Shareable abstract
A 52-year-old Caucasian male was referred to the pulmonology department in a university hospital in Riga, Latvia, due to a chest CT scan performed on an outpatient basis. Acquiring the patient's history leads us to a waterfall. And the fateful photo. https://bit.ly/3IuxAKj
A 52-year-old Caucasian male was referred to the pulmonology department in a tertiary hospital in Riga, Latvia, due to a chest computed tomography (CT) scan performed on an outpatient basis.
Task 1
Describe the chest CT scan shown in figure 1.
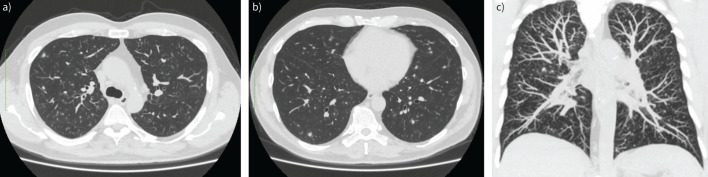
FIGURE 1.
a–c) Chest computed tomography (CT) scan (maximum intensity projection reconstruction) prior to hospitalisation. Reproduced courtesy of Ilze Priedīte (Pauls Stradins Clinical University Hospital, Latvia).
FIGURE 1.
a–c) Chest computed tomography (CT) scan (maximum intensity projection reconstruction) prior to hospitalisation. Reproduced courtesy of Ilze Priedīte (Pauls Stradins Clinical University Hospital, Latvia).
Answer 1
The chest CT scan shows bilateral atypical pneumonia with small centrilobular intrapulmonary nodules and infiltrative foci. Granulomatous lung disease or mycobacterial infection cannot be ruled out.
Upon primary examination, the patient complains of a low-intensity dry cough and general weakness.
The first complaints started 6 weeks prior to hospitalisation, 1 week after the patient's return from the Democratic Republic of the Congo (DRC), when the patient developed a fever of up to 40°C and nasal congestion for 5 days followed by a low-grade fever for six more days. The only treatment was over-the-counter nonsteroidal anti-inflammatory drugs. A fortnight later, the patient experienced a sudden recurrence of fever (up to 39°C) accompanied by diarrhoea six times a day. 2 days later a dry cough and rhinitis appeared. Most symptoms, except the cough and malaise, resolved in 4 days without specific therapy. Following this episode, the patient's general practitioner ordered blood, urine and stool tests (table 1), which revealed marked eosinophilia and moderately elevated liver function tests and C-reactive protein. Blood parasites were negative, stool microscopy for ova and parasites (O&P) yielded Blastocystis hominis and Dientamoeba fragilis cysts, which are frequently seen as commensal or opportunistic protozoans. A considerable weight loss of 9 kg from the beginning of symptoms was noted by the patient. A CT scan was ordered, which subsequently led to the referral to the pulmonology department.
TABLE 1.
Results of patient's laboratory investigations prior to hospitalisation
| Parameter | Value | Normal range |
| Blood | ||
| Red blood cells, ×1012 per L | 5.2 | 4.5–5.9 |
| Haemoglobin, g·L−1 | 141 | 131–175 |
| Haematocrit, % | 44 | 40–51 |
| Platelets, ×109 per L | 454 | 150–410 |
| White blood cells, ×109 per L | 28.18 | 4.0–9.8 |
| Bands, ×109 per L | 1.13 | 0–0.59 |
| Segmented neutrophils, ×109 per L | 6.06 | 1.92–8.00 |
| Eosinophils, ×109 per L | 17.05 | 0.02–0.53 |
| Basophils, ×109 per L | 0.14 | 0.00–0.20 |
| Lymphocytes, ×109 per L | 2.68 | 0.72–4.00 |
| Monocytes, ×109 per L | 1.13 | 0.08–1.21 |
| Alanine transaminase, U·L−1 | 132 | <41 |
| Aspartate transaminase, U·L−1 | 54 | <37 |
| γ-glutamyl transferase, U·L−1 | 215 | 0–73 |
| Creatinine, µmol·L−1 | 83 | 30–106 |
| C-reactive protein, mg·L−1 | 57.3 | <8.0 |
| High-sensitivity troponin I, ng·L−1 | 106.69 | <58.08 |
| Blood smear for parasites | Negative | Negative |
| Faeces | ||
| O&P | Positive for Blastocystis hominis and Dientamoeba fragilis cysts | Negative |
| Calprotectin μg·g−1 | 250.0 | <50 |
| Adenovirus antigen | Negative | Negative |
| Rotavirus antigen | Negative | Negative |
| Salmonella, Shigella spp. culture | Negative | Negative |
| Campylobacter spp. culture | Negative | Negative |
| Urine | ||
| Leukocytes, per µL | <1 | <28 |
| Red blood cells, per µL | 3 | <17 |
O&P: ova and parasites.
