Abstract
Backgrounds
The efficacy of atezolizumab/bevacizumab has never been reported in patients with metastatic/unresectable combined hepatocellular‐cholangiocarcinoma (cHCC‐CCA).
Patients and Methods
We retrospectively included patients with a histological diagnosis of unresectable/metastatic cHCC‐CCA and treated with atezolizumab/bevacizumab (2020–2022) in 7 centers. Clinical and radiological features were collected at the beginning of atezolizumab/bevacizumab. We reported the radiological response using RECIST criteria, overall survival (OS) and progression‐free survival (PFS).
Results
Sixteen patients with cHCC‐CCA were included and were predominantly male (75%) with advanced fibrosis/cirrhosis (69%). Nine patients received atezolizumab/bevacizumab as a first‐line systemic treatment, 5 as a second line, 1 as a third line and 1 as a fifth line. Severe digestive bleeding occurred in 2 patients. Among the 9 patients treated in the first line, 4 experienced radiological progression, 3 partial response and 1 had stable disease. Patients treated with atezolizumab/bevacizumab in the first line had a median OS of 13 months and a median PFS of 3 months. Among the 7 patients receiving atezolizumab/bevacizumab as a second line or more, 4 patients harbored a stable disease, 2 a partial response, and 1 a progressive disease.
Conclusions
The combination of atezolizumab and bevacizumab showed signs of anti‐tumor efficacy in patients with unresectable/metastatic cHCC‐CCA.
Keywords: advanced, atezolizumab, bevacizumab, CCA, cholangiocarcinoma, combined hepatocellular‐cholangiocarcinoma, HCC, hepatocellular carcinoma, immunotherapy, systemic treatment
Key summary.
We focused on patients with metastatic unresectable combined hepatocellular‐cholangiocarcinoma, a rare disease without any consensual systemic treatment currently validated.
Our retrospective, multicentric study included 16 patients treated with atezolizumab‐bevacizumab.
We have provided the first patient series available in the literature reporting on the safety and efficacy of atezolizumab/bevacizumab in these patients.
The radiological response to the treatment was 33% in the first line, with a median overall survival of 13 months, suggesting signs of antitumor efficacy.
INTRODUCTION
Combined hepatocellular‐cholangiocarcinoma (cHCC‐CCA) represents 3%–5% of all primary liver cancers. 1 , 2 , 3 , 4 The presence of both histological subtypes (cholangiocytes and hepatocytes) in the same lesion identified using hematin and eosin (H&E) staining is the main diagnostic criterion, whereas immunohistochemistry is considered to be useful to better identify the two contingents. 3 Risk factors associated with the development of these tumors are shared with hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), including chronic hepatitis B and hepatitis C infection, metabolic syndrome, excessive chronic alcohol consumption, and other risk factors for chronic liver diseases. 5 Moreover, cHCC‐CCA are associated with cirrhosis in various proportions according to geographic origin. In Western countries, about 50% of patients with cHCC‐CCA harbored advanced fibrosis or cirrhosis. 1 When feasible, liver surgery is the recommended standard of care for cHCC‐CCA, but is hampered by a high rate of tumor recurrence. 6 However, cHCC‐CCA is often diagnosed at an advanced stage not suitable to liver resection and requiring a systemic treatment. 7 , 8 , 9 Moreover, the rarity of this tumor, and the consequent difficulty in organizing prospective clinical studies explain the absence of phase 2 or 3 clinical trials available in the literature. Consequently, tyrosine kinase inhibitors (TKIs) and platinum‐based chemotherapy are used in clinical practice mimicking the treatment recommended for HCC and CCA, respectively. Recent retrospective data suggested that these two treatments seem to be equally effective as first‐line systemic therapy. The reported OS with conventional systemic treatment usually used for HCC or CCA (platinum‐based chemotherapy or TKI such as sorafenib) ranged from 8 to 12 months. 10 , 11 , 12
The current recommended first‐line therapy for advanced HCC is the combination of atezolizumab, a programmed death‐ligand 1 (PDL1) inhibitor, with bevacizumab, an anti‐vascular endothelial growth factor (VEGF) antibody. 13 Unfortunately, patients with cHCC‐CCA were not included in the phase 2 and 3 studies that tested AB in advanced HCC and, consequently, the efficacy and safety of AB in this context are unknown. To date, only few case reports describe the use of immunotherapies (including one with AB in third line) in patients with cHCC‐CCA. 14 , 15 Recently, three patients affected by cHCC‐CCA treated with AB were reported in a multicentric series. 16 On the other hand, immunotherapy with durvalumab in combination with standard chemotherapy has recently demonstrated its efficacy in CCA. 17
We aimed to perform a retrospective multicentric study to describe the clinical outcomes of patients with unresectable or metastatic cHCC‐CCA receiving AB.
MATERIALS ET METHODS
Selection of patients
We retrospectively included consecutive patients with unresectable or metastatic cHCC‐CCA from 7 French centers who underwent a systemic treatment with AB from July 2020 to March 2022. An independent French ethics committee approved the study (number CLEA‐2020‐124). The diagnosis was performed by an expert pathologist in liver disease in each center according to the 2018 World Health Organization (WHO) classification of tumors of the digestive system.
