Abstract
There is compelling evidence that exercise must be part of main line therapy for people with Parkinson’s disease. In this viewpoint, we outline the four key components of exercise: aerobic exercise, resistance exercise, flexibility exercise, and neuromotor exercises (posture, gait, balance, and agility) that can improve both motor and non-motor symptoms of the disease and, in the case of aerobic exercise, may delay the disease. We outline guidelines on how to change and optimize the exercise prescription at different stages of the disease.
Keywords: Aerobic exercise, exercise training, Parkinson’s disease, neuroprotection
Mounting evidence from the past three decades clearly demonstrates the benefits of exercise for people with Parkinson’s disease (PwPD) [1–4]. Exercise improves the motor and non-motor features of Parkinson’s disease (PD) [2], and may slow disease progression [5–7]. Yet, the best combination of exercises and optimum doses are still not routinely achieved by PwPD in their everyday life. In this viewpoint, we lay out: 1) the evidence supporting the exercise prescription for PwPD, 2) the current guidelines for the exercise prescription for PwPD and how to customize it for different disease stages, 3) practical tips for implementation, 4) medical clearance for exercise, and 5) exercise considerations specific to PD. We would like to stress from the outset that there is a commonly held misnomer that it does not matter what type of exercise a PwPD does, and that they are therefore free to choose whatever they would like. We will show that there are clear guidelines to adhere to if the goal is to optimize the benefit from exercise.
EVIDENCE SUPPORTING GUIDELINES FOR THE EXERCISE PRESCRIPTION FOR PD
PwPD should essentially follow the same exercise guidelines as those for their age-matched peers. As such, the exercise prescription for PwPD is very similar to the guidelines provided by the World Health Organization [8], and many other health agencies responsible for public health and diseases around the world. Of course, in PD, the case for exercise and activity as first-line therapy is even more compelling than in healthy individuals because PD is a relentlessly, and at times, insidiously, progressive disease.
Aerobic exercise
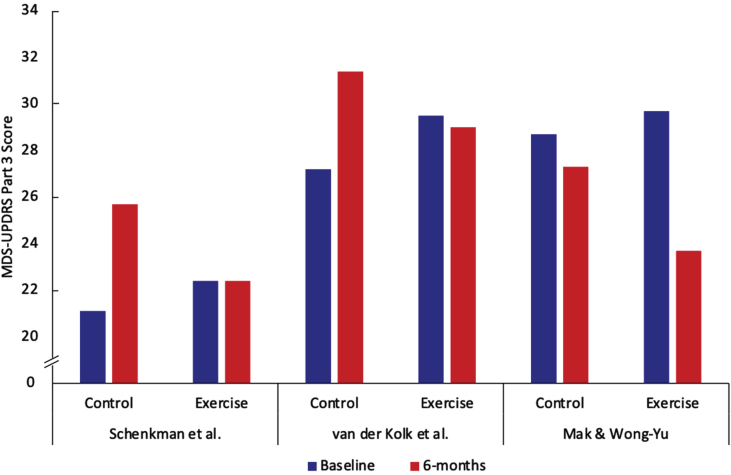
The first component of the exercise prescription is aerobic/endurance exercise [3]. In addition to potentially slowing disease progression, high intensity aerobic exercise increases overall cardiorespiratory fitness [5] which is a key vital sign [9]. There have been three studies conducted which all suggest that high-intensity (approximately 80–85% of peak heart rate) aerobic exercise has the potential to slow disease progression as well as reduce the signs and symptoms of the disease [5–7]. The data in Fig. 1 demonstrates that in all three studies there was at least a 4-point difference on the Movement Disorders Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 6 months between moderate- and high-intensity exercise. Modes of exercise varied between the three studies and included treadmill exercise [5], stationary cycling [6], and a brisk walking and balance program [7].The phase 2 clinical trial by Schenkman et al. was conducted in people who were early enough in their PD course that they did not yet need dopaminergic replacement therapy [5]. Any participants who later needed to go on medication after enrolling in the study were still tested for study outcome measures in the off-medication state. The studies by van der Kolk et al. and Mak et al. were conducted on PwPD who had already started medication and thus were further along in their disease course. Participants in the van der Kolk study were tested in the off-medication state while those in the Mak et al. study were tested in the on-medication state. PwPD in the Mak et al. study had greater motor impairment at baseline as evidenced by MDS-UPDRS part III scores on-medications being similar to the scores off-medications in the van der Kolk et al. study and higher MDS-UPDRS part III scores than the drug-naive participants in the study by Schenkman et. al. The Mak et al. study demonstrated the largest reduction in MDS-UPDRS part III of all three studies (a reduction of 6 points) in the high intensity group over six months (Fig. 1). The reason for this larger degree of symptomatic benefit may be due to the multimodal nature of the intervention that Mak et al. employed or the fact that participants with greater motor impairment may have been more likely to show improvements. Because all three studies showed a slower rate of change of the MDS-UPDRS part III in the high-intensity group despite methodologic differences (differences in disease duration and medication-status for testing), the conclusion that high-intensity exercise provides a slower rate of change is robust. A phase 3 trial on drug-naïve participants with testing conducted in the off-state is underway; this trial will definitively address whether exercise is disease modifying [10].
