Abstract
Four groups of cats, each containing four animals, were immunized at 0, 3, and 6 weeks with minimalistic immunogenic defined gene expression vector (MIDGE) vaccines containing the gene(s) for feline immunodeficiency virus (FIV) gp140, FIV gp140 and feline interleukin-12 (IL-12), FIV gp140 and feline IL-16, or FIV gp140 and a CpG motif. MIDGEs were coated onto gold beads and injected intradermally with a gene gun. A fifth group of four cats were immunized in an identical manner but with blank gold beads. All cats were challenge exposed to virulent FIV 4 weeks following the final immunization, and the course of infection was monitored. The two groups of cats immunized with the FIV gp140 gene alone or with blank gold particles became highly viremic and seroconverted as early as 4 weeks after infection. In contrast, three of four cats immunized with FIV gp140 in combination with feline IL-12 failed to become viremic or seropositive, as has been shown elsewhere (F. S. Boretti, C. M. Leutenegger, C. Mislin, et al., AIDS 14:1749–1757, 2000). Here we show the effect of IL-12 when used as an adjuvant on the viral RNA and DNA load and on the cytokine profile. In addition, the two groups of cats immunized either with gp140 and IL-16 or with gp140 and the CpG had greatly reduced viremia. Protection correlated weakly with cytotoxic T-lymphocyte activity and increased cytokine transcription of IL-12, gamma interferon, and IL-10 by peripheral blood mononuclear cells in the postchallenge period. This study extends the data on IL-12 and provides new results on CpG motifs and IL-16 used as adjuvants in the FIV cat model.
The type of immunity required to prevent infections with lentiviruses such as human immunodeficiency virus (HIV) is unknown. However, cellular immunity seems most important, based on a number of observations. Cytotoxic T lymphocytes (CTLs) play a pivotal role in controlling HIV infection in seronegative infants born to seropositive mothers (38, 61), individuals who have been repeatedly exposed but remain uninfected (5, 6, 62), and people with long-term asymptomatic HIV infections (9, 10, 70). A decline in CTL activity has a close temporal association with progression to AIDS, and studies with whole inactivated virus (31) or recombinant vaccinia virus vaccines (27) have shown an inverse correlation between CTL precursor frequencies and virus load after challenge. In addition to cytotoxic effects, CD8+ T cells downregulate HIV replication through the production of chemokines and other soluble factors, with negative correlation to disease progression (2, 7, 56).
Given the failure of conventional vaccine strategies, unconventional approaches to lentivirus immunization are being tested. Although these unconventional approaches can be directed at many aspects of the immune response, it is logical to concentrate on the earliest events in immunity, because it is these events that appear to be subverted in lentivirus immunity. The earliest antiviral defenses are innate, and these innate responses provide the scaffolding on which the subsequent adaptive immune response is built. Dendritic cells (DCs) play an important bridging role between innate and adaptive immunity. DCs interact with antigen and, once activated, present this antigen in concert with chemical signals to the cells of the adaptive immune system. DCs are important producers of interleukin-12 (IL-12), a cytokine intimately involved in the promotion and regulation of many forms of antiviral immune responses (32, 72). There is evidence of abnormal IL-12 activity in HIV infection. Cells from HIV-infected patients show a marked deficiency in their ability to produce both the p40 subunit and the p70 bioactive form of IL-12. Exogenous IL-12 supplementation in vitro restores T-cell functions, including T-cell proliferation, T-cell and natural killer (NK) cell production of IL-2 and gamma interferon (IFN-γ), and CTL and NK lytic activities (13, 15, 57). Such a defect in IL-12 production has also been described in cats infected with feline immunodeficiency virus (FIV) (53), and the use of IL-12 as an adjuvant against FIV was described recently by our laboratory (8).
IL-16 is another cytokine that may play a role in the early events in immunity and could be incorporated into a lentivirus vaccine. This CD4 chemotactic cytokine is secreted by CD8+ T lymphocytes, and the C-terminal 130-amino-acid portion will suppress HIV-1, simian immunodeficiency virus (SIV), and FIV replication in vitro (3, 47, 51, 74). IL-16 is also involved in cell-to-cell signaling between DCs and T cells (39).
CpG motifs are a new class of DNA adjuvants. They are comprised of unmethylated dinucleotides flanked by two 5′ purines and two 3′ pyrimidines. CpG DNA directly activates monocytes, macrophages, and DCs to secrete IL-12 and IFN-γ. The utility of CpGs as potent vaccine adjuvants has been demonstrated for a variety of antigens (18, 71).
DNA immunization has shown promise in several viral and nonviral infections (69). DNA vaccines also lend themselves to strategies involving codelivery of specific antigens and molecular adjuvants such as cytokines (42, 66, 65) or unmethylated CpG oligonucleotide motifs (45, 67, 25). Minimalistic immunogenic defined gene expression vectors (MIDGEs) are linear DNA constructs containing only a cytomegalovirus (CMV) promotor, a gene of interest, and a simian virus 40 (SV40) polyadenylation site but lacking an antibiotic resistance gene and are highly effective vehicles for in vivo DNA transfection. Using a gene gun, MIDGE-coated gold particles can be injected into the dermis, where DCs abound. It was found that DCs, once transfected by cutaneous genetic immunization with naked DNA, migrate into the draining lymph nodes (16). In addition, intradermal injections by gene gun seem to require 100- to 1,000-fold less DNA than the intramuscular route for induction of immune responses to target antigens (26).
The present studies were based on the concept of altering initial immune responses by delivering the antigen in DNA form directly to dermal DCs in the optimal manner and attempting to favorably alter initial events in DC processing and cellular signaling by coexpressing several relevant immunomodulatory genes. The FIV infection model in laboratory cats was used to test the approach. FIV and SIV are related lentiviruses that have identical modes of pathogenesis (19). The results obtained from these pilot experiments suggest that lentiviral immunity elicited with DNA vaccines can indeed be modulated with immunomodulators such as feline IL-12 (8) and IL-16, and with unmethylated CpG motifs, to achieve a spectrum of protection ranging from prevention of infection to transiently decreased virus loads.
MATERIALS AND METHODS
Animals, animal care, and experimental design.
Specific-pathogen-free cats 3 months of age were purchased from IFFA Crédo, Lyon, France. All animals were confirmed to be antibody and PCR negative for FIV and antibody and p27 negative for feline leukemia virus at the start of the experiment. Cats were housed in groups in a climatized animal facility, allowed to adapt to the new environment for 1 month, and assigned randomly to experimental groups.
The trial consisted of four vaccine and one mock vaccine groups with four cats each (Table 1). The DNA inoculation schedule consisted of three immunizations at 0, 3, and 6 weeks, with challenge exposure 4 weeks after the last immunization.
TABLE 1.
Summary of DNAs administered to different groups
| Group no. | No. of animals | DNA(s) | Dose per construct (ng) | Total amount of DNA (μg)/ immunization |
|---|---|---|---|---|
| 1 | 4 | Gold alone—control | ||
| 2 | 4 | gp140—control | 350 | 1.4 |
| 3 | 4 | gp140 + IL-12 | 350 | 4.2 |
| 4 | 4 | gp140 + IL-16 | 350 | 2.8 |
| 5 | 4 | gp140 + CpGs | 350 | 2.8 |
Clinical evaluation and hematology.
Cats were observed daily for gross signs of illness, and a more detailed physical examination was conducted weekly. EDTA-treated blood samples were collected periodically and at the time of each immunization for complete blood counts. Hematology parameters were evaluated in EDTA blood with an automatic electronic cell counter (CD3500; Abbott Diagnostics, Baar, Switzerland). Feline CD4+ cells and CD8+ cells were stained with labeled monoclonal antibodies Fel7 and FT2, respectively, using a modified whole-blood lysis technique (33).
DNA vaccine vectors.
