and 2D). Immunohistochemistry results obtained from HPA database also showed that HSPD1 protein level was higher in LUAD compared with normal lung tissues (Fig. 2E). Consistently, HSPD1 expression is upregulated in LUAD tumor tissues at both mRNA and protein levels among multiple databases, which therefore supports further investigations on its correlation with clinical characteristics of LUAD patients.
Figure 1.
The workflow chart of this study.
Figure 2.
Expression levels of HSPD1 in human cancers and LUAD. (A) HSPD1 mRNA expression in different TCGA tumor types in TIMER; red, tumor tissues; blue, normal adjacent tissues; purple, metastatic tissues. (B) mRNA expression of HSPD1 in normal and LUAD tissues in GEPIA2. (C) mRNA expression of HSPD1 in normal and LUAD tissues from TCGA dataset in UALCAN. (D) Protein expression of HSPD1 in normal and LUAD tissues from CPTAC dataset in UALCAN. (E) Immunohistochemistry staining images of HSPD1 protein in normal lung and LUAD tissues obtained from HPA. *p < 0.05, **p < 0.01, ***p < 0.001.
3.2. Association between HSPD1 expression and clinicopathologic features of LUAD patients
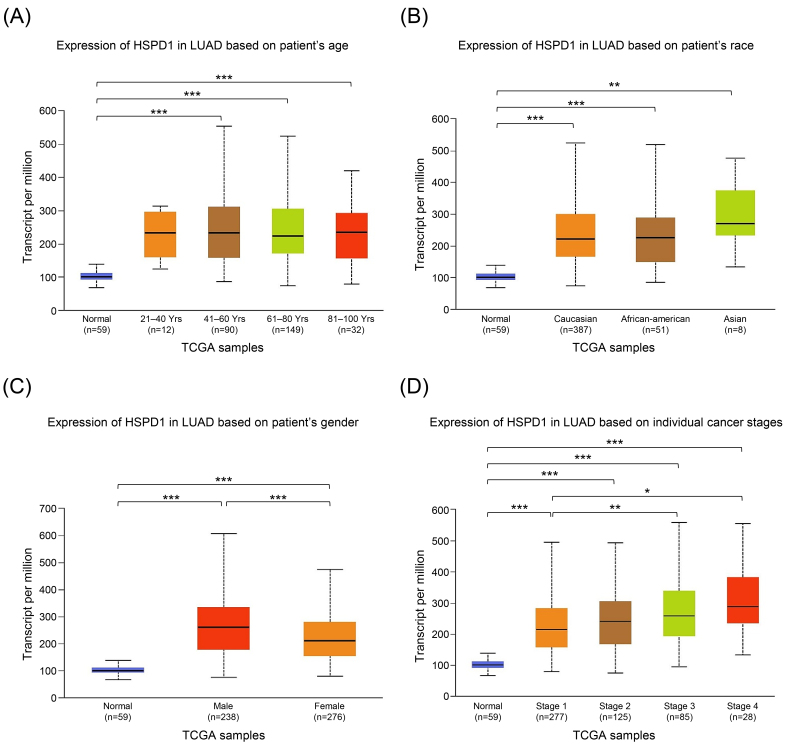
We employed UALCAN analysis to evaluate the relationship between HSPD1 mRNA expression in tumor tissues and clinicopathologic features of LUAD patients. As expected, the results showed that HSPD1 expression was significantly higher in LUAD patients compared with normal control in all subgroup analyses categorized by age, race, gender, and cancer stages (Fig. 3). While HSPD1 expression was not different among patients with various age and race groups (Fig. 3A and 3B) the significantly higher HSPD1 expression was found in male patients compared to female patients (Fig. 3C). Patients with LUAD stage 3 and stage 4 had significantly higher HSPD1 expression than those with stage 1 (Fig. 3D). This result also suggested that HSPD1 may exhibit a dose-dependent effect on the disease severity and probably determine the prognosis in LUAD patients.
Figure 3.
Relationship between HSPD1 expression and clinicopathological parameters in LUAD patients from TCGA dataset in UALCAN. HSPD1 expression in LUAD patients based on (A) age, (B) race, (C) gender, and (D) individual cancer stages. *p < 0.05, **p < 0.01, ***p < 0.001.
In this direction, we then performed univariate and multivariate Cox regression analyses of HSPD1 expression and clinicopathologic parameters for the overall survival of LUAD patients by using TIMER database. The univariate analysis showed that HSPD1 expression (HR 1.466, 0.001), stage 2 cancer (HR 2.417, 0.001), stage 3 cancer (HR 3.584, 0.001), and stage 4 cancer (HR 3.828, 0.001) were significantly associated with the higher risk of mortality in LUAD patients (Table 1). The multivariate analysis confirmed the independent prognostic value of HSPD1 expression (HR 1.382, 0.007), along with stage 2 cancer (HR 2.205, 0.001), stage 3 cancer (HR 2.715, 0.001), and stage 4 cancer (HR 2.771, 0.001) for overall survival of LUAD patients (Table 1).
Table 1.
Univariate and multivariate Cox analysis of clinicopathological parameters and HSPD1 expression for survival in LUAD patients estimated by TIMER
| Parameters | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | -value | HR (95% CI) | -value | |
| Age | 1.008 (0.992–1.024) | 0.33 | 1.008 (0.990–1.026) | 0.393 |
| Gender (Male) | 1.065 (0.796–1.424) | 0.672 | 0.956 (0.688–1.328) | 0.786 |
| Race (Black) | 2.031 (0.270–15.289) | 0.491 | 2.399 (0.313–18.364) | 0.399 |
| Race (White) | 2.788 (0.390–19.939) | 0.307 | 2.938 (0.405–21.336) | 0.287 |
| Stage 2 | 2.417 (1.68–3.476) | 0.001 | 2.205 (1.478–3.288) | 0.001 |
| Stage 3 | 3.584 (2.450–5.244) | 0.001 | 2.715 (1.796–4.104) | 0.001 |
| Stage 4 | 3.828 (2.206–6.642) | 0.001 | 2.771 (1.481–5.187) | 0.001 |
| HSPD1 | 1.466 (1.189–1.808) | 0.001 | 1.382 (1.093–1.747) | 0.007 |
HR: Hazard ratio, CI: confidence interval.
