Abstract
This study aimed to evaluate the effect of low molecular weight Acanthopanax polysaccharides on simulated digestion, probiotics, and intestinal flora of broilers in vitro. The experiments were carried out by H2O2-Vc degradation of Acanthopanax polysaccharides, in vitro simulated digestion to evaluate the digestive performance of polysaccharides with different molecular weights, in vitro probiotic evaluation of the probiotic effect of polysaccharides on lactobacilli and bifidobacteria, in vitro anaerobic fermentation and high-throughput sequencing of 16S rRNA genes to study the impact of Acanthopanax polysaccharides on the intestinal flora of broilers, and the effect of Acanthopanax polysaccharides on the short-chain fatty acids of intestines were determined by GC-MS method. The results showed that the molecular weight of Acanthopanax polysaccharide (ASPS) was 9,543 Da, and the molecular weights of polysaccharides ASPS-1 and ASPS-2 were reduced to 4,288 Da and 3,822 Da after degradation, and the particle sizes, PDIs, and viscosities were also significantly decreased. ASPS-1 has anti-digestive properties and better in vitro probiotic properties. The addition of ASPS-1 regulates the structure of intestinal microorganisms by regulating fecalibacterium to produce short-chain fatty acids, promoting the colonization of beneficial bacteria such as fecalibacterium, paraprevotella and diminishing the prevalence of detrimental bacteria such as Fusobacteria. Interestingly the ASPS-1 group found higher levels of Paraprevotella, which degraded trypsin in the gut, reducing inflammation, acted as a gut protector, and was influential in increasing the levels of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and total SCFAs in the fermented feces. Therefore, the degraded ASPS-1 can better regulate the structure of intestinal flora and promote the production of SCFAs, creating possibilities for its use as a potential prebiotic, which is conducive to the intestinal health of poultry.
Key words: Acanthopanax senticosus polysaccharide, probiotic proliferation, simulated fermentation in vitro, short chain fatty acids (SCFA), 16S rRNA
INTRODUCTION
Acanthopanax senticosus (AS), a medicinal herb with a long history and homology with food and medicine, has polysaccharides that have good pharmacological effects and application value (Yang et al., 2021). Acanthopanax polysaccharides (ASPS), the abundant active ingredients in Acanthopanax, have a variety of functions, including regulation of intestinal flora, antioxidative, antibacterial, and anti-inflammatory (Wang et al., 2023c; Zhao et al., 2023). It is worth noting that ASPSs are not degraded during gastrointestinal digestion but can be degraded by poultry fecal microorganisms, and the short-chain fatty acids produced by degradation can also regulate intestinal pH and provide energy to the organism, which is important for the health of the poultry organism. Therefore, ASPSs have great research potential in the development of prebiotic feed additives. However, the application of ASPSs is limited by their high molecular weight (Mw) and viscosity. These disadvantages also delay the transit and absorption of ASPSs in the gastrointestinal system. Therefore, many scholars have degraded polysaccharides in order to reduce their molecular weight and viscosity to promote their biological functions. H2O2-Vc degradation is widely used because of its mild conditions and greenness (Xu et al., 2019). Chen, et al. (2020) used H2O2-Vc combined with microwave to degrade Grateloupia livida, and obtained 2 polysaccharides DGLP-1 and DGLP-2 with molecular weights of 158.49±0.43 and 18.06±0.92 kDa, respectively, and antioxidant assay showed that DGLP-1, especially DGLP-2, had good antioxidant properties. Ma et al. (2021) degraded blue honeysuckle polysaccharides, and after degradation, these 2 polysaccharides showed strong anti-glycation activity, inhibited α-amylase and α-glucosidase, and exhibited competitive inhibition kinetics.
Recent research has demonstrated a strong correlation between intestinal microorganisms and the well-being of poultry. The bacterial ecosystem in the intestines of poultry plays a crucial role in the development of poultry diseases and the regulation of their immune system (Liu et al., 2018b; Fan et al., 2020). Evidence demonstrates that the active constituents included in traditional Chinese medicine, such as polysaccharides, can effectively regulate dysbiosis of intestinal flora in chicken (Liu et al., 2021b). Interpreting the regulatory effects of prebiotics on the gut microbiota from the perspective of gut microbes and metabolites has become a hot research topic. Prebiotics, which are indigestible by the host, specifically enhance the metabolic activity and proliferation of advantageous bacteria, hence promoting the well-being of the host (Ding et al., 2018; Song et al., 2020; Yaqoob et al., 2021). Medicinal plant polysaccharides are a good prebiotic, which can be fermented and metabolized by specific intestinal flora to produce short-chain fatty acids and regulate intestinal health (Wang et al., 2024).
In the present study, H2O2-Vc was used for the degradation of Acanthopanax polysaccharides to obtain ASPS-1, ASPS-2. The physicochemical properties, as well as the digestibility of artificially simulated gastrointestinal fluids, were evaluated. Then, the effect of these ASPSs as an exotic carbon source on the proliferation of Lactobacillus and Bifidobacterium in liquid culture was assessed. The effects of ASPSs on the growth and Inulin of Lactobacillus and Bifidobacterium were compared, the latter having been previously shown to be a prebiotic. SCFAs and 16S rRNA were measured in cecum feces of poultry cecum supplemented with ASPS-1 after fermentation to investigate the effect of ASPS-1 on the level of SCFAs and the structure of the intestinal flora, with a view to discovering a better prebiotic.
MATERIALS AND METHODS
Materials
A. senticosus was acquired from Limin Pharmacy in Liaocheng, China. It was then pulverised using a grinder (H8422, Hebei Huicai Technology Co., Ltd., Hebei, China) and filtered through a 60-mesh screen. MRS medium, Bifidobacterium culture medium, Pepsin (>3,000 U/mg), and trypsin (Biotechnology grade) were purchased from Shanghai Macklin Biochemical Co., Ltd. All remaining reagents were at least of analytical grade.
Preparation of ASPS
The extraction of A. senticosus polysaccharide was carried out according to the complex enzyme-assisted hot water extraction previously established in the laboratory (Wang et al., 2023b).
Content Determination
The phenol−sulfuric acid method was employed to measure the total amount of sugar, with glucose serving as a reference (DuBois et al., 1956). Reducing sugar content was determined using the DNS method (Li and McKee, 2023).
Preparation of Degraded Polysaccharides
ASPS was degraded using the H2O2-Vc method according to a previous study (Long et al., 2021). Briefly, 0.25 g of ASPS was accurately weighed, 2.5 mL of 1% H2O2 was added, and 2.5 mL of 0.5 mol/L VC was added and placed in a 50°C constant temperature water bath for degradation. The 1h product ASPS-1 and the 2 h product ASPS-2 were obtained. After the degradation was completed, the appropriate amount of NaHSO3 was added to remove the H2O2 in the solution, which produced bubbles, and then NaOH was added to adjust the pH to 7. The polysaccharide solution was put into a 500 Da dialysis bag and dialysed under running water for 12 h. The polysaccharide was then precipitated with alcohol (80%) and dried to obtain the degraded polysaccharide.
Determination of Physicochemical Properties of Polysaccharides
Molecular Weight Determination
HPGPC determined the molecular weights of ASPS, ASPS-1, and ASPS-2 (Wang et al., 2023a).
Viscosity Measurement
The polysaccharides were configured to a certain concentration of the solution to be measured, and the viscosity of the polysaccharides was determined using Ping's kinematic viscometer at room temperature.
Determination of Particle Size
An appropriate amount of ASPSs was dissolved in deionized water and configured into a polysaccharide solution with a concentration of 20 mg/mL, and the particle size and zeta potential of the ASPSs were determined using a high-sensitivity zeta potential and particle size analyzer (ZetaPALS) at 25°C, and each sample was measured 3 times.