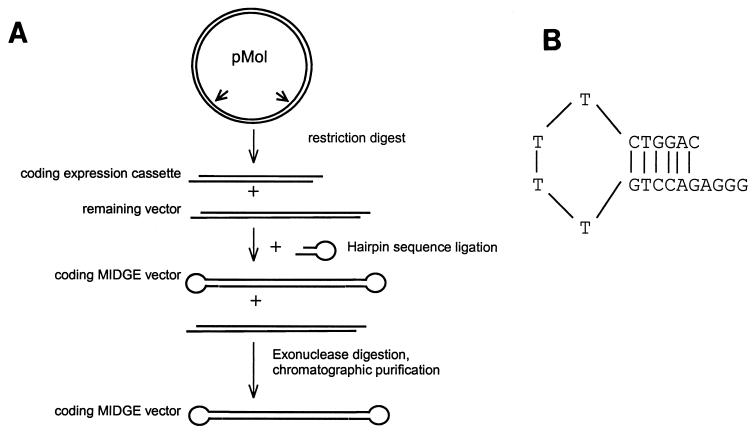
Single-stranded hairpin 5′-phosphorylated oligodesoxy-ribonucleotides (ODN) of sequence GTTCTTCGGG GCGTTCTTTT TTAAGAACGC CCC and GAAGAACGTT TTCCAATGAT TTTTCATTGG AAAAC were purchased from TIBMolBiol (Berlin) and ligated with T4 DNA ligase (MBI Fermentas; Leon Roth). After digestion of remaining product and chromatographic purification, the ODN were concentrated by precipitation in ethanol and sodium-magnesium acetate and resuspended in phosphate-buffered saline. MIDGEs (Mologen GmbH, Berlin, Germany) were synthesized as outlined in Fig. 1A. FIV-Z2 gp140 (encoding the entire SU and the extracellular part of TM), feline IL-16 (50), feline IL-12 p35 and p40 subunits (20), and the CpG motif were included in MIDGEs: the genes were cloned into the SstI and KpnI sites of plasmid pMol, a pUC19 derivative containing the CMV immediate-early promoter and the SV40 poly(A) site (Mologen, Berlin, Germany). The expression cassette consisting of the CMV promoter, the gene of interest, and an SV40 polyadenylation site was excised from the pMol vector by incubation with EcoRI (250 U/mg, 37°C, overnight). A 20-fold excess of a self-complementary oligodesoxynucleic acid sequence (5′-phosphorylated hairpin 5′AGGGGTCCAG TTTTCTGGAC3′; Metabion, Martinsried, Germany) (Fig. 1B) was added to the resulting solution and incubated with T4 ligase (MBI Fermentas, Vilnius, Lithuania) at 25 U/mg and 37°C overnight. Remaining plasmid and nonligated products were digested with HindIII (500 U/mg) and T7 polymerase (150 U/mg) at 37°C overnight. The resulting crude MIDGEs were purified by chromatography on an anionic exchange column (Merck EMD-DMAE); with sodium phosphate (pH 7.0)–0.1 M NaCl and obtained free of contamination by vector backbone, as verified by PCR.
FIG. 1.
(A) Synthesis of MIDGEs for gene expression. (B) Structure of the thymidine bridges of MIDGE constructs formed by self-complementary hairpin oligodesoxynucleic acid sequence separated by four thymidines.
Preparation of gold particles for immunization.
Cartridges containing gold particles coated with the MIDGEs were prepared according to the manufacturer's recommendations. Gold microcarriers coated with MIDGE vectors were prepared by resuspending the gold beads in 100 μl of 1 M spermidine. The suspension was briefly sonicated before adding MIDGE vector DNA. Two hundred microliters of CaCl2 was added to the DNA solution in a Vortex apparatus to precipitate the DNA onto the gold beads. The precipitate was allowed to settle for 5 min, washed three times with absolute ethanol, and resuspended in 4 ml of ethanol. The DNA-coated gold was then coated on the inner wall of Tefzel tubing. The DNA-gold particles were drawn into nitrogen-predried tubing and allowed to settle for 3 min. The ethanol was then drawn out, and the tubing was rotated for 30 s before drying the residual ethanol by a steady stream of nitrogen for 5 min with rotation. The tubing was cut into 12-mm cartridges (350 ng of DNA per construct per cartridge), which were stored at room temperature in a desiccated container until use.
Immunization procedure.
Prior to the immunizations, the fur of anesthetized cats was shaved from the lateral side of the musculus gastrocnemius, and the DNA-coated gold particles were injected intradermally at the site using the Helios gene gun (Bio-Rad, Munich, Germany) at a pressure of 500 lb/in2. Deposition of the gold particles in the epidermis was confirmed at levels greater than 95% by histological examination of punch biopsies of skin taken from the site of inoculation. The total amount of DNA injected at each immunization is given in Table 1. All animals received three immunizations at weeks 0, 3, and 6. The mock vaccine consisted of uncoated gold particles. Immunized cats showed transient reddening and hyperpigmentation of the skin, approximately 8 mm in diameter, at the site of immunization. These lesions had resolved by the time of challenge exposure, 4 weeks after the last inoculation.
Virus stock and challenge exposure.
FIV-Zurich 2 (FIV-Z2) was isolated in primary peripheral blood mononuclear cell (PBMC) cultures from the blood of a naturally infected cat showing clinical signs of disease (59). A low-in-vitro-passaged tissue culture fluid stock was created and stored at −70°C, and an aliquot was titrated in vitro for in vivo infectivity studies. Cats were challenge exposed intraperitoneally with 25 50% tissue culture infective doses, corresponding to 20 50% cat infectious doses (CID50) of this virus.
Quantitative real-time TaqMan PCR for quantitation of provirus DNA and RNA in blood.
Quantitation of FIV provirus DNA (48) and viral RNA was done using real-time PCR (TaqMan; Applied Biosystems, Foster City, Calif.). The oligonucleotide sequences for the forward primer (FIV551f) was 5′GCCTTCTCTGCAAATTTAACACCT3′, while the sequence for the reverse primer (FIV671r) was 5′GATCATATTCTGCTGTCAATTGCTTT3′. The sequence for the dual-labeled (FAM - TAMRA) probe (FIV581p) was 5′TGCGGCCATTATTAATGTGGCCATG3′. Analysis was performed on an ABI Prism 7700 sequence detector (Applied Biosystems). A cloned gag gene of FIV-Z2 was used as a standard for the TaqMan PCR. The assay could detect as few as 10 copies of a cloned FIV gag gene or of reverse-transcribed RNA from the same plasmid. Genomic DNA and viral RNA were extracted with Qiagen columns according to the manufacturer's recommendations.
Anti-TM antibody ELISA and Western blot.
Antibodies against a recombinant FIV transmembrane (TM) antigen were measured with an enzyme-linked immunosorbent assay (ELISA) under conditions described elsewhere (12). A pool of high-titered serum collected from an FIV-Z2-infected cat served as the positive control. Western blots were performed under conditions described (55) using 500 ng of gradient-purified FIV-Z2 per cellulose acetate strip.
In vitro testing of vectors for gene expression.
