Abstract
Background
Increasing evidence supports the use of transmural remission as a treatment target in Crohn's disease (CD), but it is seldom achieved in clinical practice. Tight monitoring of inflammation using fecal calprotectin with reactive treatment escalation may potentially improve these results.
Aims
To evaluate if treatment escalation based on fecal calprotectin can improve the rates of transmural remission in CD. The influence of the timing of intervention on this strategy was also evaluated.
Methods
Retrospective cohort study including 256 CD patients with 2 consecutive assessments by MRI‐enterography and colonoscopy and with regular monitoring using fecal calprotectin. For each occurrence of an elevated fecal calprotectin (≥250 μg/g), we evaluated whether a reactive adjustment of medical treatment was performed. The ratio of treatment escalation/elevated fecal calprotectin was correlated with the chances of reaching transmural remission. Early disease was defined as disease duration <18 months without previous exposure to immunomodulators and biologics.
Results
After a median follow‐up of 2 years (IQR 1–4), 61 patients (23.8%) reached transmural remission. Ratios of escalation ≥50% resulted in higher rates of transmural remission (34.2% vs. 15.1%, p < 0.001). The effect was more pronounced in patients with early disease (50.0% vs. 12.0%, p = 0.003). In multivariate analysis, a treatment escalation ratio ≥50% (OR 3.46, 95% CI 1.67–7.17, p = 0.001) and early disease intervention (OR 3.24, 95% CI 1.12–9.34, p = 0.030) were independent predictors of achieving transmural remission.
Conclusion
Tight‐monitoring and reactive treatment escalation increase the rates of transmural remission in CD. Intervention in early disease further improves these results.
Keywords: Crohn's disease, fecal calprotectin, inflammatory bowel disease, transmural remission
Tight control using fecal calprotection and early disease intervention increase the rates of transmural remission in Crohn’s disease.
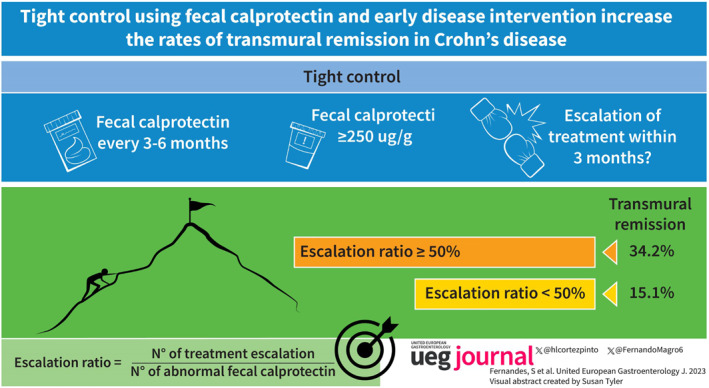
Key summary.
What is already known?
Transmural remission is associated with improved clinical outcomes compared with no remission or isolated endoscopic and radiologic remission.
The percentage of patients able to reach transmural remission with current therapies is low.
Treatment escalation based on clinical symptoms and biomarkers improves the rates of endoscopic remission in CD.
What is new here?
Tight‐monitoring and reactive treatment escalation increase the chances of reaching transmural remission of CD.
Intervention during the early stages of disease further provides better results.
INTRODUCTION
Crohn's disease (CD) is a chronic progressive disease that significantly impacts the quality of life of its patients. Untreated, it may lead to complications including strictures, fistulas, and abscesses, eventually resulting in hospitalization and surgery. 1 Current treatment strategies aim to prevent bowel damage by halting disease progression through early and effective control of inflammation. Several studies have shown that medical treatment targeting endoscopic remission improves patient quality of life and reduces the risk of relapse, hospitalization, and surgery. 2 Unfortunately, disease progression may still occur despite endoscopic remission. 3 This may result from persistent mural inflammation, which can be detected by magnetic resonance enterography (MRE) in 15%–25% of patients in endoscopic remission. 4 , 5 , 6 In a previous study, we have shown that transmural remission, defined as an absence of endoscopic and radiologic inflammation, resulted in lower rates of hospitalization, surgery, steroid dependency, and treatment escalation over 5‐years of follow‐up compared to other types of remission. 5 Although pursuing transmural remission as a treatment target may be enticing, less than 30% of patients are able to achieve it, according to data from several real‐life studies. 4 , 5 , 6 , 7 , 8 This suggests that better treatment and monitoring strategies are necessary before transmural remission can be accepted as a formal treatment target in CD. Early disease intervention with immunomodulators and biologics and tight monitoring using biomarkers have both been shown to significantly improve the chances of achieving endoscopic remission in CD. 9 , 10 However, their benefit with respect to transmural remission is unknown.