There are no known chronic illnesses, the patient denies taking any medication daily and he is a non-smoker.
Task 2
What would be the most appropriate next step in this case?
Immediately perform bronchoscopy with lavage.
Consult a tropical disease expert.
Order abdominal ultrasound.
Perform interferon-γ release assay.
Order pulmonary function tests.
Answer 2
b. Generally, if a traveller who has returned from a developing country, especially one in sub-Saharan Africa, presents to the hospital with eosinophilic fever, an infectious diseases or tropical diseases specialist, if available, should be consulted prior to commencing additional investigations.
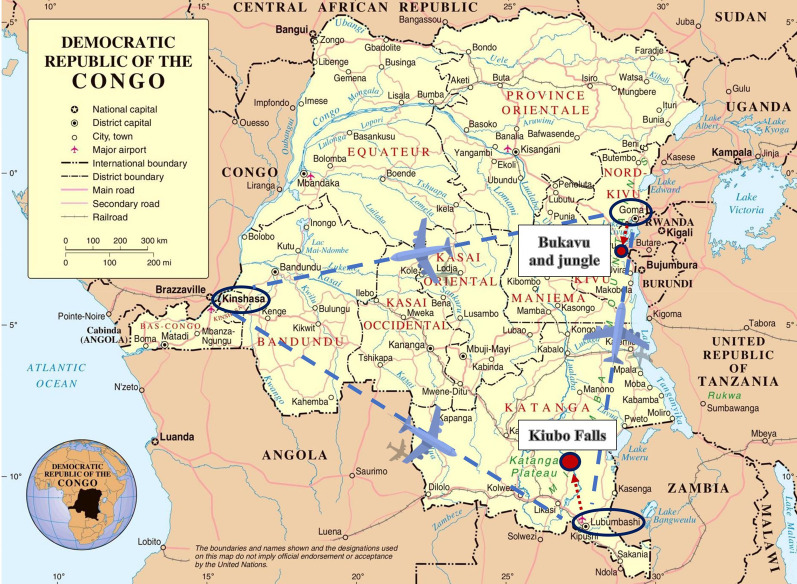
A thorough travel history was obtained. It was established that the patient had visited the DRC for 20 days in 2021. During the travel period and for 7 days upon returning to Latvia, Atovaquone/Proguanil was taken by the patient for malaria prophylaxis. The patient arrived in the DRC through N'djili International Airport in Kinshasa and then travelled further to Lubumbashi. On the seventh day of his trip, the patient visited Kiubo Falls on the Lufira river, paddling in the water to his knees, while someone took his photo. Only bottled water was used by the patient throughout the trip. He was taking showers in the hotels and brushing his teeth with tap water. In the following days, the patient travelled by plane to Goma and then by road to Bukavu, from where the patient took a guided tour of the jungle. Upon questioning about insect bites, the patient stated that there were possibly a few bites during the whole trip (no bite marks were seen upon examination). The last leg of the trip included the return to Goma, and then flights to Kinshasa and back to Riga, Latvia via Istanbul, Turkey (figure 2).
FIGURE 2.
A summary of the patient's trip. The map of the Democratic Republic of the Congo is reproduced from https://ontheworldmap.com/democratic-republic-of-the-congo/road-map-of-democratic-republic-of-the-congo.html.
Taking into consideration the patient's recent travel to sub-Saharan Africa, a suspicion of a tropical infection was raised; thus, further investigations were deemed necessary. HIV and viral hepatitides were ruled out. The autoantibody screening came back negative. Stool analysis showed positive occult blood. Stool microscopy yielded mixed protozoan species, namely Blastocystis hominis, Dientamoeba fragilis, Entamoeba coli, and Entamoeba histolytica/dispar cysts. Urinalysis revealed leukocytosis and haematuria. Blood cultures did not show any growth. Due to the chest CT findings, bronchoscopy was performed with no endobronchial pathology upon visual inspection; laboratory studies of bronchoalveolar lavage (BAL) are shown in table 2.