The following inclusion criteria were:
-
1)
Patients with a histological diagnosis of cHCC‐CCA according to 2018 WHO classification.
-
2)
Unresectable or metastatic disease.
-
3)
Systemic treatment with Atezolizumab + Bevacizumab (whatever the type of systemic treatment received before or after AB) validated by a multidisciplinary tumor board.
-
4)
Patients 18 years old or more.
The exclusion criteria were:
-
1)
Patients receiving a combination of locoregional and systemic treatment.
-
2)
Systemic treatment used in a neo‐adjuvant or an adjuvant intent together with surgery.
Treatments were decided in each individual center and previously validated by a multidisciplinary tumor board on the basis of a histological sample confirming the presence of cHCC‐CCA.
Twenty patients were screened for inclusion. After reviewing the histology and the clinical data, 4 patients were excluded because they did not meet the most recent criteria defining the histological diagnosis of cHCC‐CCAs and 16 patients were finally included in the study. Clinical data (gender, age, etiology, severity of the underlying liver disease, components of the metabolic syndrome), laboratory tests (liver function, platelets, serum alpha‐fetoprotein [AFP] and CA19.9 levels), and tumor imaging features (size, number, macrovascular invasion, extrahepatic metastasis) were collected before the beginning of AB.
Treatment posology
The dosage of AB used in patients is the standard dosage indicated in the treatment of hepatocarcinoma: atezolizumab, 1200 mg, intravenous administration, every 3 weeks in combination with bevacizumab, 15 mg/kg, intravenous administration, every 3 weeks.
Endoscopic assessment
As the study is retrospective, endoscopic screening prior to treatment was performed independently at each center, according to the current guidelines for portal hypertension in patients with cirrhosis. Endoscopic data on portal hypertension were available for all but three patients. Unavailable data concerned one patient with cirrhosis (F4), one patient with advanced fibrosis (F3) and one non‐cirrhotic patient (F0). None of these three patients experienced complications related to gastrointestinal bleeding during treatment.
Follow‐up and outcomes assessment
All patients were followed‐up from the beginning of the systemic treatment until death or the last recorded visit. The follow‐up period extended to 30th August 2023. The primary endpoint was OS, defined as the survival from the initiation of AB to death, whatever the cause. The secondary endpoints were the progression‐free survival (PFS) and the description of the tolerance and side effects of the treatment. The radiological response at the first assessment (performed 2–3 months after the beginning of AB) was collected and classified as complete response, partial response, stable disease and progression according to RECIST 1.1 criteria. 18 PFS was defined as the survival from the beginning of AB to radiological disease progression or death (whatever the cause of death) and patients alive with no progression were censored at the last follow‐up date.
Statistical analysis
Continuous variables are presented as median and interquartile range (IQR), and categorical variables as numbers and percentages. Survival curves of OS and PFS were built using the Kaplan‐Meier method with the number at risk represented under the x axis. All analyses were performed using Prism Software version 7 (GraphPad).
RESULTS
Description of the general population
We included 16 patients with unresectable/metastatic cHCC‐CCA treated by AB who were mainly male (75%) with a median age of 63 years (IQR: 42.5–71). The diagnosis was performed on tumor biopsy for 10 patients and on surgical samples for 6 patients. Thirty‐eight percent had hepatitis B virus, 25% hepatitis C virus, 44% metabolic syndrome, and 38% chronic alcohol consumption. Sixty‐nine percent of the patients had advanced fibrosis or cirrhosis confirmed by histology and, at the time of treatment, most of the cirrhotic patients were Child–Pugh A (81%). Two cirrhotic patients were classified Child–Pugh score of B7 due to the presence of radiological ascites at the time of imaging. Patients often had multiple intrahepatic liver lesions (75%) and metastases (63%) with a median largest tumor diameter of 60 mm. The extensive description of patients is reported in Table 1. In one patient, molecular profiling of the tumor was performed and we identified KRAS amplification (19 copies), CDK4 amplification (19 copies) and FGFR2 (p.Cys62Arg), SOX9 (p. Leu123Phe) and SMARCA4 (Leu783Pro) mutations. Among these 16 patients, 9 patients received AB as a first‐line systemic treatment, 5 as a second line, one as a third line, and one as a fifth line. The median follow‐up of the whole population was 15 months. Patients treated by AB in first line had a median follow‐up of 14 months those in the second line or more of 17 months.
TABLE 1.
