Abstract
Borna disease virus (BDV), a nonsegmented, negative-stranded (NNS) RNA virus, causes central nervous system (CNS) disease in a broad range of vertebrate species, including felines. Both viral and host factors contribute to very diverse clinical and pathological manifestations associated with BDV infection. BDV persistence in the CNS can cause neurobehavioral and neurodevelopmental abnormalities in the absence of encephalitis. These BDV-induced CNS disturbances are associated with altered cytokine and neurotrophin expression, as well as cell damage that is very restricted to specific brain regions and neuronal subpopulations. BDV also targets astrocytes, resulting in the development of prominent astrocytosis. Astrocytes play essential roles in maintaining CNS homeostasis, and disruption of their normal activities can contribute to altered brain function. Therefore, we have examined the effect of BDV infection on the astrocyte's physiology. We present here evidence that BDV can establish a nonlytic chronic infection in primary cortical feline astrocytes that is associated with a severe impairment in the astrocytes' ability to uptake glutamate. In contrast, the astrocytes' ability to uptake glucose, as well as their protein synthesis, viability, and rate of proliferation, was not affected by BDV infection. These findings suggest that, in vivo, BDV could also affect an important astrocyte function required to prevent neuronal excitotoxicity. This, in turn, might contribute to the neuropathogenesis of BDV.
Borna disease (BD) virus (BDV) causes central nervous system (CNS) disease in a broad range of vertebrate species (36, 43, 48, 63, 65). BDV has a nonsegmented, negative-strand (NNS) RNA genome (9, 17). Based on its unique genetic and biological features, BDV is considered to be the prototypic member of a new virus family, Bornaviridae, within the order Mononegavirales (19, 68).
Naturally occurring BDV infections were thought to be mainly restricted to horses and sheep within certain geographic regions of central Europe. Current evidence, however, indicates that the natural host range, geographic distribution, and prevalence of BDV are much broader than previously considered (36, 43, 63, 65). The genetics, immune status, and age of the host, as well as viral factors, contribute to a high degree of heterogeneity in disease symptoms and pathological manifestations associated with BDV infection. Clinical manifestations can range from dramatic to subtle or even been unapparent (32, 36, 43, 63, 65). Nevertheless, all known BDV isolates are noncytolytic and highly neurotropic (43, 48, 65). Heightened viral gene expression in limbic system structures and neuronal structural alterations within the hippocampal formation are the main histopathological hallmarks of BDV infection (32, 34, 35). Immune cell infiltrates are frequently, but not always, observed in the CNS of BDV-infected animals, and immune-mediated neuronal damage is thought to be responsible for the clinical symptoms associated with classic BD (5, 48, 65, 71). BDV affects the postnatal development of the brain monoaminergic system (61). However, the cellular and molecular mechanisms whereby BDV causes CNS disturbances in the absence of encephalitis remain largely unknown (1, 2, 11, 24, 32, 33, 40, 42, 66).
BDV persistence in the CNS is also characterized by infection of astrocytes (10, 12, 13) and development of prominent astrocytosis (32, 34, 48, 65). The reactive astrocyte response is a near universal response to brain insults, including viral infection, and represents a complex process involving profound changes in the astrocyte gene expression program (28, 57). Astrocytes play essential roles in the maintenance of a CNS microenvironment compatible with proper neuronal activity (3, 28, 69). Disturbances in astrocyte functions can induce or enhance neuronal pathology by affecting complex interactions within neuronal networks (3, 28, 37, 57). Thus, astrocyte glutamate transporters are essential to maintain low extracellular levels of glutamate, the major excitatory neurotransmitter in the CNS (64). Excessive extracellular glutamate levels would activate neuronal N-methyl-d-aspartic acid (NMDA) receptors, leading to an increase in calcium influx, which can result in neurotoxicity and cell death (25, 45, 47, 67). Both human immunodeficiency virus (HIV) and feline immunodeficiency virus (FIV) have been shown to affect glutamate uptake by astrocytes, which is likely to contribute to the neuropathogenesis of these two lentiviruses (4, 4a, 15, 25, 73, 78). We have previously documented the preparation of primary feline astrocyte (FeAst) cultures that maintain many of their normal physiological activities, including glutamate and glucose uptake (78). Using this culture system, we have examined whether BDV persistence in astrocytes leads to an impairment of their normal cell physiology, which in turn could contribute to neuronal damage and disturbances in CNS functions associated with cases of BDV infection that proceed in the absence of encephalitis. Here, we present evidence that BDV can establish a nonlytic chronic infection in primary feline cortical astrocytes that is associated with a severe and specific impairment in the astrocytes' ability to uptake glutamate. We discuss the implications of this finding with respect to the neuropathogenesis of BDV.
MATERIALS AND METHODS
Cells and viruses. (i) Enriched astrocyte cultures.
Primary cultures of cerebral cortical astrocytes were prepared from 2-day-old offspring of specific-pathogen-free (SPF) cats as described previously (55, 79) with some modification (6). Briefly, forebrains were removed aseptically from the skulls; the meninges were excised under a dissecting microscope, and neocortices were dissected. The cells were dissociated, without trypsin, by passage through needles of decreasing gauges (16G1, 19G1, and 25G1) two to three times with a 10-ml syringe. The cells were seeded at a density of 105 cells per cm2 on 12-well tissue culture plates (Costar) in Dulbecco's modified Eagle's medium (DMEM) (BioWhittaker) containing 10% fetal bovine serum (FBS) and 25 mM glucose and then incubated at 37°C in an atmosphere containing 5% CO2 at 95% humidity.
(ii) Cell lines.
C6 cells (ATCC CCL 107), derived from a rat glial tumor, were grown in DMEM containing penicillin, streptomycin, 1% glutamine, and 10% heat-inactivated FBS. C6-lacZ cells were derived from a clone of C6 cells that was stably transfected with a lacZ gene expression vector which conferred to the cells high β-galactosidase activity that can be easily detected by in situ staining for β-galactosidase. C6-lacZ cells were grown similarly to the C6 cells, but with G418 (250 μg/ml) incorporated into the medium for selection of stably transfected cells.
(iii) Virus stock.
The Giessen strain He/80 of BDV was passaged three times in Lewis rats by intracerebral inoculation. Brain homogenate from the fourth passage (BDVRp4) was prepared as described previously (16). Aliquots of BDVRp4 stock were stored at −70°C. The infectious titer (focus-forming units [FFU] per milliliter) of BDVRp4, as well as those of supernatants and whole-cell extracts (WCE) from BDV-infected FeAst, was determined by an immunofocus assay as described previously (16, 39). Preparation of WCE was done by three cycles of freezing and thawing, followed by ultrasonication on ice.
RNA analysis.
Total RNA was isolated by the TRI reagent procedure (MRC, Inc., Cincinnati, Ohio). RNA samples were dissolved in 0.5 mM EDTA and stored at −70°C. Total RNA (5 μg) from BDV-infected and mock-infected primary FeAst, as well as from C6 cells, was analyzed by Northern blot hybridization as described previously (16, 17). Briefly, RNA was size fractionated by 2.2 M formaldehyde–agarose gel electrophoresis, transferred by capillarity with 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to MagnaGraph nylon membrane (MSI, Westboro, Mass.), and UV cross-linked. Hybridization with 32P-labeled BDVp40 or GAPDH-specific probes was performed as described previously (16, 17). Methylene blue (MB) staining of the membrane after transfer and prior hybridization was used to verify that similar amounts of total RNA were loaded in all cases.