Fig. 1.
Control and exercise aerobic condition for clinical trials conducted by Schenkman et al. [5], van der Kolk et al. [6] (tested off medication), and Mak et al. [7] tested on medication. Blue depicts baseline condition and red the 6-month condition.
There is also an extensive preclinical body of research that provides possible mechanistic underpinnings for the benefit of aerobic exercise in PD [11–13]. Although each of the three high intensity aerobic studies referred to above used walking, running, or cycling [5–7], these findings likely apply to other forms of aerobic exercise performed at high intensity. Examples include but are not limited to cycling (outdoors), rowing, stair climbing machines, elliptical machines, and swimming [14]. It is important to stress that achieving 80% of peak heart rate can be hard using elliptical machines and swimming. Measuring heart rate during swimming is also more complicated than for other activities.
Resistance exercise/weight training
The second component of the exercise prescription is progressive resistance exercise, also known as weight/strength training. Studies have shown reductions in the symptoms of PD, improvements in physical function [15–18], and improvements in several aspects of cognition because of weight training programs [19–21]. An important point to stress here is that aerobic exercise and progressive resistance exercise have very different molecular and biological effects and, as such, both forms of exercise are equally important and essential [22, 23]. While high-intensity aerobic exercise training may be disease-modifying, resistance training likely allows the maintenance of function by increasing muscle mass and power, which are closely associated with mobility [24]. Resistance exercise also increases bone density [25]. There is also extensive evidence that muscle weakness should be avoided since it is associated with a higher all-cause cardiovascular and cancer risk [26] and this probably applies to PwPD.
Flexibility exercise
The third component of the exercise prescription is flexibility as loss of flexibility can lead to the inability to perform the other components of the exercise prescription and limits range of motion. Stretching exercises are encouraged [27]. Many PwPD benefit from performing flexibility exercises when they wake up to alleviate rigidity/stiffness caused by several hours of limited movement. Flexibility exercises can be performed as an activity alone to alleviate muscle rigidity/stiffness, as part of a warm-up and cool-down, or as part of activities such as yoga and Pilates. It is generally agreed that dynamic stretching is recommended before exercise or activity and static stretching is recommended after exercise. Further details of types of stretching, FITT principles and volume principles can be found on pages 158–161 of ACSM’s Guidelines for Exercise Testing and Prescription Eleventh Edition [27]. The flexibility component of the exercise prescription needs further systematic research in PwPD since it is the least studied of the four components.
Neuromotor exercise
The fourth component of the exercise prescription is balance, agility, and multi-tasking together known as neuromotor exercise. As the disease progresses, posture, gait, and balance become progressively more impaired. As such balance and mobility exercises and activities are required to counteract this. Examples of activities that help balance, mobility and multi-tasking are dancing, tai chi, yoga, table tennis, multimodal training, Nordic walking, aquatic training, exercise gaming, Qigong, Pilates, and circuit training which involve exercises targeting posture and balance [27, 28]. The underlying premise behind all these activities is that they require considerable involvement of the nervous system. As such they have the potential to improve cognition [29] and be beneficial for brain plasticity [30].
CURRENT GUIDELINES FOR THE PD EXERCISE PRESCRIPTION
Aerobic exercise and resistance training form the basis of the PD exercise prescription (PD ExRx); both can be prescribed in clear parameters specifying the frequency, intensity, time, and type (FITT) of exercise as specified in Table 1.
