Abstract
Tick-borne flaviviruses and Borrelia spp. are globally spread pathogens of zoonotic potential that are maintained by a transmission cycle at the interface between ticks and vertebrate hosts, mainly wild animals. Aside data on pathogen burden in ticks, information on the status of various hosts relative to infection is important to acquire. We reviewed how those infections have been studied in wildlife host species in the field to discuss how collected data provided relevant epidemiological information and to identify needs for further studies. The literature was screened for observational studies on pathogen or antibody detection for tick-borne Borrelia spp. and flaviviruses in wildlife host animals. Overall, Borrelia spp. were more studied (73% of case studies, representing 297 host species) than flaviviruses (27% of case studies, representing 114 host species). Studies on both Borrelia spp. and flaviviruses focused mainly on the same species, namely bank vole and yellow-necked mouse. Most studies were order-specific and cross-sectional, reporting prevalence at various locations, but with little insight into the underlying epidemiological dynamics. Host species with potential to act as reservoir hosts of these pathogens were neglected, notably birds. We highlight the necessity of collecting both demographics and infection data in wildlife studies, and to consider communities of species, to better estimate zoonotic risk potential in the One Health context.
Keywords: Tick-borne diseases, Wildlife, Borrelia, Flavivirus, Reservoir, Sentinel, Host
1. Introduction
Vector-borne and zoonotic infections are emerging threats to public health [1]. Tick-borne pathogens (TBP) are maintained at the interface between tick vectors and a large variety of vertebrate hosts permitting ticks to feed and complete their life cycles [2]. Transmission to humans occur by tick-bites on specific occasions, and understanding and quantifying this transmission risk is necessary to control diseases.
Beyond their feeding role for ticks, vertebrate species can act as pathogen reservoir or sentinel hosts. Infection and exposure data from both give precious information on dynamics of the studied TBP [3]. Whilst reservoir hosts allow the pathogen to multiply and be transmitted further, sentinel species are characterized by their ability to reflect underlying epidemiological phenomena and by being of easy access [4]. For instance, wild red and Arctic foxes have been suggested as sentinels for human and animal toxoplasma risk in Canada, based on serology and direct detection on carcasses [5]. Also, animal ecology can drive variations in risk of transmission to human [6]. Therefore, because understanding pathogens' transmission and distribution in wildlife is prerequisite to addressing risk of transmission, quantitatively tracking infection and exposure to pathogens in those hosts is necessary.
Tick-borne Borreliaceae and flaviviruses are TBP of zoonotic potential which are the most prevalent in the temperate regions of the world [7], and are thus of interest for human and veterinary public health. Tick-borne encephalitis virus (TBEV), first described in Austria in 1931, is the most common tick-borne flavivirus (TBFV) in Europe with several endemic foci in Europe and Asia and growing number of human cases [8]. Its ecology and epidemiology have early been studied in Europe, especially in Czech Republic [9]. Other TBFV have later been isolated from wild animals and their ticks, such as Meaban virus (MEAV) in Brittany, France [10], or from human cases, such as Powassan virus (POWV) in Powassan town, Canada [11]. Prominent vectors of TBFV are hard ticks Ixodes ricinus in Western and Central Europe and I. persulcatus in Eastern Europe, as well as local tick species in other parts of the world. Similarly transmitted bacterial genospecies of the Borrelia burgdorferi sensu lato (Bbsl) complex are the causative agents for Lyme disease, one of the most common TBD in the Northern Hemisphere. Other tick-borne Borrelia spp. cause relapsing fever in humans [12].
Recent reviews on tick-borne Borrelia spp. and TBFV have focused on getting information on the biology of infection in vertebrate hosts [13,14], reporting prevalence and clinical cases in human [15,16] and discussing diagnostic methodologies [17,18], the role of non-vector transmissions for TBFV [19,20], or on the importance of modelling TBP regarding climate change [21,22]. Some reviews have focused on wildlife hosts but only at a national or continental scale [23,24], or on reservoirs associated with flaviviruses in general, with little focus on TBFV [25]. Finally, a lot of research effort has focused on studying the infectious agents in the ticks [26,27].
Despite their major importance in zoonotic transmission, no study has reviewed the body of evidence in wildlife hosts from field data at a global scale. Yet, data on the level of infection and exposure in free-ranging populations are critical to model the dynamics of infectious diseases [28]. Strong inference about the transmission processes underlying those dynamics often requires a combination of approaches, notably experiments to ascertain the role of reservoir [29], but data on infection and exposure of hosts are prerequisite in most cases. When it comes to wildlife species, a strong heterogeneity in the type of field data is nevertheless expected, from local cross-sectional sampling of particular species, to broad spatial surveys over series of years. Estimating epidemiological parameters also requires accounting for potential biases in detection probabilities and host population parameters [30], which demands specific designs.
The aim of this review was thus to investigate how tick-borne Borrelia spp. and flaviviruses infection burdens have been studied in free-ranging wildlife (that is, excluding studies of TBP in ticks) to discuss how collected data provided relevant epidemiological information, and identify needs for further studies. We hypothesized that most studies would have focused on a few expected common host species sharing landscape use with humans in the Northern Hemisphere. We anticipated that monitoring of wildlife diseases would have faced difficulties due to laboratory testing constraints. Finally, we expected that few studies would have included whole communities of potential hosts because of the perceived difficulty of extensive sampling, which may also have precluded accounting for temporal and spatial variations and uncertainties in the eco-epidemiological processes.
2. Material and methods
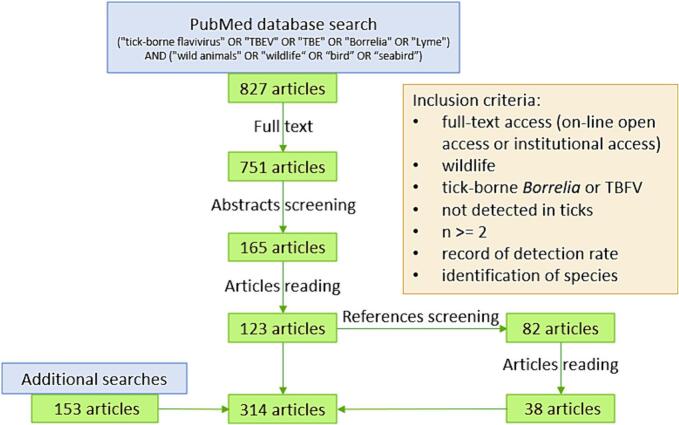
The following keyword formula was applied on PubMed to search articles: “(“tick-borne-flavivirus” OR “TBEV” OR “TBE” OR “Borrelia” OR “Lyme”) AND (“wild animals” OR “wildlife” OR “bird OR “seabird”)”. For details, see Supplementary material 1 (Material and methods). We included in this study articles that presented data on at least two free-ranging wildlife host individuals of a same species, sampled and tested for an identified tick-borne Borrelia spp. or flavivirus (Fig. 1). Additional searches were made as described in Supplementary material 1 (Material and methods). In the selected articles, we defined a ‘case study’ as a unique combination of one host species studied for one pathogen on one study area delimited over time and space. For each case study, we retrieved the following information: host species, pathogen species, prevalence, number of tested individuals, biological material for test, detection and validation methods and study area and period. The corresponding table is available in Supplementary material 2.
Fig. 1.
Diagram of inclusion and exclusion in PubMed database screening. See section Results - Literature metrics of Supplementary material 1 for details on literature search and additional searches.
3. Results
Among the 314 selected articles, we retrieved 947 case studies (Fig. 2), published between 1959 and 2022. Fig. 2 links the taxonomic order of the tested host species to the investigated pathogen. Below we present the main information on studied hosts, and their infection status. Detailed information on the literature search results is also available in Supplementary material 1 (see section Results).