Detection of viral proteins. (i) Indirect immunofluorescence (IIF).
Cells were grown on coverslips and fixed with acetone-methanol (50:50) for 5 min at room temperature. After several washes with phosphate-buffered saline (PBS), cells were blocked with PBS–10% normal goat serum for 60 min at room temperature. Double labeling of BDV antigens and the astrocytic marker glial fibrillary acidic protein (GFAP) was done by using a rat serum to BDV and a rabbit polyclonal serum to GFAP. Binding of primary antibodies was detected with species-matched secondary antibodies conjugated to either fluorescein isothiocyanate or rhodamine.
(ii) Immunoblot analysis.
WCE were prepared in 1× sodium dodecyl sulfate (SDS)–loading buffer (50 mM Tris-HCl [pH 6.5], 100 mM dithiothreitol, 2% SDS, 0.1% BPB, 10% glycerol). Extracts were analyzed by Western blotting with rabbit polyclonal antibodies to BDV nucleoprotein (NP [p40]) and phosphoprotein (P [p24]) antigens, as described previously (20, 31).
Cell proliferation and rate of protein synthesis. (i) Cell proliferation.
Viable cells were determined by trypan blue staining. The number of cells for each time point was determined in triplicate.
(ii) Rate of protein synthesis.
Cells were starved for 45 min in medium without cysteine and methionine and containing 1% dialyzed FBS. 35S-Trans label was added to the cultures (50 μCi/ml). At the indicated postlabeling times, whole-cell lysates were made by using 1× SDS–gel loading buffer. Incorporation of labeling into macromolecules was determined by precipitation with trichloroacetic acid and measurement of 35S label by liquid scintillation counting.
Glucose uptake assay.
Uptake experiments were conducted weekly after infection as described previously (78). Briefly, after the medium was removed, cells were incubated for 3 h in 0.5 ml of serum-free DMEM, containing 5 mM instead of 25 mM glucose (DMEM5), at 37°C in an atmosphere containing 5% CO2 at 95% humidity. Afterward, 0.5 ml of fresh DMEM containing [3H]2-deoxy-d-glucose (3H-2DG) (final concentration, 48 nM) was added for an additional 20-min incubation. Uptake was terminated by aspirating the uptake solution and washing the cells three times with 2 ml of ice-cold PBS. Astrocytes were then lysed by adding 0.5 ml of 10 mM NaOH containing 0.1% Triton X-100, and a 300-μl portion was assayed for 3H by liquid scintillation counting. The protein content was measured by the method of Bradford (8) in 100 μl of the remaining lysate. 3H-2DG uptake was expressed in femtomoles per milligram of protein.
Glutamate uptake assay.
The uptake of [3H]glutamate (3H-Glu) was determined by the method described by Volterra et al. (74). Briefly, after infection and supernatant collection, the medium was replaced by 0.5 ml of fresh medium containing 50 μM glutamate and 18.5 kBq (9.25 pmol) of 3H-Glu. Uptake was terminated 15 min later by removing the supernatant and washing the cells three times with 2 ml of ice-cold PBS containing 5 mM glutamate. Astrocytes were then lysed by 0.5 ml of 10 mM NaOH containing 0.1% Triton X-100, and a 300-μl portion was assayed for 3H by liquid scintillation counting. The protein content was measured (8) in 100 μl of the remaining lysate. Uptake was expressed in femtomoles per milligram of protein.
Statistical analysis and image processing.
A two-factor (BDV-uninfected versus BDV-infected cultures for 4 to 6 days) between-group analysis of variance was applied to the mean from each triplicate for each experiment. Scheffe's contrast procedures were used to perform post hoc comparisons between the infected conditions for each day. All agarose gel pictures were taken with a Stratagene Eagle eye camera. Pictures were scanned with an Agfa Studiostar scanner, and the composite images were generated with Adobe Photoshop.
RESULTS
Primary FeAst are susceptible to BDV.
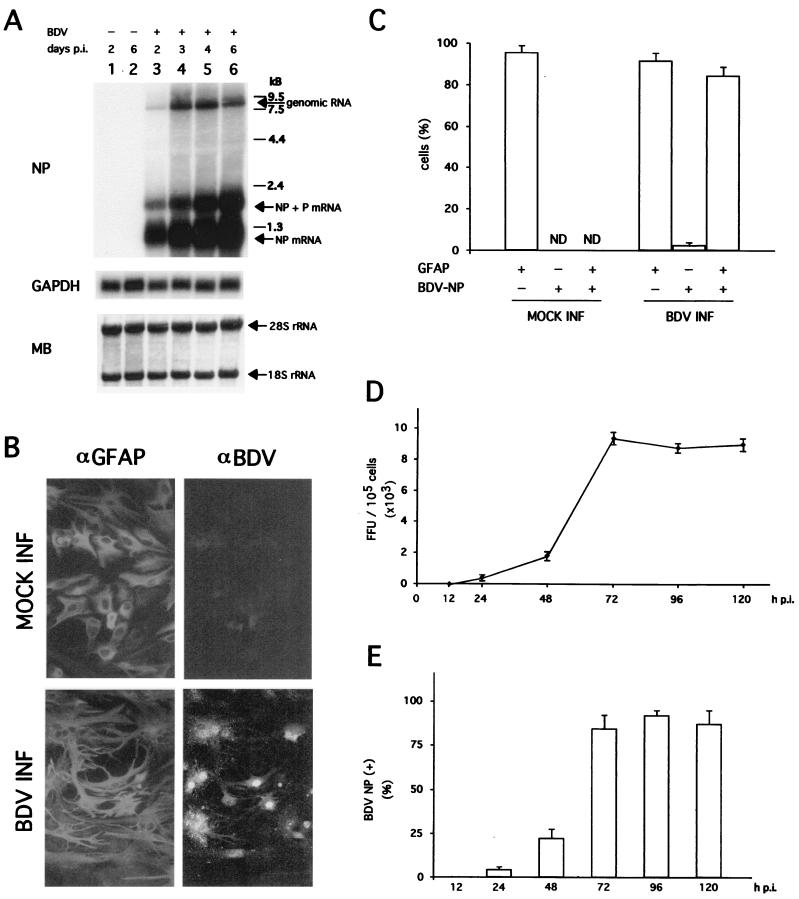
We first tested whether primary FeAst could be infected with our rat-adapted laboratory stock BDV-He80 strain without requiring adaptation passages in feline cells. This BDV-He80 viral stock corresponded to the fourth passage in brain tissue of newborn infected rats. We infected primary FeAst with BDV-He80 at a multiplicity of infection (MOI) of 0.1 FFU/cell and analyzed the synthesis of BDV RNA, as well as expression of viral antigen and production of infectious virus (Fig. 1). Both BDV genomic and subgenomic viral mRNAs were readily detected by Northern blot hybridization at 48 h postinfection (p.i.) (Fig. 1A). Levels of BDV genome and mRNA increased significantly from 48 to 72 h p.i.; thereafter, and until the last time point analyzed (6 days p.i.), levels of genome RNA remained at the same level, whereas BDV NP mRNA showed a modest, steady increase (Fig. 1A). No differences were observed between BDV- and mock-infected FeAst with respect to the levels of the housekeeping cellular mRNA GAPDH (Fig. 1A). Consistent with our previous findings (78), the majority of the cells (>90%) were positive for the astrocyte marker GFAP (Fig. 1B), reflecting a high degree of purity in our astrocyte primary cultures. About 85% of the cells in the population were positive for BDV NP antigen at 72 h p.i. as determined by immunofluorescence (IF) (Fig. 1B and C). The majority of cells that scored positive in IF for BDV antigen were also GFAP positive. However, there was a low percentage (ca. 3%) of BDV-positive cells that were not immunolabeled by the anti-GFAP antibody (Fig. 1C). The cell lineage of these GFAP-negative cells was not determined in this study. However, we have previously shown that 2 to 3% of the cells in these primary astrocyte cultures are microglia, as determined by nonspecific esterase staining (30). BDV infection of FeAst was productive, since infectious virus progeny was detected 24 h p.i. (Fig. 1D). Viral titers increased significantly between 24 and 72 h p.i., followed by a plateau effect that was maintained until 120 h p.i., the last time point analyzed (Fig. 1D). Throughout the entire kinetics experiment, BDV infectivity remained cell associated, and we were unable to detect at any time cell-free infectious particles in the tissue culture supernatant (data not shown). We also observed a correlation between the number of infected cells and production of BDV infectious progeny (Fig. 1E).