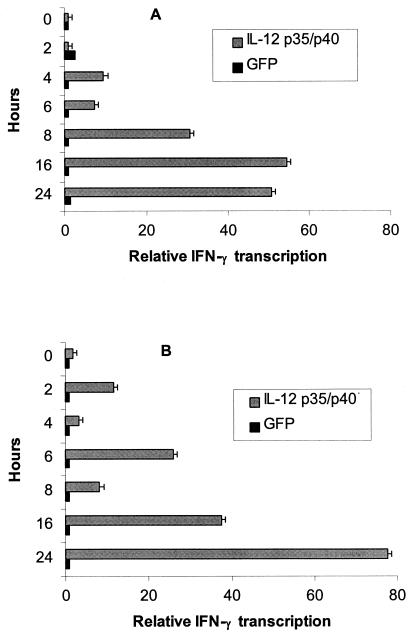
In vitro expression of biologically active IL-12 from MIDGEs expressing feline genes for the p35 and p40 chains was tested in the feline 3201 T-cell line. The two purified DNAs were cotransfected into 3201 cells with a modified PDS-1000/He ballistic device (Bio-Rad, Munich, Germany) and by electroporation. One million cells were harvested at 0, 2, 4, 6, 8, 16, and 24 h posttransfection and lysed in Qiagen lysis buffer (RNeasy blood kit; Qiagen, Basel, Switzerland). Total RNA was extracted according to the manufacturer's recommendations. Synthesis of cDNA and IFN-γ real-time TaqMan PCR were performed as described below and in reference 52. The expression of biologically active IL-12 was verified by the induction of IFN-γ transcription in transformed 3201 cells (Fig. 2). These results confirmed that both ballistomagnetic transfer and electroporation induced a significant increase in IFN-γ transcription after 16 and 24 h.
FIG. 2.
IFN-γ transcription as measured by real-time TaqMan PCR in transiently transformed feline 3201 cells by (A) electroporation and (B) balistomagnetic transfer using a modified PDS-1000/He apparatus (Bio-Rad). IFN-γ transcription was normalized against GAPDH signals. Time point zero served as the calibrator.
CpG motif activity was tested on feline spleen cells by induction of IL-12 p40 mRNA. Briefly, 5 million spleen cells were cultured with 10 μg of the CpG-MIDGEs and cultured for 18 h. Cells were harvested and tested for IL-12 p40 mRNA upregulation in comparison to control MIDGEs. Cytokine mRNA quantitation was done with quantitative real-time PCR as described below. Upregulation of IL-12-p40 mRNA verified activity of the CpG ODN in the cat.
CMV-driven IL-16 transcription was tested using the gene encoding the 130-amino-acid, biologically active IL-16 cloned behind the CMV promotor of a pCI vector designated pCI/fIL-16 (Promega, Dubendorf, Switzerland). Feline 3201 cells were transfected using polyamidoamine dendrimers according to the manufacturer's instructions (SuperFect; Qiagen, Basel, Switzerland). Upregulation of IL-16 mRNA transcripts was verified using real-time TaqMan PCR and compared to an empty pCI as described below.
Cytokine quantitation with real-time TaqMan PCR.
mRNA transcripts for feline glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the cytokines IL-4, IL-10, IL-12 p35, IL-12 p40, and IFN-γ were determined as previously described (52). Five million Ficoll Hypaque (Pharmacia, Dubendorf, Switzerland)-purified PBMCs were lysed in lysis buffer (RNeasy blood kit; Qiagen), and total RNA was extracted. Synthesis of cDNA was made from 1 to 5 μg of total RNA in a 20-μl volume using an avian myeloblastosis virus reverse transcriptase (RT) system (Promega). Eighty microliters of water was then added, and the sample was stored at −20°C until TaqMan analysis.
Ten microliters of the cDNA was subjected to real-time PCR. Calculation of cytokine transcription was quantitated from the cycle threshold (CT) values of the real-time PCR. GAPDH was used as a recorder gene to normalize the cytokine signals and for calibration. Two different types of calibrators were used: the preimmune cytokine levels of each cat, and the lowest cytokine transcription within every time point. Unless stated differently, both methods gave similar results. Cytokine gene transcription levels were expressed as an n-fold difference relative to the calibrator.
Detection of FIV-specific CTL.
Lymphocytes were collected at the time of the second vaccination, on the day of challenge, and 14 weeks postchallenge. These cells were assayed directly for CTL activity as described (21). Target cells were cultured autologous skin fibroblasts derived from biopsy material collected prior to immunization from individual cats. The target cells were infected with recombinant vaccinia viruses expressing FIV Gag or Env or wild-type vaccinia virus as a control and labeled with 51Cr. Effector cells were added at an effector-to-target (E:T) cell ratio of 20:1, and lytic activity was measured by monitoring isotype release as described previously (23).
Statistical analysis.
Statistical analysis of the data was performed with the Excel and GraphPad Prism software package version 2.0. Parameters of different vaccine groups were analyzed for statistical differences using the Mann-Whitney U test (PMWU). Changes between different time points within one group were analyzed with the Wilcoxon test (PW). Differences were considered significant if P was <0.05.
RESULTS
Gross signs of FIV infection following challenge exposure.
FIV challenge-exposed cats demonstrated no significant lymphadenopathy or fever. It has previously been reported that the FIV-Z2 strain produces only a mild acute disease, and signs of immunodeficiency occur only after several years (33).
Proviral DNA in PBMCs following challenge exposure.
The two groups of cats given either gold particles alone or FIV gp140 DNA had detectable proviral DNA in PBMCs from week 4 onward (Table 2). One of four cats in the group given FIV gp140/IL-12 DNA tested positive for proviral DNA only at week 2 postinfection. The remaining three cats were negative for PBMC-associated FIV DNA by PCR at all time points tested. One of four cats from the group given FIV gp140/IL-16 DNA was positive at weeks 4 and 6 but negative thereafter. The remaining three cats tested positive starting at week 4. All four cats that were immunized with FIV gp140/CpG DNA were positive from week 4 onward.
TABLE 2.
Qualitative provirus and viral RNA TaqMan PCR results
| Vaccine group | Cat no. |
CT valuea at wk after challenge infection:
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
2
|
3
|
4
|
5
|
6
|
8
|
10
|
12
|
14
|
16
|
|||||||||||||
| RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | RNA | DNA | ||
| Gold alone | 1 | − | − | − | − | − | − | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + |
| 2 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| 3 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| 4 | − | − | − | − | − | − | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| gp140 | 1 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + |
| 2 | − | − | − | − | − | + | − | + | + | − | + | + | − | + | − | + | − | + | − | + | − | + | |
| 3 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| 4 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| gp140/IL-12 | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 4 | − | − | − | − | − | + | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| gp140/IL-16 | 1 | − | − | − | − | − | − | − | + | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| 3 | − | − | − | − | − | − | − | + | − | − | − | + | − | + | − | + | − | + | − | − | − | − | |
| 4 | − | − | − | − | − | − | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| gp140/CpG | 1 | − | − | − | − | − | − | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + |
| 2 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| 3 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
| 4 | − | − | − | − | − | − | − | + | + | + | + | + | − | + | − | + | − | + | − | + | − | + | |
CT value of real-time PCR was <40 (−) or 40 (+).
Quantitation of proviral DNA in challenge-exposed cats.
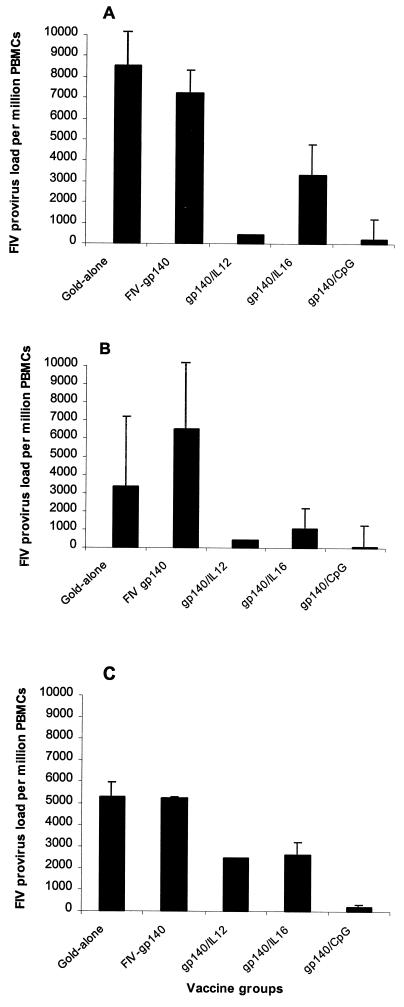
Peak copy numbers of proviral DNA occurred around weeks 4 to 6 following challenge exposure. Cats vaccinated with blank gold particles or MIDGEs with FIV gp140 alone had the highest copy number of proviral DNA. Cats immunized with MIDGEs containing FIV gp140/IL-12, FIV gp140/IL-16, or FIV gp140/CpG had 2 to 330 times less FIV provirus in their PBMCs than the former, with four animals consistently testing negative (Fig. 3). All groups of cats vaccinated with FIV gp140 with IL-12, IL-16, or CpG were significantly less viremic than cats given FIV gp140 DNA alone or blank gold particles (PMWU < 0.05; Fig. 3). Among the four groups that became uniformly viremic, cats immunized with FIV gp140/CpG DNA showed the lowest level of PBMC-associated FIV DNA, ranging between 37- and 330-fold lower level of FIV provirus compared to the FIV gp140-alone-immunized cats.