Determination of the pH of Polysaccharides
The pH value of the polysaccharides solution was measured using a P811 pH meter (Shanghai Youke Instrumentation, Shanghai, China).
In Vitro Simulated Digestion
In vitro simulation of gastrointestinal digestion was performed in 2 steps. Simulated gastric and simulated intestinal fluid formulations were slightly modified from previous studies (Wu et al., 2022). In summary, the gastrointestinal simulation buffer was produced in order to replicate the ionic concentrations of fluid seen in chickens' digestive system. The pH was modified using either HCl or NaOH solution. To simulate gastric fluid, 0.800 g of pepsin and 4.375 g of NaCl were weighed, dissolved in deionized water, and volume adjusted to 250 mL. The pH was adjusted to 1.2 with 3 mol/L of HCl solution to form an artificial simulated gastric fluid. For simulated intestinal fluid, 1.36 g of K2HPO4 was dissolved in 100 mL of distilled water, and the pH was adjusted to 6.8 with 1 mol/L NaOH solution; 2 g of trypsin was weighed, and the 2 liquids were mixed with an appropriate amount of water, and then diluted to 200 mL with water to achieve a final concentration of 0.01 g/mL, and the final concentration was 0.01 g/mL, and the final concentration was 0.22 μm micropore membrane was used for bacterial removal.
Digestive process: 20 mL of simulated gastric digest was preheated in a 37°C water bath, and 0.2 g of ASPS, ASPS-1, and ASPS-2 were added, respectively, and digested for 4 h in a 37°C water bath, during which 2 mL were removed at 0 h, 2 h, and 4h for inactivation of the enzymes and then set aside for use; at the end of gastric digestion, 20 mL of simulated small intestinal digest was added respectively to continue incubation at 37°C for 4 h. 2 mL was removed at 0, 2, and 4 h for inactivation of the enzyme and then prepared for use.
Effect of Polysaccharides on the Growth of Probiotics
Activation of Strains
Activation of Lactobacillus strains: Under the condition of aseptic operation, take the lactobacillus liquid from the frozen tube in the liquid nitrogen tank, and continue to cultivate it with 1% inoculum, and then continue to cultivate it with 1% inoculum for 12 h, and take the third generation of the bacterial liquid for spare.
Bifidobacteria activation: Under the condition of aseptic and anaerobic operation, take the bifidobacteria liquid from the frozen tube in the liquid nitrogen tank, continue to cultivate it with 1% inoculum, and then continue to cultivate it with 1% inoculum after 24 h, and then take the third generation of the bacterial liquid as a standby.
Preparation of Complex Culture Medium With ASPSs
In the MRS liquid medium, 0.1g of ASPS was added, dissolved by microthermal heat, and sterilised at 121°C for 20 min.
Proliferative Effects of Different ASPSs on Probiotics
Lactobacillus and Bifidobacterium activated for 3 generations were inoculated into MRS medium containing ASPSs at 0.2% (V/V) in 3 groups in parallel, while MRS liquid medium without ASPSs was used as a control group and incubated for 24 h at 37°C. The OD600 and pH values of the probiotics in the medium were measured consecutively.
In Vitro Fermentation
The effect of ASPS and ASPS-1 on gut microbiota was studied by fermentation of chicken faeces. The basal nutrient medium was formulated with reference to previous studies (Wang et al., 2023a). Fresh chicken faeces were collected, and mixed samples were diluted with sterile PBS and homogenized to obtain 10% (w/v) fecal slurry. After removing the residual solids from the turbid liquid by filtration through gauze, the supernatant was collected as a fecal medium. Before fermentation, the fecal medium was mixed with sterile basal medium supplemented with 1% ASPS and ASPS-1 in a ratio of 1: 9 as 2 experimental groups, respectively. A blank group was created by adding the same weight of basal nutrient growth media to the sample, whereas a positive group was created by adding 1% inulin.
Short Chain Fatty Acids (SCFA) Sequencing
In vitro fermentation broth SCFA concentrations were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS) according to Chen et al (Chen, et al., 2019).
Fecal Microbiota Bioinformatics Analysis
By high-throughput sequencing of the 16S rRNA genes in the V3 and V4 regions, the fecal fermentation broth microbiota composition and abundance were examined (Modrackova, et al., 2021).
Libraries Generated and Illumina Sequencing
Following the manufacturer's instructions, NEB Next Ultra DNA Library Prep Kit (Illumina) sequencing libraries were created with index codes. Libraries were evaluated using the Agilent 5400 (Agilent Technologies, Beijing, China). Finally, an Illumina platform sequenced the library to create 250 bp paired-end reads.
Bioinformatics Analysis
The analysis was done using QIIME2, referring to the tutorial (https://docs.qiime2.org/2019.1). The final feature list and feature sequences were obtained after quality control, trimming, denoising, splicing, and chimera removal using the QIIME2 dada2 plugin (Benjamin et al., 2015).
Statistical Analysis
The data were analyzed with SPSS statistical software version 24.0. Duncan's range tests analyzed one-way ANOVA. The results were shown as the mean ± SD. Graphing was performed using OriginPro 2021 software. Gut microbiome analysis was completed using the Wekemo Bioincloud (https://www.bioincloud.tech).
RESULTS
Analysis of Physicochemical Properties of ASPS, ASPS-1, and ASPS-2
The results of the physicochemical properties of the ASPS, ASPS-1 and ASPS-2 are shown in Table 1. By determining the molecular weights of the 3 polysaccharides, it can be seen that the molecular weight of polysaccharide ASPS before degradation was 9543 Da, and the molecular weights of polysaccharides ASPS-1 and ASPS-2 after degradation were 4288 Da and 3822 Da, respectively. The particle size of the degraded polysaccharide was significantly reduced from 773.35 ± 21.15 nm to 255.70 ± 11.61 nm and 190.30 ± 14.50 nm, respectively. Due to the smaller molecular weight and particle size, the viscosity of the polysaccharide is reduced. The viscosities of degraded polysaccharides ASPS-1, ASPS-2 were 3.644 mm/s2, 2.432 mm/s2 at 200 mg/mL concentration, respectively. PDI of degraded polysaccharides ASPS-1 and ASPS-2 becomes smaller. The above results indicate that H2O2-Vc degradation can effectively change the physicochemical properties of polysaccharides.
Table 1.
Analysis of physicochemical properties of ASPS, ASPS-1 and ASPS-2.
| ASPS | ASPS-1 | ASPS-2 | |
|---|---|---|---|
| Molecular weight (Da) | 9543 | 4288 | 3822 |
| Particle size (nm) | 773.35 ± 21.15 | 255.70 ± 11.61 | 190.30 ± 14.50 |
| PDI | 0.838 ± 0.020 | 0.419 ± 0.008 | 0.376 ± 0.059 |
| Viscosity (mm/s2) | 7.749 | 3.644 | 3.432 |
| pH | 7.26 | 6.93 | 7.20 |
Data is represented by mean ± SD, which is the mean of 3 replicates.
In Vitro Determination of Simulated Digestion
The in vitro digestive properties of ASPS, ASPS-1 and ASPS-2 were explored by simulating gastric and small intestinal digestion and the results are shown in Table S1. There was no significant change in the total sugar content and a significant increase in the reducing sugar content after 4 h of gastric digestion, probably because some of the glycosidic bonds of the ASPSs were broken to become monosaccharides or oligosaccharides and a small amount of polysaccharides were degraded to reducing sugars in the acidic environment; During the digestion phase in the small intestine, there was no significant change in the total sugar content and a significant increase in the reducing sugar content of ASPS and ASPS-2, indicating that they were able to be digested and absorbed, whereas there was no significant change in the reducing sugar content of ASPS-1. The above results indicate that ASPS-1 has good antidigestive properties and has the potential to be developed as a prebiotic.