FIG. 1.
(A) Synthesis of BDV RNA in primary FeAst. Primary cat astrocytes were infected with BDV-He80 at an MOI of 0.1 FFU/cell. At the indicated times, total RNA from mock- and BDV-infected cells was analyzed by Northern blot hybridization. BDV genomic and NP mRNAs were detected with a 32P-labeled NP DNA probe. As a control, the same blot was also hybridized with a probe for the housekeeping cellular mRNA GAPDH. MB staining of the membrane after transfer and prior hybridization was used to verify that similar amounts of total RNA were loaded in all cases. The positions of the 28S and 18S rRNAs are indicated. (B) Detection of BDV NP antigen and GFAP expression in primary FeAst. BDV-infected cells (INF) and mock-infected controls were fixed at 96 h p.i. and analyzed by indirect immunofluorescence. Cells were simultaneously labeled with a rabbit antiserum to GFAP (αGFAP) and a rat serum to BDV (αBDV). (C) The percentage of cells expressing one or both antigens was determined by counting cells (total of 250) from five or six different fields. Shown are the average and standard deviation from two independent experiments. ND, not determined. (D) Kinetics of BDV multiplication in primary FeAst. Cells were infected with BDV-He80 at an MOI of 0.1 FFU/cell. At the indicated times p.i., BDV infectivity in supernatant and in WCE was determined. Only cell-associated infectivity was detected. Values correspond to the average and standard deviation of two independent experiments. (E) Number of BDV-infected cells at different times p.i. FeAst were infected at an MOI of 0.1 FFU/cell, and at the indicated times after infection, cells were fixed and examined for expression of BDV NP antigen by IIF. Values correspond to the average and standard deviation of two independent experiments.
BDV establishes a nonlytic chronic infection in primary FeAst.
Once we verified that primary FeAst were susceptible to BDV, we examined whether BDV could establish a long-term persistent infection in these cells. Primary FeAst were infected with BDV-He80 at an MOI of 0.1 FFU/cell, and 5 days p.i., cells were subcultured at a 1/3 ratio. After 15 days, these cells (FeAst/BDVp1) were subcultured again (1/3 ratio). Subsequent cell passages were done every 20 days, with passage 5 (p5) (100 days p.i.) as the end point of the experiment. As a control, we used mock-infected FeAst cells that were handled in an identical manner to the BDV-infected cells.
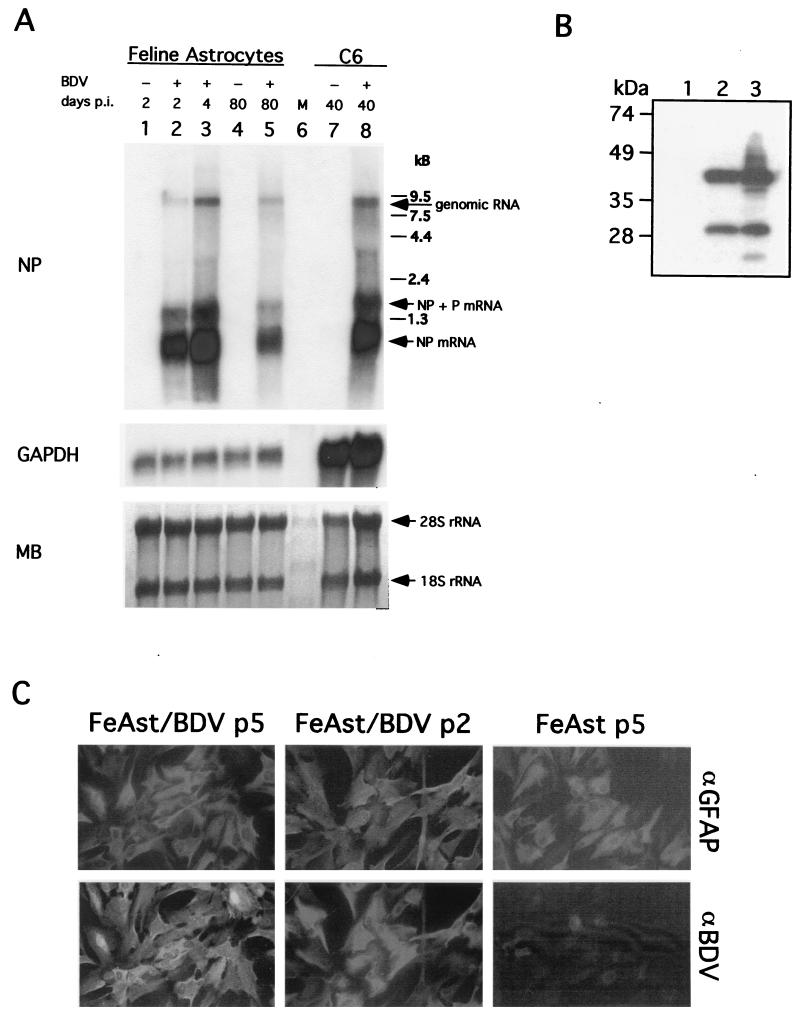
Viral genome and subgenomic NP mRNA were readily detected at 80 days p.i. (four cell passages, FeAst/BDVp4) by Northern blot hybridization (Fig. 2A). Likewise, BDV NP and P gene products were detected in FeAst/BDV cells (Fig. 2B). Levels of both BDV genomic and mRNA species in FeAst/BDVp4 were slightly decreased compared to those detected at in BDV-infected FeAst at day 4 p.i., as well as in C6 cells persistently infected with BDV-He80 for 40 days (C6BVp10) (compare Fig. 1A and 2A). IF results showed that most of the cells within the BDV persistently infected FeAst cell population expressed both GFAP and BDV NP antigen (Fig. 2C and Table 1). These results indicated that primary FeAst can sustain long-term BDV replication and transcription. We observed a small, but consistent, percentage of cells that were positive for viral antigen, but negative for GFAP. This subpopulation of GFAP-negative cells did not increase from passages 1 to 5, indicating that they were not being selected under the tissue culture conditions used in our study (Table 1). The percentage of GFAP-negative cells in BDV persistently infected FeAst was similar to that observed in mock-infected control FeAst, suggesting that BDV infection did not affect the expression of this astrocyte marker.
