SUMMARY
This review summarizes the recent Global Meningococcal Initiative (GMI) regional meeting, which explored meningococcal disease in North America. Invasive meningococcal disease (IMD) cases are documented through both passive and active surveillance networks. IMD appears to be decreasing in many areas, such as the Dominican Republic (2016: 18 cases; 2021: 2 cases) and Panama (2008: 1 case/100,000; 2021: < 0.1 cases/100,000); however, there is notable regional and temporal variation. Outbreaks persist in at-risk subpopulations, such as people experiencing homelessness in the US and migrants in Mexico. The recent emergence of β-lactamase-positive and ciprofloxacin-resistant meningococci in the US is a major concern. While vaccination practices vary across North America, vaccine uptake remains relatively high. Monovalent and multivalent conjugate vaccines (which many countries in North America primarily use) can provide herd protection. However, there is no evidence that group B vaccines reduce meningococcal carriage. The coronavirus pandemic illustrates that following public health crises, enhanced surveillance of disease epidemiology and catch-up vaccine schedules is key. Whole genome sequencing is a key epidemiological tool for identifying IMD strain emergence and the evaluation of vaccine strain coverage. The Global Roadmap on Defeating Meningitis by 2030 remains a focus of the GMI.
Keywords: Meningococcal, Neisseria species, Meningitis, North America, Vaccination, Antibiotic resistance
Introduction
Neisseria meningitidis is an obligate human gram-negative commensal/pathogen that resides in the pharyngeal mucosa.1, 2 Following entry into the bloodstream, N. meningitidis can cause invasive meningococcal disease (IMD), primarily manifesting as meningitis and/or septicemia.2 A key component that contributes to the virulence of N. meningitidis is the polysaccharide capsule; based on its biochemical composition, 12 serogroups have been characterized, of which six (A, B, C, W, X and Y) most commonly cause IMD.3 IMD is a severe disease that causes significant morbidity and mortality in children, young adults, and the elderly (aged >74 years).4, 5
Established in 2009, the Global Meningococcal Initiative (GMI) is an international multidisciplinary group of experts dedicated to promoting the prevention of IMD through education, research, and international cooperation. Since its inception, 14 roundtable meetings have been held to discuss the global and region-specific epidemiology, surveillance, and control of IMD, and provide recommendations for IMD prevention6–8.
The most recent regional meeting, held virtually in March 2022, was attended by members of the GMI steering committee, as well as delegates from across North America and the United Kingdom. With a regional focus on North America, the objectives of this meeting were to: (i) review meningococcal epidemiology and immunization schedules; (ii) promote whole genome sequencing (WGS), particularly for evaluating strain coverage of meningococcal serogroup B (MenB) vaccines; (iii) further assess the impact of the COVID-19 pandemic and population lockdowns on IMD epidemiology and vaccine schedules; (iv) explore the Neisseria gene pool; (v) revisit the World Health Organization (WHO) roadmap for defeating meningitis by 2030; (vi) discuss the regional increases in meningococcal antibiotic resistance; and (vii) explore the current evidence for meningococcal vaccines to provide herd protection.
This review summarizes the key discussion points, providing an overview of the epidemiology, surveillance, prevention, and control of IMD in the North America region.
The surveillance, epidemiology, and prevention of IMD in the North America region
Surveillance of IMD
Disease surveillance is essential for the detection of IMD cases, outbreaks, and trends within a geographical region.9 Laboratory confirmation of detected cases also allows for the collection of data on the circulation, distribution, and evolution of specific meningococcal serogroups and strains.9 Across the North America region, surveillance systems put in place by national health ministries vary.
IMD is a nationally notifiable disease in the US, Canada, several Caribbean countries, Mexico, and Costa Rica. In the US, several active and passive surveillance systems are in place, including Active Bacterial Core Surveillance (ABCs), the National Notifiable Diseases Surveillance System (NNDSS) and Enhanced Meningococcal Disease Surveillance (EMDS).10 EMDS provides a comprehensive view of US meningococcal disease epidemiology.11 Equally, national surveillance systems are utilized in Canada, including the Canadian Notifiable Disease Surveillance System (CNDSS) or the National Enhanced IMD Surveillance Systems (eIMDSS), but limited clinical data are collected.12 A separate, active national surveillance program, the Canadian Immunization Monitoring Program ACTive (IMPACT), operates through 12 pediatric tertiary care hospitals, collecting data from defined catchment areas for adverse events following vaccination, as well as cases on vaccine-preventable infectious diseases including IMD.13
Established by the Pan-American Health Organization in 1993, the Regional System for Vaccines (SIREVA) II network is a regional, passive surveillance program that obtains data on disease-causing strains of Streptococcus pneumoniae, Haemophilus influenzae, and N. meningitidis.14 Costa Rica, Cuba, El Salvador, Guatemala, Mexico, Panama, and the Dominican Republic are among the 19 countries that participate in this network.14 In Costa Rica, the characterization of meningococci is conducted by two principal national laboratories/networks (National Network Laboratories Hospitals and National Reference Center of Bacteriology [NRCB] – IN-CIENSA) and involves antimicrobial resistance surveillance testing.14 Forming part of the SIREVA-GIVEBPVac network, in Mexico a passive laboratory-based surveillance network consisting of 25–30 hospitals receive clinical isolates, but under-reporting remains an issue.15, 16 Data on the number of cases of N. meningitidis in countries included in the SIREVA II network are obtained from national reference laboratories; however, the exact burden of IMD is not available for all participating countries as several countries lack a well-established surveillance system.14
Although IMD has been documented in Guatemala, it is not a notifiable disease and surveillance systems are limited. In Guatemala City, active hospital-based surveillance for acute bacterial diseases at the pediatric departments in three major referral hospitals recorded 1021 meningitis cases over 10 years (1996–2005).17 However, with high antibiotic use in the population, the already limited capacity to detect cases is further restricted. Some of these limitations may be overcome using multiplexed, in vitro, diagnostic syndromic Panel testing, which is used for the rapid detection of the most common bacterial, viral, and fungal pathogens causing central nervous system infections (FilmArray Meningitis-Encephalitis panel [BioFire]). However, no cases have been detected at Roosevelt Hospital (a large public health center where the Filmarray MenPanel has been implemented for case identification). Overall, the surveillance system in most Central American countries, like Guatemala, are limited by the lack of systematic case detection and laboratory capacity. Additionally, there are currently no data on N. meningitidis colonization in different populations (e.g. Indigenous people, adolescents etc.).
Continual strengthening of N. meningitidis case detection in health centers and regional hospitals is of importance. In the Dominican Republic, the reporting of cases to the national surveillance system between 2016 and 2021 has been prompt (within 1 day or less).18 By coordinating with the National Health Service to train health professionals and increase the diagnostic capacity of the National Public Health Laboratory Dr. Defilló, the aim is to ensure that 80% of suspected cases are sampled and 100% of cases are notified in a timely manner. Sensitivity of an enhanced IMD surveillance system could be assessed by indicators of suspected syndromic versus confirmed cases, and rates of identification of N. meningitidis compared with other established systems.
Incidence of IMD
The reported incidence rates of IMD were relatively consistent and low across the North America region, ranging from 0.01 per 100,000 in Mexico to < 0.5 per 100,000 in Costa Rica (see Table 1). However, age-, population- and time-related variations in meningococcal disease incidence were reported. A general trend observed across the region was a notable decline in IMD incidence in recent years. However, IMD outbreaks were still observed.19
Table 1.
Epidemiology of and control strategies for invasive meningococcal disease across the North America region.
| Country/region | Surveillance system (Y/N) | Epidemiology (key points) | Control strategies (vaccines only) |
|---|---|---|---|
| Canada | Y |
|
|
| US | Y |
|
|
| Mexico | Y* |
|
|
| Cuba | N* |
|
|
| Dominican Republic | N* |
|
|
| Panama | N* |
|
|
| Guatemala | N* (meningitis is reportable) |
|
|
| El Salvador | N* |
|
|
| Costa Rica | Y* |
|
|
Part of the SIREVA II network (regional laboratory-based passive surveillance program that collects quality laboratory data on disease-causing meningococcal strains). IMD, invasive meningococcal disease; CC, clonal complex; ST, sequence type; NmY, Neisseria meningitidis serogroup Y; NIP, national immunization program; MenC, meningococcal serogroup C; MenB, meningococcal serogroup B. This table summarises the epidemiology and control strategies of countries from the North American region that were discussed during the 2022 Global Meningococcal Initiative meeting.