Table 1.
The exercise prescription for Parkinson’s disease
| FITT | FITT Recommendations for people with parkinson’s disease | |||
| Aerobic | Resistance | Flexibility | Neuromotor | |
| Frequency | 3–4 days / week | 2–3 days / week | 2–3 days / week with daily being most effective | 2–3 days / week |
| Intensity | High-intensity (80–85% HRmax) for mild – to – moderate PD; Moderate intensity (60–65% HRmax) for deconditioned individuals or those with more advanced PD; attempt to progress to 80%–85% HRmax | 30%–60% of 1RM for beginners; 60–80% 1RM for advanced | Full extension, rotation, or stretch to the point of slight discomfort | N/A |
| Time | ≥30 minutes accumulated high-intensity exercise (not including warm up/cool down or rest-intervals) Progress to total of 150 min/week | 1–3 sets of 8–12 repetitions Progress to 2–3 hours/week | Hold static stretch for 10–30 s; 2–4 repetitions of each exercise | 30–60 min |
| Type | Prolonged, rhythmic activities using large muscle groups (e.g., walking, running, cycling, swimming, rowing, elliptical) | Major muscle groups of upper and lower body – challenging all major muscle groups on nonconsecutive days. Avoid free weights for those in advanced disease stage, use weight machines, body weight, resistance bands instead | Slow static stretches for all major muscle groups working on increasing range of motion. | Exercises involving motor skills (e.g., balance, agility, coordination, gait, dual tasks) multidirectional step training and instability training |
FITT, Fitness, Intensity, Time, Type; HRmax, maximum heart rate; PD, Parkinson’s Disease; 1RM, one repetition maximum.
In addition, the PD ExRx must include recommendations to engage in activities that enhance flexibility, as well as balance/agility/multi-tasking (neuromotor). These latter activities are not so easily prescribed within the traditional parameters of frequency, intensity, time, and type but suggestions are provided in Table 1. It is very important to point out that there is significant evidence that dose does matter for the neuromotor component. A rigorous meta-analysis showed exercise can prevent falls in community-dwelling older people [31]. Exercise programs that challenge balance and are of a higher dose have larger effects [31]. The analysis also showed promise for PwPD and cognitive impairment. In addition, it has been shown that the amount of walking and dual tasking can be increased with PwPD when systematically progressed over a ten-week period [32]. Future studies conducted over at least 6 months and preferably longer are needed to work out the dose guidelines for neuromotor exercise. The prescription presented here is consistent with that developed by the American College of Sports Medicine (ACSM) [33] and the Parkinson Disease Foundation which provides an easy to read one page summary [34]. It is also consistent with a very extensive review of best physical therapy practice as summarized in Table 1 of the clinical practice guidelines from the American Physical Therapy Association [35].
It is important to inform PwPD that many activities also provide health related benefits and can provide greater enjoyment and motivation for some people (e.g., tango-dancing, boxing, and ping-pong). Table 2 outlines the differences between exercise, activities, and traditional rehabilitation therapy. It is important to counsel PwPD that activity classes alone are complementary but not sufficient to fulfill the entire exercise prescription. Many people need additional encouragement and guidance to develop an intense aerobic and resistance program. This approach is consistent with that advocated by Fox and colleagues who reviewed different components of physical interventions and found benefits for PwPD [36].
Table 2.