FIG. 3.
Provirus DNA loads as determined with real-time TaqMan PCR on genomic DNA extracted from Ficoll-purified PBMCs at weeks 6 (A), 8 (B), and 12 (C) after challenge infection. The data are expressed as the means ± standard error (SE) for four cats per group.
FIV RNA levels in plasma following challenge exposure.
The duration of plasma-associated viremia (i.e., viral RNA) was very short in all cats. The peak in plasma viral RNA occurred in the sixth week postinfection, and four of four of the FIV gp140 DNA and two of four of the blank gold particle groups were positive (Table 2). This brief plasma-associated viremia was identical to that found in earlier studies with the same challenge stock of FIV-Z2 (17). All cats were consistently negative for plasma viral RNA in the real-time RT-PCR assay after week 7 postinfection. The three provirus-negative cats in the FIV gp140/IL-12 group were consistently negative for FIV viral RNA. Viral RNA could not be detected at any time point in all four cats of the FIV gp140/IL16 group. Among the four cats in the FIV gp140/CpG group which showed the lowest levels of FIV provirus, three tested positive for FIV viral RNA at weeks 5 and 6 postchallenge.
CD4 and CD8 cell numbers following challenge exposure.
After challenge of all cats with FIV-Z2, no significant differences were found in hematological parameters and lymphocyte subsets between the different vaccine groups and/or the control group (data not shown). Similar results were found previously in the early phase after experimental infection with FIV-Z2 (49).
Humoral and cellular immunity following DNA immunization and challenge exposure.
FIV-specific antibodies were not detected postimmunization and prior to challenge exposure by ELISA (TM peptide) or by Western blot analysis against purified FIV-Z2 (Table 3). Previous studies have demonstrated both of these assays to be highly sensitive for FIV-specific antibodies in naturally and experimentally infected cats (24, 49, 64). The Western blot results and antibodies against TM after challenge correlated closely. By week 6 postchallenge, seven of eight control cats and all cats in the gp140/IL-16 and gp140/CpG groups showed evidence of infection, as indicated by seroconversion against the TM peptide. Three of four cats in the gp140/IL-12 group were consistently negative for antibodies against the TM peptide and in Western blots. One of four cats in the gp140/IL-16 group tested positive once at week 8 and then repeatedly negative for TM antibodies.
TABLE 3.
Antibody responses to FIV TM peptide (TM) and reactivity of plasma samples by Western blotting (WB) on the day of challenge and at intervals following challenge
| Vaccine group | Cat no. | Response at wk postchallenge:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0
|
4
|
5, TM | 6, TM | 7, TM | 8
|
10, TM | 12
|
14, TM | 16
|
|||||||
| WB | TM | WB | TM | WB | TM | WB | TM | WB | TM | |||||||
| Gold alone | 1 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| 2 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | |
| 3 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | |
| 4 | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | |
| gp140 | 1 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | |
| 3 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
| 4 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | |
| gp140 + IL-12 | 1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 3 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| 4 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | |
| gp140 + IL-16 | 1 | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − |
| 2 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
| 3 | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | |
| 4 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
| gp140 + CpG | 1 | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + |
| 2 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
| 3 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
| 4 | − | − | − | − | − | + | + | + | + | + | + | + | + | + | + | |
No FIV-specific CTL activity was observed postimmunization in cats receiving FIV gp140 DNA alone or blank gold particles. FIV Env-specific effector CTL responses were detected in the PBMCs of two of four cats immunized with FIV gp140/IL-12 at the time of the second vaccination (26.1 and 6% lysis). This activity was short-lived, as it was undetectable by the day of challenge. No significant CTL activities were observed after challenge. Low-level Env-specific CTL activity was detected in one FIV gp140-inoculated cat on the day of challenge (5.2% at an E:T ratio of 20:1). Following challenge, Env- and Gag-specific CTL activity was detected in this animal (7.8 and 13.8% lysis, respectively).
Cytokine responses in vaccinated cats.
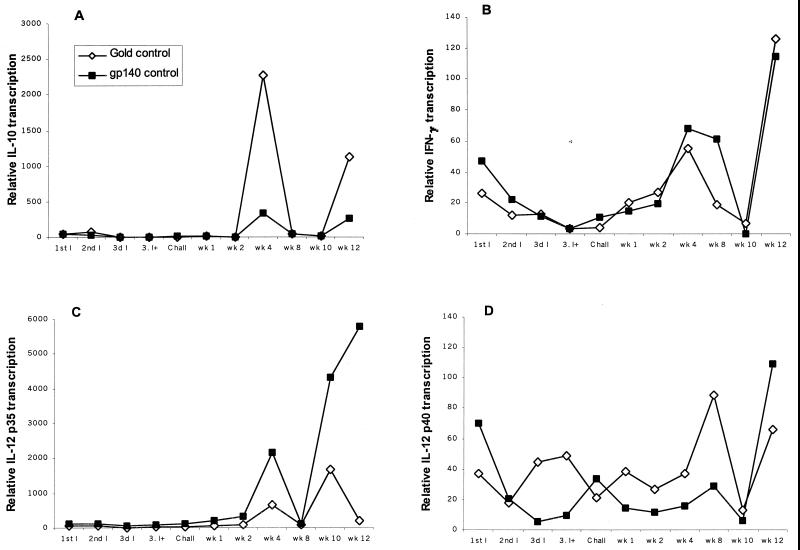
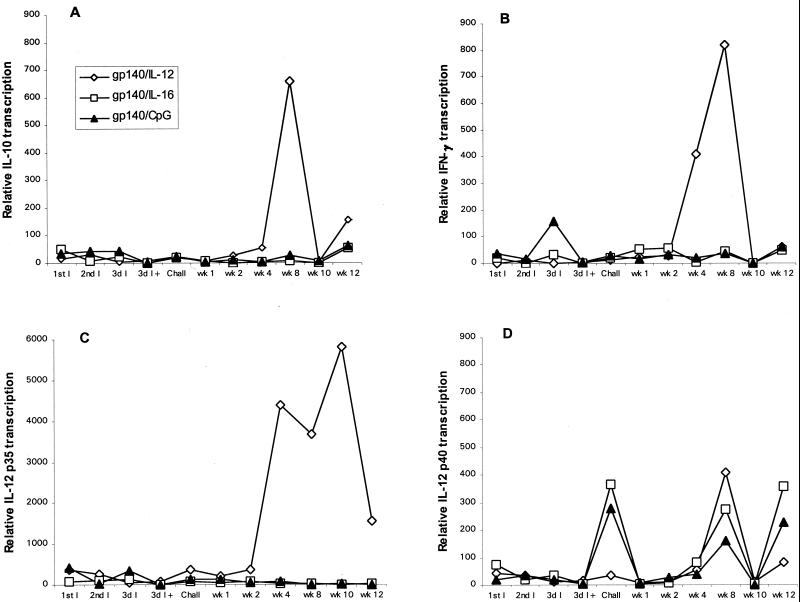
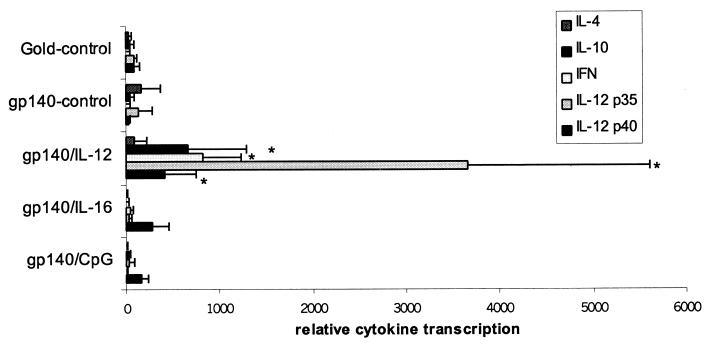
Cats that were mock immunized or given FIV gp140 DNA developed similar cytokine profiles in their PBMCs (Fig. 4). There was increased transcription of IL-10 at week 4 and increased IFN-γ transcription at weeks 4 and 8. Increased IL-12 p35 and IL-12 p40 transcription was observed at weeks 8 and 10. IL-4 transcripts were detected at low levels at early time points and increased modestly at week 8 (data not shown). FIV gp140/IL-16-immunized cats had increased IL-12 p40 transcription at the time of challenge exposure and at weeks 8 and 12 postinfection. Compared to cats given blank gold particles or FIV gp140 DNA, all other cytokine transcripts were depressed. Cats in the FIV gp140/CpG group showed increased IFN-γ transcription following the second immunization. There was increased transcription of IL-12 p40 at the time of challenge and at weeks 8 and 12 postinfection, while transcription of IL-10, IL-4, and IL-12 p35 was depressed but still detectable at low levels. The FIV gp140/IL-12 DNA immunization group exhibited similar cytokine profiles as the groups immunized with blank gold particles or FIV gp140 but with elevated transcription levels (Fig. 5). IL-12 p35 and p40, IL-10, and IFN-γ were elevated at week 8 by factors of 7, 17, 21, and 36, respectively (all PMWU < 0.05; Fig. 6). IL-4 transcripts in cats of the FIV gp140/IL-12 group were elevated at week 8 to the same extent as detected in cats of the control groups.