TABLE 2.
Results of patient's bronchoalveolar lavage investigations
| Parameter | Value | Normal range |
| Cytosis, per µL | 290 | |
| Alveolar macrophages, % | 82 | |
| Eosinophils, % | 14 | |
| Gram stain | Negative | |
| Acid-fast bacilli (Ziehl–Neelsen stain) | Negative | |
| MTB DNA | Negative | Negative |
| Mycobacterium spp. culture: liquid medium | Pending | |
| Mycobacterium spp. culture: solid medium | Pending | |
| O&P | Negative | Negative |
MTB: Mycobacterium tuberculosis; O&P: ova and parasites.
Based on the transaminitis and urinalysis, an abdominal CT scan with intravenous contrast was performed, showing mild hepatosplenomegaly and a cystic lesion, up to 2.5 cm in diameter, in the upper pole of the left kidney.
Task 3
Why was an O&P test ordered in a BAL sample?
The patient has amoebiasis, and BAL should be routinely examined prior to administration of antiparasitic agents.
To exclude pulmonary ascariasis, as roundworms are frequently missed in stool microscopy.
As the patient travelled to the DRC, where filarial infections are endemic, BAL is the only appropriate sample to measure filarial burden.
To find parasites that are seen exclusively in BAL samples.
To exclude possible paragonimiasis and disseminated strongyloidiasis.
Answer 3
e. Both of these parasites or their eggs could be found in BAL samples, particularly Paragonimus spp. eggs, which are mainly isolated from respiratory samples not faeces.
Task 4
What would your next actions be, based on the available data?
Order additional stool microscopy and microscopic urinalysis for O&P.
Order three consecutive blood smears for parasites.
Start empirical treatment with ivermectin and albendazole.
Perform liver biopsy.
Repeat the chest CT scan.
Answer 4
a. At least three stool and urine samples should be evaluated as the patient's anamnestic and clinical data suggest parasitic infection.
As the index of suspicion for tropical parasitic infection was high, repeated stool and urine samples for O&P were ordered the next day, with the stool sample finally yielding parasite eggs (see figure 3), the urine sample tested negative. Upon repeated thorough questioning, the patient recalled developing a transient itchy rash on both shins the same evening after visiting Kiubo Falls, which was alleviated by taking loratadine and resolved in 2 days.
FIGURE 3.
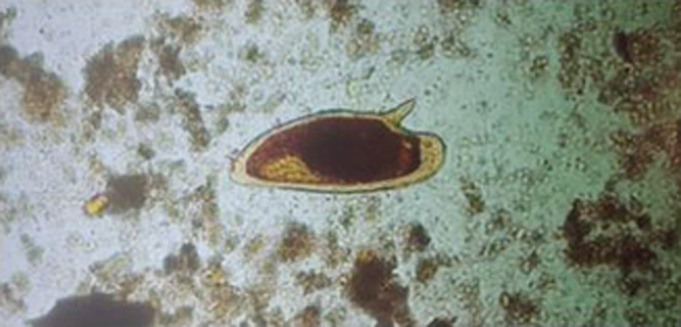
Parasite egg found on stool microscopy. Reproduced courtesy of the Latvian Centre of Infectious Diseases, Riga East University Hospital, Latvia.
Due to the presence of Entamoeba histolytica/dispar cysts on stool microscopy, an intestinal protozoa multiplex real-time PCR assay along with Entamoeba histolytica antigen test were ordered to clarify the species. Both results came back negative for Entamoeba histolytica.
Task 5
What is the name of this rash the patient had developed? And what is the final diagnosis in this case?
Answer 5
The rash is known as cercarial dermatitis or swimmer's itch. The diagnosis is acute schistosomiasis or Katayama fever (syndrome).
Based on the anamnestic and clinical data the final diagnosis of Katayama fever or acute schistosomiasis due to Schistosoma mansoni was made. Acute symptoms were preceded by cercarial dermatitis.
Task 6
How would you treat this patient?
Prescribe short-term pulsed steroids, for example, prednisolone 40 mg·kg−1, and praziquantel.
Prescribe praziquantel alone.