The difference between exercise, activities, and therapies
| Important Considerations | Exercise | Activities | Physical/Occupational/Speech Therapy |
| When should this be used? | Life-long – needs to be adapted into a lifestyle change | Life-long – needs to be adapted into a lifestyle change | Once specific motor or speech/swallow deficits arise. |
| What parts of the body or which symptom will benefit? | Heart Lungs BoneBrain | HeartLungsBoneBrain (different circuits) | PT: balance, freezing, sit-to-stand, transfers, turning, dystoniaOT: tremor, micrographia, dexteritySLP: hypophonia, dysphagia |
| Frequency | Nearly daily | As wanted | Burst and spaced – “Dental Model” |
| Focus on specific PD symptoms | No | No | Yes |
| Examples | Aerobic (cycling, running, swimming, brisk walking, rowing, elliptical)ResistanceFlexibilityNeuromotor | DanceBoxingYogaTai ChiPing-pongAquatic activitiesNordic skiingIrish Dancing | Specific problem-based protocols as administered by therapist expert in PD |
| How to prescribe or what to do? | Specific dose with specified frequency, intensity, time, type | Harder to specify dose. Explore what person enjoys. Consider referring to local group classes | Dose often defined in terms of number of sessions. Should be defined as when a plateau has been reached in performance |
We suggest weighting the dosage of the exercise prescription differentially based on personal need, disease stage and, to some extent, personal preference. For people early in their disease without evidence of frailty or imbalance, and desiring to slow down the disease, the emphasis should be on high-intensity aerobic exercise (e.g., 3–4 days a week of aerobic; 2 days of resistance; 1 day of neuromotor). For people who are showing signs of frailty, the emphasis should be on a higher dose of resistance training (e.g., 2–3 days a week of aerobic; 3 days of resistance; 1 day of neuromotor). For people with balance deficits who are at high risk of falling, neuromotor exercise should comprise a greater proportion of the prescription and should be paired with periodic physical therapy. People looking to maintain function should have a balanced plan of all prescription components that does not overwhelm them but rather facilitates compliance.
WHAT WE TELL PEOPLE WITH PARKINSON’S IN OUR CLINIC
Persuading people to take part in activities and exercise is one of the hardest challenges facing medicine. It is well established that a physician advising a patient to obtain the recommended number of minutes of exercise per week is ineffective in achieving that goal. In fact, walking with a doctor has even been suggested as one solution [37]. A detailed review of the literature on successful behavioral interventions is currently being conducted [38]. It is important to educate PwPD who are struggling to stay active, that several epidemiological studies have shown that increased activity, in general, is beneficial. It can prolong life expectancy and improve the quality of life in people with PD [39, 40]. It is also the case that maintaining muscle strength predicts longevity [41]. In other words, some activity is much better than no activity, but there are compelling reasons to follow the guidelines for the exercise prescription as we have outlined.
We suggest discussing the following strategies with PwPD who are eager to begin an exercise program or need encouragement to maintain it.
-
1)
Scheduling. When exercise is one of the first items to be scheduled, it is likely people will do it. When exercise is an afterthought, it rarely happens.
-
2)
Accountability to a friend or exercise buddy. There is a significant benefit for many people to the social aspect of exercise and activity. The social aspect of activity is linked to improved quality of life [42].
-
3)
Identifying a great place to work out. Exercise in a class or with a group at a club or fitness center can provide a level of accountability that many people need to help them maintain consistency. An increasing number of bespoke exercise centers/classes specialize in offerings for PwPD.
-
4)
Creating a clear exercise space where a person lives. Exercising at home requires no time for travel. Identifying an activity location with appropriate equipment can save time and increase convenience.
-
5)
Exercise/Activity Data. Some people benefit from keeping detailed records or logs of their exercise or activity schedule. There are also a wide variety of devices that can track activity levels and heart rate as well as software that can track activity and performance change over time. Some PwPD may find tracking such data to be motivational.
-
6)
Personalized instruction. Personal trainers may be able to design a program for PwPD based on the ExRx guidelines, although generally this is costly and not covered by insurance. Although physical therapy is traditionally geared toward treatment of a specific deficit (Table 2), physical therapists (who are more likely to be covered by insurance) can help PwPD get started on their own PD ExRx.
GENERAL CONSIDERATIONS FOR EXERCISE
-
1)
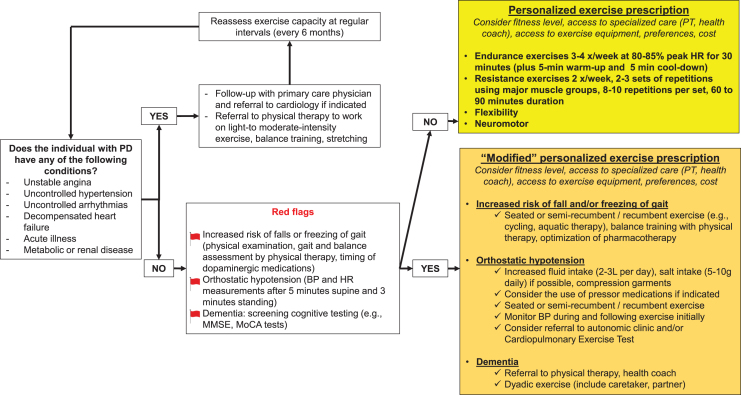
Most people do not need clearance. There are no contraindications to exercise for typical newly diagnosed PwPD beyond those that apply to the general population. The (ACSM) pre-participation health screening can be used to clear many patients for exercise without any additional testing. We have adapted their health screening for use with PwPD (Fig. 2).