Effect of Polysaccharides on the Growth of Probiotics
The probiotic properties of ASPSs were shown in Tables S2 and S3, and the number of viable bacteria was significantly higher (P < 0.05) than that of ASPSs in the media supplemented with ASPS-1 and ASPS-2, indicating that ASPSs could be utilized by Lactobacillus and Bifidobacterium, and that the growth promotion of probiotic bacteria by ASPS-1 and ASPS-2 was better than that of ASPSs. Combined with the analysis of the physicochemical properties of ASPSs, it can be seen that polysaccharides with smaller particle size and molecular weight are more easily decomposed and utilized by probiotics.
During the incubation process, the pH of all 3 groups of media supplemented with ASPSs decreased with increasing incubation time. Since lactic acid is produced by lactobacilli and bifidobacteria during growth, the medium is acidic, and the pH value tends to decrease. In addition, as can be seen from Tables S2 and S3, the pH values of the ASPS-1 and ASPS-2 groups were lower than those of the ASPS group at the same time points, and the OD600 was higher than that of the ASPS group, suggesting that the pro-biotic properties of the ASPS-1 and ASPS-2 groups were better than those of the ASPS group.
Changes in pH, Total and Reducing Sugars During Fermentation
In this study, we investigated the effect of fermentation degradation of polysaccharides on the regulation and utilization of intestinal flora in poultry by using an in vitro anaerobic fermentation model of fecal flora and determined the changes in pH, total and reducing sugars content during the fermentation process. The results of pH, total sugar and reduced sugar content of the fermentation broth at different times are shown in Table S4.
Table S4 shows that there was no significant change in the pH value between the ASPS group and the blank control group. However, in the ASPS-1 and Inulin groups, the pH value stabilized after initially decreasing sharply at 6 h. Additionally, the total sugar and reducing sugar content also decreased rapidly in these groups. This suggests that gut microorganisms were able to use ASPS-1 and Inulin as a source of carbon for growth and produce various metabolites, including organic acids and short-chain fatty acids. These metabolites led to a decrease in the pH value throughout the fermentation system. The aforementioned findings suggest that the deteriorated polysaccharide ASPS-1 can be employed as a carbon source by the gut microbiota to stimulate acid production.
Short Chain Fatty Acids Sequencing
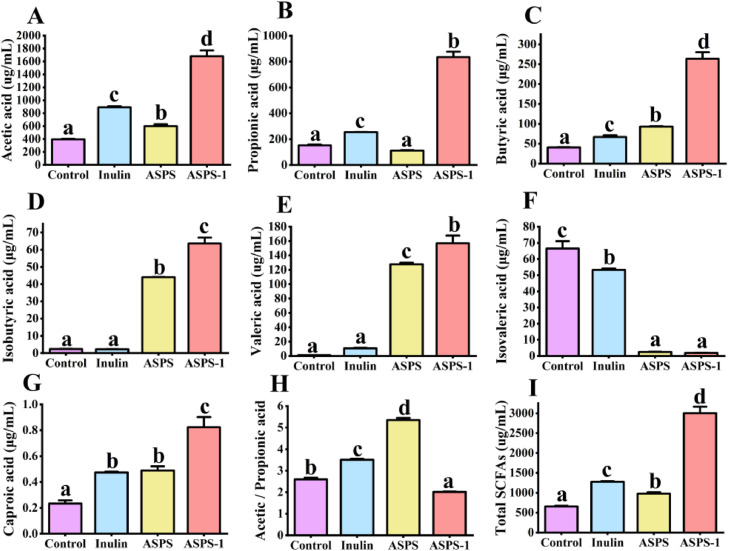
The gut microbiota has the ability to use carbohydrates and generate several metabolites, including short-chain fatty acids and organic acids. Short chain fatty acids (SCFA), also known as volatile fatty acids, are organic fatty acids consisting of 1 to 6 carbon atoms, which are one of the important metabolites of intestinal flora and are mainly produced from non-digestible carbohydrates by anaerobic bacterial fermentation in the colon. Short chain fatty acids have a significant impact on preserving the integrity of the intestinal barrier and regulating immunological responses (Hu et al., 2018). In this study, chicken cecum feces were fermented for 24 h to produce acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid, caproic acid, acetic acid/propionic acid and the total acid content shown in Figure 1.
Figure 1.
Effect of anaerobic fermentation of chicken manure with ASPSs polysaccharides on the cumulative concentration of SCFAs.
The top 3 SCFAs in the fecal samples were acetic acid, propionic acid, and butyric acid (Figure 1). The levels of acetic and propionic acids were significantly increased in the ASPS-1 group compared to the Control group (p < 0.05). The addition of ASPS-1 increased the acetic and propionic acid content of fermented chicken cecum feces by 4.2-fold and 5.5-fold, whereas the positive control, Inulin, only increased by 2.3-fold and 1.7-fold correspondingly. Conversely, the ASPS group experienced a 1.5-fold rise in acetic acid levels, but propionic acid levels remained unchanged. The ASPS-1 group had the lowest acetic acid/propionic acid ratio and the highest total SCFAs, suggesting that ASPS-1 had a more pronounced impact on SCFAs compared to the ASPS group. Furthermore, the levels of butyric, isobutyric, valeric, and hexanoic acids were considerably higher in the ASPS and ASPS-1 groups, while the level of isovaleric acid was lower, compared to the Control group.
Structure of the Fecal Microbiota
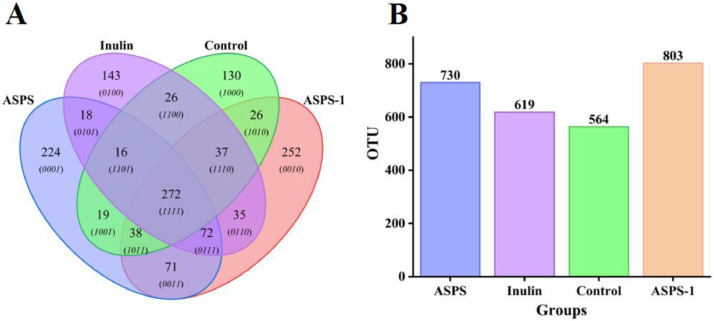
Venn Diagram Analysis
The fecal microbiota makeup in each group was evaluated using high-throughput sequencing. Figure 2A displays a Venn diagram illustrating the relative abundances of OTUs at the genus level. The ASPS, Inulin, Control, and ASPS-1 groups had a total of 730, 619, 564, and 803 operational taxonomic units (OTU), respectively (Figure 2B). There were 272 shared OTUs among the ASPS, Inulin, Control and ASPS-1 groups (Figure 2A). The species of unique OTUs of ASPS-1 and ASPS were 251 and 224, respectively, which were much higher than those of the Inulin, the Control group. It indicated that Acanthopanax polysaccharides were effective in improving species richness and that ASPS-1 was more effective than ASPS.
Figure 2.
Venn diagram of OTUs (1 means included, 0 means not included.) (A); Total number of OTUs (B).