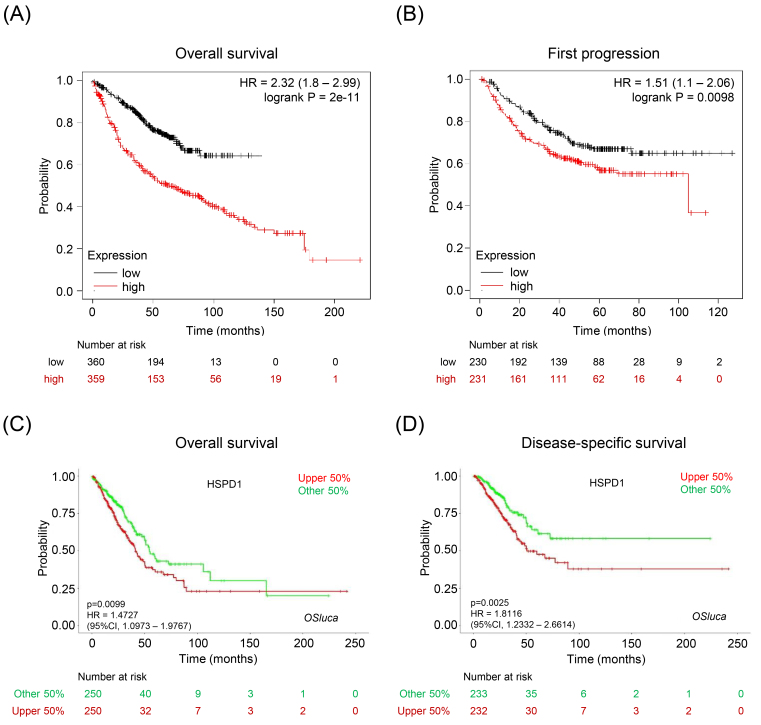
To further validate the prognostic impact of HSPD1 expression in LUAD, we performed survival analysis by using two databases. In KM plotter database, LUAD patients with high HSPD1 expression had significantly shorter overall survival (HR 2.32, Logrank 0.0001) (Fig. 4A) and time to first progression (HR 1.51, Logrank 0.0098) (Fig. 4B) than those with low HSPD1 expression. Data from OSluca also demonstrated that high HSPD1 expression was significantly associated with poor overall survival (HR 1.47, 0.0099) (Fig. 4C) and disease-specific survival (HR 1.81, 0.0025) (Fig. 4D) of LUAD patients. These cross-validation findings among multiple analyses and databases support a crucial role of HSPD1 overexpression as a key player in LUAD disease pathogenesis and progression.
Figure 4.
Relationship between HSPD1 expression and survival outcome of LUAD patients. KM survival curves for (A) overall survival and (B) first progression survival of LUAD patients in KM plotter. KM survival curves for (C) overall survival and (D) disease-specific survival of LUAD patients in OSluca.
3.3. Survival prognosis, interaction network, and enrichment analysis of HSPD1-correlated genes related to LUAD
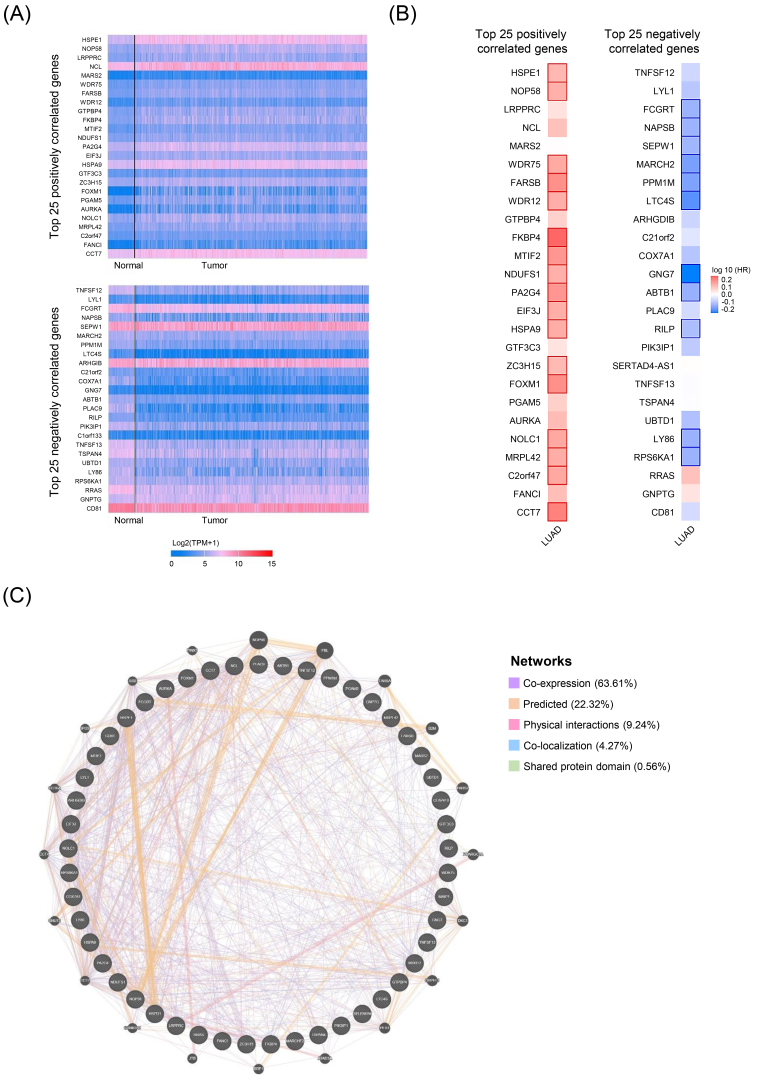
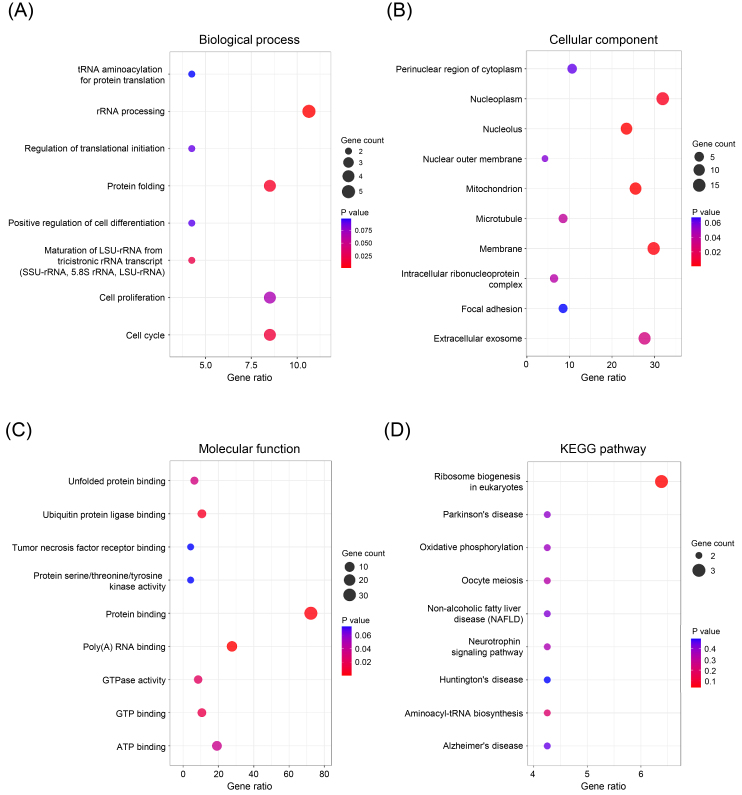
In order to investigate the potential roles of HSPD1 in LUAD carcinogenesis, top 25 genes that are positively and negatively correlated with HSPD1 expression were retrieved from UALCAN database. Expression heatmaps of HSPD1-correlated genes are represented in Fig. 5A and details of these genes are summarized in Supplementary Table S1. Then, the prognosis values of HSPD1-correlated genes in LUAD patients was explored by the survival heatmap using GEPIA2. As shown in Fig. 5B, 17 genes of the top 25 positively correlated genes had high HR ( 0.05), whereas 11 genes with low HR ( 0.05) were found among the top 25 negatively correlated genes. The interaction network data obtained from GeneMANIA showed that these HSPD1-correlated genes were connected with each other by co-expression (63.61%), predicted interaction (22.32%), physical interaction (9.24%), co-localization (4.27%), and shared protein domain (0.56%) (Fig. 5C). Furthermore, we performed GO and KEGG pathway enrichment analysis using DAVID. Based