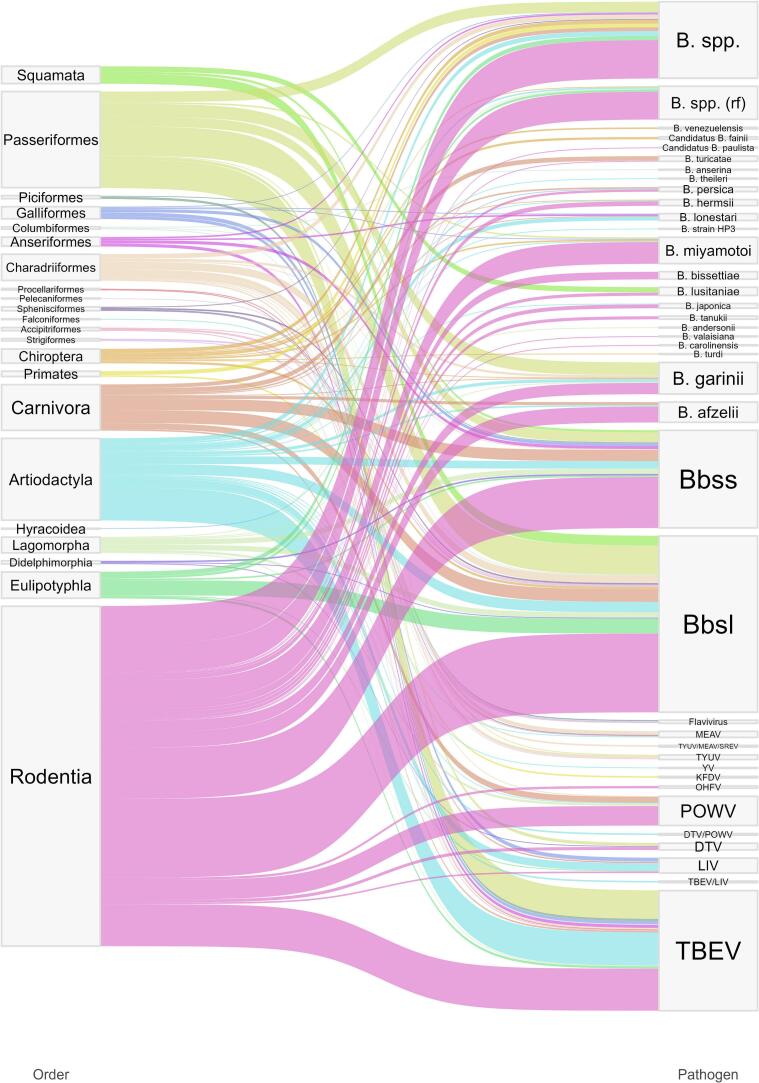
Fig. 2.
Sankey diagram pairing case studies by pathogen and taxonomic order. Width of the ribbon is proportional to the number of cases for this taxonomic order-pathogen couple. rf = relapsing fever. Data are available in Supplementary material 2.
3.1. Studied hosts and pathogens
The case studies gathered information from 349 host species belonging to 22 taxonomic orders (Fig. 2, Supplementary material 2). Mammals represented 75% (710/947) of all case studies, 78% (537/689) of Borrelia studies and 67% (173/258) of TBFV studies. Birds represented 23% (214/947) of all studies, 19% (129/689) of Borrelia studies and 33% (85/258) of TBFV studies. In terms of taxonomic order, Rodentia was the most investigated, representing around a half (53%, 363/689) of the Borrelia studies and a third (35%, 91/258) of the TBFV studies, followed by Passeriformes (13.5%, 128/947), Artiodactyls (11.5%, 109/947) and Carnivores (6.4%, 61/947).
Borrelia spp. were more studied (73% of case studies, in 297 host species) than flaviviruses (27% of case studies, in 114 host species), with B. burgdorferi sensu lato and TBEV and POWV being the most investigated pathogens. Whilst most Borrelia studies (71%) looked for pathogen's genome with molecular techniques to identify ongoing infections, 89% of flaviviruses studies used serological methods. ELISA were the most used serological techniques (89% of flaviviruses cases, and 38% of overall cases). Finally, the median sample size was at 40 (minimum 2, IQR 14–119, maximum 3186), threshold value we used to present summary measures in the following paragraphs.
3.2. Infection and exposure in hosts
When considering all Borrelia species, the overall direct detection (of whole or part of the infectious agent) and seroprevalence tended to uniformize between 15% and 30% as the number of tested individuals increased (Fig. S2A). In studies with over 40 tested individuals, the percentage of active infections nevertheless ranged from 0.4% for B. lusitaniae in Eastern grey squirrel (Sciurus carolinensis) to 90% for Bbsl in cotton mouse (Peromyscus gossypinus) [31]. Regarding only relapsing-fever associated Borrelia, direct detection ranged from 0.2% in house mouse (Mus musculus) for an unknown relapsing-fever Borrelia [32] to 50% for B. miyamotoi in wild turkey (Meleagris gallopavo) [33]. In mammals, including rodents, artiodactyls and carnivores, the level of direct detection and seroprevalence of Borrelia spp. were between 20% and 25% (Fig. 3A and 3B).
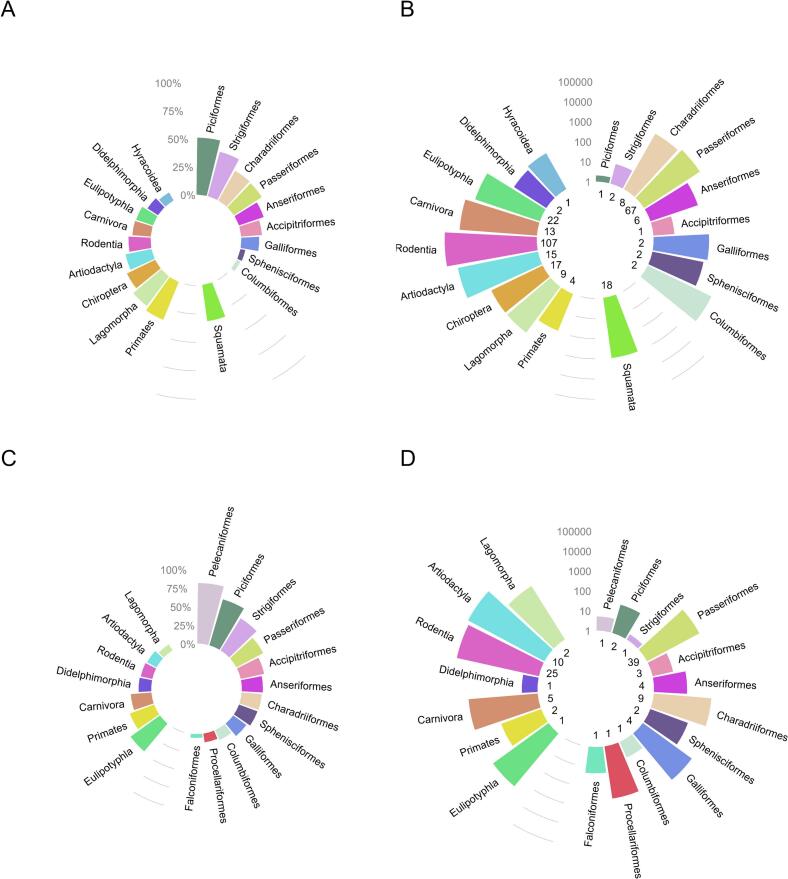
Fig. 3.
A-D: Investigation efforts for TBP by taxonomic orders, expressed as average percentage of positives and number of individuals tested, by taxonomic Order. Both serological and “active infection” data are presented. Top row shows for Borrelia spp. (A) the average percentage of animals positive to Borrelia by order, (B) the number of tested animals in each order. Bottom row shows for TBFV (C) the percentage of animals positive to TBFV by order, (D) the number of tested animals in each order. Most publications regarding TBFV used an indirect methodology (antibody detection), direct detection was used in only 11% of TBFV cases. (B) and (D) show the number of tested individuals on a log10 scale, and the number of different host species tested in each order is shown at the bottom of each bar. Orders are grouped by class, Mammalia are shown to the left, Aves to the right, and for Borrelia spp., Lepidosauria at the bottom. Data are available in Supplementary material 2.