Across the North America region, several outbreaks of serogroup B (NmB) and C (NmC) have occurred over the past 20–30 years. In the Canadian province of Quebec, outbreaks of NmC occurred in 1988 and 2001 with further outbreaks reported across the country starting in 2000.20, 21, 22 These outbreaks contributed to the introduction of routine meningococcal serogroup C (MenC) vaccines to the National Immunization Programs (NIPs) in Canada. In the US, outbreaks account for 5% of all meningococcal disease cases.23 Recent US outbreaks have occurred within specific populations, including NmB cases among university students, NmC outbreaks among men who have sex with men (MSM) and outbreaks of multiple serogroups among people experiencing homelessness (PEH)24–26. An NmC (sequence type [ST]−11) outbreak also occurred in Tijuana (Mexico) in 2010, resulting in 19 cases of IMD with a median age of 16 years.27
The introduction of vaccinations, both in routine care and for outbreak control, has potentially, partly contributed to the reduction in IMD incidence across the North America region. In the US, the incidence of IMD declined from 1.3 cases per 100,000 people in 1996 to 0.11 cases per 100,000 people in 2019.28 In Canada, incidences have been between 0.45 and 0.75 IMD cases per 100,000 (2002–2011) (Table 1). However, meningococcal disease is also low or declining in other areas of North America without routine meningococcal vaccinations. For example, the incidence rate of IMD in Costa Rica has fluctuated but remained at low levels since 2006, and in 2021 was reported as < 0.5 per 100,000 people.29 Following a peak in IMD incidence in the Dominican Republic in 2016 (18 cases), a gradual decline in incidence has been observed through to 2021 (2 cases).18 IMD incidence has also declined steadily in Panama from 2008 (1 case per 100,000) to 2021 (< 0.1 cases per 100,000).30
Several populations are particularly affected by IMD. In the US, the highest rates of IMD occur in children aged < 2 years and adults aged > 85 years, with an additional peak observed among adolescents and adults aged 16–25 years.28, 31 High incidence rates were also observed among PEH (19.8 times higher compared with non-PEH); incidence among PEH remained high even after the exclusion of outbreak cases (12.8 times higher compared with non-PEH).32, 33
IMD among children occurs across the North America region34,.16, 18, 29 In Costa Rica, IMD cases have primarily occurred in individuals aged < 1 years (n = 15), 15–29 years (n = 20) and 30–49 years (n = 19).29 A similar trend is present in the Dominican Republic: the 1–4 years age group is most affected by meningococcal disease; however, 3 out of 10 deaths occur in the 40–49-year age group.18
Between 2010 and 2014, 155 cases of IMD were reported in Mexico.35 IMD cases continue to occur in Mexico, particularly near the US border, which is an area with a large population of migrants. Confirmed IMD cases have been reported in northern Mexico, including Tijuana (52 cases; 2005–2018) and Nuevo León (10 cases; August 2018–March 2019).36, 37 A notable increase in the number of IMD cases in 2019 was also reported in the southern states of Mexico, with case numbers increasing from 0 in 2015 to 8 in Chiapas, 8 in Tabasco and 9 in Guerrero.38 Both geographical and seasonal epidemic patterns of meningococcal disease have been observed in the Dominican Republic.18 Highest incidence rates occur in the months of October to December, and in the provinces of Barahona and Duarte.18
Serogroup distribution
The predominant reported serogroups in the North America region are B, C and W (Fig. 1). However, serogroup information from surveillance systems is lacking throughout Central America and Caribbean countries, which limits the ability to assess serogroup distribution across the entire North America region.
Fig. 1.
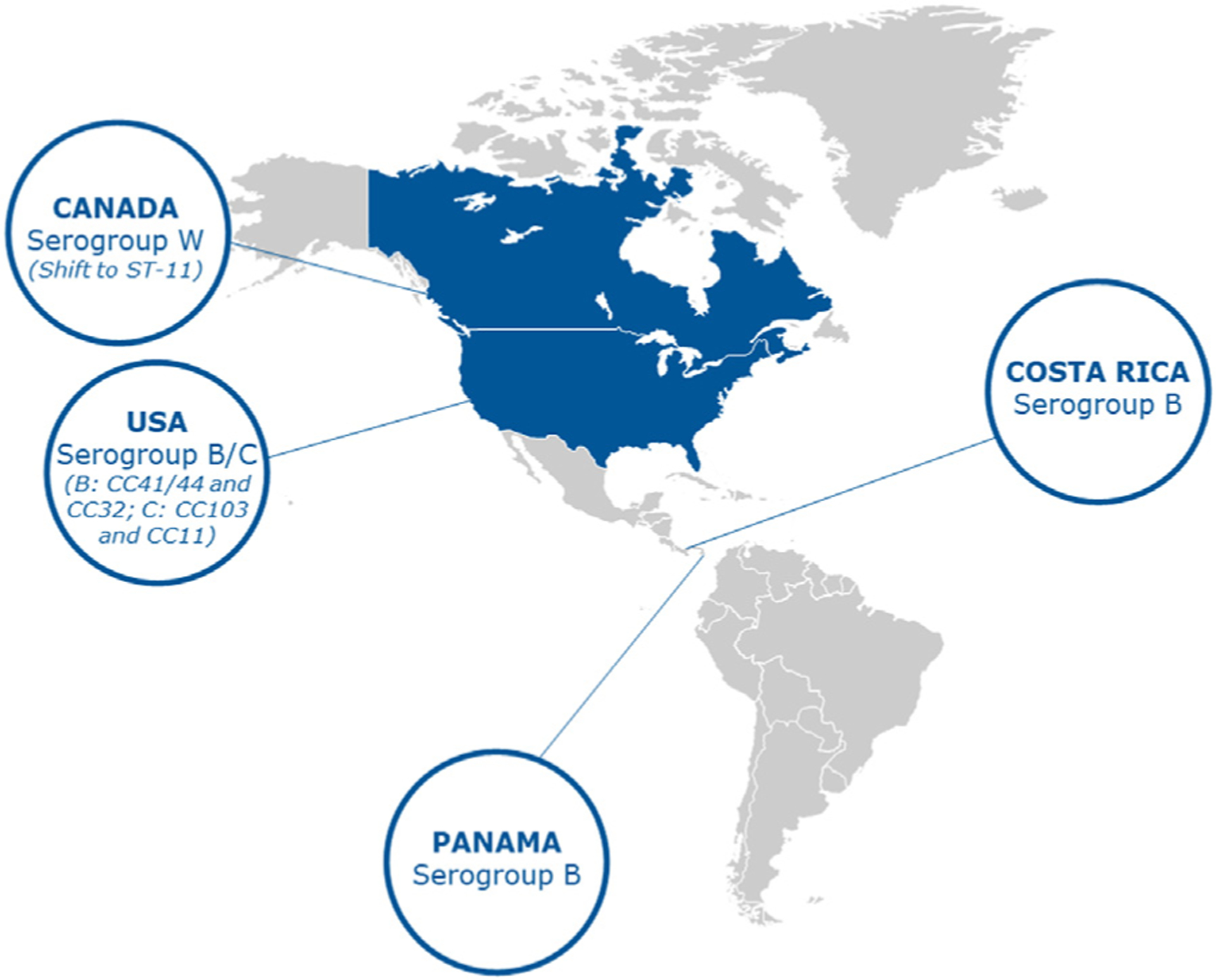
Predominant serogroup and clonal complex distribution across North America.
Despite an overall decline in the incidence of NmB,39 this serogroup continues to predominate in several countries. In the US, NmB was the most common cause of IMD in 2019 (26%).40 Between 2015–2019 the most common NmB clonal complexes (CC) causing invasive disease within the US were CC-41/44 (34%) and CC-32 (30%).41 In Canada, national laboratory surveillance data shows that 565 cases of culture-confirmed IMD were found in 2015–2020, of which just under 40% were NmB.42 Invasive NmB strains in Canada are diverse and display geographical differences. Between 2015 and 2020, of the 215 NmB isolates analysed by MLST, 41.9% and 28.8% belonged to the clonal complexes of CC-41/44 and CC-269, respectively; however, this is likely skewed due to previous hyperendemic disease in Quebec.43 In Costa Rica, NmB has been the most prevalent. Based on National Reference center for Bacteriology (NRCB) data in Costa Rica, NmB was the predominant serogroup between 2006 and 2015 across all age groups (n = 78).29
NmC also accounts for a large proportion of cases in the North America region. In the Dominican Republic, NmC accounted for most cases reported between 2016 and 2021 (n = 7/12).18 Of the 52 confirmed IMD cases reported in Tijuana (Mexico) between 2005 and 2008, 61.5% were attributable to NmC.36 NmC accounted for 23% of all IMD cases in the US in 2019.40 In Costa Rica, in 2006–2021 NmC was only detected in individuals aged 5–49 years.29 In Panama, after a prolonged period of NmC dominance from 2006 to 2012, all documented invasive isolates were NmB between 2013 and 2021.44
Serogroup W (NmW) continues to circulate in the North American region. In Western Canada (2015–2020), NmW accounted for ~50% of IMD cases, displaying a shift in the dominant invasive sequence type from ST-22 to ST-11.45 Of the 163 NmW ST-11 isolates analysed from 2015 to 2020, 135 (82.8%) were found to form three major clusters with 75, 36, and 24 isolates, respectively. Ninetyone percent of the isolates from the largest cluster were recovered from Western Canada, 61% of the isolates from the second largest cluster were recovered from Central Canada, while 92% of the isolates from the third cluster were found to harbor the penA allele 9 and displayed reduced susceptibility towards penicillin G. Since 2018, NmW has emerged in Costa Rica29 and in Mexico, where it accounted for 11.5% of confirmed IMD cases reported in Tijuana between 2005 and 2018 and 30% of confirmed meningococcal infection cases in Nuevo León between August 2018 and March 2019.36, 37 The incidence of NmW has remained low and stable in the US since the late 1990s, with an incidence of 0.01 cases per 100,000 people during 2015–201928, 31, 46–51.