-
2)
Autonomic and cardiovascular health. Although many PwPD have similar peak heart rates to those observed in people who do not have PD, some PwPD have a reduced peak heart rate, which is secondary to autonomic dysfunction [43, 44]. Blood pressure increase during exercise may also be blunted in these individuals. Screening for orthostatic hypotension should be completed before prescribing exercise in PwPD. The presence of orthostatic hypotension may require adaptation of the exercise regimen (e.g., exercise in the recumbent or seated positions such as cycling, rowing, swimming may be preferred), specific autonomic evaluation and treatments to allow safe participation in exercise, and careful monitoring of the first exercise sessions as some PwPD with autonomic failure may develop exercise-induced hypotension. Exercise training is likely still beneficial for these people. It is important to note that age-adjusted peak heart rates will be inaccurate for PwPD with autonomic dysfunction and a cardiopulmonary exercise test may be important to identify chronotropic incompetence.
-
3)
Postural instability and gait impairments. It is important to maintain both an active lifestyle but also to minimize the likelihood of falling. This is one reason that many physicians refer people to specialized programs which can monitor gait and balance over the course of the disease. They can provide specific exercises that are tailored to the needs of PwPD.
Fig. 2.
Exercise prescription flowchart for people with Parkinson’s disease.
PD SPECIFIC CONSIDERATIONS FOR EXERCISE
There are at least four other considerations unique to PD and exercise:
-
1)
Medication: It is important for people with PD to make sure they are adequately medicated to engage in their exercise program. While it may well be the case that exercise can raise endogenous levels of dopamine, people with PD should be encouraged to take extra PD medication prior to or during exercise if needed [45].
-
2)
Dystonia: One unfortunate complication of exercise can be exercise-induced dystonia which needs specialized treatment. This occurs most frequently as paroxysmal exercise-induced dystonia, which clusters among younger PD patients and may suggest a genetic etiology [46, 47].
-
3)
Tremor: While there is evidence that exercise can have a positive long-term effect on tremor [48], for some patients resting tremor may be exacerbated during or immediately after exercise, possibly due to muscle fatigue. This would be considered an acute effect of exercise. One possible idea is to suggest to people who have increased tremor after an exercise session, that they take part in a cool-down following exercise in which they focus on relaxation techniques.
-
4)
Deep brain stimulation. There are currently no contraindications to exercising with DBS. However, DBS can aggravate dynamic postural control, especially in patients with impaired balance at baseline [49]. A comprehensive assessment of balance function post-surgery could be valuable and suggest the need for physical therapy before starting an exercise program.
CONCLUSION
If a pill could be created that mimicked the effects of exercise, everyone would take it. The emerging field of exercise mimetics is clear witness to this fact [50]. The scientific evidence and the overwhelming and compelling testimony of PwPD make it clear that exercise should be first-line therapy for people with early PD with the goal of creating a lifestyle change. Exercise should continue to be part of the treatment regimen as the disease advances and additional treatments are required. Some physicians have even gone so far as to suggest that the purpose of prescribing medication is to enable people to exercise and take part in activities. This is an opinion with which we concur. This is especially the case since consistent exercise may reduce hospitalization risk [51].
