Abstract
Background:
Neural tube defects (NTDs) encompass a variety of distinct types. We assessed if the preventive effect of folic acid (FA) varied by NTD type and infant sex.
Methods:
We examined all pregnancies with NTD status confirmation from a pregnancy-monitoring system in selected locations in northern and southern regions of China between 1993 and 1996. Women who took 400 μg of FA daily during 42 days after last menstrual period were considered FA users. We analyzed NTD prevalence by FA use status, NTD type, geographic region, and infant sex.
Results:
Among 626,042 pregnancies, 700 were affected by an NTD. Among FA nonusers, 65 pregnancies (8.8 per 10,000) in the north and 51 pregnancies (1.2 per 10,000) in the south were affected by one of the two rare NTDs, that is, craniorachischisis, iniencephaly. FA use prevented occurrence of these two rare NTDs and reduced the prevalence of spina bifida (SB) by 78% (from 17.9 to 3.9 per 10,000) in the north and 51% (from 2.4 to 1.2 per 10,000) in the south. Among FA users, SB prevalence, including SB with high lesion level, was significantly reduced in both geographic regions. FA use reduced prevalence of anencephaly and encephalocele by 85% and 50%, respectively in the north, while it did not reduce the prevalence of these two NTDs in the south. There was a greater reduction in NTD prevalence in female than in male infants and fetuses.
Conclusions:
This is the first study to show that FA prevents the entire spectrum of NTD types.
Keywords: congenital anomaly, folic acid, neural tube defects (NTDs), vitamin supplement
1 |. INTRODUCTION
Neural tube defects (NTDs) are major birth defects of the brain and spine that occur early in pregnancy as a result of improper closure of the embryonic neural tube leading to death or varying degrees of disability (Williams et al., 2015). Neural tube formation is completed by the 28th day after conception (Copp & Greene, 2013; Moore, 2006), often before a pregnancy is recognized. As a group, NTDs rank among the most common birth defects—affecting an estimated 260,000 infants worldwide in 2015 (Blencowe, Kancherla, Moorthie, Darlison, & Modell, 2018; Zaganjor et al., 2016). NTDs encompass a variety of distinct phenotypes whose prevalence varies across geographic regions. For example, the prevalence of NTDs in the northern China province of Hebei was five-fold higher than in two southern provinces of Zhejiang and Jiangsu (50 vs. 10 per 10,000 pregnancies; Berry et al., 1999). This finding is likely due to regional differences in dietary folate intake (Hao et al., 2003) and genetic variations (Crider, Zhu, et al., 2011; Yan et al., 2012; Zhang et al., 2013), as there are lower blood folate concentrations and higher MTHFR TT frequencies in the northern region compared to the southern region of China (Crider, Zhu, et al., 2011; Hao et al., 2003; Yan et al., 2012; Zhang et al., 2013). In the United States, the two most common NTD types are anencephaly and spina bifida, with estimated prevalence of 2.6 and 4 per 10,000 births, respectively (Mai et al., 2019; Williams et al., 2015). Craniorachischisis and iniencephaly are rare forms in countries with low overall NTD prevalence. Compared with a U.S. population-based surveillance program in metropolitan Atlanta (CorreaVillasenor et al., 2003), the ratios of prevalence for two rare types of NTDs in northern China were markedly increased, at 81-fold for craniorachischisis and 25-fold for iniencephaly (Moore et al., 1997). In addition, previous studies, have documented a higher prevalence of NTDs in female than in male infants and fetuses (Källén et al., 1994; Liu, Li, et al., 2018; Poletta et al., 2018).
Periconceptional use of folic acid (FA) has been shown to reduce the risk of NTDs (Berry et al., 1999; Crider, Qi, Devine, Tinker, & Berry, 2018; Czeizel & Dudas, 1992; MRC, 1991). In 1992, the U.S. Public Health Service issued a recommendation that all women who could become pregnant consume 400 μg FA daily for NTD prevention (CDC, 1992). Previous studies have shown that the preventive effect of FA can vary by NTD type and location. A study in the United States reported a 23% decline in spina bifida prevalence and a 11% decline in anencephaly prevalence (from 2.6 to 2.0 and 1.2 to 1.0 per 10,000 births, respectively) after mandatory fortification of enriched grain products with FA (Honein, Paulozzi, Mathews, Erickson, & Wong, 2001). In Canada, one study found the highest decrease for spina bifida at 53% and lesser decreases for anencephaly and encephalocele at 38% and 31%, respectively, after food fortification with FA (De Wals et al., 2007). A study in Australia reported reductions for anencephaly, encephalocele, and spina bifida at 32%, 34%, and 23%, respectively after the promotion of FA supplements and voluntary fortification (Bower, D’Antoine, & Stanley, 2009). In addition, the impact of spina bifida is determined by size and location of the malformation, with higher level lesions presenting with more extensive nerve damage. Several studies have noted a change in the spina bifida phenotype with FA fortification (De Wals et al., 2008; Eldridge et al., 2018). This study aims to expand knowledge on whether the preventive effect of periconceptional exposure to FA varies by NTD type, including spina bifida lesion level, or infant sex.
2 |. METHODS
2.1 |. Study population
This study included participants of a population-based pregnancy-monitoring system known as the Perinatal Health Care (PHC) Surveillance System in selected locations in northern and southern regions in China (Berry et al., 1999). Pregnancies in the PHC dataset included any births delivered between January 1, 1993 and December 31, 1996 that could be confirmed as either having or not having an NTD. From October 1, 1993 to September 30, 1995, the Chinese Ministry of Health conducted a public health campaign to prevent NTDs with FA. During this time, all women who attended mandatory premarital examinations in locations covered by the PHC system were asked to purchase and consume 400 μg of FA in the form of a pill once a day until the end of the first trimester of pregnancy. Each woman’s pill usage was collected monthly. All women who took pills provided oral informed consent for participation in the campaign and collection of photographs (Berry et al., 1999). Additional information on the public health campaign and the findings are available elsewhere (Berry et al., 1999). The current activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (U.S. Department of Human and Health Services, 2018).
2.2 |. FA supplementation
Women were categorized into three FA exposure groups—no exposure, complete exposure, incomplete exposure. Exposure was defined based on FA use during a 42-day window between last menstrual period (LMP) and 42 days after LMP, with the assumption that conception happened around 14 days after LMP and the neural tube completes closure at about 28 days after conception. We defined compliance as the average monthly percentage of FA pills that were taken as compared with the number that could have been taken one pill per day. The complete exposure group includes women who took 400 μg of FA daily during this 42-day exposure window with a compliance rate of at least 80%. The no exposure group includes women who took no FA before LMP and during the 60 days after LMP. To ensure the unexposed group was unlikely to have any exposure within the critical window, 60 instead of 42 days after LMP was used to account for variations in menstrual cycle length and timing of conception. The incomplete exposure group includes all the remaining women and were excluded from further analyses. Women with complete exposure and no exposure are designated as FA users and FA nonusers, respectively. Between 1993 and 1996, when data for this study were collected in China, FA supplements were not commercially available, and foods were not fortified with FA in the study locations.