Description of the patient's features in the whole population and in patients treated in first‐line by atezolizumab/bevacizumab.
| Variables | Available data | Whole population (n = 16) | First‐line treatment A/B (n = 9) |
|---|---|---|---|
| Clinical characteristics | |||
| Male a | 16 | 12 (75%) | 8 (89%) |
| Age (years old) b | 16 | 63 (42.50–71.00) | 57 (30.50–66.00) |
| Hepatitis B virus a | 16 | 6 (38%) | 4 (44%) |
| Hepatitis C virus a | 16 | 4 (25%) | 1 (11%) |
| Metabolic syndrome a | 16 | 7 (44%) | 4 (44%) |
| Tobacco use a | 16 | 7 (44%) | 4 (44%) |
| Chronic alcohol intake a | 16 | 6 (38%) | 3 (33%) |
| Body mass index b | 16 | 23 (19.50–26.00) | 22 (19.75–24.25) |
| Diabetes type 2 a | 16 | 5 (31%) | 3 (33%) |
| Arterial hypertension a | 16 | 7 (44%) | 3 (33%) |
| Dyslipidemia a | 16 | 5 (31%) | 4 (44%) |
| Performance status a 0/1 | 16 | 14 (88%) | 9 (100%) |
| Advanced fibrosis/cirrhosis (F3/F4) a | 16 | 11 (69%) | 6 (67%) |
| Child‐Pugh A a | 16 | 9 (81%) | 6 (100%) |
| Biochemical characteristics | |||
| Total bilirubin μmol/l b | 16 | 13 (7.45–17.00) | 14 (9.00–16.75) |
| Albumin g/l b | 16 | 37.50 (33.50–40.90) | 38 (35.00–40.85) |
| Time of prothrombin (%) b | 16 | 78.50 (76–90) | 78 (74.00–82.75) |
| Platelets × 103 G/l b | 16 | 212 (169–271) | 229 (174–298) |
| Creatinine μmol/l b | 16 | 72.50 (65.00–99.50) | 78 (65.00–104.00) |
| Serum AFP b | 16 | 59.50 (1.40–380.65) | 3.9 (1.00–225.25) |
| Serum CA 19‐9 b | 9 | 2 (0.23–3.13) | 1.52 (0.025–6.72) |
| Tumor characteristics | |||
| Multiple intrahepatic lesions a | 16 | 12 (75%) | 7 (78%) |
| Tumor macrovascular invasion a | 16 | 5 (31%) | 4 (44%) |
| Metastases a | 16 | 10 (63%) | 6 (67%) |
| Size of biggest nodule (mm) b | 16 | 60 (29–128) | 54 (35–120) |
| Radiological response at first imaging | 15 | ||
| Progression | 5 (33%) | 4 (44%) | |
| Partial response | 5 (33%) | 3 (33%) | |
| Stable disease | 5 (33%) | 1 (11%) | |
| Median overall survival | 16 | 17 months | 13 months |
| Median progression free survival | 16 | 7.5 months | 3 months |
Note: The % of Child‐Pugh A patient was calculated on advanced fibrosis/cirrhosis patients. CA19‐9, AFP are represented as times above normal (median [IQR]), AFP, alpha‐fetoprotein; CA19‐9, carbohydrate antigen 19‐9; A/B: atezolizumab/bevacizumab.
n (%).
Median (interquartile range).
Atezolizumab/bevacizumab as a first line treatment of unresectable/metastatic cHCC‐CCA
Among the 9 patients who received AB as a first‐line treatment, eight were men (89%) with a median age of 57 years. The most frequent underlying liver disease was hepatitis B (44%), followed by chronic alcohol intake (33%). The patients had advanced fibrosis/cirrhosis in 67% of the cases. The patients had multiple intra‐hepatic tumors and extrahepatic metastases in 78% and 67% of the cases, respectively. The median time on treatment was 6.4 months. The detailed features of patients are reported in Table 2.
TABLE 2.
Individual description of patients treated by atezolizumab/bevacizumab.
| Patient | Age (years) | Gender | Fibrosis | Etiology | AFP/CA19‐9 level a | Metastases | Size of biggest nodule (mm | Multiple nodules | Macro‐vascular invasion | Previous systemic treatment | Radiological response | Time under A/B (months) | Length of follow‐up (months) | Alive/dead at last news |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First line | ||||||||||||||
| 1 | 77 | M | F0 | NASH | 175/0.03 | Yes | 19 | Yes | No | No | Progression | 3.2 | 4.0 | Dead |
| 2 | 26 | M | F3 | HBV | 376/NA | Yes | 210 | No | Yes | No | Progression | 2.5 | 12.6 | Dead |
| 3 | 63 | M | F4 | NASH + OH | 94/NA | No | 57 | Yes | Yes | No | Partial response | 6.4 | 14 | Dead |
| 4 | 32 | M | F4 | HBV | 0.3/0.02 | No | 50 | Yes | No | No | Stable disease | 15.6 | 17 | Alive |
| 5 | 41 | M | F2 | HBV | 1.85/NA | Yes | 48 | Yes | Yes | No | Progression | 2.3 | 6 | Dead |
