Abstract
High-resolution computed tomography (HRCT) plays a pivotal role in the diagnosis and management of interstitial lung diseases (ILDs), particularly given the approval of antifibrotic agents for conditions like idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Diagnosing fibrotic pulmonary disorders through HRCT involves a detailed and methodical examination. The identification of specific lung tissue changes, including ground-glass opacities and reticulation, along with signs of fibrosis like honeycombing, traction bronchiectasis and lung volume loss, establishes clear HRCT patterns indicative of various ILDs. The reliability of these patterns in predicting pathological conditions depends largely on the clinical context. For instance, when a usual interstitial pneumonia pattern is present, the predictive value of this diagnosis is so high that a lung biopsy is considered to be redundant. This review intends to delineate the HRCT signs of fibrosis, elucidate the specific radiological patterns of fibrotic lung diseases, and identify the clinical circumstances under which these patterns emerge. Additionally, we introduce and discuss novel imaging techniques that hold promise for the diagnosis, screening and early detection of ILDs.
Shareable abstract
Imaging plays a central role in diagnosing and managing fibrosing lung diseases. A methodical evaluation of HRCT is critical for identifying various lung fibroses. https://bit.ly/3UfNOfO
Educational aims
To describe the radiological signs of fibrosis such as honeycombing, traction bronchiectasis and volume loss on chest HRCT.
To describe specific chest HRCT patterns of pulmonary fibrosis.
To describe the most frequent chest HRCT patterns of pulmonary fibrosis associated with specific fibrotic ILDs.
To describe new imaging modalities which can help to recognise and treat fibrotic ILDs at an early stage.
Introduction
High-resolution computed tomography (HRCT) is an important tool for the diagnosis and assessment of patients with interstitial lung diseases (ILDs) in combination with clinical information in the context of a multidisciplinary discussion [1, 2]. The recent approval of antifibrotic agents for idiopathic pulmonary fibrosis (IPF) and for non-IPF ILDs that present with a progressive phenotype, namely progressive pulmonary fibrosis (PPF), has made the early and correct recognition of these conditions very important [1]. It is now well accepted that in the appropriate clinical scenario (i.e. considering the patient's age and exclusion of other causes of pulmonary fibrosis), the identification of a definite or probable usual interstitial pneumonia (UIP) pattern can lead to the diagnosis of IPF without the need to proceed to a surgical lung biopsy [1].
Apart from diagnostic information, HRCT can also provide prognostic information. The presence of a UIP-like pattern in non-IPF ILDs, such as connective tissue disease (CTD)-associated ILD, fibrotic hypersensitivity pneumonitis (f-HP) and unclassifiable ILD, was associated with an IPF-like progression in a recent pharmaceutical trial [3]. The presence of HRCT signs of fibrosis such as honeycombing and traction bronchiectasis was found to be associated with worse prognosis in CTD-ILD and f-HP [4–6].
In a serious and complex disease such as ILD, an ideal screening test should reliably identify ILD even before symptoms develop. Chest HRCT is the most commonly used test for the diagnosis of ILD. However, it is not offered as a screening tool to all patients and, therefore, there is a need to develop and validate more innovative screening modalities.
In this review we aim to describe the signs of fibrosis on HRCT, the distinct radiological patterns of fibrosing lung diseases and the clinical entities in which these patterns may be observed. Finally, we aim to describe new imaging modalities that can be used for screening and early diagnosis of ILDs.
HRCT protocol
Patients suspected of having ILD should undergo a nonenhanced, volumetric HRCT scan during deep inspiration, with a slice thickness not exceeding 1.5 mm [7]. To indirectly diagnose small airways disease through the visualisation of air trapping, an additional acquisition in expiration may be useful. Furthermore, scanning in the prone position can assist in distinguishing minimal lung fibrosis from dependent atelectasis.
Key signs of lung fibrosis on HRCT
The use of HRCT has markedly transformed how ILDs are diagnosed and managed, with a particular emphasis on the detection and assessment of fibrotic ILDs. An essential advantage of HRCT is the ability to distinguish predominantly fibrotic from non-fibrotic forms. This process depends on identifying three key indicators of fibrosis: honeycombing, traction bronchiectasis and lung volume loss. These are discussed in detail in the following sections.
Honeycombing
In pathology, honeycombing is defined as cystic, fibrotic airspaces that are frequently lined by bronchiolar epithelium [8]. These spaces often contain mucin and a varying number of inflammatory cells. They are encased in dense collagen, which is accompanied by varying degrees of inflammation. Scarring in this context is marked by irregular and thick collagen deposits that lead to the obliteration of alveolar structures [9].
On HRCT, honeycombing is defined as clustered cystic spaces with thick walls (figure 1a). These spaces are usually uniform in diameter, ranging from 3 to 10 mm, but can extend up to 25 mm [10]. It is worth noting that honeycomb cysts on HRCT correspond to visibly apparent cysts in gross pathological specimens. A single layer of 2–3 contiguous subpleural cysts could also be defined as honeycombing [11]. Honeycombing is particularly central in diagnosing UIP on HRCT. The presence of subpleural, basal honeycombing is considered a typical UIP pattern [1]. Variable distribution of honeycombing is observed in f-HP [12].
FIGURE 1.
a) Axial computed tomography (CT) of a patient with usual interstitial fibrosis displaying a cluster of thin-walled cysts at the lung periphery, known as honeycombing. b) Axial CT of a patient with emphysema demonstrating grouped areas of centrilobular emphysema, which may resemble honeycombing. Unlike honeycombing, centrilobular emphysema appears as very thin-walled structures without associated volume loss or traction bronchiectasis. c) Axial CT of a patient with extensive traction bronchiectasis, where the grouped traction bronchiectasis and bronchiolectasis might mimic honeycombing. d) Minimum intensity projection of the same section confirming the presence of traction bronchiectasis.
Distinguishing honeycombing from emphysema and traction bronchiectasis presents a diagnostic challenge. Areas of emphysema, either centrilobular or paraseptal, superimposed on interstitial fibrosis, can closely resemble honeycombing (figure 1b). The presence of emphysema may complicate the identification of HRCT findings such as subpleural sparing and fine reticulation which are crucial for distinguishing UIP from other fibrotic patterns, for example, nonspecific interstitial pneumonia (NSIP) [13]. Likewise, severe traction bronchiectasis may mimic the appearance of honeycombing, requiring careful observation and differentiation for accurate diagnosis and prognosis (figure 1c). Multiplanar reconstruction techniques in HRCT are instrumental in differentiating between traction bronchiectasis and honeycombing (figure 1d).
In non-IPF fibrotic lung diseases, such as f-HP and rheumatoid arthritis (RA)-associated ILD, the presence of honeycombing significantly influences patient outcomes, mirroring the outcome of IPF [6, 14].
Traction bronchiectasis
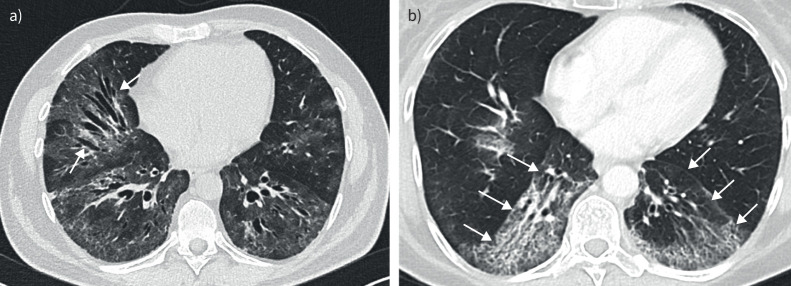
Traction bronchiectasis represents an irreversible bronchial and bronchiolar dilatation caused by surrounding retractile fibrosis (figure 2a) [10]. Traction bronchiectasis is characterised by the presence of varicose or “beaded” dilation of the airways, which is particularly notable in the peripheral regions of the lungs. In these regions the bronchi have less cartilaginous support and are more susceptible to distortion [15]. In HRCT, traction bronchiectasis is seen in conjunction with other indicators of fibrosis such as reticulation and ground-glass opacification. This combination helps distinguish traction from “freestanding” bronchiectasis, where airway dilatation occurs independently of fibrosis and is the consequence of a bronchial disease [16]. The presence and severity of traction bronchiectasis have been recognised as significant prognostic markers in various fibrosing ILDs, often correlating with poorer patient outcomes [5].