2.3 |. Neural tube defects
NTD-affected pregnancies in both geographic regions were identified via a birth defects surveillance system established in 1992 (Li et al., 2003). The system recorded detailed information on infants and fetuses with external birth defects including descriptions and measurements of the defects as well as photographs of the defects for approximately 85% of cases. NTDs included in our study are anencephaly, spina bifida, encephalocele, as well as the rare forms, craniorachischisis, and iniencephaly. Figure 1 shows examples of each NTD type using photographs from our study and further description of the main NTD types is contained in Table S1.
FIGURE 1.
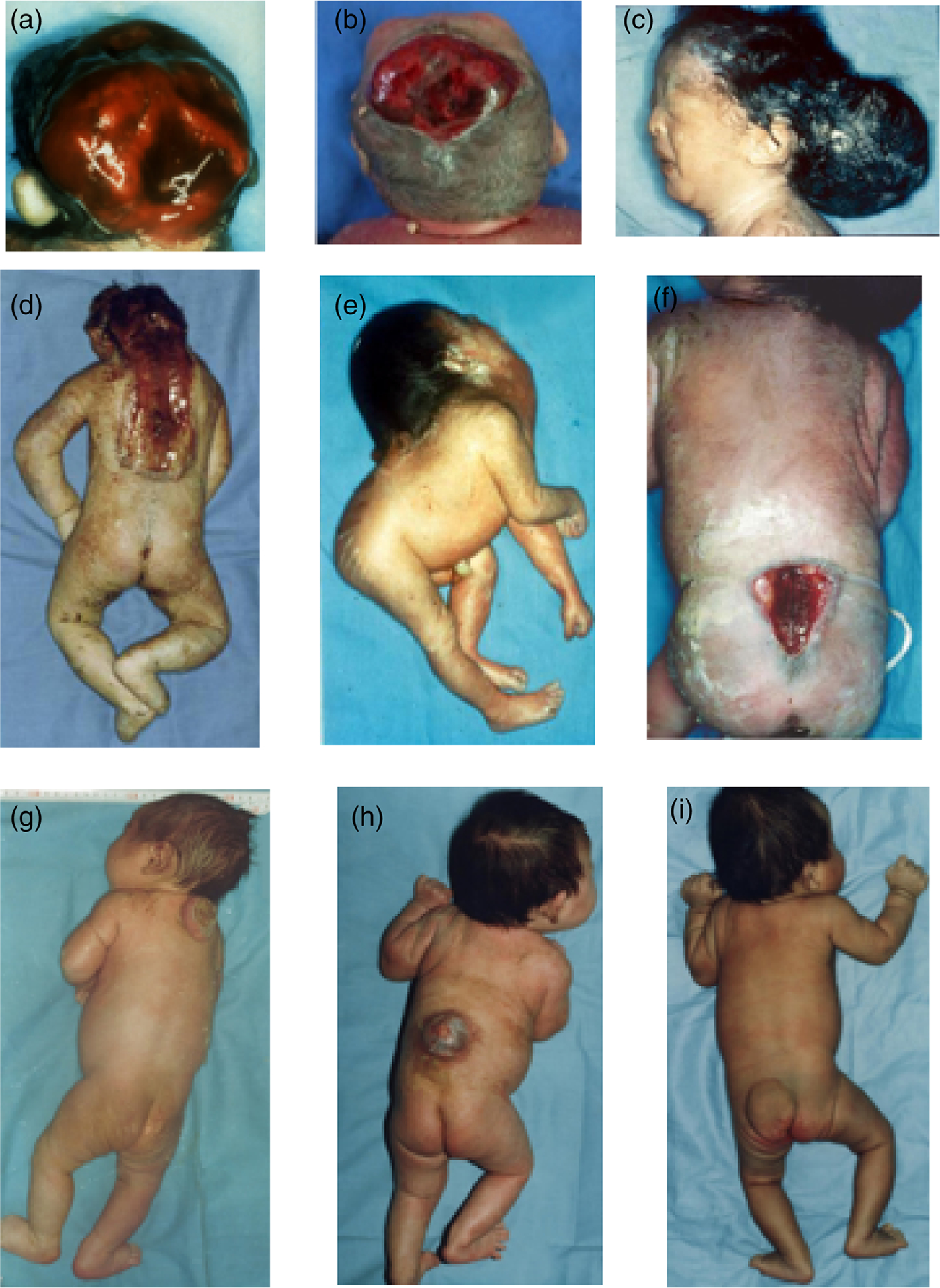
Types of neural tube defects, CDC-Beijing Medical University Collaborative Project, 1993–1996. Infant A—anencephaly, subtype holoanencephaly. Infant B—anencephaly, subtype meroanencephaly. Infant C—occipital encephalocele. Infant D—Craniorachischisis. Infant E—iniencephaly. Infant F—spina bifida, rachischisis, highest level sacral, and open lesion. Infant G—spina bifida, unspecified type, highest level cervical, open lesion. Infant H—spina bifida, unspecified type, highest level thoracic, open lesion. Infant I—spina bifida, unspecified type, highest level lumbar, closed lesion
Spina bifida were further classified into high and low-level lesions, according to the highest level of the lesion on the spine. Cervical and thoracic lesions were considered high level lesions; lumbar and sacral lesions were considered low level. In addition, NTDs were classified as “open” or “closed.” “Open” lesions had exposed neural tissue or were covered by meninges and “closed” lesions were covered by normal-appearing skin.
2.4 |. Sex difference
After excluding observations with missing, unknown or ambiguous sex in the PHC dataset, the NTD prevalence was calculated by infant sex and NTD type, among FA users and nonusers, to determine if FA changed the NTD prevalence ratio (PR) between male and female offspring.
2.5 |. Statistical analysis
NTD prevalence was calculated as the number of NTD-affected pregnancies divided by the total number of pregnancies with NTD status confirmation. We stratified data by geographic region, NTD type and FA use status, and calculated difference in NTD prevalence to assess whether they are significantly different from 0 at the .05 level. Within each stratum, Poisson regression was conducted to estimate male to female PRs and the corresponding 95% confidence intervals (CIs). Poisson regression was also conducted for all NTDs combined in each geographical region to estimate PRs and the corresponding 95% CIs for FA users vs. nonusers. All statistical analyses were performed using the SAS software (version 9.4).
3 |. RESULTS
3.1 |. NTD-affected pregnancies count by NTD type and FA use
The PHC dataset from 1993 to 1996 has 634,380 known pregnancy outcomes—about 99% were live births and the remainder were fetal deaths. After removing 9 births with multiple NTDs and 8,329 nonsingleton births including 16 NTD-affected pregnancies, 700 NTD-affected pregnancies were reported among the remaining 626,042 (Figure 2). Among the NTD-affected pregnancies, 34% were live births, 64% were fetal deaths, and 2% had unknown birth status. As shown in Figure 2, in addition to women in the public health campaign, we included an additional 379,660 FA nonusers, including 440 NTD-affected pregnancies. In the north, there were 91,856 pregnancies with NTD status confirmation—10,186 with complete FA exposure, 73,813 with no FA exposure, and the remainder with incomplete FA exposure. In the south, there were 534,186 pregnancies—50,109 with complete FA exposure and 433,595 with no FA exposure. Among public health campaign participants who took FA during the entire 42 days after LMP, compliance was high, with a mean of 94%, a median of 98% and the fifth percentile of 72.5%.