on GO terms, HSPD1-correlated genes were significantly enriched in biological processes, including “rRNA processing”, “protein folding”, “cell cycle”, and “maturation of LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA)” (Fig. 6A). Significantly enriched GO cellular components were “nucleoplasm”, “membrane”, “extracellular exosome”, “mitochondrion”, “nucleolus”, “microtubule” and “intracellular ribonucleoprotein complex” (Fig. 6B). Significantly enriched GO terms for molecular function include “protein binding”, “poly(A) RNA binding”, “ATP binding”, “ubiquitin protein ligase binding”, “GTP binding”, “GTPase activity”, and “unfolded protein binding” (Fig. 6C). Interestingly, KEGG pathway analysis demonstrated that HSPD1-correlated genes were significantly involved in “ribosome biogenesis in eukaryotes” pathway (Fig. 6D).
Figure 5.
HSPD1-correlated genes in LUAD and their interaction network. (A) Expression heatmaps of top 25 genes that are positively and negatively correlated with HSPD1 in normal and LUAD tissues visualized by UALCAN. (B) Survival heatmaps of HSPD1-correlated genes in LUAD created by GEPIA2. Heatmaps show hazard ratio for different genes in overall survival of LUAD patients. Framed blocks represent statistical significance in prognostic analysis. (C) Interaction network of HSPD1-correlated genes constructed by GeneMANIA.
Figure 6.
GO and KEGG pathway enrichment analysis of HSPD1-correlated genes in LUAD. GO terms for biological process (A), cellular component (B), and molecular function (C), and KEGG pathway (D) assigned to HSPD1-correlated genes by using DAVID online tool.
3.4. Correlation between HSPD1 and ribosome biogenesis marker genes expression in LUAD
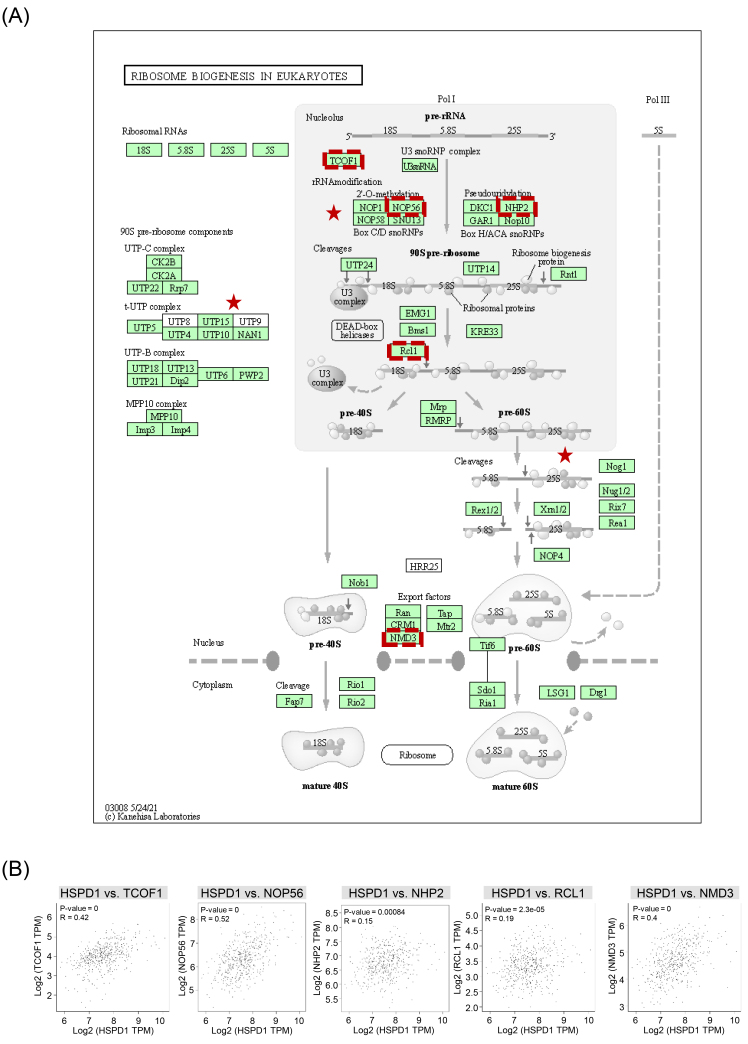
We further assessed whether HSPD1 expression was related to ribosome biogenesis in LUAD. Details of ribosome biogenesis in eukaryotes pathway and components regulated by HSPD1-correlated genes are represented in Fig. 7A. Correlation between HSPD1 and ribosome biogenesis marker gene expression, including TCOF1, NOP56, NHP2, RCL1, and NMD3 was analyzed in LUAD dataset in GEPIA2. As shown in Fig. 7B, HSPD1 expression was significantly positive correlated with all of these marker genes, indicating the potential involvement of HSPD1 in ribosome biogenesis pathway in LUAD.