FIG. 2.
BDV persists in primary FeAst. (A) Detection of BDV RNA both genome and mRNA in FeAst at 80 days p.i. (passage 4). RNA was extracted from BDV-infected FeAst (lanes 2, 3, and 5) at 2, 4, and 80 days p.i. and from mock-infected control FeAst (lanes 1 and 4) at 2 and 80 days. RNA (5 μg each) was analyzed by Northern blot hybridization with probes for BDV NP and rat GAPDH. As a comparison, RNA was also extracted from C6 cells persistently infected with BDV. Differences in the hybridization signal with the GAPDH probe reflect nucleotide differences between cat and rat species in the GAPDH gene. MB staining of the membrane is shown. The positions of the 28S and 18S rRNAs are indicated. (B) Detection of BDV NP and P. WCE from FeAst-p5 (lane 1), FeAst/BDV-p5 (lane 2), and C6BV-p10 (lane 3) were analyzed by Western blotting as described in Materials and Methods. (C) Detection by IIF of BDV NP antigen in FeAst at 40 and 100 days p.i. BDV-infected and mock-infected control FeAst were fixed at days 40 and 100 p.i. and analyzed by IIF with double labeling with antibodies to GFAP (αGFAP) and BDV NP (αBDV).
TABLE 1.
Expression of BDV antigens and GFAP in FeAst persistently infected with BDVa
| Antigen | % of cells positive at day p.i.:
|
||
|---|---|---|---|
| 40 (passage 2) | 60 (passage 3) | 100 (passage 5) | |
| BDV NP | 86 (79–91) | 92 (84–95) | 88 (83–94) |
| GFAP | 94 (88–96) | 89.5 (81–93) | 91 (87–93) |
| BDV NP + GFAP | 79.5 (73–86) | 87 (83–92) | 82 (76–93) |
| BDV NP − GFAP | 6.5 (9–2) | 5 (7–4) | 6 (8–3) |
At 40, 60, and 100 days p.i., corresponding to passages 2, 3, and 5, respectively, BDV-infected FeAst were fixed and analyzed by IIF with antibodies to BDV and GFAP. The total percentages of cells expressing viral antigens or GFAP, as well as double-positive cells, are indicated. Numbers correspond to the average and range obtained from counting cells in three independent fields. The range, indicated in parentheses, corresponds to the lowest and highest numbers of positive cells observed in any of the three fields examined for each case.
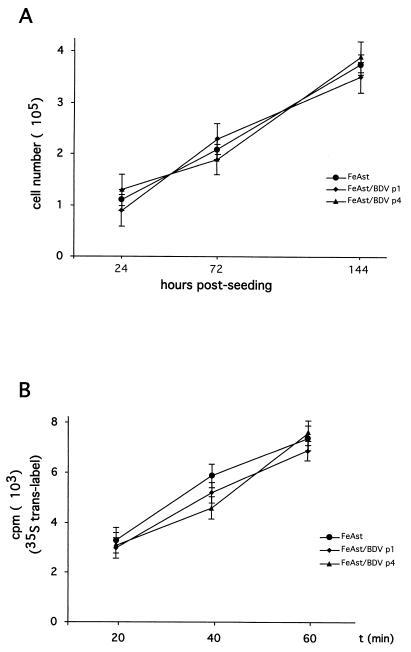
We next evaluated whether BDV persistence has a noticeable impact on the survival and growth of FeAst cells. For this, we compared the rates of cell growth and protein synthesis between FeAst/BDV at 20 (p1) and 60 (p3) days p.i. and those of mock-infected control cells. Both BDV persistently infected cells and control cells exhibited the same kinetics of cell growth (Fig. 3A) and rates of protein synthesis (Fig. 3B).
FIG. 3.
Cell growth and synthesis of proteins are not impaired in FeAst persistently infected with BDV. (A) Cell growth. Mock-infected (p4) and BDV-infected FeAst p1 and p4 were seeded (105 cells/dish) into 35-mm-diameter polylysine-treated tissue culture dishes. At the indicated postseeding times, the numbers of viable cells were determined by using trypan blue staining. For each sample and time point, cell numbers were determined in triplicate. Shown are average values and standard deviations of two independent experiments. (B) Rate of protein synthesis. Cells were labeled with 35S-Trans label for the amount of time indicated. Incorporation of 35S label into proteins was determined by trichloroacetic acid precipitation and Cerenkov counting with a scintillation counter. Each time point was determined in triplicate. Values correspond to the average and standard deviation.
Infectivity associated with FeAst persistently infected with BDV.
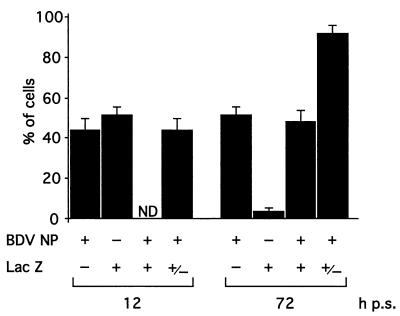
A characteristic feature of BDV-infected cultured cells is the extremely low levels, or complete lack, of cell-free virus (CFV). Likewise, the infectivity detected in FeAst/BDV cultures was cell associated and remained at similar levels (8 × 103 to 10 × 104 FFU/105 cells) from passages 1 to 5 (100 days of persistence). We did not detect CFV in the tissue culture supernatant at any time throughout the entire course of the observation period of the BDV persistently infected FeAst (data not shown). This finding led us to consider whether BDV-susceptible cells could become infected when cocultured with BDV persistently infected FeAst. For this, we cocultured FeAst/BDV and C6-lacZ cells at a 1:1 ratio. The percentage of cells within the population positive for both LacZ and BDV NP antigen increased from 0 to about 50% between 12 and 72 h after seeding of the cells (Fig. 4). Concomitantly, the majority (>90%) of the cells within the population became BDV NP positive at 72 h postseeding (Fig. 4).
FIG. 4.
BDV-susceptible cells become infected by cocultivation with BDV-infected FeAst. C6-lacZ cells and BDV-infected FeAst were seeded at a ratio of 1:1. At the indicated postseeding (p.s.) time points, cells expressing BDV antigens and LacZ were determined by IIF.
BDV affects glutamate uptake in FeAst.