FIGURE 2.
a) Axial computed tomography (CT) of a patient with nonspecific interstitial pneumonia, illustrating extensive ground-glass opacities alongside traction bronchiectasis. Notably, the bronchi exhibit varicose dilation without peripheral tapering, indicated by arrows. b) Axial CT of a patient with nonspecific interstitial pneumonia demonstrates a marked volume loss. There is a noticeable shift of the oblique fissures towards the posterior (arrows), deviating from their typical placement in healthy lungs.
Misclassification of traction bronchiectasis can occur in several scenarios: it may be confused with honeycomb cysts, appear prominent due to widespread ground-glass opacification, or occur transiently in acute and subacute inflammatory diseases, like organising pneumonia, where airway dilation can be reversible [16]. Therefore, dilated bronchi within regions where organising pneumonia is suspected should be referred to simply as “dilated bronchi” rather than bronchiectasis.
Volume loss
Although lung volume reduction is not a specific indicator of a specific fibrotic ILD pattern on HRCT, it serves as an important supportive feature, particularly when other signs like honeycombing or definitive traction bronchiectasis are absent.
In conditions such as UIP and NSIP, a notable decrease in lung volume is often most apparent in the lower zones of the lungs. This reduction is often discernible through the altered positioning of the lung's oblique fissures, which, under normal circumstances, extend from the posterior near the aortic arch to proximity with the anterior chest wall near the diaphragm [13]. The assessment of lung volume loss in HRCT also involves analysing the spatial relationship of the right and left oblique fissures on axial images (figure 2b). In lungs affected by fibrosis, these fissures tend to shift towards the back, differing from their standard positioning in healthy lungs [13].
However, care must be taken in interpreting lung volume reduction in HRCT, as it may occasionally result from non-fibrotic factors such as inadequate patient breath-hold during the scan or changes in body position. Using prone and expiratory computed tomography (CT) imaging techniques can be beneficial in distinguishing genuine pathological volume loss from alterations due to positioning or imaging techniques. An additional straightforward intervention to enhance the quality of inspiration images is for the CT radiographer to personally coach the patient on the specific type and duration of breath-hold required for the scan, rather than solely depending on recorded instructions.
Ancillary indicators of fibrosis in HRCT
Areas exhibiting increased opacity while maintaining the integrity of the underlying lung structure are referred to as “ground-glass opacities” (GGOs). These opacities are indicative of a range of pulmonary conditions. When GGOs are associated with subtle fibrotic changes, such as pronounced reticulation and traction bronchiectasis, they present with a granular texture [13]. Nonetheless, GGOs may also signal diverse pathological conditions, including viral pneumonitis, pulmonary oedema or acute exacerbations in patients with existing interstitial fibrosis.
The specificity of GGOs for the diagnosis of ILDs is limited, as they can appear in conditions like UIP, NSIP and f-HP. Occasionally, GGOs can be observed in early-stage ILD or can be associated with gravity dependence. Distinguishing these conditions is important and can be achieved by the performance of HRCT in the prone position, enabling a clearer determination of whether the density changes are disease related. Differentiating GGOs indicative of fine fibrosis from those suggestive of inflammation is also essential. GGOs embedded within regions showing reticulation and traction bronchiectasis typically represent fine fibrosis. In contrast, GGOs that are separate from fibrotic areas are frequently inflammatory in nature [15]. The occurrence of isolated GGOs, devoid of reticular changes, could point to inflammatory processes, and the emergence of new bilateral GGOs in patients with fibrosing ILD warrants consideration of a possible acute exacerbation of ILD [17]. Interpreting GGOs necessitates a detailed and nuanced approach, considering the full clinical and radiological context.
Reticular abnormalities are identified by a distinct pattern of dense linear markings, suggesting the presence of fibrosis or other types of interstitial changes [16]. These reticular patterns are frequently observed in HRCT scans of patients with fibrosing illnesses and tend to coincide with other radiographic signs such as GGOs, traction bronchiectasis and honeycombing. For instance, UIP is typically characterised by reticular opacities that coexist with traction bronchiectasis and honeycombing, mainly affecting the lungs’ peripheral and basal areas. These imaging findings align closely with UIP histopathological characteristics. The role of reticular abnormalities in diagnosing UIP is underscored by research indicating that patient age is a significant factor in predicting UIP, especially in the absence of honeycombing on HRCT. Patients over 75 years of age exhibiting moderate-to-extensive reticular changes have a high likelihood of UIP, as confirmed by surgical lung biopsy [18].
It is important to note that reticular patterns are not exclusively indicative of pulmonary fibrosis. Misinterpretations can occur, particularly when external factors like incomplete lung inflation during imaging come into play [18]. Using prone CT imaging can assist in distinguishing between changes due to patient positioning and actual pathological alterations.
CT patterns in fibrosing lung diseases
The combination of specific parenchymal findings, such as GGOs or reticulation, along with signs of fibrosis (e.g. honeycombing, traction bronchiectasis and/or volume loss), and the distribution of these finding define distinct HRCT disease patterns (table 1). The predictive value of these HRCT patterns varies depending on the clinical context. In the case of a definite or probable UIP pattern, the correlation is so strong that, in the appropriate clinical setting, a biopsy may be deemed unnecessary due to the high predictive value for the corresponding histological diagnoses. However, for other CT diagnoses like NSIP or f-HP, the predictive value for matching the histological diagnosis is not as robust.
TABLE 1.
Radiological characteristics of specific fibrotic patterns
| Parenchymal findings | Extra-parenchymal findings | Distribution | Important to note | |
| Typical UIP pattern | Honeycombing Reticular abnormalities Varying degree of ground-glass attenuation |
±Traction bronchiectasis | Subpleural with a basal predominance | The absence of features suggestive of an alternative diagnosis |
| Probable UIP pattern | Absent honeycombing Reticular abnormalities ±Mild ground-glass attenuation |
Peripheral traction bronchiectasis or traction bronchiectasis | Subpleural with a basal predominance No subpleural sparing |
The absence of features suggestive of an alternative diagnosis |
| Indeterminate for UIP pattern | Subtle reticular abnormalities ±Ground-glass opacification superimposed on a fine reticular pattern |
Do not suggest any specific aetiology | HRCT features of pulmonary fibrosis that do not meet the “UIP” or “probable UIP” patterns and does not explicitly suggest an “alternative diagnosis” | |
| Fibrotic NSIP | Ground-glass opacities: mostly bilateral and symmetrical; immediate subpleural sparing is a relatively specific sign Reticular opacities and irregular linear opacities (sometimes, minor subpleural reticulation) |
Traction bronchiectasis Loss of volume |
Symmetrical, subpleural patterns with an apicobasal gradient | Upper lobe or unilateral dominance suggests a diagnosis other than NSIP |
| Fibrotic hypersensitivity pneumonitis | Centrilobular nodules Air trapping Ground-glass Mosaic attenuation Three density pattern Reticular abnormalities and/or honeycombing |
Traction bronchiectasis | Random both axially and craniocaudally or Mid-lung zone predominant or Relatively spared in the lower lung zones |
UIP: usual interstitial pneumonia; NSIP: nonspecific interstitial pneumonia; HRCT: high-resolution computed tomography.
UIP pattern
The UIP pattern on HRCT constitutes the hallmark feature of IPF but is also observed in a variety of other diseases, such as CTDs (especially RA) and f-HP. Typical radiological UIP can predict histological UIP and thus prevents the need for lung biopsy in the appropriate clinical setting. In the 2018 IPF guidelines, the use of the four HRCT categories (UIP, probable UIP, indeterminate for UIP and CT findings suggestive of alternative diagnosis) was advocated and, despite some discussion, maintained in the 2022 IPF guideline update [1, 8]. The main difference between typical UIP and probable UIP is the presence of honeycombing. The typical UIP pattern is defined by the presence of reticular abnormalities and honeycombing in a basal and peripheral predominance with the absence of changes to suggest another diagnosis (e.g. extensive ground glass, consolidations, centrilobular nodules) (figure 3a and b) [1]. Reticular abnormalities with associated peripheral traction bronchiolectasis with a basal and peripheral distribution and the absence of changes to suggest another diagnosis is defined as probable UIP (figure 3c and d).