Figure 7.
Ribosome biogenesis in eukaryotes pathway and its correlation with HSPD1 in LUAD. (A) Ribosome biogenesis in eukaryotes pathway obtained from KEGG. Red stars denote components regulated by HSPD1-correlated genes. Genes that were selected for further correlation analysis are indicated by red dotted boxes. (B) Correlation of HSPD1 with TCOF1, NOP56, NHP2, RCL1, and NMD3 in LUAD analyzed by GEPIA2.
3.5. Relationship between HSPD1 expression and immune cell infiltration in LUAD
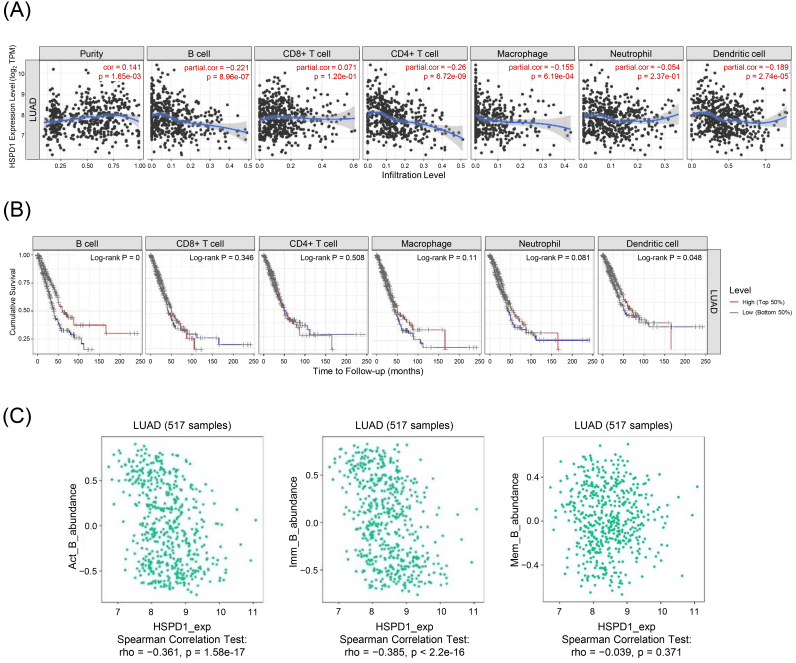
Aberrant ribosome biogenesis is not only involved with tumorigenesis, e.g., MDM2/p53-mediated ribosome biogenesis checkpoint [42, 43, 44], but also regulates immune responses via multiple mechanisms, such as interferon- (IFN--mediated inflammation and NF-B signaling pathway [45]. To this end, we sorted to elucidate the potential oncoimmunogenic role of HSPD1 expression on the TIICs in LUAD by using TIMER deconvolution analysis. As shown in Fig. 8A HSPD1 expression level was negatively correlated with B cells (0.221, 8.96 10 - 7), CD4+ T cells (0.26, 6.72 10 - 9), macrophages (0.155, 6.19 10 - 4), and dendritic cells (0.189, 2.74 10 - 5). KM survival curves of each immune cell infiltration showed that low infiltration levels of B cells and dendritic cells were associated with poor prognosis in LUAD patients (Log-rank 0 and 0.048, respectively) (Fig. 8B). Univariate Cox regression analysis demonstrated the significant associations of B cells (HR 0.024, 0.001), dendritic cells (HR 0.553, 0.047), and HSPD1 (HR 1.466, 0.001) with overall survival of LUAD patients. The prognostic significance of B cells (HR 0.006, 0.001) and HSPD1 (HR 1.415, 0.03) were confirmed in multivariate analysis (Table 2). In addition, correlations between HSPD1 gene expression and the abundance of different subsets of B cells were also observed in the TISIDB database. Data showed that HSPD1 expression was negatively correlated with activated B (Act B) cells (rho 0.361, 1.58 10 - 17) and immature (Imm B) B cells (rho 0.385, 2.20 10 - 16), but was not significantly correlated with memory B (Mem B) cells (rho 0.039, 0.371) (Fig. 8C).
Figure 8.
Relationship between HSPD1 expression and immune cell infiltration in LUAD. (A) Correlation of HSPD1 expression with immune cell infiltration in LUAD patients evaluated by TIMER. (B) KM survival curves of each type of immune cells in LUAD patients obtained from TIMER. (C) Correlation of HSPD1 expression with activated B (Act B) cells, immature B (Imm B) cells, and memory B (Mem B) cells in LUAD from TISIDB.
Table 2.