In the present study, we evaluated the potential benefits of a tight monitoring strategy using fecal calprotectin. In addition, we evaluated the influence of the timing of intervention in the response to this strategy.
MATERIAL AND METHODS
Study design
This was a single center retrospective cohort study including consecutive patients with CD referred to our Inflammatory Bowel Disease Center between January 2015 and January 2022. Inclusion criteria included a baseline and follow‐up assessment of disease activity using concurrent (≤3 months) colonoscopy and MRE, active disease on both baseline examinations, and at least 3 measurements of fecal calprotectin between assessments. Patients with incomplete/low quality MRE or colonoscopy, or with a surgical resection between assessments were excluded. Clinical data were retrieved from a local patient database and included demographics, disease characteristics, disease course, current and previous treatments, and endoscopic and radiological reports. Study procedures are presented in supplementary Figure S1. The study was approved by the local Ethics Committee.
Monitoring using fecal calprotectin
Fecal calprotectin was requested every 3–6 months according to our local practice. Additional testing could be requested by the attending physicians if necessary. All tests were performed locally using a point‐of‐care test (BÜHLMANN, Quantum‐Blue fCAL‐High‐Range) with a range of quantification between 100 and 1800 μg/g.
Magnetic resonance enterography and colonoscopy
MREs were performed and read by experienced radiologists using a standard protocol including 4–6 h fasting, luminal distention with 1.5–2 L of water and mannitol (2.5%) solution, and intravenous hyoscine to reduce peristalsis. Sequences included conventional axial and coronal T1 and T2‐weighted images, T2 fast spin echo with and without fat suppression and post‐contrast fat‐suppressed 3D T1‐weighted breath‐hold gradient ecoimages with acquisitions at 30, 60, and 90 s in the coronal orientation and at 110 s in the axial orientation. Colonoscopies were performed locally by experienced endoscopists.
The median time between colonoscopy and MRE was 1 (0–2) months.
Adjustments of medical therapy
We recorded any changes in medical therapy occurring between the baseline and follow‐up assessments including introduction, dose/interval adjustment, or switch between immunomodulators (azathioprine, 6‐mercaptopurine, or methotrexate) and biologics (infliximab, adalimumab, vedolizumab, or ustekinumab). Treatment decisions including maximum escalation regimens were left to the attending physicians and included: 2.5 mg/kg/day of azathioprine, 1.5 mg/kg/day of 6‐mercaptopurine, 25 mg/weekly of methotrexate, 15 mg/kg every 4 weeks of Infliximab, 80 mg every week of adalimumab, 300 mg every 4 weeks of vedolizumab, and 90 mg every 4 weeks of ustekinumab. In addition, in patients under Infliximab or Adalimumab therapeutic drug monitoring (reactive or proactive) could be requested at the discretion of the attending physician.
Study definitions and outcomes
The main objective of the study was to evaluate the association between the degree of treatment escalation in response to an elevated fecal calprotectin (i.e. treatment escalation ratio), and the rates of transmural remission in CD. For each abnormal fecal calprotectin (≥250 μg/g) between the baseline and follow‐up assessment of endoscopic and radiologic activity, we evaluated if a reactive adjustment of medical treatment occurred within the following 3 months. Cases with a decreasing trend of fecal calprotectin were still considered positive if ≥ 250 μg/g. The treatment escalation ratio was calculated as the quotient of the number of treatment escalations from the total number of abnormal fecal calprotectins (eg. a patient with 2 treatment escalations out of 4 abnormal fecal calprotectins would be classified as having a treatment escalation ratio of 0.5). The treatment escalation ratio was correlated with the rates of transmural remission at the follow‐up examinations. Radiologic remission was defined as a normal bowel wall thickness (≤3 mm) without T2 mural intensity or increased contrast enhancement on T1 gadolinium sequences. Endoscopic remission was defined as the absence of mucosal ulcers (>5 mm) in any gastrointestinal segment (non‐operated patients) or as a Rutgeerts score < i2 (previously operated patients). Transmural remission was defined as combined radiologic and endoscopic remission.
As a secondary objective, we evaluated whether acting in an early stage of disease would influence the effects of the treatment escalation ratio over transmural remission. In accordance with the Paris consensus, we defined early disease as a disease duration <18 months, without previous exposure to immunomodulators and biologics. 11
Statistical analysis
Continuous variables were expressed as medians (interquartile range) and compared using the Mann‐Whitney U test. Categorical variables were described using frequencies and percentages and compared using the chi‐square test. The correlation between variables was performed using Spearman's rho. Logistic regression was used to investigate factors associated with the studied endpoint. Variables with a p value < 0.1 in univariate analysis were used in the multivariate analysis. Results were expressed as odds‐ratio (OR) with 95% confidence interval (95%CI). The significance level was chosen at 0.05. Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) v26.0.