FIGURE 3.
a) Axial and b) sagittal computed tomography (CT) reformations of a patient with typical usual interstitial pneumonia (UIP), exhibiting reticular abnormalities, traction bronchiectasis, and honeycombing with a basal and subpleural predominance. Importantly, there are no features suggestive of an alternative diagnosis such as extensive ground-glass opacities, consolidations, or centrilobular nodules. c) Axial and d) sagittal CT reformations of a patient displaying a probable UIP pattern characterised by reticular abnormalities and traction bronchiectasis, absent honeycombing, and a basal and subpleural predominance, without any indicative changes of other diagnoses. e) Axial and f) sagittal CT reformations of a patient with nonspecific interstitial pneumonia presenting as extensive ground-glass opacities predominantly in basal and peripheral areas. g) Axial and h) sagittal CT reformations of a patient with fibrotic hypersensitivity pneumonia demonstrate extensive air trapping (*), a sign of small airway disease, along with fibrosis indicators such as extensive reticular abnormalities and ground-glass opacities, with distorted lung architecture typically located randomly or with a mid-lung predominance.
The guideline experts considered merging “typical UIP” and “probable UIP” into a single category because of similar disease behaviour and the high positive predictive value (PPV) for histological confirmation of UIP in patients with probable UIP [1]. However, as the PPV between the UIP pattern and probable UIP pattern for a UIP histology still differs and patients with probable UIP might have better survival, the authors decided to keep this distinction [1]. Importantly, the updated diagnostic algorithm for the diagnosis of IPF emphasises that patients with a probable UIP pattern on HRCT and an appropriate clinical context are now managed similarly to patients with a typical UIP pattern and an IPF diagnosis without the need for histological confirmation in most cases [1].
It is essential to note that UIP constitutes a radiological finding which is not pathognomonic for IPF but can also be present in other ILD entities (e.g. RA-ILD or f-HP). Consequently, alternative causes for an ILD must be excluded prior to establishing the IPF diagnosis and the patient case should have been discussed in a multidisciplinary ILD board.
NSIP pattern
The most common findings of the NSIP pattern include GGO, traction bronchiectasis, lobar volume loss and non-septal reticular abnormalities, which are typically present in the lower and peripheral lung zones (figure 3e and f) [19–21]. “Subpleural sparing” is a relatively specific sign of NSIP; however, it is only found in about 21–30% of patients [20, 22]. Contrary to the UIP pattern, the NSIP pattern is not pathognomonic but only indicative for pathological NSIP. Signs of fibrosis (e.g. volume loss and/or traction bronchiectasis) are frequently associated with NSIP whereas honeycombing is rarely observed [23, 24].
The NSIP pattern can be present in a variety of disease entities including CTD-ILDs, f-HP and drug-induced ILDs. Taking the clinical context into account, a radiological NSIP pattern can sometimes omit the need for lung biopsy in patients with these conditions, although the diagnostic accuracy of HRCT for NSIP is more limited compared with UIP/IPF [20].
Pleuroparenchymal fibroelastosis
Pleuroparenchymal fibroelastosis (PPFE) is a rare type of lung fibrosis observed either as isolated idiopathic disease or (more commonly) in combination with other fibrosing lung diseases such as IPF, fibrotic hypersensitivity pneumonia or in CTD-ILDs [25–28]. In histology, PPFE is characterised by a pleural and subpleural elastotic fibrosis and intra-alveolar fibrosis with a predominance of the lung apices [25]. On HRCT, PPFE is characterised by multifocal areas of subpleural consolidations in the lung apices that is associated with traction bronchiectasis and a volume loss of the upper lobes leading to an upwards shift of the hilar structures [25, 29].
Radiological signs of PPFE on HRCT should be considered when diagnosing a fibrosing ILD. If present, they must be monitored carefully, as it has been shown that the presence and progression of PPFE-like lesions are associated with mortality, independent of other established measures of disease progression [30, 31].
CT manifestations of specific fibrosing lung diseases
IPF
With an estimated prevalence of ∼8 cases per 100 000, IPF is the most common of all idiopathic interstitial pneumonias [32]. It is a chronic and progressive ILD. The cause is unknown. Risk factors include age, male sex, genetic predisposition and a history of smoking [33].
HRCT plays a central role in the diagnosis of IPF and its significance in the diagnostic algorithm of IPF has been further emphasised in the latest revision of the American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Asociación Latinoamericana de Tórax (ALAT) IPF guideline [1]. As described earlier, the distinctive radiological and histological pattern of IPF is the UIP pattern. IPF is a diagnosis of exclusion; therefore, other possible causes must be ruled out, especially those entities that can also present a UIP pattern on HRCT. The differential diagnoses comprise idiopathic NSIP, drug-induced ILD, CTD-ILD, pneumoconiosis, fibrosing sarcoidosis, f-HP and unclassifiable ILD. Due to the complexity of the differential diagnosis IPF should always be diagnosed by an experienced, multidisciplinary ILD board.
f-HP
Hypersensitivity pneumonitis (HP) is a heterogenous ILD that is characterised by an uncontrolled immune response to an inhaled antigen of organic dust. Two recently published guidelines for the diagnosis of HP have emphasised that the presence of fibrosis on HRCT has a major impact on prognosis and as a result suggest classifying HP as non-fibrotic and fibrotic [12, 34]. According to the proposed CHEST guideline, a confident HP diagnosis can be made with the combination of an identified antigen and a typical HP pattern on HRCT, which stresses the importance of HRCT in establishing the diagnosis [35]. In contrast, the ATS/JRS/ALAT guideline requires lymphocytosis on bronchoalveolar lavage cytology for a confident HP diagnosis [12].
f-HP has various appearances on HRCT. The ATS/JRS/ALAT guideline proposes categorising findings on HRCT as “typical HP”, “compatible with HP” and “indeterminate for HP” [12]. “Typical HP” in f-HP requires both the HRCT pattern of lung fibrosis and radiological signs of small airway disease. Lung fibrosis in f-HP features irregular linear opacities with distortion of the lung architecture located randomly or mid-lung predominantly or relatively spared in the lower lung zones (figure 3g and h) [12, 36, 37]. Traction bronchiectasis and honeycombing may be seen, but are not predominant as opposed to IPF [12]. If honeycombing is present, it can be observed more frequently in the upper lung zones, although occasionally it can be observed in the lower lung zones [36, 38].
Abnormalities indicative of small airway disease include irregular shaped, centrilobular ground-glass nodules and/or mosaic attenuation due to air trapping, which can be seen when comparing HRCT images on inspiration and expiration [34, 39]. These HRCT findings correspond to bronchiolocentric inflammation or fibrosis which results in the obstruction of small airways [39]. GGOs correspond to extensive interstitial inflammation with increased density and preserved visibility of lung vessels and bronchial walls; when these opacities are distributed randomly, the pattern is called “mosaic pattern” [39, 40].
A highly specific feature for f-HP is the concurrent appearance of three different densities of lung parenchyma on HRCT, i.e. normal lung tissue, GGOs (high attenuation) and regions of decreased attenuation (lucent lung) in a lobular distribution, known as the “three density pattern” (formerly called the “head-cheese sign”), a term which was introduced by the ATS/JRS/ALAT guidelines [12, 39, 40]. These radiological features were also regarded as typical for f-HP by an international modified Delphi survey in 2017 [41].
“Compatible with HP” refers to the finding when the HRCT pattern and/or distribution varies from that of the “typical HP” pattern; “indeterminate for HP” refers to the finding when there is a lone pattern which is not accompanied of any other feature suggestive of HP [12].
The presence of emphysema can be observed in f-HP patients and was independent of smoking and occupational history [42, 43].
CTD-ILDs and interstitial pneumonia with autoimmune features
CTDs constitute a heterogenous group of different disease entities. Lung involvement in CTD mostly manifests as an ILD and has a major impact on morbidity and prognosis [44, 45]. RA, systemic sclerosis (SSc) and inflammatory myositis are the most prevalent CTD-ILDs [40]. In addition, 10–20% of ILD patients have clinical features of an underlying autoimmune disease but do not meet established criteria for a CTD. For these patients, some of whom will develop an autoimmune disease in the course of time, the ERS/ATS has proposed the term “interstitial pneumonia with autoimmune features” (IPAF) [46, 47]. It is important to note that IPAF is not a clinical entity but was introduced to better classify those patients for research purposes.