FIGURE 2.
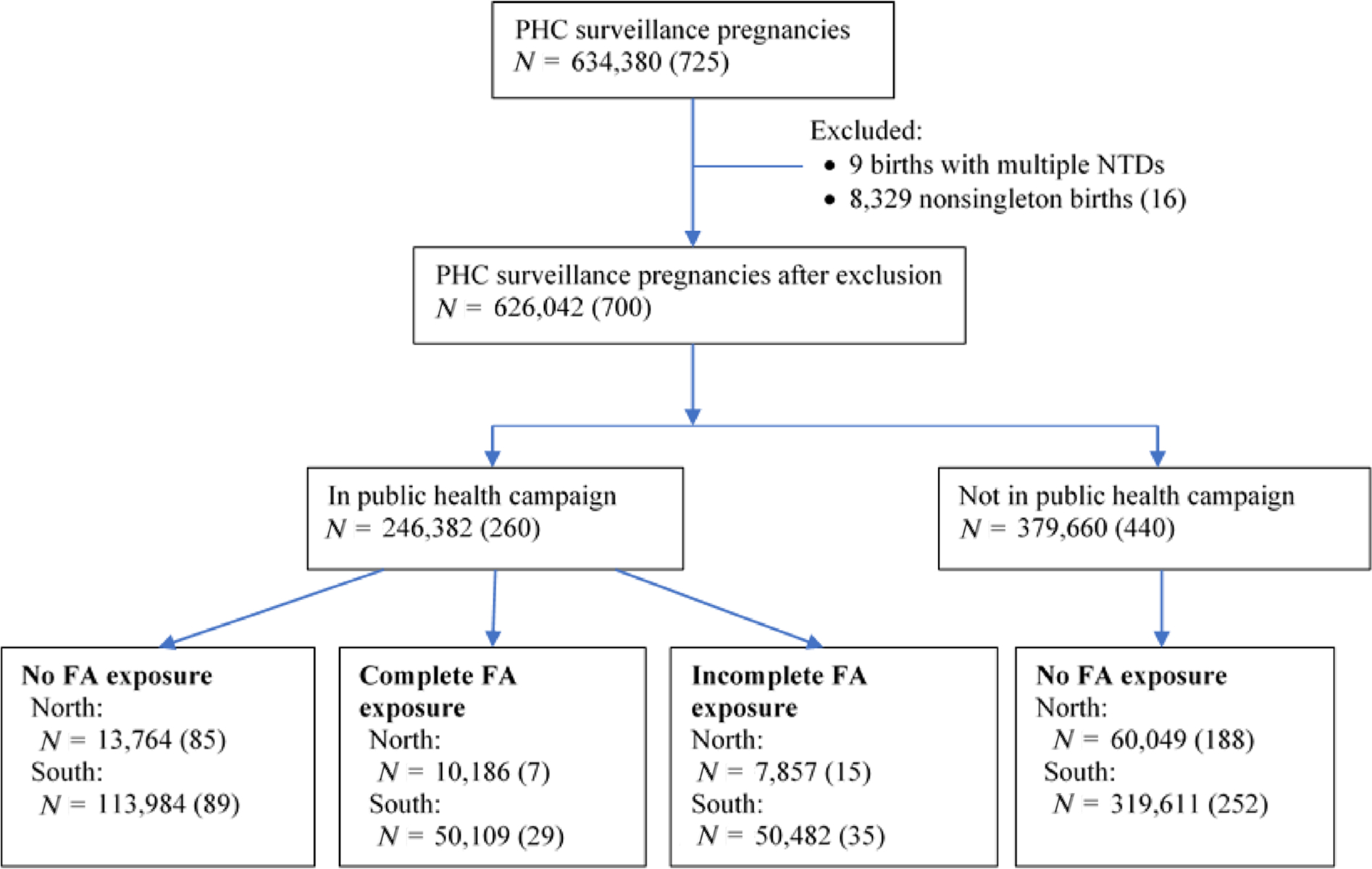
Study population flow chart and folic acid (FA) exposure group in north and south China, 1993–1996. “PHC” stands for “perinatal health care”; “N” refers to number of pregnancies with known birth outcomes which could be confirmed as either having or not having an NTD. Number in parenthesis is the count of NTD-affected pregnancies. Complete FA exposure group includes women who took 400 μg of folic acid daily between last menstrual period (LMP) and 42 days after LMP with a compliance rate of at least 80%. No FA exposure group includes women who took no folic acid before LMP and during the 60 days after LMP. Incomplete FA exposure group includes all the remaining women, who, for example, started earlier but stopped taking folic acid either before LMP or during the 42-day exposure window, started taking folic acid during the 42-day exposure window, or took folic acid during the 42-day exposure window but with a compliance rate below 80%. Women with incomplete exposure were excluded from further analysis
Table 1 shows the number of NTD-affected pregnancies by NTD type, FA use status, and geographic region, among women with either complete FA exposure or no FA exposure. There were 280 NTD-affected pregnancies in the north and 370 in the south. In the north, among women who did not take FA, spina bifida represented 48% (132/273) of total NTD-affected pregnancies. The spina bifida level of lesion was classified as high in 69 NTD-affected pregnancies and low in 59. An additional 21% of infants or fetuses with NTDs had craniorachischisis, followed by anencephaly at 17%. Among FA nonusers, there were 65 NTD-affected pregnancies of the two rare NTD types, that is, craniorachischisis, iniencephaly. Among women who took FA, there were 7 NTD-affected pregnancies; there were no pregnancies affected by craniorachischisis or iniencephaly.
TABLE 1.