Univariate and multivariate Cox analysis of infiltrating immune cells and HSPD1 expression for survival in LUAD patients estimated by TIMER
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | -value | HR (95% CI) | -value | |
| B cell | 0.024 (0.004–0.142) | 0.001 | 0.006 (0.000–0.076) | 0.001 |
| CD8+ T cell | 0.299 (0.083–1.074) | 0.064 | 1.195 (0.190–7.526) | 0.850 |
| CD4+ T cell | 0.346 (0.078–1.541) | 0.164 | 32.942 (2.091–518.996) | 0.013 |
| Macrophage | 0.577 (0.094–3.539) | 0.552 | 1.524 (0.114–20.382) | 0.750 |
| Neutrophil | 0.366 (0.055–2.452) | 0.300 | 0.415 (0.009–18.781) | 0.651 |
| Dendritic cell | 0.553 (0.308–0.993) | 0.047 | 0.879 (0.233–3.310) | 0.849 |
| HSPD1 | 1.466 (1.189–1.808) | 0.001 | 1.415 (1.124–1.781) | 0.003 |
HR: Hazard ratio, CI: confidence interval.
4. Discussion
HSPD1 has been reported to play a role in the development and progression of many cancers, including LUAD [16, 27]. Its overexpression is associated with poor prognosis in LUAD patients [25, 26] and chemoresistance in NSCLC cells [27]. Additionally, HSPD1 is widely recognized for its interaction with the immune system. Extracellular or secreted HSPD1 can modulate the activation and function of various kinds of immune cells [15]. TIICs serve as key modulators in TME, and their composition is related to the prognosis and therapeutic response of LUAD patients [6, 7, 8, 9, 10, 11]. Therefore, HSPD1 is an interesting molecule for further investigations of its potential as a biomarker for predicting prognosis and immune response and as a therapeutic target for LUAD.
A previous study using immunohistochemistry has reported an increased expression of HSPD1 and its significant correlation with the prognosis of patients with LUAD [25]. Overexpression of HSPD1 has been shown to correlate with HSP10 expression, and LUAD patients with high expression of HSP60 and HSP10 proteins show poor overall survival [26]. Nevertheless, these studies aimed to assess the prognostic impact of HSPD1 for LUAD patients, but the role of HSPD1 in cancer development and progression has not been fully explored. To our knowledge, only a study by Parma et al. has demonstrated the role of HSPD1 in promoting lung cancer development through the modulation of mitochondrial metabolism [27]. However, the precise role and underlying mechanism of HSPD1 in LUAD carcinogenesis remain largely unknown, and its association with the immune response has not been reported.
Recently, several omics datasets and bioinformatics tools have become publicly available, enabling further comprehensive studies of cancers. In this study, we thus applied an integrated bioinformatic approach to gain a more comprehensive insight into prognostic and functional significance of HSPD1 in LUAD. We first investigated the expression and prognostic significance of HSPD1 in LUAD using several databases. A significantly increased expression of HSPD1 in LUAD tissue was found at both mRNA and protein levels in multiple databases. The HSPD1 expression was significantly associated with cancer stages and was an independent prognostic factor for survival in LUAD patients. The patients with high HSPD1 expression had a poorer prognosis than those with low HSPD1 expression, confirming the prognostic significance of HSPD1 expression in LUAD. Further analyses also showed that most of HSPD1-positively correlated genes were significantly predictive of poor overall survival, while the favorable outcome of LUAD patients was associated with HSPD1-negatively correlated genes. Our findings were consistent with previous studies [25, 26, 27] in supporting the role of HSPD1 as a prognostic marker for LUAD. Additionally, our findings provided novel insights into the potential underlying mechanism of HSPD1 in LUAD progression and metastasis through regulation of ribosome biogenesis, and demonstrated a possible relationship between HSPD1 and immune cell infiltration in LUAD.
GO and KEGG pathway enrichment analyses of HSPD1-correlated genes highlighted a potential role of HSPD1 involved in ribosome biogenesis in LUAD. Several studies have demonstrated that ribosome biogenesis and ribosomal protein (RP) play important roles in tumorigenesis and cancer progression. An increase in ribosome biogenesis is associated with tumor growth, progression, and drug resistance in many cancers [46, 47, 48]. In LUAD, an elevated level of phosphorylated-RPS6 was associated with metastasis and an unfavorable prognosis [49, 50]. Knockdown of RPS6 suppressed the growth of NSCLC cells through induction of the cell cycle arrest [51]. A recent study has reported an increased expression of RPL32 in LUAD tissues. Its overexpression was associated with poor prognosis. Knockdown of RPL32 induced ribosomal stress, resulting in the accumulation of p53 and inhibition of lung cancer cell proliferation [52]. Furthermore, it has been shown that treatment with triptolide, an extract from the Chinese herb Tripterygium wilfordii, induced cell cycle arrest and apoptosis in NSCLC cells through perturbation of ribosome biogenesis [53]. These findings indicated the crucial roles of RPs and ribosome biogenesis in LUAD development. Although a direct function of HSPD1 in ribosome biogenesis has not been clearly elucidated, previous studies have demonstrated their relationship in cancer development. It has been shown that knockdown of HSPD1 results in the downregulation of RPs and a decrease in protein synthesis, leading to growth inhibition of ovarian cancer cells and glioblastoma cells [54, 55]. Therefore, the positive correlation between HSPD1 and ribosome biogenesis marker gene expressions observed in our analyses suggested that HSPD1 may exert a tumor-promoting activity in LUAD through the regulation of ribosome biogenesis.
HSPD1 is well known for its modulatory activity on many types of immune cells [15]. Composition of TIICs is related with clinical outcomes and therapeutic response in LUAD patients [6, 7, 8, 9, 10, 11]. Therefore, it was of interest to investigate an association between HSPD1 expression and TIICs in LUAD. Among several types of TIICs, our analyses showed that HSPD1 expression was negatively correlated with infiltration level of B cell, particularly activated B cells and immature B cells. A low abundance of tumor-infiltrating B cells was strongly associated with poor overall survival of the patients, suggesting a protective role of B cells in LUAD. There is increasing evidence demonstrating that B cells play an important role in anti-tumor immunity in lung cancers [56]. A decrease in tumor-infiltrating B cells was associated with poor survival in LUAD patients [8]. The presence of B cells in tertiary lymphoid structures in TME was positively correlated with tumor-specific antibody response, high level of activated CD4+T cells and low level of regulatory T cells in NSCLS patients [57, 58, 59]. Recently, it has been shown that depletion of B cells by anti-CD20 antibody accelerates tumorigenesis and tumor progression in a murine lung cancer model through suppression of tumor infiltration of immune cells [59]. Hence, it was possible that HSPD1 expression might affect B cell-mediated immune response, thereby regulating anti-tumor immunity in LUAD.