Prevention and control strategies
The prevention and control strategies implemented by health ministries vary across the North America region. When used at an early stage and successfully implemented to achieve high coverage, meningococcal vaccines can effectively control out-breaks/epidemics. As previously discussed, several outbreaks of NmB and NmC have occurred across the North America region and province-wide vaccine immunization campaigns have been launched in response to these outbreaks. For example, provincial immunization campaigns launched in response to NmC (serotype 2a electrophoretic type-15 [C:2a ET-15]; ST-11) outbreaks in Quebec resulted in 84% (1992) and 82% (2001) vaccination coverage in target populations (6 months to 20 years and 2 months to 20 years, respectively)52–54. MenB vaccination was used in 2014 with success in one region in Quebec to control an outbreak that had persisted since 2003.55
The US and Canada have robust NIPs, recommending routine meningococcal vaccinations to several risk groups; however, there are differences between the two countries. In the US, several meningococcal group A, C, W and Y conjugate (MenACWY) and meningococcal group B (MenB) vaccines are licensed.56 The US Advisory Committee on Immunization Practices recommends routine MenACWY vaccination for adolescents aged 11–12 years, with a booster dose at 16 years.57 In Canada, available meningococcal vaccines include two monovalent MenC conjugate vaccines (conjugated to cross-reactive material197 protein [MenC–CRM] or tetanus toxoid protein [MenC-TT]), three quadrivalent MenACWY conjugate vaccines (serogroups ACWY conjugated to diphtheria toxoid protein [Men-ACWY-DT], cross-reactive material197 protein [Men-ACWY-CRM], or tetanus toxoid protein [Men-ACWY-TT]), and 2 MenB vaccines (4CMenB and MenB-fHBP [subfamily A and B factor-H binding protein]).58 The National Advisory Committee on Immunization (NACI) in Canada recommends routine vaccinations with MenC for healthy infants and children, and with MenC or MenACWY for healthy adolescents and young adults.58 Recommended immunization schedules vary according to provinces and territories; however, the most common approach is one MenC dose at 12 months of age and one MenACWY dose at 12–15 years of age.59 Beyond outbreak control and use in high-risk populations, in the US MenB vaccines are recommended on the basis of shared clinical decision-making, with 28% of 17-year-olds having received at least one dose of a MenB vaccine as of 2020.60 In Canada, MenB vaccines are considered on an individual basis for children two months of age and older.58
In Cuba, the use of meningococcal vaccines is regulated, controlled, and monitored by the Center for State Control of Drugs and Medical Devices (CECMED).61 Regardless of whether a vaccine is for use in the country or for export, a rigorous regulatory process from development, preclinical and clinical trials to batch release and laboratory testing is overseen by the CECMED.62 At present, three meningococcal vaccines are available in Cuba (meningococcal B and C vaccine [VA-MENGOC-BC], meningococcal A, C, W vaccine [MenACW] and meningococcal A and W vaccine [MenAW]). The NIP includes MenBC vaccination using a 2-dose schedule in infants, the first dose at 3 months and the second at 5 months of age.63
The US, Canada and Cuba are the only countries in North America where routine meningococcal vaccines are included in the NIP.63 In many countries in this region, vaccines are only available via private health services; however, there are exceptions, such as in Trinidad and Tobago where meningococcal vaccines are provided under specific circumstances; for instance for individuals making religious pilgrimage overseas. In Panama, routine meningococcal vaccines are not recommended as part of the NIP but the introduction of meningococcal vaccination for outbreak control significantly decreased the average number of bacterial meningitis cases per year from 60 to around 15 in Hospital del Niño (1991–2020).64
Due to the overcrowded conditions in migrant camps, the city of Tijuana is a hot spot for the transmission of meningococcal and other infectious diseases. Several programs, such as the Integral Plan for Health Attention of Migrant Population, have been developed to provide health coverage and access to quality, essential health services medicines and vaccines for refugees and migrants. It is important that meningococcal vaccinations are included in these measures.
Defeating meningitis by 2030: updates on the global roadmap
Following the launch of the WHO Global Roadmap on Defeating Meningitis by 2030 in September 2021, in several regions of the world several steps have been made towards three primary goals: i) elimination of bacterial meningitis epidemics, ii) reduction of cases of vaccine-preventable bacterial meningitis by 50% and deaths by 70%, and iii) reduction of disability and improvement of quality of life after meningitis.65 Regarding prevention and epidemic control, routine vaccination programs against NmA have been successfully introduced in 13 out of 26 of the African meningitis belt countries to date. The aim is to introduce further routine vaccination programs for serogroups ACWY/ACWXY in at least 5 meningitis belt countries by 2023. Alongside prevention, the timely diagnosis and treatment of bacterial meningitis is critical to improve disease outcomes, including vaccine-preventable deaths, and minimize disease spread.66 Several studies investigating alternative diagnostic methods, such as blood PCR performed on venous dry blood spots, and the reasons underpinning the low frequency of lumbar punctures in patients with suspected meningitis are planned or in progress. The WHO are collaborating with the Programme for Appropriate Technology in Health (PATH) and the Centers for Disease Control and Prevention (CDC) to identify promising technologies for multiplex diagnostic testing, as well as developing evidence-based treatment and care guidelines. For global identification of the main meningitis pathogens, surveillance (such as through the Global Antimicrobial Resistance Surveillance System), molecular characterization, and genomic analysis (Global Meningitis Genome Partnership) strategies are under development.
Challenges in meningococcal disease management and treatment
Antibiotic resistance
The treatment and prevention of IMD relies on N. meningitidis’ susceptibility to antibiotics, particularly β-lactam antibiotics.61 Although resistance in N. meningitidis is rare, expansion of meningococcal strains with reduced susceptibility to penicillin G and/or ciprofloxacin (commonly used for post-exposure prophylaxis) is of concern68–70.
Historically, low rates of resistance to clinically relevant drugs have been reported among meningococcal isolates in the US.71, 72 However, expansion of an antibiotic-resistant NmY CC23, ST-3587 strain has been reported, with detection of cases across the US.73 This strain harbors penicillin resistance due to the acquisition of ROB-1 β-lactamase, with a subset of isolates also displaying resistance to ciprofloxacin due to gyrA mutations.73 The characterization of a β-lactamase-positive, ciprofloxacin-resistant meningococcal isolate from a 5-month-old child of Latino ethnicity in January 2020 prompted an expanded assessment conducted by the CDC.74 As a result, 33 penicillin-resistant (blaROB-1 - positive) isolates, including 11 isolates with ciprofloxacin resistance, were identified from cases reported during 2013–2020.67 Of the 11 dual-resistant strains, eight were reported from individuals of Latino ethnicity. Most cases caused by this strain were in infants aged < 1 year and adults aged > 45 years.75 Dual-resistant cases continue to be detected in the US in 2022, with most penicillin-resistant isolates tested in 2021 being CC23, ST-3587 though testing is still in process.42, 75 While most of these isolates are NmY, at least two isolates were identified as non-groupable (NmNG) by slide agglutination, though they appeared to be genetically derived from NmY. This has been reported for one isolate previously.74
In response to the expansion of resistant strains in the US, the CDC has requested expedited shipment of all NmY isolates and other isolates with genetic or state lab concerns from US state partners for whole-genome sequencing and surveillance antimicrobial susceptibility testing, which can inform prophylaxis recommendations.39 Additionally, all N. meningitidis isolates from US states undergo sequencing and cataloging into the Bacterial Meningitis Genomic Analysis Platform (BMGAP), a web-based system that can be used to check for specific genetic mutations or acquisitions of genes conferring antimicrobial resistance.76
The presence of this penicillin- and ciprofloxacin-resistant strain has also been reported in El Salvador. All six penicillin- and ciprofloxacin-resistant, culture-confirmed IMD cases in El Salvador between 2017 and 2019 were caused by NmY and classified as ST-3587, CC23.77 Based on core-genome single nucleotide polymorphism alignment, maximum likelihood phylogeny analysis demonstrated high similarity between these six strains, indicating a common origin of the Salvadorian strains. Further global dissemination has been demonstrated; the ST-3587/CC23 strain accounted for all blaROB-1 -positive NmY isolates identified in Canada and France between 2017 and 2018.78, 79 Furthermore, phylogenetic analysis revealed that 33 blaROB-1 -containing isolates from the US and 12 blaROB-1 -containing isolates from six other countries (including Canada, Mexico, and France), formed a single clade.80
Antibiotic resistant strains have been identified in other areas of the Americas, including Costa Rica. In 2019 and 2020, 78 penicillin-resistant isolates, as well as the first cases of penicillin-resistant, cefotaxime-non-susceptible (PENR CTXNS) strains of NmY were reported in Costa Rica.81 The first ciprofloxacin-resistant strain in Costa Rica was reported in 2021.29
Vaccine hesitancy
Despite the availability of vaccines in certain countries of North America, data on uptake are not available from most countries. In Canada, MenC immunization in infants is at 89% in British Columbia and 91% nationally.82, 83 The coverage for MenACWY vaccination among adolescents in British Columbia was 79% in 2019, falling from > 90% in 2005.84 A potential underlying factor behind this decrease in coverage may be attributed to vaccine hesitancy.
Vaccine hesitancy is complex and context-specific, with temporal and geographical variations. Although confidence, complacency and convenience have been identified as key issues driving vaccine beliefs, further understanding of the root cause of these beliefs is required.85 Several modifiable factors may address vaccine hesitancy, and can be broadly categorized under contextual influences, individual and group influences, and vaccine-specific issues. Several studies, including a 2018 survey of Canadian parents that assessed associations between parents’ knowledge, attitudes and beliefs toward vaccination, as well as a 2014 systematic literature review to examine factors underlying concerns regarding administration of multiple injections during childhood vaccination visits, have demonstrated the importance of healthcare providers in influencing peoples’ vaccine decision-making, with every encounter being an opportunity for discussion.86, 87 To reduce vaccine hesitancy, healthcare providers should be trained and use each health encounter as an opportunity to listen to specific concerns, use motivational interviewing, proactively correct misconceptions, and offer clear recommendations to their patients.86, 87
The impact of the COVID-19 pandemic and lockdown on IMD epidemiology and vaccine schedules
The COVID-19 pandemic impacted the incidence of, and vaccination strategies against, meningococcal disease.6 Across several regions, the incidence rates of IMD and associated mortality fell significantly following the onset of stringent Covid-19 control and national lockdown measures in 2020.6 For example, noticeable reductions in IMD cases have been recorded in Mexico, falling from 48 cases in 2019 to 12 in 2020.6
While there are some exceptions, such as Trinidad and Tobago where meningococcal vaccinations are only delivered if requested by the healthcare provider, routine immunization in countries where meningococcal vaccines are included in the NIP typically occurs during infancy, childhood, or adolescence.88 These young populations were heavily affected by the stringent Covid-19 lockdown measures introduced globally, with reported increases in obesity and myopia, a range of socioeconomic effects and rises in orphanhood89–92. For example, between 2020 and 2021, over 140,000 children in the US experienced the death of a parent or grandparent caregiver.93 The closure of schools interrupted, delayed, reorganized, or completely suspended routine immunizations.50, 94 Of note, meningococcal quadrivalent and MenB vaccination programs have been impacted by Covid-19 control measures in 50 to 75% of countries that provide these vaccines.95 With the easing of restrictions, this disruption to immunization and resultant reduction in vaccine coverage has the potential to lead to disease outbreaks, such as measles, polio and IMD.50, 94 Therefore, there are strong recommendations for the implementation of catch-up vaccination programs following the Covid-19 pandemic.96 Due to a decline in childhood vaccination coverage, a delay in vaccination during 2020 and concerns of wavering meningococcal herd protection in England and France, there are calls to introduce a boost vaccination strategy to protect against potential rebound epidemics.96, 97
Meningococcal immunization: vaccine strain coverage estimations
Sequence analysis for MenB vaccine strain coverage
For N. meningitidis, polysaccharide conjugate vaccines are available for all major disease-associated capsular groups, except for NmB, due to the poor immunogenicity of the MenB capsular polysaccharide.98, 99 Bexsero (4CMenB), a multi-component vaccine, includes the fHbp, NadA, NHBA and PorA peptide/protein antigens, the latter as part of an outer membrane vesicle.100 These vary in terms of strain distribution, cross-reactivity, and surface expression; thus, 4CMenB vaccine strain coverage varies within and between MenB strains.101 Advances have been made in the sequencing techniques used to estimate MenB vaccine strain coverage.