In rodent studies testing more than 40 individuals, the average percentage of Borrelia direct detection was 11.7% (median 5.5%, IQR 2–15%) and average seroprevalence was almost twice higher (mean 21.8%, median 15%, IQR 9.2–31.5%) (Table 1, Fig. S3). Variations in prevalence across studies were observed not only for different species but also within species, such as for Bbsl direct detection in yellow-necked mouse tissues varying from 5.8% in Hungary in 2014–2015 [34] to 23% in Germany in 2012–2014 [35]. In artiodactyl studies with more than 40 sampled animals, average prevalence of Borrelia DNA detection was 14.4% (median 12%, IQR 7–16.3%), while average seroprevalence was 23.9% (median 18.5%, IQR 8.8–31.4%) (Table 1, Fig. S3). They were of 12.3% (median 8.3%, IQR 4.5–14%) and 23% (median 15.4%, IQR 7.6–26.4%) in birds (Table 1, Fig. S3). Among birds, Columbiformes, Passeriformes, Charadriiforms and Galliformes were the most studied in terms of number of tested individuals (Fig. 3B), with Charadriiforms and Passeriformes reaching 29% of seroprevalence (Fig. 3A). Among the least studied species, Chiropters presented both seropositivity (36%) and pathogen prevalence (25%) of rather high level (Fig. 3A).
Table 1.
Prevalence of direct detection and seroprevalence by taxonomic order, extracted from studies with more than 40 individuals. n = number of cases studies, median values, mean, range and inter-quartile range (IQR). Source data is available in Supplementary material 2. See plots of distributions in Fig. S3.
| Studies with ≥40 tested individuals in: | Rodents (n = 253) | Artiodactyls (n = 90) | Birds (n = 59) | |
|---|---|---|---|---|
| Borrelia | Direct detection |
n = 148 median 5.5% mean 11.7% range 0.2–90% IQR 2–15% |
n = 16 median 12% mean 14.4% range 0.8%–49.7% IQR 7–16.3% |
n = 23 median 8.3% mean 12.3% range 1–51% IQR 4.5–14% |
| Seroprevalence |
n = 47 median 15% mean 21.8% range 0.7–89% IQR 9.2–31.5% |
n = 28 median 18.5% mean 23.9% range 0.8–69% IQR 8.8–31.4% |
n = 15; median 15.4% mean 23% range 3–78.6% IQR 7.6–26.4% |
|
| TBFV | Seroprevalence | n = 47 median 5.8% mean 9.4% range 0.4–44.4% IQR 3.5–10.5% |
n = 45 median 5.3% mean 9.7% range 0.07–62.7% IQR 2.2–10.2% |
n = 20 median 19% mean 20.8% range 0.5–68.1% IQR 3.1–33.3% |
The overall average seroprevalence for tick-borne flaviviruses ranged from 8% for MEAV to 51% for Tyuleniy virus (TYUV) (Fig. S2B), but with heterogeneous sample sizes. Among studies with more than 40 tested individuals, seroprevalences ranged from 0.07% for MEAV in red deer (Cervus elaphus) [36] to 68% for TYUV/MEAV/Saumarez Reef virus (SREV)-like in European herring gull (Larus argentatus) [37]. For TBFV of the mammalian group, seroprevalence ranged from 0,1% for TBEV/LIV in roe deer (Capreolus capreolus) [38] to 63% for TBEV in European bison (Bison bonasus) [39].
In rodent studies with at least 40 samples, the average TBFV-seropositivity was 9.4% (median 5.8%, IQR, range 3.5–10.5%) (Table 1, Fig. S3), lower than for Borrelia. The average seroprevalence in artiodactyls was 9.7% (median 5.3%, IQR 2.2–10.2%) and was more than twice as much in birds (mean 20.8%, median 19%, IQR 3.1–33.3%) (Table 1, Fig. S3). Most studied avian orders were Passeriformes, Charadriiforms, Galliformes and Procellariforms, with highest seroprevalence for Passeriformes (about 40%), followed by Charadriiforms (27%) and Galliformes (21%), when Procellariforms tended to have less seropositive individuals (Fig. 3C). Whilst 80% of Pelecaniformes had antibodies to TYUV, for this order only 8 European shags (Phalacrocorax aristotelis) were tested on a single location (in Brittany, France) in the eighties [37], highlighting the weight small scale studies could have. Exceptionally, mammalian Eulipotyphla were well-studied by PCR techniques and showed high rates of viremia (of about 50%) [40].
4. Discussion
4.1. Early research papers (decades 1950s–1980s) shaped research on rodents as reservoirs
Rodentia was the most investigated order, representing more than half (363/689) of the Borrelia case studies and more than a third (91/258) of the TBFV case studies. White-footed mouse (Peromyscus leucopus) was investigated in the first published studies from Northern America, and has been regarded since the eighties as the most common Borrelia spp. reservoir host in wildlife in Northern America [41]. Regarding TBFV, a series of studies were also initially conducted in Northern America. The first published studies looked at Powassan virus (POWV) in Peromyscus genus, only present in Northern America, and secondly in Microtus and Mus genera, present on the continent. In addition, in Canada, a series of studies from the same authors presented rodents as primary reservoirs for POWV. For this, species such as Tamias, Urocitellus and Callospermophilus chipmunks and Tamiasciurus and Sciurus squirrels were investigated, with average seroprevalence of 15% [[42], [43], [44], [45], [46], [47], [48], [49], [50]]. Groundhogs (Marmota monax) sampled in the same studies reported the highest seroprevalence (around 43%) [[46], [47], [48]]. In parallel, several studies on the eco-epidemiology of TBFV were conducted in the former Union of Soviet Socialist Republics but were not published in English, although some articles are available with translation systems such as those of NAMRU-3. Rodents were later sampled on other continents and TBFV detections were several times successful in Europe in Apodemus, Microtus and Myodes genera [42], and in Micromys, Mus and Sciurus for Borrelia [51,52]. In Europe, bank vole (Myodes glareolus) and yellow-necked mouse (Apodemus flavicollis) were recognized as reservoirs for TBEV [53]. Bbsl was detected in Tamias, Tamiasciurus and Neotoma in North America [54]. Furthermore, Gerbillus, Mastomys, Praomys and Apodemus, Cricetulus, Eothenomys, Meriones, Mus, Niviventer and Rattus bore relapsing fever associated Borrelia respectively in Africa and in Asia. Notably, Eastern grey squirrels (Sciurus carolinensis) similarly carried Borrelia DNA, both in their native countries and where they are invasive in Europe.
4.2. Deer, broadly studied as sentinels
The second most investigated order by the number of tested individuals was Artiodactyla. Artiodactyls were mainly represented by the Cervidae family, in terms of numbers of tested animals (82%, 26,090/31,862) and investigated species (56%, 9/16). Deer are considered incompetent reservoirs for Bbsl by some authors because of borreliacidal properties of their sera [55]. Yet, studies showed that the complement borreliacidal activity depends on strain and also exists on other Borrelia strains for other vertebrates, including rodents [56]. Despite their poor competence as reservoirs, deer are frequently met across the world and can be infected by feeding adult ticks they commonly host [57]. In Cervidae, species locally present in America, Europe and Asia showed similar level of TBP contact, with average seroprevalence of 18%. Restraint home ranges, of no more than 10km2 among the different deer species [58,59], allowed investigating infection processes at small spatial scales. Moreover, surveillance activities and pathogen detection can be facilitated through deer hunting, allowing widespread sampling programmes on a regular basis for reasonable costs and working efforts [60]. As such, deer could help identifying endemic or emerging risk areas and give information on underlying processes, and were repeatedly considered as sentinels. Other game artiodactyls identified in this review, such as wild boar (Sus scrofa), offer the same kind of possibilities for sentinel surveillance.
4.3. Birds, reservoirs, have been overlooked
Birds were also reported as tick-borne Borrelia spp. reservoirs in the literature [61]. Contrary to rodents, the significance of birds as reservoirs of TBP may have been overlooked, considering that whilst birds represented about 19% (129/689) of Borrelia case studies and 33% (85/258) of TBFV case studies, these came from only 12% (28/228) and 18% (16/89) of the articles. In those, pathogen presence was often screened in birds caught in a banding configuration. For instance, in one article, Newman et al. captured birds on the University of California's campus in 2003–2004 [62]. Their wide-range study allowed detecting Bbsl in 23 species out of 53 caught species. Mistnetting is a common field method efficient for capturing birds, hence for detecting potential reservoirs. However, this approach is mostly favourable for Passeriformes, and passerines was the avian order the most often studied as potential reservoir for TBP. In addition, for migratory species or those exploiting large areas during their life cycle, the infection status might not always reflect the local infection processes. Nevertheless, combined with movement information, they can unravel critical information on pathogen dissemination over long distances and between various ecosystems [63]. We hypothesized that limited availability of data on birds might be due to logistical difficulties related to blood sampling in the wild (e. g. training and legal requirements, ethical concerns for endangered species and practical implementation). However, antibodies against Gadgets Gully virus and SREV were extensively studied in a collection of Australian seabirds' sera [64], as a unique occurrence of exposure information on rare TBFV associated with birds.