RESULTS
General characteristics and demographics
We included 256 patients in the analysis, 134 (52.3%) males, with a median age at the first assessment of 35.0 years (20.0–48.0), and a median disease duration of 5 years (1.0–14.0). Ileal disease was present in 147 patients (57.4%) and non‐stricturing/non‐penetrating phenotype in 106 patients (41.4%). Previous bowel surgery was present in 58 patients (22.7%). Baseline therapy included immunomodulators in 114 patients (44.5%) and biologics in 60 patients (23.4%). One hundred and five patients (41.0%) were naïve to immunosuppressants and biologics. Detailed demographics and disease characteristics are presented in Table 1.
TABLE 1.
Patient's characteristics at baseline.
| Total n = 256 | Transmural remission n = 61 (23.8) | No transmural remission n = 195 (76.2) | p | |
|---|---|---|---|---|
| Age at first assessment, years | 35 (20–48) | 33 (18.5–48) | 36 (20–48) | 0.511 |
| Disease duration, years | 5 (1–14) | 3 (1–12) | 6 (1–15) | 0.297 |
| Time between assessments, years | 2 (1–4) | 3 (2–6) | 2 (1–4) | 0.001 |
| Early disease (%) | 61 (23.8) | 21 (34.4) | 40 (20.5) | 0.038 |
| Male gender (%) | 134 (52.3) | 26 (42.6) | 108 (55.4) | 0.106 |
| Active smoker (%) | 72 (28.1) | 13 (21.3) | 59 (30.3) | 0.195 |
| Disease location | ||||
| Ileal (%) | 147 (57.4) | 31 (50.8) | 116 (59.5) | 0.341 |
| Colonic (%) | 7 (2.7) | 1 (1.6) | 6 (3.1) | |
| Ileo‐colonic (%) | 102 (39.8) | 29 (47.5) | 73 (37.4) | |
| Upper gastrointestinal disease (%) | 46 (18.0) | 8 (13.1) | 38 (19.5) | 0.340 |
| Perianal disease (%) | 54 (21.1) | 11 (18.0) | 43 (22.1) | 0.591 |
| Disease behavior | ||||
| Non‐stricturing non‐penetrating (%) | 106 (41.4) | 33 (54.1) | 73 (37.4) | 0.026 |
| Stricturing (%) | 99 (38.7) | 15 (24.6) | 84 (43.1) | |
| Penetrating (%) | 51 (19.9) | 13 (21.3) | 38 (19.5) | |
| Previous surgery (%) | 58 (22.7) | 15 (24.6) | 43 (22.1) | 0.727 |
| Treatment at baseline | ||||
| No treatment (%) | 105 (41.0) | 28 (45.9) | 77 (39.5) | 0.376 |
| Immunomodulators (%) | 114 (44.5) | 27 (44.3) | 87 (44.6) | 1.0 |
| Biologic (%) | 60 (23.4) | 8 (13.1) | 52 (26.7) | 0.037 |
| C‐reactive protein at baseline (mg/L) | 5.7 (1.6–13.3) | 4.8 (1.8–9.4) | 6.1 (1.5–14.2) | 0.135 |
| Fecal calprotectin at baseline (ug/g) | 662 (352–1268) | 645 (331–1129) | 674 (359–1490) | 0.364 |
| Fecal calprotectin assessements/year | 3 (2–4) | 3 (2–3) | 3 (2–4) | 0.619 |
Note: Early disease was defined as disease duration <18 months without exposure to immunomodulators or biologics. Continuous variables are expressed as median (interquartile range). Significant values are highlighted in bold.
Transmural remission
After a median follow‐up of 2 years, 1 , 2 , 3 , 4 121 patients (47.3%) reached endoscopic remission, 77 patients (30.0%) reached radiologic remission, and 61 patients (23.8%) reached transmural remission. Baseline characteristics were similar between patients with and without transmural remission. However, there was a longer time between assessments (3 years 2 , 3 , 4 , 5 , 6 versus 2 years, 1 , 2 , 3 , 4 p = 0.001), a higher prevalence of early disease (34.4% vs. 20.5%, p = 0.038), and non‐stricturing non‐penetrating phenotype (54.1% vs. 37.4%, p = 0.026), and less biological use at baseline (13.1% vs. 26.7%, p = 0.037) in patients with transmural remission. These results are also shown in Table 1.
Fecal calprotectin
A median of 6 4 , 5 , 6 , 7 , 8 , 9 , 10 assessments of fecal calprotectin were performed by each patient during the study, corresponding to a median of 3 2 , 3 , 4 assessments/year. There was a significant correlation between fecal calprotectin at the time of reassessment and endoscopic remission (rho = 0.754, p < 0.001), radiologic remission (rho = 0.354, p < 0.001), and transmural remission (rho = 0.456, p < 0.001). A correlation between fecal calprotectin and C‐reactive protein at all timepoints is presented in supplementary Figure S2.