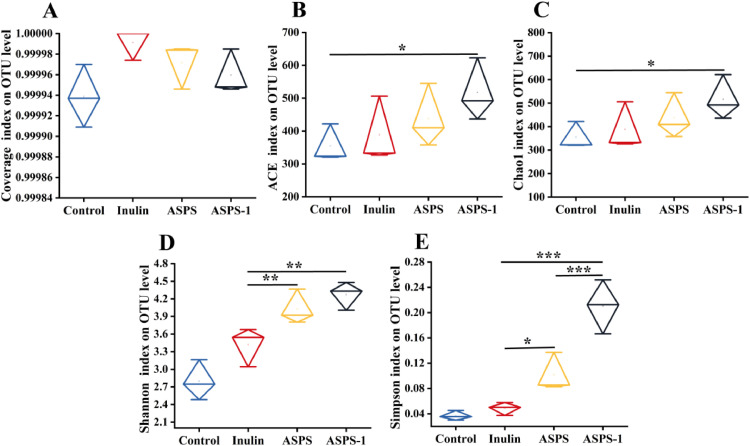
α-Diversity Analysis
The Coverage index determines whether the sequencing results accurately reflect the status of the microorganisms in the sample (Figure 3A). We used 4 commonly used indices to measure alpha diversity: the ACE, Chao1, Shannon, and Simpson index (Figures 3B–3E). Figure 3A demonstrates that the coverage of each group exceeds 0.999. The ACE and Chao1 indices were considerably greater in the ASPS-1 group compared to the Control group (P < 0.05). The Shannon index exhibited a substantial increase in both the ASPS-1 and ASPS groups compared to the Inulin group (P < 0.01). The Simpson index exhibited a significantly greater value in the ASPS-1 group compared to the Inulin group (P < 0.001). Additionally, it was significantly higher in the ASPS group compared to the Inulin group (P < 0.05), and showed a very significant difference between the ASPS-1 group and the Inulin group (P < 0.001).
Figure 3.
Alpha diversity indexs data of the gut microbiota in each group. Coverage (A), ACE (B), Chao1 (C), Shannon (D), and Simpson index (E). Note: *P < 0.05, **P < 0.01, ***P < 0.001.
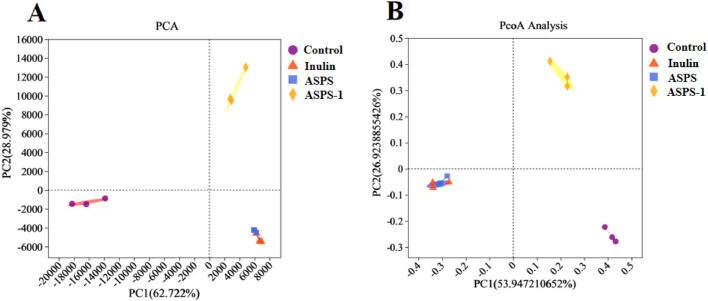
β-Diversity Analysis
We used PCA and PcoA analyses to investigate and distinguish differences in flora composition between samples. Figure 4 shows the results of the analysis. Figure 4A shows the results of PCA analysis, the explanatory power of PC1 and PC2 axes were 62.722% and 28.979%, respectively. Figure 4B shows the results of PcoA analysis, with PC1 and PC2 explaining 53.947% and 26.924% of the variation in the composition of the samples, respectively. The ASPS group and the inulin group almost overlapped; the ASPS-1 group was farther away from the other groups. PCA and PcoA demonstrated that the samples within the group were closer together, indicating better reproducibility of the samples.
Figure 4.
β-Diversity analysis for the different gut microbiota in each group, PCA (A) and PcoA (B).
Effects of ASPSs on Key Phylotypes of Fecal Microbiota
Phylum Level Analysis
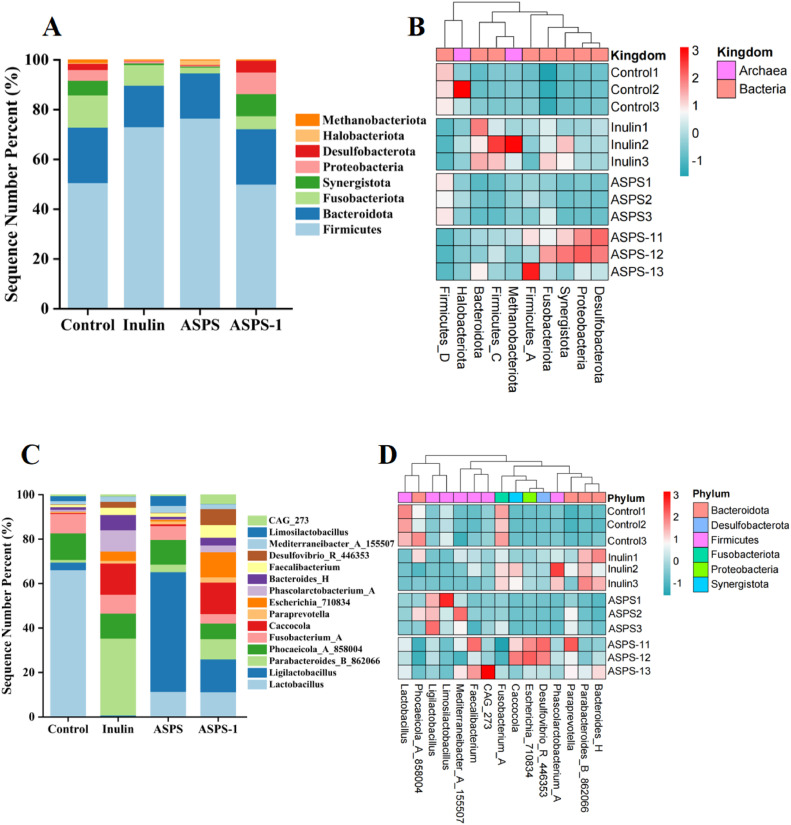
Among the 24 phylums identified, the top 8 phylums in terms of abundance were selected for analysis, and the results are shown in Figure 5A, in which Firmicutes and Bacteroidetes were the dominant phylums, followed by Fusobacteria and Synergistota. The abundance of Firmicutes was higher in the ASPS-1 group compared to the Control group, and the diversity of species was evenly distributed. The ASPS-1 group exhibited a higher prevalence of Bacteroidetes (32%), with propionic acid being the primary fermentation product of Bacteroidetes. This finding aligns with the results obtained for SCFAs. The presence of Fusobacteria was considerably lower in the ASPS-1 group compared to the Control group. Among the four sample groups, the ASPS-1 group had the highest relative abundance of cecum flora Synergistota. Proteobacteria and Desulfobacterota were also more highly represented in the ASPS-1 group than in the other groups. From Figure 5B, Halobacteriota, and Methanobacteriota are archaea Archaea, and the remaining 6 phylum are bacteria. The distribution of flora in the ASPS-1 group was similar to that of the positive control group, with the greatest difference from the Control group.
Figure 5.
The relative abundance of the gut microbiota at the phylum level (A); Phylum level cluster analysis heat map (B); The relative abundance of the gut microbiota at the genus level (C); Genus level cluster analysis heat map (D).
Genus Level Analysis
A total of 249 genus were detected at the genus level, and the top 15 bacterial genera with higher abundance were taken and analyzed and the results are shown in Figure 5CD, with 7 genus Phascolarctobacterium_ACAG273, fecalibacterium, Mediterraneibacter_A_155507, Limosilactobacillus, Ligilactobacillus and Lactobacillus belonging to the Firmicutes, four genus of Bacteroides_H, Parabacteroides_B_862066, Paraprevotella and Phocaeicola_A_858004belonging to the Bacteroidetes. Limosilactobacillus and Ligilactobacillus were regulated in the ASPS-1 group compared to the other 3 groups, and the number of genus tended to be homogeneous. Interestingly, we found a significant increase in fecalibacterium in the ASPS-1 group, one of the significant manufacturers of butyric acid. Caccocola, which is more predominant in the ASPS-1 group, belongs to Synergistota. Paraprevotella is more abundant in the ASPS-1 group. The bacterial genus composition did not differ significantly between the ASPS group and the control group. Additionally, the ASPS-1 group exhibited greater efficacy compared to the Inulin group.