Due to the low incidence of IMD, vaccine efficacy evaluations are impractical. Instead, the immunogenicity of meningococcal vaccines can be assessed using data generated by in vitro laboratory assays.102 For MenB, the human complement serum bactericidal antibody assay (hSBA) has been the gold standard for determining killing by post-vaccination sera. However, the incompatibility of hSBA with certain strains, along with comprehensive validation requirements have limited the practical use of this assay for testing large isolate panels.102 Therefore, to determine 4CMenB strain coverage, the Meningococcal Antigen Typing System (MATS) was developed with reference to hSBA.103 For MATS, a standard lysed suspension is prepared from an isolate and applied to three ELISA plates – one each for NHBA, fHbp and NadA. The relative potency (RP) is then calculated versus a reference strain.103 In a previous study assessing 1052 strains collected from England, Wales, France, Germany, Italy and Norway (July 2007–June 2008), MATS predicted that 78% of strains would be killed by post-vaccination sera (95% CI: 63, 90; range of point estimates 73–87% in individual country panels).104 To overcome the labor intensity, limited availability and viable culture requirements of MATS, genotypic alternatives have been developed, including genetic (g)MATS and the Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) index.105, 106
MenDeVAR is likely to supersede gMATS as it also covers Trumenba (MenB-fHbp) and is ‘live’ on PubMLST.org. However, in a recent assessment of MATS, gMATS and MenDeVAR in invasive MenB isolates, gMATS provided a prediction for a greater proportion of isolates (76.5% versus 63.3%) and was in better agreement with MATS. To further the application of sequence analysis, improvements for non-culture typing/whole-genome sequencing (WGS) and the incorporation of peptide expression are important considerations.
In 2010, the meningococcal antigen surface expression (MEASURE) assay was developed to quantify surface expression of fHbp variants on intact isolates to predict susceptibility to bactericidal killing and assess strain coverage by MenB-fHbp. A 30 pg of surface-expressed fHbp/μg of total cell protein was associated with a 91.2% probability that the isolate would be killed by the MenB-fHbp immune sera.107
Vaccine match against circulating strains
MenB vaccines (4CMenB and MenB-fHbp) target specific antigens among NmB isolates. Collectively the peptides contained include fHbp (peptides A05/3.45, B01/1.55, and B24/1.1), NHBA (peptide 2), NadA (peptide 8) and PorA VR2 (4).100 The presence of antigens and corresponding peptides identified in N. meningitidis NmB strains (collected during 2015–2019) that match the antigens in licensed MenB vaccines in the US has been analyzed. Among NmB isolates, over 98% of isolates harboured intact fHbp, NHBA and PorA. The fourth antigen, NadA, was present in 30% of isolates.41 fHbp subfamily A (variants 2 and 3) and PorA displayed the highest degree of match among hyperinvasive lineages (CC32, CC41/44 and CC269). The analysis identified 67 unique fHbp peptide sequences, 68 unique NHBA peptide sequences and 140 unique PorA peptide sequences. For fHbp, peptide 1.1 was predominant across subfamily B (variant 1) and 2.19 was predominant among subfamily A (variants 2 and 3). For NHBA, peptide 2 and peptide 5 were the predominant peptide sequences. fHbp peptides matched by US licensed MenB vaccines include peptides 1.1 (24.2%) and 3.45 (3.2%). NHBA peptide 2 (17.8%) is included in the 4CMenB vaccine. Retrospective analyses of the temporal peptide distribution indicated that between 2000 and 2019 the prevalence of fHbp peptide 1.1 has declined, whereas the prevalence of NHBA peptide 2 has increased.41 Analyses of antigen peptides that were predicted to be covered by MenB vaccines were conducted using gMATS for MenB-4C strain coverage and data from clinical studies conducted in vaccinated individuals using hSBA for Men-fHbp strain coverage108–110. Through gMATS analysis, 39–57% of circulating NmB strains would match antigens in MenB-4C and through immunogenicity analyses, approximately 50% of circulating strains would match antigens in MenB-fHbp.41 In Canada, the potential of 4CMenB to cover strains circulating from 2006 to 2009 was considered through the characterization of 157 isolates using MATS. Overall predicated coverage was 66%111. In another study investigating isolate susceptibility to vaccines, 91.2% of the Canadian MenB isolates were found to express fHbp levels indicative of susceptibility to the MenB-fHbp vaccine112.
Recent developments in sequencing: IMD surveillance and control
Enhancing meningococcal genomic surveillance through culture-free WGS
The identification of N. meningitidis and their molecular characterization is important for the successful study, analysis, and control of IMD. Multi Locus Sequence Typing (MLST) based on the alleles of seven housekeeping genes is used to discriminate between circulating N. meningitidis for both long-term epidemiology studies and outbreak investigations113. Further increase in typing resolution is achieved by WGS of the bacterial genome to allow single nucleotide polymorphism (SNP)-analysis on the genome level114. However, obtaining high-quality sequence data applicable for the assembly of bacterial genome is limited to cultured bacteria, while the direct sequencing of clinical specimens produces mainly unusable low-quality sequence data due to low bacterial and high human DNA abundances. To enhance the detection and molecular characterization of meningococcal strains from clinical specimens, selective whole genome amplification (SWGA) has been developed115. SWGA is a culture-free, targeted DNA enrichment strategy that has been successfully tested on meningococci-positive clinical specimens, such as cerebrospinal fluid (CSF) and urine. Following DNA extraction, denaturation and neutralization, multiple-displacement amplification is performed using several components, including SWGA primers and a phi29 polymerase115 or its thermostable version EquiPhi20116. The SWGA primers have a binding preference for meningococcal genomes with an almost two-order-of-magnitude higher coverage on N. meningitidis DNA compared with human DNA. The resulting output is enriched meningococcal DNA specimens and a 10-fold increase in the number of clinical specimens that can be successfully sequenced via WGS. The total average success rate of full molecular typing is > 50%, with higher numbers of genomes per μL leading to higher success rates115, 116.
In the sub-Saharan African meningitis belt, SWGA has been used to assess meningococcal clinical samples for enhanced meningococcal molecular surveillance. The data demonstrated that cases associated with CC11 have significantly decreased from 2011117 to 2019116. CC181 and CC10217 have emerged as the dominant clonal complexes alongside CC11 in this region, and the further application of SWGA has allowed the phylogeny of these strains to be mapped across Africa116, 117. Through identifying appropriate primers for amplification, SWGA could also be applied to other meningitis pathogens, such as H. influenzae. The integration of SWGA into genomic surveillance workflows may improve data quality and representativeness when cultures are not available, thereby strengthening public health surveillance and outbreak investigations.
Exploring the Neisserial gene pool
With the identification of further antibiotic-resistant N. meningitidis strains, identifying the potential antibiotic gene resistance pool through sequence analysis could provide valuable insights. As a genetically diverse genus, certain Neisseria species are genetically well-characterized (e.g. N. meningitidis), whereas many are not, due to discordance between WGS and phenotyping-based nomenclature118. There is an ongoing effort to refine species nomenclature using genomics, and further species have recently been discovered using WGS, including Neisseria viridiae and Neisseria basseii118. Due to the highly plastic genomes of Neisseria, the role of intra- and inter-species horizontal gene transfer (HGT) has been well documented119–121. In 2016, two human cases (sporadic) of a novel Neisseria brasiliensis species (animal commensal species) were identified in Brazil122. Genomic analyses identified the isolates as distinct strains of the same species, closely related to Neisseria iguanae. Both genomes contained an intact capsule gene cluster (cps) that was similar in gene organization and sequence identity to N. meningitidis. Isolate 1 carried capsule genes similar to N. meningitidis capsular group X (containing Nm group X sialic acid acetyltransferase) and isolate 2 carried capsule genes similar to capsular group B (containing Nm group B sialic acid transferase). The two cases each acquired a different combination of Nm-like cps genes, likely through multiple HGT steps. However, whether these HGTs occurred in the animal or human host remains unclear. The identification and characterization of these strains demonstrates virulence gene exchange among different Neisseria species.
In the Neisseria genus, antibiotic resistance is primarily found in gonococci and occurs via both point mutation and recombination123; mtrCDE and penA are frequently associated with antibiotic resistance123. However, the antibiotic resistance gene content among non-human Neisseria is yet to be explored.