| 6 | 18 | M | F0 | HBV | 1/3 | Yes | 80 | Yes | No | No | Progression | 2.2 | 6 | Alive |
| 7 | 75 | M | F4 | NASH | 3.9/NA | Yes | NA | NA | No | No | Partial response | 22.8 | 22.8 | Alive |
| 8 | 63 | F | F4 | NASH + OH | 7142/11 | Yes | 160 | Yes | Yes | No | NA | 1 | 1.3 | Dead |
| 9 | 57 | M | F3 | HCV | 1/NA | No | 22 | Yes | No | No | Partial response | 16.1 | 16.1 | Alive |
| Second line | ||||||||||||||
| 10 | 79 | F | F1 | HCV | 1.8/0.3 | No | 21 | Yes | No | Gemcitabine + Oxaliplatin | Stable disease | 4 | 20 | Dead |
| 11 | 67 | F | F4 | HBV | 0.7/NA | Yes | 60 | Yes | No | Sorafenib | Stable disease | 9 | 11 | Dead |
| 12 | 47 | F | F4 | HBV | 25/1.5 | Yes | 118 | Yes | No | Sorafenib | Stable disease | 7 | 31 | Dead |
| 13 | 66 | M | F4 | NASH + OH | 383/3.5 | No | 80 | Yes | No | Gemcitabine + cisplatin | Partial response | 24 | 24 | Alive |
| 14 | 79 | M | F0 | NASH + OH | 378/NA | Yes | 151 | Yes | No | Gemcitabine + oxaliplatin | Stable disease | 13.5 | 22 | Dead |
| Third line | ||||||||||||||
| 15 | 66 | M | F4 | HCV | 714/3 | No | 131 | No | Yes | Gemcitabine + cisplatin > 5FU + oxaliplatin | Progression | 2 | 3.9 | Dead |
| Fifth line | ||||||||||||||
| 16 | 44 | M | F4 | HCV + OH | 8642/2 | Yes | 12 | No | No | Sorafenib > Gemcitabine + oxaliplatin >5FU + irinotecan > paclitaxel | Partial response | 15.5 | 37 | Alive |
Abbreviations: A/B, atezolizumab/bevacizumab; F, female; HBV, hepatitis B virus; HCV, hepatitis C virus; M, male; NA, not available; NASH, non‐alcoholic steatohepatitis; OH, alcohol related liver disease.
Time fold of the upper limit of the normal.
At the first imaging evaluation under treatment, four patients (44%) experienced progression, three patients (33%) experienced partial response and one patient (11%) experienced stable disease. For one patient (11%), radiological response could not be assessed because the patient had digestive bleeding (without any available endoscopic data about the cause of bleeding) together with ascites development after the second cycle, leading to treatment discontinuation and death. Among the three patients with partial response, one patient developed tumor progression after 6 months of partial response, whereas the remaining two patients were classified as responders after a follow‐up of 16 and 23 months.
Regarding adverse events, none of the patients died due to the treatment. Digestive bleeding occurred in 11% of patients, grade 1 anorexia in 33% of patients, grade 1 and grade 2 asthenia in 11% and 11% of patients, respectively, grade 1 thrombocytopenia in 11% of patients, grade 2 eczema in 11% of patients and grade 2 proteinuria in 11% of patients. Among the seven patients who discontinued the treatment drug, with a median time of discontinuation of 2.5 months, five discontinued due to radiological progression, one patient due to symptomatic progression and one patient due to digestive bleeding and appearance of ascites.
Patients treated with AB in the first line had a median OS of 13 months (Figure 1a) with a 12‐month survival rate of 56%. The PFS was 3 months (Figure 1b). Among these 9 patients, two received a second‐line systemic therapy including one patient treated with regorafenib alone and one patient by a combination of regorafenib and pembrolizumab. These two patients developed tumor progression at the first radiological evaluation.
FIGURE 1.
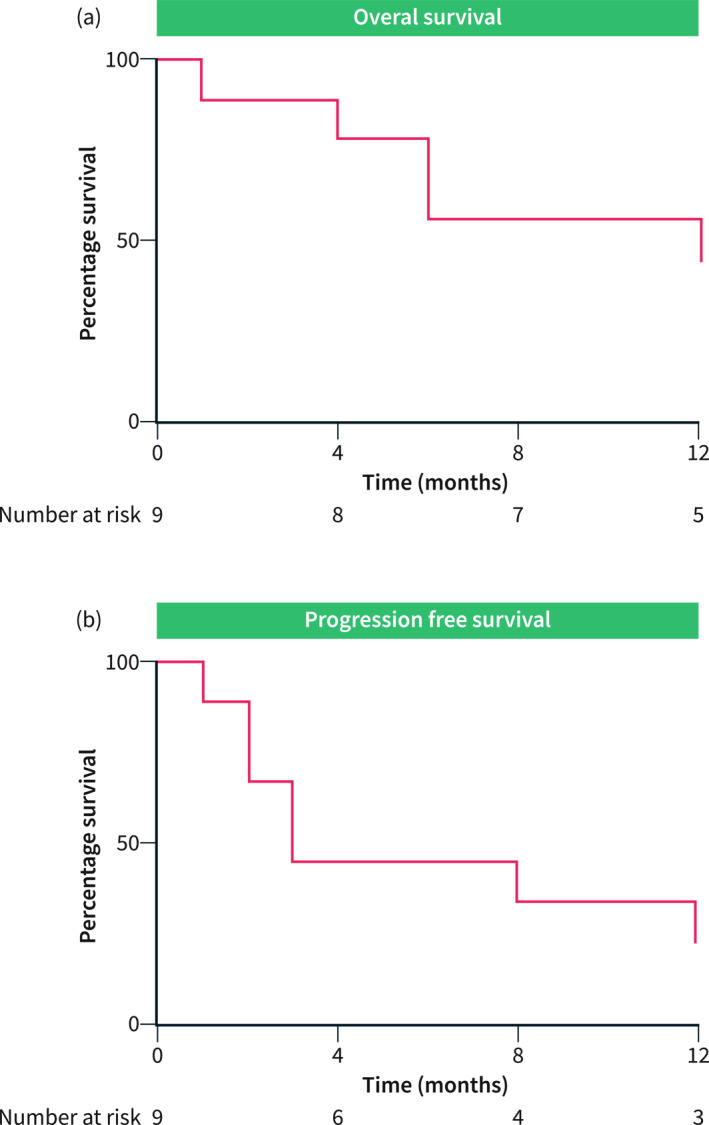
Overall survival and Progression‐free survival in patients treated with atezolizumab/bevacizumab as a first‐line systemic treatment. (a) Overall survival was represented using Kaplan‐Meier curve with the number at risk under the X axis. Median overall survival was 13 months. (b) Progression‐free survival was represented using Kaplan‐Meier curve with the number at risk under the X axis. The median progression‐free survival was 3 months.