4.4. Least studied species with growing interest
Other vertebrate species of interest have been less studied. Recently, new species of tick-borne Borrelia spp. have been identified in vertebrates from other orders. For example, squamates (lizards and snakes) were investigated for Borrelia in America, Africa and Europe, and 18 species associated with Bbsl. Anti-Bbsl antibodies were detected in raccoon (Procyon lotor) in the USA and raccoon dog (Nyctereutes procyonoides) in Asia, as well as where they are invasive in Europe or Asia. In India, primates were found seropositive to Kyanasur Forest Disease virus (KFDV), a TBFV close to POWV [65]. In the UK, an isolated clinical case of lethal borreliosis was reported in a Pipistrellus spp. bat [66]. Neotropical bats have been identified to carry particular strains of relapsing fever Borrelia and Bbsl in Central and Southern America. The studies in Central America had been conducted following up previous investigations for Borrelia in those bats, published in 1968 by Marinkelle & Grose [67].
4.5. Diagnostic tests: current versus past infections
Direct and indirect detection methods were at different use depending on the pathogen. Long-lasting in the organisms, Borrelia spp. were often sought by PCR (60% of case studies overall). Skin and blood were used for spirochetes PCR detection, allowing positive samples to be sequenced and species identified, although the performance of direct Borrelia detection in blood is questioned [68,69]. Determination of Borrelia subspecies was useful in understanding the role of hosts in disease cycles [70]. Different Borrelia have been preferentially detected in different hosts. For example, B. lusitaniae was the only Bbsl genospecies found in lizards in Europe.
In contrast, the short viremia noted after flaviviruses infection, as demonstrated by experimental infections [71], makes direct viral detection challenging especially in wildlife. PCR was therefore rarely used for flaviviruses (in only 11% of TBFV studies), but allowed sequencing of TBEV subtypes that clustered with TBEV-Eur (European) in Croatia, Slovakia and the Netherlands [[72], [73], [74]], TBEV-Eur and TBEV-Sib (Siberian) in Finland [75], TBEV-Eur, TBEV-Sib and TBEV-FE (Far Eastern) in Russia [76] and TBEV-Him (Himalayan) in China [77], and evidenced co-infection in Russia [76]. Identifying flaviviruses will help understanding further the ecology and dynamics of pathogens and will assist disease surveillance with phylogenetics providing information on circulation and evolution of TBP [78].
However, flaviviruses infections induce immune response with neutralizing antibodies that could be detected lifelong in blood in human [79] and up to 168 days after experimental infection in bank voles [80]. Serological information in combination with metadata can thus provide precious information on flaviviruses transmission [81]. However, some issues in serological data analysis arise as matters of discussion. For instance, whilst ELISA seropositivity threshold was generally reproduced from manufacturer, some authors proposed cut-off determination by fitting on observed data to optimize sensitivity and specificity [82]. Others even chose to present raw ELISA results, without indicating cut-off values, and thus, nor prevalence [83].
Cross-reactivity in wide-range immunoassays may also hamper data interpretation [84]. For instance, anti-MEAV antibodies were surprisingly detected in Cervidae [36], whilst MEAV belongs to the seabird group in TBFV phylogeny [85]. Antibodies against LIV have been unusually identified in seabirds Fratercula arctica and Hydrobates leucorhous [86]. Both of these reports may result from cross-reactivity of antibodies against other flaviviruses. To address this question, some commercial TBEV ELISA have been compared for their reliability according to the TBEV-strain and for the at-risk cross-reactivity [87]. Because TBEV and LIV as well as Turkish Sheep Encephalomyelitis virus (TSEV) and Spanish Sheep Encephalomyelitis virus (SSEV) belong to the TBEV-serocomplex [65], they can particularly easily be mistaken in serology. Hence, some authors are careful in results presentation, such as Bournez et al. who delivered their final TBEV micro-neutralization serology results under the “anti-TBEV/LIV” label [38].
Cross-reactivity may however appear useful for screening large samples collections when investigating exposure to rare pathogens. Publications about other tick-borne flaviviruses than TBEV and POWV were marginal and specific detection for these anti-TBFV antibodies was not always commercially available. Arnal et al. found MEAV-seropositivity in Larus michahellis michahellis egg yolks in France using flavivirus-ELISA cross-reactivity properties. This was done by first screening all yolks with the ID-Vet ID Screen® kit designed for the mosquito-borne flavivirus West Nile virus (WNV), then by searching and detecting the virus in local ticks, and finally by detecting anti-MEAV antibodies using serum neutralization tests (SNT) [88]. Similarly, through both COMPAC® WNV-ELISA and exclusion via SNT, Jurado-Tarifa et al. investigated flaviviruses in Spanish birds of prey and detected evidence of exposure to flaviviruses different from the mosquito-borne WNV and Usutu (USUV) viruses and from MEAV in Circus pygargus, Falco tinnunculus, Hieraaetus pennatus and Tyto alba [89]. Ytrehus et al. showed presence of both LIV and TBEV antibodies in Southern Norway in Alces alces and Capreolus capreolus with ELISA kits for TBEV, hemagglutination for LIV and SNT for validation, and they got differentiable results for the two pathogens [90]. In the UK where TBEV was thought to be absent, Holding et al. employed in cervids the FSME/TBE All-Species Progen® ELISA and an hemagglutination assay specific to LIV [60]. They recorded similar levels of LIV and TBEV seroprevalence, and sequenced TBEV in tick for confirmation of its detection.
4.6. Study designs and eco-epidemiological parameters
Implemented study designs varied in spatial and temporal dimensions, resulting in varying quality in data acquisition. Most publications conducted cross-sectional studies (57%, 180/314), and 20 did not inform a study period. Some studies conducted repeated sampling without taking into consideration the sampling period in data presentation. Spatial resolution varied from locality to country scale, with often provinces chosen as spatial scale. Inconstancy came from different focuses, either on locally targeted area or over broader-scale exposure. In each study, sample sizes were usually limited, with median sample size at 40. Overall, the way data were collected did not allow taking geography as well as other confounding factors into account. A total of 41 publications marked their captured individuals but marking techniques were not always specific and trustable. For example, some authors simply relied on scars made by biopsy or on feather clipping for individual identification. The aim of marking was sometimes only to avoid resampling, with immediate release in case of recapture. Although 24 publications used individual marks and repeated capture occasions, capture-mark-recapture data was not systematically analysed using relevant techniques. It is however an important specific sampling approach that permits accounting for detection uncertainty. For instance, seroprevalence and capture-mark-recapture data were recently used in a Borrelia host system [91]. Similar approaches were also used in other wildlife and infection systems, such as brucellosis in Alpine ibex (Capra ibex) [92].
Moreover, metadata informing basic demographics were lacking in almost all studies, whereas data on age structure can be key in understanding susceptibility to pathogen at the individual level [93] and transmission processes at the population level. Mammals and birds or squamates were not studied together in the same areas. Designs tended to investigate a single taxonomic order at once by setting field material only fit for a group of species, whilst theoretical definitions of reservoir and sentinel suggest that reservoirs are communities rather than species [29]. The underlying community of hosts was seldomly considered. Furthermore, although sometimes convenient, getting material from hunted, road-killed or rescued animals implies biases in detection that should be accounted for. Such sampling strategies often lead to small sample sizes and difficult interpretation. Moreover, as recommended by Yoccoz et al. on biodiversity monitoring, sampling designs and data analyses need to account for uncertainty on host detectability and spatial variability, for better estimating the state and rates of change of relevant ecological communities [94]. Thereby, acquiring infection and exposure data in wildlife should target communities of species in longitudinal studies with for instance capture-marking-recapture or patch-occupancy modelling.