Therapeutic drug monitoring and steroids
One hundred and seventeen patients (45.7%) performed at least one assessment of trough levels of Infliximab and/or Adalimumab within the last year of follow‐up. These patients presented with higher rates of transmural remission (29.9% vs. 18.7%, p = 0.040). Fifty‐two patients (20.3%) required at least one course of steroids within the last year of follow‐up. These patients presented with similar rates of transmural remission compared with patients with steroid‐free disease (17.3% vs. 25.5%, p = 0.220).
Tight monitoring
Adjustments of medical therapy followed 40% (0–75) of abnormal fecal calprotectin assessments: thiopurines were initiated in 47 patients, dose increased in 22; methotrexate was started in 5 patients; TNF antagonists were introduced in 97 patients and optimized with dose escalation or interval shortening in 72 patients; 17 patients switched to ustekinumab, and 7 performed interval decrease; 13 patients started vedolizumab, and 12 required interval reduction.
There was a significant correlation between the ratio of treatment escalation/elevated fecal calprotectin and the rates of transmural remission (Figure 1a). Transmural remission was present in 17.0% of patients with ratio <25%, in 11.8% of patients with a ratio ≥25–50%, in 40.0% of patients with a ratio ≥50–75%, and in 29.9% of patients with a ratio ≥75%. Therefore, an escalation ratio ≥50% provided the best chances of reaching transmural remission (34.2% vs. 15.1%, p < 0.001) (Figure 1b).
FIGURE 1.
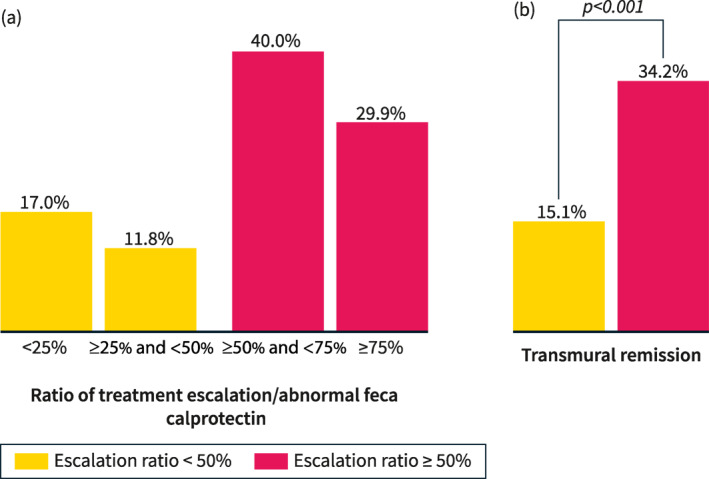
The percentage of patients reaching transmural remission according to different ratios of treatment escalation per abnormal fecal calprotectin (a) and considering a cutoff ≥50% versus <50% (b).
Intervention in early disease
At the time of the first assessment, 61 patients (23.8%) presented with a disease duration <18 months and no previous exposure to biologics or immunosuppressants. The benefits of an escalation ratio ≥50% were lower in patients without early disease (27.2% vs. 15.8%, p = 0.071) compared to patients with early disease (48.6% vs. 12.0%, p = 0.005) (Figure 2).
FIGURE 2.
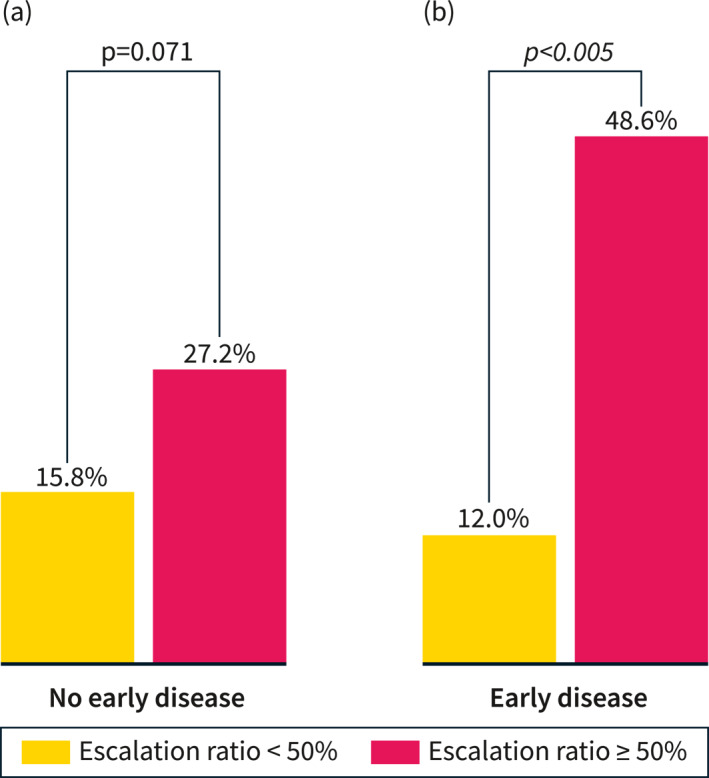
Rates of transmural remission in patients with early and late disease considering a ratio of treatment escalation per abnormal fecal calprotectin ≥50% versus <50%. Early disease was defined as disease duration <18 months without exposure to immunomodulators or biologics.