Count of NTD-affected pregnancies, neural tube defects (NTD) prevalence per 10,000 pregnancies and percent reduction by NTD type, folic acid (FA) use status, and geographical region in north and south of China, 1993–1996
| North |
South |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No FA exposure |
Complete FA exposure |
No FA exposure |
Complete FA exposure |
|||||||||||
| (N =73,813) |
(N = 10,186) |
(N = 433,595) |
(N = 50,109) |
|||||||||||
| NTD type | Count |
Prevalence per 10,000 pregnancies | Count |
Prevalence per 10,000 pregnancies | % prevalence reduction with FA use | Count |
Prevalence per 10,000 pregnancies | Count |
Prevalence per 10,000 pregnancies | % prevalence reduction with FA use | ||||
| N % | N % | N % | N % | |||||||||||
| All NTDs | 273 | 100 | 37.0 | 7 | 100 | 6.9 | 81 | 341 | 100 | 7.9 | 29 | 100 | 5.8 | 26 |
| Anencephaly | 47 | 17 | 6.4 | 1 | 14 | 1.0 | 85 | 127 | 37 | 2.9 | 15 | 52 | 3.0 | −2 |
| Craniorachischisis | 57 | 21 | 7.7 | 0 | 0 | 0.0 | 100 | 44 | 13 | 1.0 | 0 | 0 | 0.0 | 100 |
| Iniencephaly | 8 | 3 | 1.1 | 0 | 0 | 0.0 | 100 | 7 | 2 | 0.2 | 0 | 0 | 0.0 | 100 |
| Encephalocele | 29 | 11 | 3.9 | 2 | 29 | 2.0 | 50 | 58 | 17 | 1.3 | 8 | 28 | 1.6 | −19 |
| Total spina bifida | 132 | 48 | 17.9 | 4 | 57 | 3.9 | 78 | 105 | 31 | 2.4 | 6 | 21 | 1.2 | 51 |
| High lesion level | 69 | 25 | 9.4 | 2 | 29 | 2.0 | 79 | 36 | 11 | 0.8 | 1 | 3 | 0.2 | 76 |
| Low lesion level | 59 | 22 | 8.0 | 1 | 14 | 1.0 | 88 | 67 | 20 | 1.5 | 5 | 17 | 1.0 | 35 |
| Closed NTDs | 65 | 24 | 8.8 | 3 | 43 | 2.9 | 67 | 88 | 26 | 2.0 | 10 | 34 | 2.0 | 2 |
| Open NTDs | 208 | 76 | 28.2 | 4 | 57 | 3.9 | 86 | 253 | 74 | 5.8 | 19 | 66 | 3.8 | 35 |
Note: The prevalence of neural tube defect (NTD) was calculated as the number of NTD-affected pregnancies divided by the total number of pregnancies with known birth outcomes, which could be confirmed as either having or not having an NTD. For spina bifida in the north, there were 4 and 1 NTD-affected pregnancies of unknown lesion level among folic acid nonusers and users, respectively. For spina bifida in the south, there were 2 NTD-affected pregnancies of unknown lesion level among folic acid nonusers. Women with incomplete exposures to folic acid were excluded from the table.
In the south, among women who did not take FA, anencephaly was the most prevalent, representing 37% (127/341) of total NTD-affected pregnancies, followed by spina bifida (31%) in 105 pregnancies (36 with high and 67 with low lesion levels). There were 51 NTD-affected pregnancies with one of two rare NTD types. Among women who took FA, there were 29 NTD-affected pregnancies. As in the north, there was no occurrence of the two rare NTD types among mothers who took FA.
Among subtypes of spina bifida in the north, high lesion level accounted for about 50% of all infants and fetuses with spina bifida among FA users (2 of 4) and nonusers (69 of 132). In the south, percentage of infants and fetuses with high lesion level decreased from 34% (36 of 105) among FA nonusers to 17% (1 of 6) among FA users. Among subtypes of anencephaly in the south, the percentage of a less extensive form of anencephaly (meroanencephaly) increased among FA users (73% vs. 52% among FA nonusers); the percentage of holoanencephaly, the more extensive form of anencephaly, was lower among FA users than nonusers (20% vs. 30%). In the north, there was only one anencephaly among FA users, and it was of a nonspecified type.
Table S2 shows the number of NTD-affected pregnancies with open and closed NTD phenotypes. When both geographic regions were combined, open NTDs accounted for 74% of all NTDs. Encephalocele and closed spina bifida made up 92% of closed NTDs. Anencephaly, craniorachischisis, and open spina bifida made up 99% of the open NTDs.
3.2 |. Prevalence and prevalence difference by NTD type
Figure 3 and Table 1 show NTD prevalence by NTD type, FA use status, and geographic region. Note that the denominator used for reporting NTD prevalence in this study is per 10,000 pregnancies with known birth outcomes, which could be confirmed as either having or not having an NTD. In the north, the prevalence of all NTDs decreased from 37 per 10,000 pregnancies among FA nonusers to 6.9 per 10,000 among FA users (81% reduction). Among the different NTD types, spina bifida had the highest prevalence of 17.9 per 10,000 among FA nonusers. Among FA users, the prevalence of spina bifida was 3.9 per 10,000, a 78% reduction compared to FA nonusers. The two rare NTD types had 100% reduction in prevalence with FA use, followed by anencephaly with 85% reduction.
FIGURE 3.
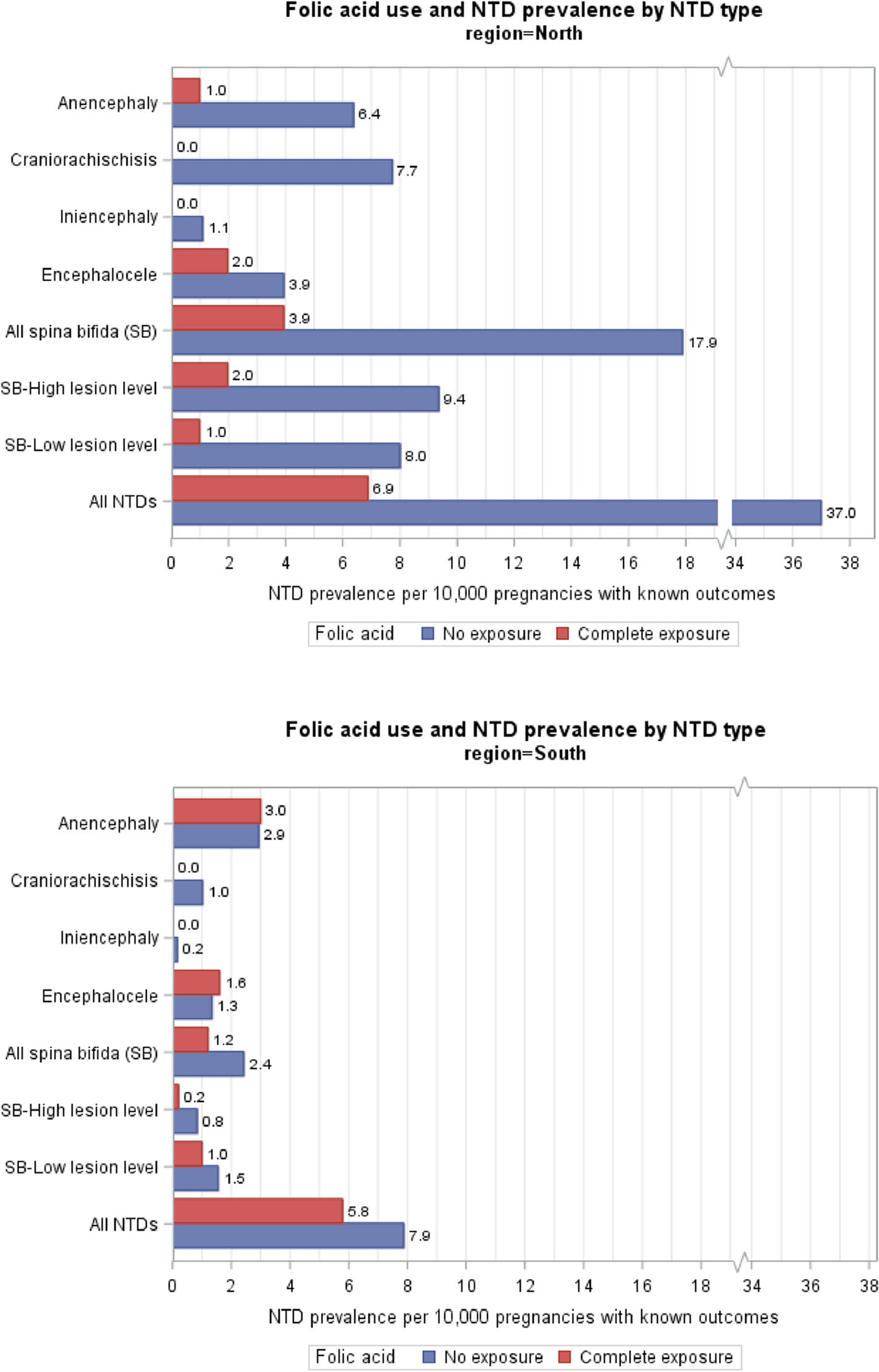
Folic acid use and neural tube defects (NTDs) prevalence by NTD type in north and south of China, 1993–1996
In the south, the prevalence of all NTDs decreased from 7.9 per 10,000 among FA nonusers to 5.8 per 10,000 among users (26% reduction). Anencephaly had the highest prevalence of 2.9 per 10,000 among FA nonusers. It remained similar for FA users. Spina bifida had the second highest prevalence of 2.4 per 10,000 among nonusers. Among FA users, the prevalence of spina bifida was 1.2 per 10,000, a 51% reduction compared to with FA nonusers.