4.7. Complementarity of laboratory and field data from hosts and ticks
In our review, some infectious agents received less attention, such as TYUV, MEAV and SREV seabird-flaviviruses. Whilst such agents may be less pathogenic, they can still be useful to understand transmission processes. In addition, their virulence could vary between host species and they could emerge as possible pathogens of concern. For example, TUYV was unexpectedly found pathogenic and deadly in seabirds in a study conducted by Berezina et al. in 1974 [97]. Therefore, combining data on pathogenicity from the field and the laboratory is still needed.
In the literature, models and experimental infections have attempted to characterize reservoir competence [95]. Especially, xenodiagnoses are used to detect host reservoir capacities and tick vector competences in experimental settings [96]. Ticks from the field have been extensively examined for pathogens, with methods ranging from PCR to metagenomics. Studies on ticks can be complementary to data from hosts insofar as they analyse tick-host interactions. Their deployment is less invasive on hosts than blood or tissue sampling. They need less expertise in the field and are subject to less strict ethical protocols. Such studies can serve as early detection methods when the pathogen is poorly known. For instance, whilst data on TBP hosts in Australia were limited, studying pathogens in ticks demonstrated circulation of Borrelia in squamates and monotremes [97]. In addition, identification of ticks' life stages allows to infer ticks' contamination capacity, although other ways of transmission such as vertical transmission in vertebrate hosts have been reported for some agents [98]. However, accounting for the whole local host community and their infection status remains as important.
5. Conclusion
Borrelia and tick-borne flaviviruses have been studied in a wide range of wild animal species, with rodents and artiodactyls having been the most investigated. Some infectious agents, rare TBFV or particular Borrelia strains, were little studied and more information on their infectivity is still needed. Diagnostic detection methods varied with the targeted pathogens. Selected publications showed that study designs were highly heterogenous, both in data collection and analysis. Key information on host age and precise location was often unavailable; 58% (200/342) of the publications conducted fieldwork only at one time point, and very few studies used quantitative methods to analyse collected data. Quantifying transmission necessitates gathering empirical data from populational studies, including hosts demographics and identifying which species or community act as reservoirs and sentinels. Whilst we acknowledge that gathering this information is difficult, this review highlights the necessity to estimate eco-epidemiological parameters in wildlife infection studies. The subject discussed in this review concerns a multidisciplinary scientific community (ecology, epidemiology, microbiology, parasitology, modelling and public health), emphasizing that generating and interpreting zoonotic infection data from wildlife in a rigorous way is important in building One Health programmes.
Credit authorship contribution statement
Armelle Poisson: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. Thierry Boulinier: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing. Laure Bournez: Methodology, Writing – review & editing. Gaëlle Gonzalez: Methodology, Writing – review & editing. Camille V. Migné: Methodology, Writing – review & editing. Sara Moutailler: Methodology, Writing – review & editing. Bruno Faivre: Methodology, Writing – review & editing. Raphaëlle Métras: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Younjung Kim and anonymous reviewers for comments on earlier versions of the manuscript. This work was funded by Agence Nationale de la Recherche (ANR) under the ANRJCJC MoZArt project, grant number ANR-22-CE35-0003. Support is also acknowledged from ANR ECOPATHS project, grant number ANR-21-CE35-0016, and from IPEV ECOPATH-1151 project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2024.100747.
Contributor Information
Armelle Poisson, Email: armelle.poisson@cefe.cnrs.fr.
Raphaëlle Métras, Email: raphaelle.metras@inserm.fr.
Appendix A. Supplementary data
Supplementary material 1
Supplementary material 2
Data availability
The data that support this study are available as Supplementary material 2. Source data are provided with this paper.
References
- 1.Woolhouse M.E.J. Population biology of emerging and re-emerging pathogens. Trends Microbiol. 2002;10:s3–s7. doi: 10.1016/S0966-842X(02)02428-9. [DOI] [PubMed] [Google Scholar]
- 2.Estrada-Peña A., De La Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antivir. Res. 2014;108:104–128. doi: 10.1016/j.antiviral.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Becker D.J., Seifert S.N., Carlson C.J. Beyond infection: integrating competence into reservoir host prediction. Trends Ecol. Evol. 2020;35:1062–1065. doi: 10.1016/j.tree.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halliday J.E.B., Meredith A.L., Knobel D.L., Shaw D.J., Bronsvoort B.M.D.C., Cleaveland S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface. 2007;4:973–984. doi: 10.1098/rsif.2007.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouchard É., Sharma R., Hernández-Ortiz A., Buhler K., Al-Adhami B., Su C., Fenton H., Gouin G.G., Roth J.D., Rodrigues C.W., Pamak C., Simon A., Bachand N., Leighton P., Jenkins E. Are foxes (Vulpes spp.) good sentinel species for toxoplasma gondii in northern Canada? Parasit. Vectors. 2022;15:115. doi: 10.1186/s13071-022-05229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eby P., Peel A.J., Hoegh A., Madden W., Giles J.R., Hudson P.J., Plowright R.K. Pathogen spillover driven by rapid changes in bat ecology. Nature. 2023;613:340–344. doi: 10.1038/s41586-022-05506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riccardi N., Antonello R.M., Luzzati R., Zajkowska J., Di Bella S., Giacobbe D.R. Tick-borne encephalitis in Europe: a brief update on epidemiology, diagnosis, prevention, and treatment. Eur. J. Intern. Med. 2019;62:1–6. doi: 10.1016/j.ejim.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Amicizia D., Domnich A., Panatto D., Lai P.L., Cristina M.L., Avio U., Gasparini R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum. Vaccin. Immunother. 2013;9:1163–1171. doi: 10.4161/hv.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubálek Z. History of arbovirus research in the Czech Republic. Viruses. 2021;13:2334. doi: 10.3390/v13112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chastel C., Main A.J., Guiguen C., Le Lay G., Quillien M.C., Monnat J.Y., Beaucournu J.C. The isolation of Meaban virus, a new Flavivirus from the seabird tick Ornithodoros (Alectorobius) maritimus in France. Arch. Virol. 1985;83:129–140. doi: 10.1007/BF01309911. [DOI] [PubMed] [Google Scholar]
- 11.McLean D.M., Donohue W.L. Powassan virus: isolation of virus from a fatal case of encephalitis. Can. Med. Assoc. J. 1959;80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 12.Rebaudet S., Parola P. Epidemiology of relapsing fever borreliosis in Europe. FEMS Immunol. Med. Microbiol. 2006;48:11–15. doi: 10.1111/j.1574-695X.2006.00104.x. [DOI] [PubMed] [Google Scholar]