Prescribe one dose of praziquantel, and concomitant metronidazole for 7 days, followed by a luminal agent.
Prescribe praziquantel followed by ivermectin.
Symptomatic treatment only, as schistosomiasis is a self-limiting disease.
Answer 6
b. This patient presents to the hospital more than 6 weeks after potential freshwater exposure, without any severe symptoms of Katayama fever; thus, praziquantel monotherapy is warranted.
The patient was discharged on oral praziquantel 40 mg·kg−1 as a single dose, the treatment course had to be repeated 6 weeks later as unhatched eggs and immature schistosomulae are relatively resistant to the medication. It was recommended that sequential stool examinations for O&P be performed. Except for general malaise following the first praziquantel dose, attributable to rapid parasite death, the patient made an uneventful recovery, stool samples turned negative for schistosome eggs on the first follow-up examination, and the full blood count showed marked decrease in eosinophil count (table 3). Both Mycobacterium spp. cultures from BAL were negative after 6 weeks of incubation.
TABLE 3.
Follow-up laboratory investigations after treatment with praziquantel
| Parameter | Value (after treatment) | Normal range | ||
| 2 weeks after the first dose | Before the second dose | 6 weeks after the second dose | ||
| Blood | ||||
| White blood cells, ×109 per L | 17.89 | 12.35 | 10.62 | 4.0–9.8 |
| Eosinophils, ×109 per L | 9.10 | 5.11 | 3.22 | 0.02–0.53 |
| Alanine transaminase, U·L−1 | 40 | 32 | 36 | <41 |
| Aspartate transaminase, U·L−1 | 26 | 30 | 37 | <37 |
| C-reactive protein, mg·L−1 | 11.1 | 4.5 | 3.7 | <8.0 |
| Faeces | ||||
| O&P | Positive for Blastocystis hominis and Entamoeba coli cysts | Positive for Blastocystis hominis cysts | N/A | Negative |
| Urine | ||||
| O&P | Negative | N/A | N/A | Negative |
N/A: not applicable; O&P: ova and parasites.
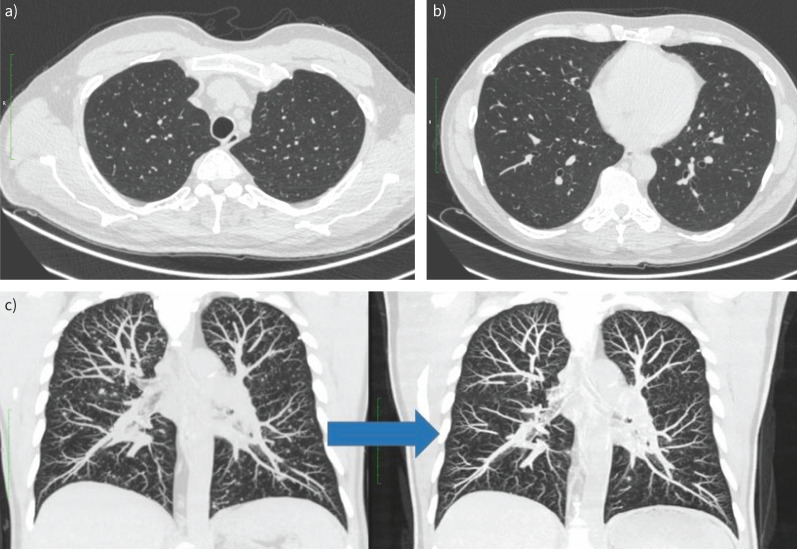
A follow-up chest CT 2 months after the treatment showed marked improvement compared with the previous CT, with infiltrative foci decreasing in size and intensity, and most of them fully resorbing (figure 4).
FIGURE 4.
a–c) The follow-up chest computed tomography (CT) scan 2 months after treatment. c) The left image is from the scan taken at presentation, the right image is from the scan 2 months after treatment. Reproduced courtesy of Ilze Priedīte (Pauls Stradins Clinical University Hospital, Latvia).
Discussion
Parasitic lung diseases are caused by several parasites either related to the transient passage of the parasites in the lung or as a result of an immunological reaction (table 4). Although these infections are primarily seen among residents of developing countries, as recently stated in the European Respiratory Review [2], globalisation, increased international travel and immigration may lead to the presentation of patients in well-developed countries as well.
TABLE 4.