Atezolizumab/bevacizumab as a second line (or more) treatment of unresectable/metastatic cHCC‐CCA
Seven patients with cHCC‐CCA received AB as a second line or more (see Table 2 for the full description of clinical, biological and imaging data). The median time from the end of the previous treatment and the start of AB treatment was 37 days.
Five patients received AB as second‐line treatment. Two patients had previously received sorafenib in the first line, while the remaining 3 have previously received gemcitabine and platinum‐based regimens. One patient developed a polymyalgia rheumatic and one syndrome of inappropriate antidiuretic hormone secretion under AB but did not require a permanent withdrawal of the treatment. The radiological response at the first imaging evaluation identified a stable disease in 4 patients and a partial response in two patients. One patient was still alive with a persistent partial response under AB after 24 months of follow‐up, an example of radiological response is shown in Figure 2.
FIGURE 2.
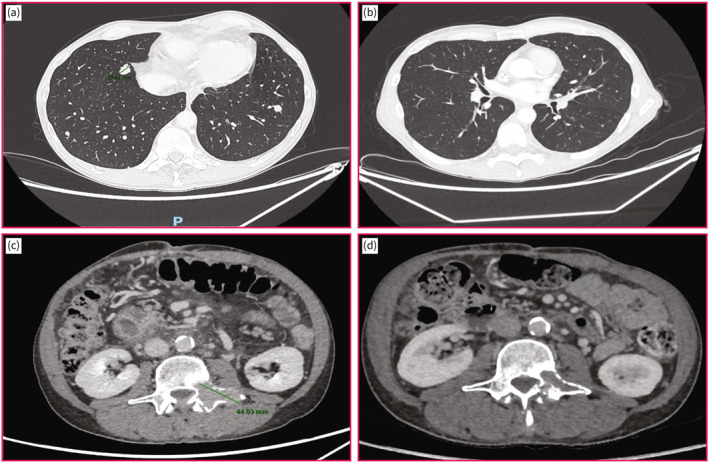
Radiological response at 12 weeks of atezolizumab/bevacizumab. (a) Pre‐treatment lung metastasis indicated by red arrows. (b) Diseappearance of lung metastasis at 12 weeks of atezolizumab/bevacizumab. (c) Pre‐treatment vertebral metastasis indicated by a red arrow. (d) Reduction in size of vertebral metastasis at 12 weeks of atezolizumab/bevacizumab.
One patient was treated with AB in the third line and was previously treated by Gemcitabine + Cisplatin thus 5‐fluorouracil (5‐FU) + Oxaliplatin treatments before (Table 2). This patient presented with progressive disease at the first imaging evaluation together with bleeding from esophageal varices; he did not receive any further treatment for cHCC‐CCA and died 3.9 months after the start of AB. The last patient received AB in the fifth line after previous treatments by sorafenib, gemcitabine + oxaliplatin, 5FU + irinotecan and paclitaxel (Table 2). The patient had a partial response at the first radiological control and discontinued the treatment after 15.5 months due to persistent radiological response. After 37 months of follow‐up, including 15.5 months under AB and 22 months after AB discontinuation, no tumor progression was observed at imaging.
DISCUSSION
In the current study, we provided the first series available in the literature on the efficacy and toxicity of atezolizumab/bevacizumab in the treatment of non‐resectable or metastatic cHCC‐CCA. We described nine patients who received AB as first‐line systemic treatment and seven patients who received AB as a further line of therapy. In the patients treated as first‐line, at first radiological control, four patients had radiological progression, three patients had a partial response and one patient had stable disease. The reported median OS was 13 months and the median PFS was 3 months. Among the 7 patients who received second‐line AB or more, at the first radiological control, four patients had stable disease, two had partial responses and one had progressive disease.