FIG. 4.
Cytokine transcription in PBMCs of cats in the two control groups (◊, gold-immunized cats; ■, gp140-immunized cats). Transcription of (A) IL-10, (B) IFN-γ, (C) IL-12 p35, and (D) IL-12 p40 was measured with quantitative real-time TaqMan PCR. PBMCs were collected at each date of immunization, at challenge, and at weeks 2, 4, 8, 10, and 12. Each cytokine signal is normalized against the GAPDH signal and then calibrated against the preimmune normalized cytokine signal. Bars represent mean normalized cytokine signals for four cats per control group. For standard deviations at week 8, see Fig. 6. I, immunization; 3d I+, third immunization plus 2 days; wk, week.
FIG. 5.
Cytokine transcription in PBMCs of cats vaccinated with gp140/IL-12, gp140/IL-16, or gp140/CpG motif. Transcription of (A) IL-10, (B) IFN-γ, (C) IL-12 p35, and (D) IL-12 p40 was measured with quantitative real-time TaqMan PCR. PBMCs were collected at each date of immunization, at challenge, and at weeks 2, 4, 8, 10, and 12. Each cytokine signal is normalized against the GAPDH signal and then calibrated against the preimmune normalized cytokine signal. Bars represent means of normalized cytokine signals for four cats per vaccine group. For standard deviations at week 8, see Fig. 6. For abbreviations, see the legend to Fig. 4.
FIG. 6.
Transcription of cytokines at week 8 after challenge infection. Each cytokine signal is normalized against the GAPDH signal and then calibrated against the lowest normalized cytokine signal at week 8. Bars represent means ± SE of normalized cytokine signals for four cats per vaccine group and control group. ∗, results for cats from the gp140/IL-12 group significantly different from results for control cats (PMWU < 0.05).
DISCUSSION
DNA immunization targeted at dermal DCs was protective in cats immunized with FIV gp140 DNA coexpressed with IL-12, IL-16, or CpG. The most dramatic effect was observed with FIV gp140 DNA coexpressed with feline IL-12 p35/p40 DNAs (8). Three intradermal inoculations of FIV gp140/IL-12 DNAs elicited complete protection in three of four cats. Protection in all groups was observed in the absence of a detectable virus-specific humoral immune response and only weakly correlated with cellular immunity. Cats vaccinated with FIV gp140/IL-12 DNAs had demonstrable CTL activity during the immunizations, suggesting an adjuvant effect of feline IL-12 (8). However, the responses were short-lived, could not be improved with the third DNA immunization, and had waned by the time of challenge infection. As no IL-12 control group was included in the study design, it is unclear how IL-12 alone would affect the immune status of the animals. However, other studies clearly show no evidence of a nonspecific adjuvant effect when IL-12 was used without antigen in different animal models (37, 54). Increased IL-12 (p40 and p35 subunits), IFN-γ, and IL-10 mRNA transcription in PBMCs was detected only after challenge infection in cats of the FIV gp140/IL-12 group and at a time when CTL activity was undetectable, suggesting a gp140/IL-12-specific cytokine response in these cats.
The poor correlation between CTL activity and protection in the three cats described above was both supportive and contrary to the literature on FIV vaccines. Flynn and colleagues induced CTL activity with synthetic peptide vaccines from the Gag protein, but cats were not protected from challenge (21). Similarly, cats immunized with a peptide derived from the V3 domain of Env developed both Env-specific CTLs and antibodies recognizing Env by ELISA but were not protected (22). However, Yamamoto and colleagues (68) have shown that major histocompatibility complex-restricted protection against FIV infection can be conferred to naive cats by adoptive transfer of lymphocytes from vaccinated cats. Whole inactivated FIV vaccines indicate a role for both cellular and humoral mechanisms in immune protection (34). Recent studies with replication-defective FIV proviral DNA have demonstrated protection (35), but in contrast to whole inactivated FIV vaccines, which induced high levels of virus-specific cytotoxicity and neutralization, the defective viral genomes induced immunity in the absence of a virus-specific humoral immune response. Coadministration of the feline IFN-γ gene as an immunomodulator increased CTL activities (35), although both the virus-specific and nonspecific CD8+ T-cell-mediated responses were enhanced. Favorable immune responses may also be facilitated by CD8 T-cell-derived antiviral factors, which have been partially characterized in FIV infection (11, 14, 51, 63).
The bias toward CTL induction rather than antibody production in cats immunized with FIV gp140 and IL-12 DNAs could have been a property of the intradermal localization of the DNA or of the coexpression of IL-12 and FIV gp140 or both. IL-12 influences the differentiation of precursor Th cells to the Th1 phenotype, which has been exploited in a number of immunizing models: protection against leishmaniasis can be evoked in highly susceptible mice with parasite extracts given in combination with IL-12 (1). The effective use of IL-12 as an adjuvant has been demonstrated with several other pathogens, inducing protection against tuberculosis (54), herpes simplex virus type 2 in a mouse model (65), Coccidioides immitis in mice (37), cutaneous leishmaniasis in rhesus monkeys (41), and tumor antigens (58). Recently an SIV-HIV chimera with inserted IL-12 subunits was shown to produce biologically active IL-12. These IL-12-producing chimeras are considered candidates for a live attenuated vaccine to induce effective cellular immunity against HIV-1 (46).