Speculatively, it is possible that HSPD1 overexpression could potentially contribute to the occurrence of abnormal ribosome biogenesis and disrupt immune responses, resulting in limited B cell and CD4+ T cell infiltration. However, some limitations of this study should be noted. First, our analyses were based on the limited datasets publicly available online on internet. More datasets would help strengthen the findings of this study. Second, bioinformatic analyses are susceptible to various confounding factors, and the obtained data may not be entirely accurate, necessitating experimental validations. Examining the effects of HSPD1 using gene knockdown and overexpression experiments in relevant cell lines or animal models, followed by comprehensive molecular and cellular analyses in response to subsequent changes in specific signaling pathways and its interaction, would provide direct evidence of the functional role of HSPD1 in aberrant ribosome biogenesis and dysregulated immune responses. Further studies are warranted to assess clinical significance of HSPD1 in LUAD development and immunotherapy responses
In summary, HSPD1 expression was significantly higher in LUAD tissues. Its overexpression was associated with poor survival in patients with LUAD. HSPD1 had a potential role involved in the dysregulation of ribosome biogenesis. HSPD1 expression was negatively correlated with B cell infiltration and might be a predictive biomarker for prognosis and immunotherapy response in LUAD.
Author contributions
Conception: SA.
Interpretation or analysis of data: SA, KS, and JN.
Preparation of the manuscript: SA, SC, and SP.
Revision for important intellectual content: SA, SC, and SP.
Supervision: SC and SP.
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/CBM-220442.
Supplementary Material
Acknowledgments
This work (Grant No. RGNS 65-164) was supported by Office of the Permanent Secretary, Ministry of Higher Education, Science, Research and Innovation (OPS MHESI), Thailand Science Research and Innovation (TSRI) and Mae Fah Luang University. Sutatip Pongcharoen received research grant from the National Science, Research and Innovation Fund (NSRF, grant no. R2565B001 and R2566B001 as a continual grant).
References
- [1]. Mattiuzzi C. and Lippi G., Current Cancer Epidemiology, J Epidemiol Glob Health 9 (2019), 217–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Zappa C. and Mousa S.A., Non-small cell lung cancer: current treatment and future advances, Transl Lung Cancer Res 5 (2016), 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Denisenko T.V., Budkevich I.N. and Zhivotovsky B., Cell death-based treatment of lung adenocarcinoma, Cell Death Dis 9 (2018), 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Sosa Iglesias V., Giuranno L., Dubois L.J., Theys J. and Vooijs M., Drug Resistance in Non-Small Cell Lung Cancer: A Potential for NOTCH Targeting? Front Oncol 8 (2018), 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Jochems C. and Schlom J., Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity, Exp Biol Med (Maywood) 236 (2011), 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Suzuki K., Kachala S.S., Kadota K., Shen R., Mo Q., Beer D.G., Rusch V.W., Travis W.D. and Adusumilli P.S., Prognostic immune markers in non-small cell lung cancer, Clin Cancer Res 17 (2011), 5247–5256. [DOI] [PubMed] [Google Scholar]
- [7]. Liu X., Wu S., Yang Y., Zhao M., Zhu G. and Hou Z., The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer, Biomed Pharmacother 95 (2017), 55–61. [DOI] [PubMed] [Google Scholar]
- [8]. Lee H.E., Luo L., Kroneman T., Passow M.R., Del Rosario K.M., Christensen M.R., Francis M.E., O’Shaughnessy J.W., Blahnik A.J., Yang P. and Yi E.S., Increased Plasma Cells and Decreased B-cells in Tumor Infiltrating Lymphocytes are Associated with Worse Survival in Lung Adenocarcinomas, J Clin Cell Immunol 11 (2020). [PMC free article] [PubMed] [Google Scholar]
- [9]. Sun J., Zhang Z., Bao S., Yan C., Hou P., Wu N., Su J., Xu L. and Zhou M., Identification of tumor immune infiltration-associated lncRNAs for improving prognosis and immunotherapy response of patients with non-small cell lung cancer, Journal for Immunotherapy of Cancer 8 (2020), e000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Schoenhals J.E., Seyedin S.N., Anderson C., Brooks E.D., Li Y.R., Younes A.I., Niknam S., Li A., Barsoumian H.B., Cortez M.A. and Welsh J.W., Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation, Translational Lung Cancer Research 6 (2017), 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Li L., Lu G., Liu Y., Gong L., Zheng X., Zheng H., Gu W. and Yang L., Low Infiltration of CD8+ PD-L1+ T Cells and M2 Macrophages Predicts Improved Clinical Outcomes After Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Carcinoma, Frontiers in Oncology 11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Kampinga H.H., Hageman J., Vos M.J., Kubota H., Tanguay R.M., Bruford E.A., Cheetham M.E., Chen B. and Hightower L.E., Guidelines for the nomenclature of the human heat shock proteins, Cell Stress Chaperones 14 (2009), 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Aluksanasuwan S., Sueksakit K., Fong-Ngern K. and Thongboonkerd V., Role of HSP60 (HSPD1) in diabetes-induced renal tubular dysfunction: regulation of intracellular protein aggregation, ATP production, and oxidative stress, Faseb J 31 (2017), 2157–2167. [DOI] [PubMed] [Google Scholar]
- [14]. Cappello F., Conway de Macario E., Marasà L., Zummo G. and Macario A.J., Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy, Cancer Biol Ther 7 (2008), 801–809. [DOI] [PubMed] [Google Scholar]