Vaccine cross-protection against gonorrhea
There is potential for the meningococcal vaccine to infer cross-protection against other genetically related species, such as N. gonorrhoeae, which shares around ~85% of its genetic sequence with N. meningitidis.124 To target a meningococcal epidemic in Cuba, a nationwide mass vaccination campaign with the MenB vaccine, VA-MENGOC-BC, was conducted from 1989 to 1990, followed by a second vaccination campaign in 1991. The incidence of gonorrhea in Cuba increased from 1970 to 1989; however, following the mass VA-MENGOC-BC immunization program, a notable and persistent decrease in gonorrhea incidence was recorded. These preliminary data suggest that A-MENGOC-BC could induce a degree of cross-protection against gonorrhea.125
Herd protection versus direct protection for meningococcal vaccines
Herd protection is a key contributor to decreasing invasive disease. In the UK, the introduction of MenC vaccines resulted in a decreased incidence of meningococcal disease across all age groups, including target and non-target (> 18 years) populations126. Moreover, a 66% reduction in NmC meningococcal carriage among older adolescents was reported127. Similarly, in Canada, the introduction of the MenC conjugate vaccine during 2001–2005 resulted in a sustained reduction in NmC disease, with the largest decrease observed in the 15–24 year age group (83%; 0.27 to 0.05 per 100 000 per year) who were not vaccinated in all 8 provinces. This study suggested that the introduction of the vaccine induced herd protection through the reduced transmission of N. meningitidis.128 These reductions in carriage rates demonstrate successful establishment of herd protection with conjugate vaccines.
In the meningitis belt of Africa, IMD cases peak during the dry season, which has resulted in numerous periodic epidemics over past decades. The Meningitis Vaccine Project was established to rapidly induce herd protection with the aim of vaccinating the target population (1–29 years) with the capsular group A conjugate vaccine (MenAfriVac) to achieve > 90% coverage129. To determine the impact of herd protection, carriage surveys of the target population before and after mass vaccination were performed in three districts in Burkina Faso130. Prior to vaccination, overall carriage was 3.98% and NmA carriage was 0.39%131. Following vaccination (89.7% coverage), no group A carriers were identified in the entire 22,093 samples. The results demonstrated persistent elimination of group A carriage in vaccinated and unvaccinated populations from 3 weeks to 13 months post-mass vaccination. In a 7-year follow-up study of this nationwide mass vaccination campaign in Burkina Faso, zero carriers of serogroup A meningococci were detected among over 13,700 specimens collected, indicating long-term effectiveness of MenAfriVac on carriage132.
Data to support herd protection with quadrivalent MenACWY conjugate vaccines is less robust with a recent meta-analysis concluding that a minimal, non-significant carriage reduction was observed with MenACWY vaccines133. However, indirect protection has been observed in several studies134. In response to a national ST-11 MenW IMD outbreak in England, an emergency adolescent vaccination program with MenACWY was implemented in September 2015. Poisson models were used to estimate the indirect effects of the adolescent MenACWY program in children eligible for 4CMenB but not MenACWY. Over four years, the 4CMenB vaccine was estimated to have directly prevented 98 cases and the MenACWY program to have indirectly prevented between 114 and 899 cases135. Recently, an ecologic analysis was conducted to assess the impact of this program between 2015 and 2019136. In August 2019, vaccine uptake was 37–41% in adolescents (18 years) immunized in primary care and 71–86% in younger teenagers routinely vaccinated in school. It was estimated that the program provided direct protection against 19 cases of NmY and 25 cases of NmW. However, this study suggested potential herd protection with MenACWY. The estimated indirect protection provided by the program was much greater than direct, preventing 60–106 cases of NmY and 25–1193 cases of NmW. In addition, Carr et al. recently reported the carriage of groups C, W, and Y decreased 65% following the introduction of the MenACWY vaccination program in the UK134. As such, MenACWY vaccines appear to confer significant herd protection.
The available data do not suggest that MenB vaccination confer herd protection. For example, 4CMenB vaccination of Australian adolescents did not have a substantial effect on meningococcal carriage137. Therefore, the control of invasive disease incidence cannot rely upon indirect protection from MenB vaccination.
Conclusion
Across the North America region, IMD prevention and control strategies vary, with meningococcal vaccines not widely approved or available as part of the NIP in most countries. In Canada, MenC and MenB vaccines are licensed and routine MenC immunization is included as part of the NIP. In the US, MenACWY conjugate vaccines are licensed and routinely included as part of the NIP and MenB vaccines are recommended for individuals at increased risk of NmB disease, as well as for 16–23 year-olds based on “shared clinical decision making”. The general trend of reducing IMD rates that has been observed across the North American region may, in part, be due to vaccine uptake in the area. As such, increasing the inclusion of meningococcal vaccines into the NIPs of North American countries beyond the US, Canada, and Cuba, is an important milestone that should remain a focus of public health authorities. Additionally, the need to increase vaccine access for at-risk populations, such as PEH and migrants, should not be overlooked.
The potential for vaccine hesitancy to impede uptake remains an issue in some regions of North America and is a concern underpinned by issues surrounding confidence, complacency and convenience. Increasing our understanding of the cause of vaccine hesitancy is key as these insights can help to inform effective solutions.
The incidence of IMD is relatively low throughout North America, with higher incidence rates in specific populations (e.g. youth and PEH). Active and passive surveillance system networks are in place, but as IMD is not notifiable in all countries, the true burden of IMD in North America is not completely understood. Based on the limited available data, NmB, NmC and NmW appear most prevalent; however, notable geographical and temporal variation in dominant serogroups has been observed between North American countries. For example, NmB dominates in the US and Costa Rica, whereas NmC dominates in the Dominican Republic. Expansion of the NmW ST-11, NmB ST-41/44 in Western Canada and NmY CC23 clonal complexes has also been observed in several countries. The emergence and dissemination of antibiotic resistant NmY is an ongoing concern, with reported cases in the USA, Costa Rica, Canada and France.
Disease surveillance is key for the identification of IMD cases and outbreaks, as well as the recognition of trends in transmission and incidence. This information can help identify and avoid potential public health emergencies and guide public health strategies. However, several countries in the North American region, such as Guatemala, appear to lack well-established surveillance systems. Furthermore, there is evidence to suggest that established systems, such as eIMDSS in Canada, may collect limited clinical data. Ensuring that more efficient and effective disease surveillance systems, including for IMD, are developed and sufficiently resourced should be taken as a priority by public health authorities.
During the Covid-19 pandemic, the reported incidence of IMD declined throughout 2020, and prevention and control strategies for IMD were impacted across the globe. The implementation of catch-up immunization programs could help to limit potential disease outbreaks that may occur with the easing of restrictions.
A high level of herd protection with monovalent conjugate vaccines has been demonstrated, and a degree of herd protection has also been observed with quadrivalent MenACWY vaccines.138, 139 However, group B protein-based vaccines must rely purely on direct protection140. Sequence analysis has provided valuable insight into the coverage of MenB vaccines and further insights into the potential pool of antibiotic resistance within the Neisseria species. Recent advancement in sequencing, such as culture-free WGS, have the potential to enhance meningococcal genomic surveillance. Such developments have the potential to further our understanding of meningococcal epidemiology, which could support more informed and effective IMD prevention strategies.
The Global Roadmap on Defeating Meningitis by 2030 is the first resolution endorsed by the World Health Assembly on the prevention and control on meningitis. The framework outlines actionable steps and milestones for meningitis prevention, treatment, and surveillance, as well as improved support for individuals affected by the disease. Continuing to follow this flagship global strategy will support the ultimate target of universal health coverage and a world free of meningitis. Furthermore, updated recommendations on the use of meningococcal vaccines from the SAGE Working Group on meningococcal vaccines and vaccination will help to guide more effective vaccination programs, with the intent of reducing the global burden of meningitis.
Acknowledgements
Medical writing support for the development of this manuscript, under the direction of the authors, was provided Matthew Gunther of Ashfield MedComms, an Inizio company. Medical writing support was funded by Sanofi Pasteur. All authors discussed and agreed to the objectives of this manuscript and contributed throughout its production. All authors read and approved the final manuscript.
Conflicts of interest
Edwin Asturias has received research funding from Pfizer and performs consultant work for Curevac, Inobio, and Merck thru his institution. Xilian Bai performs contract research on behalf of UKHSA for GSK, PATH, Pfizer and Sanofi Pasteur. Ray Borrow performs contract research on behalf of UKHSA for GSK, PATH, Pfizer and Sanofi Pasteur. Ener Cagri Dinleyici performs contract work for the Eskisehir Osmangazi University funded by GSK, Sanofi Pasteur and Pfizer. Gabriela Echaniz- Aviles has received support for research projects from GSK and Pfizer. Jay Lucidarme performs contract research on behalf of UKHSA for GSK, PATH, Pfizer and Sanofi Pasteur. Lee H. Harrison has served on advisory boards and/or made scientific presentations for GSK, Pfizer, Sanofi, and Merck, for which he does not receive any personal fees. H. Marjuki has performed whole genome sequencing activity partially funded through a cooperative agreement between CDC and GSK and Pfizer. Manish Sadarangani is supported via salary awards from the BC Children’s Hospital Foundation and the Michael Smith Foundation for Health Research. Dr Sadarangani has been an investigator on projects funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. Marco A. P. Sáfadi reports research grants and personal fees for advisory boards from GSK, Pfizer, and Sanofi. David S. Stephens reports research grants from the US National Institutes of Health. He has no industry conflicts to report. M.K. Taha performs contract work for the Institut Pasteur funded by GSK, Pfizer and Sanofi Pasteur. M.K. Taha has a patent NZ630133A Patent with GSK “Vaccines for serogroup X meningococcus” issued. J.A. Vázquez performs contract work for the Institute of Health Carlos III funded by GSK and Pfizer and he has received personal fees from Pfizer and Sanofi Pasteur Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
References
- 1.Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol 2012; 799:1–20. doi: 10.1007/978-1-61779-346-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schubert-Unkmeir A. Molecular mechanisms involved in the interaction of Neisseria meningitidis with cells of the human blood–cerebrospinal fluid barrier. Pathog Dis 2017:75. doi: 10.1093/femspd/ftx023. [DOI] [PubMed] [Google Scholar]
- 3.Parikh SR, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect 2020;81 (4):483–98. doi: 10.1016/j.jinf.2020.05.079. [DOI] [PubMed] [Google Scholar]
- 4.Sharip A, et al. Population-based analysis of meningococcal disease mortality in the United States: 1990–2002. Pediatr Infect Dis J 2006; 25 (3):191–4. doi: 10.1097/01.inf.0000202065.03366.0c. [DOI] [PubMed] [Google Scholar]
- 5.Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health 2016; 59 (2 suppl):S3–S11. doi: 10.1016/j.jadohealth.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Alderson MR, et al. Surveillance and control of meningococcal disease in the COVID-19 era: a global meningococcal initiative review. J Infect 2022; 84 (3):269–89. doi: 10.1016/j.jinf.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aye MMA, et al. Meningococcal disease surveillance in the Asia-Pacific region (2020): the global meningococcal initiative. J Infect 2020; 81 (5):698–711. doi: 10.1016/j.jinf.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Bai X, et al. Prevention and control of meningococcal disease: updates from the global meningococcal initiative in Eastern Europe. J infect 2019; 79 (6):528–41. doi: 10.1016/j.jinf.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Meningitis fact sheet. Accessed May 24, 2022. https://www.who.int/news-room/fact-sheets/detail/meningitis.