HRCT findings in patients with CTD-ILDs are highly variable and even differ within each disease entity. Generally, the most prevalent radiological pattern in CTD-ILDs is the NSIP pattern, except for in RA-ILD where the UIP pattern is more frequently observed and is a predictor of poor outcome [48–51]. Other common patterns include the organising pneumonia (OP) pattern and lymphocytic interstitial pneumonia (LIP), and some patients present with a variety of overlapping patterns [40, 50]. Compared with idiopathic interstitial pneumonias, GGOs and coexisting extra-parenchymal abnormalities (e.g. pleural/pericardial effusions and oesophageal dilatation) are more common in CTD-ILDs [52]. While there are no definitive CT characteristics that can solely differentiate UIP caused by CTD-ILD from IPF, three signs are more frequently observed in CTD-ILDs compared with idiopathic ILDs:
1) the “anterior upper lobe sign”, where signs of lung fibrosis are present in the anterior upper lobes alongside similar findings in the posterior lower lobes;
2) the “straight edge sign”, characterised by fibrosis limited to the lung bases with distinct demarcation in the craniocaudal plane and minimal extension along the lungs' lateral margins in coronal images; and
3) the “exuberant honeycombing sign”, identified when honeycombing accounts for more than 70% of the fibrotic areas [2].
RA-associated ILD
The most prevalent HRCT pattern in patients with RA-associated ILD is UIP, which is observed in 60% of patients, followed by NSIP [53]. The UIP pattern seen in RA-ILD patients does not differ from UIP in IPF patients and it has been reported that in this case the prognosis is similar between these two clinical entities [51, 54]. However, as for IPF, radiological UIP can predict histological UIP and, in the appropriate clinical setting, obviate the need for lung biopsy [51, 55, 56].
SSc-associated ILD
Up to 50% of SSc patients suffer from an ILD (SSc-ILD) which, besides pulmonary hypertension, is the leading cause of mortality [57–59]. There is evidence that in SSc-ILD the extent of fibrosis on HRCT in isolation or in combination with lung function test parameters can provide significant prognostic information [50]. A staging system has been devised (UK Raynaud's and Scleroderma Association Staging System) that uses HRCT and forced vital capacity (FVC) data to stratify the severity of disease and has been validated for prognostication. When ILD extent was obviously less than, or obviously more than, 20% of the total lung volume on rapid assessment, ILD could be defined as “mild” or “extensive”, respectively. In cases with an “indeterminate” disease extent (i.e. expert HRCT scoring would be required to classify disease), the FVC threshold of 70% can be used to define the ILD as mild or extensive. The distinction between mild and extensive lung disease was shown to be strongly predictive of mortality and subsequent disease progression.
In most cases HRCT shows a NSIP pattern which is accompanied by GGOs and distinct traction bronchiectasis [40]. UIP and OP patterns have also been described but are less frequent [60–63]. PPF may affect a third of SSc-ILD patients with implications for mortality [64].
Combined pulmonary fibrosis and emphysema
In about 54% of patients with idiopathic interstitial pneumonia, lung fibrosis coexists with pulmonary emphysema [65]. This condition is known as combined pulmonary fibrosis and emphysema (CPFE). CPFE is predominantly found in males with a history of smoking and is characterised by severe dyspnoea, despite normal airflow and volume levels, coupled with a markedly reduced diffusing capacity of the lungs for carbon monoxide (DLCO) [65]. Indeed, a recent study suggests that in IPF patients with ≥10% emphysema on HRCT, changes in DLCO might reflect disease progression more accurately than declines in FVC [66]. On HRCT, CPFE manifests as varying degrees of centrilobular, paraseptal, or panacinar emphysema, often observed alongside a UIP pattern. However, CPFE has also been noted in conjunction with other types of lung fibrosis [65]. Crucially, the presence of emphysema can complicate the diagnosis of the specific fibrosis pattern, as differentiating between emphysema and honeycombing can sometimes be challenging [67].
COVID-19-related fibrotic-like changes
In the late stages of the coronavirus disease 2019 (COVID-19) pandemic, some patients experienced long-lasting symptoms and had a prolonged recovery [68]. The pulmonary fibrosis that develops after COVID-19 pneumonia (post-COVID pulmonary fibrosis) and the residual abnormalities seen in the control CT examinations of many patients considered to have recovered, have been referred to as ILD after COVID-19 (post-COVID ILD) [69].
From the second week of COVID-19 pneumonia, the reticular pattern with associated dilatation of bronchi, irregular interlobular or septal thickening gradually increased; in the first months after the outbreak of the disease this had been indicated as the development of fibrosis. After the first wave of the pandemic, it became clear that fibroblast proliferation and a honeycombing pattern may develop, potentially leading to the development of macroscopic fibrosis [70]. A possible explanation for the development of fibrosis could be the acute respiratory distress syndrome driven by the viral infection [71, 72] or the use of mechanical ventilation in patients admitted to the intensive care unit or a possible combination of these two events. However, it is still too early to classify this as irreversible fibrosis [73].
In the first large study of hospitalised patients with COVID-19, lung damage caused by severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Wuhan was associated with viral antigen-induced cytokine release syndrome, drug-induced lung toxicity, high airway pressure and hyperoxia-induced acute lung injury, and mechanical ventilation [74]. The first review articles considered post-COVID ILD to be a chronic inflammatory or primary, genetically influenced and age-related fibroproliferative process (as in IPF) [74].
In an Italian study, pulmonary fibrosis was found in 3% of patients [75]. A review demonstrated that patients with a greater risk for post-COVID-19 pulmonary fibrosis include those who are older, males, smokers and those who have comorbidities [76]. In another Chinese study, 32 confirmed COVID-19 patients were divided into two groups based on signs of fibrosis, and follow-up CT showed fibrosis in 14 patients, and no fibrosis was detected in 18 patients [77]. Compared with the non-fibrosis group, the fibrotic group was older, and the mean value of C-reactive protein and interleukin-6 was also higher, they required longer hospitalisation, received steroid shock therapy and antiviral therapy [77]. Interstitial thickening, irregular margins, coarse reticular pattern, and parenchymal bundles appeared during the course of the disease and may be signs of pulmonary fibrosis. Irregular margins and parenchymal bundles predicted early development of pulmonary fibrosis [77]. However, several methodological problems can be identified in articles calculating the frequency of post-COVID pulmonary fibrosis [69, 78, 79]. First, the authors mainly followed cases with a severe course, which is not representative of a disease where the majority of those affected are asymptomatic or show only mild clinical symptoms. Second, the diagnosis of fibrosis was typically made on the basis of CT, without histological verification. A long-term follow-up report has shown that residual abnormalities commonly seen in at-risk groups at 3-month follow-up show continuous regression until the end of the first year, and no progression occurs [80].
According to the joint recommendation of the European Society of Radiology (ESR) and European Society of Thoracic Imaging (ESTI) [81], correct terminology should be used. In the 6 months following the acute phase, both the degree of parenchymal involvement and the proportion of GGOs decreases. The decreasing density of the GGOs can be called “low-density GGO” [81]. In fibrotic processes, GGO is also a consequence of subtle fibrosis that often begins below even the spatial resolution of CT. Similar to bronchiectasis, fibrosis also indicates an irreversible, potentially progressive process; therefore, the ESR/ESTI instead recommend the use of the term “fibrotic-like changes” to describe reticulations and dilated bronchi passing through consolidates and GGOs until their final, irreversible nature is confirmed [81].
In summary, although post-COVID pulmonary fibrosis is a real phenomenon, its frequency is lower than in previous studies. Regression of post-COVID radiological changes might be slow, even lasting for more than a year.
Interstitial lung abnormalities
Interstitial lung abnormalities (ILAs) are frequently observed as incidental findings on CT scans, particularly in older individuals (over 60 years of age), with a prevalence of 4–9% in smokers and 2–7% in nonsmokers [82]. ILAs can occur in never-smokers [83] and it is a relatively common incidental finding in participants undergoing low-dose CT screening for lung cancer [84]. Incident ILA has been associated with age, high attenuation area at baseline, the MUC5B promoter single nucleotide polymorphism, ever-smoking and an IPF polygenic risk score [85].
The abnormalities include ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis, honeycombing and non-emphysematous cysts. According to the 2020 position paper of the Fleischner Society [82], ILAs refer to CT findings that are potentially compatible with ILD in patients without previous clinical suspicion of ILD. The definition of ILAs is a purely radiological term, without taking the clinical signs into consideration, based on the incidental identification of CT abnormality [86]. Differentiation between ILAs and clinical/subclinical ILD must be based on clinical evaluation. Accordingly, subclinical ILD and abnormalities identified during screening for ILD in high-risk groups (e.g. CTD-ILD) cannot be considered as ILAs because they are not incidental [86].