Factors associated with transmural remission
In multivariate analysis, a longer time between the baseline and follow‐up assessment (OR 1.32 95%CI 1.12–1.56, p = 0.001), stricturing phenotype (OR 0.39 95%CI 0.18–0.84, p = 0.017, using non‐stricturing non‐penetrating disease as reference), early disease (OR 3.24, 95%CI 1.12–9.34, p = 0.030), and a treatment escalation ratio ≥50% (OR 3.46, 95% CI 1.67–7.17, p = 0.001) were independent predictors of reaching transmural remission. A detailed presentation of the univariate and multivariate analysis is presented in Table 2.
TABLE 2.
Multivariate analysis for predicting transmural remission.
| Predictive factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio [95% CI] | p | Odds ratio [95% CI] | p | |
| Male versus female sex | 1.671 [0.935–2987] | 0.083 | 1.376 [0.708–2.675] | 0.346 |
| Active smoker | 0.624 [0.315–1.238] | 0.177 | ||
| Age at first assessment, years | 0.996 [0.979–1.013] | 0.662 | ||
| Disease duration, years | 0.989 [0.959–1.019] | 0.454 | ||
| Time between assessments, years | 1.250 [1.088–1.437] | 0.002 | 1.321 [1.124–1.552] | 0.001 |
| Early disease | 2.034 [1.081–3.828] | 0.028 | 3.235 [1.120–9.339] | 0.030 |
| Disease location | ||||
| Ileal | Reference | |||
| Colonic | 0.624 [0.072–5.374] | 0.667 | ||
| Ileo‐colonic | 1.487 [0.828–2.688] | 0.184 | ||
| Upper gastrointestinal disease | 0.624 [0.274–1.421] | 0.261 | ||
| Perianal disease | 0.778 [0.373–1.62] | 0.503 | ||
| Disease phenotype | ||||
| Non‐stricturing non‐penetrating | Reference | |||
| Stricturing | 0.395 [0.199–0.785] | 0.008 | 0.386 [0.177–0.842] | 0.017 |
| Penetrating | 0.757 [0.357–1.605] | 0.468 | ||
| Previous surgery | 1.153 [0.588–2.262] | 0.679 | ||
| Treatment at baseline | ||||
| No treatment | Reference | |||
| Immunomodulators | 1.042 [0.554–1.959] | 0.899 | ||
| Biologics a | 0.423 [0.179–1.001] | 0.050 | 1.588 [0.432–5.829] | 0.486 |
| Fecal calprotectin assessements/year | 0.914 [0.741–1.128] | 0.404 | ||
| Number of biologics used | ||||
| 0 | Reference | |||
| 1 | 1.883 [0.901–3.932] | 0.092 | 1.632 [0.629–4.233] | 0.314 |
| 2 | 0.844 [0.267–2.673] | 0.773 | ||
| 3 | 0 | 0.999 | ||
| Use of TDM within the last year | 1.855 [1.037–3.319] | 0.037 | 1.207 [0.560–2.603] | 0.632 |
| Use of steroids within the last year | 0.612 [0.279–1.341] | 0.220 | ||
| Treatment escalation ratio ≥50% | 2.919 [1.600–5.325] | <0.001 | 3.464 [1.673–7.172] | 0.001 |
Note: Early disease was defined as disease duration <18 months without exposure to immunomodulators or biologics. Escalation ratio was defined as the proportion of treatment escalation per abnormal fecal calprotectin (≥250 μg/g). Significant predictors are highlighted in bold.
Abbreviation: TDM, therapeutic drug monitoring.
Including combination with immunomodulators.