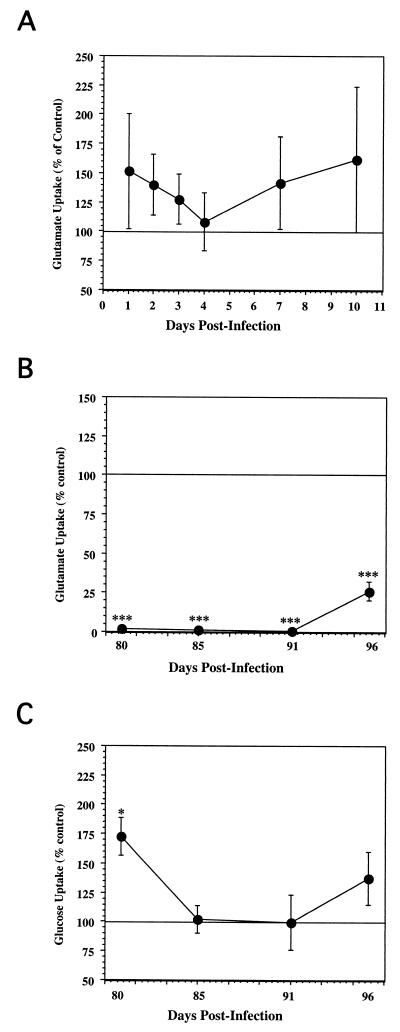
To assess the effects of BDV infection on astrocyte function, we performed a glutamate uptake assay. As a baseline, we measured glutamate uptake in mock-infected cells, which averaged 8,619.07 ± 2,238.11 fmol/mg/15 min, a value within the same range obtained under similar conditions with human astrocytes (29). We then determined the ratio of glutamate uptake between infected and mock-infected control cells that were identically and simultaneously processed. During the first 10 days p.i., which we designated as the acute phase of the infection, BDV did not cause any significant alteration in the 3H-Glu uptake (Fig. 5A), although the infected cells showed a tendency toward increased 3H-Glu uptake during this time. These results were in marked contrast to the findings with the persistently infected cultures (Fig. 5B). We observed a very dramatic and sustained effect on the glutamate uptake in astrocyte cultures persistently infected with BDV. Glutamate uptake was almost completely inhibited in FeAst/BDVp4 cells, reaching only 2 and 1% at 80 and 91 days p.i., respectively, compared to that in controls. A strong inhibition of glutamate uptake was still observed at day 96 p.i. (27% of the control value). These results were statistically significant for all time points to the P < 0.001 level (Fig. 5B).
FIG. 5.
(A) Effects of BDV acute infection on FeAst glutamate uptake. At the indicated time points after infection with BDV, the 3H-Glu uptake assay was performed, as described in Materials and Methods. Results are expressed as the ratio of 3H-Glu uptake of infected cultures over that of control cultures. (B) Effects of BDV persistent infection on FeAst glutamate uptake. At the indicated time points after infection with BDV, the 3H-Glu uptake assay was performed, as described in Materials and Methods. Results are expressed as the ratio of 3H-Glu uptake of infected cultures over the value of the control cultures ± standard error of the mean. Results were analyzed by analysis of variance. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001. (C) Effects of BDV persistent infection on astrocyte glucose uptake. At the indicated time points with BDV, the ability of the feline astrocyte cultures to remove 3H-2DG from the culture supernatant was determined, as described in Materials and Methods. Results are expressed as the ratio of [3H]glucose uptake of infected cultures over the value of the control cultures.
To determine if the effect of persistent BDV infection on glutamate uptake was selective or represented a general BDV-mediated impairment of the astrocyte physiology, we examined the effects of a chronic BDV infection on glucose uptake (3H-2DG uptake). The 3H-2DG uptake assay was performed with FeAst/BDVp4 cells over four time points (Fig. 5C). Mock-infected control cultures had an average 3H-2DG uptake value of 351.14 ± 88.25 fmol/mg of protein (12 independent assays), which was similar to the value reported for murine astrocytes (299 to 630 fmol/mg of protein). 3H-2DG uptake increased significantly on day 80 (172% of the control value), but was not significantly different from that of the mock-infected control cells on days 85, 91, and 96 p.i. These data indicated that chronic BDV infection did not significantly impair glucose uptake.
DISCUSSION
This study provides, for the first time, evidence that a rat-adapted strain of BDV can establish a nonlytic productive persistent infection in feline cortical astrocytes that is associated with a selective and nearly complete inhibition of the astrocyte's ability to uptake glutamate, an important function required to prevent neuronal excitotoxicity (46).
Diverse mechanisms are likely to contribute to BDV-induced CNS disturbances. Classic BD, both naturally occurring and experimentally induced, is an immune-mediated biphasic behavioral disease that is characterized by a strong immune cell inflammatory response within the CNS (58). In contrast, neonatally infected rats do not have inflammation and are free of clinical signs of classic BD, namely severe neurological disturbances together with high morbidity and mortality. Nonetheless, these rats, referred to as BDV/PTI-NB, exhibit distinct cognitive, neurobehavioral, and specific neurodevelopmental abnormalities (1, 2, 11, 24, 40), which are associated with specific neurochemical alterations (42, 60, 61). The cellular and molecular bases of these BDV-induced CNS disturbances are not well understood. BDV appears to have a specifically enhanced affinity for areas with a high density of aspartate and glutamate receptors in the hippocampal formation, which might contribute to impaired brain function (22). Brains of BDV/PTI-NB rats exhibit a chronic upregulation in the expression levels of several proinflammatory cytokines (66). Altered cytokine expression in the CNS can contribute to the BDV interference with neuroplasticity processes in specific cell populations seen in BDV/PTI-NB rats, thus leading to disturbances in cognitive function (33). Increased cytokine expression by CNS resident cells, mainly microglia and astrocytes, could also modulate astrocyte function, as well as affect the supply of factors required for survival of selective neuronal populations within the cortex and limbic system structures (33).
Astrocytes are the most common cell type in the brain and play a key role in maintaining the appropriate microenvironment in the CNS required for normal neuronal activity (3, 27). One of the most important functions of astrocytes is to regulate the level of extracellular glutamate, a major excitatory neurotransmitter (64). Glutamate accumulates as a consequence of neuronal activity (64). Excessive levels of extracellular glutamate often result in neuron toxicity and death (64). Thus, the astrocyte's function of controlling levels of extracellular glutamate in the brain is essential to maintaining the health of the neuron. Evidence indicates that glutamate-mediated neurotoxicity may play a major role in CNS disorders, including virus-induced diseases (25, 45, 47, 67). Astrocytes are the main cellular target of BDV in the CNS; hence it would be plausible that the disruption of the astrocyte's ability to prevent glutamate-mediated neurotoxicity could contribute to BDV-induced CNS dysfunction in the absence of encephalitis.