ILAs are non-dependent abnormalities affecting more than 5% of any lung zone (the upper, middle and lower lung zones are demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein) [86]. Where there is evidence of clinically significant disease (e.g. respiratory symptoms or physical examination findings possibly attributable to ILD, extensive disease present in three or more lung zones on CT), a potentially clinically significant ILD must be considered instead of ILA [86].
Studies have shown that fibrotic ILAs characterised by the presence of signs of fibrosis, such as traction bronchiectasis or honeycombing, might be an important precursor to IPF or other progressive fibrotic ILDs [85, 87].
Based on the recommendation of the Fleischer Society, follow-up CT scans of patients with ILAs must be decided according to the presence of risk factors for progression (non-fibrotic ILA with basal and peripheral predominance, UIP or probable UIP pattern) [86]. In cases of active monitoring, the CT scan must be repeated at 12–24 months or sooner if there is clinical or physiological progression [86]. A recent study suggested that follow-up CT at 3-year intervals may be appropriate to monitor radiological progression in patients with initially detected ILA [87]. In addition, a high-risk patient with extensive fibrotic ILA or ILAs with honeycombing may benefit from an earlier follow-up CT scan [87].
ILA is considered as a relevant comorbidity that can be an increased risk factor for rapid disease acceleration or an acute exacerbation [86]. ILA status was shown to be a significant risk factor for radiological ILA progression after surgical resection in patients with lung cancer [88, 89]. Evidence has shown that despite the increased use of the term ILA among thoracic-trained radiologists, non-thoracic general radiologists have not begun to use the term [90].
PPF
Patients with lung fibrosis may exhibit a clinical phenotype termed “progressive pulmonary fibrosis” (PPF). According to the latest guideline from the ATS/ERS/JRS/ALAT on IPF/PPF, this condition is characterised by meeting at least two of the following three criteria within the past 12 months, after cardiac and other causes have been ruled out: 1) worsening of respiratory symptoms, 2) physiological evidence of disease progression (such as declining FVC or DLCO), or 3) radiological evidence of increased fibrosis [1]. The guideline authors emphasise that PPF is not a diagnosis, but rather a clinical phenotype that has been shown to be associated with worse prognosis and thus opens doors for antifibrotic therapy in these patients [1].
Radiological evidence of PPF includes one of the following according to the ATS/ERS/JRS/ALAT guideline [1]:
increased extent or severity of traction bronchiectasis and bronchiolectasis,
new GGO with traction bronchiectasis,
new fine reticulation,
increased extent or increased coarseness of reticular abnormality,
new or increased honeycombing, or
increased lobar volume loss.
Progression of fibrosis according to the abovementioned criteria is mostly determined visually by the thoracic radiologist, but quantitative assessment (e.g. by using machine learning algorithms) should be considered as it provides a more objective and reproducible result [1].
New diagnostic modalities
The gold standard imaging method for ILDs is the HRCT technique; in addition, photon counting CT (PCCT) can open new ways in which to further understand the disease. Additional imaging methods such as lung ultrasound, magnetic resonance imaging (MRI) and molecular imaging (e.g. positron emission tomography (PET)) have also attracted attention in recent years based on the results of studies in ILD to provide additional prognostic, functional and molecular information [91]. These noninvasive imaging methods can provide new information to improve the treatment of ILD and to recognise the molecular characteristics, with the help of personalised treatment methods.
Photon counting detector CT or PCCT
The result of the developments in CT technology over the past decade has been PCCT, which provides improved image quality and reduced radiation exposure. PCCT uses a new X-ray detector, the photon counting detector (PCD), instead of the traditional energy integrating detectors (EIDs). PCDs are made of compounds (e.g. silicon, cadmium telluride) that convert each X-ray photon directly into an electrical pulse, enabling a smaller detector pixel size and the resolution of the energy of the photons, thereby reducing noise, increasing spatial resolution, radiation hardening and the X-ray dose can also be reduced [92]. Due to the energy resolution capability of PCD detectors, spectral-based imaging (e.g. monoenergetic imaging or water/iodine imaging) became possible for the first time. PCCT can produce ultra-high-resolution (UHR) CT images with a minimum slice thickness of 0.25 mm [93].
The recognition of ILD patterns requires a very high spatial resolution, since the most important signs (e.g. intralobular reticulations, bronchiectasis, honeycombing) are depicted at the level of the secondary lobules, due to the advantages of PCCT, a better recognition of the patterns is possible, even in the case of an early-stage ILD. In patients with ILD, PCCT was able to visualise the more distal bronchial distributions, or the third/fourth/fifth order bronchial walls were also more visible [94]. In a study involving a patient with RA, the reticulation and bronchiectasis were better visualised in the UHR series compared with the HRCT, while the evaluation of GGO and honeycombing did not differ between the two techniques, and it was also associated with a lower effective radiation dose [95, 96].
PCD-CT is used as an alternative to conventional CT in chest imaging, opening up new perspectives. Compared with conventional EID CT systems, PCCT can reduce radiation dose while maintaining image quality and diagnostic performance [96].
In summary, the advantages of PCCT compared with conventional CT and HRCT are higher spatial resolution, finer lesions, better evaluation of lung nodule volume and better visibility of pulmonary vessels, which is also important in imaging ILDs.
PET/CT
The use PET/CT in the management of lung fibrosis is an area of active investigation. The predominant tracer used in PET scans is 18F-fluorodeoxyglucose (FDG), a glucose analogue labelled with radioactive fluorine. This tracer is selectively taken up by cells with high glucose metabolism, which can signify active disease areas. PET/CT scans using this tracer might, therefore, identify regions of heightened metabolic activity in the lungs, thereby providing valuable insights into the pathophysiology and assisting in the clinical management of lung fibrosis. Notably, elevated FDG uptake in patients IPF has been linked to disease severity, prognosticating progression-free survival and correlating with a higher mortality risk [85, 86]. The ability of FDG-PET/CT to predict treatment response, however, is questionable [87].
Novel PET tracers currently in the research phase appear promising for evaluating the activity and progression of ILDs. Among these, fibroblast activation protein inhibitor (FAPI) PET imaging is particularly noteworthy. Fibroblast activation protein serves as a marker of wound healing and is actively involved in the remodelling processes of the extracellular matrix. Studies have shown that FAPI uptake correlates with the progression of SSc-ILD, independent of the extent of involvement observed in CT scans and lung function tests [97]. Additionally, changes in tracer uptake have been found to align with responses to treatment, such as with nintedanib, indicating its potential utility in monitoring disease progression and treatment efficacy in ILD patients [98].
MRI
Despite the fact that HRCT is still the number one imaging method for examining ILDs, new developments in MRI have improved the visualisation of coarser parenchymal abnormalities and inflammatory activity; however, there is still no evidence for the use of chest MRI in IPF, apart from cystic lung diseases. The low proton density of the lung limits the detectable signal for magnetic resonance in addition to disturbing susceptibility and motion artefacts. With new sequences, better acquisition techniques and improved image resolution, the lung has become better visualised; however, the spatial resolution of magnetic resonance is invariably lower compared to CT [34].
On T2-weighted sequences, interstitial changes increase the signal intensity of the parenchyma and show a signal similar to the muscles of the chest wall, so infiltrates, mucus, fluid collections and inflammatory bronchial wall thickening might be observed on T2-weighted images. Alveolitis can also give a high signal on T2-weighted sequences, which can be followed as a sign of therapeutic response during control examinations, and can help distinguish active inflammatory or stable fibrotic abnormalities. After the administration of contrast material (on 3D-gradient echo (GRE) sequences), the inflamed areas show a higher signal intensity in 1- or 3-minute sequences, and then show signs of washout [35–37].
Another MRI technique that has been proposed is the use of late gadolinium enhancement, which has been shown to help differentiate CPFE from pure emphysema [99].
Since a few years ago, low-field strength (0.55 T) superconductive magnetic resonance devices developed with artificial intelligence have been available; this configuration provides improved B0-field homogeneity and, in this connection, extended T2* and reduced susceptibility gradients. Dynamic sequences (e.g. FFE, TRUFI) allow the movement of the diaphragm to be measured quantitatively.
Thoracic magnetic resonance elastography (MRE) is another technique that may be a promising biomarker for ILDs in the future. This noninvasive technique can quantify the topographical distribution of shear stiffness in tissues, which is widely used in the evaluation of liver fibrosis. In ILD, the shear stiffness of the fibrotic parenchyma was found to be higher in some reports [38].