DISCUSSION
Current expert‐based recommendations support the benefits of early intervention, tight monitoring, and treat‐to‐target strategies in CD, associating them with several clinical benefits including increasing rates of endoscopic remission, sustained clinical remission, and improvement in the quality of life. 9 , 10 , 12 , 13 However, to date there have been no studies evaluating the potential benefits of these strategies with respect to transmural remission. In the present study, we demonstrated that patients with a higher rate of treatment escalation for each abnormal fecal calprotectin result (tighter control of inflammation) presented the best chances of obtaining transmural remission. The benefits of tight‐control were first explored in an open‐label randomized controlled trial published by Colombel et al. 10 Patients were randomly assigned to escalate treatment based on symptoms (clinical management) or based on symptoms and biomarkers (tight‐control). A significantly higher proportion of patients in the tight‐control group achieved the primary endpoint of endoscopic remission with no deep ulcers at week 48 (45.9% vs. 30.3%, p = 0.010). Of note, escalation involved the use of adalimumab 40 mg every other week, weekly, or combined with azathioprine. As 27% of patients in the tight‐control group were on the maximum‐allowed medication at week 36, the study may have underestimated the true potential of this strategy. Of note, fecal calprotectin was the main driver for treatment escalation. 14 Several studies have supported the accuracy of fecal calprotectin as a surrogate marker for endoscopic activity in CD. 15 Nevertheless, the limitations of using fecal calprotectin as a surrogate marker for radiologic and transmural remission should be considered. For example, in this study, only 6.7% of patients with normal fecal calprotectin (<250 μg/g) showed signs of endoscopic activity, but radiologic inflammation could still be detected in 36.9% of these patients. This suggests that fecal calprotectin, being a measure of mucosal neutrophils, may be useful as part of an initial strategy to assess treatment response and endoscopic remission, but may not be sufficient to evaluate healing of the outer layers of the bowel. Selecting a lower cutoff of fecal calprotectin (e.g. <50 μg/g) could reduce the percentage of wrongly identified patients to only 5.6%. Similar results have been reported by other authors. In a study looking at the correlation between fecal calprotectin and MRE, the median fecal calprotectin was significantly lower in patients with absent disease (80 μg/g [19–165]) compared to mild‐moderate disease (198 μg/g [101–486], p < 0.0001) and severe disease (360 μg/g [170–760]), p < 0.0001). 16 In another study, patients with radiologic remission, assessed by bowel ultrasound, presented lower values of fecal calprotectin compared to patients without radiologic remission (45.45 ± 31.26 μg/g vs. 391.68 ± 362.12 μg/g, p < 0.001). 8 In the alternative, a different modality could be employed to directly assess radiologic response and remission. Bowel ultrasound is a non‐invasive, inexpensive, highly acceptable, and well‐tolerated imaging tool with similar accuracy compared to MRE. 17 , 18 Several studies have suggested that bowel ultrasound can be used to guide treatment decisions and follow treatment response in CD. 18 , 19 , 20 , 21 , 22 In a recent multicenter prospective study, treatment optimization in symptomatic patients with evidence of inflammation by fecal calprotectin, C‐reactive protein, or bowel ultrasound was an independent predictor for achieving radiologic remission. 20 Future studies should evaluate the feasibility of using both fecal calprotectin and bowel ultrasound as part of a tight‐control and treat‐to‐target strategy in CD. Finally, we found that the effectiveness of the tight monitoring strategy was higher in patients with early disease. These results could be expected, considering that patients with early disease are more likely to have an inflammatory, non‐complicated disease and therefore a higher response to anti‐inflammatory medications. In fact, two recent meta‐analysis have shown higher rates of clinical and endoscopic remission in patients with a short duration of disease. 9 , 23
Our study represents the first attempt at identifying potential treatment strategies to improve the rates of transmural remission. We included a robust sample size evaluated using fecal calprotectin, colonoscopy and MRE. Nevertheless, we acknowledge some limitations of our study, including retrospective design and absence of formal scores to classify and stratify the clinical, endoscopic, and radiological activity. As this was a retrospective study with multiple assessments of biomarkers, we could not produce concomitant clinical assessments for all of the timepoints. However, it is not our common practice to escalate patients based only on symptoms, especially if concomitant biomarkers are within the normal range. Furthermore, previous studies have highlighted the low correlation between clinical symptoms and objective markers of inflammation. 24 Another potential limitation of our study is the inclusion of a significant percentage of patients with isolated ileal disease (57.4%). Several studies have suggested that fecal calprotectin may be less sensitive to detect inflammation in the small bowel. 25 , 26 , 27 However, other studies have reported good accuracy of fecal calprotectin in this setting, especially those using capsule endoscopic as reference. 28 , 29 Likewise, MRE may also be less sensitive for detecting active disease in the large bowel, especially if adequate rectal distention using water enemas cannot be achieved. 30 Nevertheless, isolated colonic disease was uncommon in our cohort (2.7%); therefore, this should have minimal impact on our results.
In conclusion, we demonstrate that a tight monitoring strategy based on fecal calprotectin can improve the rates of transmural remission, especially in the early disease stage. Our results may strengthen the support of transmural remission as a treatment target in CD.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest or funding to declare related to the present study.