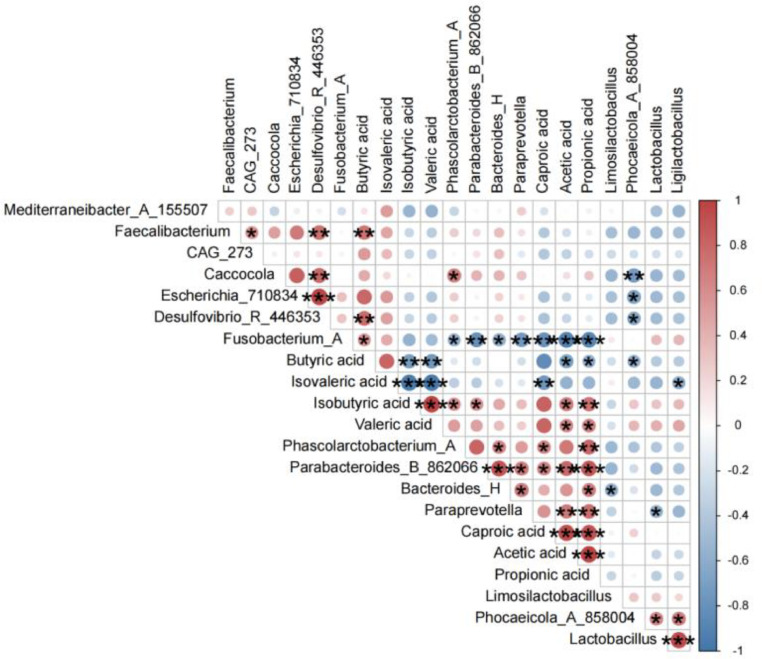
Relevance of SCFAs With Major Communities of the Gut Microbiota
The relationship between the 7 SCFAs and the major communities of intestinal microorganisms of 15 genus was analyzed at the genus level and calculated using the Pearson method, and the results are shown in Figure 6. Fecalibacterium and butyric acid were significantly positively correlated (P < 0.05), and the increase of fecalibacterium in the community promoted the increase of butyric acid levels. Many genus of Bacteroidetes are capable of producing short-chain fatty acids, and in Figure 6, Paraprevotella, Parabacteroides_B_862066 and Bacteroides_H of Bacteroides are closely correlated with acetic acid, propionic acid, and butyric acid. Fusobacterium_A was negatively correlated with acetic acid, propionic acid, valeric acid and caproic acid.
Figure 6.
Correlation of SCFAs and key communities of gut microbiota at the genus level. (Positive correlation in red, negative correlation in blue. *P < 0.05, **P < 0.01, ***P < 0.001.).
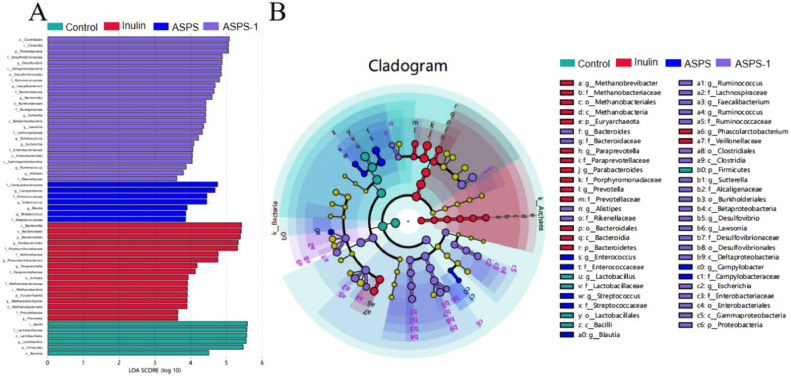
LEfSe Analysis
In the LEfSe analysis, the species of difference varied among the 4 groups. Figure 7A shows that there were 6 differential species in the control group, Lactobacillus from Firmictes; 17 differential species in the inulin group, including 5 genera from 2 phyla Bacteroidetes and Euryarchaeota; 7 differential species in the ASPS group, from 5 genera; and 25 differential species in the ASPS-1 group, including 9 genera from Proteobacteria. Firmicutes and the Lactobacillus order branch were the main components of the Control group of fecal microorganisms; in Inulin, the methanobacteria of Euryarchaeota were the more abundant species; Enterococcaceae showed high abundance in the ASPS group; Proteobacteria and Desulfobacterota abundance was higher in the ASPS-1 group. These species had a significant impact on the microbiota and their abundance differed significantly from the other groups. Fecalibacterium was found among the differential genera of ASPS-1. A classification unit display map of the group differences based on the classification hierarchy tree of the LEfSe taxonomy cladogram is presented in Figure 7B. The cladogram (Figure 7B) depicts the hierarchical arrangement of taxonomic levels, including the kingdom, phylum, class, order, family, and genus. The lines connecting these levels symbolize the links between them. Each circular node represents a distinct species. A yellow node indicates that there is no significant difference between the groups. Nodes of different colors indicate that the species is a distinctive microorganism of the corresponding group color and is more abundant in that group. The colored sectors indicate the lower taxonomic divisions of the characterized microorganisms.
Figure 7.
Distribution histogram based on LDA scores. LDA score (lg) > 2 (A). Cladogram. The circle indicates the level of phylogeny from the kingdom to the genus. The diameter of each circle is directly proportional to the abundance of the species, and the yellow circle represents no differences in microbial communities in the Control, Inulin, ASPS and ASPS-1 groups (B).
DISCUSSION
Highly productive modern broilers have been threatened by pathogens that ultimately inhibit production. Therefore, the immunization status of broilers is one of the most important factors in determining broiler production. Plant polysaccharides have been reported to exert immunomodulatory effects in immunosuppressed chickens (Meng et al., 2017; Yang et al., 2021), and can improve the growth performance of broilers, as well as regulating intestinal flora (Wu, 2018; Zhang et al., 2022b; Yang et al., 2024). H2O2-Vc degradation can change the physicochemical properties of polysaccharides, such as reducing the molecular weight of polysaccharides, lowering the viscosity and particle size, etc., so that the polysaccharides can better perform their biological activities, such as improving antioxidant capacity (Chen et al., 2016; Ma et al., 2021; Al-Wraikat et al., 2022). In this study, ASPS-1 and ASPS-2 were obtained by H2O2-Vc degradation of ASPS and the physicochemical properties, in vitro simulated digestive properties and probiotic properties of ASPS, ASPS-1 and ASPS-2 were evaluated, and it was found that the molecular weights, viscosities, particle sizes, and PDIs of ASPS-1 and ASPS-2 were decreased after degradation. Moreover, ASPS-1 has good antidigestive and probiotic properties, is not easily degraded in gastrointestinal digestive fluid, and can effectively promote the proliferation of Lactobacillus and Bifidobacterium. Gut microorganisms are closely associated with short-chain fatty acids (SCFAs), which are produced in the cecum (Hua et al., 2018), so ASPS and ASPS-1 were further added to broiler cecum feces for fermentation and to investigate their effects on SCFAs production and cecum microbial community, with the aim of discovering a polysaccharide with good immune-modulating effects.
The results showed that H2O2-Vc degradation could effectively change the physicochemical properties of polysaccharides, and the molecular weights, viscosities, particle sizes, and PDIs of ASPS-1 and ASPS-2 were decreased. PDI was used to indicate the molecular weight distribution of the polymers. The PDI of the polysaccharides ASPS-1 and ASPS-2 after degradation was smaller, indicating a more homogeneous molecular weight distribution (Zhang et al., 2022a). Both ASPS-1 and ASPS-2 were effective in promoting the proliferation of Lactobacillus and Bifidobacterium, with ASPS-1 having good antidigestive and probiotic properties.