Conclusion
Imaging plays a central role in diagnosing and managing fibrosing lung diseases. A methodical evaluation of HRCT is critical for identifying various lung fibroses. Crucially, diagnosing pulmonary fibrosis necessitates an integrated approach that combines clinical and radiological evidence and, when necessary, histological findings. This final diagnosis should be made in a multidisciplinary team meeting.
Key points
Chest HRCT has a central role in the diagnosis and treatment of fibrotic ILDs.
In the appropriate clinical setting, chest HRCT can lead to the diagnosis of a specific fibrotic ILD after a multidisciplinary team discussion, sometimes obviating the need for lung biopsy.
The presence of specific radiological signs of fibrosis has prognostic value.
More advanced radiological techniques could increase the diagnostic accuracy of fibrotic ILDs and could be used for screening and early detection of fibrotic ILDs.
Early diagnosis and early initiation of antifibrotic treatment to slow the rate of progression is of paramount importance in patients with IPF and PPF.
Self-evaluation questions
- Regarding the HRCT pattern of UIP, which of the following statement(s) is/are correct?
- a) Is associated with the diagnosis of IPF when other causes of fibrosis are excluded
- b) Is the most frequent HRCT pattern in CTD-associated ILD
- c) Is associated with a prognosis similar to IPF when associated with non-IPF fibrotic ILDs
- d) Cannot be observed in cases of fibrotic HP
- Regarding the presence of the radiological sign of honeycombing, which of the following statement(s) is/are correct?
- a) Is a sign of pulmonary fibrosis on HRCT
- b) Is required for the definition of a typical/definite UIP pattern
- c) In combination with traction bronchiectasis, can be observed in fibrotic NSIP
- d) Could be difficult to define in the presence of radiological emphysema
- Which of the following is/are true?
- a) The “three density” sign is highly suggestive of the diagnosis of fibrotic HP
- b) In SSc-associated ILD, the pattern of fibrotic NSIP is more frequent than UIP
- c) The pattern of fibrosis observed in COVID-related fibrotic ILD can regress over time, unlike classic fibrotic ILD
- d) ILAs are frequently encountered as incidental findings on CT scans which do not require any follow-up
- Which of the following is/are true?
- a) FDG-PET/CT examination plays an important role in the characterisation of possible malignant lesions, especially in the presence of UIP pattern
- b) SSc-ILD extent on HRCT provides prognostic information
- c) Ultra-high-resolution (UHR) CT images allow better recognition of a fibrotic pattern even in cases of early-stage ILD
- d) ILAs never present with a typical/definite UIP pattern
Suggested answers
1. a and c.
2. a, b and d.
3. a, b and c.
4. a, b and c.
Acknowledgements
GPT 4.0 was used for language correction.
Footnotes
Conflict of interest: M. Storman and C. Lederer have no conflicts of interests that relate to this article. D.L. Tarnoki and A.D. Tarnoki declare they have received funding outside the present work from the Bólyai scholarship of the Hungarian Academy of Sciences, from ÚNKP-20-5 and ÚNKP-21-5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund, and from the Hungarian National Laboratory (under the National Tumor Biology Laboratory project, NLP-17). G.A. Margaritopoulos has received speaking fees from Boehringer Ingelheim. H. Prosch has received speaking fees from AstraZeneca, BMS, Boehringer Ingelheim, Janssen, MSD, Novartis, Roche, Sanofi, Siemens and Takeda, and has received research support from AstraZeneca, Boehringer Ingelheim, Siemens and the EU Commission (EU4Health, Horizon Europe Health).
Support statement: For A.D. Tarnoki and D.L. Tarnoki, this project was implemented with support from the National Research, Development and Innovation Fund of the Ministry of Culture and Innovation under the National Laboratories Program (National Tumor Biology Laboratory (2022-2.1.1-NL-2022-00010)) Grant Agreement with the National Research, Development and Innovation Office.
References
- 1.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung JH, Cox CW, Montner SM, et al. CT features of the usual interstitial pneumonia pattern: differentiating connective tissue disease-associated interstitial lung disease from idiopathic pulmonary fibrosis. AJR Am J Roentgenol 2018; 210: 307–313. doi: 10.2214/AJR.17.18384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. doi: 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 4.Walsh SL, Sverzellati N, Devaraj A, et al. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014; 69: 216–222. doi: 10.1136/thoraxjnl-2013-203843 [DOI] [PubMed] [Google Scholar]
- 5.Walsh SL, Sverzellati N, Devaraj A, et al. Chronic hypersensitivity pneumonitis: high resolution computed tomography patterns and pulmonary function indices as prognostic determinants. Eur Radiol 2012; 22: 1672–1679. doi: 10.1007/s00330-012-2427-0 [DOI] [PubMed] [Google Scholar]
- 6.Salisbury ML, Gu T, Murray S, et al. Hypersensitivity pneumonitis: radiologic phenotypes are associated with distinct survival time and pulmonary function trajectory. Chest 2019; 155: 699–711. doi: 10.1016/j.chest.2018.08.1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hobbs S, Chung JH, Leb J, et al. Practical imaging interpretation in patients suspected of having idiopathic pulmonary fibrosis: official recommendations from the Radiology Working Group of the Pulmonary Fibrosis Foundation. Radiol Cardiothorac Imaging 2021; 3: e200279. doi: 10.1148/ryct.2021200279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 9.Katzenstein AL, Mukhopadhyay S, Myers JL. Diagnosis of usual interstitial pneumonia and distinction from other fibrosing interstitial lung diseases. Hum Pathol 2008; 39: 1275–1294. doi: 10.1016/j.humpath.2008.05.009 [DOI] [PubMed] [Google Scholar]
- 10.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 11.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med 2018; 6: 138–153. doi: 10.1016/S2213-2600(17)30433-2 [DOI] [PubMed] [Google Scholar]
- 12.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e36–e69. doi: 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob J, Hansell DM. HRCT of fibrosing lung disease. Respirology 2015; 20: 859–872. doi: 10.1111/resp.12531 [DOI] [PubMed] [Google Scholar]
- 14.Adegunsoye A, Oldham JM, Bellam SK, et al. CT honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann Am Thorac Soc 2019; 16: 580–588. doi: 10.1513/AnnalsATS.201807-443OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horst C, Gholipour B, Nair A, et al. Differential diagnoses of fibrosing lung diseases. BJR Open 2019; 1: 20190009. doi: 10.1259/bjro.20190009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torres P, Rabahi MF, Moreira M, et al. Importance of chest HRCT in the diagnostic evaluation of fibrosing interstitial lung diseases. J Bras Pneumol 2021; 47: e20200096. doi: 10.36416/1806-3756/e20200096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 18.Calandriello L, Walsh SL. The evolution of computer-based analysis of high-resolution CT of the chest in patients with IPF. Br J Radiol 2022; 95: 20200944. doi: 10.1259/bjr.20200944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumikawa H, Johkoh T, Fujimoto K, et al. Pathologically proved nonspecific interstitial pneumonia: CT pattern analysis as compared with usual interstitial pneumonia CT pattern. Radiology 2014; 272: 549–556. doi: 10.1148/radiol.14130853 [DOI] [PubMed] [Google Scholar]
- 20.Silva CI, Müller NL, Hansell DM, et al. Nonspecific interstitial pneumonia and idiopathic pulmonary fibrosis: changes in pattern and distribution of disease over time. Radiology 2008; 247: 251–259. doi: 10.1148/radiol.2471070369 [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Hunninghake G, King TE Jr, et al. Idiopathic nonspecific interstitial pneumonia: report of an American Thoracic Society project. Am J Respir Crit Care Med 2008; 177: 1338–1347. doi: 10.1164/rccm.200611-1685OC [DOI] [PubMed] [Google Scholar]
- 22.Park IN, Jegal Y, Kim DS, et al. Clinical course and lung function change of idiopathic nonspecific interstitial pneumonia. Eur Respir J 2009; 33: 68–76. doi: 10.1183/09031936.00158507 [DOI] [PubMed] [Google Scholar]
- 23.Flaherty KR, Toews GB, Travis WD, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002; 19: 275–283. doi: 10.1183/09031936.02.00182002 [DOI] [PubMed] [Google Scholar]
- 24.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest 2003; 124: 1215–1223. doi: 10.1378/chest.124.4.1215 [DOI] [PubMed] [Google Scholar]