Figure 4 shows the prevalence difference between FA users and nonusers and the corresponding CIs by NTD type. In the north, the prevalence difference of each NTD type was below zero. Except for encephalocele there was a statistically significant reduction in prevalence among FA users. In the south, prevalence difference for all spina bifida, spina bifida with a high lesion level, and open NTDs were less than zero and statistically significant, also indicating a substantial reduction of prevalence among FA users. In both geographic regions, there was a greater reduction in the prevalence of open NTDs than closed NTDs with FA use.
FIGURE 4.
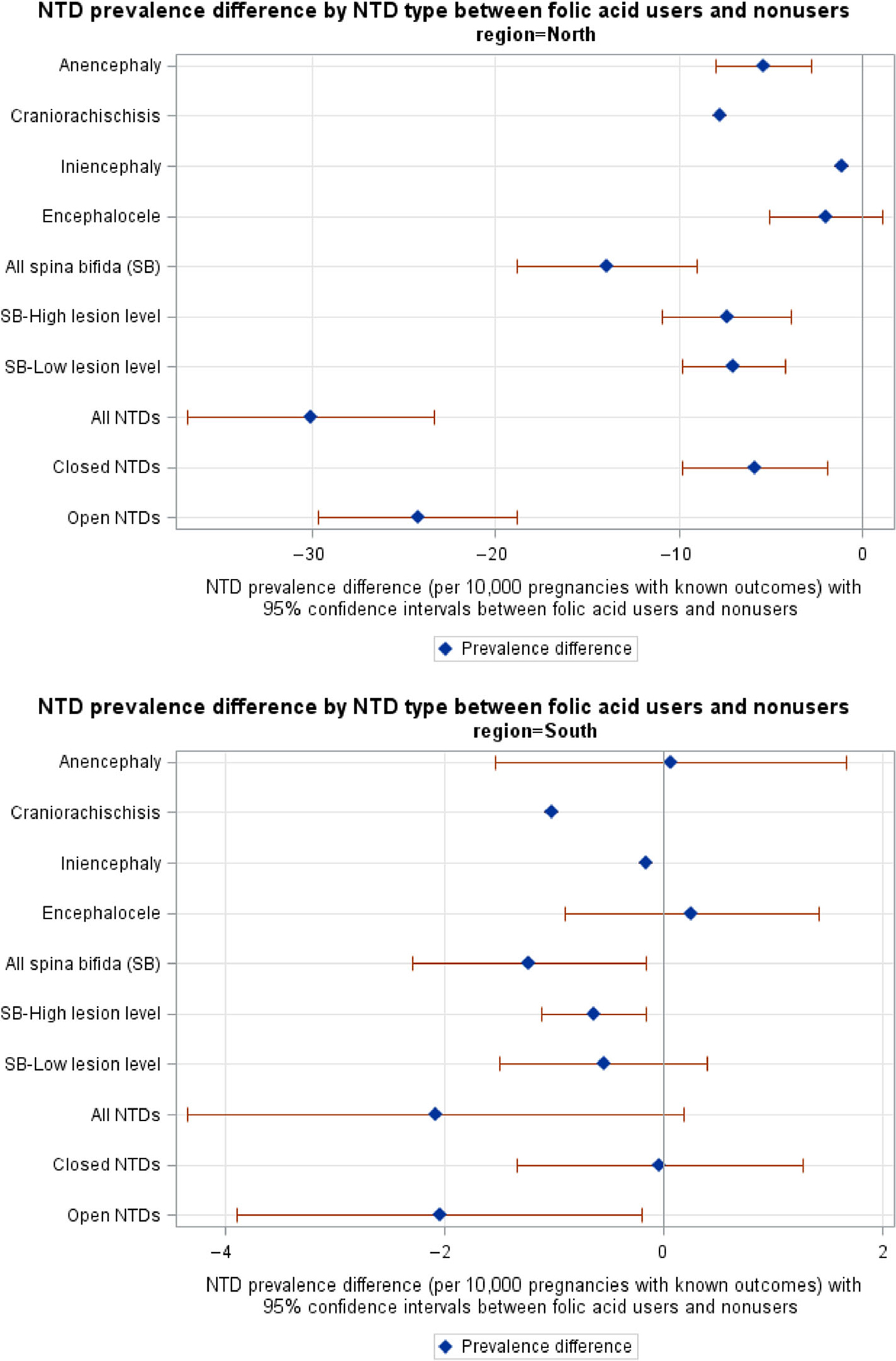
Prevalence difference between folic acid users and nonusers and the corresponding 95% confidence intervals by neural tube defects (NTDs) type in north and south of China, 1993–1996
Poisson regression showed that FA use significantly reduced the prevalence of all NTDs in the north with PR of 0.19 (95% CI: 0.09, 0.39). In the south, the PR was 0.74 (95% CI: 0.50, 1.08). The ratio of PRs in the north versus the south was 0.25 (95% CI: 0.11, 0.58), indicating FA had a greater influence in lowering NTD prevalence in the north with higher baseline NTD prevalence than the south.