Research has always focused on the link between gut microbes and the health of poultry species health, particularly their immune system and energy metabolism (Fathima et al., 2022; Jia et al., 2023). Polysaccharides, which are a dietary component, have been widely described in the literature for their ability to impact intestinal flora and generate SCFAs (Chen et al., 2022; Hu et al., 2023). In vitro anaerobic fermentation by gut microorganisms and enteric fermentation are similar in terms of bacterial numbers, composition, diversity, and metabolic products are similar (Duan et al., 2023). Gut microorganisms use undigested carbohydrates to create organic acids and short-chain fatty acids, which modify intestinal pH (Krautkramer et al., 2021). Therefore, we further performed in vitro fecal fermentation of chicken cecum with ASPS and ASPS-1 and showed that ASPS-1 increased the concentration of SCFAs. SCFAs participate in the body's energy metabolism as substrates for glucose, cholesterol, and lipid metabolism and play an important role in regulating the body's energy balance and diseases such as inflammatory bowel disease (Guo et al., 2021; Liu et al., 2021a). The most actively fermenting parts of the gastrointestinal tract are the cecum and proximal colon, which have the highest concentrations of SCFAs. It is well known that the higher the concentration of SCFAs in the gastrointestinal tract, the lower the pH in the intestinal lumen, which inhibits the colonization of some harmful microorganisms and ultimately accelerates the function of beneficial bacteria (Zhang et al., 2020; Dai et al., 2022). According to our study, ASPS-1 produces large amounts of SCFAs during cecal fecal fermentation, which could have a significant impact on the development of its biological functionality. ASPSs are catabolized and transformed by the intestinal flora as an energy source in fermented cecum feces, thereby altering the abundance of the flora and modulating the levels of SCFAs.
We analyzed the effects of ASPS and ASPS-1 on the intestinal flora in fermented manure, and the results showed that the addition of ASPS-1 for fermentation had a significant effect on the composition of the fecal microbiota. At the OTU level, the ASPS-1 group had the highest species abundance with 803 OTUs, including 251 unique OTUs. α-Diversity was employed to examine the composition of the fecal microbiota in the species, which indicates the abundance and uniformity of species in the samples (Fang, et al., 2015). The Coverage index determines whether the sequencing results accurately reflect the status of the microorganisms in the sample (Figure 3A). We used 4 commonly used indices to measure alpha diversity: the ACE, Chao1, Shannon, and Simpson index (Figures 3B–3E). Figure 3A demonstrates that the coverage of each group exceeds 0.999, implying that the likelihood of undiscovered sequences in the sample is exceedingly minimal. A catch rate over 99.9% was achieved for all bacterial samples, ensuring precise and dependable outcomes. The α-analysis showed that the addition of ASPS to fermented manure was effective in increasing the dominant OTUs of the fecal microbiota, with ASPS-1 being more effective. β-Diversity was employed to quantify the level of similarity in colony composition among groups and to examine variations in colony composition between samples (Li et al., 2019). Principal Component Analysis (PCA) utilizes the similarity coefficient matrix of the samples to identify the principal components. It employs variance decomposition to represent the distinctions among multiple datasets on a 2-dimensional coordinate plot. The axes of this plot are determined by the 2 eigenvalues that most accurately capture the variance (Wang et al., 2012). The results also demonstrated that the inclusion of ASPS-1 in the experimental group caused a modification in the composition of the fecal microbiota during in vitro fermentation.
The 2 main phylums in the fecal microbiota are Firmicutes and Bacteroidetes, in agreement with the results of existing studies (Wu et al., 2021). At the genus level, Caccocola, which is more predominant in the ASPS-1 group, belongs to Synergistota, an anaerobic bacterium that obtains energy for life activities through the degradation of amino acids, is widely distributed in the environment, and is a part of the normal microbiota of animals, and has a role in stabilizing intestinal homeostasis (Vartoukian et al., 2009). Paraprevotella is more abundant in the ASPS-1 group, and Paraprevotella strains have been reported as intestinal symbionts that efficiently degrade trypsin in the large intestine (Li et al., 2022b). Abnormally elevated levels and activity of trypsin may disrupt the intestinal mucosal barrier and promote intestinal disease (Jablaoui et al., 2020); paraprevotella colonization protects IgA from degradation by trypsin and reduces intestinal disease. Therefore, the presence of trypsin-degrading commensal colonization may have a role in preserving the balance and stability of the intestines and safeguarding against infection by pathogens (Qiu et al., 2006). The preceding analysis indicates that low molecular weight ASPS-1 exerts a more pronounced effect on fecal microorganisms and is capable of effectively regulating intestinal flora. It promotes the colonization of beneficial bacteria while reducing the number of harmful bacteria, which reduces the occurrence of diseases.
Prior research has indicated that Bacteroidetes rely on a diverse array of glycoside hydrolases and carbohydrate metabolism pathways (Liu et al., 2018a). They have the ability to generate various SCFAs including acetic, propionic, and butyric acids (Gangadoo et al., 2018). Acetic acid, in particular, serves as a secondary energy source for intestinal epithelial cells and provides energy for muscle and brain tissues. Additionally, it can hinder the growth of harmful bacteria, possess anti-inflammatory properties, and effectively alleviate inflammation in the intestinal tract (Morrison and Preston, 2016). Propionate regulates gut microbiota homeostasis (He et al., 2020). Butyric acid promotes the activity of beneficial bacilli in the intestinal tract and accelerates metabolism (Sokol et al., 2008). The investigation of the correlation between the production of SCFAs and the key genera revealed a positive correlation between the genera that produce SCFAs and the production of acetic, propionic, and butyric acids. The ASPS-1 group effectively promoted the proliferation of beneficial bacteria such as fecalibacterium, Paraprevotella, Parabacteroides_B_862066 and Bacteroides_H; and reduced the abundance of harmful bacteria such as Fusobacteria. Combined with the LEfSe differential flora analysis, the ASPS-1 group found fecalibacterium in 25 differential flora, and fecalibacterium was significantly correlated in the correlation analysis with short-chain fatty acids; therefore, ASPS-1 produces short-chain fatty acids by regulating fecalibacterium (Li et al., 2020; Tian et al., 2022).
The LEfSe method was employed to assess statistically significant variations between groups at every taxonomic level of the species (Wang et al., 2021). The LDA score histogram depicted in Figure 7A illustrates the biomarkers that possess statistical significance and unveils the essential communities within each group (Li et al., 2022a). The preceding investigation shown that the introduction of various components of ASPSs had a notable impact on the crucial species of the fecal microbiota, particularly ASPS-1. Proteobacteria were found in the ASPS-1 group, many bacteria in the Proteobacteria phylum regulated fecal homeostasis (Chen et al., 2022). The results of the above studies showed that the addition of different ASPSs components had different effects on the intestinal microorganisms of broilers, while low molecular weight ASPS-1 could effectively promote the increase of SCFAs-producing bacteria and regulate the intestinal flora balance at the same time. These findings provide a compelling rationale for the development of ASPS-1 as a prebiotic.
CONCLUSIONS
The experiments used the chemical method of H2O2+Vc to successfully degrade the high molecular weight Acanthopanax polysaccharides into low molecular weight polysaccharides, and the degraded Acanthopanax polysaccharides had lower molecular weight and viscosity. In vitro, digestive simulation showed that the low molecular weight spikenard polysaccharide ASPS-1 was less susceptible to degradation by gastrointestinal digestive juices. In vitro, probiotic experiments showed that compared with ASPS, low molecular weight ASPS-1 had a better probiotic effect on Lactobacilli and Bifidobacteria. In vitro simulated digestion showed that the low molecular weight polysaccharide ASPS-1 was more favorable to be utilized by the intestinal flora. Combined 16S rRNA and SCFAs analysis showed that ASPS-1 increased intestinal short-chain fatty acid content, decreased intestinal pH, and increased the diversity and abundance of intestinal flora by regulating fecalibacterium. These results studies favor the application of low molecular weight Acanthopanax polysaccharides in broiler digestive tract health.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China, grant number: 32172901 (funder: Xiuling Chu).
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103807.