- 25.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–748. doi: 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumikawa H, Johkoh T, Egashira R, et al. Pleuroparenchymal fibroelastosis-like lesions in patients with interstitial pneumonia diagnosed by multidisciplinary discussion with surgical lung biopsy. Eur J Radiol Open 2020; 7: 100298. doi: 10.1016/j.ejro.2020.100298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SI, Chae EJ, Song JS, et al. Pleuroparenchymal fibroelastosis in patients with idiopathic pulmonary fibrosis. Respirology 2020; 25: 1046–1052. doi: 10.1111/resp.13796 [DOI] [PubMed] [Google Scholar]
- 28.Enomoto Y, Nakamura Y, Colby TV, et al. Radiologic pleuroparenchymal fibroelastosis-like lesion in connective tissue disease-related interstitial lung disease. PLoS One 2017; 12: e0180283. doi: 10.1371/journal.pone.0180283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe K, Ishii H, Kiyomi F, et al. Criteria for the diagnosis of idiopathic pleuroparenchymal fibroelastosis: a proposal. Respir Investig 2019; 57: 312–320. doi: 10.1016/j.resinv.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 30.Gudmundsson E, Zhao A, Mogulkoc N, et al. Pleuroparenchymal fibroelastosis in idiopathic pulmonary fibrosis: survival analysis using visual and computer-based computed tomography assessment. EClinicalMedicine 2021; 38: 101009. doi: 10.1016/j.eclinm.2021.101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudmundsson E, Zhao A, Mogulkoc N, et al. Delineating associations of progressive pleuroparenchymal fibroelastosis in patients with pulmonary fibrosis. ERJ Open Res 2023; 9: 00637-2022. doi: 10.1183/23120541.00637-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duchemann B, Annesi-Maesano I, Jacobe de Naurois C, et al. Prevalence and incidence of interstitial lung diseases in a multi-ethnic county of Greater Paris. Eur Respir J 2017; 50: 1602419. doi: 10.1183/13993003.02419-2016 [DOI] [PubMed] [Google Scholar]
- 33.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017; 389: 1941–1952. doi: 10.1016/S0140-6736(17)30866-8 [DOI] [PubMed] [Google Scholar]
- 34.Fernández Pérez ER, Travis WD, Lynch DA, et al. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report. Chest 2021; 160: e97–e156. doi: 10.1016/j.chest.2021.03.066 [DOI] [PubMed] [Google Scholar]
- 35.Fernández Pérez ER, Travis WD, Lynch DA, et al. Executive summary: diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report. Chest 2021; 160: 595–615. doi: 10.1016/j.chest.2021.03.067 [DOI] [PubMed] [Google Scholar]
- 36.Vasakova M, Morell F, Walsh S, et al. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med 2017; 196: 680–689. doi: 10.1164/rccm.201611-2201PP [DOI] [PubMed] [Google Scholar]
- 37.Salisbury ML, Myers JL, Belloli EA, et al. Diagnosis and treatment of fibrotic hypersensitivity pneumonia. Where we stand and where we need to go. Am J Respir Crit Care Med 2017; 196: 690–699. doi: 10.1164/rccm.201608-1675PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva CI, Muller NL, Lynch DA, et al. Chronic hypersensitivity pneumonitis: differentiation from idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia by using thin-section CT. Radiology 2008; 246: 288–297. doi: 10.1148/radiol.2453061881 [DOI] [PubMed] [Google Scholar]
- 39.Hamblin M, Prosch H, Vašáková M. Diagnosis, course and management of hypersensitivity pneumonitis. Eur Respir Rev 2022; 31: 210169. doi: 10.1183/16000617.0169-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kreuter M, Costabel U, Herth FJF, et al., eds. Seltene Lungenerkrankungen. 2nd Edn. Heidelberg, Springer Berlin, 2021. [Google Scholar]
- 41.Morisset J, Johannson KA, Jones KD, et al. Identification of diagnostic criteria for chronic hypersensitivity pneumonitis: an international modified Delphi survey. Am J Respir Crit Care Med 2018; 197: 1036–1044. doi: 10.1164/rccm.201710-1986OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baqir M, White D, Ryu JH. Emphysematous changes in hypersensitivity pneumonitis: a retrospective analysis of 12 patients. Respir Med Case Rep 2018; 24: 25–29. doi: 10.1016/j.rmcr.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soumagne T, Chardon ML, Dournes G, et al. Emphysema in active farmer's lung disease. PLoS One 2017; 12: e0178263. doi: 10.1371/journal.pone.0178263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garen T, Lerang K, Hoffmann-Vold AM, et al. Mortality and causes of death across the systemic connective tissue diseases and the primary systemic vasculitides. Rheumatology (Oxford) 2019; 58: 313–320. doi: 10.1093/rheumatology/key285 [DOI] [PubMed] [Google Scholar]
- 45.Li L, Zuo X, Luo H, et al. Mortality trend of inpatients with connective tissue diseases: 2005-2014. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017; 42: 927–933. doi: 10.11817/j.issn.1672-7347.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 46.Lederer C, Buschulte K, Hellmich B, et al. Interstitielle Lungenerkrankungen: Klassifikation, Differenzialdiagnostik und therapeutische Ansätze bei einer heterogenen Gruppe chronischer Lungenerkrankungen [Interstitial lung diseases: classification, differential diagnosis and treatment approaches in a heterogeneous group of chronic lung disorders]. Inn Med (Heidelb) 2023; 64: 247–259. doi: 10.1007/s00108-023-01476-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015; 46: 976–987. doi: 10.1183/13993003.00150-2015 [DOI] [PubMed] [Google Scholar]
- 48.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47: 588–596. doi: 10.1183/13993003.00357-2015 [DOI] [PubMed] [Google Scholar]
- 49.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35: 1322–1328. doi: 10.1183/09031936.00092309 [DOI] [PubMed] [Google Scholar]
- 50.Jee AS, Sheehy R, Hopkins P, et al. Diagnosis and management of connective tissue disease-associated interstitial lung disease in Australia and New Zealand: a position statement from the Thoracic Society of Australia and New Zealand. Respirology 2021; 26: 23–51. doi: 10.1111/resp.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yunt ZX, Chung JH, Hobbs S, et al. High resolution computed tomography pattern of usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease: relationship to survival. Respir Med 2017; 126: 100–104. doi: 10.1016/j.rmed.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 52.Hwang JH, Misumi S, Sahin H, et al. Computed tomographic features of idiopathic fibrosing interstitial pneumonia: comparison with pulmonary fibrosis related to collagen vascular disease. J Comput Assist Tomogr 2009; 33: 410–415. doi: 10.1097/RCT.0b013e318181d551 [DOI] [PubMed] [Google Scholar]
- 53.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients – an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019; 58: 2031–2038. doi: 10.1093/rheumatology/kez177 [DOI] [PubMed] [Google Scholar]
- 54.Matson S, Lee J, Eickelberg O. Two sides of the same coin? A review of the similarities and differences between idiopathic pulmonary fibrosis and rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2021; 57: 2002533. doi: 10.1183/13993003.02533-2020 [DOI] [PubMed] [Google Scholar]
- 55.Assayag D, Elicker BM, Urbania TH, et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology 2014; 270: 583–588. doi: 10.1148/radiol.13130187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Assayag D, Lee JS, King TE Jr. Rheumatoid arthritis associated interstitial lung disease: a review. Medicina (B Aires) 2014; 74: 158–165. [PubMed] [Google Scholar]
- 57.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. doi: 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- 58.Rahaghi FF, Hsu VM, Kaner RJ, et al. Expert consensus on the management of systemic sclerosis-associated interstitial lung disease. Respir Res 2023; 24: 6. doi: 10.1186/s12931-022-02292-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoffmann-Vold AM, Maher TM, Philpot EE, et al. Assessment of recent evidence for the management of patients with systemic sclerosis-associated interstitial lung disease: a systematic review. ERJ Open Res 2021; 7: 00235-2020. doi: 10.1183/23120541.00235-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kreuter M, Behr J, Bonella F, et al. S1-Leitlinie Interdisziplinäre Diagnostik interstitieller Lungenerkrankungen im Erwachsenenalter [Consensus guideline on the interdisciplinary diagnosis of interstitial lung diseases]. Pneumologie 2023; 77: 269–302. doi: 10.1055/a-2017-8971 [DOI] [PubMed] [Google Scholar]