The clinical management of patients with cHCC‐CCA who are unresectable or metastatic relies on a limited amount of reliable evidence and is often based on the local experience of centers. Recently, several single‐center or multicenter studies have assessed the efficacy of systemic treatments with platinum‐based regimens or TKIs in these patients. 10 , 11 , 12 , 19 , 20 A study from Asia suggested that the TKI was less effective than gemcitabine/cisplatin regimens reporting a lower median survival (3.5 months) compared to gemcitabine/cisplatin (11.9 months) and fluorouracil/cisplatin (10.2 months); however, the results were biased due to a small number of patients in the TKI arm. 10 In contrast, a western monocentric cohort described a median OS of 11.5 months for gemcitabine/platinum therapy and an OS of 9.6 months for sorafenib. 20 Moreover, a Korean study suggests that patients, mainly infected by hepatitis B, had similar outcomes when treated either with TKI or platinum‐based chemotherapy. 19 Our study recently published showed that western patients with unresectable/metastatic cHCC‐CCA have a similar outcome with a median OS of 8.3 months for TKI compared to 11.9 months for platinum‐based chemotherapy even after adjustment for potential confounders. Altogether, these studies suggested that TKI and platinum‐based chemotherapy seem to have comparable efficacy in unresectable/metastatic cHCC‐CCA.
The advent of first‐line systemic immunotherapies in advanced HCC, currently offering a combination of atezolizumab/bevacizumab or durvalumab/tremelimumab as first‐line treatment, has significantly improved patient survival compared to TKI treatment, opening up new therapeutic perspectives. Similarly, in CCA, treatments with a backbone of immunotherapy combined with conventional chemotherapy also showed improved survival compared with conventional chemotherapy (gemcitabine/cisplatin/durvalumab and gemcitabine/cisplatin/pembrolizumab). Considering that by definition cHCC‐CCA consists of both histotypes, it seems reasonable to imagine that immunotherapy is the most logical treatment option in patients with unresectable/metastatic cHCC‐CCA. 13 , 17 , 21 , 22 In the present study, we described a retrospective multicenter cohort of patients with histologically proven cHCC‐CCA treated with AB. Currently, this is the only series disposable in the literature on a combination of atezolizumab and bevacizumab using the WHO 2018 classification to define cHCC‐CCA. In addition, this series was collected at the national level and enrolled patients from tertiary university centers in a Western country.
Data about immunotherapy and cHCC‐CCA in the literature relies mostly on two case reports and a small case series. 14 , 15 , 16 The first case report presents a patient with metastatic cHCC‐CCA treated by pembrolizumab as a third‐line treatment after sorafenib and regorafenib. This patient experienced a complete radiological response persistent after 18 months of immunotherapy. 14 The second case report described a patient treated with AB after a systemic treatment by gemcitabine and cisplatin followed by Lenvatinib. This patient harbored a stable disease at imaging under AB treatment with a PFS of 7.8 months. 15 Recently a series of seven patients who received immunotherapy was described, of these only three received atezolizumab plus bevacizumab, the median OS from the start of immunotherapy for all patients was 17.8 months. 16 A Korean study retrospectively examined the efficacy and safety of ICIs in 25 cHCC‐CCA patients. The median PFS was 3.5 months and the OS was 8.3 months. Most patients were treated with nivolumab; only 2 patients in the series were treated with AB. The shorter survival compared to ours is probably due to the fact that their patients were mostly treated with nivolumab monotherapy and that all but one of the included patients had been previously treated with other types of chemotherapy. 23
In the current study, we showed that AB was associated with a median PFS of 3 months and median OS of 13 months in patients treated in the first line. In addition, radiological response was observed in 3 patients (33%) among the 9 treated in the first line by AB and, as observed in other solid tumors, these radiological responses last for a long time. In contrast, our last study reported a median OS of 8.3 months for patients treated by TKI with 10% radiological response and 11.9 months for patients treated by platinum‐based regimens with 15% radiological response. 11 However, the small number of patients treated by AB in the current study impaired us to make a meaningful comparison with our previous series. Although OS and PFS in patients treated with AB second‐line or more could not be accurately described because of the small number of patients in these heterogeneous subgroups, radiological response was observed in 2 of 7 heavily pretreated patients and was associated with prolonged survival.
Despite the signal of efficacy of AB in patients with cHCC‐CCA, severe adverse events occurred in two patients: digestive bleeding in one patient with AB as a first‐line treatment and variceal bleeding in patients with AB as a third‐line treatment. As cHCC‐CCA occurred frequently on cirrhosis, especially in Western countries (69% of advanced fibrosis/cirrhosis in our study), upper endoscopy to screen for esophageal varices and subsequent prophylaxis by B‐blockers, if required, should be mandatory in patients with chronic liver disease. Considering that among the nine patients who received atezolizumab/bevacizumab as first‐line treatment, progression or death occurred in 5 patients (56%) among the 9 patients at the time of the first imaging, treatment with AB still has limitations. In contrast, a subset of patients seems to benefit from this treatment, including 33% of radiologic response with one patient still alive more than 23 months after the start of treatment.
However, our study is purely descriptive and has inherent limitations. First, it is a retrospective study and has bias related to patient selection and heterogeneity in terms of clinical care. Another limitation of our study is that we have not access to imaging to perform a centralized reviewing. However, radiological response at the first evaluation was evaluated during multidisciplinary tumor board for each patient. Moreover, the small number of patients analyzed impaired an in‐depth subgroup analysis to identify factors related to a better response to AB and didn't allow a comparison with other retrospective series of patients treated by TKI or platinum‐based regimens.