- 13.Anderson C., Brissette C.A. The brilliance of Borrelia: mechanisms of host immune evasion by Lyme disease-causing spirochetes. Pathogens. 2021;10:281. doi: 10.3390/pathogens10030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone E.T., Pinto A.K. T cells in tick-borne Flavivirus encephalitis: a review of current paradigms in protection and disease pathology. Viruses. 2023;15:958. doi: 10.3390/v15040958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y., Zhou G., Cao W., Xu X., Zhang Y., Ji Z., Yang J., Chen J., Liu M., Fan Y., Kong J., Wen S., Li B., Yue P., Liu A., Bao F. Global seroprevalence and sociodemographic characteristics of Borrelia burgdorferi sensu lato in human populations: a systematic review and meta-analysis. BMJ Glob. Health. 2022;7 doi: 10.1136/bmjgh-2021-007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wondim M.A., Czupryna P., Pancewicz S., Kruszewska E., Groth M., Moniuszko-Malinowska A. Epidemiological trends of trans-boundary tick-borne encephalitis in Europe, 2000–2019. Pathogens. 2022;11:704. doi: 10.3390/pathogens11060704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou E., Minor A., Cady N.C. Quantitative multiplexed strategies for human Lyme disease serological testing. Exp. Biol. Med. 2021;246:1388–1399. doi: 10.1177/15353702211003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gudowska-Sawczuk M., Mroczko B. Selected biomarkers of tick-borne encephalitis: a review. Int. J. Mol. Sci. 2021;22:10615. doi: 10.3390/ijms221910615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elbaz M., Gadoth A., Shepshelovich D., Shasha D., Rudoler N., Paran Y. Systematic review and Meta-analysis of foodborne Tickborne encephalitis, Europe, 1980–2021. Emerg. Infect. Dis. 2022;28 doi: 10.3201/eid2810.220498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martello E., Gillingham E.L., Phalkey R., Vardavas C., Nikitara K., Bakonyi T., Gossner C.M., Leonardi-Bee J. Systematic review on the non-vectorial transmission of tick-borne encephalitis virus (TBEv) Ticks Tick-Borne Dis. 2022;13 doi: 10.1016/j.ttbdis.2022.102028. [DOI] [PubMed] [Google Scholar]
- 21.Zannou O.M., Ouedraogo A.S., Biguezoton A.S., Abatih E., Coral-Almeida M., Farougou S., Yao K.P., Lempereur L., Saegerman C. Models for studying the distribution of ticks and tick-borne diseases in animals: a systematic review and a Meta-analysis with a focus on Africa. Pathogens. 2021;10:893. doi: 10.3390/pathogens10070893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voyiatzaki C., Papailia S.I., Venetikou M.S., Pouris J., Tsoumani M.E., Papageorgiou E.G. Climate changes exacerbate the spread of Ixodes ricinus and the occurrence of Lyme Borreliosis and tick-borne encephalitis in Europe—how climate models are used as a risk assessment approach for tick-borne diseases. Int. J. Environ. Res. Public Health. 2022;19:6516. doi: 10.3390/ijerph19116516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Defaye B., Moutailler S., Pasqualini V., Quilichini Y. A systematic review of the distribution of tick-borne pathogens in wild animals and their ticks in the Mediterranean rim between 2000 and 2021. Microorganisms. 2022;10:1858. doi: 10.3390/microorganisms10091858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mubemba B., Mburu M.M., Changula K., Muleya W., Moonga L.C., Chambaro H.M., Kajihara M., Qiu Y., Orba Y., Hayashida K., Sutcliffe C.G., Norris D.E., Thuma P.E., Ndubani P., Chitanga S., Sawa H., Takada A., Simulundu E. Current knowledge of vector-borne zoonotic pathogens in Zambia: a clarion call to scaling-up “one health” research in the wake of emerging and re-emerging infectious diseases. PLoS Negl. Trop. Dis. 2022;16 doi: 10.1371/journal.pntd.0010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pandit P.S., Doyle M.M., Smart K.M., Young C.C.W., Drape G.W., Johnson C.K. Predicting wildlife reservoirs and global vulnerability to zoonotic Flaviviruses. Nat. Commun. 2018;9:5425. doi: 10.1038/s41467-018-07896-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hussain S., Hussain A., Aziz U., Song B., Zeb J., George D., Li J., Sparagano O. The role of ticks in the emergence of Borrelia burgdorferi as a zoonotic pathogen and its vector control: a global systemic review. Microorganisms. 2021;9:2412. doi: 10.3390/microorganisms9122412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansford K.M., Wheeler B.W., Tschirren B., Medlock J.M. Questing Ixodes ricinus ticks and Borrelia spp. in urban green space across Europe: a review. Zoonoses Public Health. 2022;69:153–166. doi: 10.1111/zph.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keeling M.J., Rohani P. Princeton University Press; 2011. Modeling Infectious Diseases in Humans and Animals. [DOI] [Google Scholar]
- 29.Viana M., Mancy R., Biek R., Cleaveland S., Cross P.C., Lloyd-Smith J.O., Haydon D.T. Assembling evidence for identifying reservoirs of infection. Trends Ecol. Evol. 2014;29:270–279. doi: 10.1016/j.tree.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClintock B.T., Nichols J.D., Bailey L.L., MacKenzie D.I., William L., Kendall A.B. Franklin. Seeking a second opinion: uncertainty in disease ecology: uncertainty in disease ecology. Ecol. Lett. 2010;13:659–674. doi: 10.1111/j.1461-0248.2010.01472.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark K. Borrelia species in host-seeking ticks and small mammals in northern Florida. J. Clin. Microbiol. 2004;42:5076–5086. doi: 10.1128/JCM.42.11.5076-5086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X.-A., Tian F., Li Y., Zhang X.-L., Jiang B.-G., Liu B.-C., Zhang J.-T., Tian S., Ding H., Li S., Li H., Fang L.-Q., Liu W. Molecular detection and identification of relapsing fever Borrelia in ticks and wild small mammals in China, Emerg. Microbes Infect. 2022;11:2632–2635. doi: 10.1080/22221751.2022.2134054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott M.C., Rosen M.E., Hamer S.A., Baker E., Edwards H., Crowder C., Tsao J.I., Hickling G.J. High-prevalence Borrelia miyamotoi infection among wild turkeys (Meleagris gallopavo) in Tennessee. J. Med. Entomol. 2010;47:1238–1242. doi: 10.1603/ME10075. [DOI] [PubMed] [Google Scholar]
- 34.Szekeres S., Coipan E.C., Rigó K., Majoros G., Jahfari S., Sprong H., Földvári G. Eco-epidemiology of Borrelia miyamotoi and Lyme borreliosis spirochetes in a popular hunting and recreational forest area in Hungary. Parasit. Vectors. 2015;8:309. doi: 10.1186/s13071-015-0922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obiegala A., Król N., Oltersdorf C., Nader J., Pfeffer M. The enzootic life-cycle of Borrelia burgdorferi (sensu lato) and tick-borne rickettsiae: an epidemiological study on wild-living small mammals and their ticks from Saxony, Germany. Parasit. Vectors. 2017;10:115. doi: 10.1186/s13071-017-2053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Bocanegra I., Paniagua J., Gutiérrez-Guzmán A.V., Lecollinet S., Boadella M., Arenas-Montes A., Cano-Terriza D., Lowenski S., Gortázar C., Höfle U. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet. Res. 2016;12:249. doi: 10.1186/s12917-016-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chastel C., Guiguen C., Le Lay G., Monnat J.Y., Hardy E., Kerdraon G., Beaucournu J.C. Arbovirus serological survey among marine and non-marine birds of Brittany. Bull. Soc. Pathol. Exot. Filiales. 1985;78:594–605. [PubMed] [Google Scholar]
- 38.Bournez L., Umhang G., Faure E., Boucher J.-M., Boué F., Jourdain E., Sarasa M., Llorente F., Jiménez-Clavero M.A., Moutailler S., Lacour S.A., Lecollinet S., Beck C. Exposure of wild ungulates to the Usutu and tick-borne encephalitis viruses in France in 2009–2014: evidence of undetected Flavivirus circulation a decade ago. Viruses. 2019;12:10. doi: 10.3390/v12010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krzysiak M.K., Anusz K., Konieczny A., Rola J., Salat J., Strakova P., Olech W., Larska M. The European bison (bison bonasus) as an indicatory species for the circulation of tick-borne encephalitis virus (TBEV) in natural foci in Poland. Ticks Tick-Borne Dis. 2021;12 doi: 10.1016/j.ttbdis.2021.101799. [DOI] [PubMed] [Google Scholar]