3.3 |. Sex difference
Table 2 shows the count of NTD-affected pregnancies and prevalence by sex, geographic region, and FA use status, as well as the male-to-female PRs by NTD type and the corresponding 95% CIs. Male infants and fetuses accounted for about 40% of all NTD-affected pregnancies in both the north and the south. Among FA nonusers, the male-to-female PRs were 0.54 in the north and 0.65 in the south, and both 95% CIs were below 1, showing that there was a statistically significant female excess among NTD-affected pregnancies. For example, for craniorachischisis, the male-to-female ratios were 0.41 (13/29) in the south and 0.26 (13/44) in the north, and both 95% CIs were below 1. Among FA users in the north, the number of NTD-affected pregnancies were small, that is, four females and four males. As a result, the male-to-female PR reported in Table 2 may not be stable.
TABLE 2.
Count of neural tube defect (NTD)-affected pregnancies and NTDs prevalence by sex, geographical region and folic acid (FA) use status, and male to female prevalence ratio by NTD type in north and south China, 1993–1996
| NTD type | Count | Prevalence per 10,000 pregnancies | Count | Prevalence per 10,000 pregnancies | Male to female prevalence ratio and 95% confidence interval |
|---|---|---|---|---|---|
| North: FA nonusers | Male (N = 38,921) | Female (N = 34,466) | |||
| Anencephaly | 16 | 4.11 | 29 | 8.41 | 0.49 (0.27, 0.90) |
| Craniorachischisis | 13 | 3.34 | 44 | 12.77 | 0.26 (0.14, 0.49) |
| Iniencephaly | 1 | 0.26 | 7 | 2.03 | 0.13 (0.02, 1.03) |
| Encephalocele | 14 | 3.60 | 14 | 4.06 | 0.89 (0.42, 1.86) |
| Spina bifida | 58 | 14.90 | 72 | 20.89 | 0.71 (0.50, 1.01) |
| All NTDs | 102 | 26.21 | 166 | 48.16 | 0.54 (0.43, 0.70) |
| North: FA users | Male (N = 5,343) | Female (N = 4,804) | |||
| Anencephaly | 1 | 1.87 | 0 | 0.00 | — |
| Craniorachischisis | 0 | 0.00 | 0 | 0.00 | — |
| Iniencephaly | 0 | 0.00 | 0 | 0.00 | — |
| Encephalocele | 1 | 1.87 | 1 | 2.08 | 0.90 (0.06, 14.37) |
| Spina bifida | 3 | 5.61 | 1 | 2.08 | 2.70 (0.28, 25.93) |
| All NTDs | 5 | 9.36 | 2 | 4.16 | 2.25 (0.44, 11.59) |
| South: FA nonusers | Male (N = 226,841) | Female (N = 205,316) | |||
| Anencephaly | 64 | 2.82 | 55 | 2.68 | 1.05 (0.73, 1.51) |
| Craniorachischisis | 13 | 0.57 | 29 | 1.41 | 0.41 (0.21, 0.78) |
| Iniencephaly | 1 | 0.04 | 6 | 0.29 | 0.15 (0.02 1.25) |
| Encephalocele | 27 | 1.19 | 31 | 1.51 | 0.79 (0.47, 1.32) |
| Spina bifida | 33 | 1.45 | 70 | 3.41 | 0.43 (0.28, 0.65) |
| All NTDs | 138 | 6.08 | 191 | 9.30 | 0.65 (0.53, 0.81) |
| South: FA users | Male (N = 25,590) | Female (N = 24,376) | |||
| Anencephaly | 7 | 2.74 | 7 | 2.87 | 0.95 (0.33, 2.72) |
| Craniorachischisis | 0 | 0.00 | 0 | 0.00 | — |
| Iniencephaly | 0 | 0.00 | 0 | 0.00 | — |
| Encephalocele | 5 | 1.95 | 3 | 1.23 | 1.59 (0.38, 6.64) |
| Spina bifida | 1 | 0.39 | 5 | 2.05 | 0.19 (0.02, 1.63) |
| All NTDs | 13 | 5.08 | 15 | 6.15 | 0.83 (0.39, 1.74) |
Note: The prevalence for each type of NTD was calculated as the number of NTD-affected pregnancies divided by the total number of pregnancies with known birth outcomes which could be confirmed as either having or not having an NTD. Two thousand twenty-nine (0.3%) infants and fetuses with unknown sex and 170 (0.03%) with ambiguous sex were excluded from the analysis for sex difference. Women with incomplete exposure to folic acid were excluded from the table.
4 |. DISCUSSION
Our study is the first study to examine the effectiveness of FA supplementation for rare types of NTDs, that is, craniorachischisis, iniencephaly. FA use was most effective at preventing the rare NTD types. FA use significantly reduced the prevalence of spina bifida, including spina bifida with high lesion level in both geographic areas, though FA use had variable effects on reducing the risks of other more common NTD types. Comparing with FA nonusers, the female excess in NTD prevalence decreased among FA users in both geographic regions.
Our results are consistent with previous findings. Multiple factors have been associated with NTDs, and it is widely accepted that ≤5 per 10,000 live births is the target population rate representing optimal prevention with FA fortification and supplementation (Crider, Bailey, et al., 2011). Berry and colleagues found that among women in China with periconceptional use of FA with more than 80% compliance, the prevalence of NTDs was 7 and 6 per 10,000 pregnancies in the north and south, respectively (Berry et al., 1999) which are in agreement with results from this current study. Note that Berry and colleagues reported findings based on data from the public health campaign, a subset of the PHC surveillance data used in our study.
Craniorachischisis and iniencephaly are rare in countries with low NTD prevalence. Similar to findings from northern China, EUROCAT data documented an increased birth prevalence of craniorachischisis and iniencephaly in higher NTD prevalence areas of the United Kingdom and Ireland for the period 1980–1987 (Dolk et al., 1991); however, there are no previous reports of the impact of FA on these NTDs. In the current study, the count for these NTDs dropped to zero among FA users in both geographic areas. The ranges of prevalence reduction varied for the more common NTD types. Our study shows that the prevalence reduction of spina bifida ranged from 51% to 78% in the two geographic regions with FA use. The prevalence reductions of anencephaly and encephalocele seen with FA use were 85% and 50%, respectively in the north, though their prevalence in the south were similar between FA users and nonusers.
Our findings could provide valuable information on possible etiologic and pathogenetic mechanisms of NTDs. The total prevention of craniorachischisis and iniencephaly in fetuses and infants of women with complete FA exposure was somewhat surprising given that these two NTDs are thought to be dissimilar in pathogenesis. Iniencephaly has been included among NTDs in part due to many cases having accompanying spina bifida or encephalocele but this defect has also been postulated to originate from a split notochord (Maertens, Blackburn, & Snow, 1994). The findings indicate that these two defects likely have some pathogenetic similarities related to FA. In contrast, two NTDs that are thought to be highly related and often combined for study, anencephaly and craniorachischisis, showed dissimilar outcomes related to FA especially in the low prevalence southern region.