In the future, different strategies could be pursued in the treatment of cHCC‐CCA. First, biomarker‐driven therapy (e.g., FGFR inhibitor in FGFR2 fusion, IDH1 inhibitor in IDH1 mutations) used in clinical practice for CCA could be tested in patients with unresectable/metastatic cHCC‐CCA. 24 , 25 , 26 A recent study reported that in a large series of cHCC‐CCA, about 25% of the mutations were possible therapeutic targets. The mutations considered were: BRCA2 (8.2%), ERBB2 (5.5%), IDH1 (4.1%), BRAF (4.1%), FGFR2 (4.1%) and MET (2.7%). 8 Moreover, new systemic treatments have been validated in CCA, such as the combination of gemcitabine cisplatin with durvalumab, an anti‐PD1 antibody, or in advanced HCC, such as the combination of durvalumab and tremelimumab, an anti‐PD1 and anti‐CTLA4 antibody. 17 , 21 Interesting in the recent phase three studies evaluating the combination of gemcitabine/cisplatin/pembrolizumab in unresectable biliary tumors, eight (2%) patients in the pembrolizumab group and five (1%) patients in the placebo group had cHCC‐CCA. Unfortunately, individual data on these patients are not yet available but this treatment option represents one of the possible future options. 22 These treatments should be also tested in patients with unresectable/metastatic cHCC‐CCA.
In conclusion, we described a Western multicentric cohort of cHCC‐CCA treated by atezolizumab + bevacizumab showing a signal of anti‐tumor efficacy in terms of radiological response and long‐term survival.
AUTHOR CONTRIBUTIONS
Contributions to conception and design: Elia Gigante and Jean‐Charles Nault; acquisition of data and/or analysis and interpretation of data: Elia Gigante, Marie Lequoy, Mohamed Bouattour, Giuliana Amaddeo, Hélène Regnault, Marianne Ziol, José Ursic Bedoya, Nathalie Ganne‐Carrié, Karine Bouhier‐Leporrier, Valérie Paradis drafting, revising, and the manuscript content: Elia Gigante and Jean‐Charles Nault; and final approval of the version to be published: all the authors.
CONFLICT OF INTEREST STATEMENT
EG received travel and congress fees from Bayer, Gilead, Roche and Ipsen. Honoraria from Astra Zeneca, Roche and Sanofi. JCN received research grants from Bayer and Ipsen. HR received consulting fees for Boston. EA received travel and honoraria (board) from Bayer, BMS, Boston, IPSEN, Astrazeneca, Servier, Roche, Incyte, AAA, MSD. KBK received fees from Ipsen, AAA, BMS. NGC received travel and congress fees, Consulting fees or honoraria for lectures, presentations, speakers' bureaus from Abbvie, Bayer, Gilead, Ipsen, and Roche. JUB received travel and congress fees from Gilead, Abbvie, Astellas and Ipsen. The other authors declare no specific conflict of interest regarding this study.
ETHICS APPROVAL
According to the French laws, this study protocol was reviewed and approved by our Ethical Committee (Comité Local d’Ethique pour la Recherche Clinique des HUPSSD Avicenne‐Jean Verdier‐René Muret, 125 rue de Stalingrad 93009 BOBIGNY Cedex), approval number CLEA‐2020‐124. Written consent was not necessary because patients were initially informed of the possibility of using anonymized clinical and biological data collected during routine care in the welcome booklet for different university hospitals and the possibility of opposing the use of their data. Our research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
CONSENT FOR PUBLICATION
All the data are anonymized in the current manuscript.
ACKNOWLEDGMENTS
Thanks to the Paris Liver Cancer Group for the support. No specific funding was used for this study.
Gigante E, Bouattour M, Bedoya JU, Regnault H, Ziol M, Assenat E, et al. Atezolizumab and bevacizumab for non‐resectable or metastatic combined hepatocellular‐cholangiocarcinoma: a multicentric retrospective study. United European Gastroenterol J. 2024;12(4):429–39. 10.1002/ueg2.12503
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author and will be available after reasonable request.