BDV dramatically altered glutamate uptake by long-term persistently infected primary FeAst. This BDV-mediated impairment in glutamate uptake by astrocytes was not observed during the initial phase of infection (first 10 days after infection). However, the numbers of BDV-infected cells and levels of BDV replication were similar in both cases. This finding suggests that the impairment in glutamate uptake probably results from a combination of slow progressive changes in astrocyte cell physiology induced during BDV persistence. A variety of nonlytic viruses have been shown to be capable of affecting cell-differentiated functions (21). Therefore, it is possible that BDV infection can directly interfere with the activity of the glutamate transporters. The role of a soluble factor secreted by BDV-infected cells in the inhibition of glutamate uptake was not examined. Therefore, we cannot formally rule out the possibility that production of cytokines, or other soluble factors, by the minor fraction of BDV-infected cells that were GFAP negative could be responsible for the effect on glutamate uptake by astrocytes. Thus, for example both interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) have been shown to affect glutamate uptake by astrocytes (14, 41, 56, 77). Interestingly the expression of these two cytokines is upregulated in the CNS of PTI-NB rats (66). Significant changes in temporal expression of IL-1 and TNF have been reported in BDV/PTI-NB rats (60). This raises the possibility that, also in vivo, glutamate uptake can be affected at different levels at early and late stages of BDV infection. Arachidonic acid and reactive oxygen intermediates, which can be produced by astrocytes and microglia in response to infection, are also known to suppress the astrocyte's ability to scavenge glutamate from the environment (26, 70, 75, 76).
Cell growth and synthesis of macromolecules were not altered in BDV-infected compared to control mock-infected FeAst, indicating that BDV-mediated abrogation of glutamate uptake was not a consequence of a generalized cytotoxicity caused by BDV infection. Recent evidence indicates that glutamate and astrocytes are pivotal components of the coupling of synaptic activity with energy metabolism (38, 54). Glutamate transporters mediate glutamate-induced uptake of glucose in astrocytes. The electrochemical gradient of Na+ that drives the activity of these transporters also triggers the glycolytic processing of glucose, which results in astrocyte release of lactate, the preferred energy substrate of activated neurons (54). Therefore, an impaired astrocyte's ability to uptake glutamate not only can contribute to NMDA-mediated neurotoxicity, but also can compromise the supply of substrate for energy production in neurons. Whether glucose metabolism and lactate production are affected in BDV persistently infected FeAst remains to be determined. On the other hand, the impaired energy metabolism of astrocytes will significantly affect their ability to maintain low levels of extracellular glutamate (23). Glucose uptake was not altered in FeAst persistently infected with either BDV (this report) or FIV (30). These findings illustrate that a noncytolytic virus persistent infection can selectively target and disrupt specific components of interconnected cellular functions.
BDV has been recently implicated in “staggering disease” (SD), a neurological disorder affecting domestic cats in several parts of the world (49, 52, 59, 62). A high percentage (>40%) of cats with SD have been found to be BDV seropositive (49, 52, 59). Both viral antigen and RNA have been detected in brain tissue of diseased cats (51, 53). Moreover, SPF cats inoculated with BDV isolated from a cat with SD developed disease (50). These findings, together with the failure to detect other infectious agents in the CNS of SD cats, suggest a possible causative role of BDV in SD. BDV load in the CNS of SD cats is significantly lower than that observed in BDV infections of other species. More intriguingly, astrocytes, rather than neurons, appear to be the main virus target cell in the feline CNS (18). The mechanisms underlying neuronal damage observed in the brains of cats with SD are unknown, and whether altered astrocyte physiology is a contributing factor remains to be determined. Our findings with BDV-infected primary FeAst make plausible the hypothesis that altered glutamate uptake by astrocytes could contribute to neuronal damage associated with SD in cats.
Previous studies have shown that two lentiviruses, FIV and HIV, can establish a persistent nonlytic infection in astrocytes (7, 72, 78) and lead to a severe impairment, either directly or indirectly, in the glutamate uptake function of astrocytes (4a, 44, 45, 47, 78). The results presented here have shown that BDV, an NNS RNA virus, can also specifically abrogate glutamate uptake by astrocytes. Together, these findings suggest a possible common pathogenic mechanism for causing neuronal damage by viruses that cause a nonlytic persistent infection of astrocytes.
ACKNOWLEDGMENTS
J.-N.B. and C.L. contributed equally to this work.
This work was supported by National Institute of Mental Health AIDS Center grant MH47680 (T.R.P.) and National Institutes of Health grants RR10712 (T.R.P.) and NS12428 (J.C.T.).
Footnotes
Publication 13322-NP from The Scripps Research Institute.
REFERENCES
- 1.Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Brain Res. 1995;90:45–53. doi: 10.1016/0165-3806(96)83485-7. [DOI] [PubMed] [Google Scholar]
- 2.Bautista J R, Schwartz G J, de la Torre J C, Moran T H, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste E N. Inflammatory cytokines within the central nervous system: sources, function, mechanism of action. Am J Physiol. 1992;236:C1–C16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste E N, Shrikant P, Patton H K, Benos D J. Neuroimmunologic mechanisms for disease in AIDS: the role of the astrocyte. In: Gendelman H E, Lipton S A, Epstein L, Swindells S, editors. The neurology of AIDS. New York, N.Y: Chapman and Hall; 1998. pp. 130–154. [Google Scholar]
- 4a.Billaud J N, Selway D, Yu N, Phillips T R. Replication of feline immunodeficiency virus in astrocytes is envelope dependent: implications for glutamate uptake. Virology. 2000;266:180–188. doi: 10.1006/viro.1999.0079. [DOI] [PubMed] [Google Scholar]
- 5.Bilzer T, Stitz L. Immunopathogenesis of virus diseases affecting the central nervous system. Crit Rev Immunol. 1996;16:145–222. doi: 10.1615/critrevimmunol.v16.i2.20. [DOI] [PubMed] [Google Scholar]
- 6.Bode L, Ferszt R, Czech G. Borna disease virus infection and affective disorders in man. Arch Virol Suppl. 1993;7:159–167. doi: 10.1007/978-3-7091-9300-6_13. [DOI] [PubMed] [Google Scholar]