Comparison of the most common parasite-induced eosinophilic pneumonias
| Katayama fever | Löffler syndrome | Tropical pulmonary eosinophilia | |
| Causative agents | Schistosoma spp. | Ascaris lumbricoides, hookworms (Ancylostoma duodenale, Necator americanus), Strongyloides stercoralis, etc. | Wuchereria bancrofti, Brugia malayi |
| Mode of transmission | Skin contact with contaminated freshwater |
Ascaris: fecal–oral Hookworms and Strongyloides: walking barefoot on contaminated soil |
Mosquito bites |
| Incubation period | 1–12 weeks | 10–16 days | N/A |
| Symptoms | Fever, chills, dry cough, nausea, abdominal pain, malaise, myalgia, urticaria | Fever, malaise, cough, wheezing, and dyspnoea | Fever, malaise, anorexia or weight loss, and respiratory symptoms like non-productive cough, wheezing, dyspnoea and chest pain which are predominantly nocturnal |
| Radiographic features | Widespread, ill-defined bronchocentric nodules | ||
| Diagnosis | a) Eggs in stool, urine or tissue biopsy samples, or b) Antibody assays, or c) Antigen or PCR tests (not widely available) |
a) Eggs in stool, or b) Larva of Strongyloides stercoralis in stool, sputum or BAL, or c) Antigen or PCR tests (not widely available) |
a) History of residence or travel to a filarial endemic region b) Paroxysmal and nocturnal cough with dyspnoea c) Leukocytosis with peripheral blood eosinophilia >3000 cells per mm3 d) Elevated serum IgE and filarial antibody titres e) Pulmonary infiltrates on chest radiography, and f) Clinical improvement with DEC |
| First-line treatment | Supportive; steroids for severe cases, except strongyloidiasis | ||
| Praziquantel | Albendazole or mebendazole | DEC | |
Schistosomiasis is a waterborne helminthiasis affecting ∼250 million people worldwide [3], mainly in low- and middle-income countries, such as in Africa, parts of Asia and South America [2]. After the first exposure, previously non-immune travellers may be asymptomatic or may develop symptoms of acute schistosomiasis or Katayama fever (syndrome), typically 1–12 weeks after heavy exposure. The patient in question developed typical symptoms of Katayama fever 3 weeks after exposure. A transient pruritic papular rash, known as cercarial dermatitis or “swimmer's itch”, that could precede Katayama fever was also experienced by the patient after paddling in the river. It is associated with penetration of the skin by infective parasite larvae called cercariae that are released into freshwater by infected aquatic snails. Nearly all patients with Katayama fever have eosinophilia and an elevated serum IgE level.
The diagnosis typically relies on finding parasite eggs in stool, urine or tissue biopsy samples (depending on species and clinical manifestations of the disease), although shedding of eggs usually does not commence until ∼6 weeks after exposure, as schistosomes need to mature to adulthood while navigating from the skin, via the lungs to the mesenteric veins of the intestine (Schistosoma mansoni and Schistosoma japonicum, causing hepatosplenic disease) or bladder (Schistosoma haematobium, causing urinary schistosomiasis) [4, 5]. This clinical dilemma is well reflected in our patient, as the primary stool examinations did not yield any parasite eggs, supporting the recommendation that, ideally, a series of at least three samples should be submitted, with collection every other day because of the intermittent nature of parasite shedding [6]. Haematuria, seen upon hospitalisation, led to a false presumption of possible urinary schistosomiasis, as both Schistosoma mansoni and Schistosoma haematobium are found in the DRC; thus, repetitive urine samples for O&P were ordered, yielding negative results. The cystic lesion in the upper pole of the left kidney seen on the CT scan was subsequently diagnosed to be papillary renal cell carcinoma and was most probably responsible for haematuria.
Patients returning from tropical countries should be evaluated for possible concomitant bacterial and parasitic co-infections, as they could be asymptomatic or “masked” by other pathogens. As our patient returned from the DRC, a country endemic for malaria, West African (or Gambian) trypanosomiasis and filariasis, thin and thick blood smears were ordered to exclude possible blood parasites. Repetitive stool samples yielded mixed protozoan species, including Entamoeba histolytica/dispar cysts. As cysts and trophozoites of pathogenic Entamoeba histolytica, which requires antiparasitic treatment, are morphologically identical to the non-pathogenic Entamoeba dispar, it is usually impossible to distinguish them through microscopic examination; thus, additional tests, namely intestinal protozoa multiplex real-time PCR assay along with Entamoeba histolytica antigen, were ordered. Both tests were negative for Entamoeba hystolytica confirming that non-pathogenic Entamoeba dispar was the protozoan found on stool microscopy.