We look forward to the future in which the guidelines that are being developed and revised are used to create a personalized exercise prescription for individuals with PD [52], based on evidence-based data which is accumulating rapidly. The personalized prescription would be crafted on: 1) an initial assessment of medical needs by a physician, and 2) an initial assessment of physical and functional ability by a physical therapist or neuromotor trained specialist along with consideration of: fitness level, goals of the person, disease severity, motor fluctuations, cognitive impairment, balance, agility, and mobility, access to specialized care (PT, neuromotor specialist, health coach or lifestyle navigator), access to exercise equipment (home, fitness center), and exercise and activity preference.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Numbers U01NS113851 and K23NS123506. Research is also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. It is also supported by a generous philanthropic gift in honor of Howard Gilbert and a gift from the JCS Family Foundation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1]. Ahlskog JE (2018) Aerobic exercise: Evidence for a direct brain effect to slow Parkinson disease progression. Mayo Clin Proc 93, 360–372. [DOI] [PubMed] [Google Scholar]
- [2]. Feng YS, Yang SD, Tan ZX, Wang MM, Xing Y, Dong F, Zhang F (2020) The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci 245, 117345. [DOI] [PubMed] [Google Scholar]
- [3]. Alberts JL, Rosenfeldt AB (2020) The universal prescription for Parkinson’s disease: Exercise. J Parkinsons Dis 10, S21–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Omar Ahmad S, Longhurst J, Stiles D, Downard L, Martin S (2023) A meta-analysis of exercise intervention and the effect on Parkinson’s disease symptoms. Neurosci Lett 801, 137162. [DOI] [PubMed] [Google Scholar]
- [5]. Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, Josbeno DA, Christiansen CL, Berman BD, Kluger BM, Melanson EL, Jain S, Robichaud JA, Poon C, Corcos DM (2018) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, Bloem BR (2019) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18, 998–1008. [DOI] [PubMed] [Google Scholar]
- [7]. Mak MKY, Wong-Yu ISK (2021) Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: A randomized controlled trial. J Parkinsons Dis 11, 1431–1441. [DOI] [PubMed] [Google Scholar]
- [8].World Health Organization, https://www.who.int/news-room/fact-sheets/detail/physical-activity, Accessed 10/06/2023.
- [9]. Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisløff U; American Heart Association Physical Activity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Functional Genomics and Translational Biology; Stroke Council (2016) Importance of assessing cardiorespiratory fitness in clinical practice: A case for fitness as a clinical vital sign: A scientific statement from the American Heart Association. Circulation 134, e653–e699. [DOI] [PubMed] [Google Scholar]
- [10]. Patterson CG, Joslin E, Gil AB, Spigle W, Nemet T, Chahine L, Christiansen CL, Melanson E, Kohrt WM, Mancini M, Josbeno D, Balfany K, Griffith G, Dunlap MK, Lamotte G, Suttman E, Larson D, Branson C, McKee KE, Goelz L, Poon C, Tilley B, Kang UJ, Tansey MG, Luthra N, Tanner CM, Haus JM, Fantuzzi G, McFarland NR, Gonzalez-Latapi P, Foroud T, Motl R, Schwarzschild MA, Simuni T, Marek K, Naito A, Lungu C, Corcos DM, SPARX3-PSG Investigators (2022) Study in Parkinson’s disease of exercise phase 3 (SPARX3): Study protocol for a randomized controlled trial. Trials 23, 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD (2009) Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord 15 Suppl 3, S42–45. [DOI] [PubMed] [Google Scholar]
- [12]. Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO (2011) Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeneration. Eur J Neurosci 33, 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ, Boklewski J, McCormick K, Williams NI, Stanton GB, Greenough WT, Cameron JL (2010) Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience 167, 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. de Almeida FO, Santana V, Corcos DM, Ugrinowitsch C, Silva-Batista C (2022) Effects of endurance training on motor signs of Parkinson’s disease: A systematic review and meta-analysis. Sports Med 52, 1789–1815. [DOI] [PubMed] [Google Scholar]
- [15]. Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC (2006) High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord 21, 1444–1452. [DOI] [PubMed] [Google Scholar]