- 7.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 9.Briese T, Schneemann A, Lewis A J, Park Y S, Kim S, Ludwig H, Lipkin W I. Genomic organization of Borna disease virus. Proc Natl Acad Sci USA. 1994;94:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone K M, Moench T R, Lipkin W I. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J Neuropathol Exp Neurol. 1991;50:205–214. doi: 10.1097/00005072-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Carbone K M, Park S W, Rubin S A, Waltrip II R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone K M, Rubin S A, Sierra-Honigmann A M, Lederman H M. Characterization of a glial cell line persistently infected with Borna disease virus (BDV): influence of neurotrophic factors on BDV protein and RNA expression. J Virol. 1993;67:1453–1460. doi: 10.1128/jvi.67.3.1453-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbone K M, Trapp B D, Griffin J W, Duchala C S, Narayan O. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J Neuropathol Exp Neurol. 1989;48:631–644. doi: 10.1097/00005072-198911000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Chao C C, Hu S, Ehrlich L, Peterson P K. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 15.Conant K, Major E O. Astrocytes as mediators of CNS injury in AIDS. In: Gendelman H E, Lipton S A, Epstein L, Swindells S, editors. The neurology of AIDS. New York, N.Y: Chapman and Hall; 1998. pp. 147–155. [Google Scholar]
- 16.Cubitt B, de la Torre J C. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J Virol. 1994;68:1371–1381. doi: 10.1128/jvi.68.3.1371-1381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cubitt B, Oldstone C, de la Torre J C. Sequence and genome organization of Borna disease virus. J Virol. 1994;68:1382–1396. doi: 10.1128/jvi.68.3.1382-1396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degiorgis M-P, Berg A-L, Hård af Segerstad C, Mörner T, Johansson M, Berg M. Borna disease in a free-ranging lynx (Lynx lynx) J Clin Microbiol. 2000;38:3087–3091. doi: 10.1128/jcm.38.8.3087-3091.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Torre J C. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J Virol. 1994;68:7669–7675. doi: 10.1128/jvi.68.12.7669-7675.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Torre J C, Gonzalez-Dunia D, Cubitt B, Mallory M, Mueller-Lantzsch M, Grasser F A, Hansen L A, Masliah E. Detection of Borna disease virus antigen and RNA in human autopsy brain samples from neuropsychiatric patients. Virology. 1996;223:272–282. doi: 10.1006/viro.1996.0479. [DOI] [PubMed] [Google Scholar]
- 21.de la Torre J C, Oldstone M B A. The anatomy of viral persistence: mechanisms of persistence and associated disease. Adv Virus Res. 1996;46:311–343. doi: 10.1016/s0065-3527(08)60075-5. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich D E, Schedlowski M, Bode L, Ludwig H, Emrich H M. A viro-psycho-immunological disease-model of a subtype affective disorder. Pharmacopsychiatry. 1998;31:77–82. doi: 10.1055/s-2007-979305. [DOI] [PubMed] [Google Scholar]
- 23.Di Monte D A, Tokar I, Langston J W. Impaired glutamate clearance as a consequence of energy failure caused by MPP(+) in astrocytic cultures. Toxicol Appl Pharmacol. 1999;158:296–302. doi: 10.1006/taap.1999.8717. [DOI] [PubMed] [Google Scholar]
- 24.Dittrich W, Bode L, Ludwig H, Kao M, Schneider K. Learning deficiencies in Borna disease virus-infected but clinically healthy rats. Biol Psychiatry. 1989;89:818–828. doi: 10.1016/0006-3223(89)90122-4. [DOI] [PubMed] [Google Scholar]
- 25.Dreyer E B, Kaiser P K, Offermann J T, Lipton S A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 26.Dreyer E B, Lipton S A. The coat protein gp120 of HIV-1 inhibits astrocyte uptake of excitatory amino acids via macrophage arachidonic acid. Eur J Neurosci. 1995;7:2502–2507. doi: 10.1111/j.1460-9568.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 27.Eddleston M, de la Torre J C, Xu J Y, Dorfman N, Notkins A, Zolla-Pazner S, Oldstone M B. Molecular mimicry accompanying HIV-1 infection: human monoclonal antibodies that bind to gp41 and to astrocytes. AIDS Res Hum Retrovir. 1993;93:939–944. doi: 10.1089/aid.1993.9.939. [DOI] [PubMed] [Google Scholar]
- 28.Eddleston M, Mucke L. Molecular profile of reactive astrocytes: implications for their role in neurological disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine S M, Angel R A, Perry S W, Epstein L G, Rothstein J D, Dewhurst S, Gelbard H A. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- 30.Fu Z F, Weihe E, Zheng Y M, Schafer M K-H, Sheng H, Corisdeo S, Rauscher III F J, Koprowski H, Dietzschold B. Differential effects of rabies and Borna disease viruses on immediate-early- and late-response gene expression in brain tissues. J Virol. 1993;67:6674–6681. doi: 10.1128/jvi.67.11.6674-6681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez-Dunia D, Cubitt B, Grässer F A, de la Torre J C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J Virol. 1997;71:3208–3218. doi: 10.1128/jvi.71.4.3208-3218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Dunia D, Sauder C, de la Torre J C. Borna disease virus and the brain. Brain Res. 1997;44:647–664. doi: 10.1016/S0361-9230(97)00276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez Dunia D, Watanabe M, Syan S, Mallory M, Masliah E, de la Torre J C. Synaptic pathology in Borna disease virus persistent infection. J Virol. 2000;74:3441–3448. doi: 10.1128/jvi.74.8.3441-3448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosztonyi G, Ludwig H. Borna disease—neuropathology and pathogenesis. In: Koprowski H, Lipkin W I, editors. Borna disease virus. Berlin, Germany: Springer-Verlag; 1995. pp. 39–74. [PubMed] [Google Scholar]
- 35.Gosztonyi G, Ludwig H. Borna disease of horses. An immunohistological and virological study of naturally infected animals. Acta Neuropathol. 1984;64:213–221. doi: 10.1007/BF00688111. [DOI] [PubMed] [Google Scholar]
- 36.Hatalski C G, Lewis A J, Lipkin W I. Borna disease. Emerg Infect Dis. 1997;3:129–135. doi: 10.3201/eid0302.970205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatten M E, et al. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- 38.Hertz L, Dringen R, Schousboe A, Robinson S R. Astrocytes: glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- 39.Herzog S, Rott R. Replication of Borna disease virus in cell cultures. Med Microbiol Immunol. 1980;168:153–158. doi: 10.1007/BF02122849. [DOI] [PubMed] [Google Scholar]
- 40.Hornig M, Weissenbock H, Horscroft N, Lipkin W I. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu S, Martella A, Anderson W R, Chao C C. Role of cytokines in lipopolysaccharide-induced functional and structural abnormalities of astrocytes. Glia. 1994;10:227–234. doi: 10.1002/glia.440100309. [DOI] [PubMed] [Google Scholar]
- 42.Lipkin W I, Carbone K M, Wilson M, Duchala C S, Narayan O, Oldstone M B A. Neurotransmitter abnormalities in Borna disease. Brain Res. 1988;475:366–370. doi: 10.1016/0006-8993(88)90627-0. [DOI] [PubMed] [Google Scholar]
- 43.Lipkin W I, Hatalski C G, Briese T. Neurobiology of Borna disease virus. J Neurovirol. 1997;3:S17–S20. [PubMed] [Google Scholar]
- 44.Lipton S A. HIV-related neuronal injury. Potential therapeutic intervention with calcium channel antagonists and NMDA antagonists. Mol Neurobiol. 1994;8:181–196. doi: 10.1007/BF02780669. [DOI] [PubMed] [Google Scholar]
- 45.Lipton S A. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci. 1992;15:75–79. doi: 10.1016/0166-2236(92)90013-x. [DOI] [PubMed] [Google Scholar]