- 10.Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. Chapter 8: meningococcal. Accessed May 24, 2022. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt08-mening.html. [Google Scholar]
- 11.Centers for Disease Control and Prevention. Enhanced meningococcal disease surveillance report, 2019. Accessed May 24, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf.
- 12.Public Health Agency of Canada. Invasive meningococcal disease: surveillance. Accessed May 24, 2022. https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/invasive-meningococcal-disease/surveillance.html.
- 13.Sadarangani M, Scheifele DW, Halperin SA, et al. The impact of the meningococcal serogroup C conjugate vaccine in Canada between 2002 and 2012. Clin Infect Dis 2014; 59 (9):1208–15. doi: 10.1093/cid/ciu597. [DOI] [PubMed] [Google Scholar]
- 14.Ibarz-Pavón AB, Lemos AP, Gorla MC, Regueira M, SIREVA Working Group II, Gabastou JM. Laboratory-based surveillance of Neisseria meningitidis isolates from disease cases in Latin American and Caribbean countries, SIREVA II 2006–2010. PLoS One 2012; 7 (8):e44102. doi: 10.1371/journal.pone.0044102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gobierino De México. SINAVE - national epidemiological surveillance system. Accessed May 24, 2022. https://www.gob.mx/salud/acciones-y-programas/sistema-nacional-de-vigilancia-epidemiologica#:~:text=El%20Sistema%20Nacional%20de%20Vigilancia,%C3%BAtil%20para%20la%20salud%20p%C3%BAblica.
- 16.GIVEBPVac (Grupo Interinstitucional para La Vigilancia de Enfermedades Bacterianas Prevenibles por Vacunación) 2019. Accessed July 05, 2022. https://www.insp.mx/lineas-de-investigacion/medicamentos-en-salud-publica/sireva.html.
- 17.Dueger EL, et al. Culture- and antigen-negative meningitis in Guatemalan children. Rev Panam Salud Publica 2008; 24 (4):248–55. doi: 10.1590/s1020-49892008001000004. [DOI] [PubMed] [Google Scholar]
- 18.Data Obtained from Gobierno De La República Dominicana Salud Pública. Accessed May 24, 2022. https://msp.gob.do/web/.
- 19.Adjorlolo S, Egbenya DL. A twin disaster: addressing the COVID-19 pandemic and a cerebrospinal meningitis outbreak simultaneously in a low-resource country. Glob Health Action 2020; 13 (1):1795963. doi: 10.1080/16549716.2020.1795963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whalen CM, et al. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. JAMA 1995; 273 (5):390–4. [PubMed] [Google Scholar]
- 21.Squires SG, et al. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 1999, through 31 December, 2001. Can Commun Dis Rep 2004; 30 (3):17–28. [PubMed] [Google Scholar]
- 22.De Wals P Epidemiology and control of meningococcal disease in Canada: a long, complex, and unfinished story. Can J Infect Dis Med Microbiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mbaeyi SA, et al. Epidemiology of meningococcal disease outbreaks in the United States, 2009–2013. Clin Infect Dis 2019; 68 (4):580–5. doi: 10.1093/cid/ciy548. [DOI] [PubMed] [Google Scholar]
- 24.Oliver SE, Mbaeyi SA. A review of global epidemiology and response to meningococcal disease outbreaks among men who have sex with men, 2001–2018. Curr Epidemiol Rep 2018; 5:321–30. doi: 10.1007/s40471-018-0170-z. [DOI] [Google Scholar]
- 25.Soeters HM, McNamara LA, Blain AE, et al. University-based outbreaks of meningococcal disease caused by serogroup B, United States, 2013–2018. Emerg Infect Dis 2019; 25 (3):434–40. doi: 10.3201/eid2503.181574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mbaeyi SA, Blain A, Whaley MJ, Wang X, Cohn AC, MacNeil JR. Epidemiology of meningococcal disease outbreaks in the United States, 2009–2013. Clin Infect Dis 2019; 68 (4):580–5. doi: 10.1093/cid/ciy548. [DOI] [PubMed] [Google Scholar]
- 27.Chacon-Cruz E, et al. An outbreak of serogroup C (ST-11) meningococcal disease in Tijuana, Mexico. Ther Adv Vaccines 2014; 2 (3):71–6. doi: 10.1177/2051013614526592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Data Obtained from National Notifiable Diseases Surveillance System. Accessed May 24, 2022. https://www.cdc.gov/nndss/index.html.
- 29.Data Obtained from National Reference Center for Bacteriology (NRCB), IN-CIENSA. Accessed May 24, 2022. https://www.inciensa.sa.cr.
- 30.Data Obtained from Department of Epidemiology, Gobierno Nacional De Panamá (2022). Accessed May 24, 2022. https://www.minsa.gob.pa/.
- 31.Data Obtained from Active Bacterial Core Surveillance. Accessed May 24, 2022. https://www.cdc.gov/abcs/index.html#:~:text=Active%20Bacterial%20Core%20surveillance%20(ABCs)%20is%20an%20active%20laboratory%2D,pathogens%20of%20public%20health%20importance.
- 32.Data Obtained from EMDS 2016–2019 Surveillance Data. Accessed May 24, 2022. https://www.cdc.gov/meningococcal/surveillance/index.html.
- 33.Abstract presented as CSTE 2022 #.
- 34.Data Obtained from Ministerio de Salud Pública y Asistencia Social. National Epidemiological Bulletin. 2001, No. 17. Accessed May 24, 2022. https://www.mspas.gob.gt/. [Google Scholar]
- 35.Dirección General de Epidemiología. Anuario De Morbilidad. Secretaría de Salud de México, 2015. https://epidemiologia.salud.gob.mx/anuario/html/incidencia_casos.html.
- 36.Chacon-Cruz E, Roberts C, Rivas-Landeros RM, Lopatynsky-Reyes EZ, Almada-Salazar LA, Alvelais-Palacios JA. Pediatric meningitis due to Neisseria meningitidis, Streptococcus pneumoniae and Group B Streptococcus in Tijuana, Mexico: active/prospective surveillance, 2005–2018. Ther Adv Infect Dis 2019; 6:2049936119832274. doi: 10.1177/2049936119832274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez-Elizondo MT, Garza-González E, Echániz-Aviles G, Flores-Treviño S, Carnalla-Barajas MN, Camacho-Ortiz A. Increased incidence of Neisseria meningitidis infections in Nuevo León, Mexico. Aumento de la incidencia de infecciones por Neisseria meningitidis en Nuevo León, México. Salud Publica Mex 2020; 62 (2):120–1. doi: 10.21149/10790. [DOI] [PubMed] [Google Scholar]
- 38.Solórzano-Santos F, Echaniz-Avilés G. Aumento en casos de meningitis meningocócica en los estados del sur de México. Salud Publica Mex 2021; 63 (2):165–6. doi: 10.21149/11725. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Meningococcal disease surveillance. Accessed May 24, 2022. https://www.cdc.gov/meningococcal/surveillance/index.html.
- 40.Enhanced meningococcal disease surveillance report, 2019. Accessed July 06, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf.
- 41.CDC unpublished data: Manuscript in preparation.
- 42.Canada National Surveillance Data, presented at the Global Meningococcal Initiative 10th Regional Meeting: Meningococcal Disease in North America, March 21–24, 2022.
- 43.NML unpublished data.
- 44.Data obtained from Gorgas Memorial Institute. Accessed May 24, 2022. http://www.gorgas.gob.pa/.
- 45.Tsang R, Hoang L, Tyrrell GJ, et al. Increase in Neisseria meningitidis serogroup W invasive disease in Canada: 2009–2016. Can Commun Dis Rep 2017; 43 (7–8):144–9 Published 2017 Jul 6. doi: 10.14745/ccdr.v43i78a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease—United States, 1996–2015. Clin Infect Dis 2018; 66 (8):1276–81. [DOI] [PubMed] [Google Scholar]
- 47.Enhanced meningococcal disease surveillance report, 2019. Accessed August 09, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2019.pdf.
- 48.Enhanced meningococcal disease surveillance report, 2016. Accessed August 09, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report.pdf.
- 49.Enhanced meningococcal disease surveillance report, 2015. Accessed August 09, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2015.pdf.
- 50.Enhanced meningococcal disease surveillance report, 2017. Accessed August 09, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2017.pdf.
- 51.Enhanced meningococcal disease surveillance report, 2018. Accessed August 09, 2022. https://www.cdc.gov/meningococcal/downloads/NCIRD-EMS-Report-2018.pdf.