The advantage of using IL-12 DNA as an adjuvant is that the actual cytokine dose required for an effect is rather low, avoiding possible side effects due to prolonged treatment. Compartmentalized expression, which was done in this study, may enhance the adjuvant activity of IL-12 and minimize undesirable systemic toxicity (28). Compartmentalization of the IL-12 effect was evident in the present study; IL-12 and IFN-γ mRNA transcription was not observed in peripheral blood cells during the period of intradermal injections in cats of the FIV gp140/IL-12 group. The intradermal route of DNA immunization may also have been important. Injections of DNA-coated gold particles into the epidermal skin layer bring the vaccine formulations in more direct contact with DCs, which are thought to be involved in the process of antigen presentation and in the development of the primary and secondary immune response (4, 29, 36, 43).
The findings reported herein demonstrate that IL-16 DNA, when injected intradermally with FIV gp140 DNA, helped to mediate partial resistance to FIV infection. It is unclear whether this effect was due to the antiviral or immunomodulatory activity of IL-16, and there is evidence for both in the literature. Human CD4 cells transfected with IL-16 cDNA in vitro were resistant to HIV-1 infection (73, 74). However, the supernatant from these cultures also contained strong chemotactic activity for T cells and monocytes. It was shown previously that exogenous IL-16 inhibits the replication of HIV, SIV, and FIV (3, 47, 51). The antilentivirus activity of IL-16 appears to be multifaceted. In addition to its exogenous effect on CD4 receptors, IL-16 may act intracellularly on lentivirus replication via an internal motif involved in specific intracellular protein-protein interactions (40, 60). This motif is conserved in the feline IL-16 cDNA (51).
Immunomodulation within the very early phase of immune induction with IL-16 and CpG motifs appeared to induce partial protection, as reflected by reduced virus loads. Interestingly, the type of partial protection was greatly different between the two groups. Cats of the gp140/IL-16 group never tested positive for viral RNA in the periphery, whereas cats of the gp140/CpG group showed even lower FIV provirus load, but three of four tested positive for viral RNA. Differences seen in cytokine transcription between these groups during immunizations and after challenge exposure argue for the induction of different mechanisms of virus-specific immunity.
Cats immunized with FIV gp140/CpG were also partially protected. Therefore, bacterial (CpG) DNA appears to have significant immunomodulatory effects on the early lentivirus immune response in a mammalian system. This beneficial effect may involve cytokines elicited by synthetic CpG motifs, which include IL-12 and IFN-γ. These cytokines promote the development of Th1-dependent cytotoxic T-cell responses (30, 44). In support of this mechanism, partially protected cats showed increased IL-12 p40 and IFN-γ transcription in PBMCs during the immunization phase. No B-cell responses were observed during the immunization phase, as measured by the lack of virus-specific antibodies in cats immunized with FIV gp140/CpG.
The modulation of immune responses in this study agrees with results from previous molecular adjuvant studies in FIV, HIV-1, and SIV and highlight the capacity of cytokine gene adjuvants to modulate early immune responses in vivo. Controlling the magnitude and direction of the immune response in this phase could be advantageous in the outcome of a subsequent infection. Thus, our data support the idea that modulation of the immune response within the early phase of immunity with appropriate vaccine formulations could lead to a promising vaccine approach.
ACKNOWLEDGMENTS
This project was supported by a grant from the Swiss National Science Foundation (31-49605.96), by the European Concerted Action on FIV Vaccination, by a grant from the United Bank of Switzerland on behalf of a customer, and from Virbac SA, Cedex, Nice, France. J.N.F. is a Biotechnology and Biological Sciences Research Council (BBSRC) UK Advanced Fellow. C.M.L. is a recipient of a Swiss National Science Foundation grant (823A-0534469).
We thank Paul Luciw for critical comments on the manuscript. The cat food was kindly donated by Sieber-Hegner, Zürich, Switzerland. We thank our technical staff for providing the differential cell counts and Peter Fidler for his help with the cats.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Amiel C, Darcissac E, Truong M J, Dewulf J, Loyens M, Mouton Y, Capron A, Bahr G M. Interleukin-16 (IL-16) inhibits human immunodeficiency virus replication in cells from infected subjects, and serum IL-16 levels drop with disease progression. J Infect Dis. 1999;179:83–91. doi: 10.1086/314550. [DOI] [PubMed] [Google Scholar]
- 3.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 4.Behboudi S, Chao D, Klenerman P, Austyn J. The effects of DNA containing CpG motif on dendritic cells. Immunology. 2000;99:361–366. doi: 10.1046/j.1365-2567.2000.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernard N F, Yannakis C M, Lee J S, Tsoukas C M. Human immunodeficiency virus (HIV)-specific cytotoxic T lymphocyte activity in HIV-exposed seronegative persons. J Infect Dis. 1999;179:538–547. doi: 10.1086/314621. [DOI] [PubMed] [Google Scholar]
- 6.Beyrer C, Artenstein A W, Rugpao S, Stephens H, VanCott T C, Robb M L, Rinkaew M, Birx D L, Khamboonruang C, Zimmerman P A, Nelson K E, Natpratan C. Epidemiologic and biologic characterization of a cohort of human immunodeficiency virus type 1 highly exposed, persistently seronegative female sex workers in northern Thailand. Chiang Mai HEPS Working Group. J Infect Dis. 1999;179:59–67. doi: 10.1086/314556. [DOI] [PubMed] [Google Scholar]
- 7.Bisset L R, Rothen M, Joller-Jemelka H I, Dubs R W, Grob P J, Opravil M. Change in circulating levels of the chemokines macrophage inflammatory proteins 1 alpha and 1 beta, RANTES, monocyte chemotactic protein-1 and interleukin-16 following treatment of severely immunodeficient HIV-infected individuals with indinavir. AIDS. 1997;11:485–491. doi: 10.1097/00002030-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Boretti F S, Leutenegger C M, Mislin C, König S, Schroff M, Junghans C, Fehr D, Huettner S W, Habel A, Flynn J N, Aubert A, Pedersen N C, Wittig B, Lutz H. Protection against FIV challenge infection by genetic vaccination using minimalistic DNA constructs for FIV env gene and feline IL-12 expression. AIDS. 2000;14:1749–1757. doi: 10.1097/00002030-200008180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broström C, Visco-Comandini U, Yun Z, Sönnerborg A. Longitudinal quantification of human immunodeficiency virus type 1 DNA and RNA in long-term nonprogressors. J Infect Dis. 1999;179:1542–1548. doi: 10.1086/314757. [DOI] [PubMed] [Google Scholar]
- 11.Bucci J G, English R V, Jordan H L, Childers T A, Tompkins M B, Tompkins W A. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. J Infect Dis. 1998;177:18–25. doi: 10.1086/513822. [DOI] [PubMed] [Google Scholar]
- 12.Calzolari M, Young E, Cox D, Davis D, Lutz H. Serological diagnosis of feline immunodeficiency virus infection using recombinant transmembrane glycoprotein. Vet Immunol Immunopathol. 1995;46:83–92. doi: 10.1016/0165-2427(94)07008-u. [DOI] [PubMed] [Google Scholar]