Supporting information
Supporting Information S1
Figure S1
Figure S2
Fernandes SR, Bernardo S, Saraiva S, Gonçalves AR, Moura Santos P, Valente A, et al. Tight control using fecal calprotectin and early disease intervention increase the rates of transmural remission in Crohn's disease. United European Gastroenterol J. 2024;12(4):451–8. 10.1002/ueg2.12497
Samuel Raimundo Fernandes was responsible for designing the study, collecting and analysing the data, and writing the manuscript. Luís Correia, Helena Cortez‐Pinto, and Fernando Magro were responsible for designing the study and reviewing the manuscript. Sónia Bernardo, Sofia Saraiva, Ana Rita Gonçalves, Ana Valente and Paula Moura Santos reviewed the manuscript. All authors approved the final version of the article including the authorship list.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long‐term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8(4):244–250. 10.1097/00054725-200207000-00002 [DOI] [PubMed] [Google Scholar]
- 2. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta‐analysis: mucosal healing is associated with improved long‐term outcomes in Crohn's disease. Aliment Pharmacol Ther. 2016;43(3):317–333. 10.1111/apt.13475 [DOI] [PubMed] [Google Scholar]
- 3. Laharie D, D'Haens G, Nachury M, Lambrecht G, Bossuyt P, Bouhnik Y, et al. Steroid‐free deep remission at one year does not prevent Crohn's disease progression: long‐term data from the TAILORIX trial. Clin Gastroenterol Hepatol. 2022;20(9):2074–2082. 10.1016/j.cgh.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 4. Serban ED. Treat‐to‐target in Crohn's disease: will transmural healing become a therapeutic endpoint? World J Clin Cases. 2018;6(12):501–513. 10.12998/wjcc.v6.i12.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandes SR, Serrazina J, Botto IA, Leal T, Guimarães A, Garcia JL, et al. Transmural remission improves clinical outcomes up to 5 years in Crohn's disease. United Eur Gastroenterol J. 2023;11(1):51–59. 10.1002/ueg2.12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lafeuille P, Hordonneau C, Vignette J, Blayac L, Dapoigny M, Reymond M, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn's disease. Aliment Pharmacol Ther. 2021;53(5):577–586. 10.1111/apt.16232 [DOI] [PubMed] [Google Scholar]
- 7. Castiglione F, Imperatore N, Testa A, De Palma GD, Nardone OM, Pellegrini L, et al. One‐year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026–1039. 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 8. Castiglione F, Imperatore N, Testa A, de Sire R, Nardone OM, Ricciolino S, et al. Exploring the concept of deep remission in Crohn's disease: correlation between transmural healing and biomarkers. Therap Adv Gastroenterol. 2022;15:17562848221110643. 10.1177/17562848221110643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ungaro RC, Aggarwal S, Topaloglu O, Lee WJ, Clark R, Colombel JF. Systematic review and meta‐analysis: efficacy and safety of early biologic treatment in adult and paediatric patients with Crohn's disease. Aliment Pharmacol Ther. 2020;51(9):831–842. 10.1111/apt.15685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, et al. Effect of tight control management on Crohn's disease (CALM): a multicenter, randomised, controlled phase 3 trial. Lancet. 2017;390(10114):2779–2789. 10.1016/s0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
- 11. Peyrin‐Biroulet L, Billioud V, D'Haens G, Panaccione R, Feagan B, Panés J, et al. Development of the Paris definition of early Crohn's disease for disease‐modification trials: results of an international expert opinion process. Am J Gastroenterol. 2012;107(12):1770–1776. 10.1038/ajg.2012.117 [DOI] [PubMed] [Google Scholar]
- 12. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. 2015;385(9976):1406–1417. 10.1016/s0140-6736(14)61908-5 [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Vermeire S, D'Haens G, Panés J, Dignass A, Magro F, et al. Treat to target versus standard of care for patients with Crohn's disease treated with ustekinumab (STARDUST): an open‐label, multicentre, randomised phase 3b trial. Lancet Gastroenterol Hepatol. 2022;7(4):294–306. 10.1016/s2468-1253(21)00474-x [DOI] [PubMed] [Google Scholar]
- 14. Reinisch W, Panaccione R, Bossuyt P, Baert F, Armuzzi A, Hébuterne X, et al. Association of biomarker cutoffs and endoscopic outcomes in Crohn's disease: a post hoc analysis from the calm study. Inflamm Bowel Dis. 2020;26(10):1562–1571. 10.1093/ibd/izaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta‐analysis. J Gastrointestin Liver Dis. 2018;27(3):299–306. 10.15403/jgld.2014.1121.273.pti [DOI] [PubMed] [Google Scholar]