Appendix. Supplementary materials
REFERENCES
- Al-Wraikat M., Liu Y., Wu L., Ali Z., Li J. Structural characterization of degraded lycium barbarum L. leaves’ polysaccharide using ascorbic acid and hydrogen peroxide. Polymers. 2022;14:1404. doi: 10.3390/polym14071404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin J.C., Paul J.M., Michael J.R., Andrew W.H., Jo J.A., Susan P.H. DADA2: High resolution sample inference from amplicon data. bioRxiv. 2015 [Google Scholar]
- Chen B., Shi M., Cui S., Hao S., Hider R., Zhou T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from Sargassum fusiforme by degradation. Int. J. Biol. Macromolecules. 2016;92:715–722. doi: 10.1016/j.ijbiomac.2016.07.082. [DOI] [PubMed] [Google Scholar]
- Chen P., Lei S., Tong M., Chang Q., Zheng B., Zhang Y., Zeng H. Effect of polysaccharide fractions from Fortunella margarita on the fecal microbiota of mice and SCFA production in vitro. Food Sci. Human Wellness. 2022;11:97–108. [Google Scholar]
- Chen P., You Q., Li X., Chang Q., Zhang Y., Zheng B., Hu X., Zeng H. Polysaccharide fractions from Fortunella margarita affect proliferation of Bifidobacterium adolescentis ATCC 15703 and undergo structural changes following fermentation. Int. J. Biol. Macromolecules. 2019;123:1070–1078. doi: 10.1016/j.ijbiomac.2018.11.163. [DOI] [PubMed] [Google Scholar]
- Chen S., Liu H., Yang X., Li L., Qi B., Hu X., Ma H., Li C., Pan C. Degradation of sulphated polysaccharides from Grateloupia livida and antioxidant activity of the degraded components. Int. J. Biol. acromolecules. 2020;156:660–668. doi: 10.1016/j.ijbiomac.2020.04.108. [DOI] [PubMed] [Google Scholar]
- Dai D., Qi G., Wang J., Zhang H., Qiu K., Han Y., Wu Y., Wu S. Dietary organic acids ameliorate high stocking density stress-induced intestinal inflammation through the restoration of intestinal microbiota in broilers. J. Anim. Sci. Biotechnol. 2022;13:124. doi: 10.1186/s40104-022-00776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Li D., Bai S., Wang J., Zeng Q., Su Z., Xuan Y., Zhang K. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poult. Sci. 2018;97:874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Duan H., Yu Q., Ni Y., Li J., Fan L. Effect of Agaricus bisporus polysaccharides on human gut microbiota during in vitro fermentation: an integrative analysis of microbiome and metabolome. Foods. 2023;12:859. doi: 10.3390/foods12040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois M., Gilles K.A., Hamilton J.K., Rebers P.t., Smith F. Colorimetric method for determination of sugars and related substances. Analyt. Chem. 1956;28:350–356. [Google Scholar]
- Fan Q., Abouelezz K., Li L., Gou Z., Wang Y., Lin X., Ye J., Jiang S. Influence of mushroom polysaccharide, nano-copper, copper loaded chitosan, and lysozyme on intestinal barrier and immunity of lps-mediated yellow-feathered chickens. Animals. 2020;10:594. doi: 10.3390/ani10040594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R.-s., Dong Y.-c., Chen F., Chen Q.-h. Bacterial diversity analysis during the fermentation processing of traditional Chinese yellow rice wine revealed by 16S rDNA 454 pyrosequencing. J. Food Sci. 2015;80:M2265–M2271. doi: 10.1111/1750-3841.13018. [DOI] [PubMed] [Google Scholar]
- Fathima S., Shanmugasundaram R., Adams D., Selvaraj R. Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods (Basel, Switzerland) 2022;11:1401. doi: 10.3390/foods11101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadoo S., Dinev I., Chapman J., Hughes R., Van T., Moore R., Stanley D. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl. Microbiol. Biotechnol. 2018;102:1455–1466. doi: 10.1007/s00253-017-8688-4. [DOI] [PubMed] [Google Scholar]
- Guo C., Wang Q., Zhang S., Zhang X., Du Z., Li M., Ding K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Int. J. Biol. Macromolecules. 2021;181:357–368. doi: 10.1016/j.ijbiomac.2021.03.137. [DOI] [PubMed] [Google Scholar]
- He J., Zhang P., Shen L., Niu L., Tan Y., Chen L., Zhao Y., Bai L., Hao X., Li X., Zhang S., Zhu L. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21176356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Lin S., Zheng B., Cheung P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018;58:1243–1249. doi: 10.1080/10408398.2016.1245650. [DOI] [PubMed] [Google Scholar]
- Hu W., Di Q., Liang T., Zhou N., Chen H., Zeng Z., Luo Y., Shaker M. Effects of in vitro simulated digestion and fecal fermentation of polysaccharides from straw mushroom (Volvariella volvacea) on its physicochemical properties and human gut microbiota. Int. J. Biol. Macromolecules. 2023;239 doi: 10.1016/j.ijbiomac.2023.124188. [DOI] [PubMed] [Google Scholar]
- Hua Y., Yingping X., Guohong G., Li J., Wang J., Li D. Microbial community and short-chain fatty acid profile in gastrointestinal tract of goose. Poult. Sci. 2018;97:1420–1428. doi: 10.3382/ps/pex438. [DOI] [PubMed] [Google Scholar]
- Jablaoui A., Kriaa A., Mkaouar H., Akermi N., Soussou S., Wysocka M., Wołoszyn D., Amouri A., Gargouri A., Maguin E., Lesner A., Rhimi M. Fecal serine protease profiling in inflammatory bowel diseases. Front. Cell. Infect. Microbiol. 2020;10:21. doi: 10.3389/fcimb.2020.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B., Lin H., Yu S., Liu N., Yu D., Wu A. Mycotoxin deoxynivalenol-induced intestinal flora disorders, dysfunction and organ damage in broilers and pigs. J. Hazard. Mater. 2023;451 doi: 10.1016/j.jhazmat.2023.131172. [DOI] [PubMed] [Google Scholar]
- Krautkramer K.A., Fan J., Bäckhed F. Gut microbial metabolites as multi-kingdom intermediates. Nature Rev. Microbiol. 2021;19:77–94. doi: 10.1038/s41579-020-0438-4. [DOI] [PubMed] [Google Scholar]
- Li H., McKee L. Measuring enzyme kinetics of glycoside hydrolases using the 3,5-dinitrosalicylic acid assay. Methods Mol. Biol (Clifton, N.J.) 2023;2657:15–25. doi: 10.1007/978-1-0716-3151-5_2. [DOI] [PubMed] [Google Scholar]
- Li S., Qi Y., Chen L., Qu D., Li Z., Gao K., Chen J., Sun Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromolecules. 2019;124:931–937. doi: 10.1016/j.ijbiomac.2018.11.271. [DOI] [PubMed] [Google Scholar]
- Li X., Guo R., Wu X., Liu X., Ai L., Sheng Y., Song Z., Wu Y. Dynamic digestion of tamarind seed polysaccharide: indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydrate Polymers. 2020;239 doi: 10.1016/j.carbpol.2020.116194. [DOI] [PubMed] [Google Scholar]
- Li J., Xiao Y., Fan Q., Yang H., Yang C., Zhang G., Chen S. Dietary bacitracin methylene disalicylate improves growth performance by mediating the gut microbiota in broilers. Antibiotics (Basel, Switzerland) 2022;11:818. doi: 10.3390/antibiotics11060818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Watanabe E., Kawashima Y., Plichta D.R., Wang Z., Ujike M., Ang Q.Y., Wu R., Furuichi M., Takeshita K., Yoshida K., Nishiyama K., Kearney S.M., Suda W., Hattori M., Sasajima S., Matsunaga T., Zhang X., Watanabe K., Fujishiro J., Norman J.M., Olle B., Matsuyama S., Namkoong H., Uwamino Y., Ishii M., Fukunaga K., Hasegawa N., Ohara O., Xavier R.J., Atarashi K., Honda K. Identification of trypsin-degrading commensals in the large intestine. Nature. 2022;609:582–589. doi: 10.1038/s41586-022-05181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Chang R., Zhang X., Wang Z., Wen J., Zhou T. Non-isoflavones diet incurred metabolic modifications induced by constipation in rats via targeting gut microbiota. Front. Microbiol. 2018;9:3002. doi: 10.3389/fmicb.2018.03002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang X., Ou S., Arowolo M., Hou D., He J. Effects of achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polymers. 