- [15]. Quintana F.J. and Cohen I.R., The HSP60 immune system network, Trends Immunol 32 (2011), 89–95. [DOI] [PubMed] [Google Scholar]
- [16]. Yun C.W., Kim H.J., Lim J.H. and Lee S.H., Heat Shock Proteins: Agents of Cancer Development and Therapeutic Targets in Anti-Cancer Therapy, Cells 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Fucarino A. and Pitruzzella A., Role of HSP60/HSP10 in Lung Cancer: Simple Biomarkers or Leading Actors? J Oncol 2020 (2020), 4701868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Wu J., Liu T., Rios Z., Mei Q., Lin X. and Cao S., Heat Shock Proteins and Cancer, Trends in Pharmacological Sciences 38 (2017), 226–256. [DOI] [PubMed] [Google Scholar]
- [19]. Tsai Y.-P., Yang M.-H., Huang C.-H., Chang S.-Y., Chen P.-M., Liu C.-J., Teng S.-C. and Wu K.-J., Interaction between HSP60 and β-catenin promotes metastasis, Carcinogenesis 30 (2009), 1049–1057. [DOI] [PubMed] [Google Scholar]
- [20]. Campanella C., Bucchieri F., Ardizzone N.M., Marino Gammazza A., Montalbano A., Ribbene A., Di Felice V., Bellafiore M., David S., Rappa F., Marasà M., Peri G., Farina F., Czarnecka A.M., Conway de Macario E., Macario A.J., Zummo G. and Cappello F., Upon oxidative stress, the antiapoptotic Hsp60/procaspase-3 complex persists in mucoepidermoid carcinoma cells, Eur J Histochem 52 (2008), 221–228. [DOI] [PubMed] [Google Scholar]
- [21]. Marino Gammazza A., Campanella C., Barone R., Caruso Bavisotto C., Gorska M., Wozniak M., Carini F., Cappello F., D’Anneo A., Lauricella M., Zummo G., Conway de Macario E., Macario A.J. and Di Felice V., Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence, Cancer Lett 385 (2017), 75–86. [DOI] [PubMed] [Google Scholar]
- [22]. Zhou C., Sun H., Zheng C., Gao J., Fu Q., Hu N., Shao X., Zhou Y., Xiong J., Nie K., Zhou H., Shen L., Fang H. and Lyu J., Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth, Cell Death Dis 9 (2018), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Pei W., Tanaka K., Huang S.C., Xu L., Liu B., Sinclair J., Idol J., Varshney G.K., Huang H., Lin S., Nussenblatt R.B., Mori R. and Burgess S.M., Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation, NPJ Regen Med 1 (2016), 16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Osterloh A., Geisinger F., Piédavent M., Fleischer B., Brattig N. and Breloer M., Heat shock protein 60 (HSP60) stimulates neutrophil effector functions, J Leukoc Biol 86 (2009), 423–434. [DOI] [PubMed] [Google Scholar]
- [25]. Xu X., Wang W., Shao W., Yin W., Chen H., Qiu Y., Mo M., Zhao J., Deng Q. and He J., Heat shock protein-60 expression was significantly correlated with the prognosis of lung adenocarcinoma, J Surg Oncol 104 (2011), 598–603. [DOI] [PubMed] [Google Scholar]
- [26]. Tang Y., Yang Y., Luo J., Liu S., Zhan Y., Zang H., Zheng H., Zhang Y., Feng J., Fan S. and Wen Q., Overexpression of HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor prognosis in non-small cell lung cancer patients, Cancer Biomark 30 (2021), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Parma B., Ramesh V., Gollavilli P.N., Siddiqui A., Pinna L., Schwab A., Marschall S., Zhang S., Pilarsky C., Napoli F., Volante M., Urbanczyk S., Mielenz D., Schrøder H.D., Stemmler M., Wurdak H. and Ceppi P., Metabolic impairment of non-small cell lung cancers by mitochondrial HSPD1 targeting, J Exp Clin Cancer Res 40 (2021), 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Sangiorgi C., Vallese D., Gnemmi I., Bucchieri F., Balbi B., Brun P., Leone A., Giordano A., Conway de Macario E., Macario A.J., Cappello F. and Di Stefano A., HSP60 activity on human bronchial epithelial cells, Int J Immunopathol Pharmacol 30 (2017), 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S., Li B. and Liu X.S., TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells, Cancer Res 77 (2017), e108–e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Li B., Severson E., Pignon J.C., Zhao H., Li T., Novak J., Jiang P., Shen H., Aster J.C., Rodig S., Signoretti S., Liu J.S. and Liu X.S., Comprehensive analyses of tumor immunity: implications for cancer immunotherapy, Genome Biol 17 (2016), 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Tang Z., Kang B., Li C., Chen T. and Zhang Z., GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis, Nucleic Acids Res 47 (2019), W556–w560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B. and Varambally S., UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses, Neoplasia 19 (2017), 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Pontén F., Jirström K. and Uhlen M., The Human Protein Atlas–a tool for pathology, J Pathol 216 (2008), 387–393. [DOI] [PubMed] [Google Scholar]
- [34]. Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson Å., Kampf C., Sjöstedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C.A., Odeberg J., Djureinovic D., Takanen J.O., Hober S., Alm T., Edqvist P.H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J.M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J. and Pontén F., Proteomics. Tissue-based map of the human proteome, Science 347 (2015), 1260419. [DOI] [PubMed] [Google Scholar]
- [35]. Györffy B., Surowiak P., Budczies J. and Lánczky A., Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer, PLoS One 8 (2013), e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Yan Z., Wang Q., Lu Z., Sun X., Song P., Dang Y., Xie L., Zhang L., Li Y., Zhu W., Xie T., Ma J., Zhang Y. and Guo X., OSluca: An Interactive Web Server to Evaluate Prognostic Biomarkers for Lung Cancer, Front Genet 11 (2020), 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Warde-Farley D., Donaldson S.L., Comes O., Zuberi K., Badrawi R., Chao P., Franz M., Grouios C., Kazi F., Lopes C.T., Maitland A., Mostafavi S., Montojo J., Shao Q., Wright G., Bader G.D. and Morris Q., The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function, Nucleic Acids Res 38 (2010), W214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Huang da W., Sherman B.T. and Lempicki R.A., Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources, Nat Protoc 4 (2009), 44–57. [DOI] [PubMed] [Google Scholar]