- [16]. David FJ, Rafferty MR, Robichaud JA, Prodoehl J, Kohrt WM, Vaillancourt DE, Corcos DM (2012) Progressive resistance exercise and Parkinson’s disease: A review of potential mechanisms. Parkinsons Dis 2012, 124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Corcos DM, Robichaud JA, David FJ, Leurgans SE, Vaillancourt DE, Poon C, Rafferty MR, Kohrt WM, Comella CL (2013) A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 28, 1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Kelly NA, Ford MP, Standaert DG, Watts RL, Bickel CS, Moellering DR, Tuggle SC, Williams JY, Lieb L, Windham ST, Bamman MM (2014) Novel, high-intensity exercise prescription improves muscle mass, mitochondrial function, and physical capacity in individuals with Parkinson’s disease. J Appl Physiol (1985) 116, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. David FJ, Robichaud JA, Leurgans SE, Poon C, Kohrt WM, Goldman JG, Comella CL, Vaillancourt DE, Corcos DM (2015) Exercise improves cognition in Parkinson’s disease: The PRET-PD randomized, clinical trial. Mov Disord 30, 1657–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Silva-Batista C, Corcos DM, Roschel H, Kanegusuku H, Gobbi LT, Piemonte ME, Mattos EC, MT DEM, Forjaz CL, Tricoli V, Ugrinowitsch C (2016) Resistance training with instability for patients with Parkinson’s disease. Med Sci Sports Exerc 48, 1678–1687. [DOI] [PubMed] [Google Scholar]
- [21]. Gollan R, Ernst M, Lieker E, Caro-Valenzuela J, Monsef I, Dresen A, Roheger M, Skoetz N, Kalbe E, Folkerts AK (2022) Effects of resistance training on motor- and non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. J Parkinsons Dis 12, 1783–1806. [DOI] [PubMed] [Google Scholar]
- [22]. Shulman LM, Katzel LI, Ivey FM, Sorkin JD, Favors K, Anderson KE, Smith BA, Reich SG, Weiner WJ, Macko RF (2013) Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol 70, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Furrer R, Hawley JA, Handschin C (2023) The molecular athlete: Exercise physiology from mechanisms to medals. Physiol Rev 103, 1693–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Gamborg M, Hvid LG, Thrue C, Johansson S, Franzen E, Dalgas U, Langeskov-Christensen M (2023) Muscle strength and power in people with Parkinson disease: A systematic review and meta-analysis. J Neurol Phys Ther 47, 3–15. [DOI] [PubMed] [Google Scholar]
- [25]. Amato A, Baldassano S, Vasto S, Schiro G, Davi C, Drid P, Dos Santos Mendes FA, Caldarella R, D’Amelio M, Proia P (2022) Effects of a resistance training protocol on physical performance, body composition, bone metabolism, and systemic homeostasis in patients diagnosed with Parkinson’s disease: A pilot study. Int J Environ Res Public Health 19, 13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Lopez-Bueno R, Andersen LL, Koyanagi A, Nunez-Cortes R, Calatayud J, Casana J, Del Pozo Cruz B (2022) Thresholds of handgrip strength for all-cause, cancer, and cardiovascular mortality: A systematic review with dose-response meta-analysis. Ageing Res Rev 82, 101778. [DOI] [PubMed] [Google Scholar]
- [27]. Zhang M, Li F, Wang D, Ba X, Liu Z (2023) Exercise sustains motor function in Parkinson’s disease: Evidence from 109 randomized controlled trials on over 4,600 patients. Front Aging Neurosci 15, 1071803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Dai S, Yuan H, Wang J, Yang Y, Wen S (2023) Effects of aquatic exercise on the improvement of lower-extremity motor function and quality of life in patients with Parkinson’s disease: A meta-analysis. Front Physiol 14, 1066718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. da Silva FC, Iop RDR, de Oliveira LC, Boll AM, de Alvarenga JGS, Gutierres Filho PJB, de Melo L, Xavier AJ, da Silva R (2018) Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS One 13, e0193113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Petzinger GM, Holschneider DP, Fisher BE, McEwen S, Kintz N, Halliday M, Toy W, Walsh JW, Beeler J, Jakowec MW (2015) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: Targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Sherrington C, Michaleff ZA, Fairhall N, Paul SS, Tiedemann A, Whitney J, Cumming RG, Herbert RD, Close JCT, Lord SR (2017) Exercise to prevent falls in older adults: An updated systematic review and meta-analysis. Br J Sports Med 51, 1750–1758. [DOI] [PubMed] [Google Scholar]
- [32]. Conradsson D, Nero H, Lofgren N, Hagstromer M, Franzen E (2017) Monitoring training activity during gait-related balance exercise in individuals with Parkinson’s disease: A proof-of-concept-study. BMC Neurol 17, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].ACSM (2022) Brain health and brain-related disorders. In ACSM’s Guidelines for Exercise Testing and Prescription, Liguori G, ed. Wolters Kluwer, Philadelphia. [Google Scholar]
- [34].Parkinson Disease Foundation, https://www.parkinson.org/sites/default/files/documents/parkinsons-exercise-recommendations-infographic.pdf, Accessed 10/06/2023.