2018;10:1233. doi: 10.3390/polym10111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li Q., Yang Y., Guo A. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.736739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Li S., Wang X., Xing T., Li J., Zhu X., Zhang L., Gao F. Microbiota populations and short-chain fatty acids production in cecum of immunosuppressed broilers consuming diets containing γ-irradiated Astragalus polysaccharides. Poult. Sci. 2021;100:273–282. doi: 10.1016/j.psj.2020.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X., Hu X., Zhou S., Xiang H., Chen S., Li L., Liu S., Yang X. Optimized degradation and inhibition of α-glucosidase activity by gracilaria lemaneiformis polysaccharide and its production in vitro. Marine Drugs. 2021;20:13. doi: 10.3390/md20010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C., Bai J., Shao C., Liu J., Zhang Y., Li X., Yang Y., Xu Y., Wang L. Degradation of blue honeysuckle polysaccharides, structural characteristics and antiglycation and hypoglycemic activities of degraded products. Food Res. Int. (Ottawa, Ont.) 2021;143 doi: 10.1016/j.foodres.2021.110281. [DOI] [PubMed] [Google Scholar]
- Meng Q., Pan J., Liu Y., Chen L., Ren Y. Anti‑tumour effects of polysaccharide extracted from Acanthopanax senticosus and cell‑mediated immunity. Exp. Therap. Med. 2017;15:1694–1701. doi: 10.3892/etm.2017.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrackova N., Copova I., Stovicek A., Makovska M., Schierova D., Mrazek J., Sabolova M., Vlkova E., Hradsky O., Bronsky J., Nevoral J., Neuzil-Bunesova V. Microbial shifts of faecal microbiota using enteral nutrition in vitro. J. Functional Foods. 2021;77 [Google Scholar]
- Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z., Hingley S.T., Simmons G., Yu C., Sarma J.D., Bates P., Weiss S.R. Endosomal proteolysis by cathepsins is necessary for murine coronavirus mouse hepatitis virus type 2 spike-mediated entry. J. Virol. 2006;80:5768–5776. doi: 10.1128/JVI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol H., Pigneur B., Watterlot L., Lakhdari O., Bermúdez-Humarán L., Gratadoux J., Blugeon S., Bridonneau C., Furet J., Corthier G., Grangette C., Vasquez N., Pochart P., Trugnan G., Thomas G., Blottière H., Doré J., Marteau P., Seksik P., Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Li Q., Everaert N., Liu R., Zheng M., Zhao G., Wen J. Effects of inulin supplementation on intestinal barrier function and immunity in specific pathogen-free chickens with Salmonella infection. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skz396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Geng Y., Xu T., Zou X., Mao R., Pi X., Wu W., Huang L., Yang K., Zeng X., Sun P. Digestive characteristics of hericium erinaceus polysaccharides and their positive effects on fecal microbiota of male and female volunteers during in vitro fermentation. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.858585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartoukian S.R., Palmer R.M., Wade W.G. Diversity and morphology of members of the phylum “Synergistetes” in periodontal health and disease. Appl. Environ. Microbiol. 2009;75:3777–3786. doi: 10.1128/AEM.02763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu Q., Ye F., Tang H., Xiong Y., Wu Y., Wang L., Feng X., Zhang S., Wan Y., Huang J. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express. 2021;11:31. doi: 10.1186/s13568-021-01190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Bai R., Hu D., Chen W., Xiao Z., Huang J., Wan X., Yang J., Yu X. Structure analysis and in vitro evaluation of probiotic properties for polysaccharides from Phellinus baumii extracted with phosphotungstic acid assistance. LWT. 2023;188 [Google Scholar]
- Wang X., Su Y., Su J., Xue J., Zhang R., Li X., Li Y., Ding Y., Chu X. Optimization of enzyme−assisted aqueous extraction of polysaccharide from acanthopanax senticosus and comparison of physicochemical properties and bioactivities of polysaccharides with different molecular weights. Molecules. 2023;28:6585. doi: 10.3390/molecules28186585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zhou X., Shu Z., Zheng Y., Hu X., Zhang P., Huang H., Sheng L., Zhang P., Wang Q., Wang X., Li N. Regulation strategy, bioactivity, and physical property of plant and microbial polysaccharides based on molecular weight. Int. J. Biol. Macromolecules. 2023;244 doi: 10.1016/j.ijbiomac.2023.125360. [DOI] [PubMed] [Google Scholar]
- Wang Y., Sheng H.-F., He Y., Wu J.-Y., Jiang Y.-X., Tam N.F.-Y., Zhou H.-W. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags. Appl. Environ. Microbiol. 2012;78:8264–8271. doi: 10.1128/AEM.01821-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Zheng Y., Lai Z., Hu X., Wang L., Wang X., Li Z., Gao M., Yang Y., Wang Q., Li N. Effect of monosaccharide composition and proportion on the bioactivity of polysaccharides: a review. Int. J. Biol. Macromolecules. 2024;254 doi: 10.1016/j.ijbiomac.2023.127955. [DOI] [PubMed] [Google Scholar]
- Wu D.T., Liu W., Yuan Q., Gan R.Y., Hu Y.C., Wang S.P., Zou L. Dynamic variations in physicochemical characteristics of oolong tea polysaccharides during simulated digestion and fecal fermentation in vitro. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018;97:3489–3493. doi: 10.3382/ps/pey220. [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhang H., Zhang R., Cao G., Li Q., Zhang B., Wang Y., Yang C. Serum metabolome and gut microbiome alterations in broiler chickens supplemented with lauric acid. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang X., Yan X., Zhang J., Wang L., Xue H., Jiang G., Ma X., Liu X. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromolecules. 2019;135:706–716. doi: 10.1016/j.ijbiomac.2019.05.166. [DOI] [PubMed] [Google Scholar]
- Yang B., Li X., Mesalam N., Elsadek M., Abdel-Moneim A. The impact of dietary supplementation of polysaccharide derived from Polygonatum sibiricum on growth, antioxidant capacity, meat quality, digestive physiology, and gut microbiota in broiler chickens. Poultry science. 2024;103 doi: 10.1016/j.psj.2024.103675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Shan C., Ma X., Qin Y., Ju A., Duan A., Li W., Zhang Y. Immunomodulatory effect of Acanthopanax senticosus polysaccharide on immunosuppressed chickens. Poult. Sci. 2021;100:623–630. doi: 10.1016/j.psj.2020.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob M.U., El-Hack M.E.A., Hassan F., El-Saadony M.T., Khafaga A.F., Batiha G.E., Yehia N., Elnesr S.S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cai K., Mishra R., Jha R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020;99:4776–4785. doi: 10.1016/j.psj.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Chen C., Huang Q., Li C., Fu X. Preparation and characterization of Sargassum pallidum polysaccharide nanoparticles with enhanced antioxidant activity and adsorption capacity. Int. J. Biol. Macromolecules. 2022;208:196–207. doi: 10.1016/j.ijbiomac.2022.03.082. [DOI] [PubMed] [Google Scholar]
- Zhang S., Zhu C., Xie H., Wang L., Hu J. Effect of gan cao (Glycyrrhiza uralensis Fisch) polysaccharide on growth performance, immune function, and gut microflora of broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R., Yang F., Bai Y., Zhao J., Hu M., Zhang X., Dou T., Jia J. Research progress on the mechanisms underlying poultry immune regulation by plant polysaccharides. Front. Vet. Sci. 2023;10 doi: 10.3389/fvets.2023.1175848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.