- 46.Lipton S A, Rosenberg P A. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 47.Lipton S A, Sucher N J, Kaiser P K, Dreyer E B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig H, Bode L. Borna disease virus, new aspects on infection, disease, diagnosis and epidemiology. Rev Sci Tech Off Int Epizoot. 2000;19:259–288. doi: 10.20506/rst.19.1.1217. [DOI] [PubMed] [Google Scholar]
- 49.Lundgren A L, Czech G, Bode L, Ludwig H. Natural Borna disease in domestic animals others than horses and sheep. Zentbl Vetmed Reihe B. 1993;40:4. doi: 10.1111/j.1439-0450.1993.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 50.Lundgren A L, Johannisson A, Zimmermann W, Bode L, Rozell B, Muluneh A, Lindberg R, Ludwig H. Neurological disease and encephalitis in cats experimentally infected with Borna disease virus. Acta Neuropathol. 1997;93:391–401. doi: 10.1007/s004010050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lundgren A L, Lindberg R, Ludwig H, Gosztonyi G. Immunoreactivity of the central nervous system in cats with a Borna disease-like meningoencephalomyelitis (staggering disease) Acta Neuropathol. 1995;90:184–193. doi: 10.1007/BF00294319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundgren A L, Ludwig H. Clinically diseased cats with non-suppurative meningoencephalomyelitis have Borna disease virus-specific antibodies. Acta Vet Scand. 1993;34:101–103. doi: 10.1186/BF03548230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundgren A L, Zimmermann W, Bode L, Czech G, Gosztonyi G, Lindberg R, Ludwig H. Staggering disease in cats: isolation and characterization of the feline Borna disease virus. J Gen Virol. 1995;95:2215–2222. doi: 10.1099/0022-1317-76-9-2215. [DOI] [PubMed] [Google Scholar]
- 54.Magistretti P J, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy K D, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller L G, Fahey J M. Interleukin-1 modulates GABAergic and glutamatergic function in brain. Ann N Y Acad Sci. 1994;739:292–298. doi: 10.1111/j.1749-6632.1994.tb19831.x. [DOI] [PubMed] [Google Scholar]
- 57.Mucke L, Eddleston M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993;7:1226–1232. doi: 10.1096/fasebj.7.13.8405808. [DOI] [PubMed] [Google Scholar]
- 58.Narayan O, Herzog S, Frese K, Scheefers H, Rott R. Behavioral disease in rats caused by immunopathological responses to persistent Borna disease virus. Science. 1983;220:1401–1402. doi: 10.1126/science.6602380. [DOI] [PubMed] [Google Scholar]
- 59.Nowotny N, Weissenböck H. Description of feline nonsuppurative meningoencephalomyelitis (“staggering disease”) and studies of its etiology. J Clin Microbiol. 1995;33:1668–1669. doi: 10.1128/jcm.33.6.1668-1669.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plata-Salaman C R, Ilyin S E, Gayle D, Romanovitch A, Carbone K M. Persistent Borna disease virus infection of neonatal rats causes brain regional changes of mRNAs for cytokines, cytokine receptor components and neuropeptides. Brain Res Bull. 1999;49:441–451. doi: 10.1016/s0361-9230(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 61.Pletnikov M V, Rubin S A, Schwartz G J, Carbone K M, Moran T H. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res Dev Brain Res. 2000;119:179–185. doi: 10.1016/s0165-3806(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 62.Reeves N A, Helps C R, Gunn-Moore D A, Blundell C, Finnemore P L, Pearson G R, Harbour D. Natural Borna disease virus infection in cats in the United Kingdom. Vet Rec. 1998;143:523–526. doi: 10.1136/vr.143.19.523. [DOI] [PubMed] [Google Scholar]
- 63.Richt J A, Pfeuffer I, Christ M, Frese K, Bechter K, Herzog S. Borna disease virus infection in animals and humans. Emerg Infect Dis. 1997;3:343–352. doi: 10.3201/eid0303.970311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothstein J D, Dykes-Hoberg M, Pardo C A, Bristol L A, Jin L, Kuncl R W, Kanai Y, Hediger M A, Wang Y, Schielke J P, Welty D F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 65.Rott R, Becht H. Natural and experimental Borna disease in animals. In: Koprowski H, Lipkin W I, editors. Borna disease. Berlin, Germany: Springer; 1995. pp. 17–30. [DOI] [PubMed] [Google Scholar]
- 66.Sauder C, de la Torre J C. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J Neuroimmunol. 1999;96:29–45. doi: 10.1016/s0165-5728(98)00272-0. [DOI] [PubMed] [Google Scholar]
- 67.Savio T, Levi G. Neurotoxicity of HIV coat protein gp120, NMDA receptors, and protein kinase C: a study with rat cerebellar granule cell cultures. J Neurosci Res. 1993;34:265–272. doi: 10.1002/jnr.490340303. [DOI] [PubMed] [Google Scholar]
- 68.Schneemann A, Schneider P A, Lamb R A, Lipkin W I. The remarkable coding strategy of borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology. 1995;95:1–8. doi: 10.1006/viro.1995.1311. [DOI] [PubMed] [Google Scholar]
- 69.Schousboe A, Sonnewald U, Civenni G, Gegelashvili G. Role of astrocytes in glutamate homeostasis. Implications for excitotoxicity. Adv Exp Med Biol. 1997;429:195–206. doi: 10.1007/978-1-4757-9551-6_14. [DOI] [PubMed] [Google Scholar]
- 70.Sorg O, Horn T F, Yu N, Gruol D L, Bloom F E. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol Med. 1997;3:431–440. [PMC free article] [PubMed] [Google Scholar]
- 71.Stitz L, Dietzschold B, Carbone K M. Immunopathogenesis of Borna disease. In: Koprowski H, Lipkin W I, editors. Borna disease. Berlin, Germany: Springer; 1995. pp. 75–92. [DOI] [PubMed] [Google Scholar]
- 72.Tornatore C, Meyers K, Atwood W, Conant K, Major E. Temporal patterns of human immunodeficiency virus type 1 transcripts in human fetal astrocytes. J Virol. 1994;68:93–102. doi: 10.1128/jvi.68.1.93-102.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vesce S, Bezzi P, Rossi D, Meldolesi J, Volterra A. HIV-1 gp120 glycoprotein affects the astrocyte control of extracellular glutamate by both inhibiting the uptake and stimulating the release of the amino acid. FEBS Lett. 1997;411:107–109. doi: 10.1016/s0014-5793(97)00674-1. [DOI] [PubMed] [Google Scholar]
- 74.Volterra A, Trotti D, Cassutti P, Tromba C, Salvaggio A, Melcangi R C, Racagni G. High sensitivity of glutamate uptake to extracellular free arachidonic acid levels in rat cortical synaptosomes and astrocytes. J Neurochem. 1992;59:600–606. doi: 10.1111/j.1471-4159.1992.tb09411.x. [DOI] [PubMed] [Google Scholar]
- 75.Volterra A, Trotti D, Racagni G. Glutamate uptake is inhibited by arachidonic acid and oxygen radicals via two distinct and additive mechanisms. Mol Pharmacol. 1994;46:986–992. [PubMed] [Google Scholar]
- 76.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci. 1994;14:2924–2932. doi: 10.1523/JNEUROSCI.14-05-02924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye Z C, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. Neuroreport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- 78.Yu N, Billaud J N, Phillips T. Effects of feline immunodeficiency virus on astrocyte glutamate uptake: implications for lentivirus-induced central nervous system diseases. Proc Natl Acad Sci USA. 1998;95:2624–2629. doi: 10.1073/pnas.95.5.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu N, Martin J L, Stella N, Magistretti P J. Arachidonic acid stimulates glucose uptake in cerebral cortical astrocytes. Proc Natl Acad Sci USA. 1993;90:4042–4046. doi: 10.1073/pnas.90.9.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]