- 61.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med 2002; 165: 1581–1586. doi: 10.1164/rccm.2106012 [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann-Vold AM, Allanore Y, Alves M, et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann Rheum Dis 2021; 80: 219–227. doi: 10.1136/annrheumdis-2020-217455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffmann-Vold AM, Fretheim H, Halse AK, et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am J Respir Crit Care Med 2019; 200: 1258–1266. doi: 10.1164/rccm.201903-0486OC [DOI] [PubMed] [Google Scholar]
- 64.Morrisroe K, Hansen D, Stevens W, et al. Progressive pulmonary fibrosis and its impact on survival in systemic sclerosis-related interstitial lung disease. Rheumatology (Oxford) 2023; in press [ 10.1093/rheumatology/kead491]. [DOI] [PubMed] [Google Scholar]
- 65.Cottin V, Selman M, Inoue Y, et al. Syndrome of combined pulmonary fibrosis and emphysema: an official ATS/ERS/JRS/ALAT research statement. Am J Respir Crit Care Med 2022; 206: e7–e41. doi: 10.1164/rccm.202206-1041ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao A, Gudmundsson E, Mogulkoc N, et al. Mortality surrogates in combined pulmonary fibrosis and emphysema. Eur Respir J 2024; 63: 2300127. doi: 10.1183/13993003.00127-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Akira M, Inoue Y, Kitaichi M, et al. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: thin-section CT findings. Radiology 2009; 251: 271–279. doi: 10.1148/radiol.2511080917 [DOI] [PubMed] [Google Scholar]
- 68.Mahase E. Covid-19: what do we know about “long covid”? BMJ 2020; 370: m2815. doi: 10.1136/bmj.m2815 [DOI] [PubMed] [Google Scholar]
- 69.Wells AU, Devaraj A, Desai SR. Interstitial lung disease after COVID-19 infection: a catalog of uncertainties. Radiology 2021; 299: E216–E218. doi: 10.1148/radiol.2021204482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grillo F, Barisione E, Ball L, et al. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect Dis 2021; 21: e72. doi: 10.1016/S1473-3099(20)30582-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cabrera-Benitez NE, Laffey JG, Parotto M, et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology 2014; 121: 189–198. doi: 10.1097/ALN.0000000000000264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thille AW, Esteban A, Fernández-Segoviano P, et al. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med 2013; 1: 395–401. doi: 10.1016/S2213-2600(13)70053-5 [DOI] [PubMed] [Google Scholar]
- 73.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20: 425–434. doi: 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med 2020; 8: 750–752. doi: 10.1016/S2213-2600(20)30222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 2020; 296: E86–E96. doi: 10.1148/radiol.2020201433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanni SE, Fabro AT, de Albuquerque A, et al. Pulmonary fibrosis secondary to COVID-19: a narrative review. Expert Rev Respir Med 2021; 15: 791–803. doi: 10.1080/17476348.2021.1916472 [DOI] [PubMed] [Google Scholar]
- 77.Yu M, Liu Y, Xu D, et al. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J Radiol 2020; 21: 746–755. doi: 10.3348/kjr.2020.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guler SA, Ebner L, Aubry-Beigelman C, et al. Pulmonary function and radiological features 4 months after COVID-19: first results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J 2021; 57: 2003690. doi: 10.1183/13993003.03690-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Gassel RJJ, Bels JLM, Raafs A, et al. High prevalence of pulmonary sequelae at 3 months after hospital discharge in mechanically ventilated survivors of COVID-19. Am J Respir Crit Care Med 2021; 203: 371–374. doi: 10.1164/rccm.202010-3823LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vijayakumar B, Tonkin J, Devaraj A, et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology 2022; 303: 444–454. doi: 10.1148/radiol.2021211746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Martini K, Larici AR, Revel MP, et al. COVID-19 pneumonia imaging follow-up: when and how? A proposition from ESTI and ESR. Eur Radiol 2022; 32: 2639–2649. doi: 10.1007/s00330-021-08317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med 2020; 8: 726–737. doi: 10.1016/S2213-2600(20)30168-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pesonen I, Johansson F, Johnsson Å, et al. High prevalence of interstitial lung abnormalities in middle-aged never-smokers. ERJ Open Res 2023; 9: 00035-2023. doi: 10.1183/23120541.00035-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Upperton S, Beirne P, Bhartia B, et al. Diagnoses and treatments for participants with interstitial lung abnormalities detected in the Yorkshire Lung Screening Trial. BMJ Open Respir Res 2023; 10: e001490. doi: 10.1136/bmjresp-2022-001490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGroder CF, Hansen S, Hinckley Stukovsky K, et al. Incidence of interstitial lung abnormalities: the MESA Lung Study. Eur Respir J 2023; 61: 2201950. DOI: 10.1183/13993003.01950-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hatabu H, Hata A. Time to start describing fibrotic interstitial lung abnormalities in the chest CT report. Radiology 2023; 306: e222274. doi: 10.1148/radiol.222274 [DOI] [PubMed] [Google Scholar]
- 87.Park S, Choe J, Hwang HJ, et al. Long-term follow-up of interstitial lung abnormality: implication in follow-up strategy and risk thresholds. Am J Respir Crit Care Med 2023; 208: 858–867. doi: 10.1164/rccm.202303-0410OC [DOI] [PubMed] [Google Scholar]
- 88.Shin YJ, Yi JG, Kim MY, et al. Radiologic progression of interstitial lung abnormalities following surgical resection in patients with lung cancer. J Clin Med 2023; 12: 6858. doi: 10.3390/jcm12216858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahn Y, Lee SM, Choi S, et al. Automated CT quantification of interstitial lung abnormality and interstitial lung disease according to the Fleischner Society in patients with resectable lung cancer: prognostic significance. Eur Radiol 2023; 33: 8251–8262. doi: 10.1007/s00330-023-09783-x [DOI] [PubMed] [Google Scholar]
- 90.Escalon JG, Podolanczuk AJ, Aronson KI, et al. Practice patterns in reporting interstitial lung abnormality at a tertiary academic medical center. Clin Imaging 2023; 104: 109996. doi: 10.1016/j.clinimag.2023.109996 [DOI] [PubMed] [Google Scholar]
- 91.Montesi SB, Caravan P. Novel imaging approaches in systemic sclerosis-associated interstitial lung disease. Curr Rheumatol Rep 2019; 21: 25. doi: 10.1007/s11926-019-0826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taguchi K, Iwanczyk JS. Vision 20/20: single photon counting X-ray detectors in medical imaging. Med Phys 2013; 40: 100901. doi: 10.1118/1.4820371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakamura Y, Higaki T, Kondo S, et al. An introduction to photon-counting detector CT (PCD CT) for radiologists. Jpn J Radiol 2023; 41: 266–282. doi: 10.1007/s11604-022-01367-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Si-Mohamed S, Boccalini S, Rodesch PA, et al. Feasibility of lung imaging with a large field-of-view spectral photon-counting CT system. Diagn Interv Imaging 2021; 102: 305–312. doi: 10.1016/j.diii.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 95.Marton N, Gyebnar J, Fritsch K, et al. Photon-counting computed tomography in the assessment of rheumatoid arthritis-associated interstitial lung disease: an initial experience. Diagn Interv Radiol 2023; 29: 291–299. doi: 10.4274/dir.2023.221959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jungblut L, Euler A, von Spiczak J, et al. Potential of photon-counting detector CT for radiation dose reduction for the assessment of interstitial lung disease in patients with systemic sclerosis. Invest Radiol 2022; 57: 773–779. doi: 10.1097/RLI.0000000000000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sollini M, Kirienko M, Gelardi F, et al. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 2021; 48: 4396–4414. doi: 10.1007/s00259-021-05475-0 [DOI] [PubMed] [Google Scholar]
- 98.Bergmann C, Distler JHW, Treutlein C, et al. 68Ga-FAPI-04 PET-CT for molecular assessment of fibroblast activation and risk evaluation in systemic sclerosis-associated interstitial lung disease: a single-centre, pilot study. Lancet Rheumatol 2021; 3: e185–e194. doi: 10.1016/S2665-9913(20)30421-5 [DOI] [PubMed] [Google Scholar]
- 99.Fleming H, Clifford SM, Haughey A, et al. Differentiating combined pulmonary fibrosis and emphysema from pure emphysema: utility of late gadolinium-enhanced MRI. Eur Radiol Exp 2020; 4: 61. doi: 10.1186/s41747-020-00187-w [DOI] [PMC free article] [PubMed] [Google Scholar]