- 13.Chehimi J, Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14:149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 14.Choi I S, Hokanson R, Collisson E W. Anti-feline immunodeficiency virus (FIV) soluble factor(s) produced from antigen-stimulated feline CD8+ T lymphocytes suppresses FIV replication. J Virol. 2000;74:676–683. doi: 10.1128/jvi.74.2.676-683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerici M, Lucey D R, Berzofsky J A, Pinto L A, Wynn T A, Blatt S P, Dolan M J, Hendrix C W, Wolf S F, Shearer G M. Restoration of HIV-specific cell-mediated immune responses by interleukin-12 in vitro. Science. 1993;262:1721–1724. doi: 10.1126/science.7903123. [DOI] [PubMed] [Google Scholar]
- 16.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 17.Cuisinier A M, Mallet V, Meyer A, Caldora C, Aubert A. DNA vaccination using expression vectors carrying FIV structural genes induces immune response against feline immunodeficiency virus. Vaccine. 1997;15:1085–1094. doi: 10.1016/s0264-410x(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 18.Davis H L, Weeranta R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 19.Dua N, Reubel G, Moore P F, Higgins J, Pedersen N C. An experimental study of primary feline immunodeficiency virus infection in cats and a historical comparison to acute simian and human immunodeficiency virus diseases. Vet Immunol Immunopathol. 1994;43:337–355. doi: 10.1016/0165-2427(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 20.Fehr D, Dean G A, Huder J, Fan Z, Huettner S, Higgins J W, Pedersen N C, Lutz H. Nucleotide and predicted peptide sequence of feline interleukin-12 (IL-12) DNA Sequence. 1997;8:77–82. doi: 10.3109/10425179709020889. [DOI] [PubMed] [Google Scholar]
- 21.Flynn J N, Cannon C A, Beatty J A, Mackett M, Rigby M A, Neil J C, Jarrett C. Induction of feline immunodeficiency virus-specific cytotoxic T cells in vivo with carrier-free synthetic peptide. J Virol. 1994;68:5835–5844. doi: 10.1128/jvi.68.9.5835-5844.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flynn J N, Cannon C A, Reid G, Rigby M A, Neil J C, Jarrett O. Induction of feline immunodeficiency virus-specific cell-mediated and humoral immune responses following immunization with a multiple antigenic peptide from the envelope V3 domain. Immunology. 1995;85:171–175. [PMC free article] [PubMed] [Google Scholar]
- 23.Flynn J N, Hosie M J, Rigby M A, Mackay N, Cannon C A, Dunsford T, Neil J C, Jarrett O. Factors influencing cellular immune responses to feline immunodeficiency virus induced by DNA vaccination. Vaccine. 2000;18:1118–1132. doi: 10.1016/s0264-410x(99)00375-8. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot J D, Hoover E A, Elder J H, Montelaro R C. Evaluation of feline immunodeficiency virus and feline leukemia virus transmembrane peptides for serological diagnosis. J Clin Microbiol. 1992;30:1885–1890. doi: 10.1128/jcm.30.7.1885-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freidag B L, Melton G B, Collins F, Klinman D M, Cheever A, Stobie L, Suen W, Seder R A. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect Immun. 2000;68:2948–2953. doi: 10.1128/iai.68.5.2948-2953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuller D H, Haynes J R. A qualitative progression in HIV type 1 glycoprotein 120-specific cytotoxic cellular and humoral immune responses in mice receiving a DNA-based glycoprotein 120 vaccine. AIDS Res Hum Retroviruses. 1994;10:1433–1441. doi: 10.1089/aid.1994.10.1433. [DOI] [PubMed] [Google Scholar]
- 27.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 28.Greenberger M J, Kunkel S L, Strieter R M, Lukacs N W, Bramson J, Gauldie J, Graham F L, Hitt M, Danforth J M, Standiford T J. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J Immunol. 1996;157:3006–3012. [PubMed] [Google Scholar]
- 29.Hartmann G, Weiner G J, Krieg A M. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci USA. 1999;96:9305–9310. doi: 10.1073/pnas.96.16.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heeg K, Zimmermann S. CpG DNA as a Th1 trigger. Int Arch Allergy Immunol. 2000;121:87–97. doi: 10.1159/000024303. [DOI] [PubMed] [Google Scholar]
- 31.Heeney J L, van Els C, de Vries P, ten Haaft P, Otting N, Koornstra W, Boes J, Dubbes R, Niphuis H, Dings M, Cranage M, Norley S, Jonker M, Bongtrop R E, Osterhaus A. Major histocompatibility complex class I-associated vaccine protection from simian immunodeficiency virus-infected peripheral blood cells. J Exp Med. 1994;180:769–774. doi: 10.1084/jem.180.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman R M, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann-Lehmann R, Holznagel E, Ossent P, Lutz H. Parameters of disease progression in long-term experimental feline retrovirus (feline immunodeficiency virus and feline leukemia virus) infections: hematology, clinical chemistry, and lymphocyte subsets. Clin Diagn Lab Immunol. 1997;4:33–42. doi: 10.1128/cdli.4.1.33-42.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosie M J, Flynn J N. Feline immunodeficiency virus vaccination: characterization of the immune correlates of protection. J Virol. 1996;70:7561–7568. doi: 10.1128/jvi.70.11.7561-7568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosie M J, Flynn J N, Rigby M A, Cannon C, Dunsford T, Mackay N A, Argyle D, Willett B J, Miyazawa T, Onions D E, Jarrett O, Neil J C. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakob T, Walker P S, Krieg A M, von Stebut E, Udey M C, Vogel J C. Bacterial DNA and CpG-containing oligodeoxynucleotides activate cutaneous dendritic cells and induce IL-12 production: implications for the augmentation of Th1 responses. Int Arch Allergy Immunol. 1999;118:457–461. doi: 10.1159/000024163. [DOI] [PubMed] [Google Scholar]
- 37.Jiang C, Magee D M, Cox R A. Coadministration of interleukin 12 expression vector with antigen 2 cDNA enhances induction of protective immunity against Coccidioides immitis. Infect Immun. 1999;67:5848–5853. doi: 10.1128/iai.67.11.5848-5853.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin X, Roberts C G, Nixon D F, Cao Y, Ho D D, Walker B D, Muldoon M, Korber B T, Koup R A. Longitudinal and cross-sectional analysis of cytotoxic T lymphocyte responses and their relationship to vertical human immunodeficiency virus transmission. ARIEL Project Investigators. J Infect Dis. 1998;178:1317–1326. doi: 10.1086/314455. [DOI] [PubMed] [Google Scholar]
- 39.Kaser A, Dunzendorfer S, Offner F A, Ryan T, Schwabegger A, Cruikshank W W, Wiedermann C J, Tilg H. A role for IL-16 in the cross-talk between dendritic cells and T cells. J Immunol. 1999;163:3232–3238. [PubMed] [Google Scholar]
- 40.Keane J, Nicoll J, Kim S, Wu D M, Cruikshank W W, Brazer W, Natke B, Zhang Y, Center D M, Kornfeld H. Conservation of structure and function between human and murine IL-16. J Immunol. 1998;160:5945–5954. [PubMed] [Google Scholar]
- 41.Kenney R T, Sacks D L, Sypek J P, Vilela L, Gam A A, Evans-Davis K. Protective immunity using recombinant human IL-12 and alum as adjuvants in a primate model of cutaneous leishmaniasis. J Immunol. 1999;163:4481–4488. [PubMed] [Google Scholar]
- 42.Kim J J, Simbiri K A, Sin J I, Dang K, Oh J, Dentchev T, Lee D, Nottingham L K, Chalian A A, McCallus D, Ciccarelli R, Agadjanyan M G, Weiner D B. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J Interferon Cytokine Res. 1999;19:77–84. doi: 10.1089/107999099314441. [DOI] [PubMed] [Google Scholar]