REFERENCES
- 1. Gigante E, Paradis V, Ronot M, Cauchy F, Soubrane O, Ganne‐Carrié N, et al. New insights into the pathophysiology and clinical care of rare primary liver cancers. JHEP Rep. 2021;3(1):100174. 10.1016/j.jhepr.2020.100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sempoux C, Kakar S, Kondo F, Schirmacher P. Combined hepatocellular‐cholangiocarcinoma and undifferentiated primary liver carcinoma. In: Arends MJ, Fukuyama M, Fukuyama M, Klimstra DS, Klimstra DS, et al., editors. WHO classification of tumours: digestive system tumours. 5th. Lyon: IARC; 2019. p. 260. [Google Scholar]
- 3. Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, et al. cHCC‐CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68(1):113–126. 10.1002/hep.29789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gigante E, Ronot M, Bertin C, Ciolina M, Bouattour M, Dondero F, et al. Combining imaging and tumour biopsy improves the diagnosis of combined hepatocellular‐cholangiocarcinoma. Liver Int. 2019;39(12):2386–2396. 10.1111/liv.14261 [DOI] [PubMed] [Google Scholar]
- 5. Beaufrère A, Calderaro J, Paradis V. Combined hepatocellular‐cholangiocarcinoma: an update. J Hepatol. 2021;74(5):1212–1224. 10.1016/j.jhep.2021.01.035 [DOI] [PubMed] [Google Scholar]
- 6. Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, et al. Surgical treatment of hepatocholangiocarcinoma: a systematic review. Liver Cancer. 2020;9(1):15–27. 10.1159/000503719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM, et al. Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell. 2019;35(6):932–947.e8. 10.1016/j.ccell.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murugesan K, Sharaf R, Montesion M, Moore JA, Pao J, Pavlick DC, et al. Genomic profiling of combined hepatocellular cholangiocarcinoma reveals genomics similar to either hepatocellular carcinoma or cholangiocarcinoma. JCO Precis Oncol. 2021;5:1285–1296. PO.20.00397. 10.1200/po.20.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nowell PC. Mechanisms of tumor progression. Cancer Res. 1986;46(5):2203–2207. [PubMed] [Google Scholar]
- 10. Kobayashi S, Terashima T, Shiba S, Yoshida Y, Yamada I, Iwadou S, et al. Multicenter retrospective analysis of systemic chemotherapy for unresectable combined hepatocellular and cholangiocarcinoma. Cancer Sci. 2018;109(8):2549–2557. 10.1111/cas.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gigante E, Hobeika C, Le Bail B, Paradis V, Tougeron D, Lequoy M, et al. Systemic treatments with tyrosine kinase inhibitor and platinum‐based chemotherapy in patients with unresectable or metastatic hepatocholangiocarcinoma. Liver Cancer. 2022;11(5):460–473. 10.1159/000525488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salimon M, Prieux‐Klotz C, Tougeron D, Hautefeuille V, Caulet M, Gournay J, et al. Gemcitabine plus platinum‐based chemotherapy for first‐line treatment of hepatocholangiocarcinoma: an AGEO French multicentre retrospective study. Br J Cancer. 2018;118(3):325–330. 10.1038/bjc.2017.413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. 10.1056/nejmoa1915745 [DOI] [PubMed] [Google Scholar]
- 14. Saint A, Benchetrit M, Novellas S, Ouzan D, Falk AT, Leysalle A, et al. Prolonged efficacy of pembrolizumab in a patient presenting a multi‐treated metastatic hepatocholangiocarcinoma. Therap Adv Gastroenterol. 2020;13:1756284820935189. 10.1177/1756284820935189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saito N, Hatanaka T, Nakano S, Hazama Y, Yoshida S, Hachisu Y, et al. A case of unresectable combined hepatocellular and cholangiocarcinoma treated with atezolizumab plus bevacizumab. Clin Case Rep. 2022;10(7):e6129. 10.1002/ccr3.6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pomej K, Balcar L, Shmanko K, Welland S, Himmelsbach V, Scheiner B, et al. Clinical characteristics and outcome of patients with combined hepatocellular‐cholangiocarcinoma—a European multicenter cohort. ESMO Open. 2023;8(1):100783. 10.1016/j.esmoop.2023.100783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Do‐Youn O, Aiwu RH, Qin S, Li‐Tzong C, Takuji O, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. 10.1056/evidoa2200015 [DOI] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19. Kim EJ, Yoo C, Kang HJ, Kim KP, Ryu MH, Park SR, et al. Clinical outcomes of systemic therapy in patients with unresectable or metastatic combined hepatocellular‐cholangiocarcinoma. Liver Int. 2021;41(6):1398–1408. 10.1111/liv.14813 [DOI] [PubMed] [Google Scholar]
- 20. Trikalinos NA, Zhou A, Doyle MBM, Fowler KJ, Morton A, Vachharajani N, et al. Systemic therapy for combined hepatocellular‐cholangiocarcinoma: a single‐institution experience. J Natl Compr Canc Netw. 2018;16(10):1193–1199. 10.6004/jnccn.2018.7053 [DOI] [PubMed] [Google Scholar]
- 21. Abou‐Alfa Ghassan K, George L, Kudo M, Chan Stephen L, Kate KR, Junji F, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070. 10.1056/evidoa2100070 [DOI] [PubMed] [Google Scholar]
- 22. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE‐966): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. 10.1016/s0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 23. Jang YJ, Kim EJ, Kim HD, Kim KP, Ryu MH, Park SR, et al. Clinical outcomes of immune checkpoint inhibitors in unresectable or metastatic combined hepatocellular‐cholangiocarcinoma. J Cancer Res Clin Oncol. 2023;149(10):7547–7555. 10.1007/s00432-023-04704-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu AX, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: the phase 3 randomized clinical ClarIDHy trial. JAMA Oncol. 2021;7(11):1669–1677. 10.1001/jamaoncol.2021.3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abou‐Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al‐Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open‐label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. 10.1016/s1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meric‐Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau HT, et al. Futibatinib, an irreversible FGFR1‐4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR aberrations: a phase I dose‐expansion study. Cancer Discov. 2022;12(2):402–415. 10.1158/2159-8290.cd-21-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author and will be available after reasonable request.