- [35]. Osborne JA, Botkin R, Colon-Semenza C, DeAngelis TR, Gallardo OG, Kosakowski H, Martello J, Pradhan S, Rafferty M, Readinger JL, Whitt AL, Ellis TD (2022) Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys Ther 102, pzab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Fox SH, Katzenschlager R, Lim SY, Barton B, de Bie RMA, Seppi K, Coelho M, Sampaio C, Movement Disorder Society Evidence-Based Medicine Committee (2018) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33, 1248–1266. [DOI] [PubMed] [Google Scholar]
- [37]. Sabgir D, Dorn J (2020) Walk with a doc-a call to action for physician-led walking programs. Curr Cardiol Rep 22, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Ahern L, Timmons PS, Lamb PSE, McCullagh DR (2022) Can behavioural change interventions improve self-efficacy and exercise adherence among people with Parkinson’s? A systematic review protocol. HRB Open Res 5, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Yoon SY, Suh JH, Yang SN, Han K, Kim YW (2021) Association of physical activity, including amount and maintenance, with all-cause mortality in Parkinson disease. JAMA Neurol 78, 1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Fang X, Han D, Cheng Q, Zhang P, Zhao C, Min J, Wang F (2018) Association of levels of physical activity with risk of Parkinson disease: A systematic review and meta-analysis. JAMA Netw Open 1, e182421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Bohannon RW (2019) Grip strength: An indispensable biomarker for older adults. Clin Interv Aging 14, 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Larson D, Yeh C, Rafferty M, Bega D (2022) High satisfaction and improved quality of life with Rock Steady Boxing in Parkinson’s disease: Results of a large-scale survey. Disabil Rehabil 44, 6034–6041. [DOI] [PubMed] [Google Scholar]
- [43]. Kanegusuku H, Silva-Batista C, Pecanha T, Nieuwboer A, Silva ND Jr., Costa LA, de Mello MT, Piemonte ME, Ugrinowitsch C, Forjaz CL (2016) Blunted maximal and submaximal responses to cardiopulmonary exercise tests in patients with Parkinson disease. Arch Phys Med Rehabil 97, 720–725. [DOI] [PubMed] [Google Scholar]
- [44]. Kanegusuku H, Correia MA, Cucato GG, Ritti-Dias RM (2022) Exercise prescription for Parkinson’s disease patients: Dealing with cardiovascular autonomic dysfunction. Rev Port Cardiol 41, 359–360. [DOI] [PubMed] [Google Scholar]
- [45]. Yu Z, Tang D (2022) Artificial neural network-assisted wearable flexible sweat patch for drug management in Parkinson’s patients based on vacancy-engineered processing of g-C(3)N(4). Anal Chem 94, 18000–18008. [DOI] [PubMed] [Google Scholar]
- [46]. Bozi M, Bhatia KP (2003) Paroxysmal exercise-induced dystonia as a presenting feature of young-onset Parkinson’s disease. Mov Disord 18, 1545–1547. [DOI] [PubMed] [Google Scholar]
- [47]. Bruno MK, Ravina B, Garraux G, Hallett M, Ptacek L, Singleton A, Johnson J, Singleton A, Hanson M, Considine E, Gwinn-Hardy K (2004) Exercise-induced dystonia as a preceding symptom of familial Parkinson’s disease. Mov Disord 19, 228–230. [DOI] [PubMed] [Google Scholar]
- [48]. Farashi S, Kiani L, Bashirian S (2021) Effect of exercise on Parkinson’s disease tremor: A meta-analysis study. Tremor Other Hyperkinet Mov (N Y) 11, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Fasano A, Aquino CC, Krauss JK, Honey CR, Bloem BR (2015) Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol 11, 98–110. [DOI] [PubMed] [Google Scholar]
- [50]. Gubert C, Hannan AJ (2021) Exercise mimetics: Harnessing the therapeutic effects of physical activity. Nat Rev Drug Discov 20, 862–879. [DOI] [PubMed] [Google Scholar]
- [51]. Kannarkat GT, Rafferty MR, Luo S, Liu H, Mills KA (2022) Effect of exercise and rehabilitation therapy on risk of hospitalization in Parkinson’s disease. Mov Disord Clin Pract 9, 494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Ellis T, Rochester L (2018) Mobilizing Parkinson’s disease: The future of exercise. J Parkinsons Dis 8, S95–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]