When NTDs were grouped into open or closed NTDs, there was a greater reduction seen with FA use in open NTDs than closed NTDs in both geographic regions of China. We also found that in the south the percentage of a less extensive form of anencephaly (meroanencephaly) increased among FA users. This suggests that while the prevalence of anencephaly among FA users in the south did not decrease compared to FA nonusers, the infants, and fetuses with anencephaly had less extensive lesions among FA users. The impact of spina bifida is determined by the size and location of the malformation with higher level lesions presenting with greater amounts of nerve damage leading to loss of muscle function and sensation. Among subtypes of spina bifida in the south, the percentage of high lesion level in infants and fetuses decreased from 34% to 17%, indicating decreased spina bifida severity with FA use.
Among FA nonusers, there was a female excess in NTD infants and fetuses consistent with other studies (Liu, Xie, et al., 2018; Poletta et al., 2018). Previous hypotheses to explain this finding include better survival among female fetuses (Källén et al., 1994) and a requirement for additional methyl groups from folates to support X chromosome inactivation and prevention of cellular deficiencies that might increase NTD risk (Juriloff & Harris, 2012). Among FA users in both geographic regions in this study, there was a greater reduction in the prevalence of NTDs in female than in male infants and fetuses. A study in Chile and Argentina had similar finding after FA fortification (Poletta et al., 2018).
4.1 |. Strengths
Our study has several strengths. We used a population-based dataset that included over 560,000 pregnancies with informative birth outcomes making it possible to study the effect of FA by NTD type. Our study is the first to be able to examine the effectiveness of FA supplementation for rarer types of NTDs. The prospective collection of FA monthly use, including start and stop dates, allowed us to accurately estimate each woman’s FA exposure as the public health campaign was the only source for FA during the study period. Finally, previous studies showed that prenatal diagnosis made a substantial impact on the prevalence of NTDs (Alembik, Dott, Roth, & Stoll, 1997; Kondo et al., 2019; Velie & Shaw, 1996). For example, in Atlanta, 32% of NTD-affected pregnancies were detected prenatally and terminated before 20 weeks’ gestation (Roberts, Moore, Cragan, Fernhoff, & Khoury, 1995). The data for our study were collected prior to extensive use of prenatal screening in these regions. As a result, reduction in NTD prevalence found in our study likely was not due to selective termination of pregnancies. It is unlikely that this unique collection of strengths could be replicated in future studies.
4.2 |. Limitations
Despite a large study population, the number of NTD-affected pregnancies was low, particularly among FA users. As a result, some prevalence estimates might have high uncertainty associated with them. For spina bifida, there could be some misclassification on lesion level as level was assigned solely by visual inspection of photographs. In addition, women were not randomly selected to take or not to take FA pills. Compared with women who took FA, those who did not were slightly older and were more likely to have had a previous pregnancy (Berry et al., 1999). An earlier study showed that a women’s age, number of previous pregnancies and socioeconomic status (i.e., education and occupation) did not change findings on the effectiveness of FA supplementation in reducing NTD prevalence (Berry et al., 1999). Finally, we do not have reliable data on additional risk factors for NTDs, such as previous pregnancies with NTDs, pregestational diabetes, or body mass index (BMI). However, in a subpopulation of this cohort there was no difference in the BMI between FA users and nonusers (Berry et al., 1999).
5 |. CONCLUSION
This is the first study to show that FA prevents the entire spectrum of NTD types, and the substantial reduction in prevalence of both rare and common NTDs expands our knowledge of the significant public health benefits of periconceptional FA use. The magnitude of the prevalence reduction with FA use was greatest and statistically significant for the rare forms of NTDs, as well as spina bifida, including spina bifida with high lesion level, in both geographic regions of China. In addition, there was a greater reduction in the prevalence of NTDs in female than in male infants and fetuses in both geographic regions.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the thousands of health workers and village doctors whose dedication and effort made the China–U.S. Collaborative Project for Neural Tube Defect Prevention project possible.
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Alembik Y, Dott B, Roth MP, & Stoll C (1997). Prevalence of neural tube defects in northeastern France, 1979–1994. Impact of prenatal diagnosis. Annales de Génétique, 40(2), 69–71 https://www.scopus.com/inward/record.uri?eid=2-s2.0-0030796621&partnerID=40&md5=85767da996e5f0bf408707a95d3c56e7 [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, … Correa A (1999). Prevention of neural-tube defects with folic acid in China. New England Journal of Medicine, 341(20), 1485–1490. 10.1056/NEJM199911113412001 [DOI] [PubMed] [Google Scholar]
- Blencowe H, Kancherla V, Moorthie S, Darlison MW, & Modell B (2018). Estimates of global and regional prevalence of neural tube defects for 2015: A systematic analysis. Annals of the New York Academy of Sciences, 1414(1), 31–46. 10.1111/nyas.13548 [DOI] [PubMed] [Google Scholar]
- Bower C, D’Antoine H, & Stanley FJ (2009). Neural tube defects in Australia: Trends in encephaloceles and other neural tube defects before and after promotion of folic acid supplementation and voluntary food fortification. Birth Defects Research. Part A: Clinical and Molecular Teratology, 85(4), 269–273. 10.1002/bdra.20536 [DOI] [PubMed] [Google Scholar]
- CDC. (1992). Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR: Recommendations and Reports, 41(Rr-14), 1–7. [PubMed] [Google Scholar]
- Copp AJ, & Greene ND (2013). Neural tube defects—Disorders of neurulation and related embryonic processes. Wiley Interdisciplinary Reviews: Developmental Biology, 2(2), 213–227. 10.1002/wdev.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Villasenor A, Cragan J, Kucik J, O’Leary L, Siffel C, & Williams L (2003). The Metropolitan Atlanta Congenital Defects Program: 35 years of birth defects surveillance at the Centers for Disease Control and Prevention. Birth Defects Research. Part A: Clinical and Molecular Teratology, 67(9), 617–624. 10.1002/bdra.10111 [DOI] [PubMed] [Google Scholar]
- Crider KS, Bailey LB, & Berry RJ (2011). Folic acid food fortification—Its history, effect, concerns, and future directions. Nutrients, 3(3), 370–384. 10.3390/nu3030370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider KS, Qi YP, Devine O, Tinker SC, & Berry RJ (2018). Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: Have we reached optimal prevention? The American Journal of Clinical Nutrition, 107(6), 1027–1034. 10.1093/ajcn/nqy065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, Gindler J, … Berry RJ (2011). MTHFR 677C->T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. American Journal of Clinical Nutrition, 93(6), 1365–1372. 10.3945/ajcn.110.004671 [DOI] [PubMed] [Google Scholar]