- 52.De Wals P, Dionne M, Douville-Fradet M, Boulianne N, Drapeau J, De Serres G. Impact of a mass immunization campaign against serogroup C meningococcus in the Province of Quebec. Canada. Bull World Health Organ 1996; 74 (4):407–11. [PMC free article] [PubMed] [Google Scholar]
- 53.De Wals P, De Serres G, Niyonsenga T. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA 2001; 285 (2):177–81. doi: 10.1001/jama.285.2.177. [DOI] [PubMed] [Google Scholar]
- 54.De Wals P, Deceuninck G, Boulianne N, De Serres G. Effectiveness of a mass immunization campaign using serogroup C meningococcal conjugate vaccine. JAMA 2004; 292 (20):2491–4. doi: 10.1001/jama.292.20.2491. [DOI] [PubMed] [Google Scholar]
- 55.De Wals P, et al. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clin Infect Dis 2017; 64:1263–7. [DOI] [PubMed] [Google Scholar]
- 56.Dinleyici EC, et al. Vaccines and routine immunization strategies during the COVID-19 pandemic. Hum Vaccin Immunother 2021; 17 (2):400–7. doi: 10.1080/21645515.2020.1804776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the advisory committee on immunization practices, United States, 2020. MMWR Recomm Rep. 2020; 69 (9):1–41. doi: 10.15585/mmwr.rr6909a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meningococcal vaccine: Canadian immunization guide (https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-13-meningococcal-vaccine.html).
- 59.Government of Canada. Recommended immunization schedules: Canadian immunization guide. Accessed July, 05 2022. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-13-recommended-immunization-schedules.html#p1c12a2.
- 60.Pingali C, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States. MMWR Morb Mortal Wkly Rep 2020; 70:1183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aguilar-Guerra TL, Fajardo-Díaz EM, Gorry C. Cuba’s national regulatory authority & COVID-19: Olga Lidia Jacobo-Casanueva MS Director, Center for State Control of Drugs and Medical Devices (CECMED). MEDICC Rev 2021; 23 (3–4):9–14. doi: 10.37757/MR2021.V23.N3.3. [DOI] [PubMed] [Google Scholar]
- 62.Romeu B, Rodríguez Y, Bendiner S. The role of regulatory sciences from the perspective of the Cuban medicines regulatory agency: the impact of COVID-19 in promoting innovation, cooperation and scientific thinking. Ther Innov Regul Sci 2021; 55 (5):1014–18. doi: 10.1007/s43441-021-00300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organisation. Immunization data. Accessed May 24, 2022. https://immunizationdata.who.int/listing.html?topic=&location=.
- 64.Data obtained from hospital del Niño. Accessed May 24, 2022. https://hn.sld.pa/.
- 65.World Health Organization. Launch of the defeating meningitis roadmap. Accessed May 24, 2022. https://www.who.int/news-room/events/detail/2021/09/28/default-calendar/launch-of-the-defeating-meningitis-roadmap.
- 66.World Health Organization. Defeating meningitis by 2030. Accessed May 24, 2022. https://www.who.int/initiatives/defeating-meningitis-by-2030.
- 67.Nadel S. Treatment of meningococcal disease. J Adolesc Health 2016; 59 (2 Suppl):S21–8. doi: 10.1016/j.jadohealth.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Oppenheim BA. Antibiotic resistance in Neisseria meningitidis. Clin Infect Dis 1997; 24 (Suppl1):S98–101. doi: 10.1093/clinids/24.supplement_1.s98. [DOI] [PubMed] [Google Scholar]
- 69.Willerton L, et al. Antibiotic resistance among invasive Neisseria meningitidis isolates in England, Wales and Northern Ireland (2010/11 to 2018/19). PLoS One 2021; 16 (11):e0260677. doi: 10.1371/journal.pone.0260677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang B, et al. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine 2019; 37 (21):2768–82. doi: 10.1016/j.vaccine.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 71.Harcout BH, et al. Population-based surveillance of Neisseria meningitidis antimicrobial resistance in the United States. Open Forum Infect Dis 2015; 2 (3):ofv117. doi: 10.1093/ofid/ofv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Potts CC, et al. Antimicrobial susceptibility survey of invasive Neisseria meningitidis, United States 2012–2016. J Infect Dis 2022:jiac046 Mar 10. doi: 10.1093/infdis/jiac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Potts CC, et al. Acquisition of ciprofloxacin resistance among an expanding clade of β-lactamase–positive, serogroup Y Neisseria meningitidis in the United States. Clin Infect Dis 2021; 73 (7):1185–93. doi: 10.1093/cid/ciab358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taormina G, et al. β-Lactamase–producing, ciprofloxacin-resistant Neisseria meningitidis isolated from a 5-month-old boy in the United States. J Pediatric Infect Dis Soc 2021; 10 (3):379–81. doi: 10.1093/jpids/piaa085. [DOI] [PubMed] [Google Scholar]
- 75.Data obtained from bacterial meningitis laboratory (Centers for Disease Control and Prevention).
- 76.Bacterial meningitis genome analysis platform. Accessed May 24, 2022. https://github.com/CDCgov/BMGAP.
- 77.Marín JEO, et al. Emergence of MDR invasive Neisseria meningitidis in El Salvador, 2017–19. J Antimicrob Chemother 2021; 76 (5):115–1159. doi: 10.1093/jac/dkab010. [DOI] [PubMed] [Google Scholar]
- 78.Tsang RSW, et al. WGS analysis of a penicillin-resistant Neisseria meningitidis strain containing a chromosomal ROB-1 β-lactamase gene. J Antimicrob Chemother 2019; 74 (1):22–8. doi: 10.1093/jac/dky391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hong E, et al. Acquisition of beta-lactamase by Neisseria meningitidis through possible horizontal gene transfer. Antimicrob Agents Chemother 2018; 62 (9):e00818–31. doi: 10.1128/AAC.00831-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Potts CC, et al. Acquisition of ciprofloxacin resistance among an expanding clade of β-lactamase-positive, serogroup Y Neisseria meningitidis in the United States. Clin Infect Dis 2021; 73:1185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.INCIENSA (Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud). Aislamientos De Neisseria meningitis Serogrupo Y resistentes a Penicilina y No Sensibles a Cefotaxime En Costa Rica. Accessed May 24, 2022. https://www.inciensa.sa.cr/vigilancia_epidemiologica/informes_vigilancia/2020/CNRB/Alerta%20N.%20meningitidis%20Y%20resistente%20a%20PEN%20no%20sensible%20a%20CTX%202020.pdf.
- 82.BC Centre for Disease Control. Immunization coverage reports. Accessed May 24, 2022. http://www.bccdc.ca/health-professionals/data-reports/immunizations.
- 83.Public Health Agency of Canada. Highlights from the 2019 Childhood National Immunization Coverage Survey (cNICS). Accessed May 24, 2022. https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/2019-highlights-childhood-national-immunization-coverage-survey.html.
- 84.BC Centre for Disease Control. Childhood immunization coverage dashboard. Accessed May 24, 2022. http://www.bccdc.ca/health-professionals/data-reports/immunizations.
- 85.Report of the SAGE working group on vaccine hesitancy. Accessed May 24, 2022. https://www.asset-scienceinsociety.eu/sites/default/files/sage_working_group_revised_report_vaccine_hesitancy.pdf.