- 43.Klinman D M, Sechler J M, Conover J, Gu M, Rosenberg A S. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 44.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 46.Kuwata T, Miura T, Haga T, Kozyrev I, Hayami M. Construction of chimeric simian and human immunodeficiency viruses that produce interleukin 12. AIDS Res Hum Retroviruses. 2000;16:465–470. doi: 10.1089/088922200309124. [DOI] [PubMed] [Google Scholar]
- 47.Lee M E, Adams J W, Villinger F, Brar S S, Meadows M, Bucur S Z, Lackey D A, 3rd, Brice G T, Cruikshank W W, Ansari A A, Hillyer C D. Molecular cloning and expression of rhesus macaque and sooty mangabey interleukin 16: biologic activity and effect on simian immunodeficiency virus infection and/or replication. AIDS Res Hum Retroviruses. 1998;14:1323–1328. doi: 10.1089/aid.1998.14.1323. [DOI] [PubMed] [Google Scholar]
- 48.Leutenegger C M, Klein D, Hofmann-Lehmann R, Mislin C, Hummel U, Böni J, Boretti F, Guenzburg W H, Lutz H. Rapid feline immunodeficiency virus provirus quantitation by polymerase chain reaction using the TaqMan fluorogenic real-time detection system. J Virol Methods. 1999;78:105–116. doi: 10.1016/s0166-0934(98)00166-9. [DOI] [PubMed] [Google Scholar]
- 49.Leutenegger C M, Hofmann-Lehmann R, Holznagel E, Cuisinier A M, Wolfensberger C, Duquesne V, Cronier J, Allenspach K, Aubert A, Ossent P, Lutz H. Partial protection by vaccination with recombinant feline immunodeficiency virus surface glycoproteins. AIDS Res Hum Retroviruses. 1998;14:275–283. doi: 10.1089/aid.1998.14.275. [DOI] [PubMed] [Google Scholar]
- 50.Leutenegger C M, Huder J B, Hofmann-Lehmann R, Lutz H. Molecular cloning and expression of feline interleukin-16. DNA Sequence. 1998;9:59–63. doi: 10.3109/10425179809050026. [DOI] [PubMed] [Google Scholar]
- 51.Leutenegger C M, Huder J B, Mislin C N, Lahrtz F, Hofmann-Lehmann R, Pedersen N C, Lutz H. Molecular characterization of feline interleukin 16: chemotactic activity and effect on feline immunodeficiency virus infection and/or replication. AIDS Res Hum Retroviruses. 2000;16:569–575. doi: 10.1089/088922200308981. [DOI] [PubMed] [Google Scholar]
- 52.Leutenegger C M, Mislin C N, Sigrist B, Ehrengruber M U, Hofmann-Lehmann R, Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet Immunol Immunopathol. 1999;71:291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy J K, Ritchey J W, Rottman J B, Davidson M G, Liang Y H, Jordan H L, Tompkins W A, Tompkins M B. Elevated interleukin-10-to-interleukin-12 ratio in feline immunodeficiency virus-infected cats predicts loss of type 1 immunity to Toxoplasma gondii. J Infect Dis. 1998;178:503–511. doi: 10.1086/515632. [DOI] [PubMed] [Google Scholar]
- 54.Lindblad E B, Elhay M J, Silva R, Appelberg R, Andersen P. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect Immun. 1997;65:623–629. doi: 10.1128/iai.65.2.623-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lutz H, Arnold P, Hubscher U, Egberink H, Pedersen N, Horzinek M C. Specificity assessment of feline T-lymphotropic lentivirus serology. Zentralbl Veterinarmed. 1988;35:773–778. doi: 10.1111/j.1439-0450.1988.tb00559.x. [DOI] [PubMed] [Google Scholar]
- 56.Mackewicz C E, Ortega H W, Levy J A. CD8+ cell anti-HIV activity correlates with the clinical state of the infected individual. J Clin Investig. 1991;87:1462–1466. doi: 10.1172/JCI115153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marschall J D, Chehimi J, Gri G, Kostman J R, Montaner L J, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–1011. [PubMed] [Google Scholar]
- 58.Mazzolini G, Qian, C. C, Xie X, Sun Y, Lasarte J J, Drozdzik M, Prieto J. Regression of colon cancer and induction of antitumor immunity by intratumoral injection of adenovirus expressing interleukin-12. Cancer Gene Ther. 1999;6:514–522. doi: 10.1038/sj.cgt.7700072. [DOI] [PubMed] [Google Scholar]
- 59.Morikawa S, Lutz H, Aubert A, Bishop D H. Identification of conserved and variable regions in the envelope glycoprotein sequences of two feline immunodeficiency viruses isolated in Zurich, Switzerland. Virus Res. 1991;21:53–63. doi: 10.1016/0168-1702(91)90071-3. [DOI] [PubMed] [Google Scholar]
- 60.Muhlhahn P, Zweckstetter M, Georgescu J, Ciosto C, Renner C, Lanzendorfer M, Lang K, Ambrosius D, Baier M, Kurth R, Holak T A. Structure of interleukin 16 resembles a PDZ domain with an occluded peptide binding site. Nat Struct Biol. 1998;5:682–686. doi: 10.1038/1376. [DOI] [PubMed] [Google Scholar]
- 61.Plaeger S, Bermudez S, Mikyas Y, Harawa N, Dickover R, Mark D, Dillon M, Bryson Y J, Boyer P J, Sinsheimer J S. Decreases CD8 cell-mediated viral suppression and other immunologic characteristics of women who transmit human immunodeficiency virus to their infants. J Infect Dis. 1999;179:1388–1394. doi: 10.1086/314746. [DOI] [PubMed] [Google Scholar]
- 62.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 63.Shimojima M, Miyazawa T, Kohmoto M, Ikeda Y, Nishimura Y, Maeda K, Tohya Y, Mikami T. Expansion of CD8alpha+beta− cells in cats infected with feline immunodeficiency virus. J Gen Virol. 1998;79:91–94. doi: 10.1099/0022-1317-79-1-91. [DOI] [PubMed] [Google Scholar]
- 64.Sibille P, Avrameas A, Moraillon A, Richardson J, Sonigo P, Pancino G, Strosberg A D. Comparison of serological tests for the diagnosis of feline immunodeficiency virus infection of cats. Vet Microbiol. 1995;45:259–267. doi: 10.1016/0378-1135(94)00128-j. [DOI] [PubMed] [Google Scholar]
- 65.Sin J I, Kim J J, Arnold R L, Shroff K E, McCallus D, Pachuk C, McElhiney S P, Wolf M W, Pompa-de Bruin S J, Higgins T J, Ciccarelli R B, Weiner D B. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. 1999;162:2912–2921. [PubMed] [Google Scholar]
- 66.Song K, Chang Y, Prud'homme G J. Regulation of T-helper-1 versus T-helper-2 activity and enhancement of tumor immunity by combined DNA-based vaccination and nonviral cytokine gene transfer. Gene Ther. 2000;7:481–492. doi: 10.1038/sj.gt.3301123. [DOI] [PubMed] [Google Scholar]
- 67.Stacey K J, Blackwell J M. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect Immun. 1999;67:3719–3726. doi: 10.1128/iai.67.8.3719-3726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tellier M C, Soos J, Pu R, Pollock D, Yamamoto J K. Development of FIV-specific cytolytic T-lymphocyte responses in cats upon immunisation with FIV vaccines. Vet Microbiol. 1997;57:1–11. doi: 10.1016/s0378-1135(97)00081-3. [DOI] [PubMed] [Google Scholar]
- 69.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 70.Wagner R, Leschonsky B, Harrer E, Paulus C, Weber C, Walker B D, Buchbinder S, Wolf H, Kalden J R, Harrer T. Molecular and functional analysis of a conserved CTL epitope in HIV-1 p24 recognized from a long-term nonprogressor: constraints on immune escape associated with targeting a sequence essential for viral replication. J Immunol. 1999;162:3727–3734. [PubMed] [Google Scholar]
- 71.Weiner G J, Liu H M, Wooldridge J E, Dahle C E, Krieg A M. Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization. Proc Natl Acad Sci USA. 1997;94:10833–10837. doi: 10.1073/pnas.94.20.10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yawalkar N, Brand C U, Braathen L R. IL-12 gene expression in human skin-derived CD1a+ dendritic lymph cells. Arch Dermatol Res. 1996;288:79–84. doi: 10.1007/BF02505048. [DOI] [PubMed] [Google Scholar]
- 73.Zhou P, Devadas K, Tewari D, Jegorow A, Notkins A L. Processing, secretion, and anti-HIV-1 activity of IL-16 with or without a signal peptide in CD4+ T cells. J Immunol. 1999;163:906–912. [PubMed] [Google Scholar]
- 74.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins A L. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3:659–664. doi: 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]