- [39]. Huang da W., Sherman B.T. and Lempicki R.A., Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists, Nucleic Acids Res 37 (2009), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Zhao T. and Wang Z., GraphBio: A shiny web app to easily perform popular visualization analysis for omics data, Front Genet 13 (2022), 957317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Ru B., Wong C.N., Tong Y., Zhong J.Y., Zhong S.S.W., Wu W.C., Chu K.C., Wong C.Y., Lau C.Y., Chen I., Chan N.W. and Zhang J., TISIDB: an integrated repository portal for tumor-immune system interactions, Bioinformatics 35 (2019), 4200–4202. [DOI] [PubMed] [Google Scholar]
- [42]. Deisenroth C. and Zhang Y., Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway, Oncogene 29 (2010), 4253–4260. [DOI] [PubMed] [Google Scholar]
- [43]. Oršolić I., Bursać S., Jurada D., Drmić Hofman I., Dembić Z., Bartek J., Mihalek I. and Volarević S., Cancer-associated mutations in the ribosomal protein L5 gene dysregulate the HDM2/ p53-mediated ribosome biogenesis checkpoint, Oncogene 39 (2020), 3443–3457. [DOI] [PubMed] [Google Scholar]
- [44]. Zisi A., Bartek J. and Lindström M.S., Targeting Ribosome Biogenesis in Cancer: Lessons Learned and Way Forward, Cancers (Basel) 14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Zhou X., Liao W.J., Liao J.M., Liao P. and Lu H., Ribosomal proteins: functions beyond the ribosome, J Mol Cell Biol 7 (2015), 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Pecoraro A., Pagano M., Russo G. and Russo A., Ribosome Biogenesis and Cancer: Overview on Ribosomal Proteins, Int J Mol Sci 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Nait Slimane S., Marcel V., Fenouil T., Catez F., Saurin J.C., Bouvet P., Diaz J.J. and Mertani H.C., Ribosome Biogenesis Alterations in Colorectal Cancer, Cells 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Penzo M., Montanaro L., Treré D. and Derenzini M., The Ribosome Biogenesis-Cancer Connection, Cells 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. McDonald J.M., Pelloski C.E., Ledoux A., Sun M., Raso G., Komaki, Wistuba R., II, Bekele B.N. and Aldape K., Elevated phospho-S6 expression is associated with metastasis in adenocarcinoma of the lung, Clin Cancer Res 14 (2008), 7832–7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Chen B., Tan Z., Gao J., Wu W., Liu L., Jin W., Cao Y., Zhao S., Zhang W., Qiu Z., Liu D., Mo X. and Li W., Hyperphosphorylation of ribosomal protein S6 predicts unfavorable clinical survival in non-small cell lung cancer, J Exp Clin Cancer Res 34 (2015), 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Chen B., Zhang W., Gao J., Chen H., Jiang L., Liu D., Cao Y., Zhao S., Qiu Z., Zeng J., Zhang S. and Li W., Downregulation of ribosomal protein S6 inhibits the growth of non-small cell lung cancer by inducing cell cycle arrest, rather than apoptosis, Cancer Lett 354 (2014), 378–389. [DOI] [PubMed] [Google Scholar]
- [52]. Xie J., Zhang W., Liang X., Shuai C., Zhou Y., Pan H., Yang Y. and Han W., RPL32 Promotes Lung Cancer Progression by Facilitating p53 Degradation, Mol Ther Nucleic Acids 21 (2020), 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Wang J., Zhang Z.Q., Li F.Q., Chen J.N., Gong X., Cao B.B. and Wang W., Triptolide interrupts rRNA synthesis and induces the RPL23-MDM2-p53 pathway to repress lung cancer cells, Oncol Rep 43 (2020), 1863–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Guo J., Li X., Zhang W., Chen Y., Zhu S., Chen L., Xu R., Lv Y., Wu D., Guo M., Liu X., Lu W. and Deng H., HSP60-regulated Mitochondrial Proteostasis and Protein Translation Promote Tumor Growth of Ovarian Cancer, Sci Rep 9 (2019), 12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Tang H., Li J., Liu X., Wang G., Luo M. and Deng H., Down-regulation of HSP60 Suppresses the Proliferation of Glioblastoma Cells via the ROS/AMPK/mTOR Pathway, Sci Rep 6 (2016), 28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Wang S.S., Liu W., Ly D., Xu H., Qu L. and Zhang L., Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer, Cell Mol Immunol 16 (2019), 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Germain C., Gnjatic S., Tamzalit F., Knockaert S., Remark R., Goc J., Lepelley A., Becht E., Katsahian S., Bizouard G., Validire P., Damotte D., Alifano M., Magdeleinat P., Cremer I., Teillaud J.L., Fridman W.H., Sautès-Fridman C. and Dieu-Nosjean M.C., Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer, Am J Respir Crit Care Med 189 (2014), 832–844. [DOI] [PubMed] [Google Scholar]
- [58]. Bruno T.C., Ebner P.J., Moore B.L., Squalls O.G., Waugh K.A., Eruslanov E.B., Singhal S., Mitchell J.D., Franklin W.A., Merrick D.T., McCarter M.D., Palmer B.E., Kern J.A. and Slansky J.E., Antigen-Presenting Intratumoral B Cells Affect CD4(+) TIL Phenotypes in Non-Small Cell Lung Cancer Patients, Cancer Immunol Res 5 (2017), 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Germain C., Devi-Marulkar P., Knockaert S., Biton J., Kaplon H., Letaïef L., Goc J., Seguin-Givelet A., Gossot D., Girard N., Validire P., Lefèvre M., Damotte D., Alifano M., Lemoine F.M., Steele K.E., Teillaud J.L., Hammond S.A. and Dieu-Nosjean M.C., Tertiary Lymphoid Structure-B Cells Narrow Regulatory T Cells Impact in Lung Cancer Patients, Front Immunol 12 (2021), 626776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.