Chest radiographs of patients with Katayama fever often demonstrate diffuse pulmonary opacities or nodules with indistinct borders, which may have a ground-glass “halo” appearance on CT [7], similar to tropical pulmonary eosinophilia and Löffler syndrome, which was briefly the working diagnosis in our case until gathering the full epidemiological history. It was ruled out after establishing that there was no barefoot contact with soil, but there was, however, exposure to freshwater.
Infections with all major Schistosoma species should be treated with praziquantel. As praziquantel is most effective against adult worms and requires the presence of a mature antibody response to the parasite, the timing of antiparasitic treatment is highly important and, in travellers, it is frequently deferred for at least 6–8 weeks after the last exposure to potentially contaminated freshwater [8]. If a patient presents with severe Katayama fever it is recommended to prescribe both anti-schistosomal drugs and pulsed corticosteroids. Steroids reduce inflammation and help suppress adverse effects from the death of parasites. As maturing schistosomes are less susceptible to therapy than adult worms, a second course of treatment is necessary, typically 4–6 weeks after the first dose [9], our patient was treated accordingly. Self-limiting malaise after the first dose was reported by our patient. Adverse effects, caused by dying schistosomes (dizziness, headache, nausea, vomiting, diarrhoea, abdominal discomfort, bloody stool, urticaria and fever), are usually mild and last about 24 h following initiation of treatment.
If the pre-treatment stool or urine examination is positive for schistosome eggs, follow-up examination at 1–2 months post-treatment is suggested to help confirm a successful cure. This was demonstrated by our patient, since stool samples turned negative on the first follow-up examination, with eosinophil count decreasing and a follow-up CT scan showing a marked improvement, indicating a treatment success.
Footnotes
Conflict of interest: The authors have nothing to disclose.
References
- 1.Vijayan VK. Tropical pulmonary eosinophilia: pathogenesis, diagnosis and management. Curr Opin Pulm Med 2007; 13: 428–433. doi: 10.1097/MCP.0b013e3281eb8ec9 [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq JA, Kim H, Memish ZA. Parasitic lung diseases. Eur Respir Rev 2022; 31: 220093. doi: 10.1183/16000617.0093-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo NC, Bezerra FSM, Colley DG, et al. Review of 2022 WHO guidelines on the control and elimination of schistosomiasis. Lancet Infect Dis 2022; 22: e327–e335. doi: 10.1016/S1473-3099(22)00221-3 [DOI] [PubMed] [Google Scholar]
- 4.Maguire JH. 288 - Trematodes (Schistosomes and Liver, Intestinal, and Lung Flukes). In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th Edn. Amsterdam, Elsevier, 2019; pp. 3451–3462. [Google Scholar]
- 5.Costain AH, MacDonald AS, Smits HH. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front Immunol 2018; 9: 3042. doi: 10.3389/fimmu.2018.03042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenstein P, Pfaller M, Yungbluth M. The use and abuse of routine stool microbiology: a College of American Pathologists Q-probes study of 601 institutions. Arch Pathol Lab Med 1996; 120: 206–211. [PubMed] [Google Scholar]
- 7.Kim KC, Weiss LM. 58 - Parasitic Infections. In: Broaddus VC, Ernst JD, Talmadge EK Jr, et al. Murray and Nadel's Textbook of Respiratory Medicine. 7th Edn. Amsterdam, Elsevier, 2022; pp. 781–796. [Google Scholar]
- 8.US Centers for Disease Control and Prevention . Parasites – Schistosomiasis: Resources for Health Professionals. Date last accessed: 15 July 2023. Date last updated: 28 October 2020. www.cdc.gov/parasites/schistosomiasis/health_professionals/index.html#print
- 9.Fenwick A, Rollinson D, Southgate V. Implementation of human schistosomiasis control: challenges and prospects. Adv Parasitol 2006; 61: 567–622. doi: 10.1016/S0065-308X(05)61013-5 [DOI] [PubMed] [Google Scholar]