- 16. Jones GR, Fascì‐Spurio F, Kennedy NA, Plevris N, Jenkinson P, Lyons M, et al. Faecal calprotectin and magnetic resonance enterography in ileal Crohn's disease: Correlations between disease activity and long‐term follow‐up. J Crohns Colitis. 2019;13(4):442–450. 10.1093/ecco-jcc/jjy187 [DOI] [PubMed] [Google Scholar]
- 17. Taylor SA, Mallett S, Bhatnagar G, Baldwin‐Cleland R, Bloom S, Gupta A, et al. METRIC study investigators. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (METRIC): a multicenter trial. Lancet Gastroenterol Hepatol. 2018;3(8):548–558. 10.1016/s2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rajagopalan A, Sathananthan D, An YK, Van De Ven L, Martin S, Fon J, et al. Gastrointestinal ultrasound in inflammatory bowel disease care: patient perceptions and impact on disease‐related knowledge. JGH Open. 2019;4(2):267–272. 10.1002/jgh3.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Novak K, Tanyingoh D, Petersen F, Kucharzik T, Panaccione R, Ghosh S, et al. Clinic‐based point of care transabdominal ultrasound for monitoring Crohn's disease: impact on clinical decision making. J Crohns Colitis. 2015;9(9):795–801. 10.1093/ecco-jcc/jjv105 [DOI] [PubMed] [Google Scholar]
- 20. Kucharzik T, Wittig BM, Helwig U, Börner N, Rössler A, Rath S, et al. TRUST study group. Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol. 2017;15(4):535–542.e2. 10.1016/j.cgh.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 21. Calabrese E, Rispo A, Zorzi F, De Cristofaro E, Testa A, Costantino G, et al. Ultrasonography tight control and monitoring in Crohn's disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol. 2022;20(4):e711–e722. 10.1016/j.cgh.2021.03.030 [DOI] [PubMed] [Google Scholar]
- 22. Allocca M, Dell'Avalle C, Furfaro F, Zilli A, D'Amico F, Peyrin‐Biroulet L, et al. Early intestinal ultrasound predicts long‐term endoscopic response to biologics in ulcerative colitis. J Crohns Colitis. 2023:jjad071. [DOI] [PubMed] [Google Scholar]
- 23. Ben‐Horin S, Novack L, Mao R, Guo J, Zhao Y, Sergienko R, et al. Efficacy of biologic drugs in short‐duration versus long‐duration inflammatory bowel disease: a systematic review and an individual‐patient data meta‐analysis of randomized controlled trials. Gastroenterology. 2022;162(2):482–494. 10.1053/j.gastro.2021.10.037 [DOI] [PubMed] [Google Scholar]
- 24. Peyrin‐Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, et al. Clinical disease activity, C‐reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 2014;63(1):88–95. 10.1136/gutjnl-2013-304984 [DOI] [PubMed] [Google Scholar]
- 25. D'Arcangelo G, Imondi C, Terrin G, Catassi G, Aloi M. Is fecal calprotectin a useful marker for small bowel Crohn disease? J Pediatr Gastroenterol Nutr. 2021;73(2):242–246. 10.1097/mpg.0000000000003151 [DOI] [PubMed] [Google Scholar]
- 26. Stawczyk‐Eder K, Eder P, Lykowska‐Szuber L, Krela‐Kazmierczak I, Klimczak K, Szymczak A, et al. Is faecal calprotectin equally useful in all Crohn's disease locations? A prospective, comparative study. Arch Med Sci. 2015;11(2):353–361. 10.5114/aoms.2014.43672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Xu M, Qian W, Ling F, Chen Y, Li S, et al. Clinical value of fecal calprotectin for evaluating disease activity in patients with Crohn's disease. Front Physiol. 2023;14:1186665. 10.3389/fphys.2023.1186665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buisson A, Mak WY, Andersen MJ, Lei D, Pekow J, Cohen RD, et al. Fecal calprotectin is highly effective to detect endoscopic ulcerations in Crohn's disease regardless of disease location. Inflamm Bowel Dis. 2021;27(7):1008–1016. 10.1093/ibd/izaa269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiang B, Dong Z, Dai C. The diagnostic and predictive value of fecal calprotectin and capsule endoscopy for small‐bowel Crohn's disease: a systematic review and meta‐analysis. Rev Esp Enferm Dig. 2021;113(3):193–201. 10.17235/reed.2020.6996/2020 [DOI] [PubMed] [Google Scholar]
- 30. Chavoshi M, Mirshahvalad SA, Kasaeian A, Djalalinia S, Kolahdoozan S, Radmard AR. Diagnostic accuracy of magnetic resonance enterography in the evaluation of colonic abnormalities in Crohn's disease: a systematic review and meta‐analysis. Acad Radiol. 2021;28((Suppl 1)):S192–S202. 10.1016/j.acra.2021.02.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Figure S1
Figure S2
Data Availability Statement
Data are available upon reasonable request.