- 40.Bakhvalova V.N., Dobrotvorsky A.K., Panov V.V., Matveeva V.A., Tkachev S.E., Morozova O.V. Natural tick-borne encephalitis virus infection among wild small mammals in the southeastern part of Western Siberia, Russia. Vector-Borne Zoonotic Dis. 2006;6:32–41. doi: 10.1089/vbz.2006.6.32. [DOI] [PubMed] [Google Scholar]
- 41.LoGiudice K., Ostfeld R.S., Schmidt K.A., Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLean D.M., MacPherson L.W., Walker S.J., Funk G. Powassan virus: surveys of human and animal sera. Am. J. Public Health Nations Health. 1960;50:1539–1544. doi: 10.2105/ajph.50.10.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLean D.M., Walker S.J., MacPherson L.W., Scholten T.H., Ronald K., Wyllie J.C., McQueen E.J. Powassan virus: investigations of possible natural cycles of infection. J. Infect. Dis. 1961;109:19–23. doi: 10.1093/infdis/109.1.19. [DOI] [PubMed] [Google Scholar]
- 44.McLean D.M., McQueen E.J., Petite H.E., Macpherson L.W., Scholten T.H., Ronald K. Powassan virus: field investigations in Northern Ontario, 1959 to 1961. Can. Med. Assoc. J. 1962;86:971–974. [PMC free article] [PubMed] [Google Scholar]
- 45.Mclean D.M., Larke R.P. Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can. Med. Assoc. J. 1963;88:182–185. [PMC free article] [PubMed] [Google Scholar]
- 46.Mclean D.M., Best J.M., Mahalingam S., Chernesky M.A., Wilson W.E. Powassan virus: summer infection cycle. Can. Med. Assoc. J. 1964;91(1964):1360–1362. [PMC free article] [PubMed] [Google Scholar]
- 47.McLean D.M., Smith P.A., Livingstone S.E., Wilson W.E., Wilson A.G. Powassan virus: vernal spread during 1965. Can. Med. Assoc. J. 1966;94:532–536. [PMC free article] [PubMed] [Google Scholar]
- 48.McLean D.M., Cobb C., Gooderham S.E., Smart C.A., Wilson A.G., Wilson W.E. Powassan virus: persistence of virus activity during 1966. Can. Med. Assoc. J. 1967;96:660–664. [PMC free article] [PubMed] [Google Scholar]
- 49.McLean D.M., Ladyman S.R., Purvin-Good K.W. Westward extension of Powassan virus prevalence. Can. Med. Assoc. J. 1968;98:946–949. [PMC free article] [PubMed] [Google Scholar]
- 50.McLean D.M., Chernesky M.A., Chernesky S.J., Goddard E.J., Ladyman S.R., Peers R.R., Purvin-Good K.W. Arbovirus prevalence in the East Kootenay region, 1968. Can. Med. Assoc. J. 1969;100:320–326. [PMC free article] [PubMed] [Google Scholar]
- 51.Frandsen F., Bresciani J., Hansen H.G. Prevalence of antibodies to Borrelia burgdorferi in Danish rodents. APMIS Acta Pathol. Microbiol. Immunol. Scand. 1995;103:247–253. [PubMed] [Google Scholar]
- 52.Pisanu B., Chapuis J.-L., Dozières A., Basset F., Poux V., Vourc’h G. High prevalence of Borrelia burgdorferi s.l. in the European red squirrel Sciurus vulgaris in France. Ticks Tick-Borne Dis. 2014;5:1–6. doi: 10.1016/j.ttbdis.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Michelitsch A., Wernike K., Klaus C., Dobler G., Beer M. Exploring the reservoir hosts of tick-borne encephalitis virus. Viruses. 2019;11:669. doi: 10.3390/v11070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hacker G.M., Brown R.N., Fedorova N., Girard Y.A., Higley M., Clueit B., Lane R.S. Spatial clustering of Borrelia burgdorferi sensu lato within populations of Allen’s chipmunks and dusky-footed woodrats in northwestern California. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore S.I., Wilson M.L., Spielman A., Telford S.R., Mather T.N. Incompetence of deer as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 1988;39:105–109. doi: 10.4269/ajtmh.1988.39.105. [DOI] [PubMed] [Google Scholar]
- 56.Kurtenbach K., Sewell H.-S., Ogden N.H., Randolph S.E., Nuttall P.A. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 1998;66:1248–1251. doi: 10.1128/IAI.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radolf J.D., Caimano M.J., Stevenson B., Hu L.T. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kjellander P., Hewison A.J.M., Liberg O., Angibault J.-M., Bideau E., Cargnelutti B. Experimental evidence for density-dependence of home-range size in roe deer (Capreolus capreolus L.): a comparison of two long-term studies. Oecologia. 2004;139:478–485. doi: 10.1007/s00442-004-1529-z. [DOI] [PubMed] [Google Scholar]
- 59.Walter W.D., Evans T.S., Stainbrook D., Wallingford B.D., Rosenberry C.S., Diefenbach D.R. Heterogeneity of a landscape influences size of home range in a north American cervid. Sci. Rep. 2018;8:14667. doi: 10.1038/s41598-018-32937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holding M., Dowall S.D., Medlock J.M., Carter D.P., Pullan S.T., Lewis J., Vipond R., Rocchi M.S., Baylis M., Hewson R. Tick-borne encephalitis virus, United Kingdom. Emerg. Infect. Dis. 2020;26:90–96. doi: 10.3201/eid2601.191085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gern L., Estrada-Peña A., Frandsen F., Gray J.S., Jaenson T.G.T., Jongejan F., Kahl O., Korenberg E., Mehl R., Nuttall P.A. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralblatt Für Bakteriol. 1998;287:196–204. doi: 10.1016/S0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- 62.Newman E.A., Eisen L., Eisen R.J., Fedorova N., Hasty J.M., Vaughn C., Lane R.S. Borrelia burgdorferi Sensu Lato spirochetes in wild birds in northwestern California: associations with ecological factors, bird behavior and tick infestation. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boulinier T., Kada S., Ponchon A., Dupraz M., Dietrich M., Gamble A., Bourret V., Duriez O., Bazire R., Tornos J., Tveraa T., Chambert T., Garnier R., McCoy K.D. Migration, prospecting, dispersal? What host movement matters for infectious agent circulation? Integr. Comp. Biol. 2016;56:330–342. doi: 10.1093/icb/icw015. [DOI] [PubMed] [Google Scholar]
- 64.Humphery-Smith I., Cybinski D.H., Byrnes K.A., T.D. St George, Seroepidemiology of arboviruses among seabirds and island residents of the great barrier reef and Coral Sea. Epidemiol. Infect. 1991;107:435–440. doi: 10.1017/S0950268800049086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carpio K.L., Thompson J.K., Widen S.G., Smith J.K., Juelich T.L., Clements D.E., Freiberg A.N., Barrett A.D.T. Differences in genetic diversity of mammalian tick-borne Flaviviruses. Viruses. 2023;15:281. doi: 10.3390/v15020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans N.J., Bown K., Timofte D., Simpson V.R., Birtles R.J. Fatal Borreliosis in bat caused by relapsing fever spirochete, United Kingdom. Emerg. Infect. Dis. 2009;15:1331–1333. doi: 10.3201/eid1508.090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marinkelle C.J., Grose E.S. Species of Borrelia from a Colombian bat (Natalus tumidirostris) Nature. 1968;218 doi: 10.1038/218487a0. 487–487. [DOI] [PubMed] [Google Scholar]
- 68.Norte A.C., Lopes De Carvalho I., Núncio M.S., Araújo P.M., Matthysen E., Albino Ramos J., Sprong H., Heylen D. Getting under the birds’ skin: tissue tropism of Borrelia burgdorferi s.l. in naturally and experimentally infected avian hosts. Microb. Ecol. 2020;79:756–769. doi: 10.1007/s00248-019-01442-3. [DOI] [PubMed] [Google Scholar]
- 69.Leonhard S., Jensen K., Salkeld D.J., Lane R.S. Distribution of the Lyme disease spirochete Borrelia burgdorferi in naturally and experimentally infected Western Gray squirrels (Sciurus griseus) Vector-Borne Zoonotic Dis. 2010;10:441–446. doi: 10.1089/vbz.2009.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.O’Keeffe K.R., Oppler Z.J., Brisson D. Evolutionary ecology of Lyme Borrelia. Infect. Genet. Evol. 2020;85 doi: 10.1016/j.meegid.2020.104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salat J., Hunady M., Schanilec P., Strakova P., Stefanik M., Svoboda P., Strelcova L., Bojcukova J., Palus M., Růžek D. Experimental and natural infections of tick-borne encephalitis virus in dogs. Viruses. 2021;13:2039. doi: 10.3390/v13102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jemeršić L., Dežđek D., Brnić D., Prpić J., Janicki Z., Keros T., Roić B., Slavica A., Terzić S., Konjević D., Beck R. Detection and genetic characterization of tick-borne encephalitis virus (TBEV) derived from ticks removed from red foxes (Vulpes vulpes) and isolated from spleen samples of red deer (Cervus elaphus) in Croatia. Ticks Tick-Borne Dis. 2014;5:7–13. doi: 10.1016/j.ttbdis.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 73.Csank T., Bhide K., Bencúrová E., Dolinská S., Drzewnioková P., Major P., Korytár Ľ., Bocková E., Bhide M., Pistl J. Detection of West Nile virus and tick-borne encephalitis virus in birds in Slovakia, using a universal primer set. Arch. Virol. 2016;161:1679–1683. doi: 10.1007/s00705-016-2828-5. [DOI] [PubMed] [Google Scholar]