- 86.Dubé E, Gagnon D, Ouakki M, et al. Measuring vaccine acceptance among Canadian parents: a survey of the Canadian Immunization Research Network. Vaccine 2018; 36 (4):545–52. doi: 10.1016/j.vaccine.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 87.Wallace AS, Mantel C, Mayers G, Mansoor O, Gindler JS, Hyde TB. Experiences with provider and parental attitudes and practices regarding the administration of multiple injections during infant vaccination visits: lessons for vaccine introduction. Vaccine 2014; 32 (41):5301–10. doi: 10.1016/j.vaccine.2014.07.076. [DOI] [PubMed] [Google Scholar]
- 88.Vuocolo S, et al. Vaccination strategies for the prevention of meningococcal disease. Hum Vaccin Immunother 2018; 14 (5):1203–15. doi: 10.1080/21645515.2018.1451287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schanzenbach D, Pitts A (2020). Estimates of food insecurity during the covid-19 crisis: results from the COVID impact survey, Week 1 (April 20–26, 2020). 2020; 1:1–12. [Google Scholar]
- 90.Wang J, et al. Progression of myopia in school-aged children after COVID-19 home confinement. JAMA Ophthalmol 2021; 139 (3):293–300. doi: 10.1001/jamaophthalmol.2020.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu L, et al. COVID-19 quarantine reveals that behavioral changes have an effect on myopia progression. Opthalmology 2021; 128 (11):1652–4. doi: 10.1016/j.ophtha.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nicola M, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 2020; 78:185–93. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Unwin HJT, et al. Global, regional, and national minimum estimates of children affected by COVID-19-associated orphanhood and caregiver death, by age and family circumstance up to Oct 31, 2021: an updated modelling study. Lancet Child Adolesc Health 2022; 6 (4):249–59. doi: 10.1016/S2352-4642(22)00005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Causey K, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modelling study. Lancet 2021; 398 (10299):522–34. doi: 10.1016/S0140-6736(21)01337-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harris RC, et al. Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: disruptions and solutions. Lancet Reg Health West Pac 2021; 10:100140. doi: 10.1016/j.lanwpc.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cohen R, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now 2021; 51 (5):418–23. doi: 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McDonald HI, et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Euro Surveill 2020; 25 (19):2000848. doi: 10.2807/1560-7917.ES.2020.25.19.2000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ali A, et al. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health 2014; 108 (1):11–20. doi: 10.1179/2047773214Y.0000000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Panatto D, et al. Neisseria meningitidis B vaccines. Expert Rev Vaccines 2011; 10 (9):1337–51. doi: 10.1586/erv.11.103. [DOI] [PubMed] [Google Scholar]
- 100.Davide S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine 2013; 30 (02):B87–97. doi: 10.1016/j.vaccine.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simões MJ, Bettencourt C, De Paola R, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Portugal. PLoS One 2017; 12 (5):e0176177 Published 2017 May 1. doi: 10.1371/journal.pone.0176177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borrow R, et al. Methods to evaluate serogroup B meningococcal vaccines: from predictions to real-world evidence. J Infect 2020; 81 (6):862–72. doi: 10.1016/j.jinf.2020.07.034. [DOI] [PubMed] [Google Scholar]
- 103.Donnelly J, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA 2010; 107 (45):19490–5. doi: 10.1073/pnas.1013758107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vogel U, et al. Predicted strain coverage of a meningococcal multicomponent vaccine (4CMenB) in Europe: a qualitative and quantitative assessment. Lancet Infect Dis 2013; 13 (5):416–25. doi: 10.1016/S1473-3099(13)70006-9. [DOI] [PubMed] [Google Scholar]
- 105.Muzzi A, et al. Genetic Meningococcal Antigen Typing System (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 2019; 37 (7):991–1000. doi: 10.1016/j.vaccine.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 106.Rodrigues CMC, et al. Meningococcal Deduced Vaccine Antigen Reactivity (MenDeVAR) Index: a rapid and accessible tool that exploits genomic data in public health and clinical microbiology applications. J Clin Microbiol 2021; 59 (1):e02120–61. doi: 10.1128/JCM.02161-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McNeil LK, et al. Predicting the susceptibility of meningococcal serogroup B isolates to bactericidal antibodies elicited by bivalent rLP2086, a novel pro- phylactic vaccine. MBio 2018; 9:e00018–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muzzi A, Brozzi A, Serino L, et al. Genetic Meningococcal Antigen Typing System (gMATS): a genotyping tool that predicts 4CMenB strain coverage worldwide. Vaccine 2019; 37 (7):991–1000. doi: 10.1016/j.vaccine.2018.12.061. [DOI] [PubMed] [Google Scholar]
- 109.Nissen MD, Marshall HS, Richmond PC, et al. A randomized, controlled, phase 1/2 trial of a Neisseria meningitidis serogroup B bivalent rLP2086 vaccine in healthy children and adolescents. Pediatr Infect Dis J 2013; 32 (4):364–71. doi: 10.1097/INF.0b013e31827b0d24. [DOI] [PubMed] [Google Scholar]
- 110.Richmond PC, Nissen MD, Marshall HS, et al. A bivalent Neisseria meningitidis recombinant lipidated factor H binding protein vaccine in young adults: results of a randomised, controlled, dose-escalation phase 1 trial. Vaccine 2012; 30 (43):6163–74. doi: 10.1016/j.vaccine.2012.07.065. [DOI] [PubMed] [Google Scholar]
- 111.Bettinger JA, et al. Diversity of Canadian meningococcal serogroup B isolates and estimated coverage by an investigational meningococcal serogroup B vaccine (4CMenB). Vaccine 2013; 32 (1):124–30. [DOI] [PubMed] [Google Scholar]
- 112.Betting JA, et al. Estimated susceptibility of Canadian meningococcal B isolates to a meningococcal serogroup B vaccine (MenB-FHbp). Vaccine 2020; 38 (8):2026–33. [DOI] [PubMed] [Google Scholar]
- 113.Maiden MC, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad USA 1998; 95:3140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whaley MJ, et al. Whole genome sequencing for investigations of meningo- coccal outbreaks in the United States: a retrospective analysis. Sci Rep 2018; 8:15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Itsko M, et al. Full molecular typing of Neisseria meningitidis directly from clinical specimens for outbreak investigation. J Clin Microbiol 2020; 58 (12):e01720–80. doi: 10.1128/JCM.01780-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Itsko M, et al. Enhancing meningococcal genomic surveillance in the meningitis belt using high-resolution culture-free whole genome sequencing. J Infect Dis 2022. jiac104. doi: 10.1093/infdis/jiac104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Topaz N, et al. Phylogenetic relationships and regional spread of meningococcal strains in the meningitis belt, 2011–2016. EBioMedicine 2019; 41:488–96. doi: 10.1016/j.ebiom.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Diallo K, MacLennan J, Harrison OB, et al. Genomic characterization of novel Neisseria species. Sci Rep 2019; 9 (1):13742 2019. doi: 10.1038/s41598-019-50203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marri PR, et al. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 2010; 5 (7):e11835. doi: 10.1371/journal.pone.0011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kong Y, et al. Homologous recombination drives both sequence diversity and gene content variation in Neisseria meningitidis. Genome Biol Evol 2013; 5 (9):1611–27. doi: 10.1093/gbe/evt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swartley JS, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A, 1997; 94 (1):271–6. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mustapha MM, et al. Two cases of newly characterized Neisseria species. Brazil. Emerg Infect Dis 2020; 26 (2):366–9. doi: 10.3201/eid2602.190191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Golparian D, et al. Genomic evolution of Neisseria gonorrhoeae since the preantibiotic era (1928–2013): antimicrobial use/misuse selects for resistance and drives evolution. BMC Genomics 2020; 21 (1):116. doi: 10.1186/s12864-020-6511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marjuki H, et al. Genetic similarity of gonococcal homologs to meningococcal outer membrane proteins of serogroup B vaccine. MBio 2019; 10 (5):e01619–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ochoa Azze RF. A meningococcal B vaccine induces cross-protection against gonorrhea. Clin Exp Vaccine Res 2019; 8 (2):110–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trotter CL, Maiden MCJ. Meningococcal vaccines and herd immunity: lessons learned from serogroup C conjugate vaccination programs. Expert Rev Vaccines 2009; 8 (7):851–61. doi: 10.1586/erv.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maiden MCJ, Stuart JM, Meningococcal Carriage Group UK. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 2002; 359 (9320):1829–31. doi: 10.1016/S0140-6736(02)08679-8. [DOI] [PubMed] [Google Scholar]
- 128.Sadarangani M, et al. The impact of the meningococcal serogroup C conjugate vaccine in Canada between 2002 and 2012. Clin Infect Dis 2014; 59 (9):1208–15. [DOI] [PubMed] [Google Scholar]
- 129.Viviani S. Efficacy and effectiveness of the meningococcal conjugate group A vaccine MenAfriVac® in preventing recurrent meningitis epidemics in Sub-Saharan Africa. Vaccines 2022; 10 (4):617. doi: 10.3390/vaccines10040617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Novak RT, et al. Serogroup A meningococcal conjugate vaccination in Burkina Faso: analysis of national surveillance data. Lancet Infect Dis 2012; 12 (10):757–64. doi: 10.1016/S1473-3099(12)70168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kristiansen PA, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis 2012; 56 (3):354–63. doi: 10.1093/cid/cis892. [DOI] [PubMed] [Google Scholar]
- 132.Mbaeyi S, et al. Meningococcal carriage 7 years after introduction of a serogroup A meningococcal conjugate vaccine in Burkina Faso: results from four cross-sectional carriage surveys. Lancet Infect Dis 2020; 20:1418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McMillan M, et al. Effectiveness of meningococcal vaccines at reducing invasive meningococcal disease and pharyngeal Neisseria meningitidis carriage: a systematic review and meta-analysis. Clin Infect Dis 2021; 73 (3):e609–19. doi: 10.1093/cid/ciaa1733. [DOI] [PubMed] [Google Scholar]
- 134.Carr JP, et al. Impact of meningococcal ACWY conjugate vaccines on pharyngeal carriage in adolescents: evidence for herd protection from the UK MenACWY programme. Clin Microbiol Infect 2022. [Epub ahead of online print]. doi: 10.1016/j.cmi.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 135.Ladhani SN, et al. First real-world evidence of meningococcal group B vaccine, 4CMenB, protection against meningococcal group W disease: prospective enhanced national surveillance, England. Clin Infect Dis 2021; 73 (7):e1661–8. doi: 10.1093/cid/ciaa1244. [DOI] [PubMed] [Google Scholar]
- 136.Campbell H, et al. Impact of an adolescent meningococcal ACWY immunisation programme to control a national outbreak of group W meningococcal disease in England: a national surveillance and modelling study. Lancet Child Adolesc Health 2022; 6 (2):96–105. doi: 10.1016/S2352-4642(21)00335-7. [DOI] [PubMed] [Google Scholar]
- 137.Marshall HS, et al. Meningococcal B vaccine and meningococcal carriage in adolescents in Australia. N Engl J Med 2020; 382 (4):318–27. doi: 10.1056/NEJMoa1900236. [DOI] [PubMed] [Google Scholar]
- 138.Ramsay ME, et al. (2003). Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis;326:365–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Campbell H, et al. (2022). Impact of adolescent meningococcal ACWY immunisation programme to control a national outbreak of group W meningococcal disease in England: a national surveillance and modelling study;6:96–105 [DOI] [PubMed] [Google Scholar]
- 140.McMillan M, et al. (2021). effectiveness of meningococcal vaccines at reducing invasive meningococcal disease and pharyngeal Neisseria meningitidis carriage: a systematic review and meta-analysis;73:e609–19 [DOI] [PubMed] [Google Scholar]
- 141.Public Health Agency of Canada. Invasive meningococcal disease. Accessed May 24, 2022. https://www.canada.ca/en/public-health/services/immunization/vaccine-preventable-diseases/invasive-meningococcal-disease/health-professionals.html#figure1.
- 142.Nationally Notifiable Infectious Diseases and Conditions (Centers for Disease Control and Prevention). Weekly cases of notifiable diseases, United States, U.S. territories, and non-U.S. Residents week ending January 2, 2021 (Week 53). Accessed May 24, 2022. https://wonder.cdc.gov/nndss/static/2020/53/2020-53-table1w.html.
- 143.Pérez AE, Dickinson F, Llanes R. Invasive meningococcal disease. Cuba, 1983–2006. Vaccimonitor 2010; 19 (3):8–14. [Google Scholar]