- Czeizel AE, & Dudas I (1992). Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. New England Journal of Medicine, 327(26), 1832–1835. 10.1056/nejm199212243272602 [DOI] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, Lowry RB, Evans JA, Van den Hof MC, … Niyonsenga T (2008). Spina bifida before and after folic acid fortification in Canada. Birth Defects Research. Part A: Clinical and Molecular Teratology, 82(9), 622–626. 10.1002/bdra.20485 [DOI] [PubMed] [Google Scholar]
- De Wals P, Tairou F, Van Allen MI, Uh SH, Lowry RB, Sibbald B, … Niyonsenga T (2007). Reduction in neural-tube defects after folic acid fortification in Canada. New England Journal of Medicine, 357(2), 135–142. 10.1056/NEJMoa067103 [DOI] [PubMed] [Google Scholar]
- Dolk H, de Wals P, Gillerot Y, Lechat MF, Ayme S, Cornel M, … Kate LT (1991). Heterogeneity of neural tube defects in Europe: The significance of site of defect and presence of other major anomalies in relation to geographic differences in prevalence. Teratology, 44(5), 547–559. 10.1002/tera.1420440508 [DOI] [PubMed] [Google Scholar]
- Eldridge C, Bandlamuri S, Andrews JG, Galindo MK, Contreras D, Flood TJ, & Rice S (2018). Postfolate spina bifida lesion level change. Birth Defects Research, 110(11), 949–955. 10.1002/bdr2.1221 [DOI] [PubMed] [Google Scholar]
- Hao L, Ma J, Stampfer MJ, Ren A, Tian Y, Tang Y, … Li Z (2003). Geographical, seasonal and gender differences in folate status among Chinese adults. Journal of Nutrition, 133(11), 3630–3635. 10.1093/jn/133.11.3630 [DOI] [PubMed] [Google Scholar]
- Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, & Wong LYC (2001). Impact of folic acid fortification of the US food supply on the occurrence of neural tube defects. Journal of the American Medical Association, 285(23), 2981–2986. 10.1001/jama.285.23.2981 [DOI] [PubMed] [Google Scholar]
- Juriloff DM, & Harris MJ (2012). Hypothesis: The female excess in cranial neural tube defects reflects an epigenetic drag of the inactivating X chromosome on the molecular mechanisms of neural fold elevation. Birth Defects Research. Part A: Clinical and Molecular Teratology, 94(10), 849–855. 10.1002/bdra.23036 [DOI] [PubMed] [Google Scholar]
- Källén B, Cocchi G, Knudsen LB, Castilla EE, Robert E, Daltveit AK, … Mastroiacovo P (1994). International study of sex ratio and twinning of neural tube defects. Teratology, 50(5), 322–331. 10.1002/tera.1420500503 [DOI] [PubMed] [Google Scholar]
- Kondo A, Akada S, Akiyama K, Arakawa M, Ichi S, Inamoto Y, … Yokomine M (2019). Real prevalence of neural tube defects in Japan: How many of such pregnancies have been terminated? Congenital Anomalies, 59(4), 118–124. 10.1111/cga.12333 [DOI] [PubMed] [Google Scholar]
- Li S, Moore C, Li Z, Berry R, Gindler J, Hong S, … Erickson J (2003). A population-based birth defects surveillance system in the People’s Republic of China. Paediatric and Perinatal Epidemiology, 17(3), 287–293. [DOI] [PubMed] [Google Scholar]
- Liu J, Li Z, Ye R, Liu J, & Ren A (2018). Periconceptional folic acid supplementation and sex difference in prevention of neural tube defects and their subtypes in China: Results from a large prospective cohort study. Nutrition Journal, 17(1), 115. 10.1186/s12937-018-0421-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xie J, Li Z, Greene NDE, & Ren A (2018). Sex differences in the prevalence of neural tube defects and preventive effects of folic acid (FA) supplementation among five counties in northern China: Results from a population-based birth defect surveillance programme. BMJ Open, 8(11), e022565. 10.1136/bmjopen-2018-022565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens P, Blackburn W, & Snow R (1994). Iniencephaly: A rostral split notochord syndrome. Pediatric Neurology, 11(2), 136–137. [Google Scholar]
- Mai CT, Isenburg JL, Canfield MA, Meyer RE, Correa A, Alverson CJ, … Kirby RS (2019). National population-based estimates for major birth defects, 2010–2014. Birth Defects Research, 111(18), 1420–1435. 10.1002/bdr2.1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA (2006). Classification of neural tube defects. In Wyszynski DF(Ed.), Neural tube defects. From origin to treatment (pp. 66–75). Oxford: Oxford University Press. [Google Scholar]
- Moore CA, Li S, Li Z, Hong SX, Gu HQ, Berry RJ, … Erickson JD (1997). Elevated rates of severe neural tube defects in a high-prevalence area in northern China. American Journal of Medical Genetics, 73(2), 113–118. [DOI] [PubMed] [Google Scholar]
- MRC. (1991). Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. The Lancet, 338(8760), 131–137. 10.1016/0140-6736(91)90133-A [DOI] [PubMed] [Google Scholar]
- Poletta FA, Rittler M, Saleme C, Campana H, Gili JA, Pawluk MS, … Lopez-Camelo JS (2018). Neural tube defects: Sex ratio changes after fortification with folic acid. PLoS One, 13(3), e0193127. 10.1371/journal.pone.0193127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts HE, Moore CA, Cragan JD, Fernhoff PM, & Khoury MJ (1995). Impact of prenatal diagnosis on the birth prevalence of neural tube defects, Atlanta, 19901991. Pediatrics, 96(5 I), 880–883 https://www.scopus.com/inward/record.uri?eid=2-s2.0-0028832419&partnerID=40&md5=b0f10d0d081850425332211a82a22a13 [PubMed] [Google Scholar]
- U.S. Department of Human and Health Services (2018). The HHS regulations for the protection of human subjects in research. 45 C.F.R. part 46.102(l)(2). [Google Scholar]
- Velie EM, & Shaw GM (1996). Impact of prenatal diagnosis and elective termination on prevalence and risk estimates of neural tube defects in California, 1989–1991. American Journal of Epidemiology, 144(5), 473–479. 10.1093/oxfordjournals.aje.a008953 [DOI] [PubMed] [Google Scholar]
- Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, … Kirby RS (2015). Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. Morbidity and Mortality Weekly Report, 64(1), 1–5 https://www.scopus.com/inward/record.uri?eid=2-s2.0-84922447495&partnerID=40&md5=180a7265e82b79295f3a387a96b024f4 [PMC free article] [PubMed] [Google Scholar]
- Yan L, Zhao L, Long Y, Zou P, Ji G, Gu A, & Zhao P (2012). Association of the maternal MTHFR C677T polymorphism with susceptibility to neural tube defects in offsprings: Evidence from 25 case-control studies. PLoS One, 7(10), e41689. 10.1371/journal.pone.0041689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, … Rosenthal J (2016). Describing the prevalence of neural tube defects worldwide: A systematic literature review. PLoS One, 11(4), e0151586. 10.1371/journal.pone.0151586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Lou J, Zhong R, Wu J, Zou L, Sun Y, … Xiong G (2013). Genetic variants in the folate pathway and the risk of neural tube defects: A meta-analysis of the published literature. PLoS One, 8(4), e59570. 10.1371/journal.pone.0059570 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