- 74.Esser H.J., Lim S.M., De Vries A., Sprong H., Dekker D.J., Pascoe E.L., Bakker J.W., Suin V., Franz E., Martina B.E.E., Koenraadt C.J.M. Continued circulation of tick-borne encephalitis virus variants and detection of novel transmission foci, the Netherlands. Emerg. Infect. Dis. 2022;28:2416–2424. doi: 10.3201/eid2812.220552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tonteri E., Jääskeläinen A.E., Tikkakoski T., Voutilainen L., Niemimaa J., Henttonen H., Vaheri A., Vapalahti O. Tick-borne encephalitis virus in wild rodents in winter, Finland, 2008–2009. Emerg. Infect. Dis. 2011;17:72–75. doi: 10.3201/eid1701.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bakhvalova V.N., Chicherina G.S., Potapova O.F., Panov V.V., Glupov V.V., Potapov M.A., Seligman S.J., Morozova O.V. Tick-borne encephalitis virus diversity in Ixodid ticks and small mammals in South-Western Siberia, Russia. Vector-Borne Zoonotic Dis. 2016;16:541–549. doi: 10.1089/vbz.2015.1834. [DOI] [PubMed] [Google Scholar]
- 77.Dai X., Shang G., Lu S., Yang J., Xu J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet plateau, China. Emerg. Microbes Infect. 2018;7:1–9. doi: 10.1038/s41426-018-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bestehorn M., Weigold S., Kern W.V., Chitimia-Dobler L., Mackenstedt U., Dobler G., Borde J.P. Phylogenetics of tick-borne encephalitis virus in endemic foci in the upper Rhine region in France and Germany. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holzmann H. Diagnosis of tick-borne encephalitis. Vaccine. 2003;21:S36–S40. doi: 10.1016/S0264-410X(02)00819-8. [DOI] [PubMed] [Google Scholar]
- 80.Tonteri E., Kipar A., Voutilainen L., Vene S., Vaheri A., Vapalahti O., Lundkvist Å. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the Bank vole (Myodes glareolus) PLoS One. 2013;8 doi: 10.1371/journal.pone.0081214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kucharski A.J., Kama M., Watson C.H., Aubry M., Funk S., Henderson A.D., Brady O.J., Vanhomwegen J., Manuguerra J.-C., Lau C.L., Edmunds W.J., Aaskov J., Nilles E.J., Cao-Lormeau V.-M., Hué S., Hibberd M.L. Using paired serology and surveillance data to quantify dengue transmission and control during a large outbreak in Fiji. eLife. 2018;7 doi: 10.7554/eLife.34848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garnier R., Ramos R., Sanz-Aguilar A., Poisbleau M., Weimerskirch H., Burthe S., Tornos J., Boulinier T. Interpreting ELISA analyses from wild animal samples: some recurrent issues and solutions. Funct. Ecol. 2017;31:2255–2262. doi: 10.1111/1365-2435.12942. [DOI] [Google Scholar]
- 83.Paillard L., Jones K.L., Evans A.L., Berret J., Jacquet M., Lienhard R., Bouzelboudjen M., Arnemo J.M., Swenson J.E., Voordouw M.J. Serological signature of tick-borne pathogens in Scandinavian brown bears over two decades. Parasit. Vectors. 2015;8:398. doi: 10.1186/s13071-015-0967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vilibic-Cavlek T., Ferenc T., Vujica Ferenc M., Bogdanic M., Potocnik-Hunjadi T., Sabadi D., Savic V., Barbic L., Stevanovic V., Monaco F., Listes E., Savini G. Cross-reactive antibodies in tick-borne encephalitis: case report and literature review. Antibodies. 2022;11:72. doi: 10.3390/antib11040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gritsun T.S., Lashkevich V.A., Gould E.A. Tick-borne encephalitis. Antivir. Res. 2003;57:129–146. doi: 10.1016/S0166-3542(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 86.Main A.J., Downs W.G., Shope R.E., Wallis R.C. Avian arboviruses of the Witless Bay seabird sanctuary, Newfoundland, Canada. J. Wildl. Dis. 1976;12:182–194. doi: 10.7589/0090-3558-12.2.182. [DOI] [PubMed] [Google Scholar]
- 87.Weissbach F.H., Hirsch H.H. Comparison of two commercial tick-borne encephalitis virus IgG enzyme-linked immunosorbent assays. Clin. Vaccine Immunol. 2015;22:754–760. doi: 10.1128/CVI.00096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arnal A., Gómez-Díaz E., Cerdà-Cuéllar M., Lecollinet S., Pearce-Duvet J., Busquets N., García-Bocanegra I., Pagès N., Vittecoq M., Hammouda A., Samraoui B., Garnier R., Ramos R., Selmi S., González-Solís J., Jourdain E., Boulinier T. Circulation of a Meaban-like virus in yellow-legged gulls and seabird ticks in the Western Mediterranean Basin. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jurado-Tarifa E., Napp S., Lecollinet S., Arenas A., Beck C., Cerdà-Cuéllar M., Fernández-Morente M., García-Bocanegra I. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Comp. Immunol. Microbiol. Infect. Dis. 2016;49:58–64. doi: 10.1016/j.cimid.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Ytrehus B., Vainio K., Dudman S.G., Gilray J., Willoughby K. Tick-borne encephalitis virus and Louping-ill virus may co-circulate in southern Norway. Vector-Borne Zoonotic Dis. 2013;13:762–768. doi: 10.1089/vbz.2012.1023. [DOI] [PubMed] [Google Scholar]
- 91.Ollivier V., Choquet R., Gamble A., Bastien M., Combes B., Gilot-Fromont E., Pellerin M., Gaillard J., Lemaître J., Verheyden H., Boulinier T. Temporal dynamics of antibody level against Lyme disease bacteria in roe deer: tale of a sentinel? Ecol. Evol. 2023;13 doi: 10.1002/ece3.10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambert S., Gilot-Fromont E., Toïgo C., Marchand P., Petit E., Rossi S., Thébault A. Combining seroprevalence and capture-mark-recapture data to estimate the force of infection of brucellosis in a managed population of alpine ibex. Epidemics. 2022;38 doi: 10.1016/j.epidem.2022.100542. [DOI] [PubMed] [Google Scholar]
- 93.Sweeny A.R., Albery G.F. Exposure and susceptibility: the twin pillars of infection. Funct. Ecol. 2022;36:1713–1726. doi: 10.1111/1365-2435.14065. [DOI] [Google Scholar]
- 94.Yoccoz N.G., Nichols J.D., Boulinier T. Monitoring of biological diversity in space and time. Trends Ecol. Evol. 2001;16:446–453. doi: 10.1016/S0169-5347(01)02205-4. [DOI] [Google Scholar]
- 95.Ostfeld R.S., Levi T., Jolles A.E., Martin L.B., Hosseini P.R., Keesing F. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS One. 2014;9 doi: 10.1371/journal.pone.0107387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Donahue J.G., Piesman J., Spielman A. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 1987;36:92–96. doi: 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- 97.Panetta J.L., Šíma R., Calvani N.E.D., Hajdušek O., Chandra S., Panuccio J., Šlapeta J. Reptile-associated Borrelia species in the goanna tick (Bothriocroton undatum) from Sydney, Australia. Parasit. Vectors. 2017;10:616. doi: 10.1186/s13071-017-2579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larsson C., Andersson M., Guo B.P., Nordstrand A., Hägerstrand I., Carlsson S., Bergström S. Complications of pregnancy and Transplacental transmission of relapsing-fever Borreliosis. J. Infect. Dis. 2006;194:1367–1374. doi: 10.1086/508425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1
Supplementary material 2
Data Availability Statement
The data that support this study are available as Supplementary material 2. Source data are provided with this paper.